-
大量使用石油、煤炭等化石燃料使大气中CO2浓度迅速增加,导致全球变暖,并引发一系列环境问题[1 − 2]. 通过碳捕集利用(CCU)将CO2催化转化为燃料或化学品,具有环境、能源、经济三重效益,前景广阔[3 − 5].
在常压条件下,CO2催化加氢的产物一般为CH4或CO[6]. CO2甲烷化可以将H2中化学能储存在CH4中,但存在能量损失[7];而选择性生成CO,则可与过剩的H2进一步通过费托合成反应,得到种类丰富的燃料或化学品,具有更高的附加值[8]. CO2加氢催化剂常用的活性组分有Ni、Ru、Cu[9]. 其中,Cu虽然具有高CO选择性,但活性和稳定性较差[2]. 而Ru具有高活性,却通常过度加氢生成CH4[10]. 研究表明,当Ru以单原子或原子簇形式存在时,也可将CO2选择性加氢为CO[11]. 但是在高温还原性气氛中,Ru单原子或原子簇会发生团聚,稳定性差[12].
构建Ru-Cu双金属活性位点是一种有效的解决策略. Cu对Ru的稀释作用限制Ru的尺寸可以提高选择性,而Ru可以活化H2并溢流活性H到Cu表面,从而提高活性[13]. 常用的双金属催化剂的制备方法为浸渍法,但难以有效控制催化剂活性组分结构,导致形成单独的单金属纳米颗粒[14]. 而微乳液法是一种可控的催化剂合成方法,可以制备出合金、核壳等结构的双金属纳米粒子[15 − 16].
本文采用微乳液法制备了组成与尺寸均匀的钌铜纳米金属粒子,将其负载在热稳定性好的Al2O3载体上,合成了双金属RuCu/Al2O3催化剂,以及采用浸渍法制备了对比材料. 然后对各材料进行了系列表征并考察了制备方法、钌铜比、负载量对催化活性与选择性的影响,以及测试了材料的稳定性.
-
在催化剂制备前,将Al2O3放入马弗炉中600 ℃下焙烧5 h进行预处理.
微乳液法催化剂过程如下:将正辛烷、CTAB、正丁醇、RuCl3溶液和Cu(NO3)2溶液按照质量比40:29:21:x:10-x(x为RuCl3溶液的质量)搅拌混合均匀,得到钌铜混合微乳液. 然后在N2保护下,滴加入一定量NaBH4进行还原反应2 h,其中,NaBH4与金属离子物质的量之比为10:1. 然后加入一定量Al2O3并搅拌1 h,再滴加与混合微乳液相同体积的破乳剂,其中破乳剂由乙醇和丙酮1:1复配而成. 最后用大量乙醇和纯水洗涤材料并干燥,在350 ℃下焙烧4 h,即可得到材料. 改变RuCl3溶液和Cu(NO3)2溶液的用量,可以得到不同金属负载量的ME-xRuyCu/Al2O3材料(x和y分别是Ru和Cu的负载量wt%).
浸渍法制备催化剂:将一定量RuCl3溶液和Cu(NO3)2溶液入烧杯,再加入一定量纯水和Al2O3,搅拌2 h,并90 ℃水浴蒸干,烘干后在350 ℃马弗炉中焙烧4 h,得到im-xRuyCu/Al2O3材料.
-
X射线衍射分析(XRD):使用德国Bruker-AXS公司的D8 Advance型X射线衍射仪,采用Cu靶(Kα1,λ = 0.15418 nm),操作电压40 kV、电流40 mA. 透射电镜-X射线能谱分析(TEM-EDS):采用美国FEI公司的Tecnai G2 F20 S-Twin+AZtecX-Max 80T型透射电子显微镜并装配X射线能谱采集样品(载于Ni网)在明场中的透射电镜图和暗场中的Mapping图. X射线光电子能谱分析(XPS):使用美国ThermoFisher Scientific公司的ESCALAB250Xi型X射线光电子能谱仪对样品Cu 2p轨道扫描精细谱. CO吸附原位红外光谱分析:使用美国Thermo Fisher公司的Nicolet IS10型傅里叶红外光谱仪对样品进行CO吸附原位红外光谱分析.
-
催化剂的性能评价是在微型固定床反应器进行催化反应,再通过气相色谱在线检测反应物与产物. 将含50 mg催化剂的石英反应管置于微型固定床反应器中,先在H2流量为30 mL·min−1气氛下400 ℃还原2 h. 然后将温度调至反应温度(250—550 ℃),将CO2、H2和N2按体积比1:4:1混合,总流量为54 mL·min−1,气流通过催化剂床层进行CO2加氢反应. 反应平衡30 min后,尾气通过气相色谱在线检测,分析条件为:色谱填充柱是TDX-01,柱温为70 ℃,气化室温度为150 ℃,TCD检测器温度为150 ℃,热丝温度为130 ℃.
-
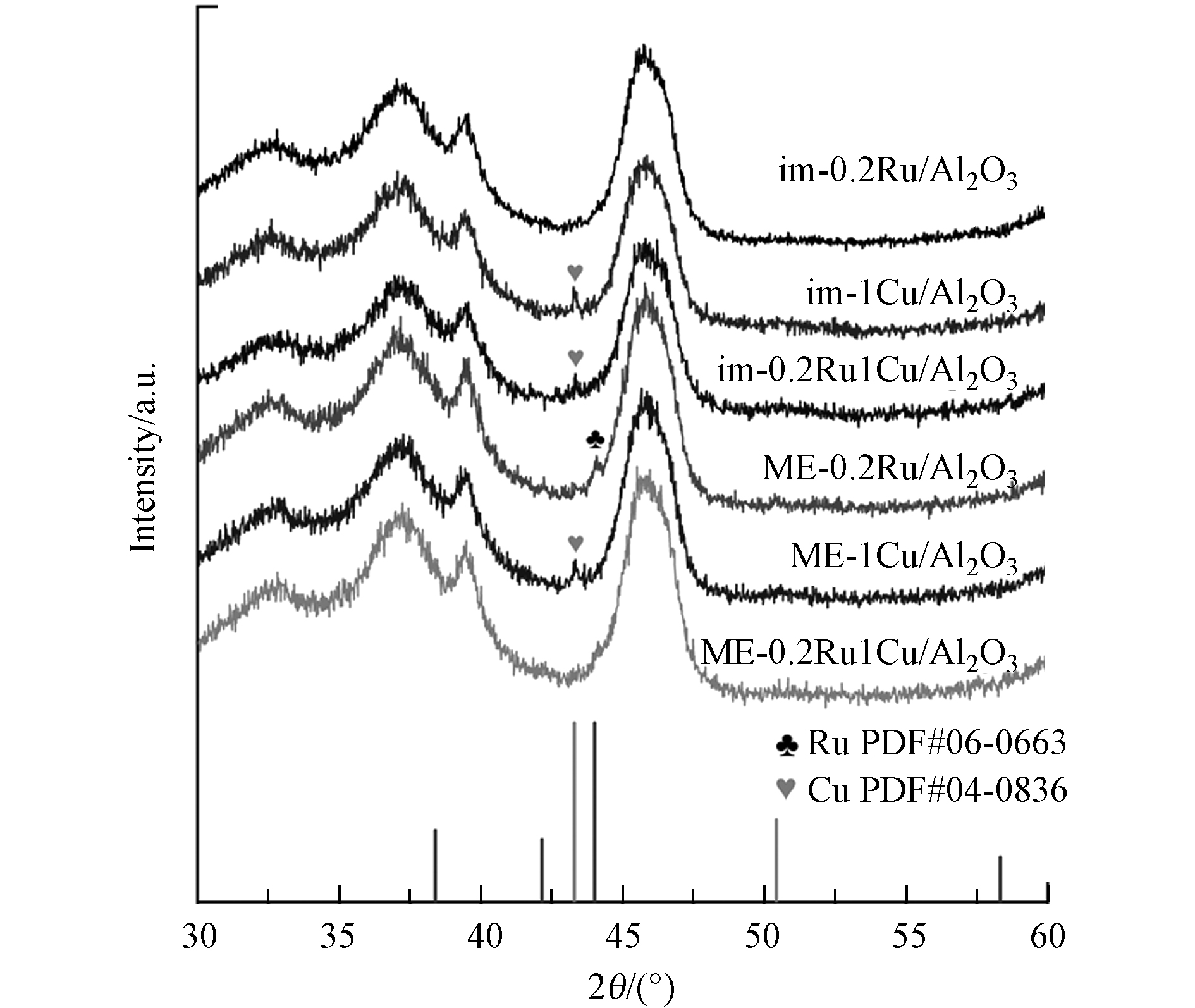
对采用浸渍法和微乳液法制备的催化剂通过XRD分析晶体结构,结果见图1. im-0.2Ru/Al2O3没有明显Ru特征峰,说明低含量的Ru通过浸渍法实现在Al2O3载体上的高度分散[17]. im-1Cu/Al2O3和im-0.2Ru1Cu/Al2O3中都观察到明显的Cu特征峰,存在单独的Cu纳米粒子,表明浸渍法制备的双金属催化剂中Ru与Cu的混合不均匀. ME-0.2Ru/Al2O3和ME-1Cu/Al2O3可以分别观察到Ru和Cu的特征峰,而ME-0.2Ru1Cu/Al2O3中没有明显的Ru和Cu的特征峰,表明微乳液法使Ru和Cu紧密接触,几乎没有形成单独的Ru或Cu纳米粒子.
-
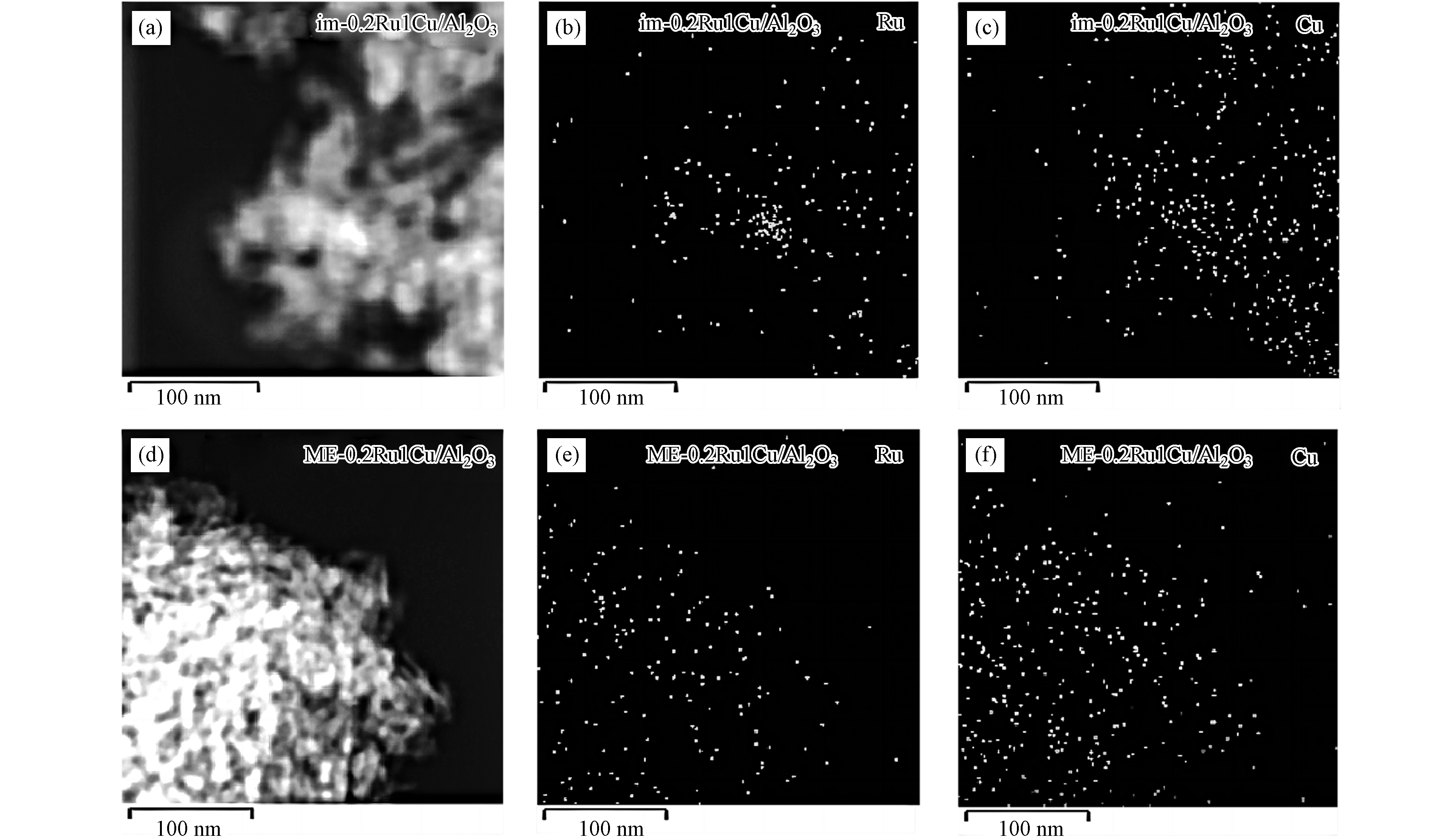
im-0.2Ru1Cu/Al2O3和ME-0.2Ru1Cu/Al2O3的HAADF-TEM图和EDS元素Mapping图如图2所示. 从Mapping图可见,采用浸渍法制备的im-0.2Ru1Cu/Al2O3催化剂中Ru元素的分布存在聚集情况,并且在同一位置与Cu的元素的信号丰度无相关性,而采用微乳液法制备的ME-0.2Ru1Cu/Al2O3催化剂中Ru与Cu元素分布都比较均匀. 以上结果表明,im-0.2Ru1Cu/Al2O3催化剂中存在团聚的单金属Ru,而ME-0.2Ru1Cu/Al2O3催化剂中Ru和Cu为均匀分布.
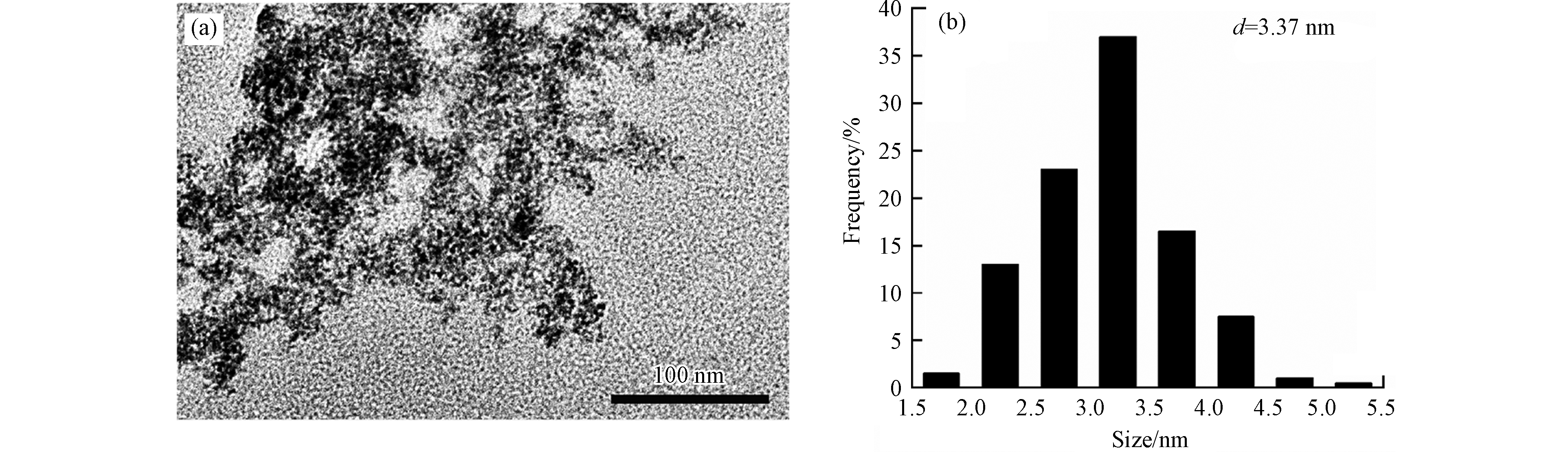
由于Al2O3载体尺寸很小且团聚现象严重,TEM难以观察其负载的金属粒子及其粒径,因而采用微乳液法制备了钌铜纳米粒子(制备过程中不加Al2O3载体并省略焙烧步骤),TEM图及粒径分布图见图3. 可以看出,钌铜纳米粒子的尺寸很小,平均粒径为3.37 nm,粒径分布较窄,表明尺寸比较均匀.
-
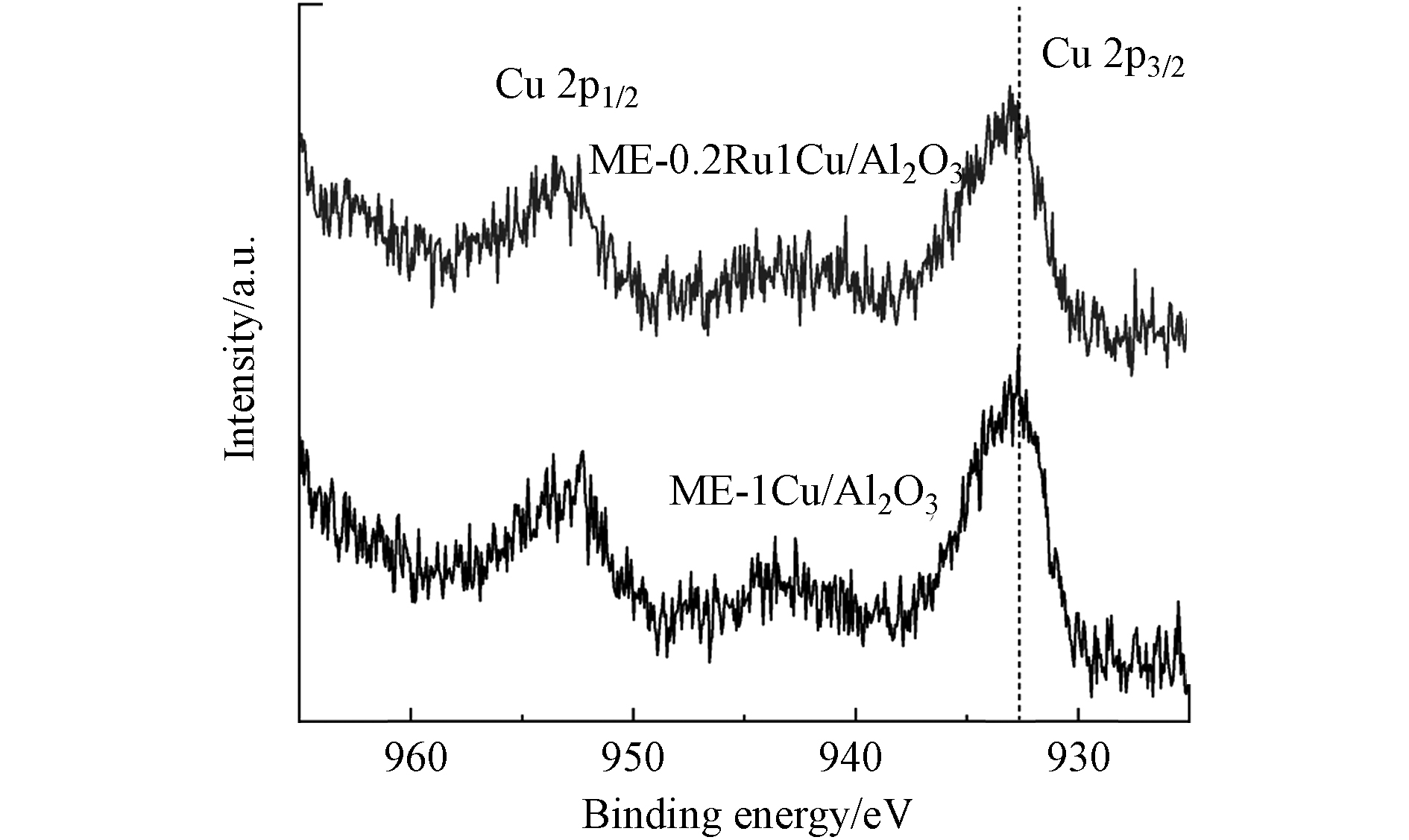
ME-1Cu/Al2O3和ME-0.2Ru1Cu/Al2O3在Cu 2p轨道的XPS图见图4. 采用微乳液法将Ru掺杂入Cu中,使Cu 2p3/2的结合能从932.7 eV向高场偏移至933.1 eV,说明存在Cu向Ru的电子传递,表明Cu与Ru存在强相互作用[18]. 而Ru 3p区域没有明显信号,可能由于Ru负载量很低,未达到检出限.
-
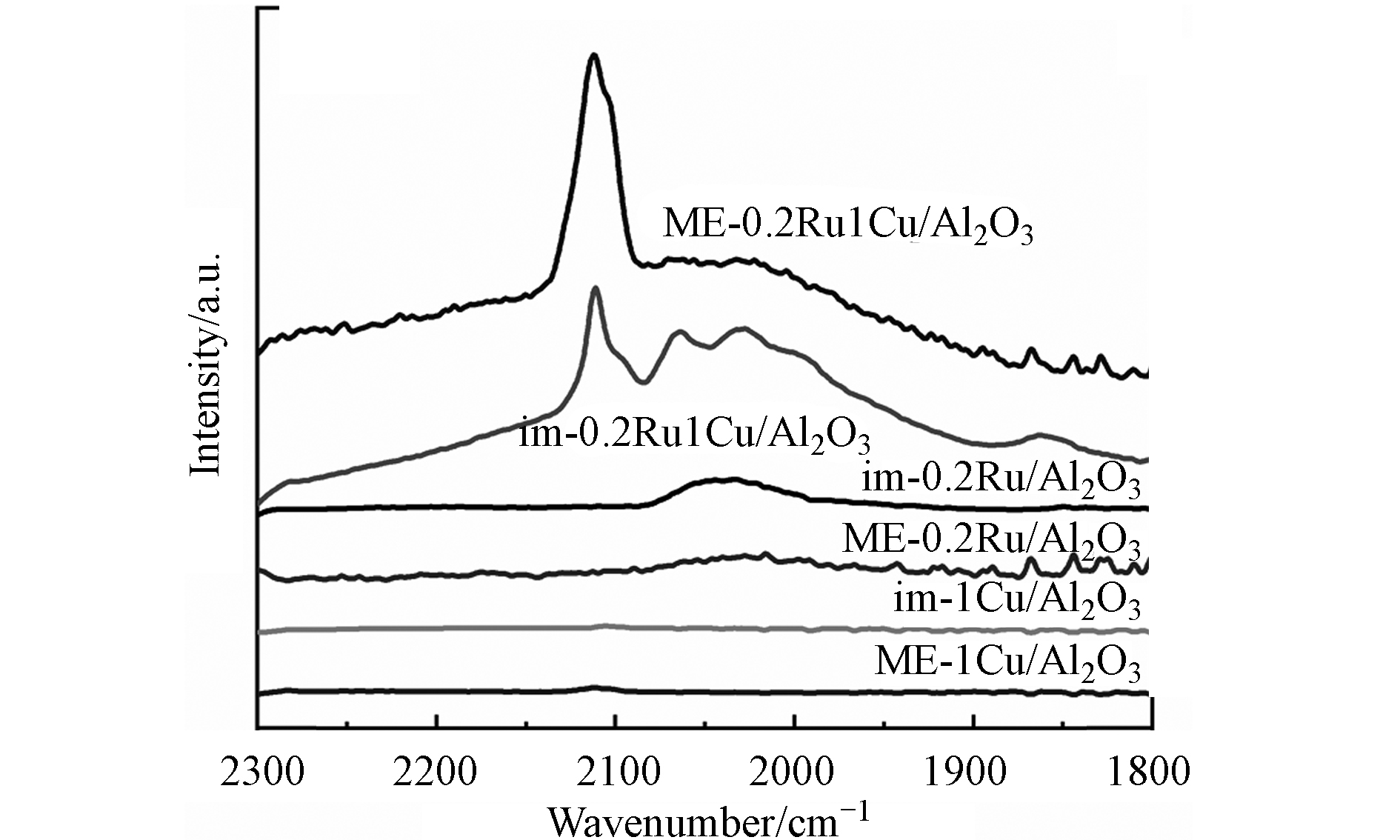
对不同方法制备的单金属和双金属催化剂进行CO吸附原位红外光谱分析,结果见图5. 位于2130 cm−1处吸收峰对应于吸附在低配位Ru原子上的Ru多羰基物种的对称振动. 2070 cm−1处峰为Ru多羰基物种的不对称拉伸振动,或单层Ru位点上线式吸附的CO. 2000—2050 cm−1宽峰为吸附在Ru纳米粒子顶部的CO. 低于2000 cm−1的峰为桥式吸附的CO[19 − 20]. 可以看出,im-0.2Ru/Al2O3与ME-0.2Ru/Al2O3只有2000—2050 cm−1之间的宽峰,说明Ru以纳米粒子形式存在. im-1Cu/Al2O3和ME-1Cu/Al2O3没有明显的吸收峰,说明Cu对CO几乎不吸附或吸附能力很弱. im-0.2Ru1Cu/Al2O3分别在2112、2064、2028、1992 cm−1处有吸收峰,说明既存在Ru纳米粒子,也存在高分散的Ru. ME-0.2Ru1Cu/Al2O3在2112 cm−1处有强峰,而其他3处的峰不明显,说明Ru的分散性较好.
-
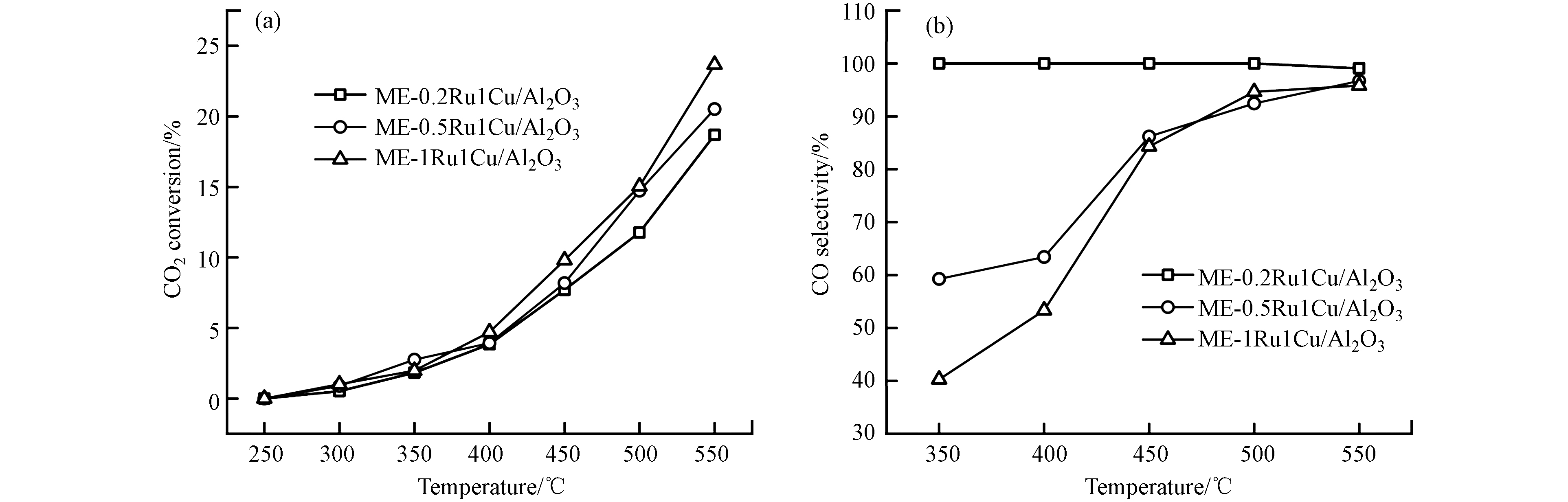
采用不同合成方法制备的单金属及双金属催化剂对CO2加氢活性和选择性的影响见图6,产物为CH4、CO和水,均未检测到甲醇. ME-0.2Ru/Al2O3在550 ℃时CO2的转化率为55.0%,对CO选择性仅为53.4%. 而ME-1Cu/Al2O3在550℃对CO2的转化率为8.5%,选择性在反应温度范围内均为100%. 可以看出,单金属Ru的催化活性高于Cu,但Ru对CO选择性较低. 在ME-1Cu/Al2O3中掺杂适量(0.2%wt)的Ru可以提高催化剂的反应活性(550 ℃时从8.5%到18.7%),而几乎不会降低CO选择性(550 ℃时100%到99.1%),表现出Ru-Cu双金属协同作用. 550 ℃时,ME-0.2Ru1Cu/Al2O3对CO的选择性为99.1%,明显高于im-0.2Ru1Cu/Al2O3(88.0%),这是由于微乳液法制备的钌铜纳米粒子成分更均匀,高分散的Ru活性位可将CO2选择性加氢为CO. 同时Ru可以活化H2并溢流活性H到Cu表面,提高催化活性. 浸渍法制备的双金属催化剂由于存在单金属Ru纳米颗粒,导致CO2过度加氢为CH4.
-
通过改变微乳液法制备过程中Ru和Cu前驱体的比例,制备了不同钌铜比的催化剂,其对催化性能的影响见图7. 随着钌铜比从1:5增加到1:1,550 ℃时催化剂活性从18.7%提高到23.7%,但CO选择性从99.1%下降到95.8%, 温度低于450 ℃时,选择性差异更显著. 随着钌铜比增加,Cu对Ru的稀释作用减弱,双金属颗粒中出现了集合态Ru,集合态Ru具有强加氢活性,导致催化活性升高而选择性降低. 当钌铜比为1:5时,在CO选择性接近100%的基础上达到了较高的反应活性,为最佳钌铜比.
-
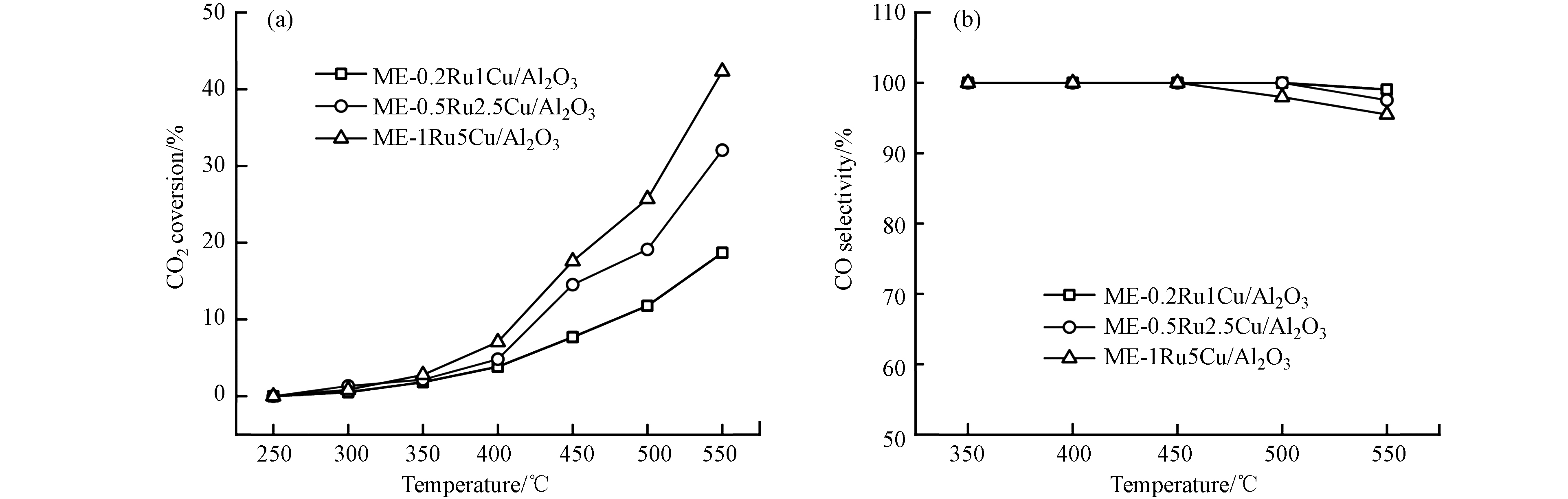
固定钌铜比不变,考察不同金属负载量对催化性能的影响,结果如图8所示. 550 ℃时,将Ru和Cu总负载量从1.2%wt.提高到6%wt.,活性从18.7%大幅提高至42.3%,说明增加金属负载量可以有效提高催化剂的活性,因为增多了活性位点. 而且当负载量较高时,CO选择性没有显著下降,这表明采用微乳液法制备催化剂时,适当提高负载量不会使制备的钌铜纳米粒子显著团聚.
-
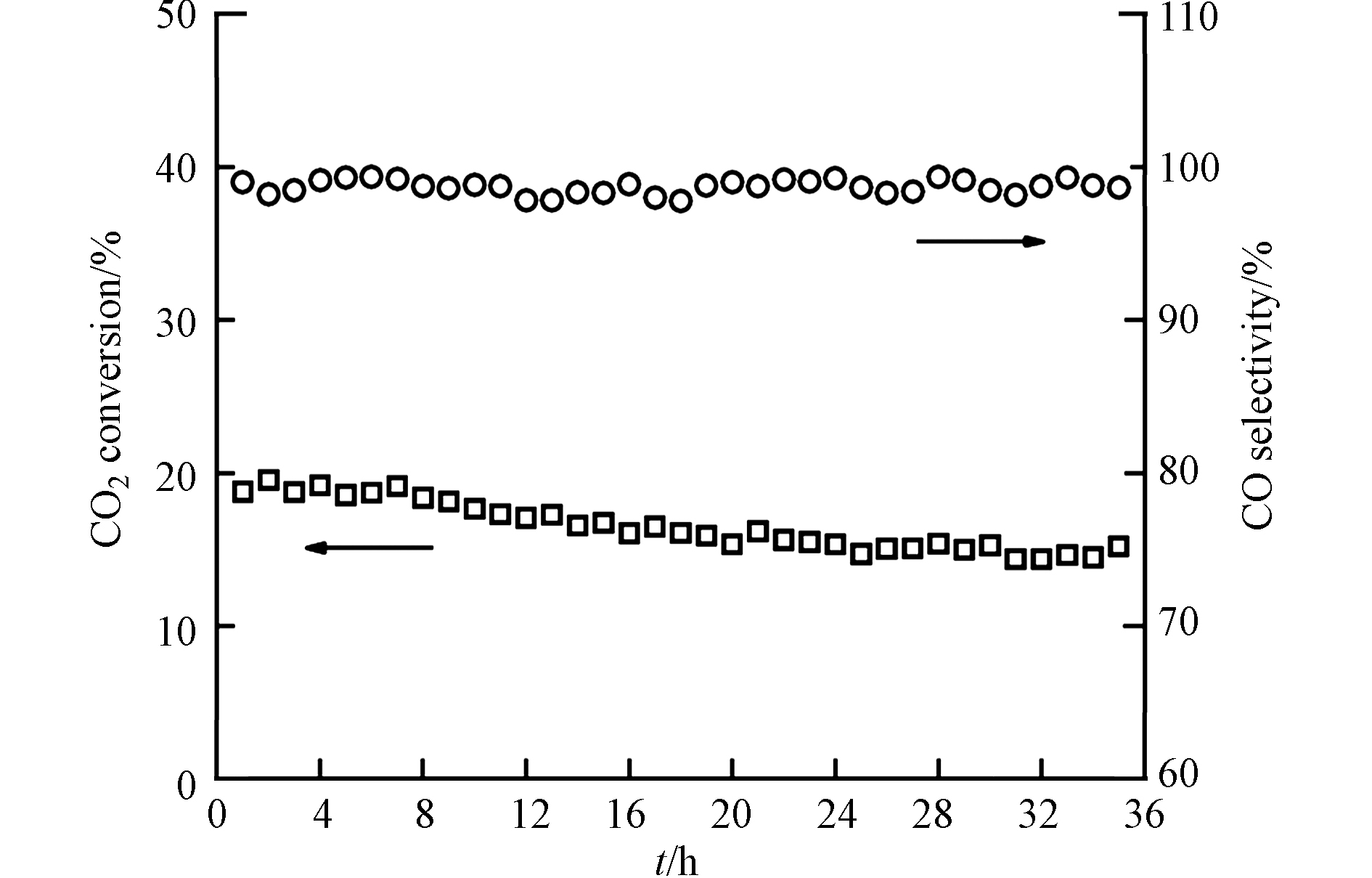
ME-0.2Ru1Cu/Al2O3催化剂在550 ℃、空速为64800 mL·h−1·g−1的条件下连续催化CO2加氢反应35 h,活性和选择性随时间的变化如图9所示. 可以看出,ME-0.2Ru1Cu/Al2O3 的稳定性良好,经过 35 h 长时间反应,CO2 转化率从 18.8% 下降至 15.2%, CO 选择性维持在 99% 左右.
-
(1)微乳液法制备的双金属催化剂中Ru和Cu紧密接触,且钌铜纳米粒子尺寸、成分均匀,对CO的选择性达到99%.
(2)浸渍法制备的双金属催化剂中存在单独的Ru和Cu纳米粒子,对CO的选择性不足90%.
(3)在Cu中掺杂适量的Ru可以提高催化剂的反应活性,而几乎不会降低CO选择性,表现出双金属协同作用.
RuCu/Al2O3对CO2选择性催化加氢
Selective catalytic hydrogenation of CO2 on bimetallic RuCu/Al2O3
-
摘要: 将温室气体CO2通过催化加氢的方式生产合成气具有重要的环境意义和经济价值. 本文分别采用微乳液法和浸渍法制备了Ru-Cu双金属催化剂. 通过不同分析手段对催化剂结构进行了表征,并考察了催化剂对CO2催化加氢的性能. 表征结果表明,在微乳液法合成的催化剂(ME-0.2Ru1Cu/Al2O3)中Ru和Cu紧密接触,且钌铜纳米粒子尺寸均匀,而浸渍法合成的催化剂(im-0.2Ru1Cu/Al2O3)存在单独的Ru和Cu纳米粒子. 催化实验结果说明,Ru对Cu的掺杂可提高其催化活性但会降低CO选择性. 采用微乳液法合成的双金属催化剂表现出比浸渍法更高的CO选择性. 此外,固定钌铜比不变,提高负载量可以有效提高活性,而CO选择性无明显下降.Abstract: Selective catalytic hydrogenation of greenhouse gas CO2 to synthesis gas has important environmental significance and economic value. In this paper, bimetallic Ru-Cu catalysts were prepared by the microemulsion and impregnation methods, respectively. The catalysts were characterized and their catalytic performances for CO2 hydrogenation were evaluated. The characterization results showed that in the catalyst prepared by the microemulsion method (ME-0.2Ru1Cu/Al2O3), Ru and Cu were in close contact in the Ru-Cu nanoparticles with uniform size, while separate Ru and Cu nanoparticles were identified in the catalyst prepared by the impregnation method (im-0.2Ru1Cu/Al2O3). The results of catalytic CO2 hydrogenation showed that doping Ru in Cu resulted in enhanced catalytic activity but reduced CO selectivity. The bimetallic catalysts prepared by the microemulsion method showed higher CO selectivity than those by the impregnation method. For the catalysts with identical Ru/Cu ratio, their activities increased with metal loading amount, while the CO selectivity did not decrease significantly.
-
Key words:
- CO2 /
- catalytic hydrogenation /
- bimetal /
- microemulsion method.
-

-
图 2 im-0.2Ru1Cu/Al2O3的HADDF-TEM图(a)及Ru(b)、Cu(c)元素Mapping图和ME-0.2Ru1Cu/Al2O3的HADDF-TEM图(d)及Ru(e)、Cu(f)元素Mapping图
Figure 2. HAADF-TEM images of (a) im-0.2Ru1Cu/Al2O3 and the corresponding EDS elemental mapping of Ru (b), Cu (c), and HAADF-TEM image (d) of ME-0.2Ru1Cu/Al2O3 and the corresponding EDS elemental mapping of Ru (e), Cu (f)
-
[1] COX P M, BETTS R A, JONES C D, et al. Erratum: Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model[J]. Nature, 2000, 408(6813): 750. [2] WANG W, WANG S P, MA X B, et al. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chemical Society Reviews, 2011, 40(7): 3703-3727. doi: 10.1039/c1cs15008a [3] RA E C, KIM K Y, KIM E H, et al. Recycling carbon dioxide through catalytic hydrogenation: Recent key developments and perspectives[J]. ACS Catalysis, 2020, 10(19): 11318-11345. doi: 10.1021/acscatal.0c02930 [4] YE R P, DING J, GONG W B, et al. CO2 hydrogenation to high-value products via heterogeneous catalysis[J]. Nature Communications, 2019, 10: 5698. doi: 10.1038/s41467-019-13638-9 [5] YIN G H, YUAN X T, DU D X, et al. Efficient reduction of CO2 to CO using cobalt-cobalt oxide core-shell catalysts[J]. Chemistry - A European Journal, 2018, 24(9): 2157-2163. doi: 10.1002/chem.201704596 [6] WANG L X, WANG L, XIAO F S. Tuning product selectivity in CO2 hydrogenation over metal-based catalysts[J]. Chemical Science, 2021, 12(44): 14660-14673. doi: 10.1039/D1SC03109K [7] YOUNAS M, LOONG KONG L, BASHIR M J K, et al. Recent advancements, fundamental challenges, and opportunities in catalytic methanation of CO2[J]. Energy & Fuels, 2016, 30(11): 8815-8831. [8] KRYLOVA A Y. Products of the Fischer-Tropsch synthesis (A review)[J]. Solid Fuel Chemistry, 2014, 48(1): 22-35. doi: 10.3103/S0361521914010030 [9] JALAMA K. Carbon dioxide hydrogenation over nickel-, ruthenium-, and copper-based catalysts: Review of kinetics and mechanism[J]. Catalysis Reviews, 2017, 59(2): 95-164. doi: 10.1080/01614940.2017.1316172 [10] XU X L, LIU L, TONG Y, et al. Facile Cr3+-doping strategy dramatically promoting Ru/CeO2 for low-temperature CO2 methanation: Unraveling the roles of surface oxygen vacancies and hydroxyl groups[J]. ACS Catalysis, 2021, 11: 5762-5775. doi: 10.1021/acscatal.0c05468 [11] AITBEKOVA A, WU L H, WRASMAN C J, et al. Low-temperature restructuring of CeO2-supported Ru nanoparticles determines selectivity in CO2 catalytic reduction[J]. Journal of the American Chemical Society, 2018, 140(42): 13736-13745. doi: 10.1021/jacs.8b07615 [12] KURNATOWSKA M, MISTA W, MAZUR P, et al. Nanocrystalline Ce1− x Ru x O2 - Microstructure, stability and activity in CO and soot oxidation[J]. Applied Catalysis B: Environmental, 2014, 148/149: 123-135. doi: 10.1016/j.apcatb.2013.10.047 [13] ZHUANG Y C, CURRIE R, MCAULEY K B, et al. Highly-selective CO2 conversion via reverse water gas shift reaction over the 0.5wt% Ru-promoted Cu/ZnO/Al2O3 catalyst[J]. Applied Catalysis A: General, 2019, 575: 74-86. doi: 10.1016/j.apcata.2019.02.016 [14] BOUTONNET M, SANCHEZ-DOMINGUEZ M. Microemulsion droplets to catalytically active nanoparticles. How the application of colloidal tools in catalysis aims to well designed and efficient catalysts[J]. Catalysis Today, 2017, 285: 89-103. doi: 10.1016/j.cattod.2016.12.047 [15] LALIK E, DRELINKIEWICZ A, KOSYDAR R, et al. A role of Au-content in performance of Pd-Au/SiO2 and Pd-Au/Al2O3 catalyst in the hydrogen and oxygen recombination reaction. The microcalorimetric and DFT studies[J]. Applied Catalysis A: General, 2016, 517: 196-210. doi: 10.1016/j.apcata.2016.03.004 [16] TOJO C, BUCETA D, LÓPEZ-QUINTELA M A. Bimetallic nanoparticles synthesized in microemulsions: A computer simulation study on relationship between kinetics and metal segregation[J]. Journal of Colloid and Interface Science, 2018, 510: 152-161. doi: 10.1016/j.jcis.2017.09.057 [17] SUN J Y, HAN Y X, FU H Y, et al. Selective hydrodechlorination of 1, 2-dichloroethane catalyzed by trace Pd decorated Ag/Al2O3 catalysts prepared by galvanic replacement[J]. Applied Surface Science, 2018, 428: 703-709. doi: 10.1016/j.apsusc.2017.09.168 [18] BAN C, YANG S, KIM H, et al. Effect of Cu addition to carbon-supported Ru catalysts on hydrogenation of alginic acid into sugar alcohols[J]. Applied Catalysis A: General, 2019, 578: 98-104. doi: 10.1016/j.apcata.2019.04.003 [19] CHEN D S, ABDEL-MAGEED D A M, DYBALLA D M, et al. Raising the CO x methanation activity of a Ru/ γ-Al2O3 catalyst by activated modification of metal-support interactions[J]. Angewandte Chemie International Edition, 2020, 59(50): 22763-22770. doi: 10.1002/anie.202007228 [20] YAN Y, WANG Q J, JIANG C Y, et al. Ru/Al2O3 catalyzed CO2 hydrogenation: Oxygen-exchange on metal-support interfaces[J]. Journal of Catalysis, 2018, 367: 194-205. doi: 10.1016/j.jcat.2018.08.026 -



 下载:
下载: