-
生物炭是一种由生物质热解转化而成的富炭材料,由于其原料来源广泛,成本低,具有优良的物理和化学性质,是一种可持续性环保型的吸附材料,已被广泛应用于重金属污染控制[1]. 近年来,研究人员在以农林废弃物[2],动物废弃物[3]和污水污泥[4 − 5]等为原料制备生物炭开展了大量的研究. 污水污泥由于其含有大量的有机物,且产量大,以污水污泥为原料制备污泥生物炭可以实现污泥的资源化,同时还可以实现重金属的污染控制,实现以废治废. 近年来研究人员对污水污泥制备生物炭开展了大量的研究[6 − 7],随着研究的深入,污泥基生物炭也显现了一些不足,如纯污泥生物炭比表面积较低,表面官能团不够丰富等限制了其实际使用,对于水溶液中污染物的去除能力有限. 因此,研究人员开始对污泥生物炭进行表面改性负载吸附材料等来提高污泥生物炭的吸附能力. Ngambia等[7]采用Mg改性生物炭制备的材料对Pb(Ⅱ)和Cd(Ⅱ)具有良好的吸附效果. Ramya等[8]将ZnO负载于污泥生物炭上催化降解初始浓度为10 mg·L−1 Cr(Ⅵ)时,Cr(Ⅵ)的去除率可达到95%. 现有的研究结果表明负载吸附材料对污泥生物炭的吸附能力具有强化的效果,因此通过负载吸附材料强化污泥生物炭吸附能力是一个具有潜力的污泥生物炭发展方向.
层状双金属氢氧化物(layer double hydroxides,简称LDHs)是由带正电荷的层板金属氢氧化物和层间的阴离子构成的一类柱状化合物[9]. 大量的研究表明,LDHs对水中污染物如磷[10]、Cr(Ⅵ)[11]、Pb(Ⅱ)[12 − 13]、染料[11,14]等均有较好的吸附去除能力,显示了其在水处理中具有较大的应用潜力. 然而,LDHs的机械强度低,在水处理过程中容易发生团聚等缺点,限制了其在水处理中的应用. 已有研究人员将LDHs负载到稳定的载体上如麦饭石[15]、陶粒[16]或沸石[17]等,结果显示能够很好地解决LDHs的不足. 然而,现有的载体价格较高,不利于其工业化应用,因此选用合适廉价载体成为了现有的研究关注点.
生物炭原料来源广泛,成本低,机械强度良好,是一种理想的廉价载体. 以生物炭作为载体负载LDHs,可以解决LDHs的不足的同时也可以提高污泥生物炭的吸附能力,有利于其在工业应用中推广. 采用生物炭负载LDHs去除水中重金属目前的研究主要是在对水中Cr(Ⅵ)的去除,在其他重金属离子如Pb(Ⅱ)和Cu(Ⅱ)的去除方面也有少量研究. Chen等[18]采用油茶壳制备的生物炭负载Ni-Al-LDHs去除水中Cr(Ⅵ),最大吸附量达271.5 mg·g−1. Wang等[19]采用油茶渣制备的生物炭负载Mn-Al-LDH用来去除水中Cu(Ⅱ),Cu(Ⅱ)主要是通过同晶型取代,即水中Cu(Ⅱ)被Mn-Al-LDH中Mn(Ⅱ)置换进去,并以Cu2.5(OH)3SO4的形式在吸附剂表面共沉淀来达到去除的目的,最大吸附量达74.07 mg·g−1,是原生物炭的7.3倍. 由已有的文献可以看出,现有的生物炭负载LDHs主要集中以农林废弃物生物炭为主,且主要使用Mn、Ni、Al等作为金属组合,对于污泥生物炭为载体的研究较少. 以污泥生物炭作为载体负载双金属氧化物,可为污泥资源化处理提供有效的处理路径,同时也可以降低其成本,有利于工业化推广.
本文以氢氧化钾改性污泥生物炭(ASB)为载体,负载LDHs并进一步焙烧制备双金属氧化物-污泥生物炭复合材料(LDO-ASB),考察了不同的制备条件对LDO-ASB的重金属吸附性能的影响,进而确定了LDO-ASB的制备条件. 同时对制备的LDO-ASB进行表征并与ASB相比,探讨了双金属氧化物的负载对污泥生物炭的重金属吸附性能的影响机制.
-
本实验中使用的污泥生物炭来自课题组已发表制备方法制备的污泥生物炭(ASB)[20]. 实验中使用的氯化锰、氯化铁、氯化铝、硝酸镉、硝酸铅和氢氧化钠等化学药剂均为分析纯,实验用水为超纯水.
实验所使用仪器包括扫描电子显微镜-X光微区分析(Zeiss Auriga,美国赛默飞世尔),傅里叶变换红外光谱仪(Is50R,美国THMOR-FILSHER),X射线衍射光谱分析仪(SmartLab,日本理学),电热恒温鼓风干燥箱(DGH-9053A,上海森信实验仪有限公司),火焰原子吸收光谱仪(Z2000,日本日立),管式热解炉(SK-G06123K,天津市中环实验电炉有限公司)等.
-
取6 g污泥生物炭(ASB)加入到100 mL含0.2 mol·L−1二价金属(M2+)和0.07 mol·L−1三价金属(M3+)的混合溶液中,采用六联磁力搅拌器搅拌混合液2 h至混合均匀,加入24%的氢氧化钠溶液调节pH稳定为10,将混合溶液置于高压反应釜中120 ℃保持24 h,待冷却后采用去离子水清洗固体物质至pH恒定,于65 ℃下烘干,得到双金属氢氧化物-污泥生物炭(LDH-ASB). 将LDH-ASB置于管式热解炉中焙烧(氮气为保护气),焙烧温度为400 ℃,得到的样品为双金属氧化物-污泥生物炭复合材料(LDO-ASB). 考察不同的二价金属(Mn2+或Mg2+)和三价金属(Fe3+或Al3+)对制备的LDO-ASB吸附Cd(Ⅱ)和Pb(Ⅱ)的影响.
-
取6 g污泥生物炭(ASB)加入到100 mL含有一定比例的Mg2+和Fe3+的混合溶液中,采用六联磁力搅拌器搅拌混合液2 h至混合均匀,加入24%的氢氧化钠溶液调节pH稳定为10,将混合溶液置于高压反应釜中120 ℃保持24 h,待冷却后采用去离子水清洗固体物质至pH恒定,于65 ℃下烘干,得到双金属氢氧化物-污泥生物炭(LDH-ASB). 将LDH-ASB置于管式热解炉中焙烧(氮气为保护气),焙烧温度为400 ℃,得到的样品为双金属氧化物-污泥生物炭复合材料(LDO-ASB). 考察在Mg2+的浓度为0.2 mol·L−1的条件下,Mg2+:Fe3+的物质的量比(2:1、2.5:1和3:1)对制备的LDO-ASB吸附Cd(Ⅱ)和Pb(Ⅱ)的影响.
-
取6 g污泥生物炭(ASB)加入到100 mL Mg2+: Fe3+为3:1的混合溶液中,采用六联磁力搅拌器搅拌混合液2 h至混合均匀,加入24%的氢氧化钠溶液调节pH值稳定为10,将混合溶液置于高压反应釜中120 ℃保持24 h,待冷却后采用去离子水清洗固体物质至pH恒定,于65 ℃下烘干,得到双金属氢氧化物-污泥生物炭(LDH-ASB). 将LDH-ASB置于管式热解炉中焙烧(氮气为保护气),焙烧温度为400 ℃,得到的样品为双金属氧化物-污泥生物炭复合材料(LDO-ASB). 以0.2—0.8 mol·L−1 Mg2+负载浓度考察不同的负载金属浓度对(Mg/Fe)LDO-ASB的吸附能力的影响.
-
取6 g污泥生物炭(ASB)加入到100 mL Mg2+: Fe3+为3:1的混合溶液中(其中Mg2+浓度为0.4 mol·L−1),采用六联磁力搅拌器搅拌混合液2 h至混合均匀,加入24%的氢氧化钠溶液调节pH稳定为10,将混合溶液置于高压反应釜中120 ℃保持24 h,待冷却后采用去离子水清洗固体物质至pH恒定,于65 ℃下烘干,得到双金属氢氧化物-污泥生物炭(LDH-ASB). 将LDH-ASB置于管式热解炉中焙烧(氮气为保护气),得到的样品为双金属氧化物-污泥生物炭复合材料(LDO-ASB). 分别以300—700 ℃的焙烧温度考察不同的焙烧温度对(Mg/Fe)LDO-ASB的吸附能力的影响.
-
采用X射线衍射仪,傅里叶变换红外光谱仪,扫描电子显微镜-X光微区和X射线光电子能谱仪分别对ASB,LDH-ASB和LDO-ASB进行表征分析. 采用火焰原子吸收光谱仪测定Cd(Ⅱ)和Pb(Ⅱ)的浓度,并按照式(1)计算Cd(Ⅱ)和Pb(Ⅱ)的吸附量.
其中
$ ,W $ 为吸附剂的用量(g),$ V $ 为Cd(Ⅱ)和Pb(Ⅱ)溶液的体积(L),$ q $ 为吸附剂分别对Cd(Ⅱ)和Pb(Ⅱ)的吸附量(mg·g−1),C0,Ce分别为吸附前和吸附后溶液中Cd(Ⅱ)和Pb(Ⅱ)的浓度(mg·L−1). -
不同的金属组合会影响双金属氧化物的晶体结构,进而对于负载后污泥生物炭复合材料的吸附性能产生相应的影响. ASB负载不同M2+(Mn2+或Mg2+)和M3+(Fe3+或Al3+)的金属组合制备的复合材料[(Mg/Al)LDO-ASB,(Mg/Fe)LDO-ASB,(Mn/Al)LDO-ASB和(Mn/Fe)LDO-ASB]对Cd(Ⅱ)和Pb(Ⅱ)的吸附量变化如图1所示. 由于Cd(Ⅱ)和Pb(Ⅱ)有相似的吸附位点[21],所以不同金属组合制备LDO-ASB对其吸附能力的影响规律是一致的. 同时,由图1也可以看出,同种金属组合制备的LDO-ASB对Pb(Ⅱ)的吸附量均强于Cd(Ⅱ),这可能是由于Pb(Ⅱ)比Cd(Ⅱ)具有更高的水合能和更大的离子半径[22]. 对比不同金属组合制备LDO-ASB的吸附量可以看出,以Mg2+为二价金属的(Mg/Al)LDO-ASB和(Mg/Fe)LDO-ASB的吸附性能较(Mn/Al)LDO-ASB和(Mn/Fe)LDO-ASB的高,说明Mg改性较Mn改性更有利于强化污泥生物炭的吸附性能. 这是由于Mg改性过程在生物炭表面刻蚀出更多通道,为生物炭提供了较大的比表面积和活性基团,从而提高了LDO-ASB的吸附性能[7]. 对比(Mg/Al)LDO-ASB和(Mg/Fe)LDO-ASB的吸附性能可以看出,(Mg/Fe)LDO-ASB对Pb(Ⅱ)和Cd(Ⅱ)吸附能力更强(吸附量分别为121.14 mg·g−1和99.30 mg·g−1),这是由于生物炭上的Fe-O的存在有助于Cd(Ⅱ)在其表面形成沉淀,促进Cd(Ⅱ)的吸附[23]. 因此选取Mg2+和Fe3+为后续复合材料固定金属组合.
-
层板金属的比例影响制备的LDHs的电荷密度,并且影响LDHs的晶体结构[24],已有的研究表明,二价金属和三价金属的比例的范围在2:1—3:1才能生成层板金属氧化物. 因此,本次考察Mg2+:Fe3+的物质的量比(2:1, 2.5:1和3:1)对(Mg/Fe)LDO-ASB的吸附能力的影响,结果如图2所示. 随着Mg2+: Fe3+的比例的增加,有利于(Mg/Fe)LDO-ASB对Pb(Ⅱ)和Cd(Ⅱ)吸附. 当Mg2+: Fe3+为3:1时,(Mg/Fe)LDO-ASB对Cd(Ⅱ)的吸附量为121.36 mg·g−1,对Pb(Ⅱ)的吸附量为143.69 mg·g−1. 因此,选择3:1的Mg2+: Fe3+制备(Mg/Fe)LDO-ASB.
-
在Mg2+: Fe3+为3:1,焙烧温度为400 ℃的条件下,不同的负载金属浓度对(Mg/Fe)LDO-ASB的吸附能力的影响,结果如图3所示. 由图3可以看出,在Mg2+负载浓度为0.4 mol·L−1时制备的材料的吸附能力达到最大,对Cd(Ⅱ)和Pb(Ⅱ)的吸附量分别为135.43 mg·g−1和165.39 mg·g−1. 随着负载金属浓度的增加,(Mg/Fe)LDO-ASB对Pb(Ⅱ)和Cd(Ⅱ)吸附性能均呈现先增加后降低的规律. 这可能是由于负载金属浓度的增加,使得(Mg/Fe)LDO-ASB的表面变得紧密. 为了验证该假设,对Mg2+的负载浓度为0.2 mol·L−1、0.4 mol·L−1、0.8 mol·L−1制备的(Mg/Fe)LDO-ASB进行表面形貌分析,分析结果如图4所示. 由Mg、Fe mapping图可以看出,随着负载金属浓度的增加,Mg、Fe映射图强度也逐渐加深,表明了Mg、Fe成功负载在了污泥生物炭的表面. 同时由SEM图看出,(Mg/Fe)LDO-ASB表面含大量的六边形状物质,这与含镁的双金属氧化物的六边形片状结构是一致的[12]. 当Mg2+的浓度为0.2 mol·L−1时,(Mg/Fe)LDO-ASB六边形片状结构密集,有较多的孔隙,但是表面光滑;当Mg2+的浓度为0.8 mol·L−1时,(Mg/Fe)LDO-ASB表面凝结在一起,出现成团现象,孔隙减少,导致(Mg/Fe)LDO-ASB的吸附性能降低. 而Mg2+的浓度为0.4 mol·L−1时,(Mg/Fe)LDO-ASB表面粗糙,且含有大量细纹隧道,细纹隧道增加了(Mg/Fe)LDO-ASB的比表面积,从而提高(Mg/Fe)LDO-ASB的吸附量.
-
焙烧温度的高低对于(Mg/Fe)LDO-ASB中的双金属氧化层的结构有一定的影响. 温度的升高有利于生物炭的再热解,以及促进双金属氢氧化物的脱水,但是过高的温度会破坏双金属氧化层的结构,从而降低生物炭的吸附性能. 图5(a)为不同的焙烧温度对(Mg/Fe)LDO-ASB吸附性能的影响. 在Mg2+:Fe3+为3:1,Mg2+浓度为0.4 mol·L−1的制备条件下,不同焙烧温度对(Mg/Fe)LDO-ASB的吸附性能先升高后降低,当焙烧温度为500、600 ℃时,(Mg/Fe)LDO-ASB的吸附性能较为接近,且达到最高. 焙烧温度为700 ℃时,(Mg/Fe)LDO-ASB的吸附能力显著降低. 对比500 ℃和700 ℃的FTIR分析图(图5(b))可以看出,700 ℃焙烧制备的(Mg/Fe)LDO-ASB中的—OH、—COOH、C=C/C=O峰强度较500 ℃的有明细的减小,说明了温度过高破坏了材料中的含氧官能团,导致其吸附能力降低. 因此,选择500 ℃作为(Mg/Fe)LDO-ASB的焙烧温度,在该温度下制备的(Mg/Fe)LDO-ASB对Cd(Ⅱ)和Pb(Ⅱ)的吸附量分别为149.72 mg·g−1和183.69 mg·g−1,较ASB的吸附能力(对Cd(Ⅱ)和Pb(Ⅱ)的吸附量分别为64.5 mg·g−1和151.1 mg·g−1)有显著的提高.
-
基于以上的制备条件的筛选,选择Mg2+和Fe3+的作为LDO-ASB的金属组合,在Mg2+:Fe3+物质的量比为3:1,Mg2+负载浓度为0.4 mol·L−1,焙烧温度为500 ℃的制备条件下,制备(Mg/Fe)LDO-ASB,分析其表面形貌、元素、晶体结构和官能团,探讨其强化污泥生物炭吸附的机制.
-
ASB和(Mg/Fe)LDO-ASB的表面形貌和元素分析结果如图6所示. 由图6可以看出,ASB表面粗糙,具有较多孔隙,孔隙较大,在负载镁、铁后,表面孔隙被镁铁氧化物填充,在(Mg/Fe)LDO-ASB表面有大量的细纹路,细纹隧道的增加有利于生物炭的比表面积的增加,可以为Cd(Ⅱ)、Pb(Ⅱ)提供更多的吸附位点. 对比ASB和(Mg/Fe)LDO-ASB的 EDS分析结果可以看出,(Mg/Fe)LDO-ASB的Mg和Fe峰的含量较ASB的占比有明显的提高,说明了Mg、Fe成功负载在了生物炭上.
-
ASB、(Mg/Fe)LDH、(Mg/Fe)LDH-ASB、(Mg/Fe)LDO-ASB的X射线衍射分析结果如图7所示. 由(Mg/Fe)LDH-ASB的分析结果可以看出,(Mg/Fe)LDH-ASB主要衍射峰包括了ASB检出的主要衍射峰二氧化硅及其硅酸盐和(Mg/Fe)LDH中检测出的主要衍射峰(Mg4Fe(OH)10Cl(H2O)3)0.6,表明镁铁双金属氢氧化物成功负载在污泥生物炭上. ASB-Mg-Fe/LDH热解后得到的ASB-Mg-Fe/LDO中,除了二氧化硅和硅铝酸盐外,检测出MgO结构,这说明高温焙烧下,镁铁双金属氢氧化物转化为氧化物展现出类氧化物复合相[25].
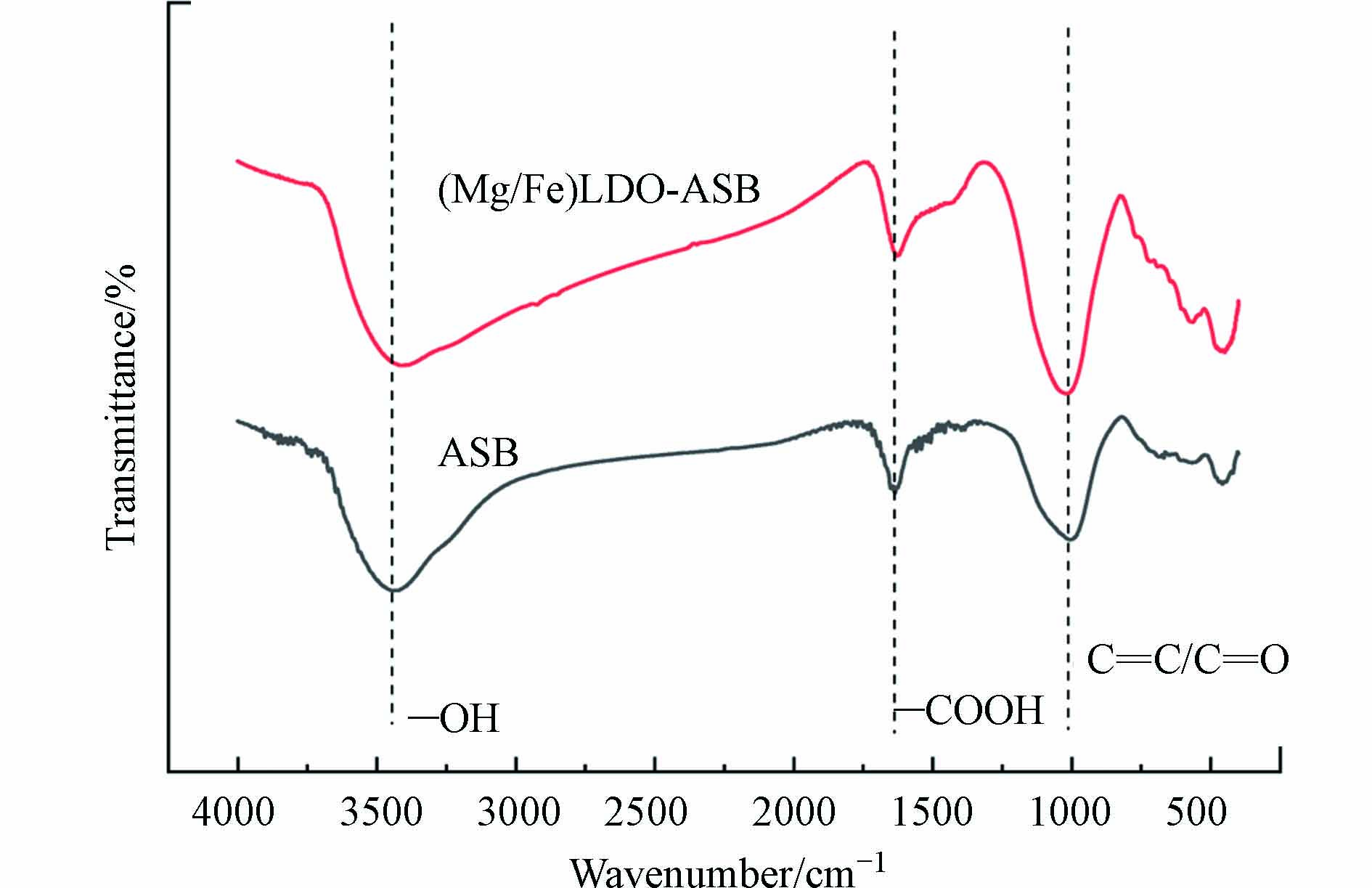
ASB和(Mg/Fe)LDO-ASB的FTIR分析结果如图8所示. 在3436 cm−1和1662 cm−1处分别为—OH和—COOH峰[26],1000—1200 cm−1处的峰为醚、酯或酚中的C=C或C=O [19]. 对比ASB和(Mg/Fe)LDO-ASB的FTIR分析图可以看出,由于LDO中含大量的—OH官能团[27],LDO负载到ASB上,导致ASB-Mg-Fe/LDO的—OH官能团较ASB的大大增加了. (Mg/Fe)LDO-ASB中—COOH峰与ASB的强度无明显差别,表明ASB在负载镁铁氧化物的过程中不影响—COOH官能团. 而中的C=C/C=O峰的强度增加了,可能是因为经过二次焙烧,增加了复合物的芳构化程度[9].
-
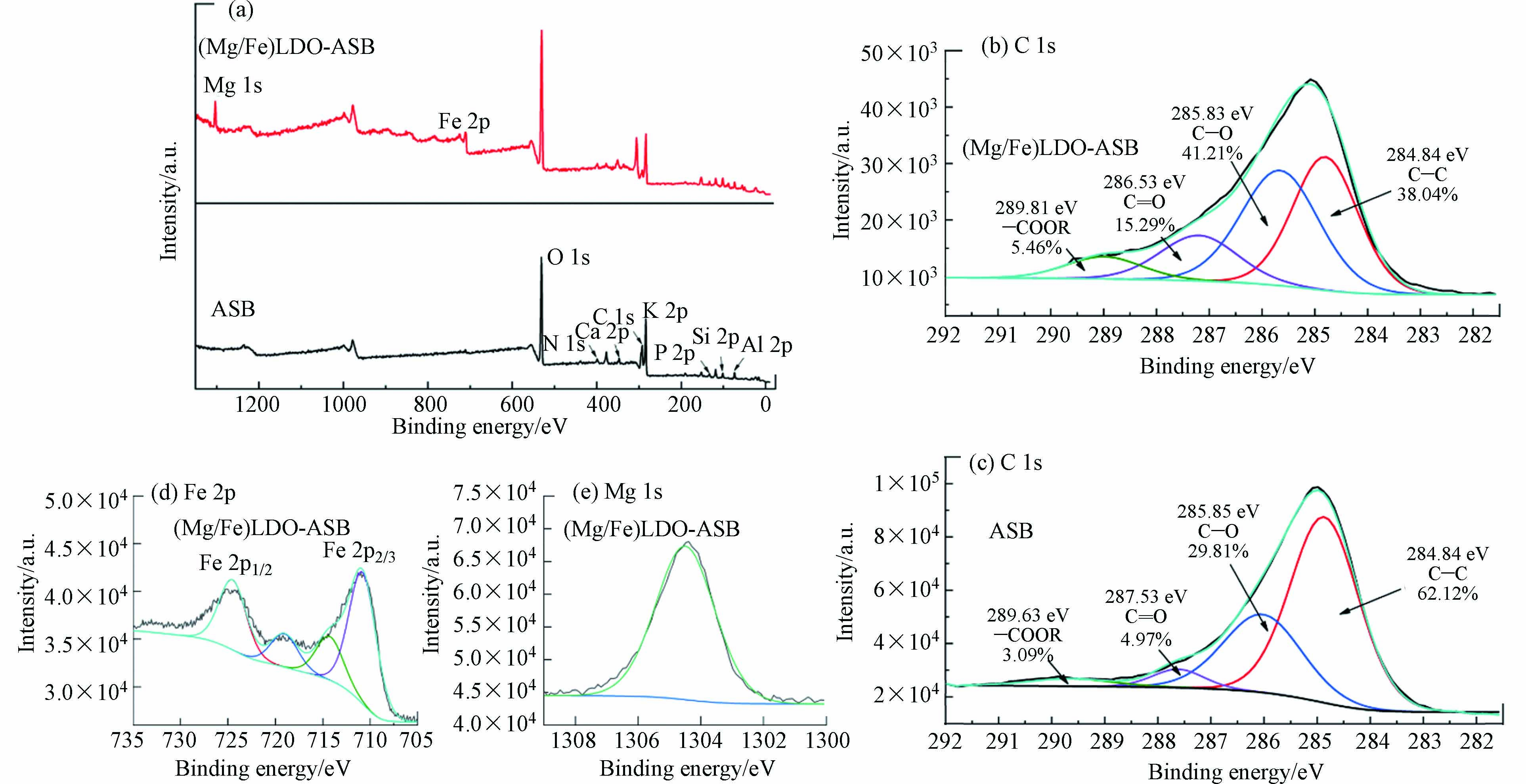
图9为ASB,(Mg/Fe)LDO-ASB的X射线光电子能谱图. ASB经负载镁铁后制备的(Mg/Fe)LDO-ASB材料中,出现了Mg、Fe的结合能峰,表明Mg、Fe成功负载在ASB上. 图9(b)和(c)分别为ASB负载前后C结合能峰的变化,样品的C1s光谱可拟合为C—C(芳环)、C—O(酚、醇、醚)、C=O(羰基)和O=C—O(羧基、碳酸根)4种结合形态[28],ASB的C—O峰面积为29.81%,(Mg/Fe)LDO-ASB的C—O峰面积为40.21%,ASB负载Mg、Fe后C—O峰的含量大大增加,ASB负载镁铁后—OH官能团增加. 图9(d)为(Mg/Fe)LDO-ASB中Fe的结合能峰,在711.4 eV和724.4 eV分别为Fe2p1/2,Fe2p3/2,且711.4 eV和724.4 eV的结合能相差13 eV,表明(Mg/Fe)LDO-ASB中存在三价铁;同时在719 eV出现了伴峰,表明(Mg/Fe)LDO-ASB中存在Fe2O3. 图9(e),(Mg/Fe)LDO-ASB中1304.5 eV处的Mg1s结合能峰为氧化镁,焙烧后Mg主要以氧化镁的形式存在,这与XRD的结果是一致的.
-
(1)Mg2+和Fe3+组合制备的LDO-ASB较其他金属组合具有更好的Cd(Ⅱ)和Pb(Ⅱ)吸附能力. (Mg/Fe)LDO-ASB的Cd(Ⅱ)和Pb(Ⅱ)吸附性能随着Mg2+:Fe3+物质的量比的变大而增加. 过高的负载金属浓度导致金属氧化物成团,孔隙减少,而焙烧温度过高破坏了LDO结构,导致其吸附官能团减少,均降低了(Mg/Fe)LDO-ASB的重金属吸附性能.
(2)(Mg/Fe)LDO-ASB的最优制备参数为:Mg2+:Fe3+物质的量比为3:1,Mg2+的负载浓度为0.4 mol·L−1,焙烧温度为500 ℃,在此条件下制备的(Mg/Fe)LDO-ASB对Cd(Ⅱ)和Pb(Ⅱ)的吸附量分别为149.72 mg·g−1和183.69 mg·g−1,较ASB的吸附能力有显著的提高.
(3)(Mg/Fe)LDO-ASB的表征结果显示,Mg和Fe分别以氧化镁和三氧化二铁的形态的负载在污泥生物炭表面,负载后,(Mg/Fe)LDO-ASB表面粗糙,含有大量的细纹隧道,增加了—OH官能团含量,进而强化了污泥生物炭的重金属吸附能力.
双金属氧化物负载对污泥生物炭吸附重金属Cd(Ⅱ)和Pb(Ⅱ)的影响
Influence of bimetallic oxide loading on Cd(Ⅱ) and Pb(Ⅱ) adsorption capacity of sludge biochar
-
摘要: 纯污泥生物炭由于其比表面积较低等导致其去除水中污染物的能力有限,需要对污泥生物炭进行改性强化其对污染物的去除. 本文采用污泥生物炭负载层状双金属氧化物强化污泥生物炭吸附水中重金属. 考察了金属组合,金属离子比例,负载金属浓度和焙烧温度对双金属氧化物(LDO)强化污泥生物炭(ASB)吸附水中重金属的影响,进而确定双金属氧化物-污泥生物炭复合材料(LDO-ASB)的制备方法. 同时对制备的LDO-ASB进行表征并与ASB相比,探讨了双金属氧化物的负载对污泥生物炭的重金属吸附性能的影响机制. 结果表明,Mg2+和Fe3+组合制备的LDO-ASB较其他金属组合具有更好的Cd(Ⅱ)和Pb(Ⅱ)的吸附能力. 在Mg2+∶Fe3+物质的量比为3∶1,Mg2+的负载浓度为0.4 mol·L−1,焙烧温度为500 ℃的条件下制备的(Mg/Fe)LDO-ASB对Cd(Ⅱ)和Pb(Ⅱ)的吸附量分别为149.72 mg·g−1和183.69 mg·g−1,较ASB的吸附能力有显著的提高. 材料的表征结果显示,Mg和Fe以氧化镁和三氧化二铁的形态负载在污泥生物炭表面,负载后污泥生物炭表面粗糙,加大了细纹隧道,同时也增加了—OH官能团的含量,进而强化了污泥生物炭的重金属吸附能力.Abstract: Due to the low specific surface area of pure sludge biochar, its ability to remove pollutants in water is limited, so it is necessary to modify sludge biochar to enhance its ability to remove pollutants. In this paper, sludge biochar was loaded with layered bimetallic oxides to enhance its adsorption of heavy metals in water. The effects of metal combination, metal ion ratio, metal concentration and roasting temperature on the adsorption of heavy metals by LDO enhanced ASB were investigated, and the preparation method of bimetallic oxide sludge biochar composite (LDO-ASB) was determined. Also, the influence of bimetallic oxide loading on the heavy metal adsorption performance of sludge biochar was discussed by comparing the characteristics of LDO-ASB and ASB. The results show that the LDO-ASB prepared by combination of Mg2+ and Fe3+ has better adsorption capacity of Cd(Ⅱ) and Pb(Ⅱ) than other metal combinations. The adsorption capacity of (Mg/Fe) LDO-ASB prepared under the conditions of 3∶1 ratio of Mg2+∶Fe3+, 0.4 mol·L−1 loading concentration of Mg2+ and 500 ℃ calcination temperature for Cd (Ⅱ) and Pb (Ⅱ) is 149.72 mg·g−1 and 183.69 mg·g−1 respectively, which is significantly higher than that of ASB. The characterization results showed that Mg and Fe were loaded on the surface of sludge biochar in the form of magnesium oxide and ferric trioxide. After loading, the surface of sludge biochar was rough, which increased the fine grain tunnel and the content of —OH functional group, thereby enhanced the heavy metal adsorption capacity of sludge biochar.
-
Key words:
- sludge biochar /
- double oxides /
- heavy metals /
- adsorption.
-

-
-
[1] 马文艳, 裴鹏刚, 高歌, 等. 微纳米粒径生物炭的结构特征及其对Cd2+吸附机制[J]. 环境科学, 2022, 43(7): 3682-3691. MA W Y, PEI P G, GAO G, et al. Structural characteristics of micro-nano particle size biochar and its adsorption mechanism for Cd2+[J]. Environmental Science, 2022, 43(7): 3682-3691(in Chinese).
[2] DENG Y Y, HUANG S, DONG C Q, et al. Competitive adsorption behaviour and mechanisms of cadmium, nickel and ammonium from aqueous solution by fresh and ageing rice straw biochars[J]. Bioresource Technology, 2020, 303: 122853. doi: 10.1016/j.biortech.2020.122853 [3] ZHANG D W, ZHANG K J, HU X L, et al. Cadmium removal by MgCl2 modified biochar derived from crayfish shell waste: Batch adsorption, response surface analysis and fixed bed filtration[J]. Journal of Hazardous Materials, 2021, 408: 124860. doi: 10.1016/j.jhazmat.2020.124860 [4] NAQVI S R, TARIQ R, SHAHBAZ M, et al. Recent developments on sewage sludge pyrolysis and its kinetics: Resources recovery, thermogravimetric platforms, and innovative prospects[J]. Computers & Chemical Engineering, 2021, 150: 107325. [5] 杨雪, 张士秋, 侯其东, 等. 生物炭的制备及其镁改性对污染物的吸附行为研究[J]. 环境科学学报, 2018, 38(10): 4032-4043. doi: 10.13671/j.hjkxxb.2018.0252 YANG X, ZHANG S Q, HOU Q D, et al. The preparation of biochar and adsorption behavior of Mg-modified biochar to pollutants[J]. Acta Scientiae Circumstantiae, 2018, 38(10): 4032-4043(in Chinese). doi: 10.13671/j.hjkxxb.2018.0252
[6] LUO X W, HUANG Z J, LIN J Y, et al. Hydrothermal carbonization of sewage sludge and in situ preparation of hydrochar/MgAl-layered double hydroxides composites for adsorption of Pb(Ⅱ)[J]. Journal of Cleaner Production, 2020, 258: 120991. doi: 10.1016/j.jclepro.2020.120991 [7] N GAMBIAA, IFTHIKRJ, SHAHIBII, et al. Adsorptive purification of heavy metal contaminated wastewater with sewage sludge derived carbon-supported Mg(Ⅱ) composite[J]. Science of the Total Environment, 2019, 691: 306-321. doi: 10.1016/j.scitotenv.2019.07.003 [8] RAMYAV, MURUAND, LAJAPTHIRAIC, et al. Activated carbon (prepared from secondary sludge biomass) supported semiconductor zinc oxide nanocomposite photocatalyst for reduction of Cr(Ⅵ) under visible light irradiation[J]. Journal of Environmental Chemical Engineering, 2018, 6((6): ): 7327-7337. doi: 10.1016/j.jece.2018.08.055 [9] YANG X. Preparation of ferric-activated sludge-based adsorbent from biological sludge for tetracycline removal[J]. Bioresource Technology, 2016, 211: 566-573. doi: 10.1016/j.biortech.2016.03.140 [10] 李嘉雯, 郝瑞霞, 李宏康, 等. 磁性Mg/Al-LDHs制备条件对其吸附除磷性能的影响[J]. 环境科学学报, 2020, 40(2): 520-526. doi: 10.13671/j.hjkxxb.2019.0425 LI J W, HAO R X, LI H K, et al. Adsorption performance of phosphorus by magnetic Mg/Al-LDHs prepared under different conditions[J]. Acta Scientiae Circumstantiae, 2020, 40(2): 520-526(in Chinese). doi: 10.13671/j.hjkxxb.2019.0425
[11] LU Y, JIANG B, FANG L, et al. High performance NiFe layered double hydroxide for methyl orange dye and Cr(Ⅵ) adsorption[J]. Chemosphere, 2016, 152: 415-422. doi: 10.1016/j.chemosphere.2016.03.015 [12] MOSTAFA M S, BAKR A S A, EL NAGGAR A M A, et al. Water decontamination via the removal of Pb (Ⅱ) using a new generation of highly energetic surface nano-material: Co+2Mo+6 LDH[J]. Journal of Colloid and Interface Science, 2016, 461: 261-272. doi: 10.1016/j.jcis.2015.08.060 [13] BO L F, LI Q R, WANG Y H, et al. One-pot hydrothermal synthesis of thrust spherical Mg-Al layered double hydroxides/MnO2 and adsorption for Pb(II) from aqueous solutions[J]. Journal of Environmental Chemical Engineering, 2015, 3(3): 1468-1475. doi: 10.1016/j.jece.2015.05.023 [14] JIA Y H, LIU Z H. Preparation of borate anions intercalated MgAl-LDHs microsphere and its calcinated product with superior adsorption performance for Congo red[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2019, 575: 373-381. [15] TAN X F, LIU Y G, GU Y L, et al. Biochar-based nano-composites for the decontamination of wastewater: A review[J]. Bioresource Technology, 2016, 212: 318-333. doi: 10.1016/j.biortech.2016.04.093 [16] 向洋, 张翔凌, 雷雨, 等. 不同合成条件对ZnAl-LDHs覆膜改性生物陶粒除磷效果的影响[J]. 环境科学, 2018, 39(5): 2184-2194. doi: 10.13227/j.hjkx.201710128 XIANG Y, ZHANG X L, LEI Y, et al. Influencing factors for phosphorus removal by modified bio-ceramic substrates coated with ZnAl-LDHs synthesized by different modification conditions[J]. Environmental Science, 2018, 39(5): 2184-2194(in Chinese). doi: 10.13227/j.hjkx.201710128
[17] 张翔凌, 黄华玲, 郭露, 等. Zn系LDHs覆膜改性人工湿地沸石基质除磷机制[J]. 环境科学, 2016, 37(8): 3058-3066. doi: 10.13227/j.hjkx.2016.08.029 ZHANG X L, HUANG H L, GUO L, et al. Mechanisms of phosphorus removal by modified zeolites substrates coated with Zn-LDHs in laboratory-scale vertical-flow constructed wetlands[J]. Environmental Science, 2016, 37(8): 3058-3066(in Chinese). doi: 10.13227/j.hjkx.2016.08.029
[18] CHEN S, HUANG Y, HAN X, et al. Simultaneous and efficient removal of Cr(Ⅵ) and methyl orange on LDHs decorated porous carbons[J]. Chemical Engineering Journal, 2018, 352: 306-315. doi: 10.1016/j.cej.2018.07.012 [19] WANG T, LI C, WANG C Q, et al. Biochar/MnAl-LDH composites for Cu(Ⅱ) removal from aqueous solution[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 538: 443-450. doi: 10.1016/j.colsurfa.2017.11.034 [20] 周佳丽, 林伟雄, 关智杰, 等. 响应曲面法优化KOH改性污泥生物炭的制备及其强化去除Pb(Ⅱ)的研究[J]. 环境科学学报, 2022, 42(8): 194-207. doi: 10.13671/j.hjkxxb.2021.0565 ZHOU J L, LIN W X, GUAN Z J, et al. Optimization of preparation of KOH-modified sludge biochar by response surface method and its enhanced Pb(Ⅱ)removal[J]. Acta Scientiae Circumstantiae, 2022, 42(8): 194-207(in Chinese). doi: 10.13671/j.hjkxxb.2021.0565
[21] BEHBAHANI E S, DASHTIAN K, GHAEDI M. Fe3O4-FeMoS4: Promise magnetite LDH-based adsorbent for simultaneous removal of Pb (Ⅱ), Cd (Ⅱ), and Cu (Ⅱ) heavy metal ions[J]. Journal of Hazardous Materials, 2021, 410: 124560. doi: 10.1016/j.jhazmat.2020.124560 [22] HERATH A, LAYNE C A, PEREZ F, et al. KOH-activated high surface area Douglas Fir biochar for adsorbing aqueous Cr(Ⅵ), Pb(Ⅱ) and Cd(Ⅱ)[J]. Chemosphere, 2021, 269: 128409. doi: 10.1016/j.chemosphere.2020.128409 [23] YANG T T, XU Y M, HUANG Q Q, et al. Adsorption characteristics and the removal mechanism of two novel Fe-Zn composite modified biochar for Cd(Ⅱ) in water[J]. Bioresource Technology, 2021, 333: 125078. doi: 10.1016/j.biortech.2021.125078 [24] PENG Y T, SUN Y Q, SUN R Z, et al. Optimizing the synthesis of Fe/Al (Hydr)oxides-Biochars to maximize phosphate removal via response surface model[J]. Journal of Cleaner Production, 2019, 237: 117770. doi: 10.1016/j.jclepro.2019.117770 [25] ZHENG X G, ZHU Q, PENG H, et al. Efficient solar-light induced photocatalytic capacity of Mg-Al LDO coupled with N-defected g-C3N4[J]. Chemical Physics Letters, 2021, 779: 138846. doi: 10.1016/j.cplett.2021.138846 [26] ZHANG W, SONG J Y, HE Q L, et al. Novel pectin based composite hydrogel derived from grapefruit peel for enhanced Cu(Ⅱ) removal[J]. Journal of Hazardous Materials, 2020, 384: 121445. doi: 10.1016/j.jhazmat.2019.121445 [27] WU J W, WANG T, WANG J W, et al. A novel modified method for the efficient removal of Pb and Cd from wastewater by biochar: Enhanced the ion exchange and precipitation capacity[J]. Science of the Total Environment, 2021, 754: 142150. doi: 10.1016/j.scitotenv.2020.142150 [28] WONGROD S, SIMON S, van HULLEBUSCH E D, et al. Changes of sewage sludge digestate-derived biochar properties after chemical treatments and influence on As(III and V) and Cd(II) sorption[J]. International Biodeterioration & Biodegradation, 2018, 135: 96-102. -




 下载:
下载: