-
目前,寻找一种可以替代传统化石燃料的可再生能源已成为能源领域的研究热点,绿色、健康、可持续发展是当前环保领域的关键词[1]. 微生物燃料电池( microbial fuel cells,MFCs) 是利用产电微生物(electroactive microorganisms,EABs)将有机污染物中的化学能转化为电能的装置[2]. MFCs 通常由两个电极室组成,在阳极室内,产电菌释放电子和质子,电子经过外电路转移到阴极释放携带的能量,而质子经过离子交换膜转移到阴极[3],在闭环条件下阳极室EABs发生电子转移产生电流,包括直接电子转移(direct electron transfer,DET)和介导电子转移(mediated electron transfer,MET)两种方式将微生物代谢产生的电子转移到阳极上[1]. 但MFCs低功率密度使其难以商业化,因此,优化MFCs的功率密度属于当前的研究热点之一. 在驱动MFCs运行的各种机制中,EABs与阳极之间的细胞外电子传递(extracellular electron transport,EET)成为最重要的机制之一,对EET过程的调控和优化已成为一种常见的策略.
为了提高MFCs的功率密度,研究人员从提高阳极性能、EABs提纯和富集等方面开发了一系列方法来增强阳极与EABs的相互作用,包括使用聚合物涂层和3D阳极材料、结构优化或掺杂导电金属离子、EABs基因工程等[4]. 其中,高性能的阳极材料因具有成本低、制备方法简单、导电性好、生物相容性好,稳定性高等[5]优点成为研究热点. 生物炭材料以稳定性高、易化学改性、成本低、易获得等优点成为公认的阳极材料. 已研究的生物碳材料有松果[6]、天然丝瓜海绵[7]和杏仁壳[8]等. 生物炭阳极材料具有均匀分布的大孔结构,有利于发电微生物的粘附,能避免堵塞问题[9].
已有研究表明,氮可以作为一种元素用于修饰阳极材料,增强产电微生物与电极之间的相互作用[10]. 据报道,He等[11]首次证明向碳纸中注入N+等离子体可以通过降低电荷转移阻力提高生物相容性,从而提高MFCs的发电能力. Wu等[12]使用氮掺杂碳材料,在原子上匹配介导电池外电子转移的扩散氧化还原物种的反应位点,证明氮的贡献. Yu等[13]将氮掺杂碳纳米颗粒引入到MFCs阳极中,证明氮可以促进产电微生物细胞外电子传递过程中黄素的分泌,增强细菌与电极之间的相互作用.
本文从改善阳极材料表面物理化学性质的角度出发,用吐司作为多孔碳前驱体,三聚氰胺为氮源,直接烧制氮掺杂三维碳材料,并将其与不掺杂氮的阳极材料和未改性的商用碳布材料进行比较. 用混合地杆菌Geobacter和希瓦氏菌Shewanella在MFCs中培养后,对其性能进行评价和分析. 三维多孔碳阳极材料与氮元素结合,可以同时提供更大的比表面积、更好的电导率和良好的生物相容性以及适应性强的混合微生物群落,使MFCs具有更好的功率密度,并进一步研究氮在促进EET过程中的作用机制.
-
吐司原材料购于昆明沃尔玛超市有限公司,主要成分是面粉、酵母、玉米油等,由碳、氮、氧等元素组成,吐司内部多孔且均匀分布,将购买的吐司切成2 cm3×2 cm3×1.5 cm3的小块于室温条件下风干一夜后保存备用,然后以三聚氰胺为氮源,在氩气气氛下,分别于700、800、900、1000、1100 ℃的管式炉中热解2 h,制备NBs材料,标记为NB700、NB800、NB900、NB1000和NB1100. 将切成小块的吐司置于1000 ℃的管式炉中热解2 h,不需要外部氮源,制成B1000. 相应地,将普通碳布型号W0S1009切成小块(1 cm2×1 cm2)制备CC电极. 将所有材料切成小块(1 cm3×1 cm3×1 cm3),用钛丝连接,制备NBs、B1000和CC阳极,最后用丙酮、异丙醇、乙醇和去离子水对所有材料进行洗涤,干燥.
-
本次研究利用热重分析仪(TGA)和差示扫描量热分析(DSC)在室温至1000 ℃,N2流量为60 mL·min−1,加热速度为2 ℃·min−1时进行测定;Brunner-Emmet-Teller (BET)测量由TriStar II(3020)仪器进行;利用X射线衍射仪(XRD)对材料的组成进行表征;扫描XPS微探针系统(PHI5000 Versaprobe-II)在15 kV、50 W条件下对吐司中成功掺杂的氮元素进行X射线光电子能谱(XPS)测定;在5 kV 5 μA的条件下,利用扫描电子显微镜(Hitachi SU8010)对电极的宏观形貌和生物膜的定殖进行表征,在进行SEM测量之前,所有材料都喷涂了铂以增强导电性;对于阳极材料的电化学表征、氮掺杂阳极促进电活性微生物EET能力的机理和循环伏安图(CV),在中国上海CHI660E电化学工作站(CHI Instrument, Shanghai, China)使用差示脉冲伏安法(DPVs)和电化学阻抗谱(EIS)测试评估,阳极材料的电化学性能测试在三电极体系中进行,三电极分别为工作电极、饱和甘汞参比电极(SCE)和铂丝对电极,在不同的扫描速率和各自的开路电压下进行了研究[14].
-
本实验使用的反应器是双室MFCs反应器,将所有组装好的阳极在磷酸缓冲盐溶液(phosphate buffer saline,PBS)中浸泡一夜,使电极材料和电解质之间充分接触,PBS溶液的主要成分为NaH2PO4·2H2O(2.77 g·L−1)、Na2HPO4·2H2O(11.55 g·L−1)、NH4Cl(0.31 g·L−1)、KCl(0.13 g·L−1). MFCs电池使用了经典的反应堆屏蔽双室,阳极室和阴极室的体积均为100 mL,阴极为市售的碳刷电极,使用前用无水乙醇和去离子水清洗干净后烘干备用. 阳极电解液的组成为NaH2PO4·2H2O (2.77 g·L−1)、Na2HPO4·2H2O(11.55 g·L−1)、NH4Cl(0.3 g·L−1)、KCl(0.13 g·L−1)、CH3COONa(2 g·L−1)、混合维生素(10 mol·L−1)和矿物质元素(12.5 mol·L−1),阴极电解液的组成为K3[Fe(CN)6](2 g·L−1)和KCl(3.715 g·L−1)[15]. 阳极液在使用前用氩气冲洗30 min,将氧气排尽.
实验开始时,从活性厌氧污泥中接种5 mL预培养细菌. MFCs连接一个1 kΩ外部加载电阻,并连接到数据采集器. 当MFCs电压降至50 mV时,更换新的阳极液和阴极液,测试电压循环稳定运行4个周期后的功率密度和极化曲线.
-
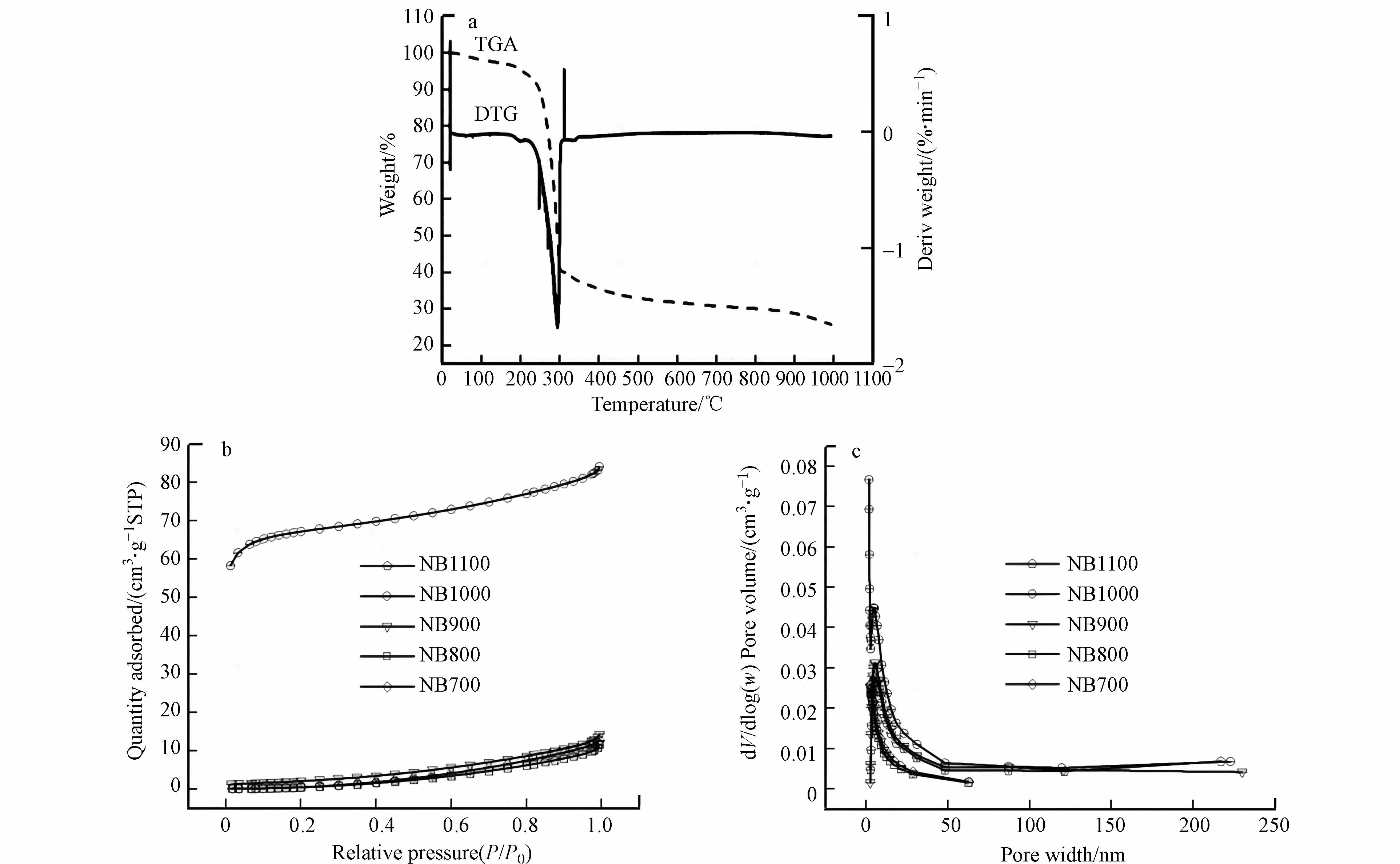
在氩气气氛下进行TGA-DSC分析,对吐司炭化过程进行监测. 如图1a所示,失重主要有3种原因,在第一阶段,200—250 ℃的温度区间内,主要是由于去除了吸附在孔结构中水分和空气;在第二阶段,250—350 ℃的温度区间内,主要是由于油脂类物质的分解,同时伴随着对孔结构中的已经吸附水分和残留空气的进一步移除;在第三阶段,325—1100 ℃的温度区间内,主要是样品的碳化,在这个期间内完成了石墨碳的形成以氮原子掺杂到碳材料表面的过程. NBs的比表面积和孔径分布见图1b、c. BET表面积在1000 ℃(216.664 m2·g−1)时达到最大值,在1100 ℃进一步升高后减小(表1). 此外,在1000 ℃时介孔数量达到最大值,有利于基体的扩散和产物的去除.
-
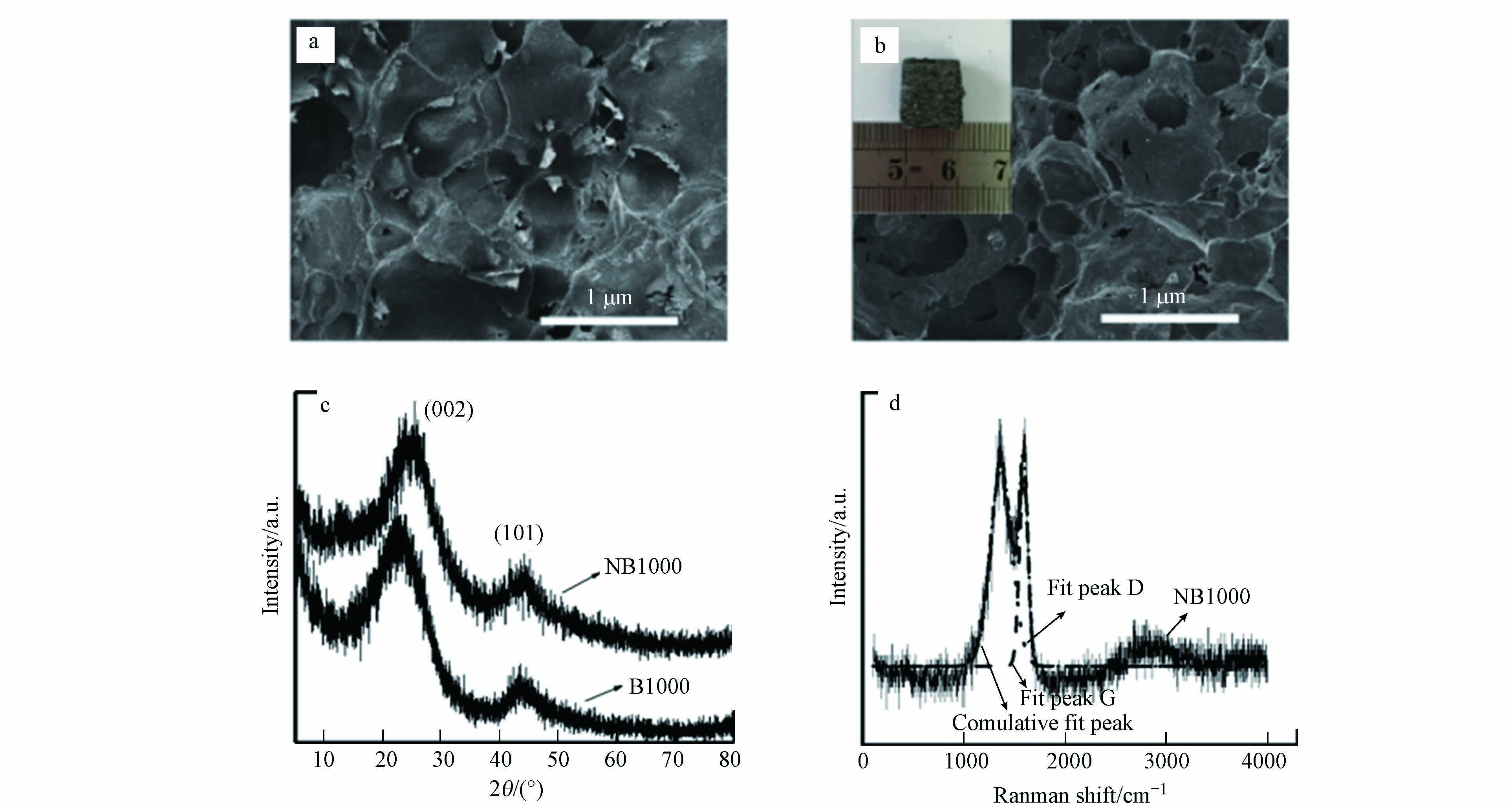
图2a和图2b分别为碳化前和1000 ℃下碳化后的吐司SEM图片. 碳化后的吐司具有孔结构均匀分布且相互连通的大孔开放结构,碳化过程中多孔结构保存良好,丰富的多孔性使电活性微生物更好的定殖,避免了代谢过程中的堵塞. 此外,该结构可以为暴露表面提供高活性的电荷储存位点,促进电子转移. NB1000和B1000的XRD谱图均显示出两个宽的XRD衍射峰,分别属于石墨碳在2θ角27.3°和43°处的晶面(002)和(101)衍射峰(图2c). 与B1000相比,NB1000的(002)发生了轻微的偏移,这是由于氮原子的掺入使晶体的规则性发生轻微的扭曲. 此外,通过拉曼光谱来确定样品的石墨化程度(图2d)[16]. 光谱中有2个峰位于1585 cm−1和1379 cm−1,峰面积比对应的ID/IG值反映了石墨化程度[17]. NB1000的ID/IG值小于1,说明样品的石墨化效果较好,这与XRD结果一致,进一步证明了对碳前驱体碳化操作的成功进行.
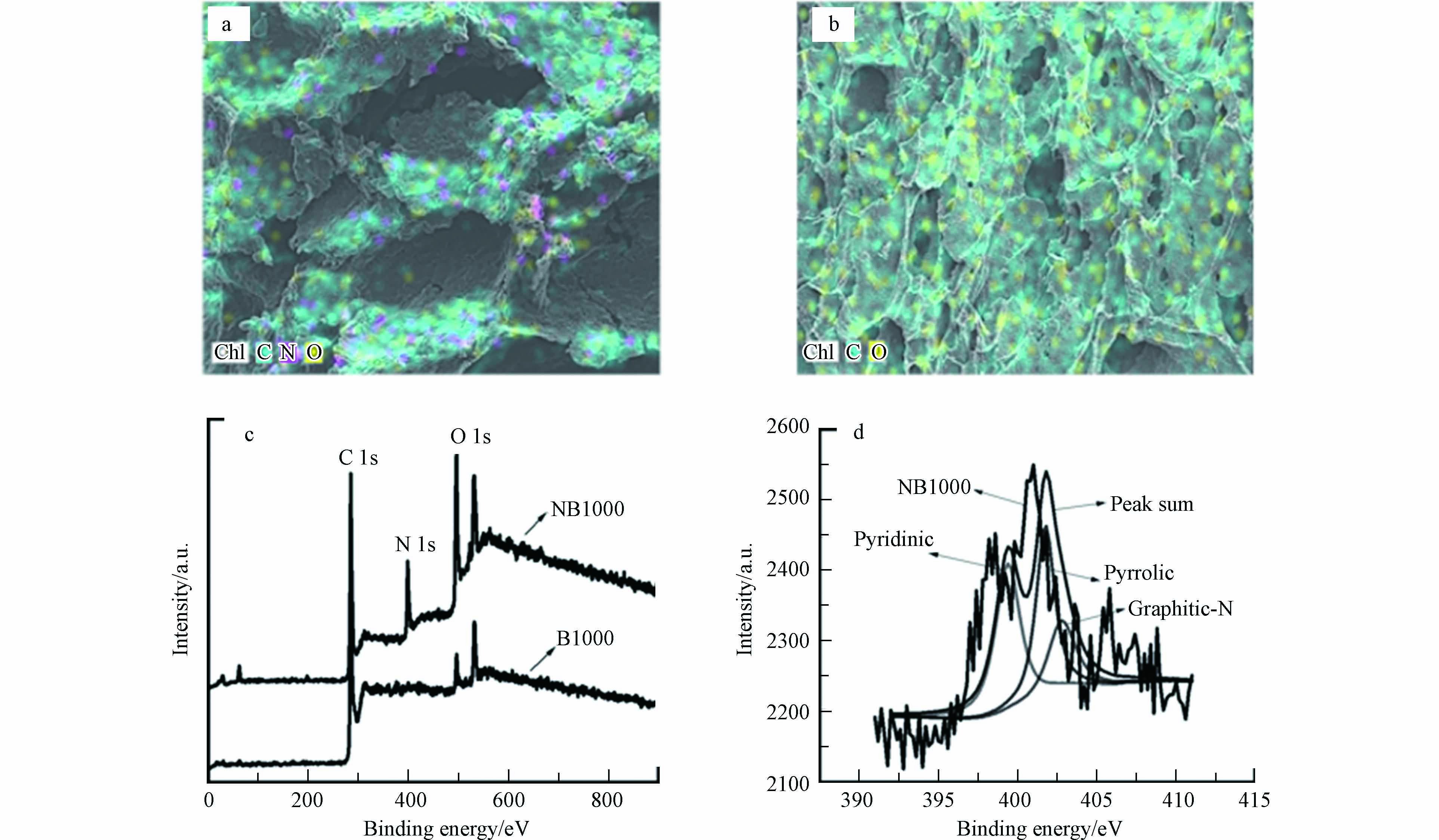
图3a和图3b中EDX模式的元素组成分析证实了三聚氰胺中的氮原子在焙烧过程中成功掺杂进入碳前驱体. 然后用XPS进一步研究合成的NB1000和B1000的元素组成,其中NB1000的氮含量为16%,高于氨通风法和热解法制备的掺氮碳材料[15,18-19]. 图3cXPS测量光谱进一步证实了NB1000中氮的存在,且与XRD和拉曼结果一致. 图3d的N1s光谱表明,NB1000中存在3种氮态,分别是吡啶氮(399 eV)、吡咯氮(401 eV)和石墨氮(401.8 eV),且3种氮态的比例相等[20-21]. 研究表明,通过氨和硝酸处理含氮官能团修饰碳材料或在碳基阳极中掺入氮元素是提高MFCs性能的有效策略[15]. 吡咯氮的存在容易使产电微生物分泌外膜c型细胞色素传递电子,从而增强电活性微生物的胞外电子传递功能[22].
-
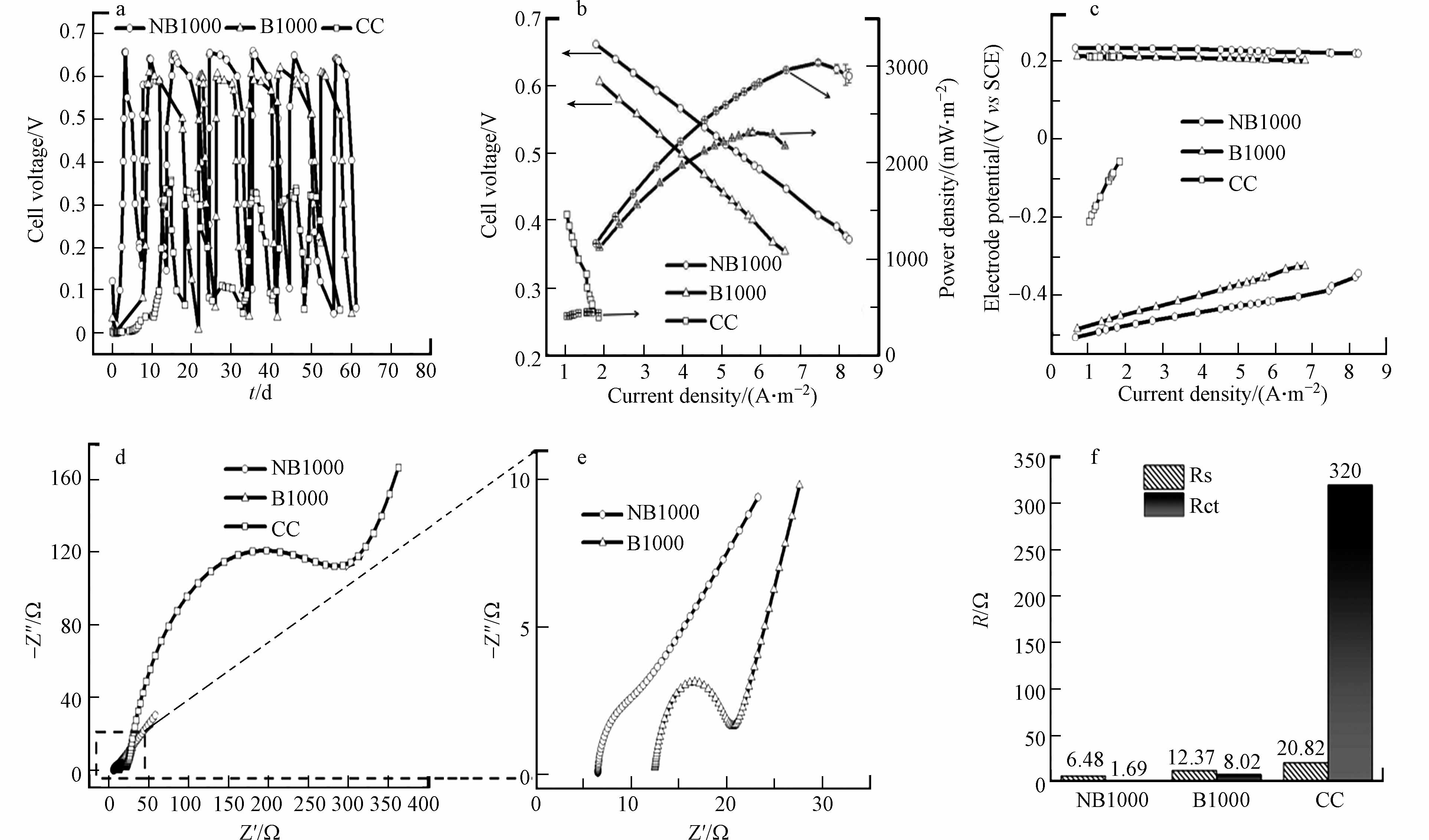
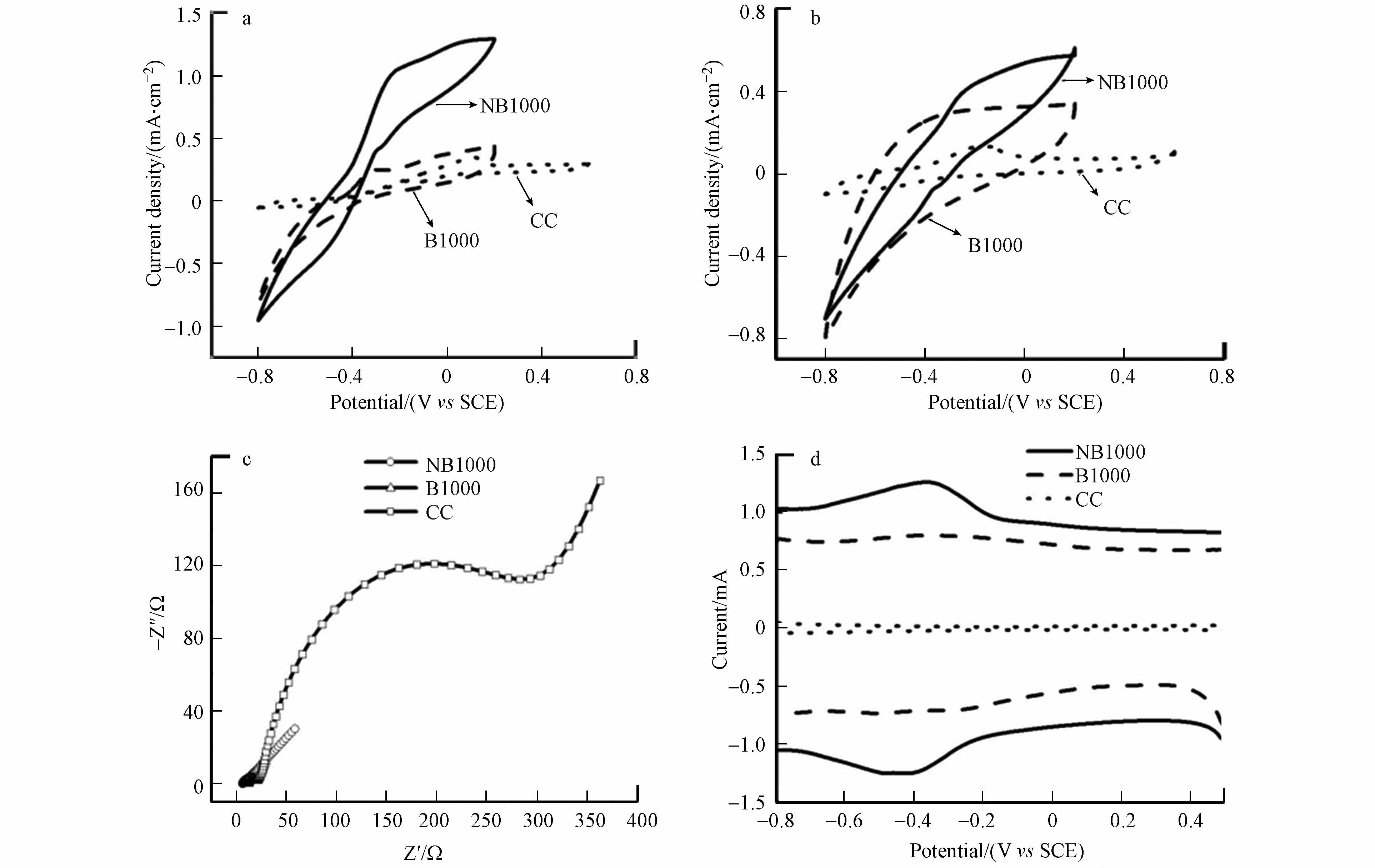
以NB1000和B1000作为阳极,接种典型的电活性微生物Shewanella oneidensis MR-1和Geobacter,以常用的plain carbon cloth (CC)电极作为对照参比,进一步研究了典型H型双室微生物燃料电池 (H-MFCs)的性能. 阴极是商用碳刷电极,包含100 mL铁氰化钾(50 mol·L−1 K3[Fe(CN)6]和50 mol·L−1 KCl),阳极室组分为90 mL PBS与乳酸钠溶液以及10 mL混菌MFCs阳极出水液. 图4a显示了MFCs的激活情况和输出电压分布图. 研究发现,配置NB1000阳极的MFCs需要大约3 d的激活和微生物富集才能达到稳定的输出电压,而配置B1000和CC阳极的MFCs分别需要9 d和12 d才能达到最大输出电压. H-MFCs表现出细胞电压的可重复性,NB1000的细胞电压最大值为(625±25) mV,分别是B1000((595±5) mV)和CC((325±5) mV)的1.05和1.92倍. 结果表明,NB1000电极的表面更适合电活性微生物的附着和生长,比B1000和CC电极具有更好的亲和力和生物相容性. 经过3次稳定循环后,测量MFC的极化和功率密度曲线(图4b)和电极电势曲线(图4c),评价其发电能力. 配备有NB1000阳极的MFCs在3个电极中具有最高的面积功率密度和最高面电流密度分别为3049.714 mW·m−2和7.4464 A·m−2,B1000阳极的面积功率密度和电流密度分别为2323.14 mW·m−2和5.77 A·m−2,CC阳极的面积功率密度和电流密度分别为465.88 mW·m−2和4.83 A·m−2,NB1000阳极的最大面积功率密度分别是B1000和CC阳极的1.31倍和6.54倍,NB1000阳极的最大面积电流密度分别是B1000和CC阳极的1.29倍和1.54倍. 图4c说明不同的MFCs中,阴极的电位几乎相同,而阳极电位有明显的变化. 与B1000和CC阳极相比,NB1000阳极产生更高的功率密度是由于阳极电位增加,这意味着在NB1000阳极中存在更低的过电势(包括活化极化和欧姆损耗),这是由于NB1000中有氮掺入. 图4d和4e所示,利用EIS测量MFCs的电阻进一步评价产电微生物附着后的阻抗大小. 由图4d和4e可以看出,在形成生物膜后的3个阳极中,NB1000阳极的欧姆电阻(Rs)和电荷转移电阻(Rct)最低,说明NB1000阳极的电极/电解质界面电子转移速度更快. 此外,图4f可以看出由于阳极材料上生物膜的形成,导致Rs和Rct降低,说明细菌与电极表面之间的电子传递速度加快[23].
-
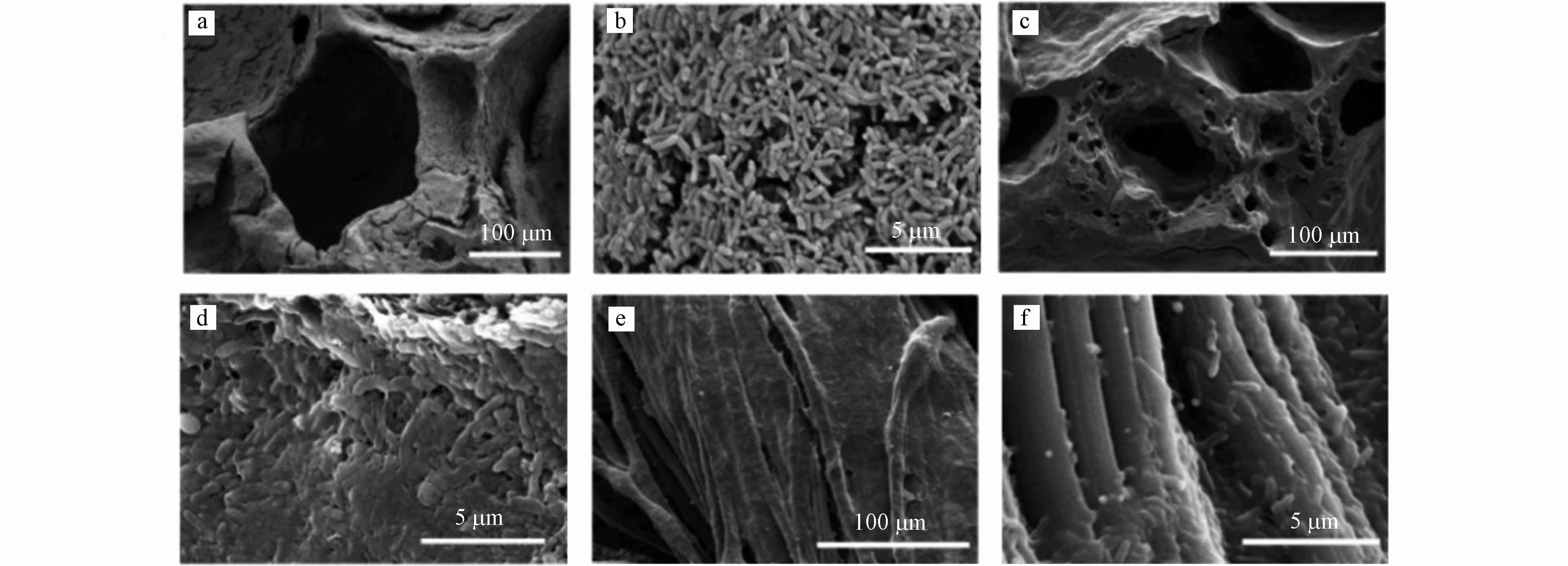
扫描电镜图像(图5a-b、5c-d和5e-f)显示了MFCs经过5个循环培养后((55±5) d),在NB1000、B1000和CC阳极表面上定殖的生物膜情况.
与B1000和CC阳极表面相比,NB1000阳极上生长的生物膜几乎覆盖了整个阳极. 而B1000和CC阳极的厚度和微生物分布均匀程度均不如NB1000阳极,这说明NB1000阳极表面对电活性微生物的更容易定殖,具有更好的生物相容性,这是由于NB1000阳极掺杂了氮.
-
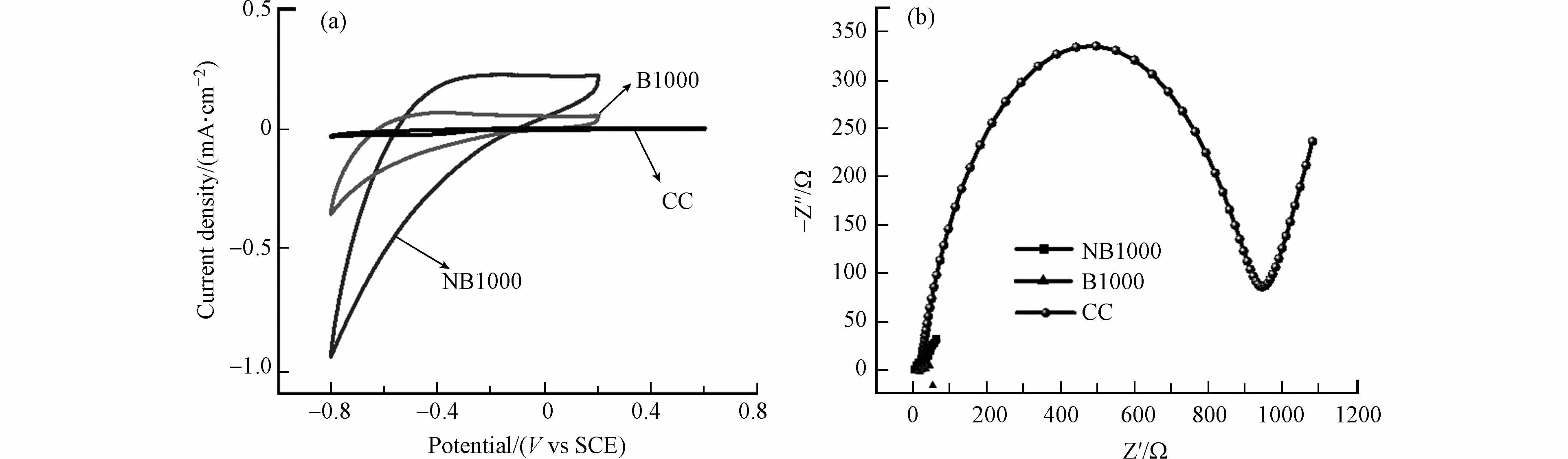
为了探讨NB1000阳极促进电极与电活性微生物间EET的机理,采用CVs(分别在有乙酸钠底物条件和无乙酸钠底物条件下)和DVPs电化学测试进行研究. 图6a显示了NB1000、B1000和CC阳极经过4个稳定的电压输出循环后在有底物条件下的CVs曲线. 结果表明,NB1000电极的电流密度为1.29 mA·cm−2,B1000电极的电流密度为0.44 mA·cm−2,CC电极的电流密度为0.30 mA·cm−2,NB1000电极的电流密度分别是B1000和CC阳极的2.9倍和4.3倍,表明NB1000阳极具有更好的电催化活性.
S形的CVs曲线是由阳极上的产电微生物Geobacter和Shewanella产生的,这表明了阳极上的电活性微生物对乙酸盐具有催化氧化作用[24]. 此外,在生物膜定殖前添加2 g·L−1乳酸和PBS溶液,CVs曲线没有呈现S形(图7),这一结果表明在阳极上定殖的产电微生物消耗的是乳酸而不是阳极材料. 乳酸耗尽后,进行无底物条件下的CVs来研究与微生物进行EET相关的氧化还原对(图6b),NB1000阳极有两对氧化还原峰,分别为−0.422 V(阴极峰)、−0.381 V(阳极峰)和 −0.0193 V(阴极峰)、−0.0482 V(阴极峰). NB1000和CC阳极均有一对氧化还原峰,分别为−0.422 V(阴极峰)和 −0.381 V(阳极峰). 为了消除包括溶液组分和背景信号等因素的干扰,用高灵敏度的DPVs对EET相关的氧化还原进行了研究测试(图6d)[25]. NB1000阳极存在两对氧化还原峰,分别为 −0.422 V(阴极峰)、−0.381 V(阳极峰)和−0.0193 V(阴极峰)、−0.0482 V(阴极峰). 其中−0.422 V(阴极峰)和−0.381 V(阳极峰)的氧化还原峰与外膜c型细胞色素OmcA[26]的电势一致. −0.019 V(阴极峰)和0.048 V(阴极峰)接近外膜c型细胞色素MtrC[27]的电位. 已有研究表明,外膜c型细胞色素OmcA和MtrC是与Geobacter和Shewanella[28]进行EET过程相关的必要蛋白[29],除外膜蛋白质外,产电微生物还会分泌电子介导体将电子转递到胞外,这也是产电微生物进行细胞外电子传递的重要途经[30]. 与NB1000阳极相比,B1000和CC阳极只出现一对氧化还原峰,这对峰与外膜c型细胞色素OmcA有关. 这一结果有力地证明了氮掺杂操作有利于电活性微生物分泌c型细胞色素,并通过c型细胞色素将电子转移到阳极上. 此外,石墨氮和吡咯氮是氮掺杂操作的主要贡献者,从分子水平上看,吡咯氮与卟啉铁(外膜c型细胞色素的结构中心)之间具有更好的亲和力[19,31].
-
(1)以吐司为碳前驱体,在1000 ℃下焙烧制备的NB1000电极具有最高的比表面积216.664 m2·g−1和均匀分布的中孔结构,因此选择1000 ℃作为最佳的碳化条件.
(2)氮掺杂的三维多孔碳阳极材料NB1000作为MFCs的阳极,最大功率密度可达3049.714 mW·m−2,分别是不掺杂氮的碳材料N1000和传统碳布阳极CC的1.31倍和6.54倍;最大面积电流密度为7.4464 A·m−2,分别是B1000和CC阳极的1.29倍和1.54倍.
(3)使用廉价的市售吐司成功制备了掺杂氮元素的三维多孔碳基材料,利用氮元素的生物相容性促进产电微生物分泌活度更高的OmcA和MtrC细胞色素从而促进细胞外电子传递. 其中,NB1000中的吡咯氮的结构中心卟啉铁具有较低的吸附能,因此吡咯氮对于产电微生物分泌外膜蛋白MtrC具有贡献作用.
氮掺杂多孔碳材料阳极制备及其在微生物燃料电池上的应用
Preparation of nitrogen-doped porous carbon anode and its application in microbial fuel cells
-
摘要: 微生物燃料电池(MFCs)作为一种可以替代传统能源的生物电化学系统引起研究者的极大兴趣,其阳极材料的构造是目前的研究热点. 本文从改善阳极材料表面物理化学性质的角度出发,用吐司作为多孔碳前驱体,三聚氰胺为氮源,直接烧制氮掺杂三维碳材料,并与不添加氮源的阳极材料和未改性的商用碳布进行比较. 制备的掺氮NB1000阳极具有较大的比表面积(216.664 m2·g−1)和优良的电导率. 利用Geobacter和Shewanella混合菌落在微生物燃料电池(MFCs)中进行培养和性能评价,NB1000阳极的微生物燃料电池最大面功率密度为3049.714 mW·m−2,电流密度为7.4464 A·m−2,分别是普通碳布阳极的6.54倍和1.54倍. 结果表明,NB1000作阳极的MFCs具有较高的功率密度,主要归因于阳极中引入氮掺杂,促进了产电微生物胞外电子传递过程所需的外膜c型细胞色素OmcA和MtrC的分泌.Abstract: Microbial fuel cells (MFCs), as a bioelectrochemical system that can replace traditional energy sources, have attracted great interest of researchers, and the construction of anode materials is the current research focus. In order to improve the physical and chemical properties of the surface of the anode material, the nitrogen-doped three-dimensional carbon material was directly sintered by using toast as the porous carbon precursor and melamine as the nitrogen source, and compared with the anode material without adding nitrogen source and the unmodified commercial carbon cloth. The nitrogen-doped NB1000 anode has a large specific surface area (216.664 m2·g−1) and excellent conductivity. A mixed Geobacter and Shewanella colony was used to culture and evaluate the performance of MFCs, and the maximum areal power density and current density of the microbial fuel cell with NB1000 anode were 3049.714 mW·m−2 and 7.4464 A·m−2. It is 6.54 times and 1.54 times that of ordinary carbon cloth anode, respectively. The results showed that the high power density of MFCs from NB1000 anode was due to the introduction of nitrogen into the anode, which promoted the secretion of c-type cytochromes OmcA and MtrC in the outer membrane, which were required for the extracellular electron transport process of electrogenic microorganisms.
-

-
图 4 (a)输出电压曲线 (b)极化曲线和功率密度曲线 (c) 电极电位(vs SCE)与电流密度的关系 (d) 生物膜形成后的Nyquist图 (e)图d的Nyquist放大图 (f) 生物膜形成后的拟合欧姆电阻和电荷转移电阻
Figure 4. (a) Output voltage curve (b) Polarization curve and power density curve (c) Relation between electrode potential (VS SCE) and current density (d) Nyquist plot after biofilm formation (e) Nyquist magnification of Figure d (f) fitted ohmic resistance and charge transfer resistance after biofilm formation
图 6 N1000,B1000和CC阳极在有底物条件下的CVs曲线(a),在无底物条件下的CVs曲线(b),稳定培养四个周期后的EIS曲线(c),在底物耗尽后于PBS溶液中的DPV曲线(d)
Figure 6. CVs curves of NB1000, B1000 and CC anodes with substrate (a), CVs curves without substrate (b), EIS curves after four cycles of stable culture (c) ,DPV curves in PBS solution after substrate depletion (d)
表 1 NBs的比表面积
Table 1. Specific surface area of NBs
样品
SamplesNB1100 NB1000 NB9000 NB800 NB700 SBET/(m2·g−1) 61.028 216.664 92.045 82.813 45.634 Note: SBET: the specific surface area. -
[1] SLATE A J, WHITEHEAD K A, BROWNSON D A C, et al. Microbial fuel cells: An overview of current technology [J]. Renewable and Sustainable Energy Reviews, 2019, 101: 60-81. doi: 10.1016/j.rser.2018.09.044 [2] 蒋杭城, 许蕾, 谢静, 等. 超声预处理对剩余污泥微生物燃料电池性能的影响 [J]. 环境化学, 2015, 34(5): 989-994. doi: 10.7524/j.issn.0254-6108.2015.05.2014091201 JIANG H C, XU L, XIE J, et al. The effect of ultrasonic pretreated excess sludge on the performance of microbial fuel cells [J]. Environmental Chemistry, 2015, 34(5): 989-994(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.05.2014091201
[3] 刘岩婉晶, 赵倩楠, 葛润蕾, 等. 微生物燃料电池阳极产电菌电子转移主要机制及其影响因素 [J]. 环境化学, 2019, 38(8): 1745-1756. doi: 10.7524/j.issn.0254-6108.2018101005 LIU Y, ZHAO Q N, GE R L, et al. Research progress on electron transfer mechanism and its influencing factors on microbial fuel cells anode exoelectrogens [J]. Environmental Chemistry, 2019, 38(8): 1745-1756(in Chinese). doi: 10.7524/j.issn.0254-6108.2018101005
[4] PALANISAMY G, JUNG H Y, SADHASIVAM T, et al. A comprehensive review on microbial fuel cell technologies: Processes, utilization, and advanced developments in electrodes and membranes [J]. Journal of Cleaner Production, 2019, 221: 598-621. doi: 10.1016/j.jclepro.2019.02.172 [5] CHEN S, PATIL S A, BROWN R K, et al. Strategies for optimizing the power output of microbial fuel cells: Transitioning from fundamental studies to practical implementation[J]. Applied Energy, 2019, 233–234: 15-28. [6] WANG R W, LIU D, YAN M, et al. Three-dimensional high performance free-standing anode by one-step carbonization of pinecone in microbial fuel cells [J]. Bioresource Technology, 2019, 292: 121956. doi: 10.1016/j.biortech.2019.121956 [7] YUAN Y, ZHOU S G, LIU Y, et al. Nanostructured macroporous bioanode based on polyaniline-modified natural loofah sponge for high-performance microbial fuel cells [J]. Environmental Science & Technology, 2013, 47(24): 14525-14532. [8] LI M Z, CI S Q, DING Y C, et al. Almond shell derived porous carbon for a high-performance anode of microbial fuel cells [J]. Sustainable Energy & Fuels, 2019, 3(12): 3415-3421. [9] ZOU L, QIAO Y, WU Z Y, et al. Tailoring Unique Mesopores of Hierarchically Porous Structures for Fast Direct Electrochemistry in Microbial Fuel Cells [J]. Advanced energy materials, 2016, 6(4): 1501535.1-1501535.6. [10] CAI T, MENG L J, CHEN G, et al. Application of advanced anodes in microbial fuel cells for power generation: A review [J]. Chemosphere, 2020, 248: 125985. doi: 10.1016/j.chemosphere.2020.125985 [11] HE Y R, XIAO X, LI W W, et al. Enhanced electricity production from microbial fuel cells with plasma-modified carbon paper anode [J]. Physical Chemistry Chemical Physics:PCCP, 2012, 14(28): 9966-9971. doi: 10.1039/c2cp40873b [12] WU X X, QIAO Y, GUO C X. et al. Nitrogen doping to atomically match reaction sites in microbial fuel cells[J]. Communications Chemistry, 2020, 68(3). [13] YU Y Y, GUO C X, YONG Y C, et al. Nitrogen doped carbon nanoparticles enhanced extracellular electron transfer for high-performance microbial fuel cells anode [J]. Chemosphere, 2015, 140: 26-33. doi: 10.1016/j.chemosphere.2014.09.070 [14] WANG R W, YAN M, LI H D, et al. FeS2 Nanoparticles decorated graphene as microbial-fuel-cell anode achieving high power density[J]. Advanced Materials, 2018, 30(22): 1800618. [15] OH S, MIN B, LOGAN B E. Cathode performance as a factor in electricity generation in microbial fuel cells [J]. Environmental Science & Technology, 2004, 38(18): 4900-4904. [16] QU L T, LIU Y, BAEK J B, et al. Nitrogen-doped graphene as efficient metal-free electrocatalyst for oxygen reduction in fuel cells [J]. ACS Nano, 2010, 4(3): 1321-1326. doi: 10.1021/nn901850u [17] ENGLERT J M, VECERA P, KNIRSCH K C, et al. Scanning-Raman-microscopy for the statistical analysis of covalently functionalized graphene [J]. ACS Nano, 2013, 7(6): 5472-5482. doi: 10.1021/nn401481h [18] ZHU N W, CHEN X, ZHANG T, et al. Improved performance of membrane free single-chamber air-cathode microbial fuel cells with nitric acid and ethylenediamine surface modified activated carbon fiber felt anodes [J]. Bioresource Technology, 2011, 102(1): 422-426. doi: 10.1016/j.biortech.2010.06.046 [19] YOU S J, MA M, WANG W, et al. 3D macroporous nitrogen-enriched graphitic carbon scaffold for efficient bioelectricity generation in microbial fuel cells [J]. Advanced Energy Materials, 2017, 7(4): 1601364. doi: 10.1002/aenm.201601364 [20] GONG K P, DU F, XIA Z H, et al. Nitrogen-doped carbon nanotube arrays with high electrocatalytic activity for oxygen reduction [J]. Science, 2009, 323(5915): 760-764. doi: 10.1126/science.1168049 [21] ZHANG Y, WANG C W, HOU H S, et al. Sodium‐ion batteries: Nitrogen doped/carbon tuning yolk‐like TiO2 and its remarkable impact on sodium storage performances [J]. Advanced Energy Materials, 2016, 7(4): 1600173.1-1600173.12. [22] ZHANG L J, HE W H, YANG J C, et al. Bread-derived 3D macroporous carbon foams as high performance free-standing anode in microbial fuel cells [J]. Biosensors & Bioelectronics, 2018, 122: 217-223. [23] HU M H, LI X, XIONG J, et al. Nano-Fe3C@PGC as a novel low-cost anode electrocatalyst for superior performance microbial fuel cells [J]. Biosensors and Bioelectronics, 2019, 142: 111594. doi: 10.1016/j.bios.2019.111594 [24] CARMONA-MARTINEZ A A, HARNISCH F, FITZGERALD L A, et al. Cyclic voltammetric analysis of the electron transfer of Shewanella oneidensis MR-1 and nanofilament and cytochrome knock-out mutants [J]. Bioelectrochemistry (Amsterdam, Netherlands), 2011, 81(2): 74-80. doi: 10.1016/j.bioelechem.2011.02.006 [25] SHARMA M, JAIN P, VARANASI J L, et al. Enhanced performance of sulfate reducing bacteria based biocathode using stainless steel mesh on activated carbon fabric electrode [J]. Bioresource Technology, 2013, 150: 172-180. doi: 10.1016/j.biortech.2013.09.069 [26] HAO L, YU J, XU X, et al. Nitrogen-doped MoS2/carbon as highly oxygen-permeable and stable catalysts for oxygen reduction reaction in microbial fuel cells [J]. Journal of Power Sources, 2017, 339: 68-79. doi: 10.1016/j.jpowsour.2016.11.041 [27] ZHAO S, LIU P, NIU Y Y, et al. A novel early warning system based on a sediment microbial fuel cell for in situ and real time hexavalent chromium detection in industrial wastewater [J]. Sensors (Basel, Switzerland), 2018, 18(2): 642. [28] HUANG Y X, LIU X W, XIE J F, et al. Graphene oxide nanoribbons greatly enhance extracellular electron transfer in bio-electrochemical systems [J]. Chemical Communications (Cambridge, England), 2011, 47(20): 5795-5797. doi: 10.1039/c1cc10159e [29] HARTSHORNE R S, REARDON C L, ROSS D, et al. Characterization of an electron conduit between bacteria and the extracellular environment [J]. Proceedings of the National Academy of Sciences of the United States of America, 2009, 106(52): 22169-22174. doi: 10.1073/pnas.0900086106 [30] WHITE G F, EDWARDS M J, GOMEZ-PEREZ L, et al. Mechanisms of bacterial extracellular electron exchange [J]. Advances in Microbial Physiology, 2016, 68: 87-138. [31] JIMÉNEZ-RAMÍREZ L E, CAMACHO-MOJICA D C, MUÑOZ-SANDOVAL E, et al. First-principles study of transition metal adsorbed on porphyrin-like motifs in pyrrolic nitrogen-doped carbon nanostructures [J]. Carbon, 2017, 116: 381-390. doi: 10.1016/j.carbon.2017.02.018 -



 下载:
下载: