-
我国北方黄河沿岸的水库水源发生了较为严重的鱼腥味问题,引起了人们的心理恐慌[1],继而加大水厂的水处理压力. 研究发现鱼腥味主要由一些胺类物质和不饱和醛类物质引起[2],在低温、贫营养化水体中,随着一些藻类如锥囊藻、针杆藻的大量生长和腐败,产生的中等强度的鱼腥味和土霉味[3],令人不适. 国内外报道的主要鱼腥味嗅味物质有三甲胺、二甲胺、2,4-庚二烯醛、2,4-癸二烯醛、2,4,7-三烯醛、2,6-壬二烯醛等[4-9]. 庚二烯醛和癸二烯醛等是多元不饱和烯醛类物质,可以由水生生物体内的多元不饱和脂肪酸先后经过脂肪氧合酶和脂氢过氧化物裂解酶的催化作用产生[10];反,反- 2,4-癸二烯醛是水体中硅藻脂氧合裂解产物[11]. 目前自来水厂混凝、沉淀、过滤等常规处理方法对以上引起水中鱼腥味的醛类物质的去除效果十分有限,增加合适的预处理或深度处理环节显得尤为必要[2].
传统水厂多采用氧化剂对原水进行预处理,分解有机物,降低CODMn等指标. 醛类嗅味物质的氧化处理技术,现阶段大多数研究都集中于β-环柠檬醛的氧化去除. 根据Jüttner等的实验[12],β-环柠檬醛在富营养化水体中主要是由微囊藻的细胞分裂过程中产生的胡萝卜素(β - carotene)氧化分解产生的,可引起水体产生草木异嗅味. 张可佳、高乃云等[13]进行了高锰酸钾氧化去除水中β-环柠檬醛的研究,并建立了相关的动力学模型,结果表明高锰酸钾氧化β-环柠檬醛的效果良好,在氧化后30 min内去除率达到90%,并且高锰酸钾与β-环柠檬醛的反应符合二级动力学反应,二级动力学常数为107.2 L−1·mol−1·s−1. 刘禧文[14]等研究发现高锰酸钾对1-辛烯-3-醇、β-环柠檬醛和2,4,6-三氯苯甲醚这3种嗅味物质均有一定去除效果,去除率在40%—55%. 饮用水处理中关于常见氧化剂对其他醛类嗅味物质去除效能的报道并不多见,值得进一步深入研究.
本研究以呼和浩特市JH饮用水厂检出频率和浓度均较高的5种醛类物质——反,反-2,4-庚二烯醛(tt24hept)、反-2-辛烯醛(t2oa)、反,反-2,4-辛二烯醛(tt24oda)、反,反-2,4-癸二烯醛(tt24dda)和 β-环柠檬醛(β-cyclo)为研究对象,选择实际水厂运用较多的高锰酸钾为氧化剂,从去除率、氧化时间等方面对氧化效果进行评价;同时开展高锰酸钾氧化各醛类嗅味物质的反应动力学研究,利用理论反应方程拟合动力学反应过程,得到理论速率常数,继而通过JH水厂原水加标实验验证高锰酸钾氧化醛类嗅味物质的效果与过程,希望能够为实际生产提供指导和经验支持.
-
实验所用试剂:反,反-2,4-庚二烯醛、反,反- 2,4 -辛二烯醛、反,反-2,4-癸二烯醛购自德国CNW公司,β-环柠檬醛、反-2-辛烯醛购自英国Alfa Aesar公司,均为色谱纯,结构式、嗅阈值等信息见表1;甲醇(CH3OH)购自Thermo Fisher Scientific公司,色谱纯;高锰酸钾(KMnO4)、五水合硫代硫酸钠(Na2S2O3·5H2O)、氯化钠(NaCl)购自北京化工厂,均为分析纯;磷酸二氢钠(NaH2PO4)购自国药集团化学试剂有限公司,分析纯;进厂原水取自呼和浩特市JH自来水厂,其主要水质指标测定如下DOC:2—3 mg·L−1,CODMn:3—4 mg·L−1,pH:6.9—7.4.
耗材与仪器:气相色谱-质谱联用仪购自日本岛津公司,型号GCMS-QP 2010 Plus;自动进样器购自德国Gerstel公司,型号MPS 2;50/30 μm DVB/CAR/PDMS SPME萃取头购自美国Supelco公司,型号SAAB-57329U;磁力搅拌器购自金坛市荣华仪器制造有限公司,78-1型;超纯水制备仪器购自法国Milli-Q公司,型号Integral 5 purification system.
-
嗅味物质初始浓度在表1中给出. 考虑到对饮用水嗅味事件的有效控制,所研究目标嗅味物质的初始浓度设定在各自嗅阈值的5 ~ 20倍范围内,同时可以保证被分析仪器稳定定量检测. tt24hept、t2oa和β-cyclo均在设定范围内. 但是tt24oda和tt24dda初始浓度为各自嗅阈值20倍时仍无法被定量检测,所以对这两种物质的初始浓度有所提高.
氧化实验:准备若干顶空瓶,称量2 g NaCl(450 ℃烘2 h)并加入1 mL 0.1 mol·L−1反应终止剂硫代硫酸钠溶液(Na2S2O3·5H2O, aq),待用. 在含有纯水的250 mL磨口锥形瓶中加入设定初始浓度(见表1)的嗅味物质,置于磁力搅拌器搅拌10 min,移取4 mL样品于顶空瓶中,立即用带有PTFE涂层的硅胶橡胶垫的瓶盖密封,待测,此样品为0 min时的样品. 然后加入2 mg·L−1氧化剂高锰酸钾(KMnO4),开始计时,分别在30、60 、120 min取样待测(个别物质在15 min内也取样). 取样完成后,采用顶空-固相微萃取(HS-SPME)进行萃取,并采用色谱-质谱联用仪(GC-MS)测定各样品的嗅味物质的浓度. 氧化实验结束后,根据各目标物的氧化效果,开展氧化动力学实验.
动力学实验:方法与氧化实验的过程相同,但取样时间均设置在反应开始后10 min以内.
氧化机理探讨实验:在5个含有纯水的250 mL磨口锥形瓶中分别加入10 μg·L−1的各嗅味物质和过量反应终止剂Na2S2O3·5H2O(aq),然后加入1 mg·L−1 KMnO4,搅拌2 h后取样分析.
实验设2组平行,均在20 ℃,pH=7(磷酸缓冲溶液调节)的条件下进行.
-
HS-SPME条件为:温度65 ℃,加热3 min,萃取30 min,解吸附3 min.
气相色谱(GC)条件:载气为高纯氦气(纯度大于99.999%),压强为50.1 kPa;流量控制方式为线速度为36.2 cm·s−1;总流量为21.1 mL·min−1;柱流量为1.01 mL·min−1;不分流进样;柱初始温度为40 ℃,保持3 min,以8 ℃·min−1升温至120 ℃,以5 ℃·min−1升温至130 ℃,再以15 ℃·min−1升温至250 ℃,保持5 min;进样口温度为240 ℃.
质谱(MS)条件:电子轰击源(EI);电子能量为70 eV;离子源温度为230 ℃;接口温度为230 ℃. 使用全扫描模式(SCAN)定性,再选择合适的特征离子,采用扫描离子模式(SIM)定量. SIM模式时5种醛类嗅味物质特征离子及保留时间见表2. 目标嗅味物质采用外标法进行定量.
-
将2 mg·L−1的KMnO4与各醛类嗅味物质反应2 h后,氧化效果如图1(a)、(b)所示,分别展示了KMnO4氧化各不同醛类嗅味物质的氧化趋势和去除率. 实验设置了空白组,从图1(a)空白组结果可以看出,反应2 h内5种醛类嗅味物质没有挥发损失. KMnO4与t2oa和tt24oda的反应中,还测定了1、3、5 min时的t2oa的浓度以及4,8,15 min时tt24oda的浓度,由图1(a)可知,反应10 min内,t2oa和tt24oda被氧化50%以上,浓度下降趋势明显,去除效果佳;在30 min后4种烯醛类嗅味物质浓度逐渐趋向平衡,而环状醛 β-cyclo浓度在60 min时趋近平衡. 图1(b)表示反应平衡后,KMnO4对5种醛类嗅味物质的氧化去除率均>75%,其中t2oa、tt24dda和 β-cyclo的去除率均>90%.
综上,KMnO4可以在短时间内去除75%以上这5种醛类嗅味物质,因此,KMnO4可以用于应对由此类醛类嗅味物质引发的突发嗅味问题.
-
由于KMnO4对5种醛类嗅味物质均具有高去除率,本研究继而开展了氧化动力学研究. 这里以KMnO4氧化tt24hept为例,详细介绍氧化动力学的计算过程. KMnO4与大多数有机物的反应为伪二级反应[13][15-16],所以先假设KMnO4氧化tt24hept的反应为伪二级反应,则其过程可以用式(1)表示.
式中,
$ k $ 为反应速率常数,单位为$\mathrm{L}\cdot {\mathrm{m}\mathrm{o}\mathrm{l}}^{-1}\cdot {\mathrm{m}\mathrm{i}\mathrm{n}}^{-1}$ ;$ \left[{\mathrm{K}\mathrm{M}\mathrm{n}\mathrm{O}}_{4}\right] $ 为反应体系中KMnO4的浓度,单位为mol·L−1;$ C $ 为反应体系中tt24hept的浓度,单位为mol·L−1.为使浓度成为反应速率方程的唯一变量,使其中一种反应物的浓度远远大于另一反应物的浓度. 实验过程中KMnO4的初始浓度远高于目标嗅味物质浓度(≥8倍),因此,KMnO4的浓度可视为常数,令
$ {k}_{{\rm{obs}}} $ 为KMnO4与醛类嗅味物质反应的伪一级反应速率常数,单位为$ {\mathrm{m}\mathrm{i}\mathrm{n}}^{-1} $ . 把式(2)带入式(1),作移项变形后等式两边求定积分,运算得式(3):式中,t表示反应开始后进行到t时刻,单位为s;
$ C\left(t\right) $ 表示在t时刻tt24hept的浓度,单位为mol·L−1;$ {C}_{0} $ 表示tt24hept的初始浓度,单位为mol·L−1.由式(3)可知,只要测定出对应时刻t对应的tt24hept浓度,即可通过线性回归求得
$ {k}_{{\rm{obs}}} $ 的值.图2展示了0.5—2 mg·L−1 KMnO4浓度下氧化tt24hept的伪一级拟合,具体参数如表3所示. 在KMnO4过量的情况下,反应体系内剩余tt24hept浓度与初始浓度比值的自然对数和其对应时刻呈现良好的线性相关关系,说明高锰酸钾氧化tt24hept的反应对tt24hept是一级反应.
根据表3,以
$ {k}_{{\rm{obs}}} $ 为纵坐标,$ \left[{\mathrm{K}\mathrm{M}\mathrm{n}\mathrm{O}}_{4}\right] $ 为横坐标关于式(2)进行拟合,结果如图3所示. 从图3看出KMnO4浓度与伪一级反应速率常数存在良好的线性关系,故高锰酸钾氧化tt24hept的反应对高锰酸钾的反应级数也是一级. 由此,此氧化反应整体是二级反应,二级反应速率常数k=5.248×104 L·mol−1·min−1.根据上述计算,同理可得KMnO4氧化t2oa、tt24oda、tt24dda、β-cyclo的伪二级反应动力学常数(见表4). 由表4可知烯醛类的嗅味物质反应速率常数比β-cyclo高一个数量级,3种二烯醛(tt24hept,tt24oda,tt24dda)反应速率常数均高于一烯醛(t2oa).
-
KMnO4对中性天然水源水中各种有机物氧化去除效果均很好,无论是低分子量、低沸点还是高分子量、高沸点有机污染物,剩余的有机污染物浓度很低[17]. KMnO4可以通过直接进行氧原子的转移而与碳碳双键(C=C)反应[18-19],很好地去除醇、醛、酚等有机污染物和致突变物质[20-21]. 本实验研究的对象为烯醛类和环醛嗅味物质,含有双键、环等不饱和键,易被KMnO4迅速氧化,且可以达到很高的去除率,如t2oa和tt24oda在10 min内被氧化去除50%. 氧化动力学实验中,通过对实验数据进行回归分析并结合研究对象中的4个烯醛类物质的结构与反应速率常数,可以发现:tt24oda比t2oa多1个碳碳双键,其反应速率常数也比t2oa的大,碳碳双键是1个化学性质活泼的原子团,由此醛类物质结构中含碳碳双键数目越多,KMnO4与它的反应速率常数就越大;tt24hept比tt24oda少1个亚甲基(—CH2—),其反应速率常数比tt24oda大,由于亚甲基是一个化学性质稳定的原子团,因此醛类物质结构中含亚甲基数目越少,KMnO4与它的反应速率常数就越大. 在判断其它烯醛类嗅味物质的氧化可处理性时,可以利用此规律进行初步估算.
其次,在pH中性条件下,KMnO4能被水中的还原性物质还原成新生态二氧化锰(MnO2)(式4):
根据MnO2的性质推测其在高锰酸钾氧化醛类嗅味物质中的作用机制:一是生成的新生态MnO2具有自催化作用,可很好的催化高锰酸钾氧化过程;二是MnO2具有较大的比表面积,可有效地吸附水中的有机物[22-23]. 以往的研究也可以证明这一推测的合理性,庞素艳等[24]发现KMnO4氧化降解酚类化合物的过程中存在着明显的自催化现象,并推测有机物吸附在MnO2表面形成络合物,比存在于溶液中更易被高锰酸钾氧化,是一种表面吸附络合催化作用.
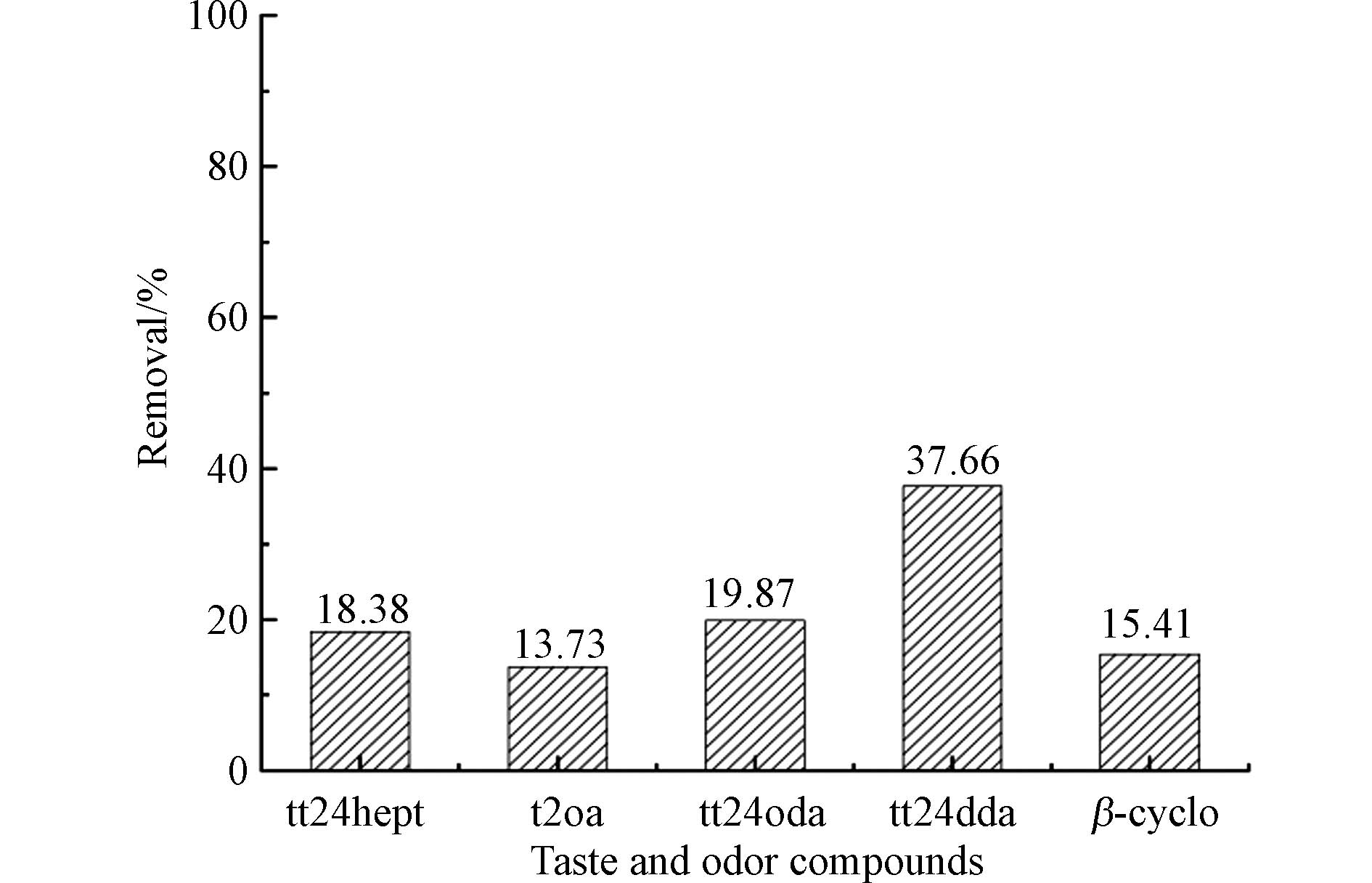
本研究中为了验证MnO2在氧化中是否产生一定协同作用,提前在反应体系加入了过量的硫代硫酸钠,投加1 mg·L−1 KMnO4后,高锰酸钾被迅速充分转化为新生态MnO2. 如图4所示,新生态MnO2反应2h后5种醛类物质的去除率为13.73% — 37.66%,且二烯醛tt24hept、tt24oda、tt24dda的去除率明显高于其他物质,这表示在反应过程中除了KMnO4氧化去除,MnO2也起到了一定催化或吸附作用. 更详细的协同作用机制仍有待深入探讨和剖析.
-
综上,实验得到KMnO4氧化五种醛类嗅味物质的氧化动力学常数,因此可以根据动力学指导生产,将式(3)变形可得式(5),即可求得不同浓度下KMnO4氧化醛类嗅味物质的理论动力学反应方程:
以tt24hept为例,KMnO4投加量为2 mg·L−1时,其氧化tt24hept的方程式如下(式6),并可根据此方程做出理论反应曲线.
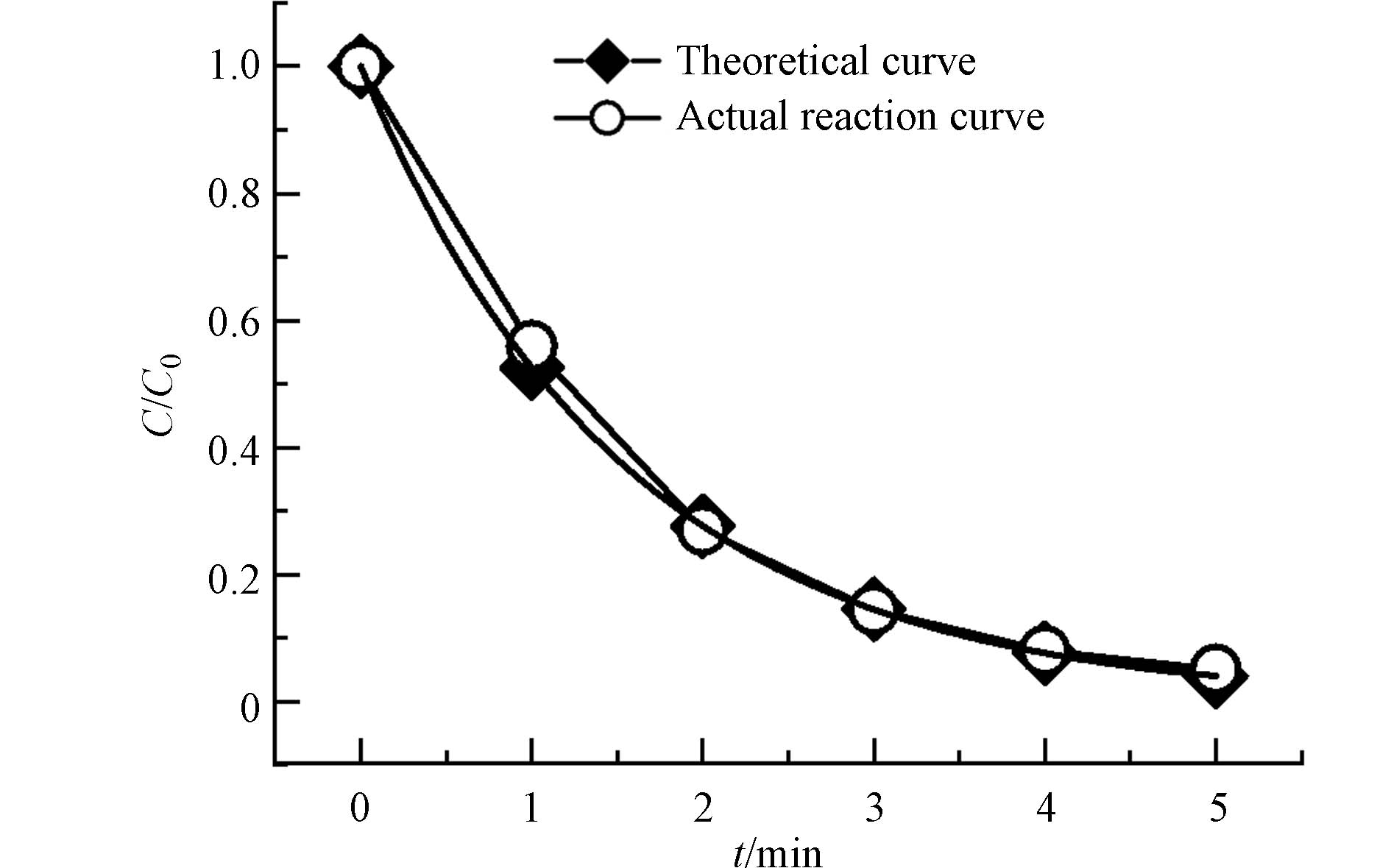
本研究选取呼和浩特市JH饮用水厂进厂原水加标实验考察理论反应曲线是否适用于水厂条件下的氧化反应. 如图5,反应开始5 min时,tt24hept仅剩余4.4%,整个氧化反应在前5 min内已基本完成. 除0时刻外,各时刻的tt24hept浓度比均高于经验反应曲线上相同时刻对应的tt24hept浓度比,且随着反应时间的增加,此差距越来越小. 这说明原水中有其它还原剂(如腐殖酸和氨氮等有机物)存在与tt24hept竞争,而后随着其它还原剂的氧化减少,KMnO4氧化tt24hept的反应受其影响减小,实际反应曲线表现为越来越接近理论反应曲线. 因此理论反应方程可以模拟原水在实际反应中tt24hept的浓度比随时间的变化,进而指导实践. 在后续的实验中发现原水条件下,理论方程对tt24oda、tt24dda和β-cyclo等3种醛类特征嗅味物质实际氧化过程也有很好的拟合度.
对于可用理论反应方程模拟KMnO4氧化醛类特征嗅味物质的过程,根据式(5),欲使水中嗅味物质降至嗅阈值(或有关标准)OTC以下,则有
所以
由不等式(7)可知,氧化剂的投加量
$ \left[{\mathrm{K}\mathrm{M}\mathrm{n}\mathrm{O}}_{4}\right] $ 与氧化时间$ t $ 和进厂原水中嗅味物质的浓度$ {\left[C\right]}_{0} $ 有关. 根据水厂条件确定氧化时间$ t $ 和进厂原水中的$ {\left[C\right]}_{0} $ ,即可得到水厂面临相应问题时的适宜投加量. 当饮用水中存在多种嗅味物质时,应当先找出最适合的氧化剂(能有效去除最多种类的嗅味物质),再分别计算出去除对应嗅味物质的投加量,然后取最大值,得到适宜投加量.由式(7)可得到KMnO4关于不同反应时间和氧化剂投加量对有关醛类特征嗅味物质的去除率. 以本研究所设定的各嗅味物质初始浓度为例,得到使各嗅味物质浓度降至嗅阈值以下所使用的最低KMnO4投加量及对应的反应时间,见表5.
-
本研究开展了高锰酸钾氧化5种醛类嗅味物质的研究,得到如下结论:
(1) KMnO4对反,反-2,4-庚二烯醛(tt24hept)、反-2-辛烯醛(t2oa)、反,反-2,4-辛二烯醛(tt24oda)、反,反-2,4-癸二烯醛(tt24dda)和β-环柠檬醛(β-cyclo)这5种醛类特征嗅味物质均有较好的氧化效果:反应30 min左右,氧化去除率均>75%.
(2)通过回归分析发现,在pH=7,20℃的条件下,KMnO4氧化与上述5种醛类特征嗅味物质的反应均符合伪二级动力学模型,并通过计算得到KMnO4与5种醛类嗅味物质反应的伪二级动力学常数:5.25×104、2.66×104、4.50×104、2.71×104 、5.37×103 L·mol−1·min−1.
(3)KMnO4氧化过程中会生成新生态MnO2,对醛类嗅味有机物也有一定的去除效果,去除率在13.73%— 37.66%.
(4)理论反应方程可以很好地模拟原水条件下,KMnO4分别氧化tt24hept、tt24oda、tt24dda和 β-cyclo 这4种醛类特征嗅味物质的反应过程. 而对于KMnO4氧化t2oa的反应,理论方程的拟合程度不高,需要进一步研究影响反应的因素,才能更好的指导生产. 同时可以根据研究结果,在确定氧化时间t和原水中嗅味物质初始浓度
$ {C}_{0} $ 的情况下,即可得到控制目标嗅味物质达到嗅阈值以下的适宜高锰酸钾投加量.
高锰酸钾氧化饮用水中醛类嗅味物质的效果及动力学研究
Study on the effect and kinetics of aldehydes oxidation by potassium permanganate in drinking water
-
摘要: 我国北方呼和浩特市以黄河为水源的JH饮用水厂近年来冬季经常有醛类嗅味物质检出,常规处理工艺如混凝、沉淀等对其去除效果有限,需要对其进行其它处理工艺的探究. 本文选择高锰酸钾对水厂检出频率和浓度均较高的反,反-2,4-庚二烯醛(tt24hept)、反-2-辛烯醛(t2oa)、反,反-2,4-辛二烯醛(tt24oda)、反,反-2,4-癸二烯醛(tt24dda)和β-环柠檬醛(β-cyclo)5种醛类嗅味物质进行氧化控制研究,探究其去除效果、氧化动力学和氧化机理. 结果表明,20 ℃,pH=7时,2 mg·L-1高锰酸钾氧化5种醛类嗅味物质30 min后,去除率达75%以上. 根据动力学分析可知,高锰酸钾氧化5种醛类嗅味物质属于伪二级动力学过程,其伪二级反应速率常数分别为5.25×104、2.66×104、4.50×104、2.71×104、5.37×103 L·mol−1·min-1,醛类嗅味物质结构中含碳碳双键数目越多、含亚甲基数目越少,反应速率常数越大. 同时,氧化过程会产生新生态二氧化锰,促进高锰酸钾对嗅味物质的控制效果. 最后,通过水厂原水加标实验效果验证,理论反应方程可为饮用水厂应对醛类物质嗅味问题提供相应的理论依据并指导生产.Abstract: Aldehyde odor substances have always been detected in recent years in winter for JH drinking water treatment plant in Hohhot city of northern China, which with the resource water from Yellow River. Conventional treatment processes such as coagulation, sedimentation and other effects on its removal are limited to control the aldehyde odorants, other treatment processes need to explore. In this paper, potassium permanganate was selected for the oxidation of five aldehyde odorants, trans,trans-2,4-heptadienal (tt24hept), trans-2-octenal (t2oa), trans,trans-2,4-octadienal (tt24oda), trans,trans-2,4-decadienal (tt24dda) and β-cyclocitral (β-cyclo), which were detected with high frequency and concentration in JH plants. The oxidation removal, kinetics and mechanism were investigated. The results showed that at 20 °C and pH=7, the removal of the five aldehyde odorants was over 75% after 30 min of oxidation by 2 mg·L−1 potassium permanganate. According to the kinetic analysis, the oxidation of the five aldehyde odorants by potassium permanganate belongs to the pseudo-second-order dynamic process, and its pseudo-second-order rate constants were 5.25×104, 2.66×104, 4.50×104, 2.71×104 and 5.37×103 L·mol-1·min−1, respectively. The higher number of carbon-carbon double bond and the lower amount of methylene presented in the structure of an aldehyde odor substance cause the higher reaction rate constant. At the same time, the oxidation process will produce new in-site manganese dioxide, which promoting the oxidation effect of potassium permanganate on the aldehyde odorants. Finally, through the spiked recovery experiments by the raw water of JH plant, it was verified that the theoretical reaction equation could provide the corresponding theoretical basis, and guide the production of the problem of aldehydes in drinking water treatment plants.
-
Key words:
- potassium permanganate /
- aldehyde odorants /
- oxidation kinetics /
- manganese dioxide.
-

-
图 1 KMnO4氧化5种醛类嗅味物质的趋势图(a)和2 h去除率(b)(嗅味物质初始浓度:tt24hept 100 μg·L−1,t2oa 50 μg·L−1,tt24oda 250 μg·L−1,tt24dda 50 μg·L−1,β-cyclo 20 μg·L−1;KMnO4浓度:2 mg·L−1)
Figure 1. Oxidation trend diagram (a) and 2 h removal rate (b) of 5 aldehydes odorous substances oxidated by KMnO4 (Initial concentration of odorous substances:tt24hept 100 μg·L−1,t2oa 50 μg·L−1,tt24oda 250 μg·L−1, tt24dda 50 μg·L−1, β-cyclo 20 μg·L−1;KMnO4:2 mg·L−1)
表 1 5种醛类嗅味物质基本信息
Table 1. Basic information of five aldehyde odorants
物质名称
Substance name英文名
English name结构式
Constitutional formula嗅阈值/(μg·L−1)
(OTC)CAS 初始浓度/(μg·L−1)
Initial concentration反,反-2,4-庚二烯醛 trans,trans-2,4-heptadienal
(tt24hept)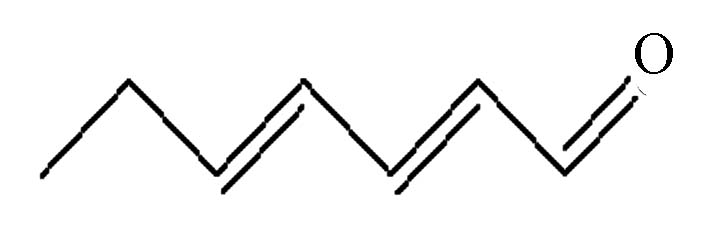
5 4313-03-5 100 反-2-辛烯醛 trans-2-octenal
(t2oa)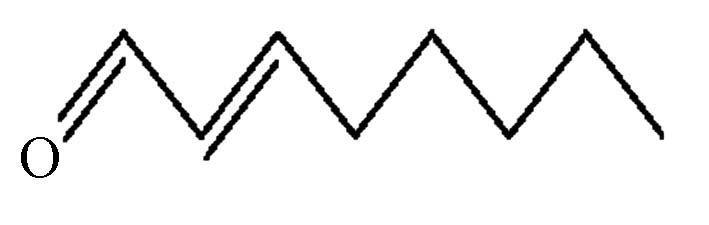
3 2548-87-0 50 反,反-2,4-辛二烯醛 trans,trans-2,4-octadienal
(tt24oda)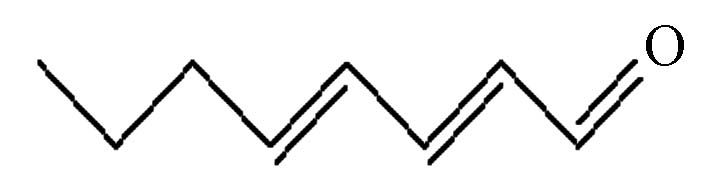
10 30361-28-5 250 反,反-2,4-癸二烯醛 trans,trans-2,4-decadienal
(tt24dda)
0.3 25152-84-5 50 β-环柠檬醛 β-cyclocitral
(β-cyclo)
3 432-25-7 20 表 2 5种醛类嗅味物质的特征离子及保留时间
Table 2. Characteristic ions and retention times of five aldehyde odorants
嗅味物质
Taste and odor compounds质荷比
m/z开始时间/min
Start time结束时间/min
Terminal time保留时间/min
Retention timett24hept 81*, 53, 39 16.41 17.77 16.895 t2oa 41*, 55, 70 15.00 20.00 16.660 tt24oda 81*, 39, 41 12.00 14.00 12.660 tt24dda 81*, 41, 67 22.83 24.26 23.335 β-cyclo 137*, 152, 123 12.00 13.50 12.767 注:*为特征离子,其余为参考离子. Note: * the characteristic ion, and the rest are reference ions.
表 3 各KMnO4浓度下氧化tt24hept的伪一级动力学参数
Table 3. Pseudo-first-order kinetic parameters of tt24hept oxidation by different dosage of KMnO4
$\left[{\mathrm{K}\mathrm{M}\mathrm{n}\mathrm{O} }_{4}\right]/(\mathrm{m}\mathrm{o}\mathrm{l}\cdot\mathrm{L}^{-1})$ 回归方程
Regression equation$ {k}_{{\rm{obs}}}/{\mathrm{m}\mathrm{i}\mathrm{n}}^{-1} $ 线性相关系数R2
Linearly dependent coefficient3.165×10−6 $\mathrm{ln}\dfrac{C}{ {C}_{0} }=-0.1488{t}+0.0173$ 0.1488 0.9956 6.329×10−6 $\mathrm{ln}\dfrac{C}{ {C}_{0} }=-0.3165{t}+0.0247$ 0.3165 0.9979 9.494×10−6 $\mathrm{ln}\dfrac{C}{ {C}_{0} }=-0.4867{t}+0.0685$ 0.4867 0.9952 1.266×10−5 $\mathrm{ln}\dfrac{C}{ {C}_{0} }=-0.6457{t}+0.0168$ 0.6457 0.9951 表 4 KMnO4氧化5种醛类嗅味物质的伪二级反应动力学常数
Table 4. Kinetic constants of pseudo-second-order reaction of KMnO4 oxidation of five aldehydes odorants
嗅味物质
Taste and odor compoundsk/(L·mol−1·min−1) tt24hept 5.25×104 t2oa 2.66×104 tt24oda 4.50×104 tt24dda 2.71×104 β-cyclo 5.37×103 表 5 KMnO4去除四种醛类特征嗅味物质的投加量参考
Table 5. Reference of KMnO4 to remove four aldehydes
嗅味物质
Taste and odor compounds初始浓度/(μg·L−1)
Initial concentration氧化时间/min
Oxidation time氧化剂量/(mg·L−1)
Oxidative dosagett24hept 100 40 0.50 tt24oda 250 60 0.50 tt24dda 50 70 0.50 β-cyclo 20 120 1.00 -
[1] ZHAO Y Y, YU J W, SU M, et al. A fishy odor episode in a North China reservoir: Occurrence, origin, and possible odor causing compounds [J]. Journal of Environmental Sciences (China), 2013, 25(12): 2361-2366. doi: 10.1016/S1001-0742(12)60317-9 [2] SUN D L, YU J W, AN W, et al. Identification of causative compounds and microorganisms for musty odor occurrence in the Huangpu River, China [J]. Journal of Environmental Sciences (China), 2013, 25(3): 460-465. doi: 10.1016/S1001-0742(12)60012-6 [3] LI X, YU J W, GUO Q Y, et al. Source-water odor during winter in the Yellow River area of China: Occurrence and diagnosis [J]. Environmental Pollution(Barking, Essex:1987), 2016, 218: 252-258. doi: 10.1016/j.envpol.2016.06.069 [4] 李霞, 魏魏, 乔莉, 等. 低温期黄河水源鱼腥味问题的预处理技术应用探讨[J]. 给水排水, 2015, 51(S1): 25-29. LI X, WEI W, QIAO L, et al. Discussion on the application of pretreatment technology for fish smell in Yellow River water source during low temperature period[J]. Water & Wastewater Engineering, 2015, 51(Sup 1): 25-29(in Chinese).
[5] WU W, TAO N P, GU S Q. Characterization of the key odor-active compounds in steamed meat of Coilia ectenes from Yangtze River by GC–MS–O [J]. European Food Research and Technology, 2014, 238(2): 237-245. doi: 10.1007/s00217-013-2098-3 [6] WU N, GU S Q, TAO N P, et al. Characterization of important odorants in steamed male Chinese mitten crab (Eriocheir sinensis) using gas chromatography-mass spectrometry-olfactometry [J]. Journal of Food Science, 2014, 79(7): C1250-C1259. doi: 10.1111/1750-3841.12511 [7] WATSON S, SATCHWLLL T. Chrysophyte odour production: Resource-mediated changes at the cell and population levels [J]. Phycologia, 2003, 42: 393-405. doi: 10.2216/i0031-8884-42-4-393.1 [8] WENDEL T, JÜTTNER F. Lipoxygenase-mediated formation of hydrocarbons and unsaturated aldehydes in freshwater diatoms [J]. Phytochemistry, 1996, 41(6): 1445-1449. doi: 10.1016/0031-9422(95)00828-4 [9] WEE J L, HARRIS S A, SMITH J P, et al. Production of the taste/odor-causing compound, trans-2, Cis-6-nonadienal, within the synurophyceae [J]. Journal of Applied Phycology, 1994, 6(4): 365-369. doi: 10.1007/BF02182152 [10] RIBALET F, WICHARD T, POHNERT G, et al. Age and nutrient limitation enhance polyunsaturated aldehyde production in marine diatoms [J]. Phytochemistry, 2007, 68(15): 2059-2067. doi: 10.1016/j.phytochem.2007.05.012 [11] MIRALTO A, BARONE G, ROMANO G, et al. The insidious effect of diatoms on copepod reproduction [J]. Nature, 1999, 402(6758): 173-176. doi: 10.1038/46023 [12] JÜTTNER F, WATSON S B, von ELERT E, et al. Β-cyclocitral, a grazer defence signal unique to the cyanobacterium Microcystis [J]. Journal of Chemical Ecology, 2010, 36(12): 1387-1397. doi: 10.1007/s10886-010-9877-0 [13] 张可佳, 高乃云, 黎雷. 高锰酸钾氧化嗅味物质β-环柠檬醛的动力学 [J]. 中南大学学报(自然科学版), 2011, 42(4): 1161-1166. ZHANG K J, GAO N Y, LI L. Kinetics of oxidation of odorant β-cyclocitral by potassium permanganate [J]. Journal of Central South University (Science and Technology), 2011, 42(4): 1161-1166(in Chinese).
[14] 刘禧文, 闫慧敏, 韩正双, 等. 水中8种典型嗅味物质的氧化去除研究 [J]. 供水技术, 2020, 14(4): 1-7. LIU X W, YAN H M, HAN Z S, et al. Research on the removal of 8 typical taste and odor compounds from source water by oxidation methods [J]. Water Technology, 2020, 14(4): 1-7(in Chinese).
[15] 廖宇, 张慧鑫, 李璐玮, 等. 饮用水中两种硫醚类嗅味物质的氧化去除 [J]. 环境化学, 2020, 39(5): 1254-1261. doi: 10.7524/j.issn.0254-6108.2019081303 LIAO Y, ZHANG H X, LI L W, et al. Removal of two typical sulfides odorants by different oxidants in drinking water [J]. Environmental chemistry, 2020, 39(5): 1254-1261(in Chinese). doi: 10.7524/j.issn.0254-6108.2019081303
[16] 徐勇鹏, 杨静琨, 王在刚. 高锰酸钾氧化去除水中三氯生动力学研究 [J]. 哈尔滨工业大学学报, 2011, 43(12): 48-52. XU Y P, YANG J K, WANG Z G. Kinetics on triclosan oxidation by potassium permanganate in drinking water [J]. Journal of Harbin Institute of Technology, 2011, 43(12): 48-52(in Chinese).
[17] 马军, 李圭白, 李晓东. 高锰酸钾除微污染效能-GC/MS分析 [J]. 中国给水排水, 1999, 15(5): 13-15. MA J, LI G B, LI X D. Removal of organic micropollutants from water by permanganate pre-oxidation-GC/MS analysis [J]. China Water & Wastewater, 1999, 15(5): 13-15(in Chinese).
[18] YAN Y E, SCHWARTZ F. Oxidative degradation and kinetics of chlorinated ethylenes by potassium permanganate [J]. Journal of Contaminant Hydrology, 1999, 37: 343-365. doi: 10.1016/S0169-7722(98)00166-1 [19] WIBERG K B, SAEGEBARTH K A. The mechanisms of permanganate oxidation. IV. hydroxylation of olefins and related reactions [J]. Journal of the American Chemical Society, 1957, 79(11): 2822-2824. doi: 10.1021/ja01568a042 [20] BECK C B. Physicochemical processes for water quality control, Walter J. Weber, Jr. (with eight contributors), Interscience, New York(1972). 640 pages. $19.95 [J]. AIChE Journal, 1973, 19(2): 413. [21] SUFFET I H, BAKER R J, YOHE T L. Pretreatment of drinking water to control organic contaminants and taste and odor[C]//Pretreatment in Chemical Water and Wastewater Treatment, 1988: 15-39. [22] 庞雅丽. 高锰酸钾与粉末活性炭联用去除水中嗅味物质[D]. 北京: 北京工业大学, 2011. PANG Y L. Study on the use of potassium permanganate in combination with powder activated carbon for the removal of taste and odor in drinking water[D]. Beijing: Beijing University of Technology, 2011(in Chinese).
[23] VEGA E, MARTIN M J, GONZALEZ-OLMOS R. Integration of advanced oxidation processes at mild conditions in wet scrubbers for odourous sulphur compounds treatment [J]. Chemosphere, 2014, 109: 113-119. doi: 10.1016/j.chemosphere.2014.02.061 [24] 庞素艳, 江进, 马军, 等. MnO2催化KMnO4氧化降解酚类化合物 [J]. 环境科学, 2010, 31(10): 2331-2335. PANG S Y, JIANG J, MA J, et al. Oxidation of phenolic compounds with permanganate catalyzed by manganese dioxide [J]. Environmental Science, 2010, 31(10): 2331-2335(in Chinese).
-




 下载:
下载: