-
世界卫生组织(WHO)数据表明,空气污染每年可导致数百万人过早死亡,已成为危害人类健康的最大环境风险之一[1]. 污染物主要包括颗粒物(particulate matters, PMs)、气态污染物(如氮氧化物(NOx)、硫氧化物(SOx)和臭氧(O3)等)、持久性有毒污染物(persistent toxic substances, PTSs)和重金属(heavy metals, HMs),其中PMs来源广、毒性强、组分复杂、形态多变,可对大气环境和人类健康带来长期的负面影响和危害. 值得注意的是,国际癌症研究机构(International Agency for Research on Cancer)基于已有研究成果将PMs列为第一类致癌化学物[2].
PMs可细分为初级颗粒物和次级颗粒物,其中由自然因素(如风沙扬尘、森林火灾等)和人类活动(如汽车尾气、煤炭燃烧等)等方式直接排放到大气环境中的颗粒物为初级颗粒物,而次级颗粒物主要是由大气环境中已存在的气态污染物经化学反应所形成. 依据空气动力学直径(aerodynamic equivalent diameter, AED)将PMs分为可吸入颗粒物PM10(AED≤10 μm)、细颗粒物PM2.5(AED≤2.5 μm)和超细颗粒物PM0.1(AED≤0.1 μm). 相较于PM10,PM2.5粒径小、比表面积大,且可负载重金属、苯系物、多环芳烃等污染物,入肺后可穿透气血屏障进入血液循环,进而对人体健康产生不良影响[3-4]. 除传统呼吸和心脑血管系统损伤外,PMs还可对机体血细胞和造血器官产生毒性效应,即造血毒性[5-6],且毒性效应与其粒径大小、化学组分相关[7]. 人群数据揭示PM2.5及其化学组分可诱发贫血(anemia)、血栓(hemophilia)和白血病(leukemia)等血液系统疾病发生发展[8-10]. 体外细胞实验和动物实验结果表明,PM2.5可促进白血病细胞生长,并加剧小鼠造血毒性的进展[11-13].
本综述简要阐述机体造血过程,结合流行病学数据与毒理学实验研究重点探讨PM2.5对人群和实验动物血液系统的毒性效应及生物学机制,以期从造血毒性与健康效应的角度为PM2.5健康风险评估提供科学思路.
-
人类和小鼠的造血发育过程具有高度保守性[14-15],其造血发育过程是指各类血细胞产生、增殖、分化、成熟和释放的过程,主要分为胚胎期造血和出生后造血.
胚胎期造血分为原始造血和定向造血两个时空相互重叠的阶段[16],不同阶段血细胞的产生、分化和成熟受到机体多种信号的调控,调控失衡将导致严重的发育缺陷或血液疾病[17]. 小鼠原始造血始于胚胎期7.5 d(embryonic day 7.5, E7.5),在卵黄囊血岛区产生原始红细胞、原始巨核细胞、原始巨噬细胞、红系-髓系祖细胞(erythro-mmyeloid progenitors, EMPs)和淋巴-髓系祖细胞(lympho-myeloid progenitors, LMPs)[18-20]. 但原始造血持续时间短暂且产生的血细胞无自我更新能力,自E9.5被定向造血代替. E9.5—E11.5在背主动脉-性腺-中肾区(aorta gonad mesonephros region, AGM区)、胎盘和胚胎头部血管内皮细胞产生的造血干细胞(hematopoietic stem cells, HSCs)随血液循环定植于胎儿肝脏并进一步分化[21-23]. 胚胎发育至E12.5,HSCs于肝脏进一步发育成熟并分化成前体血细胞(如巨核细胞、淋巴细胞前体)和终末血细胞(如红细胞、各类粒细胞、淋巴细胞等),随后进入胸腺、脾脏和骨髓,以维持终身造血活性[24]. 因此,定向造血阶段对机体血细胞的分化和成熟起着重要的作用.
出生后,骨髓成为主要造血器官. HSCs在骨髓中先分化成具有有限自我更新能力和全谱系分化潜能的造血祖细胞(hematopoietic progenitor stem cells, HPSCs)[25],之后在基因和细胞因子的调控下,HPSCs定向分化为机体所需的特定血细胞[26]. 终末血细胞伴随着强烈的细胞流动穿越骨髓-血屏障进入外周血循环,一方面为机体提供物质需求和发挥特定功能,另一方面以维持机体造血活动的动态平衡[27]. 值得关注的是,骨髓造血微环境改变将会影响HSCs静默、激活和死亡之间的动态平衡,进而诱发血液疾病[28]. 当骨髓造血功能受损或机体血细胞需求量增加,作为代偿性造血器官的脾脏可替代骨髓开启髓外造血,常见于儿童[29].
-
随着PMs造血毒性研究的不断深入,已有流行病学证据提示PMs可对不同生命阶段人群血细胞和造血器官产生损伤,且与血液疾病的发生发展相关[8,30](表1).
-
产前暴露于PMs可通过改变脐带血细胞表型分布、抑制血细胞发育进程与前体血细胞分化等方式增加儿童血液疾病患病风险[43-44],其中白血病和贫血关注度较高[45-46].
目前PM2.5与儿童白血病之间是否存在关联性尚无具体定论. 部分队列研究未发现PM2.5与儿童白血病具有显著的相关性[9,47]. 但Lee等[31]和Ou等[10]研究发现,PM2.5可增加儿童白血病患病率和白血病患儿死亡率. 研究对象的暴露窗口期、暴露源、暴露水平和暴露评估方法等因素的差异性可能是造成上述结果不同的原因.
PM2.5还可致儿童贫血发病率增高. Morales-Ancajima等[32]和Mehta等[33]研究发现PM2.5与儿童贫血相关,且高浓度PM2.5可加重儿童贫血程度. 另一项研究运用多级混合效应模型首次证明了妊娠期、出生时和儿童期3个阶段PM2.5与婴幼儿贫血之间的关系,结果显示不同阶段暴露PM2.5均可增加儿童贫血患病率[34].
-
多项研究表明,PMs可影响成人红细胞、血小板和白细胞等血细胞数量、形态和功能[37,48-50]. 如研究对比分析有无炭黑暴露的工人血液样本差异性,结果发现炭黑与嗜酸性粒细胞增加有关[39]. Hou等[40]选取河南农村队列研究空气污染物与血小板之间的关联性,发现PMs对农村成年人的血小板功能和性状有影响,但可通过体力劳动减缓. 国内研究基于外出出差的学生,收集并分析出差前、返回后1 d和返回后1周等3个时间段的血液样本,结果显示短期暴露于PM2.5可加剧血栓形成[36]. 另有研究发现,长期暴露PM2.5可增加血小板数量,且对血液凝固性有潜在的不良影响[35,38].
少数队列研究调查了空气污染物与成人白血病患病率之间的相关性,其数据量不足以明确得出PMs与成年白血病具有关联[51-52]. 一项来自丹麦的病例对照研究发现,PM2.5可通过DNA损伤、相关染色体畸变与易位等途径来增加成年人白血病患病率[5].
-
PMs对老年人红细胞和血红蛋白的影响亦受到相关学者的关注[53]. Elbarbary等[54]开展了PMs与中国老年人贫血患病率和血红蛋白水平之间关系的研究,结果显示PMs可增加老年人群贫血患病率. 另一项研究基于美国4121名老年人群队列探讨PM2.5与血红蛋白水平或(和)贫血之间的关系,发现PM2.5对血红蛋白和贫血均呈很强的剂量反应关系,其中PM2.5与血红蛋白之间的关联性是由C反应蛋白(C-reactive protein, CRP)介导的[42]. Liu等[41] 开展了PM2.5对血小板影响的研究,结果发现PM2.5可导致老年人血小板线粒体DNA低甲基化. 此外,Puett等[55]探讨了成年人白血病与空气污染物暴露的相关性,发现PM2.5可增加老年人白血病患病风险.
-
PMs穿过气-血屏障迁移至血液,与红细胞、血小板和白细胞及其细胞亚群发生相互作用[56]. 另有研究发现,PM2.5和PM0.1诱导肺脏分泌的炎症因子随血液流动至骨髓,通过干扰骨髓造血微环境影响血细胞的增殖、分化与成熟以及HSCs的功能趋向[57],进而引发血液肿瘤发生发展[27,58].
-
红细胞主要承担输送氧气的功能,其数量、形态或功能异常可导致机体不良反应[59-60]. PMs通过激活炎症细胞因子(如肿瘤坏死因子-α(TNF-α)和γ干扰素(IFN-γ)等)以抑制红系前体细胞的增殖-分化和促红细胞生成素(erythropoietin, EPO)反应性,其结果是红细胞数量降低[61]. 另一项研究将小鼠暴露于汽车尾气源PM2.5和PM0.1,发现其浓度与小鼠体内异常红细胞的数量呈线性相关[62]. 也有研究发现,PMs与小鼠红细胞畸形有关[63]. 此外,小鼠孕期暴露PMs可导致红细胞功能下降[64].
PM2.5还可影响血液中血红蛋白含量,从而引发机体贫血[65-66]. 值得关注的是,白细胞介素-6(IL-6)等炎症因子通过改变血液中铁调素(hepcidin)水平影响红细胞数量和血红蛋白浓度[67]. IL-6增加血清中循环铁调素浓度,进而减弱十二指肠、脾脏与肝脏对铁的吸收以及铁向骨髓的循环,最终导致缺铁性红细胞生成;其次,PM2.5诱发炎症导致红系前体细胞因缺铁而生成缺铁性红细胞. 但是,PM2.5通过铁调素影响红细胞的生物过程和分子机制仍有待阐明.
-
PMs及其重金属组分经呼吸道沉积于肺脏,刺激其产生并释放促氧化介质(如活性氧(reactive oxygen species, ROS))和促炎性介质(如炎症因子)至血液,导致血小板活化或形态与功能改变[68-69]. PMs与血小板生成、激活存在正相关[70]. 此结果在人群样本中得到了证实,Delfino等于冠状动脉疾病患者血液中发现PM2.5与血小板活化标志物p-选择素(p-selectin)存在关联性[71];而Yin等[72]研究报告发现,PMs可抑制血小板活化标志物分泌而发挥抗血小板作用. 另一项人群模拟试验研究表明暴露于柴油机尾气的男性志愿者血液中血小板-单核细胞聚集体增加[73].
肺脏为血小板髓外分化、成熟的重要位点,且巨核细胞可独立于血小板发挥促炎作用[74]. 基于此,Jin等[56]探讨了PM2.5与巨核细胞分化之间的关系,结果显示PM2.5促进巨核细胞发育成熟并分化为血小板,从而导致血栓形成.
-
PM2.5入肺后可激活巨噬细胞发挥吞噬清除作用,而高积累则会导致巨噬细胞损伤与凋亡[75]. PM2.5亦可促进巨噬细胞发生极化改变,具体表现为M1亚型相关的白细胞介素-12(IL-12)、IFN-γ等细胞因子富集和M2亚型相关的白细胞介素-4(IL-4)、白细胞介素-10(IL-10)和白细胞介素-13(IL-13)水平发生下降[76]. 而巨噬细胞极化可促进肺脏白细胞与血小板的结合和活化,其结果是具有止血活性的微粒释放至体循环,进而激活全身的凝血状态[77]. 此外,PM2.5削弱肺脏巨噬细胞内化作用,同时募集自然杀伤细胞和中性粒细胞等白细胞至肺脏以减少组织损伤[78-79].
PMs刺激肺泡产生炎症因子介导骨髓释放单核细胞和中性粒细胞进入体循环[80-81]. 动物实验证实,PMs亦可诱导单核细胞和各类粒细胞的累积和功能改变[82-83]. 如Xu等[84]研究发现PM2.5可诱导单核细胞和中性粒细胞聚集而产生炎症反应. 另一项研究发现,PM2.5可诱发小鼠血液毒性,当与甲醛联合暴露后可加剧影响,主要原因是PM2.5和甲醛破坏了免疫平衡[30],提示PM2.5通过激活免疫系统介导血液毒性效应及作用机制需进一步关注.
-
骨髓是各种血细胞储存库. 研究显示,PM2.5及其负载的有害物质可致骨髓的累积损伤和内皮祖细胞(endothelial progenitor cells,EPCs)释放的受损,进而诱发EPCs数量及功能发生改变[85-86]. PMs与骨髓造血干细胞(BM-HSCs)之间的关联性也得到证实,即PMs通过ROS引发BM-HSCs增殖显著减少[6],PM2.5可诱导BM-HSCs耗竭[57]. 体外细胞培养和动物实验结果表明,PM2.5可促机体炎症反应和白血病细胞因子表达,且PM2.5剂量与白血病细胞增殖呈低促高抑趋势[12].
维持骨髓造血微环境稳态对血液系统的正常运行至关重要. 最近一项研究发现,孕期暴露PM2.5与子代血液肿瘤发生有关,可表现为胎儿BM-HSCs衰老表型、DNA的双链断裂和骨髓微环境产生了高水平的蛋白水解酶,从而增加骨髓增殖性疾病风险[87]. 骨髓微环境受损可致使子代BM-HSCs通过非细胞自主机制逐渐衰老,增加骨髓增殖性疾病风险[87]. 国内一项研究发现,PM2.5通过破坏儿童骨髓造血微环境影响HSCs功能趋向[88].
-
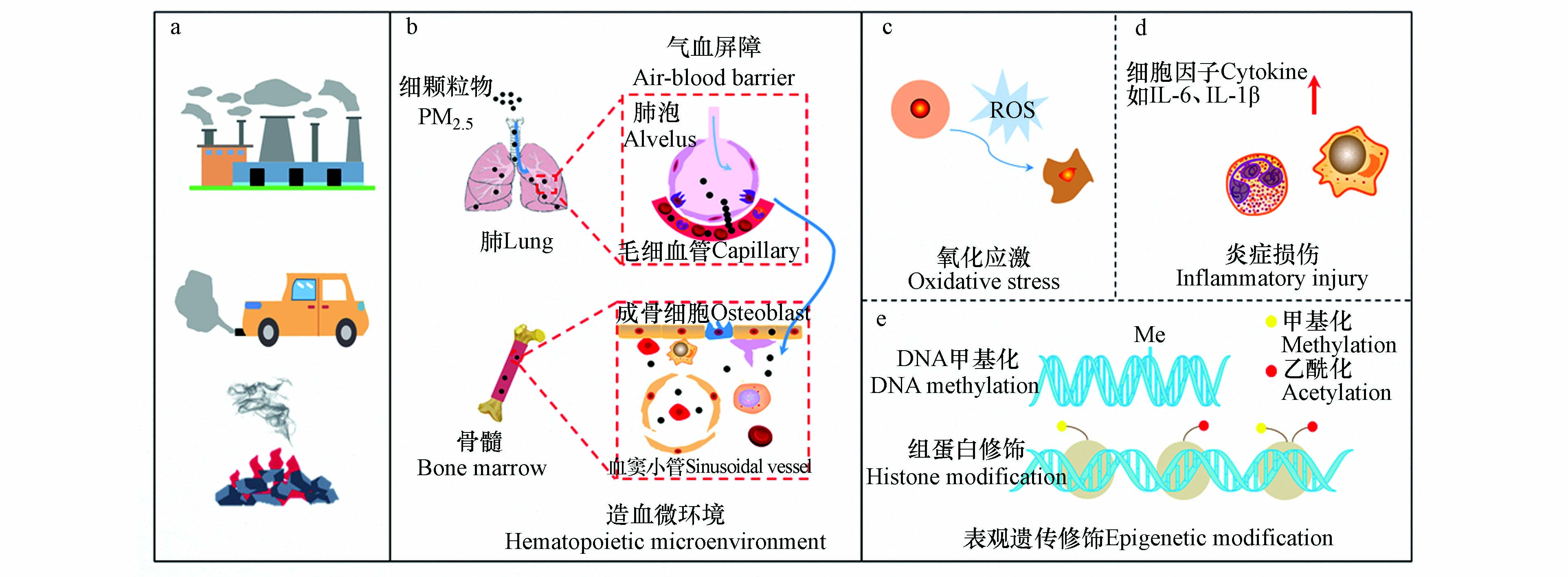
PM2.5造血毒性与氧化应激[11,87]、炎症损伤[89-90]和表观遗传修饰[91-93]等有关,且主要集中于氧化应激假说和炎症假说[27](图1).
-
造血稳态的维持依赖于HSCs和HPSCs,而HSCs功能选择取决于ROS水平高低[88]. Cui等证实,PM2.5暴露可抑制小鼠骨髓基质细胞增殖,该过程依赖于机体氧化应激反应[6]. 最近一项动物研究发现,PM2.5通过诱导孕鼠子代ROS高水平表达导致BM-HSCs衰老[88]. PM2.5亦可诱导ROS相关酶(如NAPDH氧化酶)生成以刺激ROS的生成,进而加剧白血病发展进程[11]. 值得注意的是,亚氮应激也被发现是血液系统产生毒性效应的一种机制. 如血小板活化与一氧化氮含量的下降有关,这是因为一氧化氮作为血小板活化抑制剂,其水平的降低和过氧亚硝酸盐水平的增加可能激活血小板并发生聚集效应[94]. 但是,PM2.5诱导亚氮应激引发造血毒性的生物学过程还有待进一步阐明.
-
动物研究发现,PM2.5诱发炎症反应以损害骨髓微环境稳态,进而导致血液疾病发生[89]. PM2.5所激活的炎症反应可影响红系前体细胞的增殖与分化、内源性EPO耐药性和血红蛋白浓度水平[90]. 其具体生物学过程可从3个方面解释[95],即其一炎症可能调控EPO表达量降低,进而引发红细胞的生物反应下调,最终红细胞生成不足;其二炎症诱发低氧或缺氧致使红细胞生成减少;其三炎性因子上调铁调素合成而导致贫血发生.
此外,炎症可刺激血小板活化、聚集. 动物模型发现,PM2.5暴露后IL-6、IL-1β和CRP等血浆炎症细胞因子表达增加,进而促进血管细胞粘附分子-1(VCAM-1)和细胞间黏附分子-1(ICAM-1)等细胞黏附分子的表达. 另一方面,炎症释放的细胞因子刺激巨核细胞生成而增加血小板生成量. 例如,IL-6刺激肝脏释放促血小板生成素(thrombopoietin, TPO),随后TPO受体刺激Janus激酶2(JAK2)/信号转导剂和转录激活剂3(STAT3)等信号途径,进而诱导巨核细胞的增殖与分化[96].
-
PM2.5影响全生命周期的表观遗传修饰,特别是DNA甲基化(DNA methylation, DNAm)和组蛋白修饰[97-99]. PM2.5所引起的机体DNAm标志物主要集中在血液[91]. 如,Chi等[92]在长期暴露PM2.5的人群外周血单核细胞中观察到5处胞嘧啶-鸟嘌呤二核苷酸(CpG)位点甲基化,提示与单核细胞DNAm显著相关. 也有报道PM2.5暴露与人群外周血白细胞DNAm水平增高相关[93]. Li等[100]研究发现,PM2.5导致RAP1GAP2(RAP1 GTPase activating protein 2)基因位点发生甲基化,进而调节血小板活性. 此外,Zheng等[101]研究发现,交通源PM10与卡车司机血液中白细胞的H3赖氨酸27三甲基化(H3K27me3)和H3赖氨酸36三甲基化(H3K36me3)水平呈现显著负相关,且PM2.5及其不同组分亦可影响组蛋白H3修饰[99].
-
PM2.5仍是危害人类健康的主要环境风险之一. 人群队列研究和毒理学实验显示PM2.5与血液系统疾病之间存在着显著相关性,其造血毒性研究已取得了一些进展. 考虑到PM2.5引起的血细胞或(和)造血器官受损可能受到暴露剂量、暴露频次、暴露途径、暴露评估模型等因素影响,现有研究工作仍有待加强.
(1)目前研究多集中于贫血和血栓,较少关注PM2.5促血液肿瘤(如白血病)发生过程和机制. 另外,肺脏是新发现的造血位点,且作为PM2.5直接作用靶器官,亟需关注PM2.5及其化学组分对肺脏造血的影响.
(2)PM2.5具有高度异质性,即不同地区、不同季节及不同气象条件下PM2.5化学成分具有差异性,导致PM2.5造血毒性的关键组分尚不够明确. 因此,基于生物过程和毒理机制识别关键毒性组分及其分子作用,阐明从暴露到机体特定损伤乃至健康危害的过程,有助于预防和干预PM2.5对人群产生的不良健康影响.
空气细颗粒物造血毒性与生物学机制研究进展
Research progress on hematopoietic toxicity and biological mechanism of ambient fine particulate matters
-
摘要: 近年来,空气细颗粒物(fine particulate matters,PM2.5)毒理与健康效应一直是环境化学领域的热点问题. 流行病学和毒理学研究表明,除呼吸和心脑血管系统损伤外,PM2.5还可对血细胞和造血器官产生不良影响. 为此,本文简要回顾了机体造血过程,基于流行病学数据和毒理学实验结果综述了PM2.5造血毒性,从氧化应激、炎症损伤和表观遗传修饰等方面归纳了生物学机制.Abstract: In recent years, the toxicological and health effects of ambient fine particulate matters (PM2.5) have been a hot topic in the field of environmental chemistry. In addition to respiratory and cardiovascular system damages, PM2.5 posed the adverse impacts on blood cell and hematopoietic organs. For this purpose, this paper briefly reviewed the hematopoietic process of organisms, introduced PM2.5-induced hematopoietic toxicity based on epidemiological studies and toxicological experiments, and clarified biological mechanisms, mainly focusing on oxidative stress, inflammatory damage and epigenetic modification.
-

-
表 1 PM2.5造血毒性的流行病学研究结果
Table 1. Epidemiological studies on PM2.5-induced hematopoietic toxicity
人群
Population样本量
Sample size年龄
Age groupe地区
Region暴露物质
Exposure method暴露周期
Exposure duration主要结果
Main findings文献
Reference儿童
Children1261855 — 韩国
KoreaPM2.5 2002—2012 致儿童患白血病 [31] 儿童、青少年和年轻成人
Pediatric, adolescent, and young adult2444+13459 0—39月 美国,犹他州
Utah, AmericanPM2.5 1986—2015 与儿童白血病相关 [10] 儿童
Children139368 6—59月 秘鲁,利马
Lima, PeruPM2.5 2012—2019 增加儿童贫血患病率 [32] 儿童
Children98557 <5岁 印度
IndiaPM2.5 2015—2016 导致儿童贫血 [33] 儿童
Children117511 <5岁 撒哈拉以南非洲
Sub-Saharan AfricaPM2.5 2006—2020 增加儿童贫血患病率 [34] 成人
Adults25355 ≥18岁 中国东北地区
Northeast ChinaPM2.5 2019—2021 增加血小板数量 [35] 学生
Students8 24—28岁 青岛—石家庄
Qingdao—
ShijiazhuangPMs、SO2、
CO、NO22—3周 可增加血栓形成的风险 [36] 居民
Residents82431 (42.83 ± 15.09)岁 中国南京
Nanjing ChinaPM2.5 2017—2018 影响红细胞、单核细胞的数量 [37] 成人
Adults362396 >50岁 中国台湾
Taiwan ChinaPM2.5 2001—2014 与血小板增加相关 [38] 成人
Adults118 >40岁 中国焦作
Jiaozuo ChinaCB ≥1年 增加粒细胞计数量 [39] 农村队列
Rural Cohort31282 >50岁 中国河南
Henan ChinaPMs、NO2 — 增加血小板数量 [40] 成人
Adults110 50—75岁 中国北京
Beijing ChinaPM2.5、CB、
NO2、噪声2018—2019 导致血小板低甲基化 [41] 老年人群
Older Population4121 75—84岁 美国
AmericanPM2.5、NO2 1年 增加老年人贫血患病率 [42] -
[1] World Health Organization. Ambient(outdoor) air pollution[EB/OL]. [2022-05-01]. [2] International Agency for Research on Cancer. Agents Classified by the IARC Monographs, Volumes 1-131[EB/OL]. [2022-05-01]. [3] FENG S L, GAO D, LIAO F, et al. The health effects of ambient PM2.5 and potential mechanisms [J]. Ecotoxicology and Environmental Safety, 2016, 128: 67-74. doi: 10.1016/j.ecoenv.2016.01.030 [4] YUE H F, YUN Y, GAO R, et al. Winter polycyclic aromatic hydrocarbon-bound particulate matter from peri-urban North China promotes lung cancer cell metastasis [J]. Environmental Science & Technology, 2015, 49(24): 14484-14493. [5] TAJ T, POULSEN A H, KETZEL M, et al. Exposure to PM2.5 constituents and risk of adult leukemia in Denmark: A population-based case-control study [J]. Environmental Research, 2021, 196: 110418. doi: 10.1016/j.envres.2020.110418 [6] CUI Y Q, JIA F P, HE J F, et al. Ambient fine particulate matter suppresses in vivo proliferation of bone marrow stem cells through reactive oxygen species formation [J]. PLoS One, 2015, 10(6): e0127309. doi: 10.1371/journal.pone.0127309 [7] KIM K H, KABIR E, KABIR S. A review on the human health impact of airborne particulate matter [J]. Environment International, 2015, 74: 136-143. doi: 10.1016/j.envint.2014.10.005 [8] FONGSODSRI K, CHAMNANCHANUNT S, DESAKORN V, et al. Particulate matter 2.5 and hematological disorders from dust to diseases: A systematic review of available evidence [J]. Frontiers in Medicine, 2021, 8: 692008. doi: 10.3389/fmed.2021.692008 [9] HECK J E, WU J, LOMBARDI C, et al. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life [J]. Environmental Health Perspectives, 2013, 121(11/12): 1385-1391. [10] OU J Y, HANSON H A, RAMSAY J M, et al. Fine particulate matter air pollution and mortality among pediatric, adolescent, and young adult cancer patients [J]. Cancer Epidemiology, Biomarkers & Prevention, 2020, 29(10): 1929-1939. [11] JIN X T, CHEN M L, LI R J, et al. Progression and inflammation of human myeloid leukemia induced by ambient PM2.5 exposure [J]. Archives of Toxicology, 2016, 90(8): 1929-1938. doi: 10.1007/s00204-015-1610-x [12] CHEN T T, ZHANG J, ZENG H, et al. The impact of inflammation and cytokine expression of PM2.5 in AML [J]. Oncology Letters, 2018, 16(2): 2732-2740. [13] 閤静, 郭晴, 江清英, 等. 甲醛复合PM2.5致小鼠血液毒性的研究 [J]. 中国环境科学, 2017, 37(7): 2740-2748. doi: 10.3969/j.issn.1000-6923.2017.07.040 GE J, GUO Q, JIANG Q Y, et al. Formaldehyde and PM2.5 induced hepatotoxicity in mice [J]. China Environmental Science, 2017, 37(7): 2740-2748(in Chinese). doi: 10.3969/j.issn.1000-6923.2017.07.040
[14] MEDVINSKY A, RYBTSOV S, TAOUDI S. Embryonic origin of the adult hematopoietic system: Advances and questions [J]. Development, 2011, 138(6): 1017-1031. doi: 10.1242/dev.040998 [15] IVANOVS A, RYBTSOV S, NG E S, et al. Human haematopoietic stem cell development: From the embryo to the dish [J]. Development (Cambridge, England), 2017, 144(13): 2323-2337. doi: 10.1242/dev.134866 [16] GOLUB R, CUMANO A. Embryonic hematopoiesis [J]. Blood Cells, Molecules, and Diseases, 2013, 51(4): 226-231. doi: 10.1016/j.bcmd.2013.08.004 [17] CANU G, RUHRBERG C. First blood: The endothelial origins of hematopoietic progenitors [J]. Angiogenesis, 2021, 24(2): 199-211. doi: 10.1007/s10456-021-09783-9 [18] GOMEZ PERDIGUERO E, KLAPPROTH K, SCHULZ C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors [J]. Nature, 2015, 518(7540): 547-551. doi: 10.1038/nature13989 [19] PALIS J, ROBERTSON S, KENNEDY M, et al. Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse [J]. Development, 1999, 126(22): 5073-5084. doi: 10.1242/dev.126.22.5073 [20] BÖIERS C, CARRELHA J, LUTTEROPP M, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells [J]. Cell Stem Cell, 2013, 13(5): 535-548. doi: 10.1016/j.stem.2013.08.012 [21] ORKIN S H, ZON L I. Hematopoiesis: An evolving paradigm for stem cell biology [J]. Cell, 2008, 132(4): 631-644. doi: 10.1016/j.cell.2008.01.025 [22] GEKAS C, DIETERLEN-LIÈVRE F, ORKIN S H, et al. The placenta is a niche for hematopoietic stem cells [J]. Developmental Cell, 2005, 8(3): 365-375. doi: 10.1016/j.devcel.2004.12.016 [23] LI Z, LAN Y, HE W Y, et al. Mouse embryonic head as a site for hematopoietic stem cell development [J]. Cell Stem Cell, 2012, 11(5): 663-675. doi: 10.1016/j.stem.2012.07.004 [24] LAURENTI E, GÖTTGENS B. From haematopoietic stem cells to complex differentiation landscapes [J]. Nature, 2018, 553(7689): 418-426. doi: 10.1038/nature25022 [25] SEITA J, WEISSMAN I L. Hematopoietic stem cell: Self-renewal versus differentiation [J]. Wiley Interdisciplinary Reviews:Systems Biology and Medicine, 2010, 2(6): 640-653. doi: 10.1002/wsbm.86 [26] CHENG H, ZHENG Z F, CHENG T. New paradigms on hematopoietic stem cell differentiation [J]. Protein & Cell, 2020, 11(1): 34-44. [27] SCHARF P, BROERING M F, da ROCHA G H O, et al. Cellular and molecular mechanisms of environmental pollutants on hematopoiesis [J]. International Journal of Molecular Sciences, 2020, 21(19): 6996. doi: 10.3390/ijms21196996 [28] MENDELSON A, FRENETTE P S. Hematopoietic stem cell niche maintenance during homeostasis and regeneration [J]. Nature Medicine, 2014, 20(8): 833-846. doi: 10.1038/nm.3647 [29] SHORT C, LIM H K, TAN J, et al. Targeting the spleen as an alternative site for hematopoiesis [J]. BioEssays, 2019, 41(5): 1800234. doi: 10.1002/bies.201800234 [30] GE J, YANG H L, LU X X, et al. Combined exposure to formaldehyde and PM2.5: Hematopoietic toxicity and molecular mechanism in mice [J]. Environment International, 2020, 144: 106050. doi: 10.1016/j.envint.2020.106050 [31] LEE J M, LEE T H, KIM S, et al. Association between long-term exposure to particulate matter and childhood cancer: A retrospective cohort study [J]. Environmental Research, 2022, 205: 112418. doi: 10.1016/j.envres.2021.112418 [32] MORALES-ANCAJIMA V C, TAPIA V, VU B N, et al. Increased outdoor PM2.5 concentration is associated with moderate/severe Anemia in children aged 6-59 months in Lima, Peru [J]. Journal of Environmental and Public Health, 2019, 2019: 6127845. [33] MEHTA U, DEY S, CHOWDHURY S, et al. The association between ambient PM2.5 exposure and Anemia outcomes among children under five years of age in India [J]. Environmental Epidemiology, 2021, 5(1): e125. doi: 10.1097/EE9.0000000000000125 [34] AMEGBOR P M. Early-life environmental exposures and anaemia among children under age five in Sub-Saharan Africa: An insight from the Demographic & Health Surveys [J]. The Science of the Total Environment, 2022, 832: 154957. doi: 10.1016/j.scitotenv.2022.154957 [35] ZHANG H H, CHANG Q, ZHAO Y H. Association between ambient particulate matter exposure and platelet counts in adults: A retrospective cohort study [J]. Environmental Science and Pollution Research, 2021, 28(24): 31268-31275. doi: 10.1007/s11356-021-12865-2 [36] SUN H M, LI Y T, ZHANG J Z, et al. Platelet mitochondrial DNA methylation as epigenetic biomarker of short-term air pollution exposure in healthy subjects [J]. Frontiers in Molecular Biosciences, 2022, 8: 803488. doi: 10.3389/fmolb.2021.803488 [37] LI Z Q, LI X N, SONG H, et al. Effects of short-term ambient PM2.5 exposure on the blood cell count and hemoglobin concentration among 82, 431 people in Eastern China [J]. The Science of the Total Environment, 2021, 776: 146046. doi: 10.1016/j.scitotenv.2021.146046 [38] ZHANG Z L, CHAN T C, GUO C, et al. Long-term exposure to ambient particulate matter (PM2.5) is associated with platelet counts in adults [J]. Environmental Pollution, 2018, 240: 432-439. doi: 10.1016/j.envpol.2018.04.123 [39] DAI Y F, NIU Y, DUAN H W, et al. Effects of occupational exposure to carbon black on peripheral white blood cell counts and lymphocyte subsets [J]. Environmental and Molecular Mutagenesis, 2016, 57(8): 615-622. doi: 10.1002/em.22036 [40] HOU J, DUAN Y Y, LIU X T, et al. Associations of long-term exposure to air pollutants, physical activity and platelet traits of cardiovascular risk in a rural Chinese population [J]. The Science of the Total Environment, 2020, 738: 140182. doi: 10.1016/j.scitotenv.2020.140182 [41] LIU Q, LI H Y, GUO L Q, et al. Effects of short-term personal exposure to air pollution on platelet mitochondrial DNA methylation levels and the potential mitigation by L-arginine supplementation [J]. Journal of Hazardous Materials, 2021, 417: 125963. doi: 10.1016/j.jhazmat.2021.125963 [42] HONDA T, PUN V C, MANJOURIDES J, et al. Anemia prevalence and hemoglobin levels are associated with long-term exposure to air pollution in an older population [J]. Environment International, 2017, 101: 125-132. doi: 10.1016/j.envint.2017.01.017 [43] SORDO M, MACIEL-RUIZ J A, SALAZAR A M, et al. Particulate matter-associated micronuclei frequencies in maternal and cord blood lymphocytes [J]. Environmental and Molecular Mutagenesis, 2019, 60(5): 421-427. doi: 10.1002/em.22275 [44] YEN H C, LIN C H, LIN M C, et al. Prenatal exposure to air pollution and immune thrombocytopenia: A nationwide population-based cohort study [J]. Frontiers in Pediatrics, 2022, 10: 837101. doi: 10.3389/fped.2022.837101 [45] LAVIGNE É, BÉLAIR M A, DO M T, et al. Maternal exposure to ambient air pollution and risk of early childhood cancers: A population-based study in Ontario, Canada [J]. Environment International, 2017, 100: 139-147. doi: 10.1016/j.envint.2017.01.004 [46] ACCINELLI R A, LEON-ABARCA J A. Solid fuel use is associated with anemia in children [J]. Environmental Research, 2017, 158: 431-435. doi: 10.1016/j.envres.2017.06.032 [47] RAASCHOU-NIELSEN O, ANDERSEN Z J, HVIDBERG M, et al. Air pollution from traffic and cancer incidence: A Danish cohort study [J]. Environmental Health:a Global Access Science Source, 2011, 10: 67. [48] PAN J, LI C, ZHANG X, et al. Hematological effects of ultrafine carbon black on red blood cells and hemoglobin [J]. Journal of Biochemical and Molecular Toxicology, 2020, 34(3): e22438. [49] HANTRAKOOL S, KUMFU S, CHATTIPAKORN S C, et al. Effects of particulate matter on inflammation and thrombosis: Past evidence for future prevention [J]. International Journal of Environmental Research and Public Health, 2022, 19(14): 8771. doi: 10.3390/ijerph19148771 [50] WANG S, KAUR M, LI T, et al. Effect of different pollution parameters and chemical components of PM2.5 on health of residents of Xinxiang City, China [J]. International Journal of Environmental Research and Public Health, 2021, 18(13): 6821. doi: 10.3390/ijerph18136821 [51] WINTERS N, GOLDBERG M S, HYSTAD P, et al. Exposure to ambient air pollution in Canada and the risk of adult leukemia [J]. The Science of the Total Environment, 2015, 526: 153-176. doi: 10.1016/j.scitotenv.2015.03.149 [52] KHORRAMI Z, POURKHOSRAVANI M, ESLAHI M, et al. Multiple air pollutants exposure and leukaemia incidence in Tehran, Iran from 2010 to 2016: A retrospective cohort study [J]. BMJ Open, 2022, 12(6): e060562. doi: 10.1136/bmjopen-2021-060562 [53] GAO K, CHEN X, ZHANG L N, et al. Associations between differences in anemia-related blood cell parameters and short-term exposure to ambient particle pollutants in middle-aged and elderly residents in Beijing, China [J]. The Science of the Total Environment, 2022, 816: 151520. doi: 10.1016/j.scitotenv.2021.151520 [54] ELBARBARY M, HONDA T, MORGAN G, et al. Ambient air pollution exposure association with anaemia prevalence and haemoglobin levels in Chinese older adults [J]. International Journal of Environmental Research and Public Health, 2020, 17(9): 3209. doi: 10.3390/ijerph17093209 [55] PUETT R C, POULSEN A H, TAJ T, et al. Relationship of leukaemias with long-term ambient air pollution exposures in the adult Danish population [J]. British Journal of Cancer, 2020, 123(12): 1818-1824. doi: 10.1038/s41416-020-01058-2 [56] JIN X T, YU H Y, WANG B Q, et al. Airborne particulate matters induce thrombopoiesis from megakaryocytes through regulating mitochondrial oxidative phosphorylation [J]. Particle and Fibre Toxicology, 2021, 18(1): 19. doi: 10.1186/s12989-021-00411-4 [57] ABPLANALP W, HABERZETTL P, BHATNAGAR A, et al. Carnosine supplementation mitigates the deleterious effects of particulate matter exposure in mice [J]. Journal of the American Heart Association, 2019, 8(13): e013041. doi: 10.1161/JAHA.119.013041 [58] JANTZEN K, MØLLER P, KAROTTKI D G, et al. Exposure to ultrafine particles, intracellular production of reactive oxygen species in leukocytes and altered levels of endothelial progenitor cells [J]. Toxicology, 2016, 359/360: 11-18. doi: 10.1016/j.tox.2016.06.007 [59] VAYÁ A, ALIS R, SUESCÚN M, et al. Association of erythrocyte deformability with red blood cell distribution width in metabolic diseases and thalassemia trait [J]. Clinical Hemorheology and Microcirculation, 2015, 61(3): 407-415. [60] PRETORIUS E, BESTER J, VERMEULEN N, et al. Profound morphological changes in the erythrocytes and fibrin networks of patients with hemochromatosis or with hyperferritinemia, and their normalization by iron chelators and other agents [J]. PLoS One, 2014, 9(1): e85271. doi: 10.1371/journal.pone.0085271 [61] ABU-ELMAGD M, ALGHAMDI M A, SHAMY M, et al. Evaluation of the effects of airborne particulate matter on bone marrow-mesenchymal stem cells (BM-MSCs): Cellular, molecular and systems biological approaches [J]. International Journal of Environmental Research and Public Health, 2017, 14(4): 440. doi: 10.3390/ijerph14040440 [62] WARDOYO A, JUSWONO U, NOOR J. How exposure to ultrafine and fine particles of car smoke can alter erythrocyte forms of male mice [J]. Polish Journal of Environmental Studies, 2019, 28(4): 2901-2910. doi: 10.15244/pjoes/94047 [63] WARDOYO A Y P, JUSWONO U P, NOOR J A E. A study of the correlation between ultrafine particle emissions in motorcycle smoke and mice erythrocyte damages [J]. Experimental and Toxicologic Pathology, 2017, 69(8): 649-655. doi: 10.1016/j.etp.2017.06.003 [64] ABE K C, BRANDÃO L D C, TUFIK S, et al. In utero exposure to air pollution lowers erythrocyte antioxidant defense and decreases weight in adult mice [J]. Environmental Toxicology and Pharmacology, 2011, 32(2): 315-318. doi: 10.1016/j.etap.2011.05.001 [65] KWAG Y, YE S, OH J, et al. Direct and indirect effects of indoor particulate matter on blood indicators related to Anemia [J]. International Journal of Environmental Research and Public Health, 2021, 18(24): 12890. doi: 10.3390/ijerph182412890 [66] XIE G L, YUE J, YANG W F, et al. Effects of PM2.5 and its constituents on hemoglobin during the third trimester in pregnant women [J]. Environmental Science and Pollution Research, 2022, 29(23): 35193-35203. doi: 10.1007/s11356-022-18693-2 [67] LANGER A L, GINZBURG Y Z. Role of hepcidin-ferroportin axis in the pathophysiology, diagnosis, and treatment of anemia of chronic inflammation [J]. Hemodialysis International, 2017, 21: S37-S46. doi: 10.1111/hdi.12543 [68] SIGNORELLI S S, OLIVERI CONTI G, ZANOBETTI A, et al. Effect of particulate matter-bound metals exposure on prothrombotic biomarkers: A systematic review [J]. Environmental Research, 2019, 177: 108573. doi: 10.1016/j.envres.2019.108573 [69] BACCARELLI A, ZANOBETTI A, MARTINELLI I, et al. Effects of exposure to air pollution on blood coagulation [J]. Journal of Thrombosis and Haemostasis, 2007, 5(2): 252-260. doi: 10.1111/j.1538-7836.2007.02300.x [70] TABLIN F, den HARTIGH L J, AUNG H H, et al. Seasonal influences on CAPs exposures: Differential responses in platelet activation, serum cytokines and xenobiotic gene expression [J]. Inhalation Toxicology, 2012, 24(8): 506-517. doi: 10.3109/08958378.2012.695815 [71] DELFINO R J, STAIMER N, TJOA T, et al. Air pollution exposures and circulating biomarkers of effect in a susceptible population: Clues to potential causal component mixtures and mechanisms [J]. Environmental Health Perspectives, 2009, 117(8): 1232-1238. doi: 10.1289/ehp.0800194 [72] YIN Z, XU H J, YAO X L, et al. Ambient fine particles (PM2.5) attenuate collagen-induced platelet activation through interference of the PLCγ2/Akt/GSK3β signaling pathway [J]. Environmental Toxicology, 2017, 32(2): 530-540. doi: 10.1002/tox.22257 [73] LUCKING A J, LUNDBACK M, MILLS N L, et al. Diesel exhaust inhalation increases thrombus formation in man [J]. European Heart Journal, 2008, 29(24): 3043-3051. doi: 10.1093/eurheartj/ehn464 [74] LEFRANÇAIS E, ORTIZ-MUÑOZ G, CAUDRILLIER A, et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors [J]. Nature, 2017, 544(7648): 105-109. doi: 10.1038/nature21706 [75] GANGWAR R S, VINAYACHANDRAN V, RENGASAMY P, et al. Differential contribution of bone marrow-derived infiltrating monocytes and resident macrophages to persistent lung inflammation in chronic air pollution exposure [J]. Scientific Reports, 2020, 10: 14348. doi: 10.1038/s41598-020-71144-1 [76] ZHAO Q J, CHEN H, YANG T, et al. Direct effects of airborne PM2.5 exposure on macrophage polarizations [J]. Biochimica et Biophysica Acta (BBA) - General Subjects, 2016, 1860(12): 2835-2843. doi: 10.1016/j.bbagen.2016.03.033 [77] MANNUCCI P M, HARARI S, FRANCHINI M. Novel evidence for a greater burden of ambient air pollution on cardiovascular disease [J]. Haematologica, 2019, 104(12): 2349-2357. doi: 10.3324/haematol.2019.225086 [78] ZHAO H, LI W X, GAO Y F, et al. Exposure to particular matter increases susceptibility to respiratory Staphylococcus aureus infection in rats via reducing pulmonary natural killer cells [J]. Toxicology, 2014, 325: 180-188. doi: 10.1016/j.tox.2014.09.006 [79] LIN C M, HUANG T H, CHI M C, et al. N-acetylcysteine alleviates fine particulate matter (PM2.5)-induced lung injury by attenuation of ROS-mediated recruitment of neutrophils and Ly6Chigh monocytes and lung inflammation [J]. Ecotoxicology and Environmental Safety, 2022, 239: 113632. doi: 10.1016/j.ecoenv.2022.113632 [80] GOTO Y, ISHII H, HOGG J C, et al. Particulate matter air pollution stimulates monocyte release from the bone marrow [J]. American Journal of Respiratory and Critical Care Medicine, 2004, 170(8): 891-897. doi: 10.1164/rccm.200402-235OC [81] MUKAE H, HOGG J C, ENGLISH D, et al. Phagocytosis of particulate air pollutants by human alveolar macrophages stimulates the bone marrow [J]. American Journal of Physiology-Lung Cellular and Molecular Physiology, 2000, 279(5): L924-L931. doi: 10.1152/ajplung.2000.279.5.L924 [82] BRANDT E B, KOVACIC M B, LEE G B, et al. Diesel exhaust particle induction of IL-17A contributes to severe asthma [J]. Journal of Allergy and Clinical Immunology, 2013, 132(5): 1194-1204.e2. doi: 10.1016/j.jaci.2013.06.048 [83] SIGAUD S, GOLDSMITH C A W, ZHOU H W, et al. Air pollution particles diminish bacterial clearance in the primed lungs of mice [J]. Toxicology and Applied Pharmacology, 2007, 223(1): 1-9. doi: 10.1016/j.taap.2007.04.014 [84] XU X H, JIANG S Y, WANG T Y, et al. Inflammatory response to fine particulate air pollution exposure: Neutrophil versus monocyte [J]. PLoS One, 2013, 8(8): e71414. doi: 10.1371/journal.pone.0071414 [85] HABERZETTL P, CONKLIN D J, ABPLANALP W T, et al. Inhalation of fine particulate matter impairs endothelial progenitor cell function via pulmonary oxidative stress [J]. Arteriosclerosis, Thrombosis, and Vascular Biology, 2018, 38(1): 131-142. doi: 10.1161/ATVBAHA.117.309971 [86] HABERZETTL P, LEE J, DUGGINENI D, et al. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow [J]. Environmental Health Perspectives, 2012, 120(6): 848-856. doi: 10.1289/ehp.1104206 [87] BHATTARAI G, LEE J B, KIM M H, et al. Maternal exposure to fine particulate matter during pregnancy induces progressive senescence of hematopoietic stem cells under preferential impairment of the bone marrow microenvironment and aids development of myeloproliferative disease [J]. Leukemia, 2020, 34(5): 1481-1484. doi: 10.1038/s41375-019-0665-8 [88] 阳静, 陈丽琼, 叶中绿, 等. PM2.5对儿童骨髓基质细胞增殖及细胞因子G-CSF、GM-CSF分泌的影响 [J]. 中国医学创新, 2016, 13(19): 5-9. doi: 10.3969/j.issn.1674-4985.2016.19.002 YANG J, CHEN L Q, YE Z L, et al. Effects of PM2.5 on the proliferation and secretion levels of G-CSF and GM-CSF in children’s BMSCs [J]. Medical Innovation of China, 2016, 13(19): 5-9(in Chinese). doi: 10.3969/j.issn.1674-4985.2016.19.002
[89] 何永忠, 刘丽丽, 田川, 等. PM2.5长期暴露对小鼠骨髓造血内环境的毒性效应及壳寡糖的保护作用 [J]. 中国实验血液学杂志, 2021, 29(5): 1478-1484. HE Y Z, LIU L L, TIAN C, et al. Toxic effects of long-term exposure to PM2.5 and protective effects of chitosan on bone marrow hematopoietic environment of the mice [J]. Journal of Experimental Hematology, 2021, 29(5): 1478-1484(in Chinese).
[90] BRITO J M, MACCHIONE M, YOSHIZAKI K, et al. Acute cardiopulmonary effects induced by the inhalation of concentrated ambient particles during seasonal variation in the city of São Paulo [J]. Journal of Applied Physiology (Bethesda, Md. :1985), 2014, 117(5): 492-499. doi: 10.1152/japplphysiol.00156.2014 [91] TARANTINI L, BONZINI M, APOSTOLI P, et al. Effects of particulate matter on genomic DNA methylation content and iNOS promoter methylation [J]. Environmental Health Perspectives, 2009, 117(2): 217-222. doi: 10.1289/ehp.11898 [92] CHI G C, LIU Y M, MacDONALD J W, et al. Long-term outdoor air pollution and DNA methylation in circulating monocytes: Results from the Multi-Ethnic Study of Atherosclerosis (MESA) [J]. Environmental Health:a Global Access Science Source, 2016, 15(1): 119. [93] FAN T T, FANG S C, CAVALLARI J M, et al. Heart rate variability and DNA methylation levels are altered after short-term metal fume exposure among occupational welders: A repeated-measures panel study [J]. BMC Public Health, 2014, 14: 1279. doi: 10.1186/1471-2458-14-1279 [94] CHEN S, SU Y, WANG J. ROS-mediated platelet generation: A microenvironment-dependent manner for megakaryocyte proliferation, differentiation, and maturation [J]. Cell Death & Disease, 2013, 4(7): e722. [95] FERRUCCI L, BALDUCCI L. Anemia of aging: The role of chronic inflammation and cancer [J]. Seminars in Hematology, 2008, 45(4): 242-249. doi: 10.1053/j.seminhematol.2008.06.001 [96] LIANG S, ZHAO T, HU H J, et al. Repeat dose exposure of PM2.5 triggers the disseminated intravascular coagulation (DIC) in SD rats [J]. The Science of the Total Environment, 2019, 663: 245-253. doi: 10.1016/j.scitotenv.2019.01.346 [97] SANCHEZ-GUERRA M, ZHENG Y N, OSORIO-YANEZ C, et al. Effects of particulate matter exposure on blood 5-hydroxymethylation: Results from the Beijing truck driver air pollution study [J]. Epigenetics, 2015, 10(7): 633-642. doi: 10.1080/15592294.2015.1050174 [98] BYUN H M, COLICINO E, TREVISI L, et al. Effects of air pollution and blood mitochondrial DNA methylation on markers of heart rate variability [J]. Journal of the American Heart Association, 2016, 5(4): e003218. doi: 10.1161/JAHA.116.003218 [99] LIU C, XU J H, CHEN Y H, et al. Characterization of genome-wide H3K27ac profiles reveals a distinct PM2.5-associated histone modification signature [J]. Environmental Health:a Global Access Science Source, 2015, 14: 65. [100] LI H C, CHEN R J, CAI J, et al. Short-term exposure to fine particulate air pollution and genome-wide DNA methylation: A randomized, double-blind, crossover trial [J]. Environment International, 2018, 120: 130-136. doi: 10.1016/j.envint.2018.07.041 [101] ZHENG Y N, SANCHEZ-GUERRA M, ZHANG Z, et al. Traffic-derived particulate matter exposure and histone H3 modification: A repeated measures study [J]. Environmental Research, 2017, 153: 112-119. doi: 10.1016/j.envres.2016.11.015 -



 下载:
下载:

