-
无论食品安全还是生态健康,农化残留处理都是污染治理的重要课题,其中除草剂占全球农化市场的40%以上,其污染的处理需求也更为迫切. 由尿素、硫脲和磷脲等化肥产品衍生的氮化碳(g-C3N4)属于非金属和生物安全性半导体,能吸收转化可见光资源用于环境修复[1],但其光催化活性较为温和. 已有报道将g-C3N4改性以提升除草剂的降解,包括三嗪类(阿特拉津、嗪草酮)、有机磷类(草甘膦)、酰胺类(乙草胺)、联吡啶类(百草枯)、苯氧羧酸类(2,4-D)、磺酰脲类(甲磺隆、氯嘧磺隆)和烟酸类(灭草烟)等,涉及改性剂主要有钛、锌、铋、钴和铁的氧化物,硫化镉、硫化钼、钒酸和钨酸等化合物[2 − 3].
与这些大宗非选择性除草剂相比,二苯醚类除草剂作为一种选择性原卟啉原氧化酶抑制剂,目前的销售额已稳步增长至全球除草剂市场的2%[4],其中占比最多的是氟磺胺草醚(fomesafen, FSA)、乙氧氟草醚(oxyfluorfen, OFF)和三氟羧草醚(acifluorfen, AFF)等产品,在土壤中的半衰期分别为100—240 d、30—40 d和14—60 d. 此类农药残留可导致杂草抗性日趋加重、作物产量逐渐下降,后茬作物如蔬菜、玉米和小麦的育苗受到影响,土壤酶活和微生物多样性遭到破坏;通过雨水、灌溉随地表径流进入附近池塘、湖泊和河流中,被藻类和鱼类等水生生物吸收累积并破坏生态健康[5 − 8]. 采用生物污泥等传统市政处理措施并不能彻底修复残留除草剂的污染,需增加电解工艺处理才能达标排放[9 − 11],或者从农药厂活性污泥中筛选培育芽孢杆菌、金黄杆菌和假单胞菌等用以加速降解二苯醚类除草剂的残留污染[12 − 15],仅有少数报道将金属半导体(如TiO2和ZnO)用于二苯醚类除草剂的光降解[16 − 17].
微纳米的金属半导体并不为生物所吸收,可能造成次生的生态毒理和环境风险. 若沿用非金属掺杂或修饰的有机半导体,则能在确保生物安全性的同时、提高除草剂的光降解效率,例如硫氧磷等元素掺杂以及石墨烯和碳量子点等修饰的氮化碳[18 − 20]. 碳量子点(carbon quantum dots,CQDs)具有荧光特性和光电子传导能力,兼有化学惰性、生物安全性和一定的抗菌活性,能用于传感器、光催化、发光材料、药物载体以及促进植物生长. 多数农林牧渔业废弃物含有丰富的有机碳资源,均可作为廉价友好和可持续的碳量子点原料[21]. 碳量子点与氮化碳复合的环境修复材料,已用于氟喹诺酮、氟喹啉、四环素和环丙沙星等抗生素的光降解[22 − 25],但未有除草剂等农化残留处理的报道.
本文以玉米芯为碳量子点源,用氮化碳进行修饰,合成一种非金属型光催化剂,研究了在该催化剂作用下,氟磺胺草醚、乙氧氟草醚和三氟羧草醚的降解效率,结合分子模拟结果和降解产物分布解释二苯醚类农药的光解行为,并利用玉米种子生长模型评价了光解产物的毒性,以期对该类农化污染的修复与管控提供基础数据支持.
-
玉米芯颗粒(30目)购自山东禹城市盛之源农业科技有限公司,碳酸氢铵(AR)、双氧水(30%)、对苯醌(AR)、叔丁醇(AR)和乙二胺四乙酸二钠(AR)均购自上海阿拉丁试剂公司,氟磺胺草醚、乙氧氟草醚和三氟羧草醚均为标准品,购自中国默克试剂公司,实验所用蒸馏水为去离子水. 实验用透析袋由北京百瑞极生物科技有限公司提供.
-
Tenson27型红外光谱(FTIR)分析仪,德国Bruker公司;Tecnai F30型透射电子显微镜(TEM),荷兰Philips-FEI公司;K-Alpha型X射线光电子能谱仪(XPS),美国Thermo Fisher公司;Lambda1050型紫外可见光漫反射测试,美国PerkinElmer公司;LC5090型液相色谱分析仪,浙江福立分析仪器公司;Agilent 6550型液相色谱质谱联用仪(LC-MS),美国Aglient公司.
-
玉米芯颗粒进行粉碎研磨,过200目筛,所得粉末与碳酸氢铵和蒸馏水按质量比2:1:50混合加入100 mL聚四氟乙烯内衬的反应釜,在180 ℃下水热反应6 h;取30 mL上清液装入分子量为12000—14000 DA的透析袋中透析48 h,取透析袋外液体旋蒸浓缩至30 mL后二次透析,装入分子量为3000 DA的透析袋中透析48 h,取透析袋内液体,旋蒸浓缩至5 mL即得碳量子点(CQDs)母液,用TEM观察其平均粒径为10—15 nm.
将工业尿素研磨后填充于氧化铝坩埚内(占总体积的75%—85%),加盖后放入马弗炉中,以5 ℃·min−1的升温速度从室温渐升至150 ℃干燥1 h,继续以10 ℃·min−1的速度从150 ℃渐升至650 ℃煅烧2 h,制得浅黄色g-C3N4粗粉;每1 g粗粉加入100 mL 5% H2O2溶液,经超声剥离处理4 h(超声频率为80 kHz),以6000 r·min−1离心获得白色g-C3N4.
分别取碳量子点母液0、300、600、900、1200、1500 μL与300 mg白色g-C3N4混合,加入30 mL蒸馏水,转移至100 mL聚四氟乙烯内衬的不锈钢反应釜中,在150 ℃下水热搅拌反应36 h,用0.22 μm超滤膜过滤、洗涤、干燥,制得CQDs/g-C3N4的复合光催化剂,对应的样品依次命名为CCN0、CCN1、CCN2、CCN3、CCN4和CCN5.
-
配制50 mL农药溶液(50 mg·L−1),加入30 mg CQDs/g-C3N4复合催化剂,于暗室中搅拌0.5 h达到吸附平衡后,开启氙灯(CEL-HXF300,北京中教金源)进行光照,每隔0.5 h取样,经0.22 μm滤头过滤,用液相色谱检测溶液中除草剂的浓度变化,并计算其光降解率. 实验结束后,光催化剂可进行回收循环实验. 空白对照实验同上,但未加入任何催化剂. 预实验证实在黑暗条件和实验周期内,光催化剂对农药的吸附均不超过5%,可排除农药显著去除与物理吸附有关.
根据除草剂随反应时间的光降解率变化,绘制光降解的动力学曲线,采用一阶动力学模型进行数据拟合. 为解释除草剂的光催化降解机理,基于密度泛函理论(DFT)用Gaussian 09W对农药分子构型进行优化,在B3LYP/6-311G水平上计算NPA电荷分布(Natural Population Analysis),参考文献[26]获得福井函数(Concentrated Fukui Function, CFF)和双描述符(Concentrated Dual Descriptor, CDD),用于预测二苯醚分子结构的降解反应位点. 同时收集产物进行浓缩,用LC-MS分析产物分布和推测降解途径. 应用对苯醌、叔丁醇和 EDTA-2Na分别为超氧自由基、羟基自由基和空穴自由基的捕获剂,考察光降解过程中的自由基作用机理.
-
挑选种子并采用5%左右的次氯酸钠进行消毒,30 min后用清水冲刷洗去残留药剂. 种子在清水中浸泡2—3 d,直至出现嫩芽备用. 将露白的种子铺在覆盖陶瓷托盘的纱布上(已消毒),喷洒适量的水份,待生长3—4 d后选取根长芽长大致相同的植株进行分组,每组两个培养皿,每皿放置20个. 预实验证实仅有光催化剂时与空白对照组无显著性差异,在实验周期内未对玉米种子生长发育造成不利. 因此,玉米生长实验分别设置了对照组、原药组(3 mg·L−1)及相应的处理组,分别在3、6、9 d对植物的平均根长和芽长进行测量,与空白对照组相比,计算抑制率.
-
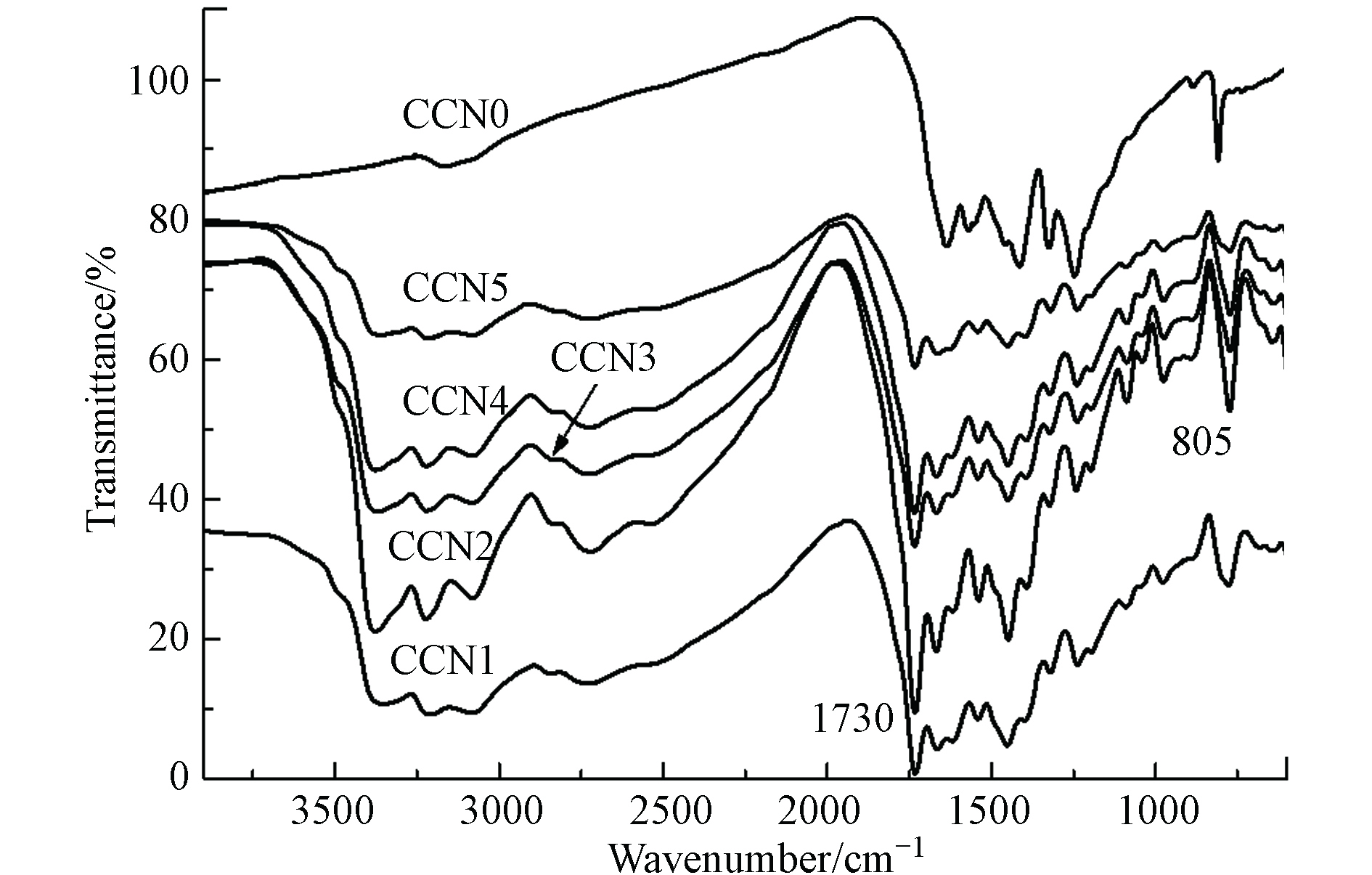
图1为红外光谱表征结果. 在未添加碳量子点(CQDs)的CCN0样品谱图中,观察到1200—1800 cm−1有石墨相氮化碳的典型多齿状吸收峰,归属于三嗪环上C=N、C—N(C)和C—N(H)的伸缩振动,805 cm−1的吸收峰归属于三嗪环上N—H的面外弯曲振动,3000—3400 cm−1的吸收峰应归属于边缘N—H氢键的伸缩振动[27 − 28]. 其余如CCN1—CCN5的样品由于水热法制备碳量子点的化学组成较为复杂,与氮化碳复合之后新出现的特征峰也较为丰富,3000—3400 cm−1的特征峰源自O—H氢键伸缩振动,1730 cm−1的强吸收峰源自C=O的伸缩振动,1000—1800 cm−1增加的吸收峰归属于生物质C=C、C—O(C)和C—O(H)的伸缩振动,600—1000 cm−1的吸收峰归属于芳烃的C—H面外弯曲振动.
通过XPS可以进一步证实碳量子点组成给C3N4带来的变化. 图2(a)展示了样品的XPS全谱,可以看到碳量子点与C3N4复合后表现出更显著的氧特征峰. 根据C1s的去卷积峰变化也可以充分证明,见图2(b). 氮化碳的高分辨率C1s光谱可以拟合为两个峰,287.9 eV 处的峰对应于 sp2-C(N—C=N),而 284.6 eV 处的峰源自石墨相碳缺陷位的 sp3 杂化 C—C 键[29 − 30]. 与C3N4 相比,复合材料的 C1s 光谱可以去卷积为3个峰,其中 286.1 eV 的额外峰通常被认为来自 C—O,即碳量子点引入的碳氧官能团[31].
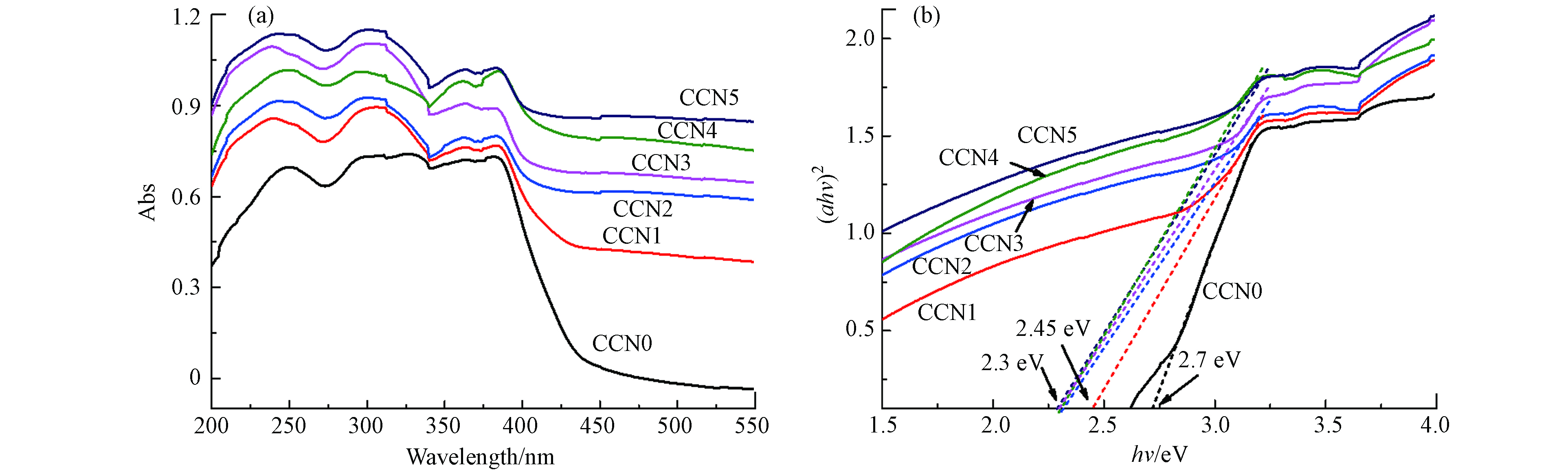
碳量子点具有上转换光致发光特征,能吸收长波长光并将其转换为短波长能量,还表现出一定的电子储存/转移特性,能阻碍光生电子和空穴的复合[32]. 比较CCN0和其余5个样品的紫外-可见漫反射光谱(图3),对于初始 C3N4 纳米片,在450 nm波长附近出现吸收边缘,对应带隙为2.7 eV,这与之前文献结果一致[33],然而随着碳量子点的引入CCN1的带隙收窄为2.45 eV,后续样品中可吸收的光子数量增多,带隙逐步降低到2.3 eV,证实玉米芯水热法制备的碳量子点与C3N4结合时显示出更好的可见光吸收能力,碳量子点的优点增强了复合系统的可见光捕获,有助于提高光催化的性能.
-
用氙灯模拟可见光照射(λ> 420 nm)评估不同样品光催化降解除草剂的活性. 如图4(a)所示,所有复合样品都表现出比纯C3N4较高的光催化活性,其中CCN-3样品最佳. 光照持续3 h,氟磺胺草醚降解最为明显达99%,乙氧氟草醚可降解91%,但三氟羧草醚只有55%,继续光照7—8 h才能达到90%以上,然而在相同条件下,三种除草剂在C3N4上的降解率仅为23%—25%. 采用一阶动力学模型可解释该降解速率与时间的关系. 如图4(b)所示,CCN-3样品处理3种除草剂时,其降解动力学常数比C3N4高约14、2.5、2倍. 此外,还研究了3种除草剂在未加任何催化剂时的光降解曲线,证实在实验周期内农药浓度均没有明显降低. 由此可知,碳量子点的引入确实有利于提高C3N4催化剂的光催化性能. 然而碳量子点的过度引入会削弱光催化效果,例如CCN-4和CCN-5样品的性能差于CCN-3,可能是由于过量碳量子点对C3N4的屏蔽效应,与C3N4竞争吸附光子[23],阻碍了后者对于可见光的吸附和转化.
-
上述数据表明碳量子点修饰确实可以改进提高C3N4的光催化活性,促使二苯醚类除草剂的光降解,但是这3种农药的降解速度又有显著的差异. 考虑到三者的母体化学结构相似,其差异可能源自于取代基的不同,分别为酰胺、羧酸和烷氧基. 利用高斯软件优化农药分子的空间结构计算NPA电荷分布,在此基础上可获得福井函数(CFF)和双描述符(CDD),这两者是DFT理论中的重要概念,已被广泛用于预测化学反应的位点[34]. 有文献表明双描述符要比福井函数更精确地判断碳原子附近反应的趋势[35 − 36]. 比较3种农药分子的CDD值分布,氟磺胺草醚和三氟羧草醚的电荷分布较为相似,如图5(a)所示,CDD值最正的C1\C2\C5\C3电荷分布有利于亲核进攻,但乙氧氟草醚的C1原子反应趋势排在C2\C5\C3之后,可能是烷氧基相较羧基和酰胺更为稳定的缘故. 其他中间的碳原子周围电荷分布几近于零,反应趋势不明显,所以不再赘述,而CDD值最负的C8/C12/C13电荷分布揭示了亲电反应的趋势,同时根据福井函数CFF分布亦可推断3种农药均在C7处易受自由基进攻而导致裂解反应.
关于二苯醚类光降解过程的研究极少,Chakraborty等报道乙氧氟草醚的光解产物可能经过了醚键断裂和脱卤等途径[37],辛柏福等用TiO2降解三氟羧草醚的过程中也证实有醚键断裂和脱卤、羟基化等产物[38]. 根据分子模拟计算和液质联用分析结果,如图5(b)所示,推断二苯醚类除草剂的光催化降解过程至少包括以下3种途径:(1)水解、脱卤和硝基还原,(2)醚键裂解和(3)羟基化. 以最易降解的氟磺胺草醚P1为例,受亲核和自由基攻击,得到酰胺水解和硝基还原的中间体P2—P5,然后醚键断裂得到苯氧基中间体P6和P7,芳环上C—H键、C—Cl键和C—F键可能发生亲电和自由基驱动的羟基化和脱卤反应[39],得到中间体如 P8—P12等,这些初级芳烃更容易降解,直至矿化成CO2和H2O.
分别向体系中加入了不同的自由基抑制剂进行3种农药的光催化降解,其中叔丁醇(TBA)、EDTA-2Na和对苯醌(PBQ)依次为羟基自由基(·OH)、空穴(h+)和超氧自由基(·O2−)的抑制剂. 图6a表明,添加TBA后,3种农药的降解率与催化剂组相差仿佛,说明·OH对于二苯醚类农药的降解没有显著作用. 然而添加另外两种试剂时,3种农药的降解率均受到显著抑制. 与无添加的催化剂组相比,加入EDTA-2Na后三氟羧草醚(AFF)、乙氧氟草醚(OFF)与氟磺胺草醚(FSA)的降解率分别下降了53%、58%和42%,加入PBQ后三者降解率分别下降了28%、30%和29%. 这说明空穴和氧自由基是二苯醚类农药降解过程中的关键活性物质,而且空穴的作用要略强于氧自由基. 同时,优选CCN3进行二苯醚类农药的光催化降解,验证该类催化剂的稳定性和重复使用性,结果见图6b. 在相应的最佳工艺条件下,CCN3在经过4次循环光催化降解实验后,仅有少许活性损耗,但仍能保持较高的光催化性能. 与第一次初始值相比,三氟羧草醚(AFF)、乙氧氟草醚(OFF)与氟磺胺草醚(FSA)的降解率在使用4次之后仅下降了3%、3%和4%.
-
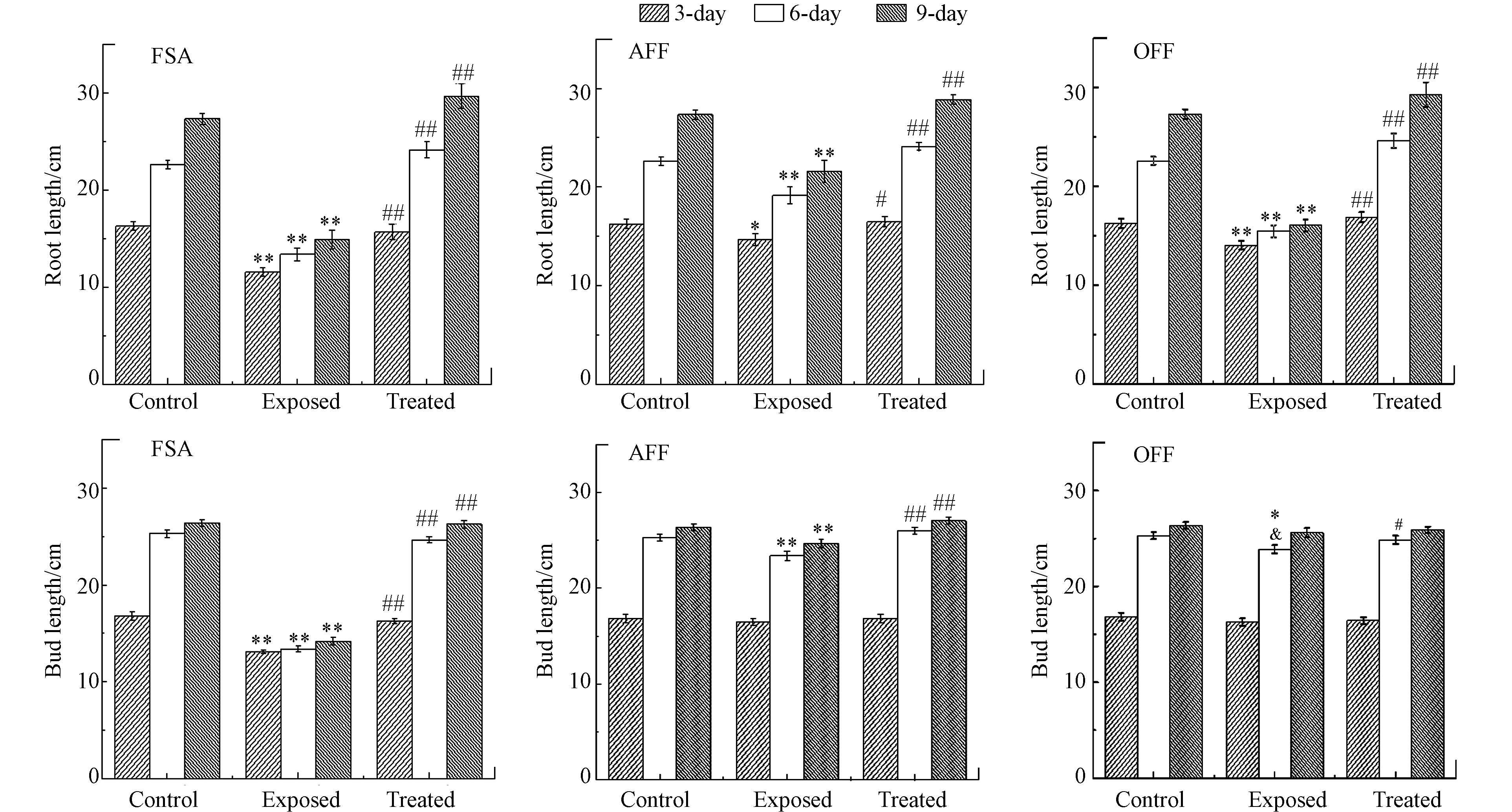
利用玉米种子的水培生长实验来探讨二苯醚类除草剂及其光解产物的毒性,结果见图7. 与空白实验组相比,可以判断氟磺胺草醚和乙氧氟草醚对种子的根长生长抑制较为明显,氟磺胺草醚毒性最高,三氟羧草醚的毒性最小,根据芽长生长情况,也可以发现氟磺胺草醚的毒性最强,但三氟羧草醚和乙氧氟草醚对芽长生长抑制不明显. 以氟磺胺草醚胁迫为例,玉米根系的生长受到抑制,根长变短、根须减少和部分根尖卷曲畸形,同时玉米叶片发育枯黄,株高和鲜重降低,可能是氟磺胺草醚抑制原卟啉原氧化酶活性,产生高水平的单线态氧与氧自由基,破坏细胞膜结构[40]. 但所有经过光照降解处理之后的液体,无论是种子根长和芽长均可以恢复至空白对照组的生长水平,由此可以判断以二苯醚为母核的这一类除草剂中,酰胺基和乙氧基取代的农药残留有一定危害,羧基取代的三氟羧草醚最具有光化学安全性.
-
(1)以氟磺胺草醚、三氟羧草醚和乙氧氟草醚为例,证实二苯醚类除草剂在可见光环境中的稳定性和毒性随取代基不同而显著差异,氟磺胺草醚最易光解但毒性最大,三氟羧草醚的光解速率最小但最为安全,而光催化降解后的产物均未影响玉米种子的生长.
(2)水介质中二苯醚类除草剂光降解过程包括脱卤、水解、醚键断裂、硝基还原和羟基化等,主要受空穴和羟基自由基影响,通过理论计算判断降解反应位点、结合液质分析的结果,合理推测二苯醚类除草剂的光解机理. 实验表明碳量子点修饰g-C3N4可有效缓解农药的生态风险.
碳量子点修饰g-C3N4缓解二苯醚类除草剂污染
Remediation of diphenyl-ether herbicides pollution by g-C3N4 modified with carbon quantum dots
-
摘要: 为加速二苯醚类除草剂在可见光环境的降解,以玉米芯制碳量子点修饰石墨相氮化碳,合成一种非金属型光催化剂. 考察在该催化剂作用下,氟磺胺草醚、三氟羧草醚和乙氧氟草醚等的光解行为及光解前后毒性. 结果表明,氟磺胺草醚在可见光照射下的光解速率最大、其次为乙氧氟草醚,光照3 h的降解率即达99%和91%,而三氟羧草醚的光解速率最低、须光照至8 h才能达到90%的降解率. 由高斯软件计算NPA(Natural Population Analysis)电荷分布得福井函数和双描述符,预测反应位点并结合液质联用分析降解产物,推测除草剂的降解过程应包括裂解、水解、脱卤、还原和羟基化等,证明了空穴和羟基自由基在其中的作用. 观察玉米种子的生长实验,发现氟磺胺草醚的毒性略高于乙氧氟草醚,但二者经光照处理后毒性均显著下降,三氟羧草醚及其光解产物显示为低毒性.Abstract: To move faster the visible-light degradation of diphenyl-ether herbicides, the corn cob-derived carbon quantum dots were prepared to modify graphitic carbon nitride (g-C3N4) and then achieved a non-metallic photocatalyst. It was well studied with the photolytic behaviors and toxicity alterations on the fomesafen, acifluorfen and oxyfluorfen. The results showed that the degradation rate of fomesafen was the highest and followed by that of oxyfluorfen. They were separately at 99% and 91% after 3 h of visible-light irradiation. And yet the degradation rate of acifluorfen was the lowest and it reached 90% even after lighted by 8 h. Using Gaussian software to calculate the charge distribution of NPA and then the Fukui function and dual descriptors were obtained to predict the reactive sites. The degradation products were analyzed with LC-MS and the photolysis process of herbicides was reasonably speculated such as scission, hydrolysis, dehalogenation, reduction and hydroxylation. The presence of vacancies and hydroxyl radicals was proved great roles in them. It was found that fomesafen performed a slightly higher toxicity than oxyfluorfen during the development of corn seeds while both of them significantly reduced to nontoxic after treatments. Finally, acifluorfen and its photolysis products showed the lowest toxicity among three herbicides.
-
Key words:
- carbon quantum dots /
- carbon nitride /
- herbicides /
- fomesafen /
- photocatalysis.
-

-
-
[1] MAMBA G, MISHRA A K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation[J]. Applied Catalysis B: Environmental, 2016, 198: 347-377. doi: 10.1016/j.apcatb.2016.05.052 [2] SAHOO S, ACHARYA R. An overview on recent developments in synthesis and molecular level structure of visible-light responsive g-C3N4 photocatalyst towards environmental remediation[J]. Materials Today: Proceedings, 2021, 35: 150-155. doi: 10.1016/j.matpr.2020.04.008 [3] ZHAO G Q, ZOU J, HU J, et al. A critical review on graphitic carbon nitride (g-C3N4)-based composites for environmental remediation[J]. Separation and Purification Technology, 2021, 279: 119769. doi: 10.1016/j.seppur.2021.119769 [4] 顾林玲, 王欣欣. 全球除草剂市场、发展概况及趋势(Ⅰ)[J]. 现代农药, 2016, 15(2): 8-12, 38. GU L L, WANG X X. The global market, development, trend of herbicide(Ⅰ)[J]. Modern Agrochemicals, 2016, 15(2): 8-12, 38 (in Chinese).
[5] SHEEBA, PRATAP SINGH V, KUMAR SRIVASTAVA P, et al. Differential physiological and biochemical responses of two cyanobacteria Nostoc muscorum and Phormidium foveolarum against oxyfluorfen and UV-B radiation[J]. Ecotoxicology and Environmental Safety, 2011, 74(7): 1981-1993. doi: 10.1016/j.ecoenv.2011.07.006 [6] POWE D K, DASMAHAPATRA A K, RUSSELL J L, et al. Toxicity implications for early life stage Japanese medaka ( Oryzias latipes) exposed to oxyfluorfen[J]. Environmental Toxicology, 2018, 33(5): 555-568. doi: 10.1002/tox.22541 [7] ABD EL-RAHMAN G I, AHMED S A A, KHALIL A A, et al. Assessment of hematological, hepato-renal, antioxidant, and hormonal responses of Clarias gariepinus exposed to sub-lethal concentrations of oxyfluorfen[J]. Aquatic Toxicology, 2019, 217: 105329. doi: 10.1016/j.aquatox.2019.105329 [8] LI Z K, GUO J, JIA K, et al. Oxyfluorfen induces hepatotoxicity through lipo-sugar accumulation and inflammation in zebrafish ( Danio rerio)[J]. Ecotoxicology and Environmental Safety, 2022, 230: 113140. doi: 10.1016/j.ecoenv.2021.113140 [9] CHAIR K, BEDOUI A, BENSALAH N, et al. Treatment of soil-washing effluents polluted with herbicide oxyfluorfen by combined biosorption-electrolysis[J]. Industrial & Engineering Chemistry Research, 2017, 56(8): 1903-1910. [10] CARBONERAS M B, RODRIGO M A, CANIZARES P, et al. Removal of oxyfluorfen from polluted effluents by combined bio-electro processes[J]. Chemosphere, 2020, 240: 124912. doi: 10.1016/j.chemosphere.2019.124912 [11] ACOSTA-SANTOYO G, RASCHITOR A, BUSTOS E, et al. Electrochemically assisted dewatering for the removal of oxyfluorfen from a coagulation/flocculation sludge[J]. Journal of Environmental Management, 2020, 258: 110015. doi: 10.1016/j.jenvman.2019.110015 [12] CUI N, WANG S G, KHORRAM M S, et al. Microbial degradation of fomesafen and detoxification of fomesafen-contaminated soil by the newly isolated strain Bacillus sp. FE-1 via a proposed biochemical degradation pathway[J]. Science of the Total Environment, 2018, 616/617: 1612-1619. doi: 10.1016/j.scitotenv.2017.10.151 [13] ZHAO H H, XU J, DONG F S, et al. Characterization of a novel oxyfluorfen-degrading bacterial strain Chryseobacterium aquifrigidense and its biochemical degradation pathway[J]. Applied Microbiology and Biotechnology, 2016, 100(15): 6837-6845. doi: 10.1007/s00253-016-7504-x [14] 陈道康, 蔡天明, 陈立伟, 等. 海藻酸钠与纳米Fe3O4联合固定化菌对三氟羧草醚的降解[J]. 环境工程学报, 2017, 11(6): 3907-3913. CHEN D K, CAI T M, CHEN L W, et al. Biodegradation of acifluorfen using joint immobilized cells of sodium alginate and Fe3O4 nanoparticles[J]. Chinese Journal of Environmental Engineering, 2017, 11(6): 3907-3913 (in Chinese).
[15] FENG Z Z, LI Q F, ZHANG J, et al. Microbial degradation of fomesafen by a newly isolated strain Pseudomonas zeshuii BY-1 and the biochemical degradation pathway[J]. Journal of Agricultural and Food Chemistry, 2012, 60(29): 7104-7110. doi: 10.1021/jf3011307 [16] 张守花, 张新海. 铜掺杂改性TiO2光催化降解三氟羧草醚废水研究[J]. 广州化工, 2017, 45(23): 90-92. ZHANG S H, ZHANG X H. Photocatalytic degradation of wastewater containing acifluorfen by copper modified titanium dioxide[J]. Guangzhou Chemical Industry, 2017, 45(23): 90-92 (in Chinese).
[17] NAVEETHA G, ATMAKURU R. Photocatalytic degradation of persistence herbicide fomesafen by using ZnO/Na2S2O8 as a catalyst/oxidant under UV radiation[J]. Applied Ecology and Environmental Sciences, 2019, 7(5): 182-189. [18] LIU X, LI C S, ZHANG Y, et al. Simultaneous photodegradation of multi-herbicides by oxidized carbon nitride: Performance and practical application[J]. Applied Catalysis B: Environmental, 2017, 219: 194-199. doi: 10.1016/j.apcatb.2017.07.007 [19] LIU X, LI C S, ZHANG B J, et al. A facile strategy for photocatalytic degradation of seven neonicotinoids over sulfur and oxygen co-doped carbon nitride[J]. Chemosphere, 2020, 253: 126672. doi: 10.1016/j.chemosphere.2020.126672 [20] JO W K, SELVAM N C S. Z-scheme CdS/g-C3N4 composites with RGO as an electron mediator for efficient photocatalytic H2 production and pollutant degradation[J]. Chemical Engineering Journal, 2017, 317: 913-924. doi: 10.1016/j.cej.2017.02.129 [21] DAS R, BANDYOPADHYAY R, PRAMANIK P. Carbon quantum dots from natural resource: A review[J]. Materials Today Chemistry, 2018, 8: 96-109. doi: 10.1016/j.mtchem.2018.03.003 [22] WANG Y F, WANG F L, FENG Y P, et al. Facile synthesis of carbon quantum dots loaded with mesoporous g-C3N4 for synergistic absorption and visible light photodegradation of fluoroquinolone antibiotics[J]. Dalton Transactions, 2018, 47(4): 1284-1293. doi: 10.1039/C7DT04360K [23] WANG W J, ZENG Z T, ZENG G M, et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light[J]. Chemical Engineering Journal, 2019, 378: 122132. doi: 10.1016/j.cej.2019.122132 [24] ASADZADEH-KHANEGHAH S, HABIBI-YANGJEH A, SEIFZADEH D, et al. Visible-light-activated g-C3N4 nanosheet/carbon dot/FeOCl nanocomposites: Photodegradation of dye pollutants and tetracycline hydrochloride[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2021, 617: 126424. doi: 10.1016/j.colsurfa.2021.126424 [25] WANG Y, LI X F, LEI W W, et al. Novel carbon quantum dot modified g-C3N4 nanotubes on carbon cloth for efficient degradation of ciprofloxacin[J]. Applied Surface Science, 2021, 559: 149967. doi: 10.1016/j.apsusc.2021.149967 [26] LIU W, LI Y Y, LIU F Y, et al. Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation[J]. Water Research, 2019, 151: 8-19. doi: 10.1016/j.watres.2018.11.084 [27] CHEN Q Y, LI S J, XU H Y, et al. Co-MOF as an electron donor for promoting visible-light photoactivities of g-C3N4 nanosheets for CO2 reduction[J]. Chinese Journal of Catalysis, 2020, 41(3): 514-523. doi: 10.1016/S1872-2067(19)63497-2 [28] 朱娜, 张洁, 吴潇潇, 等. g-C3N4/TiO2可见光催化降解硝基苯废水[J]. 火炸药学报, 2018, 41(1): 66-71. ZHU N, ZHANG J, WU X X, et al. Visible-light photocatalytic degradation of nitrobenzene wastewater by g-C3N4/TiO2[J]. Chinese Journal of Explosives & Propellants, 2018, 41(1): 66-71 (in Chinese).
[29] XIE Z J, FENG Y P, WANG F L, et al. Construction of carbon dots modified MoO3/g-C3N4 Z-scheme photocatalyst with enhanced visible-light photocatalytic activity for the degradation of tetracycline[J]. Applied Catalysis B: Environmental, 2018, 229: 96-104. doi: 10.1016/j.apcatb.2018.02.011 [30] ZHANG J R, MA Y, WANG S Y, et al. Accurate K-edge X-ray photoelectron and absorption spectra of g-C3N4 nanosheets by first-principles simulations and reinterpretations[J]. Physical Chemistry Chemical Physics: PCCP, 2019, 21(41): 22819-22830. doi: 10.1039/C9CP04573B [31] ZANG J Y, CHEN C Z, YANG Y, et al. Efficient Z-scheme g-C3N4/MoO3 heterojunction photocatalysts decorated with carbon quantum dots: Improved visible-light absorption and charge separation[J]. Research on Chemical Intermediates, 2022, 48(10): 4145-4162. doi: 10.1007/s11164-022-04804-8 [32] ASADZADEH-KHANEGHAH S, HABIBI-YANGJEH A. g-C3N4/carbon dot-based nanocomposites serve as efficacious photocatalysts for environmental purification and energy generation: A review[J]. Journal of Cleaner Production, 2020, 276: 124319. doi: 10.1016/j.jclepro.2020.124319 [33] WU M, HE X, JING B H, et al. Novel carbon and defects co-modified g-C3N4 for highly efficient photocatalytic degradation of bisphenol A under visible light[J]. Journal of Hazardous Materials, 2020, 384: 121323. doi: 10.1016/j.jhazmat.2019.121323 [34] 曹静思, 陈飞武. 芳香化合物亲核、亲电反应活性的理论预测和实验反应速率的相关性研究[J]. 有机化学, 2016, 36(10): 2463-2471. doi: 10.6023/cjoc201602026 CAO J S, CHEN F W. Theoretical study on the correlation of the experimental nucleophilic and electrophilic reaction rates of aromatic compounds with the prediction results of theoretical methods[J]. Chinese Journal of Organic Chemistry, 2016, 36(10): 2463-2471 (in Chinese). doi: 10.6023/cjoc201602026
[35] MORELL C, GRAND A, TORO-LABBÉ A. New dual descriptor for chemical reactivity[J]. Journal of Physical Chemistry A, 2005, 109(1): 205-212. doi: 10.1021/jp046577a [36] MARTÍNEZ-ARAYA J I. Why is the dual descriptor a more accurate local reactivity descriptor than Fukui functions?[J]. Journal of Mathematical Chemistry, 2015, 53(2): 451-465. doi: 10.1007/s10910-014-0437-7 [37] CHAKRABORTY S K, CHAKRABORTY S, BHATTACHARYYA A, et al. Photolysis of oxyfluorfen in aqueous methanol[J]. Journal of Environmental Science and Health, Part B, 2013, 48(11): 919-926. doi: 10.1080/03601234.2013.816586 [38] 辛柏福. 铜和锡改性纳米TiO2的制备及其光催化降解三氟羧草醚效能[D]. 哈尔滨: 哈尔滨工业大学, 2009. XIN B F. Preparation of TiO2 nanoparticles modified by copper or stannum and its applications in acifluorfen photocatalitic degradation[D]. Harbin: Harbin Institute of Technology, 2009 (in Chinese).
[39] ZENG C, WANG Y, XIAO T, et al. Targeted degradation of TBBPA using novel molecularly imprinted polymer encapsulated C-Fe-Nx nanocomposite driven from MOFs[J]. Journal of Hazardous Materials, 2022, 424: 127499. doi: 10.1016/j.jhazmat.2021.127499 [40] 白杰, 韩玉军, 祖永平, 等. 氟磺胺草醚毒害玉米的生理指标分析[J]. 玉米科学, 2014, 22(3): 81-85. BAI J, HAN Y J, ZU Y P, et al. Physiological index of corn caused by fomesafen poisoned[J]. Journal of Maize Sciences, 2014, 22(3): 81-85 (in Chinese).
-



 下载:
下载: