-
随着全球工业化的迅速发展,越来越多的有机污染物被排放到我们赖以生存的水环境中,这不仅严重威胁着水生态环境,还对人体健康带来危害。随着国家对环保要求的不断提高以及相关政策的不断出台,采用传统的方法处理难降解有机废水在经济和技术上都难以达标,因此,迫切需要一种绿色高效的方法来去除水中的有机污染物。以产生活性自由基(例如SO4·−和·OH)高效分解污染物的高级氧化技术(advanced oxidation processes,AOPs)[1-2]被认为是处理这类生化性较差的有机污染物的有效手段[3]。根据提供的能量和反应种类的不同,AOPs技术可分为光催化、电催化、声解、臭氧化、Fenton/类Fenton反应和硫酸盐基AOPs (SR-AOPs)等[4-5]。不同的AOPs技术各有优点和缺点,在实际运用中,通常需要根据不同的情况将不同工艺进行组合来提高有机污染物降解率和矿化率[6]。尽管一些AOPs技术(例如,UV/H2O2体系和UV/过一硫酸盐(PMS)体系)在没有催化剂的情况下也能正常工作,但如果添加催化剂,就可以显著减少能源和试剂(活性物质来源)的消耗[7]。因此,设计一种有效且稳定的催化剂是AOPs绿色可持续发展的关键策略。
近年来,石墨氮化碳(g-C3N4)备受科学家们的关注。g-C3N4作为一种非金属半导体光催化物具有诸多优点:较宽的带隙宽度、丰富的活性位点、化学稳定性高和合成原材料成本低廉[8-10]。2009年,Wang等[11]首次将g-C3N4作为非金属半导体应用于光催化分解水制H2,并呈现出良好的光催化活性。从此,掀起了g-C3N4应用于催化领域的研究热潮,尤其是在催化降解水中有机污染物方面。然而,其可见光利用效率低、比表面积小以及光生的电子-空穴对快速重组等缺点限制了其催化活性[12-13]。目前,研究人员已开发出多种改性方法提高g-C3N4光催化活性,如形貌调控、元素掺杂、半导体复合等[14]。其中,元素掺杂被证明是调节g-C3N4独特电子结构和分子结构的有效方法,通过元素掺杂氮化碳可以大幅扩展其光响应范围,并促进光生电荷分离[15]。元素掺杂包括金属元素(如铁(Fe)、铜(Cu)、钴(Co)等)和非金属元素(如氮(N)、氧(O)、硫(S)、磷(P)等)。与金属掺杂相比,非金属元素更易接近和进入到g-C3N4骨架中,实现缺陷调控。经过这十多年的努力,已有大量研究结果表明非金属掺杂g-C3N4在光催化氧化(降解有机污染物)中的应用的可行性[16-17]。由于非金属原子具有较高的电离能和不同的电负性,当非金属原子被引入时,就会从其他物质中获得形成共价键的电子,从而改变掺杂处周围的电子分布。一般来说,由于高电负性的掺杂原子会促使电子从相邻的C原子迁移到掺杂处,原有的化学惯性收到破坏,C原子会具有诱导极化,从而产生新的反应位点。而电负性较低的掺杂原子会增加相邻C原子的不对称自旋密度,从而增强了g-C3N4的给电子能力。然而,仅利用光催化技术难以实现有机污染物的深度矿化,因此利用非金属掺杂g-C3N4的氧化能力活化硫酸盐,产生高活性物种降解有机污染物的研究引起广泛关注[18-19]。在光催化中引入额外的活性物质,如过硫酸盐,可以将可见光光催化技术与基于过硫酸盐的高级氧化工艺(PS-AOP)互补,进而显著提高有机污染物的降解效率和矿化率[20-21]。过硫酸盐包括过一硫酸盐(PMS, HSO5−)和过二硫酸盐(PDS, S2O82−),在PMS与PDS活化特性相比中,PMS中的O—O键更容易被激活,因此选用PMS作为硫酸根自由基的前躯体更有利于水中有机污染物降解。
鉴于非金属掺杂g-C3N4复合材料在AOPs领域的应用日趋频繁,本文综述了非金属掺杂g-C3N4的制备方法及其在光催化AOPs和硫酸盐基AOPs降解有机污染物的最新研究进展,讨论了近年引起关注的非金属掺杂g-C3N4光催化耦合过硫酸盐降解有机污染物的基本机理和研究进展。最后,提出了非金属掺杂氮化碳复合材料在这些AOPs中所面临的挑战和机遇,以期为非金属石墨氮化碳材料日后在光催化协同过硫酸盐活化催化降解有机污染物的发展和应用提供有益的参考。
-
非金属掺杂石墨氮化碳是一种在g-C3N4基体中引入杂原子或缺陷以调整其电子结构,同时保持其无金属特性的物质。一般来说,非金属掺杂g-C3N4后,非金属元素会代替g-C3N4中的C、N元素。由于非金属元素的电负性与C、N原子不同,它们的引入会使电子在整个网络中发生偏移,导致电子结构的改变,从而显著地调节 g-C3N4的表面特性、光学性质和电子输运能力。非金属掺杂不仅提高了g-C3N4光生载体的氧化还原能力,还在可见光下发挥了显著的光催化活化和无外能的非光化学活化的协同作用[22]。
非金属掺杂g-C3N4的制备方法多种多样,而根据非金属元素与氮化碳结合的方法不同可以分为:水热法和溶剂热法[23-25]、热缩合法[26-27]、热聚合法[28-30]、缩聚法[31-32]等。表1列出了使用不同方法添加各种掺杂剂的相关研究。非金属掺杂元素主要是氮(N)、氧(O)、硫(S)、磷(P)等,上述元素的适当组合可以产生各种非金属掺杂g-C3N4催化剂。掺杂后的催化剂具有良好的光性能,可见光能收集明显增强。
-
水热合成技术是作为制备纳米复合材料的一种重要且有前景的技术。该合成方法具有体系均匀、反应条件温和,流动性好等优点,已被证明是一种高效的低温合成技术。Liu等[33]以三聚氰胺为前躯体,在马弗炉中煅烧得到的g-C3N4进行水热处理,合成了氧掺杂石墨氮化碳(GCNO),结果表明O掺杂可以促进染料敏化。
此外,由水热法发展而来的溶剂热法也被应用于制备非金属掺杂氮化碳,与水热法的不同之处在于其所使用的溶剂为有机溶剂,如甲醇、乙醇等,而不是水或其他无机溶剂。Wei等[34]首次利用溶剂热法将1,3,5-三氯三嗪(CC)溶解在乙腈中,同时加入适量双氰胺(DCNA),将混合物转移到高压釜中,在200 ℃下维持24h,成功制备氧自掺杂的g-C3N4纳米球。结果表明氧掺杂后形成的C—O—C键可调节g-C3N4的本征电子态和能带结构,使其具有良好的光催化性能。然而,相比于水热法,溶剂热法由于溶剂的不同,所合成的材料的物化性质差异大,且存在产品的产率较低等缺点。
-
使用三聚氰胺、尿素、硫脲、双氰胺等前体,通过热缩合方法可在400 ℃至610 ℃的温度范围内合成多孔g-C3N4[26]。热缩合方法具有操作简单,效率高,成本低,结晶度好等优点,但是所需的温度较高。Liang等[35]利用硫脲和三聚氰胺在空气氛围下热缩合制备了一系列硫掺杂石墨氮化碳(g-CNS)材料,掺S后复合材料的光生载流子复合率降低,光催化性能得到明显提升。Wang等[27]首次以琼脂三聚氰胺凝胶(AMG)为前驱体,通过一步热缩合的方法合成了新型碳掺杂介孔g-C3N4超薄纳米片(C/CNNS),由于洋葱状碳(OLC)与超薄二维纳米片结构的协同作用,光催化活性的显著增强。
-
通过在高温下将单体前驱体转化为聚合物的热聚合法是合成金属或非金属掺杂g-C3N4最常用的方法[36]。热聚合方法具有制备过程简单,反应条件易于控制等优点,但是对反应环境和设备要求较高。Zhuo等[37]以尿素为前驱体,加入少量柠檬酸,合成N掺杂石墨碳结合g-C3N4,材料保留了g-C3N4的原始骨架结构,提高了光催化性能。Wang等[38]以适量柠檬酸、尿素与双氰胺为前躯体,通过热聚合法,成功地制备出一种新型的可见光驱动N掺杂碳量子点(NCDs)/g-C3N4复合材料。掺杂后归因于NCDs独特的上转换光致发光性质、光生载流子有效分离以及带隙缩小,NCDs/g-C3N4在可见光照射下,对吲哚美辛(IDM)的光催化降解率显著高于g-C3N4和CDs/g-C3N4。
-
缩聚法是一种逐步递进的聚合合成,基于缩聚法的合成路线和微波辅助缩聚机理较简单,其被认为是一种适合用于制备非金属掺杂氮化碳的方法[31]。缩聚方法具有操作简单、安全,反应条件易于控制等优点。Chai等[39]以六氟磷酸铵(NH4PF6)为磷源,硫氰酸铵(NH4SCN)为g-C3N4前驱体,通过直接热共缩聚法成功制备了磷掺杂石墨氮化碳。结果表明,掺杂后磷原子取代了g-C3N4骨架中的碳原子,使g-C3N4的光捕获能力增强、带隙能量由2.9 eV降低到2.86 eV,有利于提高光催化性能。
-
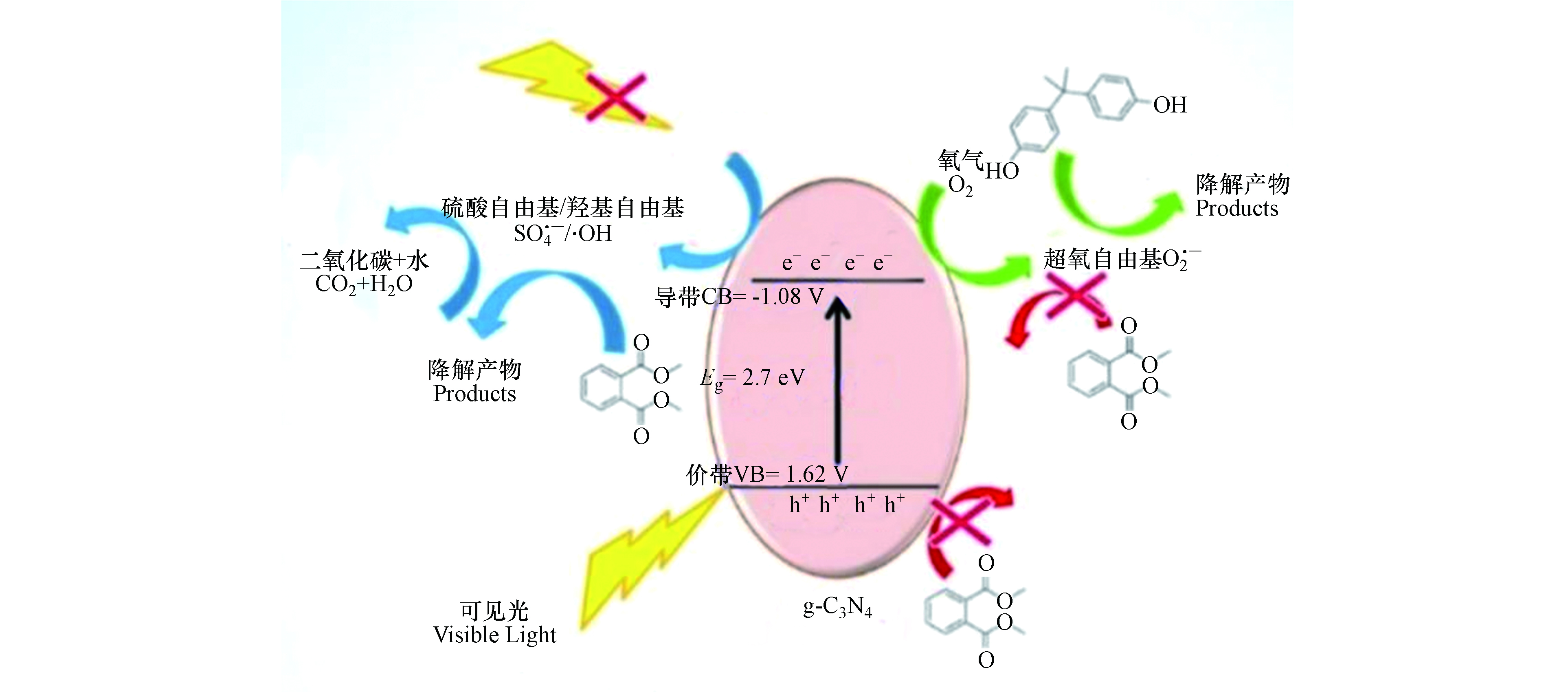
光催化降解作为一种典型的AOPs技术,具有无毒、操作方便、效率高等优点。非金属元素掺杂氮化碳能进一步缩小氮化碳的带隙宽度,增强其光吸收能力。不同元素的掺杂可以促进π-共轭电子的离域,有利于光生电子的迁移和降低电子-空穴的复合率,提高氮化碳材料的光催化性能[44, 48-49]。其中,部分非金属原子掺杂时会取代原氮化碳中的碳或氮原子如图1所示。
当可见光照射能量大于非金属掺杂氮化碳的能隙时,在催化剂上的光生电子(e−)从价带(VB)跃迁到相应的导带(CB),在价带中留下空穴(h+),其中空穴可直接氧化污染物或与H2O/OH−反应生成羟基自由基[50]。当非金属掺杂g-C3N4复合材料的CB比O2/O2·−的氧化还原电位更负时,材料CB中的光生电子则可与O2反应产生具有强氧化能力的O2·−(图2)[51]。由此产生的O2·−随后被质子化生成·OH[52]。最后,有机污染物在这些自由基的作用下被降解为CO2和H2O。具体反应如下
-
已有研究证明石墨氮化碳(g-C3N4)是活化过硫酸盐降解有机污染物的有效催化剂[53]。然而,受制于较差的电子转移能力,纯g-C3N4在活化过硫酸盐降解有机污染物的性能还有待提高[19]。为了进一步促进g-C3N4活化过硫酸盐提高体系电子迁移率,研究者们开始将非金属掺杂到氮化碳以提升其活化PMS的能力。非金属掺杂氮化碳活化PMS主要分为自由基途径和非自由基途径。其中,关于非自由基的过程,有3种主要的反应机制: (i)单线态氧(1O2):PMS自分解产生激发态氧物种1O2(式7)。研究表明非金属掺杂氮化碳可以进一步活化PMS产生1O2,同时,碳材料中的C=O基团是碳材料活化PMS体系产生1O2的关键活性位点[54-56];(ii)电子转移:非金属掺杂氮化碳作为介导载体,污染物将电子转移到PMS中,完成污染物的氧化降解[56];(iii)电荷转移表面活化配合物:PMS在催化剂表面活化成活性分子,有效氧化污染物[57]。而自由基的过程主要是SO4·−、·OH参与催化降解污染物过程。首先,非金属掺杂氮化碳表面的活性位点可传递电子分解PMS,形成SO4·−、·OH和SO5·−(式(8-10))。SO4·−可进一步与H2O反应生成·OH(式(11)),而SO5·−可通过自反应再生成SO4·−(式(12)),最后系列反应所产生的·OH和SO4·−共同作用降解有污染物。非金属掺杂氮化碳活化PMS的具体反应如下[17-19, 57]:
-
近年来,有学者将光催化与过硫酸盐高级氧化技术进行耦合,发现耦合体系降解有机污染物的效率有明显提高[58-59]。虽然PMS很难被可见光单独激活,但是通过加入同时具有可见光响应和PMS活化能力的催化剂(复合材料),可以有效吸收能量激活导带电子活化过硫酸盐。这两个技术的耦合不仅可以减少催化剂和过硫酸盐的投加量,还可以显著提高了可见光的利用效率,增强过硫酸盐活化效果和污染物去除效率。在该系统中,复合材料可以充分利用光催化剂在光照射激发下不断产生的光生电子,从而克服了传统非金属活化剂对PMS活化的电子转移能力有限的缺点[60]。Xu等[61]在可见光下的作用下利用g-C3N4高效活化PMS降解邻苯二甲酸二甲酯(DMP),实验结果显示在可见光照射下,g-C3N4介导的光催化不能直接降解DMP,但在PMS存在下,优势自由基由O2·−转化为氧化能力更强的SO4·−和·OH,导致DMP有效降解和矿化,具体机理如图3所示。
协同降解的机理基本结合了其单独光催化或单独活化PMS降解有机污染物的路径。因此,非金属掺杂g-C3N4复合材料PMS/vis体系中降解有机污染物同样与其单独应用于活化PMS降解有机污染物一样,存在自由基途径与非自由基途径,同时又结合了光催化的作用,材料在可见光的作用下产生了光生电子-空穴的同时,促进了系统内电子的转移速率,两种技术的耦合使得催化体系各种自由基与非自由基路径变得更加丰富。其中自由基途径也可分为有光照辐射辅助和材料自身特性而导致的。在可见光照射下,非金属掺杂g-C3N4在吸收大于禁带宽度的光子能量时,其VB中的e–会被入射光激发,发生e–跃迁。光生e–从g-C3N4的VB激发到CB,并在VB处留下h+,从而产生光生e–/h+对。当非金属掺杂g-C3N4的导带比O2/ O2·−的氧化还原电位更负,则材料CB中的光生电子则可激活吸附的氧分子生成超氧自由基(O2·−)[62],还可以与h+一起氧化还原PMS生成其他活性物质,例如SO4·−、·OH和SO5·−。此外,所生成的SO4·−可以氧化H2O或OH−生成·OH。同时h+也可直接降解部分有机污染物。其中,PMS还可作为电子受体捕获g-C3N4表面生成的电子,促进电子与空穴对的分离,从而协同提高光催化氧化性能和PS活化性能。同时,g-C3N4复合材料自身富电子特性引发的自由基途径,在没有光照辐射时也可发生。材料表面的离域化π电子与含氧官能团(如C=O)等活性位点也可活化PMS分解产生SO4·−进而降解有机污染物。
而非自由基途径包括过一硫酸盐可通过自身的分解产生具有催化活性的单线态氧亦可通过超氧自由基与复合材料受光照后生成光生空穴产生反应而生成,以及表面电子传递途径,即以PMS为电子受体,催化剂为传递介质,有机污染物为电子供体,电子从污染物流经磁性生物炭再传输到PMS上,PMS得到电子被激活,从而降解污染物的过程。
-
由于纯g-C3N4光生载流子的快速复合和缺乏足够的PMS活化反应位点,其对PMS活化的光催化效率还是不太理想[17, 63]。为了克服纯g-C3N4的缺陷,提高其光催化与活化PMS的能力,研究者们探索出了非金属掺杂氮化碳,发现非金属掺杂可有效调整氮化碳电子结构和延长可见光吸收[64]。在非金属掺杂后,g-C3N4表现出更好的光催化性能,在光照下可更高效地活化PMS生成活性自由基(ROS),进而促进有机污染物的降解。
-
近年来,碳纳米材料,特别是碳量子点(CQDs)凭借其独特的电子特性,被广泛应用于半导体领域,改善了半导体的导电性和电子转移行为,有利于在光照射下激活PMS产生ROS。Ming等[65]成功合成了碳量子点(CQDs)修饰的氮化碳(PCNC),将其作为一种有效的光催化剂,在可见光照射下活化PMS降解双酚A。CQDs的引入使得氮化碳(PCN)表面积增大,光吸收增强,电荷分离改善,促进了PMS的光催化活化。其中Vis-PMS/PCNC-2体系氧化过程的一级动力学常数为0.098 min−1,是Vis/PCNC-2(0.035 min−1)和PMS/PCNC-2(0.0021 min−1)之和的2.6倍。CQDs修饰的氮化碳在可见光照射下光催化活化PMS降解BPA的过程中具有较强的协同作用。Cui等[66]通过 g-CN与硼氢化钾(KBH4)的简单煅烧,获得一系列硼掺杂物和表面缺陷共修饰的石墨氮化碳(B-CNU)。电化学测试结果证明,B-CNU比g-CNU具有更好的电化学性能,在可见光的作用下可更有效地激活PMS,在9 min内2, 4-DCP几乎完全去除,TOC的去除率达到51%。ESR实验和对照实验表明,B-CNU/PMS系统中以1O2为优势活性物种,主要来源于PMS的激活。
-
近年来,研究者们发现与单元素掺杂相比,共掺杂的g-C3N4材料可结合单一元素掺杂的优点,并且比单元素掺杂的g-C3N4具有更高的活性。于是开始对二元非金属元素共掺杂g-C3N4进行了研究,将二元非金属元素共掺杂g-C3N4也应用于光催化耦合过硫酸盐降解有机污染物。Shi等[67],通过一步煅烧三聚氰胺-草酸团聚体制备了羰基修饰的g-C3N4(CO-C3N4)。引入PMS后CO-C3N4对盐酸四环素(TCH)的可见光催化降解效率有了显著提高,其表观速率常数由CO-C3N4/vis体系的0.01966 min−1提高到CO-C3N4/PMS/vis体系的0.07688 min−1。催化活性的增强归结于PMS对可见光的吸收更强,使得光生载体的分离更快,这在很大程度上有助于PMS活化为活性自由基。
-
据报道,调节g-C3N4电子能带结构的另一种方法是利用氮或碳空位进行均匀的自修饰,以有利于可见光收集、加速电荷分离和传输。Cao等[68]在NH3氛围下利用三聚氰胺和三硫氰尿酸制备了硫掺杂碳空位g-C3N4多孔纳米片(SCNNs)用于研究光催化固氮。研究表明NH3氛围有效地扩大比表面积,碳空位有效促进了N2分子的吸附和活化。这种优异的光催化性能归功于硫掺杂和碳空位的多孔片状结构为表面反应提供了许多活性位点,提高了载流子分离率。
-
除了以上的非金属元素掺杂外,近年来研究人员发现采用高导电性的非金属材料掺杂修饰氮化碳亦可加速g-C3N4上的电子转移,使其可以更高效地在可见光下活化过硫酸盐降解有机污染物。Dikdim等[69]研究了活性炭/g-C3N4光催化耦合过硫酸盐对阿特拉津的光催化降解,实验结果表明,AC的加入有效地抑制了电子空穴对的复合,提高了g-C3N4的光催化性能。10%AC/g-C3N4/Vis/PMS体系的反应速率常数(0.0376 min−1)约是g-C3N4/Vis/PMS体系(0.0128 min−1)的2.9倍。Zhang等[70]通过苝四甲酸二酐(PTCDA)与石墨氮化碳的酰胺化反应合成了一种无金属光催化剂(PI-g-C3N4),并将其应用于可见光下活化过一硫酸盐(PMS)降解双酚A(BPA)。实验结果表明,PI-g-C3N4的光催化活性高于单独的PTCDA和g-C3N4。随着PMS的加入BPA的准一级降解动力学常数从0.0057 min−1提高到0.0501 min−1。降解速率的提高可归因于PI-g-C3N4中光生电子-空穴对的更有效分离和更高电导率,促进了光生电子活化PMS产生活性自由基O2·−、1O2和h+以降解BPA。非金属掺杂g-C3N4活化过硫酸盐光催化活化降解有机污染物情况如表2。
-
综上所述,目前非金属掺杂g-C3N4复合材料的制备方法主要包括水热法和溶剂热法、热缩合法、热聚合法和缩聚法。在光催化中,非金属掺杂g-C3N4能缩小氮化碳的带隙宽度,增强其光吸收能力,降低电子-空穴的复合率,显著提高氮化碳材料的光催化性能。在活化PMS时,非金属掺杂g-C3N4可提高氮化碳的电子迁移率、增加了更多的活性位点有助于活化PMS降解有机污染物。而在光催化耦合过硫酸盐高级氧化技术中,氮化碳在非金属掺杂后表现出更好的光催化性能,更有利于在光照射下活化PMS生成ROS降解有机污染物。尽管目前学界对非金属掺杂g-C3N4复合材料通过高级氧化技术降解有机污染物已经进行了大量的研究并取得了一定成果,但仍面临诸多挑战,如:
(1)目前大部分实验的模拟废水还只是针对单一污染物,这与实际废水中的多污染物复杂组成存在较大差异,这使得模拟废水中有机污染物的去除效果说服力有所减弱。因此,建议可使用实际废水来进行催化剂的降解活性实验。
(2)关于如何提高非金属掺杂g-C3N4复合材料的催化活性和稳定性仍需进一步研究。在对氮化碳进行非金属掺杂后虽然降解效率高,但矿化率仍有提升空间。因此,优化非金属掺杂g-C3N4复合材料的合成方法,进一步提高复合材料的催化活性和稳定性具有重要意义。
(3)关于水中有机污染物的降解机理和反应中间体的毒性仍需深入研究。考虑到在某些情况下,降解过程的中间产物的毒性可能甚至比其原始化合物更大,因此对中间产物的生态毒性或人体毒性进行评估是之后相关研究不容忽视的重要内容。
非金属掺杂石墨氮化碳复合材料光催化协同活化过一硫酸盐降解有机污染物的研究进展
Research progress of nonmetallic doped graphite carbon nitride composites in photocatalytic activation of peroxymonosulfate system for organic pollutants removal
-
摘要: 石墨氮化碳(g-C3N4)是一种二维的非金属半导体聚合物,因具有较宽的带隙宽度、丰富的活性位点和原材料合成成本低廉等优点,在协同光催化和活化过一硫酸盐(PMS)降解有机污染物方面具有广阔的应用前景。非金属元素掺杂g-C3N4不仅能克服纯氮化碳可见光利用效率低、比表面积小以及光生的电子-空穴对快速复合等缺点,还能大幅扩展其光响应范围,促进激活PMS的电子迁移,提高催化活性。本文综述了非金属掺杂g-C3N4的制备方法及其耦合光催化和PMS催化降解有机污染物的最新研究进展。此外,针对非金属掺杂g-C3N4光催化协同PMS去除污染物的过程机理进行了简要讨论。最后,提出非金属掺杂g-C3N4复合材料在高级氧化技术中所面临的挑战和机遇,旨在为非金属掺杂g-C3N4耦合光催化- PMS氧化体系在可持续碳催化中的应用提供参考依据。Abstract: Graphite carbon nitride (g-C3N4), as a two-dimensional nonmetallic semiconductor polymer, has been widely used for organic pollutants removal by synergistic photocatalysis-peroxymonosulfate (PMS) oxidation coupling system due to its wide band gap, abundant active sites and low cost. Doping non-metallic elements into g-C3N4 can not only overcome the shortcomings of low visible light utilization efficiency, small specific surface area and rapid recombination of photogenerated electron-hole pairs of pure g-C3N4, and it can also expand visible light response, promote electron migration for PMS activation and improve catalytic activity. In this paper, a comprehensive overview on the preparation methods of non-metallic doped g-C3N4 and their application in the removal of organic pollutants by the synergistic effect of photocatalysis and Fenton-like catalytic/PMS activation reactions. In addition, the photocatalysis-PMS mechanisms of non-metal doped g-C3N4 for pollutant removal are also discussed. Finally, the challenges and opportunities of non-metallic doped g-C3N4 composites in advanced oxidation technology are presented, and this review helps to provide insights into the synergistic effect between non-metallic doped g-C3N4 and photocatalysis-PMS oxidation coupling system in sustainable carbocatalysis.
-

-
表 1 非金属掺杂g-C3N4的特性及应用
Table 1. The characteristic and application of non-metallic doped g-C3N4
掺杂元素
Doping
element前躯体
Precursor合成方法
Fabrication
method应用
Application带隙能量/eV
Band gap效率
(掺杂后/未掺杂)
Efficiency
(Doped/undoped)数据来源
ReferencesO 三聚氰胺、过氧化氢 水热法 降解亚甲基蓝 2.7 50%/20%(在3 h内) [33] O 1,3,5-三氯三嗪、双氰胺 溶剂热法 降解罗丹明B 2.09 0.249 min−1/0.007 min−1 [34] O 三聚氰胺、过氧化氢 水热法 降解罗丹明B 1.98 90%/20%(在30 min内) [40] O 双氰胺 水热法 光催化析氢 2.49 37.5 μmol·h−1/15.2·μmol h−1 [25] S 硫脲、三聚氰胺 热缩合 降解亚甲基蓝 2.53 60%/36%(在2 h内) [35] P 三聚氰胺 热缩合 芳烃催化 2.55 — [41] P 磷酸腺嘌呤、尿素 热缩合 光催化析氢 2.56 9524 μmol·g−1h−1/458 μmol·g−1h−1 [42] N 双氰胺、柠檬酸、尿素 热聚合 降解吲哚美辛 2.67 91.5%/16%(在90 min内) [38] N 尿素、咪唑 热聚合 降解罗丹明B 2.26 94.5%/23%(在120 min内) [43] P 磷酸氢二铵、双氰胺 热聚合 降解罗丹明B 2.63 0.0466 min−1/0.0115 min−1 [44] P 三聚氰胺、聚磷酸盐 热聚合 光催化析氢 2.31 57 μmol·h−1/6 μmol·h−1 [45] N 柠檬酸、尿素 热聚合 光催化析氢 — 64 μmol·h−1/14.8 μmol·h−1 [37] O 尿素、二水合草酸 热聚合 降解阿特拉津 100%/65%(在20 min内) [46] P 硫氰酸铵、六氟磷酸铵 缩聚法 降解罗丹明B 2.86 0.03679 min−1/0.09856 min−1 [39] B 三聚氰胺、硼酸 缩聚法 降解四环素 2.83 68% /10%(在2 h内) [47] C 无水乙醇预处理的三聚氰胺 缩聚法 降解罗丹明B 2.65 0.0362 min−1/0.0081 min−1 [32] 表 2 非金属掺杂g-C3N4活化过硫酸盐光催化降解有机污染物
Table 2. Non-metallic doping g-C3N4 activated persulfate photocatalytic degradation of organic pollutants
催化剂
Catalyst污染物
Target pollutant光源
Light
source反应条件
Reaction conditions去除效果
Removal efficiency活化降解机理(主要的活性物种)
Active degradation mechanism (Main active species)数据来源
ReferencesOCN 卡马西平 500W
氙灯Cat = 1 g·L−1;
CBZ = 5 mg·L−1;
PMS=5.0 mmol·L−198.7%
(在3 h内)氧掺杂降低了带隙,提高了光响应能力和光致载流子分离,从而提高了OCN的光催化活性能。(1O2、O2·−、SO4·−和h+) [71] OCN 吡虫啉(IMD) 500W
氙灯Cat = 0.5 g·L−1;
IMD = 3 mg·L−1;
PMS = 5.0 mmol·L−1;
T = 25 ℃;
pH = 4.2 ± 0.3约100%
(在2 h内)OCN调制电子结构促进了PMS的吸附、电子转移和氧化还原位点的形成(1O2) [21] S-doped CN(CNS) 双酚A 150W
可见光灯Cat = 0.3 g·L−1;
BPA = 50 mg·L−1;
PMS = 0.3 g·L−1;
T = 20–40 °C;
pH = 580%
(在1 h内)硫和氮共掺杂的协同作用,这可能使相邻的碳原子更活跃,带隙更窄,从而分别提高其催化活性活化PMS和光催化活性。(SO4·–) [20] S-doped CN 双酚A 150W
可见光灯Cat = 0.3 g·L−1;
BPA = 50 mg·L−1;
PMS = 0.3 mmol·L−1;
T = 40°C;
pH=540%
(在1 h内)硫掺杂使碳原子更活跃,带隙更窄,从而提高其催化活性和光催化活性。(SO4·−) [72] VCN 罗丹明B 350W
氙灯Cat = 0.15 g·L−1;
RhB = 0.04 mmol·L−1;
MB = 0.04 mmol·L−1;
MO = 0.04 mmol·L−1;
PMS = 0.5 g·L−1;
T = 20 ℃;
pH = 5.594%
(在1 h内)N空位增强了PMS的吸附,捕获光生电子使PMS还原为·OH,而由N损失而产生的缺电子C原子促进PMS氧化为O2·−。(O2·−和·OH) [73] CQDs-(PCNC) 双酚A 300W
氙灯Cat = 0.33 g·L−1;
BPA = 20 mg·L−1;
PMS = 30 mg·L−1;
T = 20 ℃;
pH = 6.795%
(在0.5 h内)CQDs的加入使氮化碳表面积增大,光吸收增强,电荷分离改善,活性位点增多有利于PMS的光催化活化。(1O2、O2·−和 h+) [65] B-CNU 2、4-二氯苯酚 35W
LED灯Cat = 0.25 g·L−1;
2,4-DCP = 0.06 mmol·L−1;
PMS = 0.5 mmol·L−1100%
(在1.5 h内)硼掺杂拓宽并强化了催化剂表面的正静电势分布并降低电阻,使得PMS向催化剂的电子转移更容易,从而有效地生成1O2(1O2) [66] PI-g-C3N4 双酚A 300W
氙灯Cat = 1 g·L−1;
BPA = 10 mg·L−1;
PMS = 5 mmol·L−196%
(在1 h内)光生电子-空穴对的有效分离效率和PI-g-C3N4具有更高的电导率,通过光生电子促进PMS活化为活性自由基(O2·−、1O2和 h+) [70] AC/CN 阿特拉津 300W
氙灯Cat = 1g·L−1;
ATZ = 5 mg·L−1;
PMS = 3 g·L−1;
pH = 5.60.0376 min−1 光催化
(·OH、SO4·−)[69] PCN/GO 马拉硫磷农药 35W
LED灯Cat = 0.6 g·L−1;
农药 = 0.1 mmol·L−1;
PMS = 0.15 mmol·L−1;
pH = 1098%
(在5 h内)GO与PCN的耦合增加了界面电荷传输并最小化了光生载流子复合,促进PMS活化(O2·−、SO4·−、h+和·OH) [74] CO-C3N4 TCH 300W
氙灯Cat = 0.2 g·L−1;
TCH = 10 mg·L−1;
PMS = 0.2 g·L−197.77%
(在1 h内)CO-C3N4对可见光的吸收更强,光产生的载流子分离更快,这在很大程度上有助于PMS活化为活性自由基(O2·−、1O2和h+) [67] -
[1] GUAN Y H, MA J, REN Y M, et al. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals [J]. Water Research, 2013, 47(14): 5431-5438. doi: 10.1016/j.watres.2013.06.023 [2] GIANNAKIS S, LIN K Y A, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs) [J]. Chemical Engineering Journal, 2021, 406: 127083. doi: 10.1016/j.cej.2020.127083 [3] Nidheesh p v, Raman r. Removal of rhodamine B from a water medium using hydroxyl and sulphate radicals generated by iron loaded activated carbon [J]. RSC Advances, 2016, 6(7): 5330-5340. doi: 10.1039/C5RA19987E [4] ESPLUGAS S, GIMÉNEZ J, CONTRERAS S, et al. Comparison of different advanced oxidation processes for phenol degradation [J]. Water Research, 2002, 36(4): 1034-1042. doi: 10.1016/S0043-1354(01)00301-3 [5] KLAVARIOTI M, MANTZAVINOS D, KASSINOS D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes [J]. Environment International, 2009, 35(2): 402-417. doi: 10.1016/j.envint.2008.07.009 [6] TROJANOWICZ M. Removal of persistent organic pollutants (POPs) from waters and wastewaters by the use of ionizing radiation [J]. Science of the Total Environment, 2020, 718: 134425. doi: 10.1016/j.scitotenv.2019.134425 [7] BUTHIYAPPAN A, ABDUL AZIZ A R, WAN DAUD W M A. Recent advances and prospects of catalytic advanced oxidation process in treating textile effluents [J]. Reviews in Chemical Engineering, 2016, 32(1): 1-47. doi: 10.1515/revce-2015-0034 [8] BICALHO H A, LOPEZ J L, BINATTI I, et al. Facile synthesis of highly dispersed Fe(II)-doped g-C3N4 and its application in Fenton-like catalysis [J]. Molecular Catalysis, 2017, 435: 156-165. doi: 10.1016/j.mcat.2017.04.003 [9] LIU J Y, XU H, XU Y G, et al. Graphene quantum dots modified mesoporous graphite carbon nitride with significant enhancement of photocatalytic activity [J]. Applied Catalysis B:Environmental, 2017, 207: 429-437. doi: 10.1016/j.apcatb.2017.01.071 [10] PEI Z X, GU J X, WANG Y K, et al. Component matters: Paving the roadmap toward enhanced electrocatalytic performance of graphitic C 3 N 4-based catalysts via atomic tuning [J]. ACS Nano, 2017, 11(6): 6004-6014. doi: 10.1021/acsnano.7b01908 [11] WANG X C, MAEDA K, THOMAS A, et al. A metal-free polymeric photocatalyst for hydrogen production from water under visible light [J]. Nature Materials, 2009, 8(1): 76-80. doi: 10.1038/nmat2317 [12] BAI X J, WANG L, ZONG R. Photocatalytic activity enhanced via g-C3N4 nanoplates to nanorods [J]. The Journal of Physical Chemistry C, 2013, 117(19): 9952-9961. doi: 10.1021/jp402062d [13] ZHANG S W, LI J X, WANG X K, et al. Rationally designed 1D Ag@AgVO3 nanowire/graphene/protonated g-C3N4 nanosheet heterojunctions for enhanced photocatalysis via electrostatic self-assembly and photochemical reduction methods [J]. Journal of Materials Chemistry A, 2015, 3(18): 10119-10126. doi: 10.1039/C5TA00635J [14] 黄建辉, 林文婷, 谢丽燕, 等. 石墨相氮化碳-碘氧化铋层状异质结的构建及其光催化杀菌性能 [J]. 环境科学, 2017, 38(9): 3979-3986. doi: 10.13227/j.hjkx.201702014 HUANG J H, LIN W T, XIE L Y, et al. Construction of graphitic carbon nitride-bismuth oxyiodide layered heterostructures and their photocatalytic antibacterial performance [J]. Environmental Science, 2017, 38(9): 3979-3986(in Chinese). doi: 10.13227/j.hjkx.201702014
[15] 王亦清, 沈少华. 非金属掺杂石墨相氮化碳光催化的研究进展与展望 [J]. 物理化学学报, 2020, 36(3): 106-119. doi: 10.3866/PKU.WHXB201905080 Wang Y Q, Shen S H. Progress and prospects of non-metal doped graphitic carbon nitride for improved photocatalytic performances [J]. Acta Physico-Chimica Sinica, 2020, 36(3): 106-119(in Chinese). doi: 10.3866/PKU.WHXB201905080
[16] ISMAEL M. A review on graphitic carbon nitride (g-C3N4) based nanocomposites: Synthesis, categories, and their application in photocatalysis [J]. Journal of Alloys and Compounds, 2020, 846: 156446. doi: 10.1016/j.jallcom.2020.156446 [17] YANG Y, LI X, ZHOU C Y, et al. Recent advances in application of graphitic carbon nitride-based catalysts for degrading organic contaminants in water through advanced oxidation processes beyond photocatalysis: A critical review [J]. Water Research, 2020, 184: 116200. doi: 10.1016/j.watres.2020.116200 [18] ZHOU Y, GAO Y, PANG S Y, et al. Oxidation of fluoroquinolone antibiotics by peroxymonosulfate without activation: Kinetics, products, and antibacterial deactivation [J]. Water Research, 2018, 145: 210-219. doi: 10.1016/j.watres.2018.08.026 [19] LIN K Y A, ZHANG Z Y, WI-AFEDZI T. Sulfur-doped carbon nitride as a non-metal heterogeneous catalyst for sulfate radical-based advanced oxidation processes in the absence of light irradiation [J]. Journal of Water Process Engineering, 2018, 24: 83-89. doi: 10.1016/j.jwpe.2018.02.018 [20] LIN K Y A, ZHANG Z Y. Degradation of Bisphenol A using peroxymonosulfate activated by one-step prepared sulfur-doped carbon nitride as a metal-free heterogeneous catalyst [J]. Chemical Engineering Journal, 2017, 313: 1320-1327. doi: 10.1016/j.cej.2016.11.025 [21] TAN J, LI Z F, LI J, et al. Visible-light-assisted peroxymonosulfate activation by metal-free bifunctional oxygen-doped graphitic carbon nitride for enhanced degradation of imidacloprid: Role of non-photochemical and photocatalytic activation pathway [J]. Journal of Hazardous Materials, 2022, 423: 127048. doi: 10.1016/j.jhazmat.2021.127048 [22] TAN J, LI Z F, LI J, et al. Graphitic carbon nitride-based materials in activating persulfate for aqueous organic pollutants degradation: A review on materials design and mechanisms [J]. Chemosphere, 2021, 262: 127675. doi: 10.1016/j.chemosphere.2020.127675 [23] LIU S Z, LI D G, SUN H Q, et al. Oxygen functional groups in graphitic carbon nitride for enhanced photocatalysis [J]. Journal of Colloid and Interface Science, 2016, 468: 176-182. doi: 10.1016/j.jcis.2016.01.051 [24] ZHANG P, LI X H, SHAO C L, et al. Hydrothermal synthesis of carbon-rich graphitic carbon nitride nanosheets for photoredox catalysis [J]. Journal of Materials Chemistry A, 2015, 3(7): 3281-3284. doi: 10.1039/C5TA00202H [25] LI J H, SHEN B, HONG Z H, et al. A facile approach to synthesize novel oxygen-doped g-C3N4 with superior visible-light photoreactivity [J]. Chemical Communications, 2012, 48(98): 12017-12019. doi: 10.1039/c2cc35862j [26] ZHANG Y, GONG H H, LI G X, et al. Synthesis of graphitic carbon nitride by heating mixture of urea and thiourea for enhanced photocatalytic H2 production from water under visible light [J]. International Journal of Hydrogen Energy, 2017, 42(1): 143-151. doi: 10.1016/j.ijhydene.2016.11.040 [27] WANG Y M, CAI H Y, QIAN F F, et al. Facile one-step synthesis of onion-like carbon modified ultrathin g-C3N4 2D nanosheets with enhanced visible-light photocatalytic performance [J]. Journal of Colloid and Interface Science, 2019, 533: 47-58. doi: 10.1016/j.jcis.2018.08.039 [28] LIU G, NIU P, SUN C H, et al. Unique electronic structure induced high photoreactivity of sulfur-doped graphitic C3N4 [J]. Journal of the American Chemical Society, 2010, 132(33): 11642-11648. doi: 10.1021/ja103798k [29] YAN J, ZHOU C J, LI P R, et al. Nitrogen-rich graphitic carbon nitride: Controllable nanosheet-like morphology, enhanced visible light absorption and superior photocatalytic performance [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2016, 508: 257-264. [30] YAN Q, HUANG G F, LI D F, et al. Facile synthesis and superior photocatalytic and electrocatalytic performances of porous B-doped g-C3N4 nanosheets [J]. Journal of Materials Science & Technology, 2018, 34(12): 2515-2520. [31] KOMOROWSKA-DURKA M, DIMITRAKIS G, BOGDAŁ D, et al. A concise review on microwave-assisted polycondensation reactions and curing of polycondensation polymers with focus on the effect of process conditions [J]. Chemical Engineering Journal, 2015, 264: 633-644. doi: 10.1016/j.cej.2014.11.087 [32] DONG G H, ZHAO K, ZHANG L Z. Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4 [J]. Chemical Communications, 2012, 48(49): 6178-6180. doi: 10.1039/c2cc32181e [33] LIU S Z, SUN H Q, ANG H M, et al. Integrated oxygen-doping and dye sensitization of graphitic carbon nitride for enhanced visible light photodegradation [J]. Journal of Colloid and Interface Science, 2016, 476: 193-199. doi: 10.1016/j.jcis.2016.05.026 [34] WEI F Y, LIU Y, ZHAO H, et al. Oxygen self-doped g-C3N4 with tunable electronic band structure for unprecedentedly enhanced photocatalytic performance [J]. Nanoscale, 2018, 10(9): 4515-4522. doi: 10.1039/C7NR09660G [35] LIANG Q, ZHANG M, LIU C H, et al. Sulfur-doped graphitic carbon nitride decorated with zinc phthalocyanines towards highly stable and efficient photocatalysis [J]. Applied Catalysis A:General, 2016, 519: 107-115. doi: 10.1016/j.apcata.2016.03.033 [36] GONçALVES D A F, ALVIM R P R, BICALHO H A, et al. Highly dispersed Mo-doped graphite carbon nitride: Potential application as oxidation catalyst with hydrogen peroxide [J]. New Journal of Chemistry, 2018, 42(8): 5720-5727. doi: 10.1039/C8NJ00316E [37] ZHOU Y J, ZHANG L X, HUANG W M, et al. N-doped graphitic carbon-incorporated g-C3N4 for remarkably enhanced photocatalytic H2 evolution under visible light [J]. Carbon, 2016, 99: 111-117. doi: 10.1016/j.carbon.2015.12.008 [38] WANG F L, CHEN P, FENG Y P, et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin [J]. Applied Catalysis B:Environmental, 2017, 207: 103-113. doi: 10.1016/j.apcatb.2017.02.024 [39] CHAI B, YAN J T, WANG C L, et al. Enhanced visible light photocatalytic degradation of Rhodamine B over phosphorus doped graphitic carbon nitride [J]. Applied Surface Science, 2017, 391: 376-383. doi: 10.1016/j.apsusc.2016.06.180 [40] CHEN M, BAI R N, JIN P, et al. A facile hydrothermal synthesis of few-layer oxygen-doped g-C3N4 with enhanced visible light-responsive photocatalytic activity [J]. Journal of Alloys and Compounds, 2021, 869: 159292. doi: 10.1016/j.jallcom.2021.159292 [41] BELLARDITA M, GARCÍA-LÓPEZ E I, MARCÌ G, et al. Selective photocatalytic oxidation of aromatic alcohols in water by using P-doped g-C3N4 [J]. Applied Catalysis B:Environmental, 2018, 220: 222-233. doi: 10.1016/j.apcatb.2017.08.033 [42] SU C Y, ZHOU Y Z, ZHANG L L, et al. Enhanced n→π* electron transition of porous P-doped g-C3N4 nanosheets for improved photocatalytic H2 evolution performance [J]. Ceramics International, 2020, 46(6): 8444-8451. doi: 10.1016/j.ceramint.2019.12.079 [43] QI H L, LIU Y N, LI C Y, et al. Precursor-reforming protocol to synthesis of porous N-doped g-C3N4 for highly improved photocatalytic water treatments [J]. Materials Letters, 2020, 264: 127329. doi: 10.1016/j.matlet.2020.127329 [44] HU S Z, MA L, YOU J G, et al. A simple and efficient method to prepare a phosphorus modified g-C3N4 visible light photocatalyst [J]. RSC Advances, 2014, 4(41): 21657-21663. doi: 10.1039/C4RA02284J [45] GUO S E, TANG Y Q, XIE Y, et al. P-doped tubular g-C3N4 with surface carbon defects: Universal synthesis and enhanced visible-light photocatalytic hydrogen production [J]. Applied Catalysis B:Environmental, 2017, 218: 664-671. doi: 10.1016/j.apcatb.2017.07.022 [46] ZHANG J, XIN B, SHAN C, et al. Roles of oxygen-containing functional groups of O-doped g-C3N4 in catalytic ozonation: Quantitative relationship and first-principles investigation [J]. Applied Catalysis B:Environmental, 2021, 292: 120155. doi: 10.1016/j.apcatb.2021.120155 [47] PREEYANGHAA M, VINESH V, SABARIKIRISHWARAN P, et al. Investigating the role of ultrasound in improving the photocatalytic ability of CQD decorated boron-doped g-C3N4 for tetracycline degradation and first-principles study of nitrogen-vacancy formation [J]. Carbon, 2022, 192: 405-417. doi: 10.1016/j.carbon.2022.03.011 [48] LI Y F, WANG S, CHANG W, et al. Preparation and enhanced photocatalytic performance of sulfur doped terminal-methylated g-C3N4 nanosheets with extended visible-light response [J]. Journal of Materials Chemistry A, 2019, 7(36): 20640-20648. doi: 10.1039/C9TA07014A [49] ZHANG S, LIU Y, GU P C, et al. Enhanced photodegradation of toxic organic pollutants using dual-oxygen-doped porous g-C3N4: Mechanism exploration from both experimental and DFT studies [J]. Applied Catalysis B:Environmental, 2019, 248: 1-10. doi: 10.1016/j.apcatb.2019.02.008 [50] LI J Q, QI Y, MEI Y Q, et al. Construction of phosphorus-doped carbon nitride/phosphorus and sulfur co-doped carbon nitride isotype heterojunction and their enhanced photoactivity [J]. Journal of Colloid and Interface Science, 2020, 566: 495-504. doi: 10.1016/j.jcis.2020.01.102 [51] ZHANG B, LI X J, ZHAO Y, et al. Facile synthesis of oxygen doped mesoporous graphitic carbon nitride with high photocatalytic degradation efficiency under simulated solar irradiation [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2019, 580: 123736. doi: 10.1016/j.colsurfa.2019.123736 [52] JIANG L B, YUAN X Z, ZENG G M, et al. A facile band alignment of polymeric carbon nitride isotype heterojunctions for enhanced photocatalytic tetracycline degradation [J]. Environmental Science:Nano, 2018, 5(11): 2604-2617. doi: 10.1039/C8EN00807H [53] GUAN C T, JIANG J, PANG S Y, et al. Facile synthesis of pure g-C3N4 materials for peroxymonosulfate activation to degrade bisphenol A: Effects of precursors and annealing ambience on catalytic oxidation [J]. Chemical Engineering Journal, 2020, 387: 123726. doi: 10.1016/j.cej.2019.123726 [54] LIANG P, ZHANG C, DUAN X G, et al. An insight into metal organic framework derived N-doped graphene for the oxidative degradation of persistent contaminants: Formation mechanism and generation of singlet oxygen from peroxymonosulfate [J]. Environmental Science:Nano, 2017, 4(2): 315-324. doi: 10.1039/C6EN00633G [55] ZHU Y, CHEN Z H, GAO Y W, et al. General synthesis of carbon and oxygen dual-doped graphitic carbon nitride via copolymerization for non-photochemical oxidation of organic pollutant [J]. Journal of Hazardous Materials, 2020, 394: 122578. doi: 10.1016/j.jhazmat.2020.122578 [56] WEI M Y, GAO L, LI J, et al. Activation of peroxymonosulfate by graphitic carbon nitride loaded on activated carbon for organic pollutants degradation [J]. Journal of Hazardous Materials, 2016, 316: 60-68. doi: 10.1016/j.jhazmat.2016.05.031 [57] MIAO J, GENG W, ALVAREZ P J J, et al. 2D N-doped porous carbon derived from polydopamine-coated graphitic carbon nitride for efficient nonradical activation of peroxymonosulfate [J]. Environmental Science & Technology, 2020, 54(13): 8473-8481. [58] WANG W J, WANG H N, LI G Y, et al. Visible light activation of persulfate by magnetic hydrochar for bacterial inactivation: Efficiency, recyclability and mechanisms [J]. Water Research, 2020, 176: 115746. doi: 10.1016/j.watres.2020.115746 [59] YANG Q, MA Y H, CHEN F, et al. Recent advances in photo-activated sulfate radical-advanced oxidation process (SR-AOP) for refractory organic pollutants removal in water [J]. Chemical Engineering Journal, 2019, 378: 122149. doi: 10.1016/j.cej.2019.122149 [60] ZHAO Y, WANG G L, LI L J, et al. Enhanced activation of peroxymonosulfate by nitrogen-doped graphene/TiO2 under photo-assistance for organic pollutants degradation: Insight into N doping mechanism [J]. Chemosphere, 2020, 244: 125526. doi: 10.1016/j.chemosphere.2019.125526 [61] XU L J, QI L Y, SUN Y, et al. Mechanistic studies on peroxymonosulfate activation by g-C3N4 under visible light for enhanced oxidation of light-inert dimethyl phthalate [J]. Chinese Journal of Catalysis, 2020, 41(2): 322-332. doi: 10.1016/S1872-2067(19)63447-9 [62] YAN W, ZHANG R, JI F, et al. Deciphering co-catalytic mechanisms of potassium doped g-C3N4 in Fenton process [J]. Journal of Hazardous Materials, 2020, 392: 122472. doi: 10.1016/j.jhazmat.2020.122472 [63] 张金水, 王博, 王心晨. 石墨相氮化碳的化学合成及应用 [J]. 物理化学学报, 2013, 29(9): 1865-1876. doi: 10.3866/PKU.WHXB201306173 ZHANG J S, WANG B, WANG X C. Chemical synthesis and applications of graphitic carbon nitride [J]. Acta Physico-Chimica Sinica, 2013, 29(9): 1865-1876(in Chinese). doi: 10.3866/PKU.WHXB201306173
[64] XIAO X, WANG Y H, BO Q, et al. One-step preparation of sulfur-doped porous g-C3N4 for enhanced visible light photocatalytic performance [J]. Dalton Transactions, 2020, 49(24): 8041-8050. doi: 10.1039/D0DT00299B [65] MING H B, WEI D L, YANG Y, et al. Photocatalytic activation of peroxymonosulfate by carbon quantum dots functionalized carbon nitride for efficient degradation of bisphenol A under visible-light irradiation [J]. Chemical Engineering Journal, 2021, 424: 130296. doi: 10.1016/j.cej.2021.130296 [66] CUI M S, CUI K P, LIU X Y, et al. Insights into the photocatalytic peroxymonosulfate activation over defective boron-doped carbon nitride for efficient pollutants degradation [J]. Journal of Hazardous Materials, 2021, 418: 126338. doi: 10.1016/j.jhazmat.2021.126338 [67] SHI Y H, LI J S, WAN D J, et al. Peroxymonosulfate-enhanced photocatalysis by carbonyl-modified g-C3N4 for effective degradation of the tetracycline hydrochloride [J]. Science of the Total Environment, 2020, 749: 142313. doi: 10.1016/j.scitotenv.2020.142313 [68] CAO S H, FAN B, FENG Y C, et al. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation [J]. Chemical Engineering Journal, 2018, 353: 147-156. doi: 10.1016/j.cej.2018.07.116 [69] DANGWANG DIKDIM J M, GONG Y, NOUMI G B, et al. Peroxymonosulfate improved photocatalytic degradation of atrazine by activated carbon/graphitic carbon nitride composite under visible light irradiation [J]. Chemosphere, 2019, 217: 833-842. doi: 10.1016/j.chemosphere.2018.10.177 [70] ZHANG J J, ZHAO X, WANG Y B, et al. Peroxymonosulfate-enhanced visible light photocatalytic degradation of bisphenol A by perylene imide-modified g-C3N4 [J]. Applied Catalysis B:Environmental, 2018, 237: 976-985. doi: 10.1016/j.apcatb.2018.06.049 [71] MENG Y, LI Z F, TAN J, et al. Oxygen-doped porous graphitic carbon nitride in photocatalytic peroxymonosulfate activation for enhanced carbamazepine removal: Performance, influence factors and mechanisms [J]. Chemical Engineering Journal, 2022, 429: 130860. doi: 10.1016/j.cej.2021.130860 [72] LIN K Y A, ZHANG Z Y. Metal-free activation of Oxone using one-step prepared sulfur-doped carbon nitride under visible light irradiation [J]. Separation and Purification Technology, 2017, 173: 72-79. doi: 10.1016/j.seppur.2016.09.008 [73] WANG G L, ZHAO Y, MA H R, et al. Enhanced peroxymonosulfate activation on dual active sites of N vacancy modified g-C3N4 under visible-light assistance and its selective removal of organic pollutants [J]. Science of the Total Environment, 2021, 756: 144139. doi: 10.1016/j.scitotenv.2020.144139 [74] SUDHAIK A, RAIZADA P, THAKUR S, et al. Peroxymonosulphate-mediated metal-free pesticide photodegradation and bacterial disinfection using well-dispersed graphene oxide supported phosphorus-doped graphitic carbon nitride [J]. Applied Nanoscience, 2020, 10(11): 4115-4137. doi: 10.1007/s13204-020-01529-1 -




 下载:
下载: