-
近年来,新兴污染物(ECs)因具有化学结构稳定、难生物降解和潜在风险等特性受到广泛关注[1- 2]. 大部分ECs在常规污水处理技术中难以去除,导致其进入水环境中,对人类健康和环境造成危害[3-4]. 基于硫酸根自由基(
${\rm{SO}}_4^{\cdot -} $ )的高级氧化技术(SR-AOTs)在去除各类EC中展现出优异的性能[5-7]. 一直以来,${\rm{SO}}_4^{\cdot -} $ 的产生方式主要是基于过硫酸盐的活化[7],然而,在实际水处理中,过硫酸盐面临着成本高且可能带来潜在毒性的问题[8]. 近年来,亚硫酸盐作为烟气脱硫的残余产物被认为是一种具有潜力的过硫酸盐替代品,亚硫酸盐可通过不同的活化方式产生活性氧物种(ROS). 目前,亚硫酸盐的活化技术主要有过渡金属活化(如Fe3+/Fe2+[9-10]、Co2+[11])、光生电子活化(如BiVO4[12])和紫外光活化[13]等. 其中,Fe3+或Fe2+活化亚硫酸盐由于成本低、环境友好、设备要求低,被认为是更具应用潜力的活化方式.在Fe3+/Fe2+活化亚硫酸盐的体系中,亚硫酸铁络合物(
${\rm{FeSO}}_3^+ $ )是产生亚硫酸根自由基(${\rm{SO}}_3^{\cdot -} $ )必要的中间体. 当溶解氧(DO)存在时,一方面${\rm{SO}}_3^{\cdot -} $ 可进一步转化为其它氧化能力更强的ROS与污染物反应;另一方面Fe2+与亚硫酸盐的络合物(${\rm{FeHSO}}_3^+ $ )可被氧化生成${\rm{FeSO}}_3^+ $ [9-10, 14]. 因此,DO对于ROS的生成转化和Fe2+/Fe3+的循环是必不可少的,但过量的DO也可能导致亚硫酸盐的快速氧化,从而降低亚硫酸盐的有效利用. Chen等研究了充氧对Fe2+/S(Ⅳ)体系中氯霉素降解的作用,发现充氧难以实现氯霉素的完全去除,过量的溶解氧还加速了亚硫酸盐的无效氧化,并且在实际废水修复中的曝气成本不容忽视[15]. Wang等利用Fe2+活化亚硫酸氢盐降解水中双氯芬酸(DCF),结果发现在pH为4、 Fe2+初始浓度为 10 μmol·L−1、亚硫酸盐初始浓度为200 μmol·L−1时,反应5 分钟DCF去除率最佳可达 90%上,但该研究没有对其中的机理进行深入探讨[10]. 一直以来,${\rm{SO}}_4^{\cdot -} $ 和/或•OH被认为是Fe3+/S(Ⅳ)体系中污染物去除的主要活性物质,但缺乏必要的ROS定性检测,而这对于理解Fe3+/S(Ⅳ)过程中的相关机理至关重要. 此外,目前很少有研究同时比较Fe2+和 Fe3+活化亚硫酸盐体系两者的联系与区别.因此,本文比较了自然复氧条件下Fe3+/S(Ⅳ)和Fe2+/S(Ⅳ)体系对污染物的降解效率,特别是对Fe3+/S(Ⅳ)体系中活性物质进行了深入分析. 由于双酚A(BPA)广泛分布于废水、地表水甚至饮用水中 [16-17],所以本研究选择BPA作为代表性EC进行研究. 通过单因素变量法考察了 Fe2+/Fe3+和亚硫酸盐投加剂量,及溶液初始pH对Fe3+/S(Ⅳ)和Fe2+/S(Ⅳ)两个体系中BPA 降解效率与动力学的影响;通过测定Fe2+的浓度变化,比较Fe3+/S(Ⅳ)和Fe2+/S(Ⅳ)体系的联系与区别. 最后,通过对体系中的活性物质进行分析,结合BPA降解的中间产物分析,提出BPA的降解机理和转化途径.
-
双酚A(BPA,纯度≥99%)、亚硫酸钠(Na2SO3,≥98%)、叔丁醇(C4H10O,≥99%)均购自Sigma-Aldrich试剂公司;5,5-二甲基-1-吡咯啉-N-氧化物(DMPO,97%)、磷酸(H3PO4,色谱级)、硫酸铁(Fe2(SO4)3·xH2O,分析纯)购于Aladdin试剂公司;甲基苯基亚砜(PMSO,98%)、甲基苯基砜(PMSO2,98%)购于Macklin试剂公司;二甲基亚砜(DMSO,99.7%)购于J&K公司;甲醇(CH4O,色谱级)购于TEDIA试剂公司;硫酸亚铁(FeSO4·7H2O,分析纯)、硫酸(H2SO4,分析纯)、氢氧化钠(NaOH,分析纯)、1,10-菲罗啉(C12H8N2,分析纯)、乙醇(C2H6O、分析纯)购于国药集团化学试剂有限公司. 实验溶剂均采用超纯水,由超纯水机(Smart-DUV,上海和泰仪器有限公司,18 MΩ·cm)制得.
-
实验反应体系均为100 mL. 首先,配置100 mL初始浓度为0.01 mmol·L−1 的BPA和所需浓度的Fe3+反应液,使用0.1 mmol·L−1 H2SO4和NaOH调节体系反应所需pH后,加入所需体积的亚硫酸钠储备液引发反应(投加的亚硫酸钠体积对反应溶液总体积的影响可忽略不计). 反应过程中用磁力搅拌器对溶液进行连续搅拌(转速为500 r·min−1),使反应溶液呈现自然复氧状态. 在预定的取样时间里从反应溶液中取出1.0 mL样品放入装有0.5 mL甲醇的液相小瓶里淬灭反应后进行定量分析.
-
BPA、PMSO和PMSO2的浓度通过高效液相色谱(Dionex Ultimate 3000)进行测定,配备Athena C18(4.6 mm× 250 mm,5 μm)的色谱柱. 流动相由甲醇(A)和0.1%磷酸水溶液(B)组成,流速为1.0 mL·min−1;测定BPA、PMSO和PMSO2 的流动相(A/B)体积比分别为70/30、20/80和20/80;检测波长分别设置为225、227和272 nm. Fe2+的浓度采用邻菲罗啉法测定[18]. BPA的降解中间产物通过LC/MS(Thermo Scientific LTQ-Orbitrap XL)进行分析,中间产物的检测采用阴离子模式,甲醇和水梯度洗脱,流速为0.6 mL·min−1. 电子自旋共振(ESR)信号由Bruker E500-9.5/12检测.
-
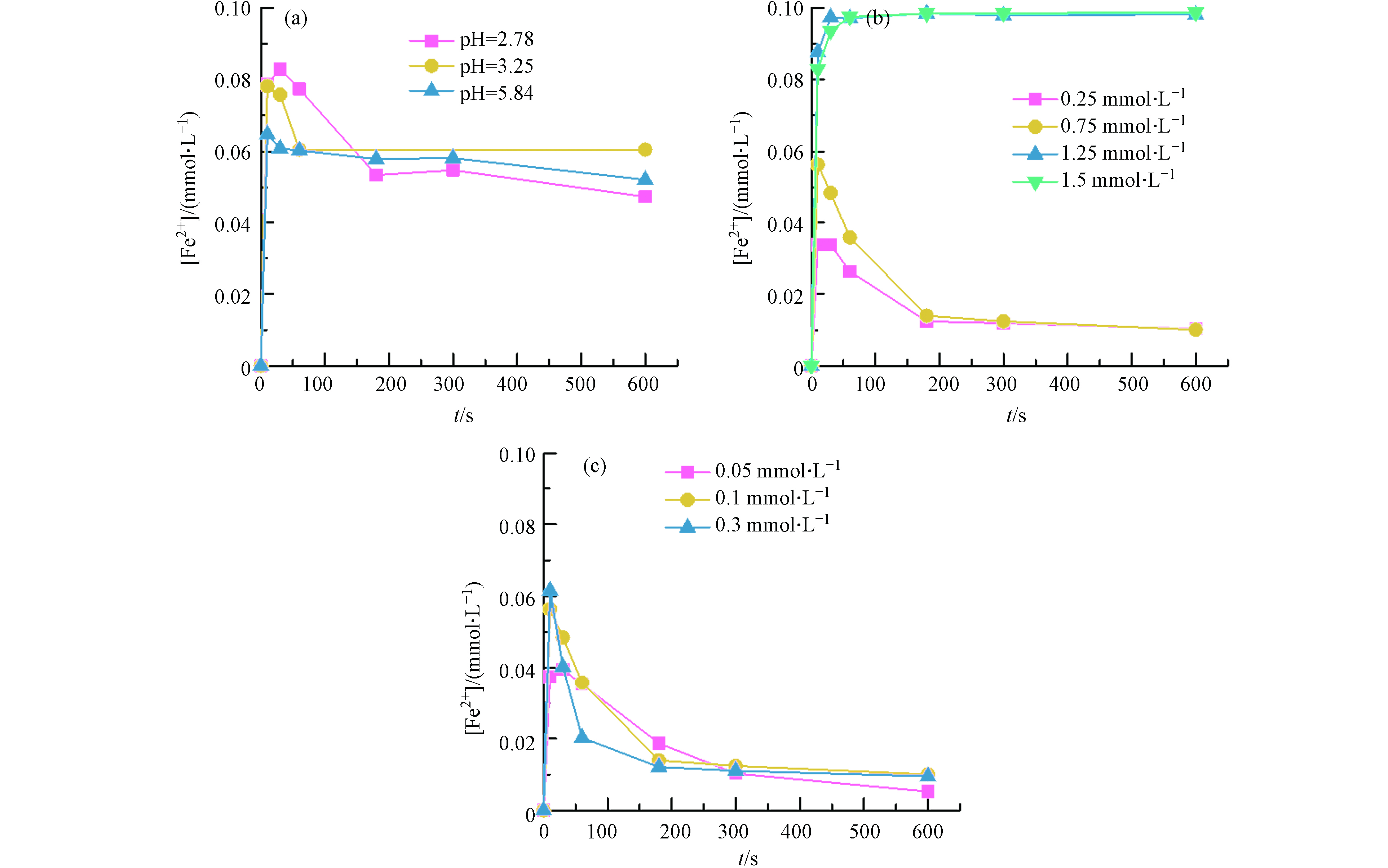
采用单因素变量法分别考察了pH值、亚硫酸盐(S(Ⅳ))浓度和Fe3+浓度对Fe3+/S(Ⅳ)体系中BPA降解的影响,结果如图1所示. 在Fe3+/S(Ⅳ)体系中,BPA的降解表现出明显的两阶段动力学特征,即初始快速去除阶段(约60 s)和后续的平台期. 图1(a)显示了溶液初始pH值(2.0—8.0)对BPA降解的影响.
结果表明,BPA只有在强酸性条件下才能有效降解,最佳pH值为3.25左右,反应60 s后BPA的去除率为53.9%;随着pH值的升高,BPA的降解速率逐渐降低,当pH 为4.21时,BPA的去除率小于13%,pH值进一步升高至中、碱性条件下BPA的降解基本可以忽略. 这可能是因为溶液pH可以影响S(Ⅳ)和Fe3+的形态分布,在pH 3—4时,S(Ⅳ)几乎以
${\rm{HSO}}_3^− $ 的形式存在(pKa1 = 1.9,pKa2 = 7.2)[19],而${\rm{HSO}}_3^− $ 更易结合Fe3+生成${\rm{SO}}_3^{\cdot -} $ 和Fe2+(反应1—2),其中${\rm{SO}}_3^{\cdot -} $ 可以作为其它氧化性更强的ROS的前驱体[20]. 此外,当pH > 4 时,Fe3+更容易沉淀,不仅减弱三价铁的氧化还原活性,同时也降低了与${\rm{HSO}}_3^− $ 结合的可能性[21].在最佳pH(3.25)条件下,继续考察了Fe3+和S(Ⅳ)的浓度变化对BPA降解的影响. Fe3+和S(Ⅳ)浓度对BPA的去除都具有双重作用. 从图1(b)中可以看出,当S(Ⅳ) 浓度从0.1 mmol·L−1增加到0.75 mmol·L−1, BPA的60 s去除率从23.3%增加到53.9%,然而当S(Ⅳ)浓度进一步增大,BPA的去除率反而降低了. 相似地,当Fe3+浓度从0.05 mmol·L−1增加到0.1 mmol·L−1时,60 s内BPA的去除也逐渐增强,当Fe3+浓度进一步增大时,BPA的降解效率下降(图1c). 这可能是由于过量的S(Ⅳ)和 Fe2+(从Fe3+反应原位生成的)对ROS产生竞争(反应3—6)[22-23],降低了ROS与BPA反应的几率,从而影响BPA的去除.
-
由于
${\rm{FeSO}}_3^+ $ 络合物为Fe3+/S(Ⅳ)体系的关键,${\rm{FeSO}}_3^+ $ 可自分解产生Fe2+和${\rm{SO}}_3^{\cdot -} $ (反应2)[20],因此,Fe2+的浓度变化能够间接反映体系中${\rm{SO}}_3^{\cdot -} $ 的生成趋势. 图2为不同条件下Fe3+/S(Ⅳ)体系中Fe2+浓度的变化趋势. 由于BPA在pH > 7时几乎不降解(图1),因此不考虑偏碱性条件下体系中Fe2+的累积情况. 由图2(a)可知,在Fe3+和S(Ⅳ)初始浓度不变的条件下,Fe2+浓度在各酸性pH条件下具有相似的变化趋势,在前10 秒左右迅速增加到峰值后降低至稳定浓度(约0.06 mmol·L−1). 这表明Fe2+在Fe3+/S(Ⅳ)体系初始阶段可能经历一个有效的生成(反应1—2)-消耗(反应7—8)过程,而该过程可能在后期受阻. 这可能是由两种原因造成的:(1)在反应的过程中S(Ⅳ)逐渐被耗尽,Fe2+/Fe3+缺乏反应受体[24];(2)溶解氧不足,使得Fe2+/Fe3+的循环受限[24]. 除此之外,还发现随着pH值的升高,Fe2+的累积量逐渐减少,实验结果表明在较高pH值下不利于${\rm{FeSO}}_3^+ $ 生成和/或分解反应的进行. 值得注意的是,无论在何种条件下,Fe2+前期的生成和转化伴随着BPA的有效降解(< 150 s),而当Fe2+浓度趋于稳定时BPA的降解也停滞了(> 150 s). 可见,污染物的降解与${\rm{FeSO}}_3^+ $ 分解成${\rm{SO}}_3^{\cdot -} $ 和Fe2+这一关键步骤紧密相关.当在最佳pH条件下改变Fe3+的初始浓度时,体系中Fe2+的浓度呈现类似变化趋势(图2c),当Fe3+浓度从0.05 mmol·L−1增至0.1 mmol·L−1时,反应10 s Fe2+浓度峰值从0.04 mmol·L−1增至0.058 mmol·L−1,然而当Fe3+浓度进一步增至0.3 mmol·L−1时,Fe2+浓度却只增加到0.06 mmol·L−1,这可能是由于S(Ⅳ)浓度有限,单方面增加Fe3+的浓度生成的FeSO3+有限,Fe2+的累积也受到制约(反应1). 当体系中存在过量Fe3+时,在反应10 s后Fe2+浓度依然下降的原因可归因于两个方面:(1)Fe2+与
${\rm{HSO}}_3^- $ 的反应速率大于Fe3+与${\rm{HSO}}_3^- $ 的反应速率(反应1,反应7);(2)生成的ROS消耗了部分Fe2+(反应3—4)[20],这也是过量Fe3+并不能有效促进BPA降解的原因.不同S(Ⅳ)浓度下Fe2+浓度的变化趋势有所不同(图2(b)),Fe2+的累积量在低S(Ⅳ)浓度下是先增加后减少,然而当S(Ⅳ)浓度超过1.25 mmol·L−1时,体系中投加的0.1 mmol·L−1的 Fe3+几乎能在前15 秒完全与
${\rm{HSO}}_3^- $ 反应转化生成Fe2+,且Fe2+的累积量在后续没有下降,可能是因为过量的S(Ⅳ)消耗了体系中大量的溶解氧,使得Fe2+向Fe3+的转化受阻[14],${\rm{SO}}_3^{\cdot -} $ 前驱体(${\rm{FeSO}}_3^+ $ )来源受限制,从而影响BPA的降解. -
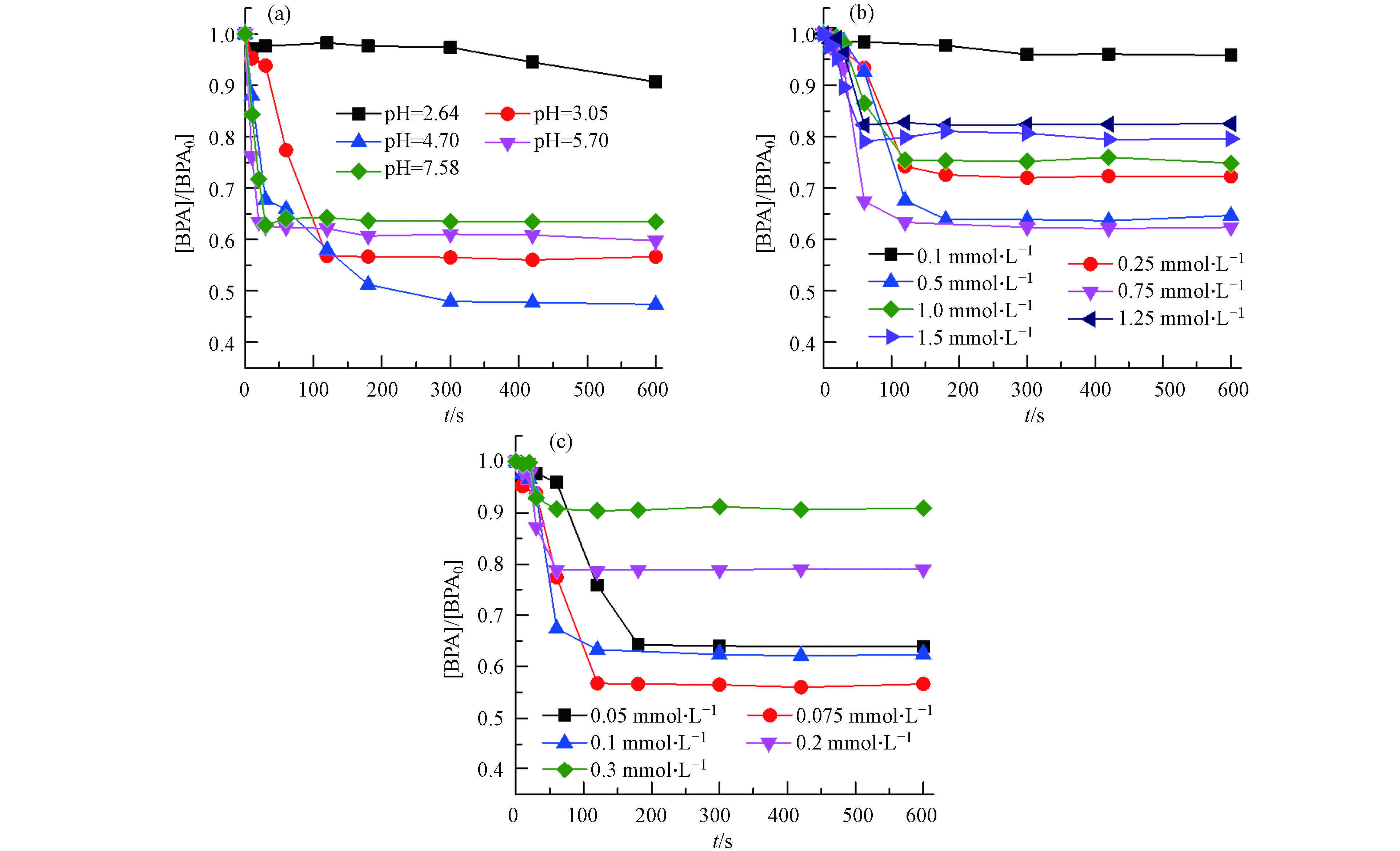
为了探索 Fe3+和Fe2+活化S(Ⅳ)的体系对有机污染物降解性能的区别与联系,同样研究了初始pH、亚硫酸盐浓度和Fe2+浓度对Fe2+/S(Ⅳ)体系降解BPA的影响,结果如图3所示. 在Fe2+/S(Ⅳ)体系中,BPA的降解总体也表现出两阶段动力学特征,即初始快速去除阶段,随后进入一个非常缓慢甚至停滞的平台期. 不同于Fe3+/S(Ⅳ)体系的是,在酸性条件下Fe2+/S(Ⅳ)体系中BPA的整体降解率有所下降,并且反应初期BPA的降解出现了短暂的缓滞. 这可能是因为Fe2+直接与
${\rm{HSO}}_3^- $ 反应生成的FeHSO3+(反应7)或是Fe2+的自氧化后再与${\rm{HSO}}_3^- $ 结合(反应1,9),都需要氧气的参与才能进一步转化生成能有效分解自由基产物的FeSO3+(反应8),这些过程可能延迟了体系中有效自由基产生时间,使得BPA的快速去除阶段延后[10, 25]. 除此之外,在自然复氧条件,Fe2+/S(Ⅳ)体系中Fe2+与${\rm{HSO}}_3^- $ 反应会消耗更多的溶解氧,同时后续自由基的转化也会竞争氧气(反应 10),体系中溶解氧的不足,自由基转化受到阻碍,从而使得在酸性条件下Fe2+/S(Ⅳ)体系中BPA整体的降解效果下降[26].图3(a)显示了不同pH对Fe2+/S(Ⅳ)体系中BPA降解的影响,与Fe3+/S(Ⅳ)体系不同的是,pH对Fe2+/S(Ⅳ)体系降解BPA的影响相对较小,pH在3.05—7.58之间,BPA的去除率均高于35%,尤其当pH 为4.7时,BPA的去除率达到了52.1%,稍低于Fe3+/S(Ⅳ)体系最优条件下BPA的降解率(53.9 %). 这可能是因为Fe2+耐受pH值比Fe3+更广泛,熊龙和李健欣等[27-28]的文章中就指出在Fe2+或Fe3+/S(Ⅳ)体系中,当pH > 4时,Fe3+开始出现沉淀,而对于Fe2+当pH值大于8,才开始出现沉淀. 值得注意的是,当pH > 3.05时,BPA在反应初期降解的缓滞期消失了,这可能是因为Fe2+被氧气氧化的速率会随着pH值的升高而加快(反应9)[29],在中碱性条件下,即使是Fe2+体系里,反应初始可能也会存在少量的Fe3+,可以瞬时引发反应降解BPA. 不同S(Ⅳ)和Fe2+浓度对Fe2+/S(Ⅳ)体系中BPA降解的影响如图3(b)、(c)所示. Fe2+和S(Ⅳ)浓度对BPA的去除同样也具有双重作用,从图3(b)中可以看出,S(Ⅳ)浓度从0.1 mmol·L−1增至0.75 mmol·L−1时,BPA的去除率从4.2%增加到37.6%,进一步提升S(Ⅳ)浓度BPA的去除率下降. 同样的变化趋势在不同Fe2+浓度的体系中也被观测到(图3c),在Fe2+浓度为0.075 mmol·L−1 时,BPA的降解率达到最大(约 43.3 %),继续增加Fe2+浓度至0.3 mmol·L−1,BPA的降解效果显著下降至9.07%. 结果表明,无论是Fe3+/S(Ⅳ)还是Fe2+/S(Ⅳ)体系,过量的Fe2+/Fe3+及S(Ⅳ)浓度都会使得污染物的去除率下降.
-
作为比较,对不同环境条件下Fe2+/S(Ⅳ)体系中的Fe2+浓度变化也进行了监测(图4). 与Fe3+/S(Ⅳ)体系的Fe2+浓度变化趋势相反,Fe2+/S(Ⅳ)体系中Fe2+浓度均呈现先快速下降,随后迅速上升的演变趋势. 这是因为Fe2+加入到体系中与
${\rm{HSO}}_3^- $ 反应生成的Fe${\rm{HSO}}_3^- $ (反应7)或是自氧化(反应9)均会使体系中Fe2+含量下降;生成FeHSO3+会进一步被氧化成${\rm{FeSO}}_3^+ $ ,${\rm{FeSO}}_3^+ $ 分解会生成Fe2+,使体系Fe2+浓度重新增多(反应1)[10]. 图4(a)显示了不同初始pH条件下Fe2+/S(Ⅳ)体系中Fe2+的变化. 由图4可见,随着pH的升高,Fe2+在反应初期的消耗量越显著,当pH值从3.05增至4.70时Fe2+最低量从0.06 mmol·L−1降至0.038 mmol·L−1;与Fe3+/S(Ⅳ)体系不同的是,即使在pH升高至7.58,体系也能观测到Fe2+浓度的变化,实验结果表明pH越高Fe2+与${\rm{HSO}}_3^- $ 的结合形成FeHSO3+或者Fe2+自氧化的量就越大,实验结果与熊和刘等的研究也相符[27, 29]. 然而,后续反应过程中Fe2+的再生却显示出不同的趋势:当pH为4.7时,Fe2+再生量最多,约0.018 mmol·L−1,当pH为7.58时,Fe2+再生量仅为0.005 mmol·L−1. 结果表明,尽管Fe2+/S(Ⅳ)体系中在中、碱性条件下也有${\rm{FeSO}}_3^+ $ 的生成或/和分解,但是相比较于酸性条件下,${\rm{FeSO}}_3^+ $ 的生成或/和分解依旧会随着pH值的升高而受阻. 尽管随着pH的升高Fe2+的初期消耗量逐渐增加,但是污染物的去除率却不是随着pH的升高而一直增加(图3),这是因为减少的Fe2+并不能完全有效转化为${\rm{FeSO}}_3^+ $ ,随着pH的升高,Fe2+的自氧化生成Fe3+作用增强[29],而在碱性条件下,铁物种易生成沉淀减少了与S(Ⅳ)络合作用,从而影响了BPA的有效降解. 实验结果表明,无论是Fe2+/S(Ⅳ)还是Fe3+/S(Ⅳ)体系,BPA降解均与${\rm{FeSO}}_3^+ $ 分解成${\rm{SO}}_3^{\cdot -} $ 和Fe2+步骤相关,而在Fe2+/S(Ⅳ)体系中这一过程进行的pH值可以拓宽到中碱性条件下.图4(b)和图4(c)显示了不同S(Ⅳ)和Fe2+浓度下Fe2+/S(Ⅳ)体系中Fe2+浓度的变化. 当投加低剂量Fe2+和S(Ⅳ)时,体系中Fe2+变化幅度较小,说明Fe2+与S(Ⅳ)之间的反应较弱,产生活性物质少(反应2,反应7—8),因此BPA的降解也较弱(图3). 随着S(Ⅳ)和Fe2+浓度的增加,体系中Fe2+变化幅度增大,BPA的去除率也逐渐升高. 但是值得注意的是,投加高剂量S(Ⅳ)(1.5 mmol·L−1)和Fe2+(0.3 mmol·L−1)到体系中,虽然Fe2+变化幅度最大,但是BPA的去除效果却不是最佳(图3). 从图4(b)中可以看出,当S(Ⅳ)含量充足时,体系里Fe2+最终能恢复至初始Fe2+投加剂量的相同水平,后续Fe2+不再变化可能是由于体系缺乏溶解氧的缘故. 而投加过量Fe2+(0.3 mmol·L−1)时(图4(c)),体系中Fe2+的增加量却低于初始投加的Fe2+的量,这可能是因为此时体系中Fe2+过量,过量的Fe2+在消耗完S(Ⅳ)后会继续与体系中自由基反应生成Fe3+(反应3—4)从而降低了体系中Fe2+的量,限制BPA的去除. 考虑到Fe2+/S(Ⅳ)和Fe3+/S(Ⅳ)体系中BPA的降解效率是FeSO3+起决定性作用,因此考虑到自然复氧条件下溶解氧的限制,后续机理研究均是围绕Fe3+/S(Ⅳ)体系而展开.
-
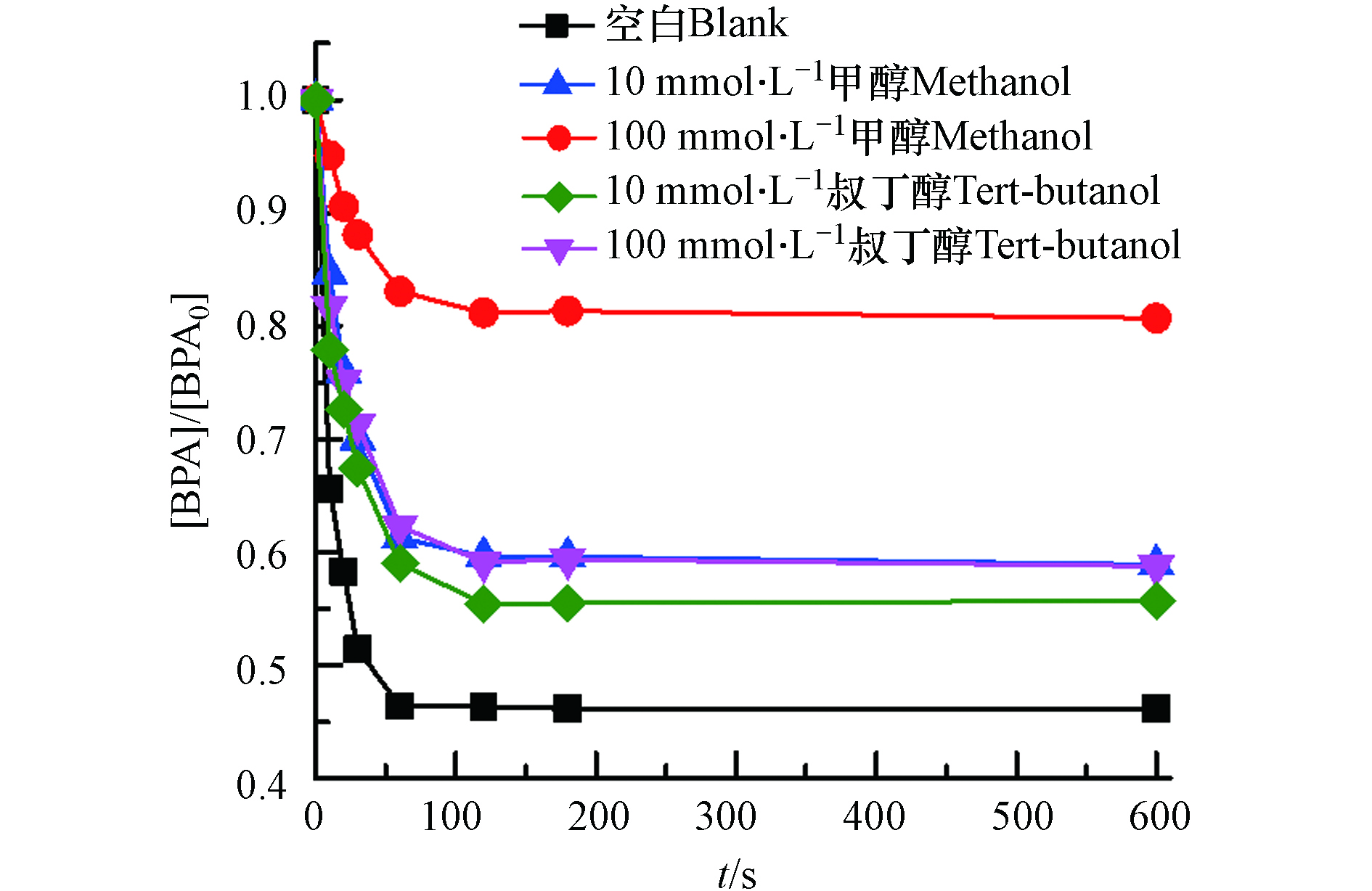
为了阐明Fe3+/S(Ⅳ)体系中的ROS对BPA降解的贡献作用,通过甲醇和叔丁醇竞争实验验证
${\rm{SO}}_4^{\cdot -} $ 和·OH的相对作用(图5). 甲醇与${\rm{SO}}_4^{\cdot -} $ (k = 2.5 × 107 mol·L−1·s−1)和·OH(k = 9.7 × 108 mol·L−1·s−1)均可快速反应,而叔丁醇和·OH反应(k = 3.8—7.6 × 108 mol·L−1·s−1)较与${\rm{SO}}_4^{\cdot -} $ 反应(k = 4.0—9.1 × 105 mol·L−1·s−1)更迅速 [30-31]. 如图5所示,在Fe3+/S(Ⅳ)体系中,BPA的去除主要受到甲醇的抑制,在体系中添加10 mmol·L−1 的甲醇后,BPA的去除率下降了15%,当体系中甲醇浓度进一步增加到100 mmol·L−1后,约有80%以上的BPA降解被抑制了. 然而,当体系中添加不同浓度的叔丁醇后,BPA的去除效率虽然均受抑制,但是抑制程度相差不大,这表明${\rm{SO}}_4^{\cdot -} $ 和·OH都是导致Fe3+/S(Ⅳ)体系中BPA降解的主要ROS,并且${\rm{SO}}_4^{\cdot -} $ 的贡献要大于·OH. 尽管100 mmol·L−1的甲醇可以抑制80%以上BPA的降解,但在初始阶段总是无法完全抑制BPA降解. 因此,推测在Fe3+/S(Ⅳ)体系中可能有其他活性物质贡献BPA的降解. -
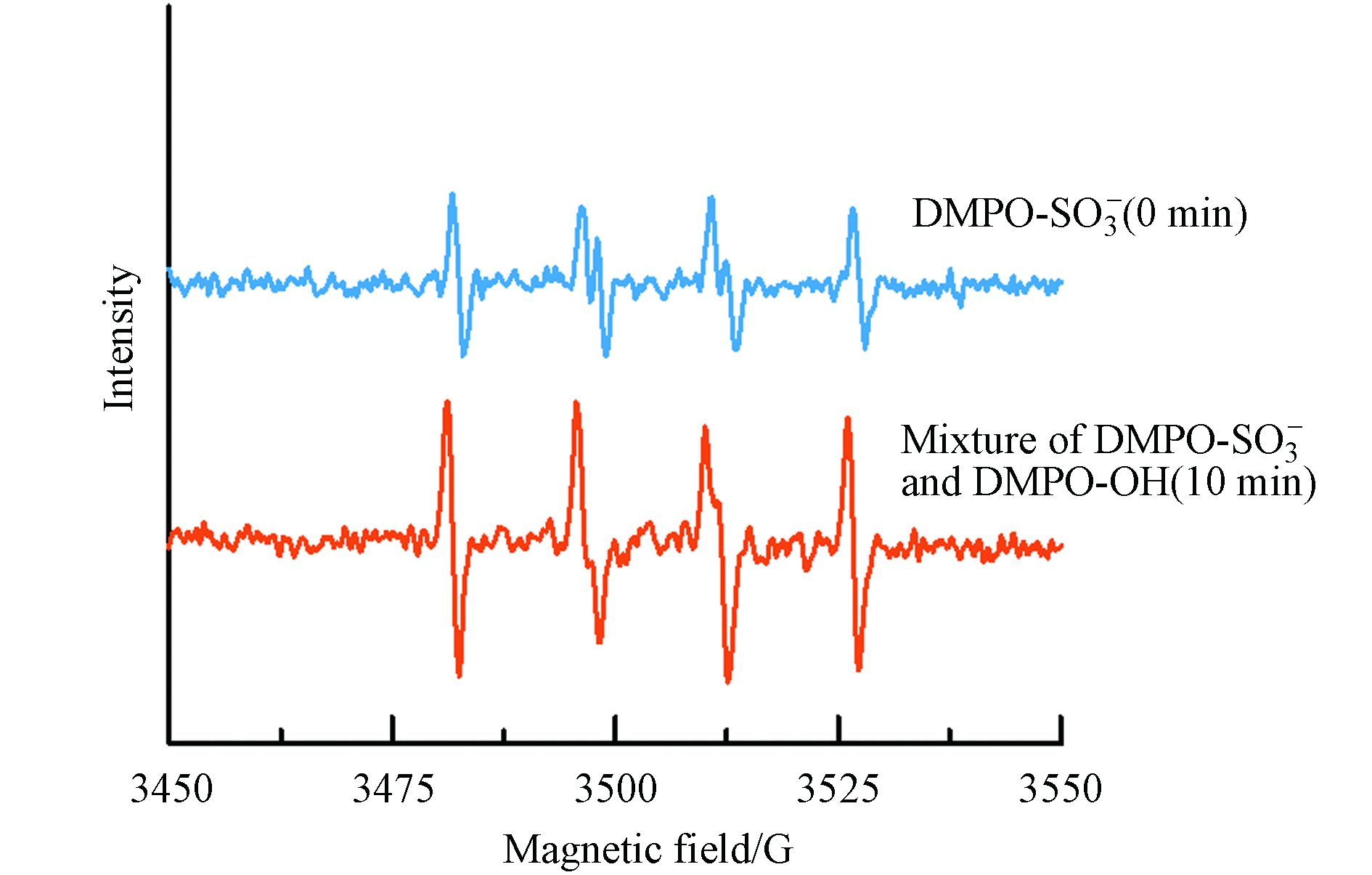
为了更进一步分析Fe3+/S(Ⅳ)体系中自由基的演变过程,在不同反应时间向体系中加入DMPO,通过ESR对体系中的自由基进行了检测(图6). 当DMPO在反应开始前(0 min)加至溶液时,反应10 min后,在Fe3+/S(Ⅳ)体系中仅观察到了DMPO-
${\rm{SO}}_3^{\cdot -} $ 的信号(αN = 14.4 G, αβ-H = 16.2 G)[32],这在Dong等的文章中也得到了相似的结果[9]. 这是因为${\rm{FeSO}}_3^+ $ 分解产生${\rm{SO}}_3^{\cdot -} $ (反应2)被DMPO捕获. 而在体系反应进行10 分钟后加入DMPO,ESR谱图显示出两端峰强度较强,中间两峰的强度较低的四重峰,这是混合了过量的DMPO-${\rm{SO}}_3^{\cdot -} $ 和少量的DMPO-·OH所致. 由于DMPO-${\rm{SO}}_3^{\cdot -} $ 和 DMPO-·OH有相似的超精细耦合常数,当体系里同时含有DMPO-${\rm{SO}}_3^{\cdot -} $ 和DMPO-·OH时,两者的信号会重叠,相同的结果也在Zamora和Villamena等的研究中呈现[33]. 上述结果证实,${\rm{SO}}_3^{\cdot -} $ 是Fe3+/S(Ⅳ)体系中的初级自由基产物,可在自然复氧条件下转化形成·OH(反应10—17). 此外,如果${\rm{SO}}_3^{\cdot -} $ 被DMPO完全捕获,几乎检测不到其他自由基的信号,说明几乎所有的其它ROS均为${\rm{SO}}_3^{\cdot -} $ 引发. 值得注意的是,ESR几乎检测不到${\rm{SO}}_4^{\cdot -} $ 的信号,这可能与DMPO-${\rm{SO}}_4^{\cdot -} $ 加成物的半衰期(95 s)较短有关[34],并且${\rm{SO}}_4^{\cdot -} $ 可以与水反应转化为·OH(反应17)[35-36]. -
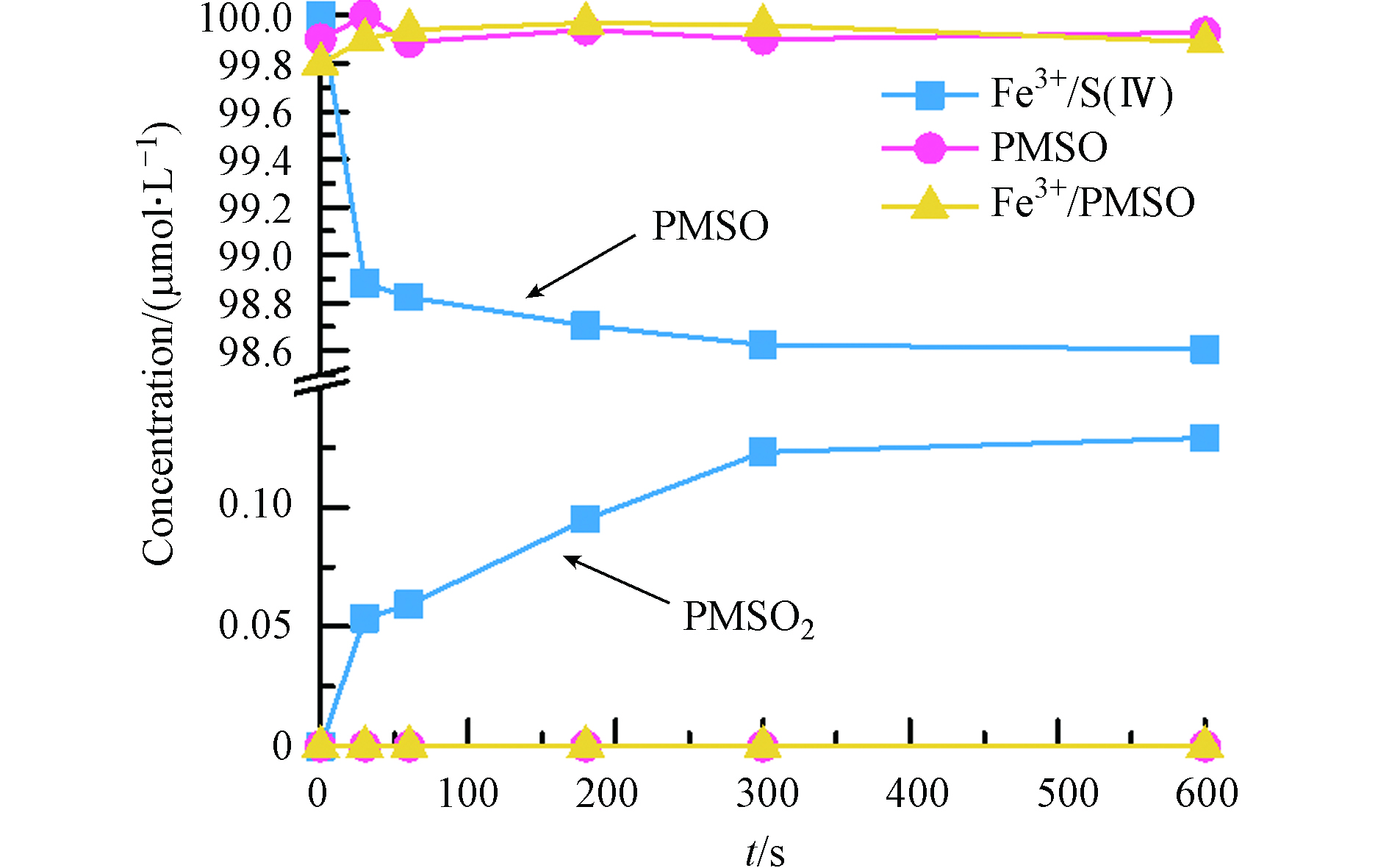
从竞争实验结果来看(2.5部分),100 mmol·L−1的甲醇不能完全抑制住BPA的降解,且有研究报道在Fe2+与过硫酸盐反应的过程中会有高价铁(Fe(Ⅳ))的产生[37-38],对体系中染污物的降解有一定的贡献. 因此,本研究对Fe3+/S(Ⅳ)体系中产生的Fe(Ⅳ)进行了定性定量分析. PMSO可作为Fe(Ⅳ)的探针化合物,因为PMSO可以被Fe(Ⅳ)氧化,通过氧原子转移生成PMSO2(k = 1.23 × 105 mol·L−1·s−1),而·OH(k = 3.08 × 109 mol·L−1·s−1)和
${\rm{SO}}_4^{\cdot -} $ (k = 3.17 × 108 mol·L−1·s−1)与PMSO反应的产物并非PMSO2[38],因此PMSO2的生成可说明体系中存在Fe(Ⅳ). Fe3+/S(Ⅳ)体系中PMSO的降解和PMSO2的生成如图7所示. 由对照实验可知,仅Fe3+存在时,PMSO无明显减少也无PMSO2的生成;PMSO的自氧化生成PMSO2的可能性也可忽略(图7),因此PMSO2的生成可归因于Fe3+与S(Ⅳ)的反应. 由图7可见,PMSO在Fe3+/S(Ⅳ)体系中反应的前30 秒可被迅速氧化为PMSO2,后续PMSO和PMSO2都进入一个缓慢反应的过程,与BPA降解趋势类似. 这可归因于${\rm{FeSO}}_3^+ $ 分解转化生成的中间物${\rm{SO}}_5^{\cdot -} $ (反应1—2,反应10),${\rm{SO}}_5^{\cdot -} $ 可通过自身结合或与${\rm{HSO}}_3^- $ /Fe2+反应产生过氧化物(${\rm{HSO}}_5^- $ 和/或${\rm{S}}_2 {\rm{O}}_8^{2-} $ )(反应12—14)[39-40],形成的部分${\rm{HSO}}_5^- $ 和${\rm{S}}_2 {\rm{O}}_8^{2-} $ 会将Fe2+氧化成Fe(Ⅳ)(反应18—19)[41-42]. 值得注意的是,在Fe3+/S(Ⅳ)体系中,每摩尔PMSO氧化产生PMSO2的产率(η)为23%,远低于100%. 这可能是因为体系中生成的${\rm{SO}}_4^{\cdot -} $ 和·OH与PMSO和PMSO2反应,消耗了体系中PMSO与PMSO2的量,${\rm{SO}}_4^{\cdot -} $ 和·OH与PMSO2的反应速率分别为2.75 × 105 mol·L−1·s−1 和3.08 × 109 mol·L−1·s−1 [37-38]. 虽然体系中自由基的存在可能干扰PMSO与PMSO2量的实际存在情况,但是PMSO2的累积足以证明Fe3+/S(Ⅳ)体系中有Fe(Ⅳ)的产生,结合甲醇的不完全抑制可说明Fe(Ⅳ)可能也贡献了BPA的降解. -
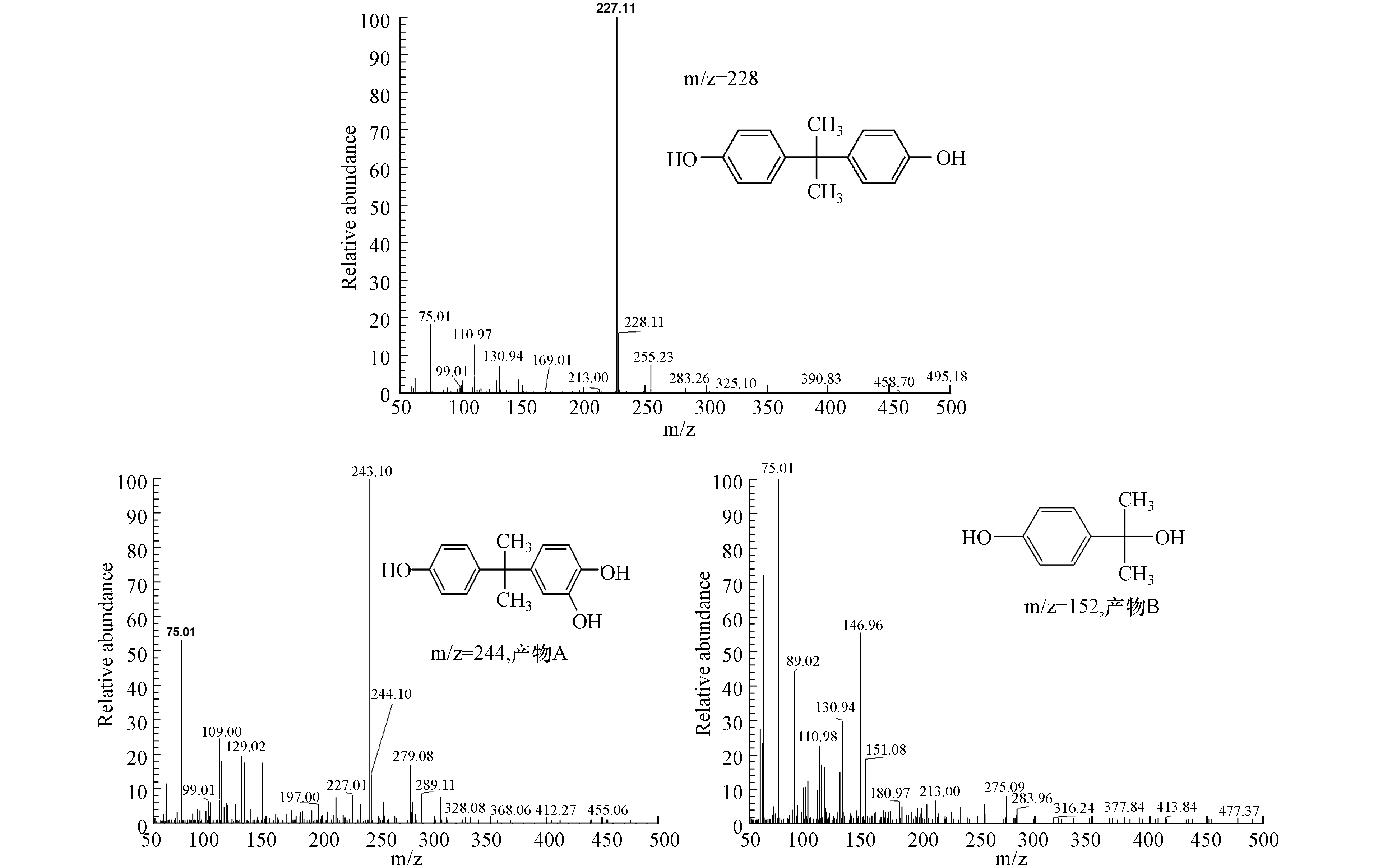
通过HPLC/MS研究了Fe3+/S(Ⅳ)体系中的BPA降解途径(图8). 从BPA降解过程中的质谱图可以清楚地看出,BPA出峰时间约为26.70 min,并且在BPA出峰时间前面检测到了两个产物峰,保留时间分别25.05 min和19.49 min. 主要的单羟基化合物(产物A,m/z 244)在体系中被检测到,这是BPA羟基化的主要产物,Darsinou等在超声波活化过硫酸盐系统降解BPA的过程中检测到相同的产物[43]. 除此之外,在体系中还检测到了2-(4-羟基苯基)-2-丙醇(产物B,m/z 152),这可归因于
${\rm{SO}}_4^{\cdot -} $ 和·OH直接对BPA 的芳环进行攻击生成相关产物的结果[44],自由基直接对BPA芳环攻击可以产生高度氧化的苯氧基BPA,高度氧化的苯氧基BPA可以通过C—C键的β断裂进一步形成产物B[44]. 因此. 由于上述鉴定的中间产物可知,BPA在Fe3+/S(Ⅳ)体系中的降解主要包括苯环的羟基化和BPA分子C—C键的β断裂两个路径. -
(1)Fe3+/S(Ⅳ)和Fe2+/S(Ⅳ)体系降解BPA的效果依赖体系中pH、Fe2+/Fe3+的浓度和S(Ⅳ)的浓度;S(Ⅳ)和铁的浓度的影响均具有双重作用.
(2)Fe3+/S(Ⅳ)体系仅在强酸性条件下(pH < 4)对BPA有较好的降解效果,而Fe2+/S(Ⅳ)体系降解BPA的有效pH可以拓宽到中性.
(3)Fe2+/S(Ⅳ)体系对溶解氧的需求更强,BPA的最佳去除率较Fe3+/S(Ⅳ)体系低,且BPA降解初期出现缓滞期.
(4)Fe2+的浓度变化在Fe3+/S(Ⅳ)和Fe2+/S(Ⅳ)体系中具有指示作用,可用来预测自由基的生成情况及污染物的降解情况;两个体系中BPA的有效降解均与亚硫酸盐铁络合物(
${\rm{FeSO}}_3^+ $ )的有效生成和分解紧密相关.(5)通过抑制实验证明了
${\rm{SO}}_4^{\cdot -} $ 和·OH是BPA在Fe3+/S(Ⅳ)体系中降解的主要活性氧物质,ESR 分析表明其它活性氧自由基均为${\rm{SO}}_3^{\cdot -} $ 转化而来.(6)首次在Fe3+/S(Ⅳ)体系中检测到高价铁(Fe(Ⅳ))的生成,并对BPA的降解起到作用.
(7)BPA在Fe3+/S(Ⅳ)体系中的降解途径主要包括苯环的羟基化和BPA分子C—C键的β断裂.
Fe3+/Fe2+活化亚硫酸盐降解水中双酚A的性能与机理
Mechanistic and performance studies on sulfite activation by Fe3+ or Fe2+ for degrading bisphenol A in water
-
摘要: 文章以典型新兴污染物双酚A (BPA)为研究对象,考察了自然复氧条件下各影响因素对Fe3+/S(Ⅳ)和Fe2+/S(IV)体系降解BPA的影响及二者的区别与联系,重点研究了Fe3+/S(IV)体系中活性物种的贡献和变化. 研究结果表明,Fe2+/S(Ⅳ)和Fe3+/S(Ⅳ)体系均能在一定程度上降解BPA,Fe3+/S(Ⅳ)体系对降解环境要求严格,限于强酸性条件下有较好的降解效能,而Fe2+/S(Ⅳ)体系降解BPA的有效pH可以拓宽至7.58,但是Fe2+/S(Ⅳ)体系对溶解氧需求更高,且BPA的最佳降解率低于Fe3+/S(Ⅳ)体系. Fe2+的浓度变化在Fe3+/S(Ⅳ)和Fe2+/S(Ⅳ)体系中具有指示作用,可用来预测自由基的生成情况及污染物的降解情况,通过对比二者体系中Fe2+浓度变化和BPA降解趋势,确定了两个体系中BPA降解均与
${\rm{FeSO}}_3^+ $ 分解成${\rm{SO}}_3^{\cdot -} $ 和Fe2+这一关键步骤相关. 此外,${\rm{SO}}_3^{\cdot -} $ 和·OH被电磁自旋共振(ESR)检测到,并且通过抑制实验和ESR实验确定了由${\rm{SO}}_3^{\cdot -} $ 衍生而来的${\rm{SO}}_4^{\cdot -} $ 和·OH均是Fe3+/S(Ⅳ)体系中BPA降解的主要贡献者. 除此之外,首次在Fe3+/S(Ⅳ)体系中发现高价态Fe(Ⅳ)的存在. 最后根据LC/MS分析表明,Fe3+/S(Ⅳ)体系中BPA降解途径主要包括苯环的羟基化和BPA分子C—C键的β断裂.Abstract: The influence of different factors on Fe3+/S(Ⅳ) and Fe2+/S(Ⅳ) processes under natural reoxygenation was investigated using a typical emerging contaminant, bisphenol A (BPA), as the target pollutant. The contribution and variation of different reactive species in Fe3+/S(Ⅳ) process were emphatically studied. Results showed that BPA could be degraded in Fe2+/S(Ⅳ) and Fe3+/S(Ⅳ) processes. However, the high efficiency of Fe3+/S(Ⅳ) process for the degradation of BPA was always achieved at strong acidic conditions, while the effective pH range of Fe2+/S(Ⅳ) process could be broadened to 7.58. The demand of dissolved oxygen in Fe2+/S(Ⅳ) process was higher than that in Fe3+/S(Ⅳ) process, leading to the lower BPA removal rate at the optimum condition in Fe2+/S(Ⅳ) process than that in Fe3+/S(Ⅳ) process. The concentration of Fe2+ could be used as an indicator for the effective generation of radical species and the degradation performance of organic pollutants. The degradation of BPA in Fe2+/S(Ⅳ) and Fe3+/S(Ⅳ) processes was closely related to the crucial step of${\rm{FeSO}}_3^+$ formation and decomposition. Both${\rm{SO}}_4^{\cdot -} $ and ·OH derived from${\rm{SO}}_3^{\cdot -} $ were assumed as the main contributors to BPA degradation based on the quenching experiments and electron spin resonance (ESR) examination. High-valent iron was for the first time reported to be present in Fe3+/S(Ⅳ) process, which also contributed to the degradation of BPA. Finally, based on LC/MS analysis, BPA degradation pathways in Fe3+/S(Ⅳ) process were found to mainly include the hydroxylation of the benzene ring and the beta-scission of the C-C bond of BPA molecule.-
Key words:
- sulfite /
- iron /
- high-valence iron /
- sulfate radical /
- hydroxyl radical /
- bisphenol A.
-

-
-
[1] PETRIE B, BARDEN R, KASPRZYK-HORDERN B. A review on emerging contaminants in wastewaters and the environment: Current knowledge, understudied areas and recommendations for future monitoring [J]. Water Research, 2015, 72: 3-27. doi: 10.1016/j.watres.2014.08.053 [2] 孙红文, 应光国. 环境中的新兴污染物研究 [J]. 环境化学, 2018, 37(8): 1681-1682. SUN H W, YING G G. Research on emerging pollutants in the environment [J]. Environmental Chemistry, 2018, 37(8): 1681-1682(in Chinese).
[3] GIULIVO M, DE ALDA ML, CAPRI E, et al. Human exposure to endocrine disrupting compounds: Their role in reproductive systems, metabolic syndrome and breast cancer. A review [J]. Environmental Research, 2016, 151: 251-264. doi: 10.1016/j.envres.2016.07.011 [4] MATAMOROS V, RODRÍGUEZ Y, ALBAIGÉS J. A comparative assessment of intensive and extensive wastewater treatment technologies for removing emerging contaminants in small communities [J]. Water Research, 2016, 88: 777-785. doi: 10.1016/j.watres.2015.10.058 [5] WANG J L, WANG S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants [J]. Chemical Engineering Journal, 2018, 334: 1502-1517. doi: 10.1016/j.cej.2017.11.059 [6] ZHOU Z, LIU X T, SUN K, et al. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review [J]. Chemical Engineering Journal, 2019, 372: 836-851. doi: 10.1016/j.cej.2019.04.213 [7] 谷得明, 郭昌胜, 冯启言, 等. 基于硫酸根自由基的高级氧化技术及其在环境治理中的应用 [J]. 环境化学, 2018, 37(11): 2489-2508. doi: 10.7524/j.issn.0254-6108.2018012102 GU D M, GUO C S, FENG Q Y, et al. Sulfate radical-based advanced oxidation processes and its application in environmental remediation [J]. Environmental Chemistry, 2018, 37(11): 2489-2508(in Chinese). doi: 10.7524/j.issn.0254-6108.2018012102
[8] LIANG C J, WANG C W. Assessing acute toxicity potential of persulfate ISCO treated water [J]. Chemosphere, 2013, 93(11): 2711-2716. doi: 10.1016/j.chemosphere.2013.08.078 [9] DONG H Y, WEI G F, YIN D Q, et al. Mechanistic insight into the generation of reactive oxygen species in sulfite activation with Fe(III) for contaminants degradation [J]. Journal of Hazardous Materials, 2020, 384: 121497. doi: 10.1016/j.jhazmat.2019.121497 [10] WANG H B, WANG S X, LIU Y Q, et al. Degradation of diclofenac by Fe(Ⅱ)-activated bisulfite: Kinetics, mechanism and transformation products [J]. Chemosphere, 2019, 237: 124518. doi: 10.1016/j.chemosphere.2019.124518 [11] YUAN Y N, ZHAO D, LI J J, et al. Rapid oxidation of paracetamol by cobalt(Ⅱ) catalyzed sulfite at alkaline pH [J]. Catalysis Today, 2018, 313: 155-160. doi: 10.1016/j.cattod.2017.12.004 [12] CHEN F, YANG Q, YAO F B, et al. Synergetic transformations of multiple pollutants driven by BiVO4-catalyzed sulfite under visible light irradiation: Reaction kinetics and intrinsic mechanism [J]. Chemical Engineering Journal, 2019, 355: 624-636. doi: 10.1016/j.cej.2018.08.182 [13] CHU Y Y, XU L J, GAN L, et al. Efficient destruction of emerging contaminants in water by UV/S(Ⅳ) process with natural reoxygenation: Effect of pH on reactive species [J]. Water Research, 2021, 198: 117143. doi: 10.1016/j.watres.2021.117143 [14] XIAO Q, YU S L. The role of dissolved oxygen in the sulfite/divalent transition metal ion system: Degradation performances and mechanisms [J]. Chemical Engineering Journal, 2021, 417: 129115. doi: 10.1016/j.cej.2021.129115 [15] CHEN X Y, MIAO W, YANG Y L, et al. Aeration-assisted sulfite activation with ferrous for enhanced chloramphenicol degradation [J]. Chemosphere, 2020, 238: 124599. doi: 10.1016/j.chemosphere.2019.124599 [16] WANG H, LIU Z H, ZHANG J, et al. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants [J]. Science of the Total Environment, 2019, 692: 107-116. doi: 10.1016/j.scitotenv.2019.07.134 [17] 宋作栋, 仇雁翎, 张华, 等. 水体中双酚类物质的赋存现状及研究进展 [J]. 环境化学, 2020, 39(6): 1496-1503. doi: 10.7524/j.issn.0254-6108.2019081413 SONG Z D, QIU Y L, ZHANG H, et al. The occurrence and research progress of bisphenol analogues in aquatic environment [J]. Environmental Chemistry, 2020, 39(6): 1496-1503(in Chinese). doi: 10.7524/j.issn.0254-6108.2019081413
[18] TAMURA H, GOTO K, YOTSUYANAGI T, et al. Spectrophotometric determination of iron(Ⅱ) with 1, 10-phenanthroline in the presence of large amounts of iron(Ⅲ) [J]. Talanta, 1974, 21(4): 314-318. doi: 10.1016/0039-9140(74)80012-3 [19] LI X C, FANG J Y, LIU G F, et al. Kinetics and efficiency of the hydrated electron-induced dehalogenation by the sulfite/UV process [J]. Water Research, 2014, 62: 220-228. doi: 10.1016/j.watres.2014.05.051 [20] GUO Y G, LOU X Y, FANG C L, et al. Novel photo-sulfite system: Toward simultaneous transformations of inorganic and organic pollutants [J]. Environmental Science & Technology, 2013, 47(19): 11174-11181. [21] KUO D T F, KIRK D W, JIA C Q. The chemistry of aqueous S(Ⅳ)-Fe-O2 system: State of the art [J]. Journal of Sulfur Chemistry, 2006, 27(5): 461-530. doi: 10.1080/17415990600945153 [22] REDDY K B, VANELDIK R. Kinetics and mechanism of the sulfite-induced autoxidation of Fe(II) in acidic aqueous solution [J]. Atmospheric Environment. Part A. General Topics, 1992, 26(4): 661-665. doi: 10.1016/0960-1686(92)90177-M [23] ABDELHALEEM A, CHU W, LIANG X L. Diphenamid degradation via sulfite activation under visible LED using Fe (III) impregnated N-doped TiO2 photocatalyst [J]. Applied Catalysis B:Environmental, 2019, 244: 823-835. doi: 10.1016/j.apcatb.2018.11.085 [24] YUAN Y N, LUO T, XU J, et al. Enhanced oxidation of aniline using Fe(III)-S(Ⅳ) system: Role of different oxysulfur radicals [J]. Chemical Engineering Journal, 2019, 362: 183-189. doi: 10.1016/j.cej.2019.01.010 [25] ZHANG L, CHEN L, XIAO M, et al. Enhanced decolorization of orange II solutions by the Fe(Ⅱ)–sulfite system under xenon lamp irradiation [J]. Industrial & Engineering Chemistry Research, 2013, 52(30): 10089-10094. [26] BUXTON G V, MCGOWAN S, SALMON G A, et al. A study of the spectra and reactivity of oxysulphur-radical anions involved in the chain oxidation of S(Ⅳ): A pulse and γ-radiolysis study [J]. Atmospheric Environment, 1996, 30(14): 2483-2493. doi: 10.1016/1352-2310(95)00473-4 [27] 熊龙. Fe(Ⅱ)/亚硫酸盐氧化体系的构建及其转化As(Ⅲ)机理研究[D]. 东营: 中国石油大学(华东), 2016. XIONG L. Construction of Fe(Ⅱ)/sulfite oxidation system and investigation of the transformation mechanisms of as(Ⅲ)[D]. Dongying: China University of Petroleum (Huadong), 2016(in Chinese).
[28] 李健欣, 汤一桢, 徐立杰, 等. Fe2+/S2O82-体系对双酚A的降解性能及优化 [J]. 环境化学, 2021, 40(11): 3580-3589. doi: 10.7524/j.issn.0254-6108.2020070905 LI J X, TANG Y Z, XU L J, et al. The performance and optimization of Fe2+/S2O82- process for bisphenol A degradation [J]. Environmental Chemistry, 2021, 40(11): 3580-3589(in Chinese). doi: 10.7524/j.issn.0254-6108.2020070905
[29] 刘颖, 郭依玮, 乔俊莲, 等. 环境pH条件下Fe2+活化过二硫酸盐降解有机污染物的效能与影响因素 [J]. 环境科学, 2022, 43(8): 4146-4153. LIU Y, GUO Y W, QIAO J L, et al. Investigation on the performance of organic contaminants degradation by Fe2+/PDS at environmentally relevant pH conditions [J]. Environmental Science, 2022, 43(8): 4146-4153(in Chinese).
[30] WANG Y B, ZHAO X, CAO D, et al. Peroxymonosulfate enhanced visible light photocatalytic degradation bisphenol A by single-atom dispersed Ag mesoporous g-C3N4 hybrid [J]. Applied Catalysis B:Environmental, 2017, 211: 79-88. doi: 10.1016/j.apcatb.2017.03.079 [31] NETA P, HUIE R E, ROSS A B. Rate constants for reactions of inorganic radicals in aqueous solution [J]. Journal of Physical and Chemical Reference Data, 1988, 17(3): 1027-1284. doi: 10.1063/1.555808 [32] KIRINO Y, OHKUMA T, KWAN T. Spin trapping with 5, 5-dimethylpyrroline-N-oxide in aqueous solution [J]. Chemical and Pharmaceutical Bulletin, 1981, 29(1): 29-34. doi: 10.1248/cpb.29.29 [33] ZAMORA P L, VILLAMENA F A. Theoretical and experimental studies of the spin trapping of inorganic radicals by 5, 5-dimethyl-1-pyrroline N-oxide (DMPO). 3. Sulfur dioxide, sulfite, and sulfate radical anions [J]. The Journal of Physical Chemistry. A, 2012, 116(26): 7210-7218. doi: 10.1021/jp3039169 [34] FINKELSTEIN E, ROSEN G M, RAUCKMAN E J. Spin trapping of superoxide and hydroxyl radical: Practical aspects [J]. Archives of Biochemistry and Biophysics, 1980, 200(1): 1-16. doi: 10.1016/0003-9861(80)90323-9 [35] GUAN Y H, MA J, LI X C, et al. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system [J]. Environmental Science & Technology, 2011, 45(21): 9308-9314. [36] CHEN L, TANG M, CHEN C, et al. Efficient bacterial inactivation by transition metal catalyzed auto-oxidation of sulfite [J]. Environmental Science & Technology, 2017, 51(21): 12663-12671. [37] DONG H Y, LI Y, WANG S C, et al. Both Fe(Ⅳ) and radicals are active oxidants in the Fe(II)/peroxydisulfate process [J]. Environmental Science & Technology Letters, 2020, 7(3): 219-224. [38] WANG Z, JIANG J, PANG S Y, et al. Is sulfate radical really generated from peroxydisulfate activated by iron(II) for environmental decontamination? [J]. Environmental Science & Technology, 2018, 52(19): 11276-11284. [39] DAS T N. Reactivity and role of SO5•- radical in aqueous medium chain oxidation of sulfite to sulfate and atmospheric sulfuric acid generation [J]. The Journal of Physical Chemistry A, 2001, 105(40): 9142-9155. doi: 10.1021/jp011255h [40] BRANDT C, van ELDIK R. Transition metal-catalyzed oxidation of sulfur(Ⅳ) oxides. Atmospheric-relevant processes and mechanisms [J]. Chemical Reviews, 1995, 95(1): 119-190. doi: 10.1021/cr00033a006 [41] LIANG J, DUAN X G, XU X Y, et al. Biomass-derived pyrolytic carbons accelerated Fe(Ⅲ)/Fe(Ⅱ) redox cycle for persulfate activation: Pyrolysis temperature-depended performance and mechanisms [J]. Applied Catalysis B:Environmental, 2021, 297: 120446. doi: 10.1016/j.apcatb.2021.120446 [42] WANG Z, QIU W, PANG S Y, et al. Further understanding the involvement of Fe(Ⅳ) in peroxydisulfate and peroxymonosulfate activation by Fe(II) for oxidative water treatment [J]. Chemical Engineering Journal, 2019, 371: 842-847. doi: 10.1016/j.cej.2019.04.101 [43] DARSINOU B, FRONTISTIS Z, ANTONOPOULOU M, et al. Sono-activated persulfate oxidation of bisphenol A: Kinetics, pathways and the controversial role of temperature [J]. Chemical Engineering Journal, 2015, 280: 623-633. doi: 10.1016/j.cej.2015.06.061 [44] LI X N, WANG Z H, ZHANG B, et al. FexCo3xO4 nanocages derived from nanoscale metal-organic frameworks for removal of bisphenol A by activation of peroxymonosulfate [J]. Applied Catalysis B:Environmental, 2016, 181: 788-799. doi: 10.1016/j.apcatb.2015.08.050 -












 下载:
下载: