-
近年来,大量的抗生素如四环素类和氟喹诺酮类等被广泛用作促进畜禽生长、预防和治疗感染的饲料添加剂[1-2]. 尤其是四环素类抗生素,它是一类广谱性抗生素,因杀菌效率高和成本低等已广泛应用于畜牧业的. 然而,30%—90%的四环素类污染物不能被畜禽所吸收消化[3],在畜禽体内未代谢的四环素类会通过尿液、粪便排泄进入水环境,未经处理的富含四环素类的废水会对环境和人体健康造成潜在的危害,还会引起一些致病菌产生抗药性基因的环境风险[2,4]. 因此,必须采取高效的治理措施对这些残留水体中的抗生素进行消减处理. 到目前为止,很多处理方法如吸附法、生物法和化学氧化法都被应用于抗生素废水的治理,其中高级氧化法因效率高和操作简单等优势受到了广泛的应用[5-7]. Fenton 法是高级氧化法的一种,是利用溶解性Fe2+与H2O2反应所生成的OH·的强氧化性把抗生素降解去除的一种均相Fenton法[8-9],但其pH适应范围窄,Fenton铁泥产量大成了最主要的直接限制因素. 而非均相Fenton法则克服了均相Fenton法的缺点,采用含铁的矿物或复合材料作为非均相Fenton法的溶解性Fe2+离子源,被广泛用于抗生素类物质的高效降解去除[5, 7]. 近年来,黄铁矿是一种有色金属矿山开采的尾矿渣,具有来源广泛和价格低廉的特点[10-12],常被用作为非均相Fenton的溶解性Fe2+离子源应用于多种有机污染物如染料和抗生素等的去除[7,13],如采用黄铁矿与过氧化氢或过硫酸盐联合高效降解水中的难降解的有机污染物如2,4,6-三硝基甲苯、罗丹明B、双氯芬酸和2,4-二氯苯酚[10-11,14-15]. 大量研究表明,黄铁矿除了提供Fenton所需的Fe2+离子源外,在反应过程中,黄铁矿经氧化释放出来的H+能较好地维持体系的酸性环境,而且,黄铁矿本身的还原性可促进Fe2+/Fe3+循环等,能实现黄铁矿Fenton对污染物的高效持续去除[13-15]. 另外,据研究表明,超声(US)不仅能通过声空化产生自由基,还能加速催化剂表面反应,实现声化学和催化化学的协同,能提升污染物的去除效果[16-18]. 而且,US也能激活H2O2分解产生OH· [17, 19],若在黄铁矿/H2O2体系引入超声或许能较大地提升抗生素的去除效率,加速黄铁矿物与抗生素的界面反应. 到目前为止,有关黄铁矿与超声联合激活H2O2降解去除水体中的抗生素的研究仍鲜见报道.
基于四环素类和氟喹诺酮类抗生素是目前应用广泛的抗生素两大主要类型,本研究以典型的四环素类抗生素金霉素(CTC)和氟喹诺酮类抗生素氧氟沙星(OFX)为主要目标污染物,采用天然黄铁矿与超声(US)联合激活H2O2降解去除水体中的抗生素CTC和OFX. 系统地考察了不同条件下金霉素的去除效果,鉴定了反应过程中的自由基组分和降解产物,评估了黄铁矿/H2O2/US体系对CTC和OFX的同步去除效果,探讨了CTC和OFX在黄铁矿/H2O2/US体系的降解反应机制,以期为利用黄铁矿Fenton体系去除水中的抗生素提供理论基础,实现尾矿渣黄铁矿“以废治废”的环境治理理念.
-
天然黄铁矿样品采自广东省大宝山矿区,先用超纯水清洗表面泥沙,研磨后过100目筛,置于超声清洗5 min,再用1 mol·L−1硝酸洗涤及清洗反应氧化层,随后用超纯水和乙醇反复冲洗后于30 ℃真空干燥备用;金霉素(CTC,99.8%)和氧氟沙星(OFX,99.8%)购自上海阿拉丁公司,其中,甲醇和乙腈等均为色谱纯,其它试剂如H2O2、叔丁醇、苯醌等购于广州化学试剂公司,本实验所用试剂均为分析纯级别以上,本实验溶液均为超纯水配制.
-
在装有100 mL浓度为40 mg·L−1 CTC的烧杯中,用0.01 mmol·L−1 HCl和NaOH溶液调节反应液的pH. 随后把烧杯放置于超声器中,采用转速为200 r·min−1的机械搅拌,往烧杯中加入一定量的黄铁矿样品和H2O2溶液,摇匀起动反应. 超声功率固定为40 W,反应时间设置为60 min,定时取样4 mL,并加入2 mL甲醇淬灭自由基反应,再过0.22 μm滤膜后用于高效液相的分析. 设置pH (3.0、5.0、7.0和9.0)、黄铁矿样品剂量(0.5、0.75、1.0、1.25 g·L−1)、CTC初始浓度(5、10、20、40 mg·L−1)、H2O2剂量(0.5、0.75、1.0、1.25 mmol·L−1)、共存离子浓度(0、1、10、100 mmol·L−1)和OFX初始浓度(0、5、10、20 mg·L−1)来考察黄铁矿/H2O2/US体系对CTC的去除效果影响. 在重复性实验中,反应后的黄铁矿用超纯水清洗后烘干,随后用于下一轮实验. 在自由基淬灭实验中,初始浓度为50 mmol·L−1 的叔丁醇和苯醌分别加入到反应液,其它条件与批量实验一致. 所有实验重复3次.
-
CTC的浓度分析采用高效液相仪(HPLC)测定,采用C18柱(250 mm × 4.6 mm, 5 μm),流动相为乙腈、甲醇和0.01 mmol·L−1草酸溶液,其体积比为22:11:67,流速为0.8 mL·min−1,柱温为30 ℃,检测波长为274 nm,样品量为10 mL [20]. OFX的流动相为乙腈和1%甲酸溶液,其体积比为20:80,流速为0.5 mL·min−1,柱温为50 ℃,检测波长为294 nm [21]. CTC的降解中间产物采用液相色谱质谱联用仪(HPLC-MS),电喷雾离子源(ESI) 为正离子检出模式,扫描范围为m/z (100—600). 总有机碳(TOC)采用TOC分析仪测定,反应液经0.22 μm微孔滤膜过滤后,溶液中的总铁采用电感耦合等离子体发射光谱仪(ICP-OES),Fe2+采用邻菲啰啉分光光度法(HJ/T 345-2007)测定;黄铁矿样品表面的形貌和元素成分采用扫描电子显微镜能谱仪(SEM-EDS)分析,其晶体结构采用X射线衍射仪(XRD)分析.
-
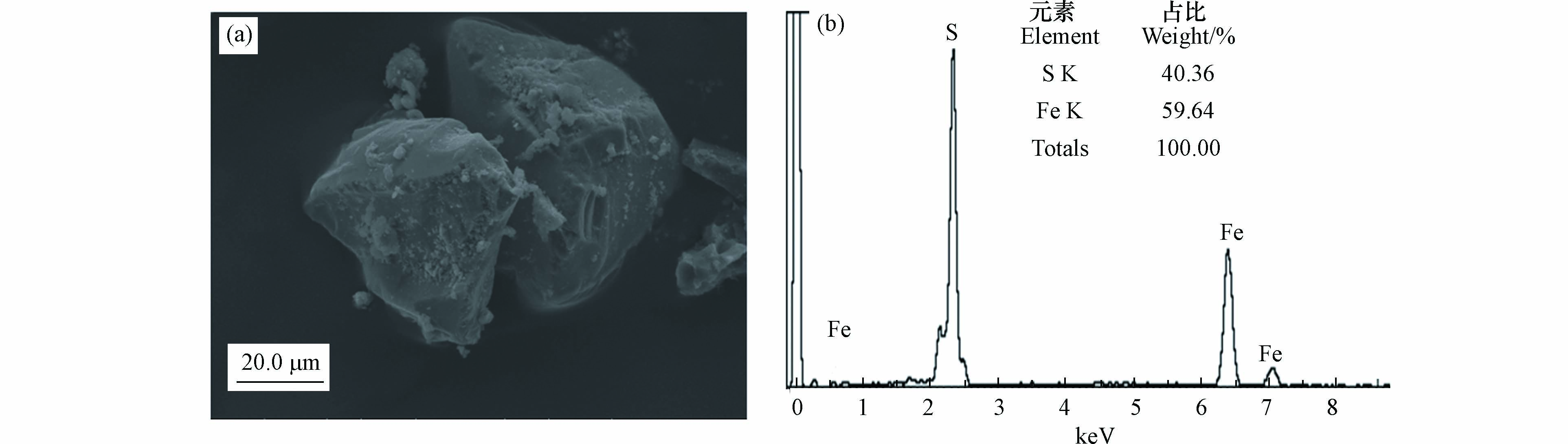
本实验所采用的天然黄铁矿样品表面的SEM-EDS分析结果如图1所示,结果表明,此黄铁矿的尺寸处于微米级水平,粒径在30—60 μm范围内,并呈现不规则的颗粒结构(图1a). 同时,矿物表面EDS能谱分析结果显示,这种黄铁矿表面的主要成分为Fe和S,其中,Fe和S的质量比重分别为40.36%和59.64%(图1b). 此黄铁矿物的Fe/S值低于理论值,表明本黄铁矿组分中还含有不可忽视的杂质或缺陷.
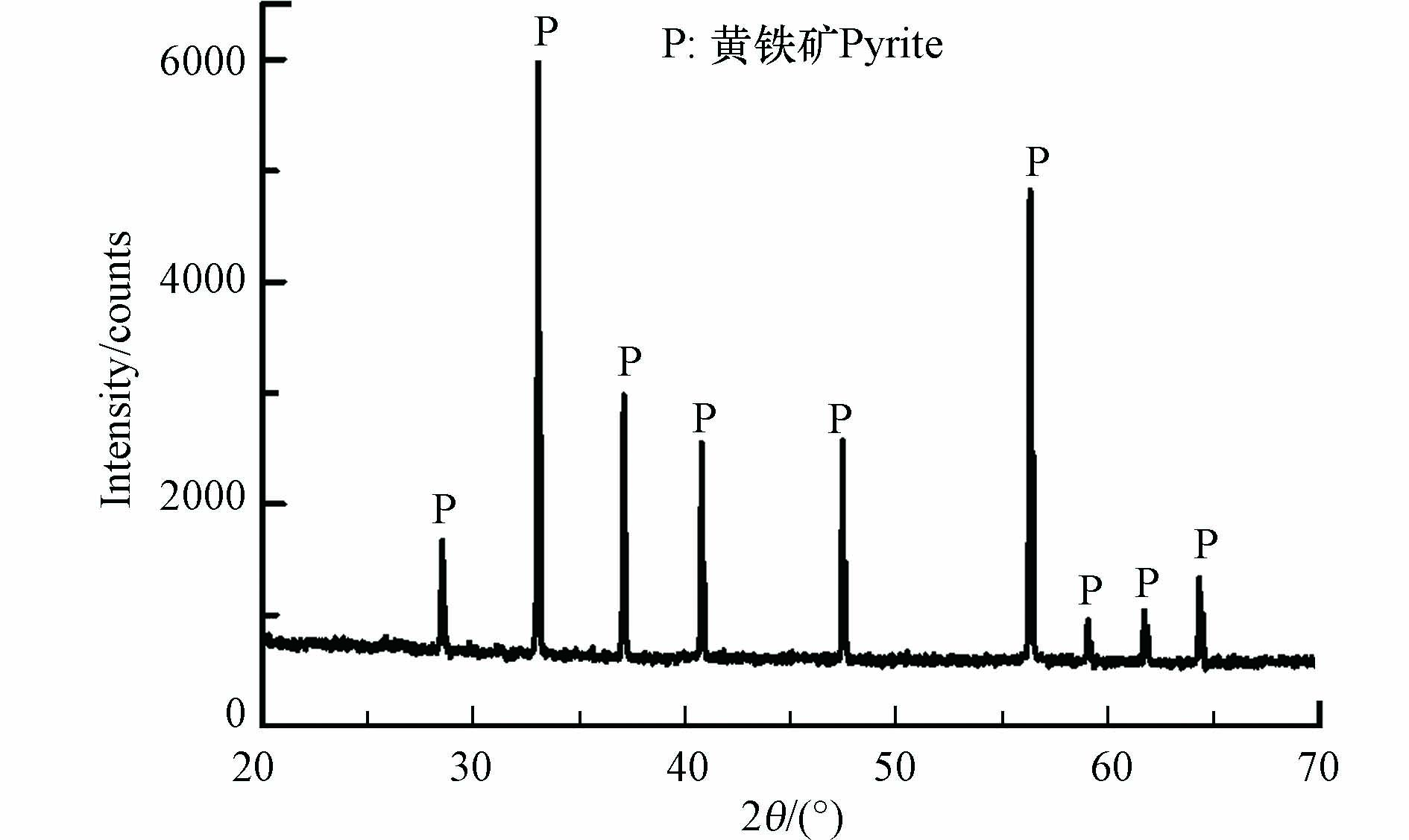
此外,采用X射线衍射(XRD)对其晶体结构进行分析,结果如图2 所示. 在XRD图谱20º—70º范围,出现9个衍射峰,与黄铁矿标准样品的XRD图谱ICDD-PDF42-1340中的(111)、(200)、(210)、(211)、(220)、(311)、(222)、(230)和(321)晶面相吻合 [12]. 本实验所采用的黄铁矿的XRD结果与前期研究工作类似[12].
-
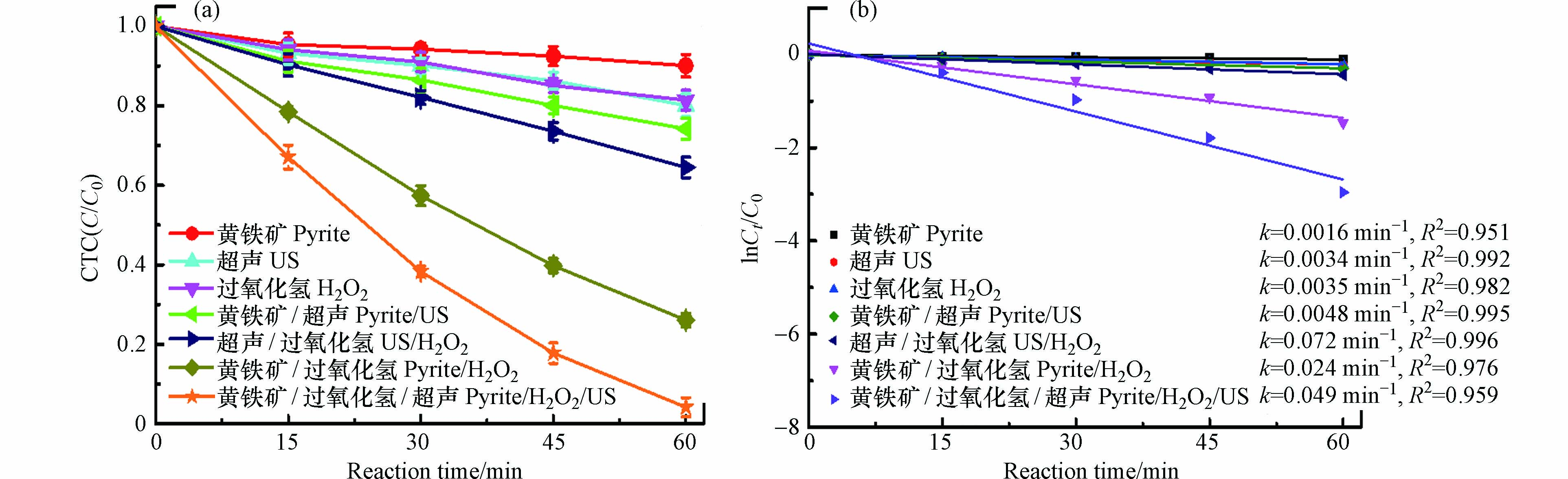
图3 为不同体系对CTC去除效果. 由图3a可知,单纯的黄铁矿对CTC的去除效果作用非常有限,CTC仅有大约10%得到去除,这主要是因为黄铁矿颗粒表面的吸附作用.
当采用超声US去除CTC时,与黄铁矿相比,CTC的去除效果稍微提升高了约9%,其去除率能达到18.6%,这是因为水溶液在超声波的作用下会生成少量如OH·的强氧化性自由基,正是由于这些自由基的存在,促进了CTC的去除 [18-19]. 另外,当CTC在H2O2溶液时,发现约有20%的CTC能得到去除,这是由于H2O2本身对CTC的氧化作用. 然而,CTC在以上3种单一黄铁矿、US和H2O2的体系下均未能实现较好的去除效果. 当黄铁矿与US联合时,CTC的去除率能达到26%;另外,当US和H2O2联合时,CTC的去除率达到了35%,而CTC的去除率在黄铁矿和H2O2联合体系下能在60 min内达到74%. 与两个二元体系黄铁矿/US和US/H2O2相比,CTC能在黄铁矿/H2O2体系下实现了较好的去除,这可能是因为黄铁矿释放来的Fe2+能有效激活H2O2产生较多数量的强氧化性OH·,较大程度地促进了CTC的去除. 当US加入到黄铁矿/H2O2体系时,CTC的去除率从74% 提高到了96%,CTC去除率的显著提升归因于黄铁矿、US和H2O2三者间的协同反应,实现了三元体系黄铁矿/H2O2/US的最优化效应. 同时,还采用准一级反应动力学模型对上述实验数据进行了拟合,结果如图3b所示. 这些实验数据具有较好的准一级反应动力学模型拟合程度,R2都在0.95以上,CTC在黄铁矿、US、H2O2、黄铁矿/US、US/H2O2、黄铁矿/H2O2和黄铁矿/H2O2/US体系下的动力学速率k分别为0.0016、0.0034、0.0035、0.0048、0.072、0.024、0.049 min−1. 以上数据表明,CTC能在黄铁矿/H2O2/US体系下实现最佳的降解去除效果.
-
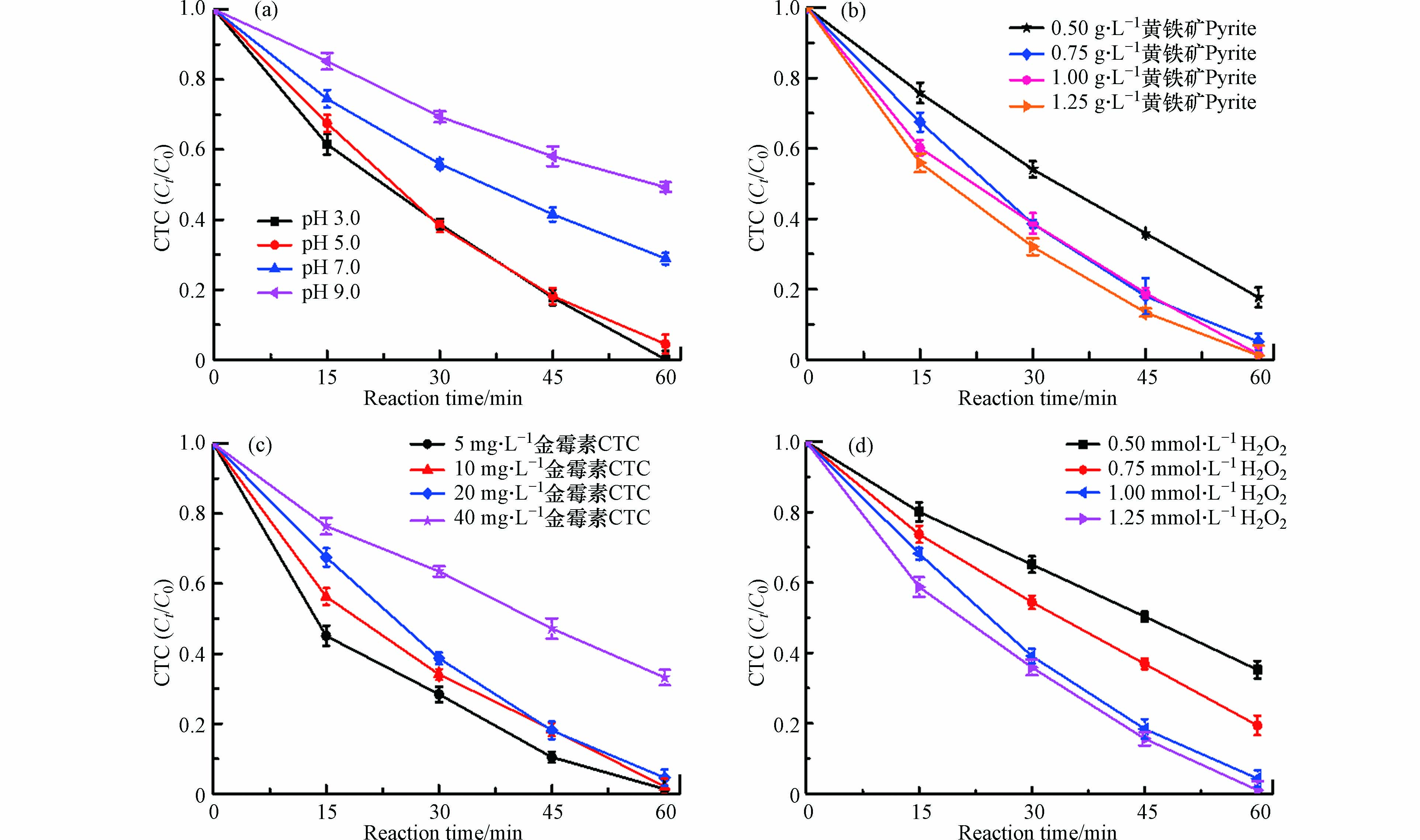
一些关键的反应条件如pH、催化剂量和H2O2浓度等都可能会影响CTC在黄铁矿/H2O2/US体系下的去除效果[2,19],因此,考察了pH、黄铁矿剂量、H2O2浓度和CTC初始浓度对CTC去除的影响,结果如图4所示.
由图4可见,在pH 3.0—9.0 范围内,随着pH的增大,CTC的去除率逐渐降低(图4a). 当pH从3.0增加到9.0时,CTC的去除率从99%降低50.7%,结果表明,高pH环境会抑制黄铁矿/H2O2/US体系对CTC去除,可能是因为酸性条件下的黄铁矿易发生氧化溶解. 有趣的是,pH在3.0—5.0范围内,CTC的去除率变化不大,均能保持在96%左右. 这是因为在这个酸性条件下,黄铁矿因被氧化释放出来H+能较好地维持该Fenton反应对CTC的高去除效果所需要的pH条件. 由图4b可知,CTC的去除率随着黄铁矿剂量的增加而增大,原因是高剂量的黄铁矿具有较多的反应位点和Fe2+的量. 不过当黄铁矿剂量高于0.75 g·L−1后,所提升的CTC的去除效果却不明显,其数值保持在95% 以上. 另外,CTC的去除率与CTC的初始浓度成反比关系(图4c),随着CTC的初始浓度的增大,其去除率呈现下降趋势. 当CTC的初始浓度从20 mg·L−1增加到40 mg·L−1,相应的去除率从95.4%降到了66.8%,这是由于体系所产生的OH·数量远满足不了高浓度CTC去除对OH·的需求量,OH·的供给呈现不足状态. 由图4d可知,CTC的去除率与H2O2浓度成正比关系,H2O2浓度的增加直接导致更多的H2O2与Fe2+反应产生大量的OH·用于CTC去除. 不过,当H2O2浓度超过1 mmol·L−1时,继续增大H2O2浓度对CTC的去除效果却不明显,这可解释为过量的H2O2会因发生自我分解和参与竞争消耗OH·而浪费掉. 因此,基于上述结果分析,CTC在黄铁矿/H2O2/US体系下的最优化条件为pH=5.0、黄铁矿剂量为0.75 g·L−1、CTC的初始浓度为20 mg·L−1和H2O2浓度为1 mmol·L−1.
-
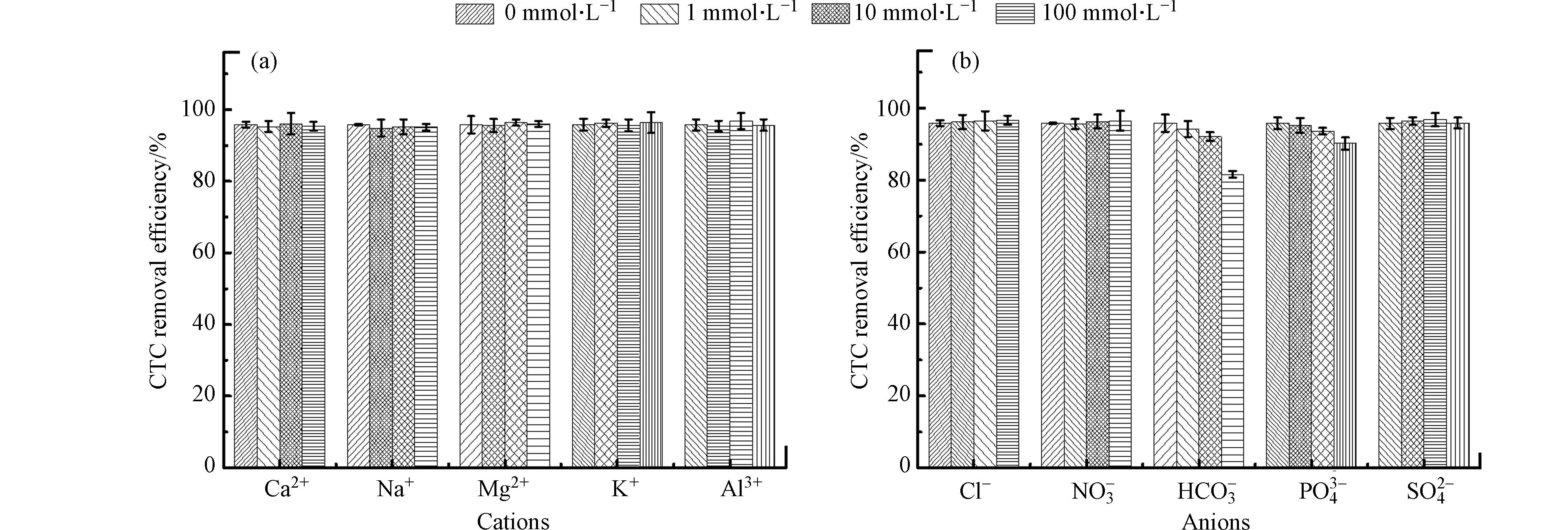
现实水环境中包括着各类共存阳离子(如Ca2+、Na+、Mg2+和Al3+等)和阴离子(如Cl−、NO3−、HCO3−和PO43-等),据研究表明,这些共存离子会对Fenton氧化体系去除有机物的效果产生不同程度影响[14-16]. 因此,本研究考察了不同浓度共存离子如Ca2+、Mg2+、Al3+、Cl−、NO3−、HCO3−和PO43-等对CTC在黄铁矿/H2O2/US体系下的去除效果影响,结果如图5所示.
由图5a可知,阳离子Ca2+、Na+、Mg2+、Na+和Al3+的加入,几乎不会影响黄铁矿/H2O2/US体系对CTC去除效果,CTC的去除率均能保持在95%以上. 这个表明,这些阳离子不会与CTC产生竞争效应. 因此,黄铁矿/H2O2/US体系在富含阳离子的环境去除CTC具有良好的稳定性. 然而,加入阴离子如HCO3−和PO43-对CTC去除效果呈现不同程度的抑制作用(图5a). 其中HCO3−的抑制作用最为明显,CTC去除率随着HCO3−浓度的增加而减小,当HCO3−浓度从0增加到100 mmol·L−1时,CTC去除率相应地从95.8% 降到了81.6%. 这导致约14%降幅的原因可能有如下两个方面:(1)HCO3−的加入会发生自我水解反应释放出大量的OH−,引起体系溶液的pH上升,使溶液中的Fe2+形成沉淀,影响到OH·的产生量;(2)HCO3−能与OH·反应产生弱氧化性的HCO3·− 和CO3·−. 另外,高浓度的PO43-也会对CTC去除产生一定的抑制作用,当PO43-浓度达到100 mmol·L−1时,CTC去除率从95.8%降到了90.2%,可解释为高浓度的PO43-会与溶液中的Fe3+形成沉淀,从而减少OH·的产生量. 其它阴离子如Cl−、NO3−和SO42-的加入对CTC去除效果影响不大,几乎可以忽略 [13]. 综上所述,除HCO3−和PO43-之外,其它大部分共存离子几乎不会影响黄铁矿/H2O2/US体系对CTC去除效果,因此,黄铁矿/H2O2/US体系具有较好的水质适用性.
-
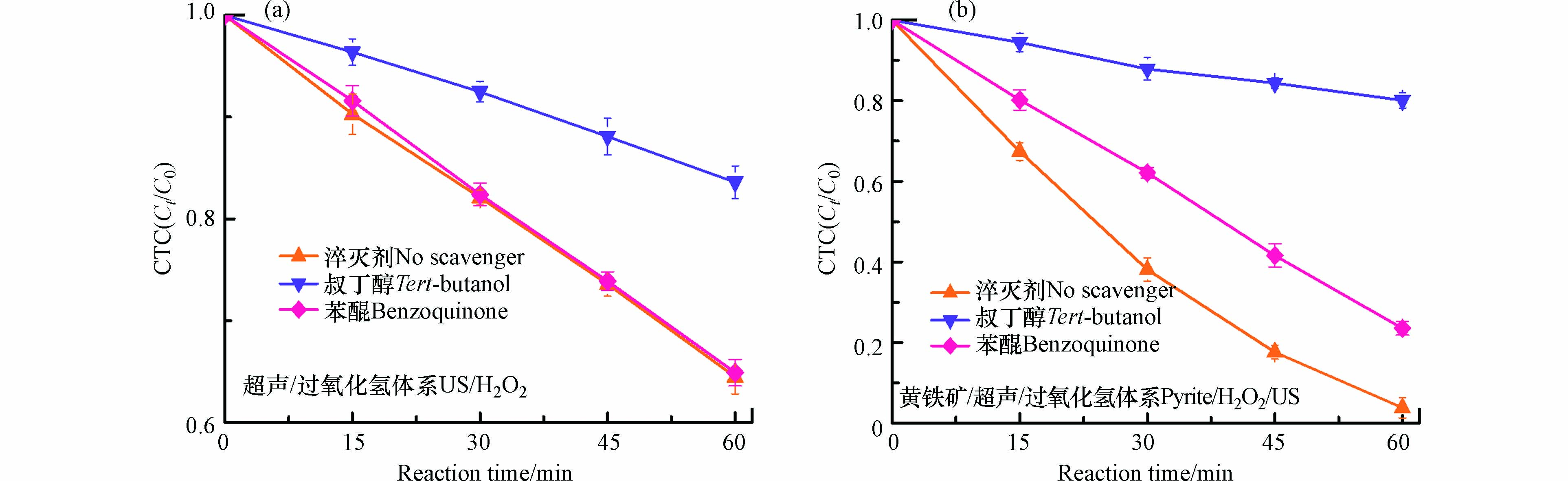
在Fenton氧化体系去除有机物的过程会有自由基如OH·和O2·−的参与,叔丁醇和苯醌常被用作OH·和O2·−的淬灭剂 [17]. 为了探讨CTC在黄铁矿/H2O2/US体系的去除机制,自由基淬灭剂叔丁醇和苯醌分别加入到两个CTC去除反应体系(US/H2O2和黄铁矿/H2O2/US)中,淬灭实验结果如图6所示. 由图6a可知,在US/H2O2体系中,加入叔丁醇会CTC的去除产生显著的抑制效果,其去除率从35.5%降至16.4%,表明OH·参与了CTC的去除过程. 而苯醌对CTC的去除效果的影响几乎可以忽略不计,上述结果,在US/H2O2体系降解CTC的过程中,OH·是起主导作用的. 然而,在黄铁矿/H2O2/US体系,叔丁醇和苯醌的加入对CTC的去除效果呈现出不同程度的影响(图6b). 苯醌的加入会使CTC的去除率下降约20 %,而叔丁醇的加入使CTC的去除率下降约76%,这表明,在黄铁矿/H2O2/US体系,OH·和O2·−都参与了CTC的降解去除过程,其中,OH·是起主导作用的,O2·−的作用次之.
-
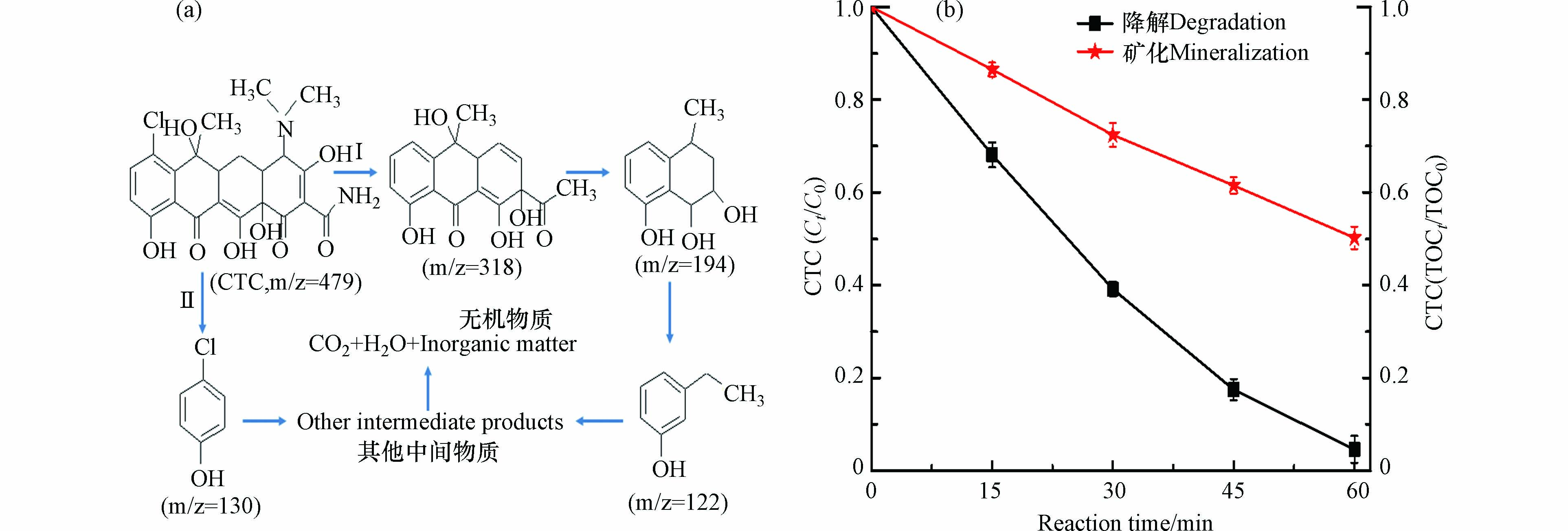
在黄铁矿/H2O2/US体系对CTC的降解过程,采用液质联用仪(LC-MS)分析了反应30 min和60 min反应液的降解中间产物. 结果发现有4种CTC的降解中间产物,其中,m/z=318是30 min反应液探测到的,在60 min反应液探测到m/z=194、m/z=122和m/z=130中间产物. 随着反应的进行,CTC降解中间产物的分子量都是逐渐变小的,这意味着CTC大分子结构已被黄铁矿/H2O2/US体系中的OH·氧化而受到破坏. 结合所测的中间产物和相关文献报道,推导出CTC在黄铁矿/H2O2/US体系的可能降解路径(图7a). 路径I:CTC首先通过脱氯、开环、脱烷基化和羟基化形成产物m/z=318,随后脱烷基化和羟基化生成产物m/z=194,进一步开环和羟基化生成产物m/z=122,接着进一步生成其它中间产物 [21];路径II,CTC通过开环、脱烷基化和羟基化生成产物m/z=130,随后再形成其它中间产物如有机小分子酸[19],最终,两路径所生的其它中间产物进一步矿化为二氧化碳、水和其它无机物质. 本研究推导的CTC路径与前期相关文献所报道的吻合 [1,21],黄铁矿/H2O2/US体系对CTC的降解和矿化变化过程如图7b所示,在反应60 min内,CTC的降解率和矿化率分别达到了95.4%和49.8%,这表明黄铁矿/H2O2/US体系能有效降解CTC.
-
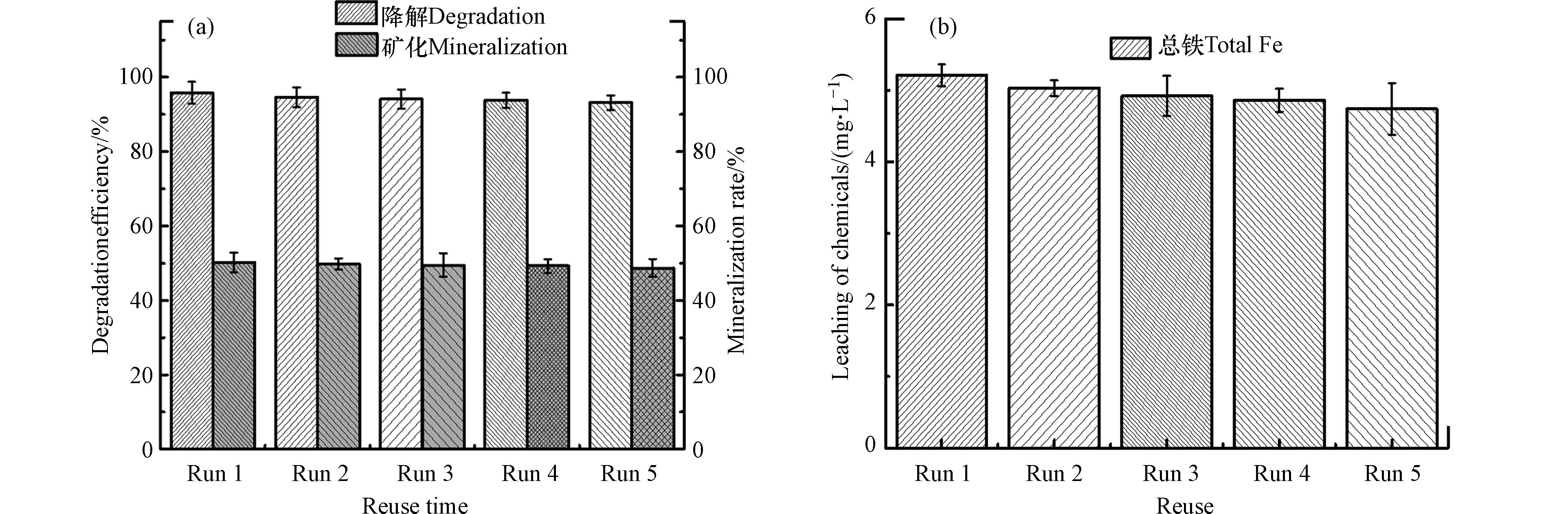
黄铁矿/H2O2/US体系对CTC的降解效果的重复稳定性结果如图8所示. 研究发现,黄铁矿/H2O2/US体系对CTC具有良好的连续降解去除效果,经过5次的连续重复使用,CTC的去除率仍然能保持在93%以上,相对应的矿化率均能保持在48%左右(图8a),这表明黄铁矿的重复性能性高. 同时,还研究了在重复实验过程中总铁的释放过程,相关结果如图8b所示,总铁的释放量随着反应次数的增加而减少,当在第5次重复实验完成时,总铁浓度达到4.74 mg·L−1,这表明黄铁矿的损耗较小,具有较好的重复性能.
-
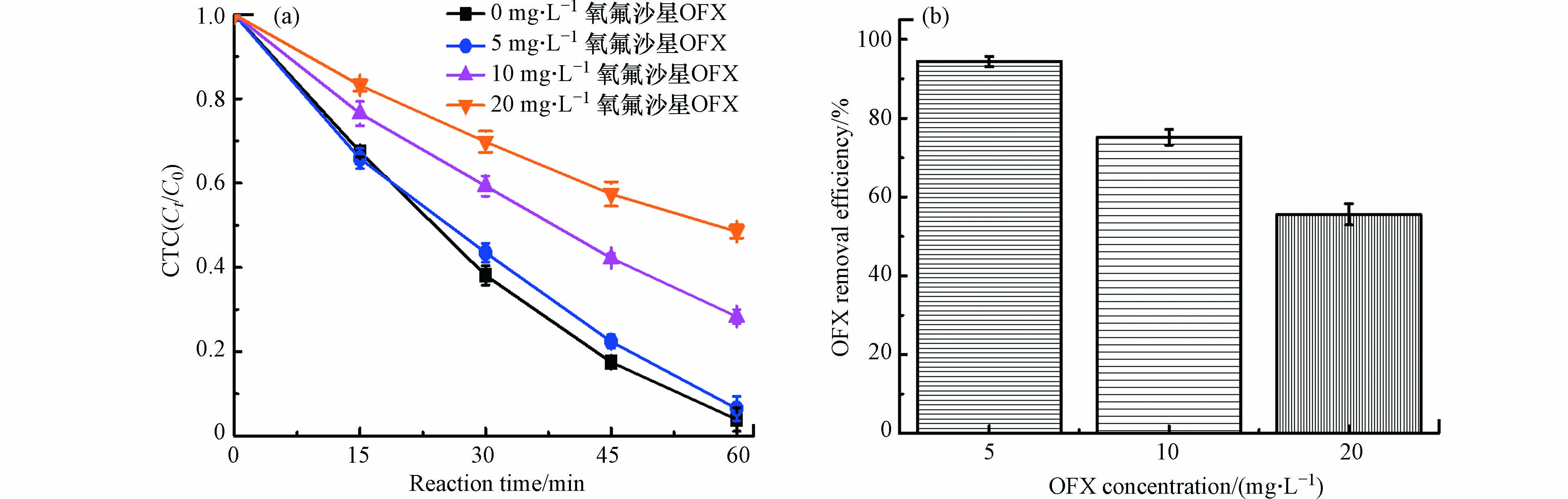
事实上,现实的环境污染都不是单一的污染,而是多种污染物质的复合污染类型 [6,21],因此,研究了黄铁矿/H2O2/US体系对两种抗生素CTC和OFX的同步去除,结果如图9所示. 研究发现,CTC的去除率随着OFX加入浓度的增加而降低,与空白相比,当OFX加入浓度为5、10、20 mg·L−1时,黄铁矿/H2O2/US体系对CTC的去除率分别降至为93.5%、71.7%和51.5%. 去除率下降可解释为OFX的加入会与CTC形成自由基的竞争,引起CTC去除效果的下降. 不过,在OFX加入浓度较低时,这种竞争作用表现不明显,当OFX加入浓度为5 mg·L−1,CTC的去除率仍能达到93.5%,这结果表明低浓度OFX的加入不会对CTC的去除产生明显的抑制效应. 同时,也研究了共存条件下的OFX去除效果,如图9b所示,当OFX加入浓度为5、10、20 mg·L−1时,黄铁矿/H2O2/US体系对OFX的去除率分别为94.4%、75.2%和55.6%,上述结果表明,在一定的条件下,两种抗生素CTC和OFX能在黄铁矿/H2O2/US体系下实现同步高效的去除.
-
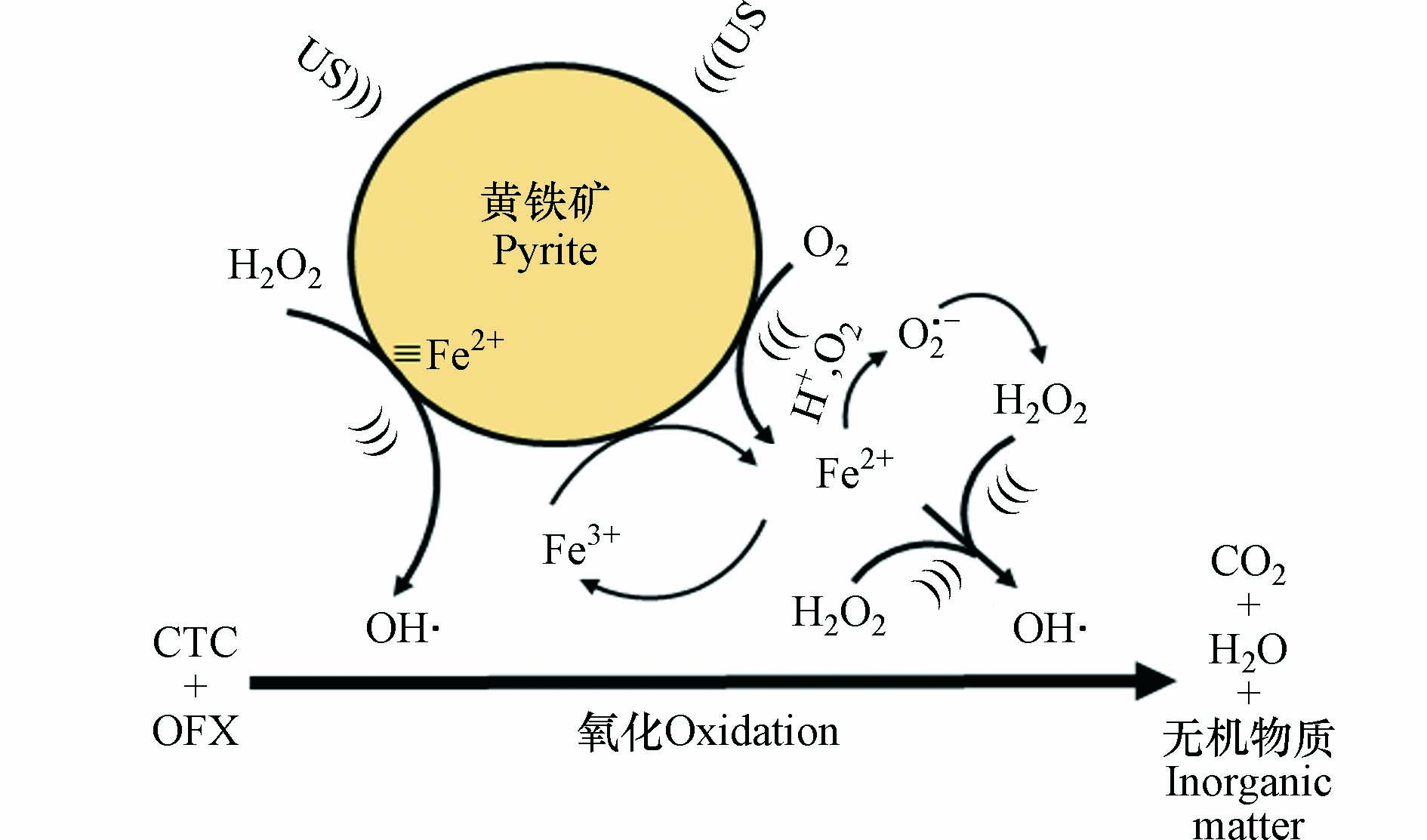
基于上述实验结果和前期文献研究 [18-19, 22],推导出黄铁矿/H2O2/US体系对两种抗生素CTC和OFX的同步去除反应机理,所述机理如图10所示.
首先,黄铁矿氧化溶出释放H+,体系pH会缓慢降低到3.80左右,此时黄铁矿释放出Fe2+ [22],超声的介入,加速了Fe2+从黄铁矿表面溶出. 所产生的Fe2+与所加入的H2O2形成Fenton反应产生大量的OH·,这些自由基攻击CTC和TC分子而氧化分解,最终矿化为二氧化碳、水和其它无机物质(反应式1—7). 在这个过程中,体系中O2·−也在起着重要的作用,而且,有超声介入的Fenton对CTC和OFX的降解去除更加高效. 在这个体系中,黄铁矿能及时把溶液中的Fe3+转化为Fe2+,促使反应持续进行,除了溶液Fe2+与H2O2的均相Fenton反应外,同时也存在着黄铁矿表面≡Fe2+与H2O2的非均相Fenton反应 [14-15],正是两者反应的协同效应促使CTC和OFX能在黄铁矿/H2O2/US体系下实现同步高效的降解去除.
-
天然黄铁矿、H2O2和超声(US)联合用于水体中典型抗生素金霉素(CTC)和氧氟沙星(OFX)的高效去除,三者实现了良好的协同效应. US实现了声化学和催化化学的协同效应. 黄铁矿/H2O2/US体系在最优化反应条件下对CTC的去除率能达到96%,CTC降解过程符合准一级动力学方程. 共存离子Ca2+、Mg2+、Cl−、NO3−和SO42-等对CTC的去除未呈现明显的抑制作用,而HCO3−和PO43-呈现较大程度的抑制作用. OH·和O2·−都参与了CTC的降解去除过程,其中,OH·起主导作用. 黄铁矿/H2O2/US体系对CTC具有良好的连续降解去除效果,其去除率均能保持在93%以上. 两种抗生素CTC和OFX能在黄铁矿/H2O2/US体系下实现同步高效的去除.
超声协同天然黄铁矿Fenton催化降解水中的金霉素和氧氟沙星
Ultrasound-assisted heterogeneous natural pyrite based Fenton-like catalytic degradation of chlortetracycline and ofloxacin in water
-
摘要: Fenton 氧化常被广泛应用于环境水体中难降解有机污染物的高效去除. 本研究以天然黄铁矿为Fenton铁源,联合超声(US)激活H2O2高效降解去除水体中的抗生素金霉素(CTC)和氧氟沙星(OFX). 采用扫描电子显微镜(SEM)和X射线衍射(XRD)分析了黄铁矿物的形貌、元素组成和晶相结构,并考察了不同条件下黄铁矿/H2O2/US对CTC的去除影响,并结合淬灭实验和降解中间产物分析,探究了黄铁矿/H2O2/US体系同步去除CTC和OFX的反应机理. 结果表明,CTC的高效去除主要归因于黄铁矿与US在激活H2O2分解产生羟基自由基的协同效应. 在最佳反应条件下,CTC能在60 min内实现约96% 的去除,且CTC降解过程符合准一级动力学方程. 羟基自由基主导着CTC的降解去除过程,CTC能通过脱氯、开环、脱烷基化和羟基化等过程降解生成一些分子较小的中间产物而去除. 经5轮连续反应后,黄铁矿/H2O2/US体系对CTC的去除率均能保持在93%以上,具有良好的稳定性. 而且,黄铁矿/H2O2/US体系还能较好地同步去除CTC和OFX. 基于上述实验结果,推导出了黄铁矿/H2O2/US体系同步高效降解去除CTC和OFX的反应机理,溶液Fe2+与H2O2的均相Fenton反应和黄铁矿表面≡Fe2+与H2O2的非均相Fenton反应的协同高效降解这两种抗生素. 本研究较好地为受抗生素污染水体的治理提供理论和技术支撑.Abstract: In recent years, Fenton oxidation is often widely used for efficient removal of refractory organic pollutants from aquatic environment. In this study, the natural pyrite was used as Fenton iron source, and combined with ultrasound (US) to activate H2O2 for the efficient degradation of antibiotics chlortetracycline (CTC) and ofloxacin (OFX) in water. The morphology, element composition and crystal phase structure of pyrite were analyzed by scanning electron microscopy (SEM) and X-ray diffraction (XRD), respectively. The effects of different reaction conditions on CTC removal by pyrite/H2O2/US system were investigated, and quenching experiment and analysis of degradation intermediates were also carried out to explore the reaction mechanism for the simultaneous removal of CTC and OFX by pyrite/H2O2/US system. The experimental results show that the efficient removal of CTC was mainly attributed to a synergistic effect of pyrite and US in activating H2O2 decomposition for the formation of OH•. About 96% of CTC could be removed within 60 min under the optimal reaction conditions, and the CTC degradation process was well fit to the pseudo-first-order kinetic equation model. OH• dominated the removal process of CTC, CTC molecule could be degraded into intermediate products with smaller molecules through dechlorination, ring opening, dealkylation and hydroxylation. The reactivity of pyrite showed a nice stability, and above 93% of CTC removal could be maintained in pyrite/H2O2/US system within five runs. Moreover, the simultaneous removal of CTC and OFX could be achieved by pyrite/H2O2/US system. The reaction mechanism of the pyrite/H2O2/US system for the simultaneous removal of CTC and OFX was proposed based on the experimental results, which involved in synergistic effect of homogeneous Fenton reaction of solution Fe2+ with H2O2 and heterogeneous Fenton reaction of pyrite surfaces Fe2+ with H2O2. This study provided theoretical and technical support for the treatment of antibiotic-contaminated water.
-
Key words:
- antibiotics /
- pyrite /
- Fenton /
- chlortetracycline(CTC) /
- ofloxacin(OFX)
-

-
-
[1] MA S J, JING J N, LIU P Y, et al. High selectivity and effectiveness for removal of tetracycline and its related drug resistance in food wastewater through schwertmannite/graphene oxide catalyzed photo-Fenton-like oxidation [J]. Journal of Hazardous Materials, 2020, 392: 122437. doi: 10.1016/j.jhazmat.2020.122437 [2] QIAO X X, LIU X J, ZHANG W Y, et al. Superior photo-Fenton activity towards chlortetracycline degradation over novel g-C3N4 nanosheets/schwertmannite nanocomposites with accelerated Fe(III)/Fe(II) cycling [J]. Separation and Purification Technology, 2021, 279: 119760. doi: 10.1016/j.seppur.2021.119760 [3] MARTÍNEZ-CARBALLO E, GONZÁLEZ-BARREIRO C, SCHARF S, et al. Environmental monitoring study of selected veterinary antibiotics in animal manure and soils in Austria [J]. Environmental Pollution (Barking, Essex:1987), 2007, 148(2): 570-579. doi: 10.1016/j.envpol.2006.11.035 [4] ZHANG Q Q, YING G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. [5] LIU J J, DIAO Z H, LIU C M, et al. Synergistic reduction of copper (II) and oxidation of norfloxacin over a novel sewage sludge-derived char-based catalyst: Performance, fate and mechanism [J]. Journal of Cleaner Production, 2018, 182: 794-804. doi: 10.1016/j.jclepro.2018.02.045 [6] DIAO Z H, JIN J C, ZOU M Y, et al. Simultaneous degradation of amoxicillin and norfloxacin by TiO2@nZVI composites coupling with persulfate: Synergistic effect, products and mechanism [J]. Separation and Purification Technology, 2022, 278: 119620. [7] NIE X, LI G Y, LI S S, et al. Highly efficient adsorption and catalytic degradation of ciprofloxacin by a novel heterogeneous Fenton catalyst of hexapod-like pyrite nanosheets mineral clusters [J]. Applied Catalysis B:Environmental, 2022, 300: 120734. doi: 10.1016/j.apcatb.2021.120734 [8] XU X R, LI X Y, LI X Z, et al. Degradation of melatonin by UV, UV/H2O2, Fe2+/H2O2 and UV/Fe2+/H2O2 processes [J]. Separation and Purification Technology, 2009, 68(2): 261-266. doi: 10.1016/j.seppur.2009.05.013 [9] GAO Y W, WANG Y, ZHANG H. Removal of Rhodamine B with Fe-supported bentonite as heterogeneous photo-Fenton catalyst under visible irradiation [J]. Applied Catalysis B:Environmental, 2015, 178: 29-36. doi: 10.1016/j.apcatb.2014.11.005 [10] MATTA R, HANNA K, CHIRON S. Fenton-like oxidation of 2, 4, 6-trinitrotoluene using different iron minerals [J]. Science of the Total Environment, 2007, 385(1/2/3): 242-251. [11] BAE S, KIM D, LEE W. Degradation of diclofenac by pyrite catalyzed Fenton oxidation [J]. Applied Catalysis B:Environmental, 2013, 134/135: 93-102. doi: 10.1016/j.apcatb.2012.12.031 [12] DIAO Z H, XU X R, LIU F M, et al. Photocatalytic degradation of malachite green by pyrite and its synergism with Cr(VI) reduction: Performance and reaction mechanism [J]. Separation and Purification Technology, 2015, 154: 168-175. doi: 10.1016/j.seppur.2015.09.027 [13] MASHAYEKH-SALEHI A, AKBARMOJENI K, ROUDBARI A, et al. Use of mine waste for H2O2-assisted heterogeneous Fenton-like degradation of tetracycline by natural pyrite nanoparticles: Catalyst characterization, degradation mechanism, operational parameters and cytotoxicity assessment [J]. Journal of Cleaner Production, 2021, 291: 125235. doi: 10.1016/j.jclepro.2020.125235 [14] FENG Y, LI H L, LIN L, et al. Degradation of 1, 4-dioxane via controlled generation of radicals by pyrite-activated oxidants: Synergistic effects, role of disulfides, and activation sites [J]. Chemical Engineering Journal, 2018, 336: 416-426. doi: 10.1016/j.cej.2017.12.011 [15] DIAO Z H, LIU J J, HU Y X, et al. Comparative study of Rhodamine B degradation by the systems pyrite/H2O2 and pyrite/persulfate: Reactivity, stability, products and mechanism [J]. Separation and Purification Technology, 2017, 184: 374-383. doi: 10.1016/j.seppur.2017.05.016 [16] DIAO Z H, DONG F X, YAN L, et al. Synergistic oxidation of Bisphenol A in a heterogeneous ultrasound-enhanced sludge biochar catalyst/persulfate process: Reactivity and mechanism [J]. Journal of Hazardous Materials, 2020, 384: 121385. doi: 10.1016/j.jhazmat.2019.121385 [17] HOU L W, WANG L G, ROYER S, et al. Ultrasound-assisted heterogeneous Fenton-like degradation of tetracycline over a magnetite catalyst [J]. Journal of Hazardous Materials, 2016, 302: 458-467. doi: 10.1016/j.jhazmat.2015.09.033 [18] DIAO Z H, LIN Z Y, CHEN X Z, et al. Ultrasound-assisted heterogeneous activation of peroxymonosulphate by natural pyrite for 2, 4-diclorophenol degradation in water: Synergistic effects, pathway and mechanism [J]. Chemical Engineering Journal, 2020, 389: 123771. doi: 10.1016/j.cej.2019.123771 [19] PULICHARLA R, BRAR S K, ROUISSI T, et al. Degradation of chlortetracycline in wastewater sludge by ultrasonication, Fenton oxidation, and Ferro-sonication [J]. Ultrasonics Sonochemistry, 2017, 34: 332-342. doi: 10.1016/j.ultsonch.2016.05.042 [20] SHAO Y Y, GAO Y, YUE Q Y, et al. Degradation of chlortetracycline with simultaneous removal of copper (II) from aqueous solution using wheat straw-supported nanoscale zero-valent iron [J]. Chemical Engineering Journal, 2020, 379: 122384. doi: 10.1016/j.cej.2019.122384 [21] FAN Y, ZHOU Z Y, FENG Y, et al. Degradation mechanisms of ofloxacin and cefazolin using peroxymonosulfate activated by reduced graphene oxide-CoFe2O4 composites [J]. Chemical Engineering Journal, 2020, 383: 123056. doi: 10.1016/j.cej.2019.123056 [22] DIAO Z H, CHU W. FeS2 assisted degradation of atrazine by bentonite-supported nZVI coupling with hydrogen peroxide process in water: Performance and mechanism [J]. Science of the Total Environment, 2021, 754: 142155. doi: 10.1016/j.scitotenv.2020.142155 -



 下载:
下载: