-
合成染料因其赋予颜色的特性,在皮革、纸张和纺织工业等各种重要工业中是必需存在的[1]. 合成染料具有复杂的组成、易于合成、稳定的化学结构和难以分解的特性[2],纺织工业(54%)排放的染料废水量最高,占世界环境中现有染料废水的一半以上[3]. 众所周知,染色工业(21%)、造纸和纸浆工业(10%)、制革和涂料工业(8%)以及染料制造业(7%)也会从各种相关工艺中产生大量染料废水[4]. 染料废水作为最难分解的废水之一,具有高色度、高生化需氧量和高溶解固体含量等特性[5]. 同时,大多数纺织染料具有生物毒性、致癌性和致畸性[6]. 传统的污水处理方法,如混凝-絮凝法[7]、离子交换法[8]、吸附-吸收法[9]、膜过滤法[10]、电絮凝法[11]等,但不能高效地处理染料废水. 为此,科研人员研发了产生·OH或SO4−·等强氧化性自由基的高级氧化技术(AOPs),可有效降解染料等难降解有机污染物[12]. AOPs中常用的芬顿氧化技术,常用于难降解废水的处理,但实际染料废水pH在6—7左右,需加入大了药剂调节适宜pH 3左右,处理成本较高[13];光催化氧化技术则存在可利用光谱范围较窄,处理时间长、效率低、催化剂易失活等缺点,针对大量染料废水处理耗时又耗力[14 − 15]. 催化臭氧氧化技术具有效率高、无二次污染,容易实现自动化和工业化处理等优点. 然而传统的催化臭氧氧化技术一般为均相氧化技术,直接将催化剂溶于溶液中,导致催化剂无法回收,处理成本偏高[16]. 因此,学者们研发了非均相催化臭氧化,催化剂常为固态金属、金属氧化物、氢氧化物等[17],或将催化剂负载于活性炭、硅藻土、分子筛等载体上,催化剂可实现回收和重复利用,处理成本大大降低.
陶瓷膜(CM)因具有耐高温高压、耐酸碱、机械强度高、使用寿命长(大于5年)、不易堵塞、运行稳定性好以及价格低廉、通量大、易于反冲洗和检修等诸多优点[18 − 19],可作为高级氧化催化剂载体,研制出反应性陶瓷膜,组成“更加有效、更加高效、更加小型化”AOPs-CM耦合水处理技术,主要有光催化氧化陶瓷膜技术[20]、芬顿氧化陶瓷膜技术[21]、催化臭氧氧化陶瓷膜耦合技术[22]等. 与其他方法相比,催化臭氧氧化耦合陶瓷膜具备效率高、无污染,易于实现自动化和工业化,陶瓷膜抗污性能等优势[23].
本研究采用新型喷涂成膜技术,将TiO2/MnO2催化剂喷涂在陶瓷膜表面,负载催化剂的反应性陶瓷膜应用在自制的反应器中,催化臭氧氧化处理以罗丹明B(RhB)溶液模拟的染料废水,探究处理过程作用机理,本研究可为染料废水的高效低耗处理提供新思路.
-
实验中所用的罗丹明B(C28H31ClN2O3)、纳米TiO2(P25)、无水乙醇(C2H5OH)、硫酸(H2SO4)、三氯化钛(TiCl3)、高锰酸钾(KMnO4)、靛蓝二磺酸钠(C16H8N2Na2O8S2)、磷酸二氢钠(NaH2PO4)、对苯二甲酸(C8H6O4)、磷酸(H3PO4)、二水磷酸钠(Na3PO4)、碘化钾(KI)、硝酸钙(Ca(NO3)2)、硫酸铝(Al2(SO4 )、硫酸镁(MgSO4)、硝酸铜(Cu(NO3)2)均为分析纯.
材料制备与表征测试设备主要有均相反应器(烟台科立化工设备有限公司)、全自动喷涂成膜设备(Film-1,中国天津嘉银纳米科技有限公司)、XRD-6000型X射线衍射仪(日本岛津)、Zeiss Sigma 300型扫描电子显微镜(德国蔡司)、TU-1901型双束紫外可见分光光度计(北京普析通用仪器有限公司)、Lumina荧光光谱仪(赛默飞世尔科技公司).
-
准确称取0.0160 g的高锰酸钾置于干净烧杯中,并移取2 mL的三氯化钛溶液一并加入到烧杯中,移取10 mL无水乙醇和40 mL去离子水加于上述溶液中,盖上保鲜膜超声15 min,转入聚四氟乙烯高压反应釜中,放入均相反应器中升温至180 ℃反应18 h,反应完成后取出冷却至室温后进行过滤,干燥后得到白色粉末样品,即为TiO2/MnO2催化剂[24].
-
实验中所用到的陶瓷膜是购自中国华怀新材料公司的矩形平板式陶瓷膜(PCFM-A-100-3-M),其主要材质为α-Al2O3. 首先通过水槽切割机将陶瓷膜切割成尺寸大小为长×宽×厚度=70 mm×150 mm×4 mm的矩形陶瓷膜,然后将切割后的陶瓷膜浸泡在丙酮:无水乙醇:去离子水(1:1:1)的混合溶液中超声1 h,随后用去离子水冲洗陶瓷膜,于105 ℃烘箱中烘干备用. 将制备好的TiO2/MnO2催化剂以浓度为0.5 g·L−1分散在无水乙醇中,超声1 h,得到TiO2/MnO2悬浮液. 利用全自动喷涂成膜设备(Film-1,中国天津嘉银纳米科技有限公司),将TiO2/MnO2悬浮液喷涂至陶瓷膜表面(分别喷涂1、3、6、9、12、15层). 在空气中干燥后,将喷涂后的陶瓷膜在马弗炉中以程序升温为1 ℃·min−1升温至100 ℃下煅烧2 h,有利于将TiO2/MnO2催化剂固化. 最后,通过环氧树脂将改性后的陶瓷膜与塑料组件粘合,得到催化臭氧氧化陶瓷膜组件.制备TiO2/MnO2负载陶瓷膜如图1所示.
-
将臭氧发生器、臭氧检测器、三口烧瓶和碘化钾溶液桶(处理臭氧尾气)用配套管道连接,检查管道是否堵塞和漏气. 考察不同催化剂种类(无催化剂,α-MnO2、纳米TiO2(P25)和TiO2/MnO2)、TiO2/MnO2催化剂浓度(0.1、0.2、0.3、0.4 g·L−1)、臭氧浓度(0、0.8、1.8、2.2 g·m−3)和初始pH(4.68、8.00、11.50)下,催化剂催化臭氧氧化降解RhB性能. 首先,将一定浓度催化剂投加在300 mL 20 mg·L−1的RhB废水中,待臭氧浓度稳定30 min后即可通入一定初始浓度的臭氧,间隔一定时间进行取样,总反应时长为40 min. 采用紫外分光光度计测量所采样品吸光度,RhB在λ=554 nm处具有最大吸收峰.
采用一级反应动力学方程式如式(1)所示进行拟合,即可得反应速率常数k值[25]:
式中,k为动力学速率常数,min−1;t为反应时间,min,C0和Ct分别为初始和处理时间为t时刻RhB浓度,mg·L−1;t时刻的浓度与初始浓度之比Ct/C0被定义为降解效率.
-
将TiO2/MnO2催化剂负载于陶瓷微滤膜(α-Al2O3),重点研究TiO2/MnO2催化剂浓度、喷涂层数等关键材料制备因素对微观陶瓷膜的孔径大小及分布均匀性的影响;在宏观上对负载型陶瓷膜对O3催化氧化效率、截留分子量和纯水通量的影响;研究建立负载型催化陶瓷膜材料制备和性能调控方法.
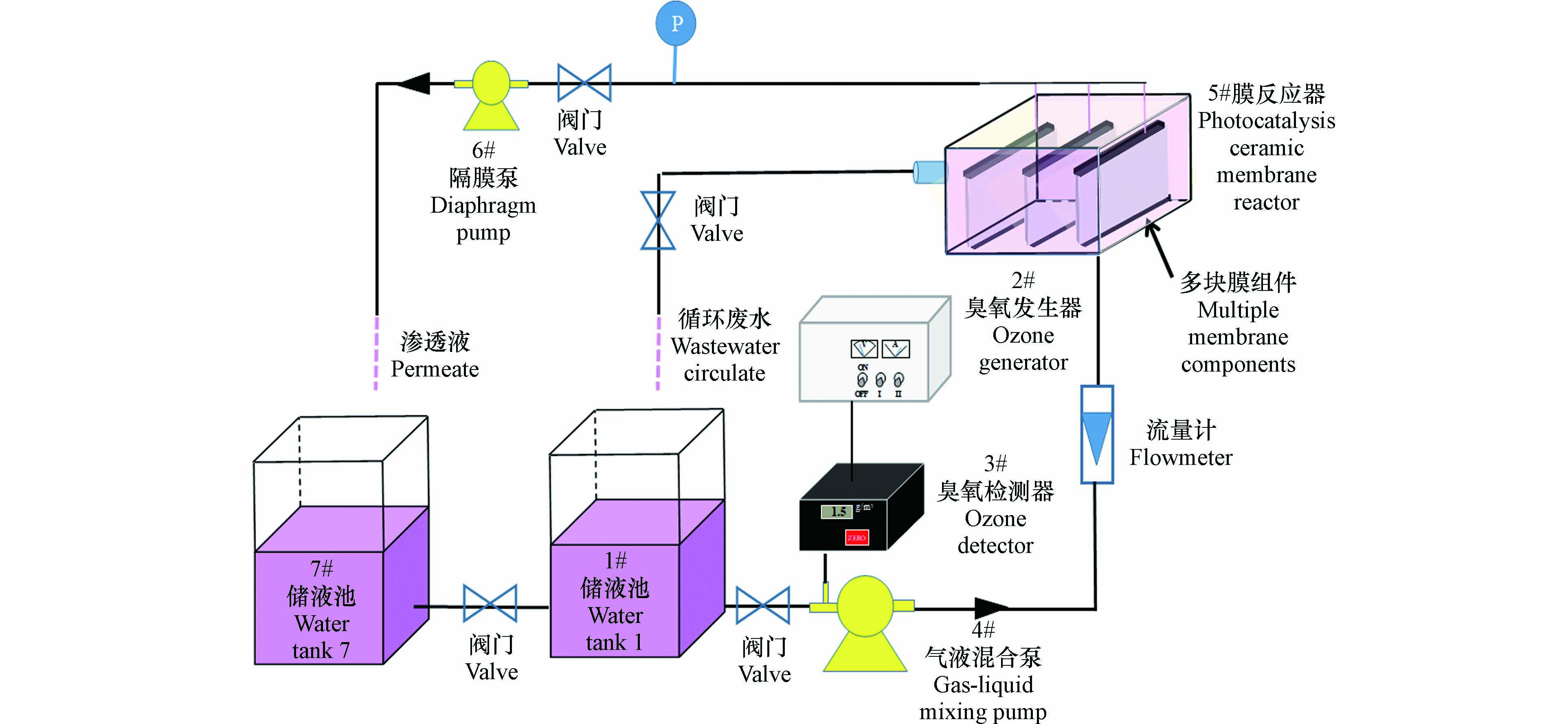
催化臭氧氧化耦合陶瓷膜实验装置如图2所示,反应器主要由臭氧发生器、臭氧检测器、气液混合泵、膜反应器、负压泵及储液池组成. 其中膜反应器是将制备的负载催化剂膜组件与混合气液在密闭条件下完成水处理的重要组成部分. 首先通入一定浓度臭氧,待浓度稳定后,在储液池1#中加入2 L的20 mg·L−1的RhB溶液,通过气液混合泵将臭氧与RhB溶液抽至膜反应器中,待循环稳定后取5 mL水样,记为C0. 然后利用负压泵对单端从膜表面将水样抽滤形成跨膜压力,记为跨膜压差. 然后分别间隔5 min取样且同时用电子天平称得质量记下时间,样品测量吸光度并记录,采用一级反应动力学方程式如式(1)所示进行拟合,即可得反应速率常数k值. 负载后的陶瓷膜渗透通量(J, kg·(m2·h)−1)表示为式(2)[26]:
其中,m为渗透质量(kg),A为陶瓷膜有效面积(m2),t为时间(h).
-
利用靛蓝二磺酸钠分光光度法和荧光分光光度计分别测定液相中的臭氧浓度和·OH浓度[27]. 臭氧与靛蓝二磺酸钠反应物质的量比为1:1的关系,因此在612 nm下测得靛蓝二磺酸钠吸光度,再通过朗伯比尔定律计算臭氧的浓度. 配制pH=2的磷酸盐缓冲液:称取32.7259 g二水磷酸钠和19 mL磷酸溶于水中,定容至1 L. 于50 mL的比色管中加入2 mL靛蓝二磺酸钠溶液(C0=0.5 g·L−1)和20 mL的磷酸盐缓冲液(pH=2),利用移液管取待测臭氧溶解液25 mL,插入检测液中,缓慢加入,摇匀放于暗处,空白组为加入相同体积蒸馏水. 在612 nm下测得吸光度,记为Ai,液相中臭氧浓度计算公式如下[28]:
式中,A0,Ai分别为空白和待测样品的吸光度;b为比色皿光程长度(1 cm),VX为取样体积),M为臭氧的摩尔质量.
将对苯二甲酸溶解于10 mmol·L−1 NaOH溶液中,使得对苯二甲酸的初始浓度为4 mmol·L−1. 利用对苯二甲酸与·OH反应生成2-羟基对苯二甲酸,2-羟基对苯二甲酸通过荧光分析仪在激发波长315 nm和发射波长425 nm下测定不同反应时间的荧光吸光度,以此确定·OH浓度.
-
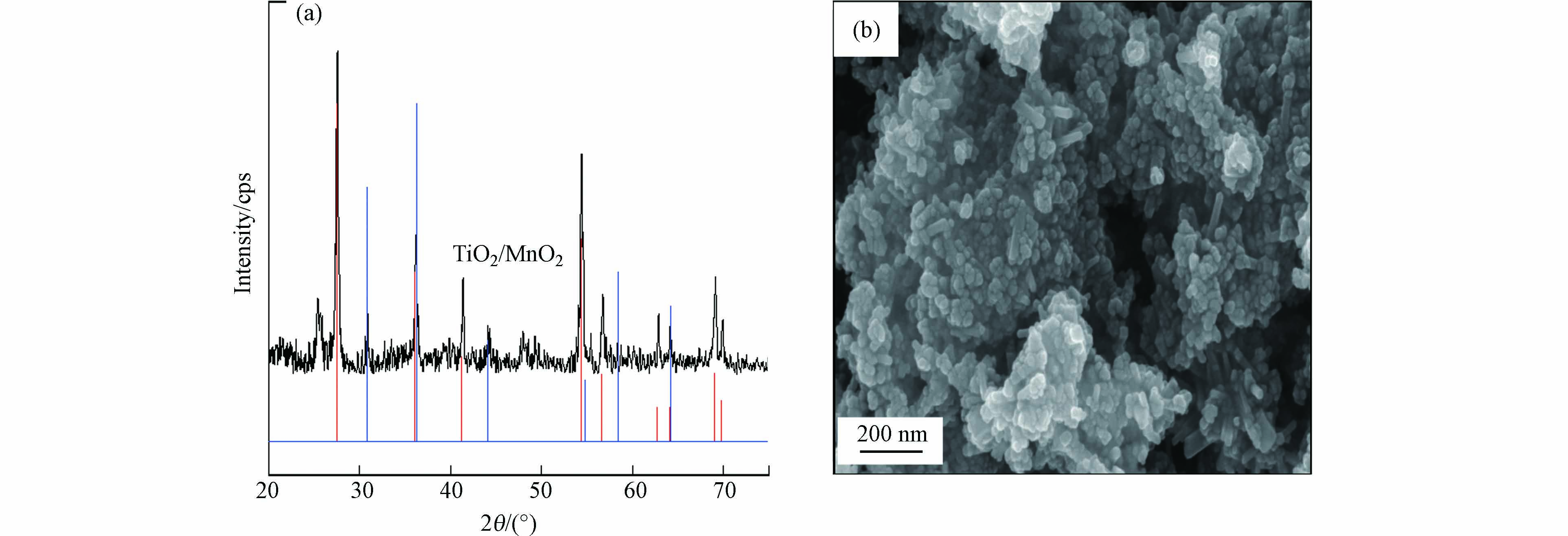
图3分别为TiO2/MnO2催化剂的XRD谱图及其对应的SEM图. 由XRD谱图结果可以看出,所制备的TiO2/MnO2催化剂中MnO2的晶型为γ型,TiO2为金红石相和锐钛矿相的混合相[24],主要特征衍射峰对应2θ位于27.3°、36.1°、41.3°、54.3°、56.7°、62.9°、69.1°和69.9°,也证明了催化剂纯度较高. 图2(b)为TiO2/MnO2催化剂的SEM图,通过水热反应得到的主要结构为棒状和不规整球状[29].
为了研究TiO2/MnO2催化剂的表面组成和化学状态,如图4所示,其显示了TiO2/MnO2催化剂上O1 s、Mn 2p和Ti 2p的主峰.
O 1s峰值位置约为529.08 eV对应于Ti—O键的晶格氧[30],氧空位的含量升高[31]. 在Ti 2p区,Ti 2p1/2和Ti 2p2/3的主峰位置分别出现在458.83 eV和464.50 eV[32]. 检测到的峰值出现在结合能643.6 eV可归属于Mn 2p3/2,表明制备样品中的元素锰以Mn4+的化学状态存在,键能为652.4 eV时的峰值对应于Mn 2p1/2,同样证明Mn4+的存在[33]. 通过使用以下公式从Mn 3s光谱估算MnO2催化剂的平均氧化状态(AOS):AOS=8.956-1.126ΔEs,其中ΔEs是双Mn 3s峰的结合能差,证明有Mn3+的存在[34]. Mn 3s对锰的氧化状态比Mn 2p更敏感,其原因可能是氧空位诱导的Mn 3s分裂能的增加[35].
-
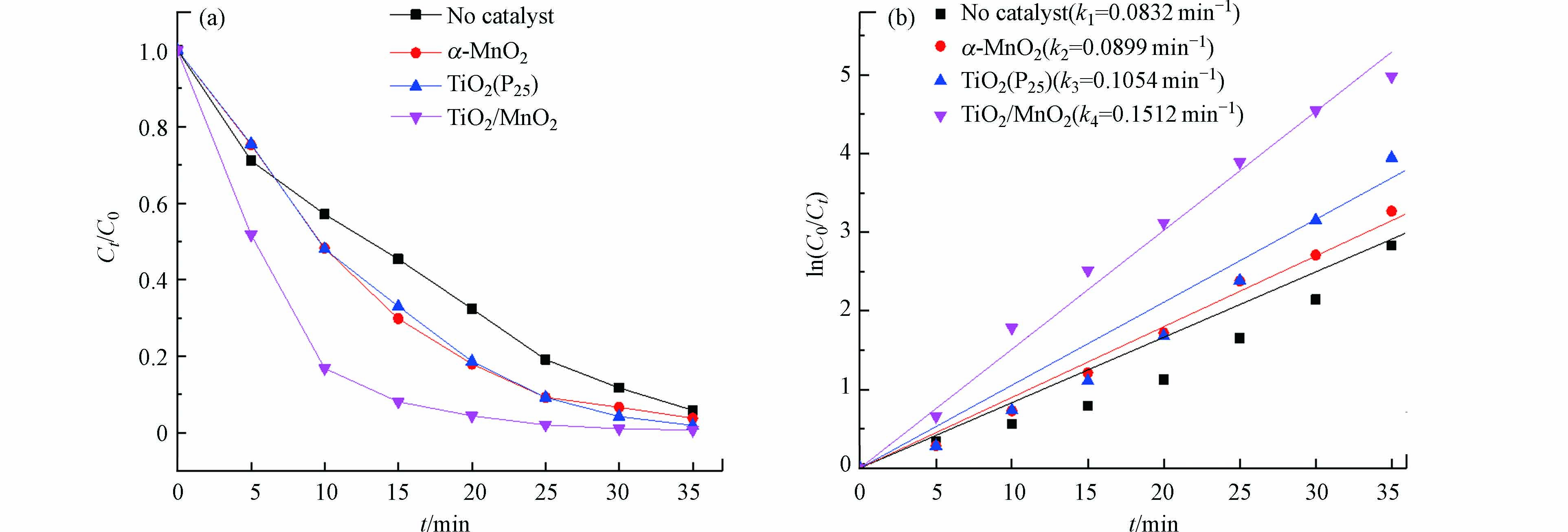
不同种类催化剂催化臭氧氧化处理RhB溶液降解速率及一级动力学结果如图5所示. 在不同种类催化剂催化下,处理RhB的反应呈现线性关系,为一级反应动力学的特征,也能清晰证明在臭氧浓度为2.5 g·m−3时仅需35 min便可将20 mg·L−1 RhB降解完全. 从5(b)图中,降解速率常数k均大于未加入催化剂反应速率常数,效果最好的TiO2/MnO2催化剂的k为0.1512 min−1,而无催化剂的降解速率常数为0.0832 min−1,降解速率常数是无催化剂的近2倍,在一定程度上减缓了臭氧的成本问题.
-
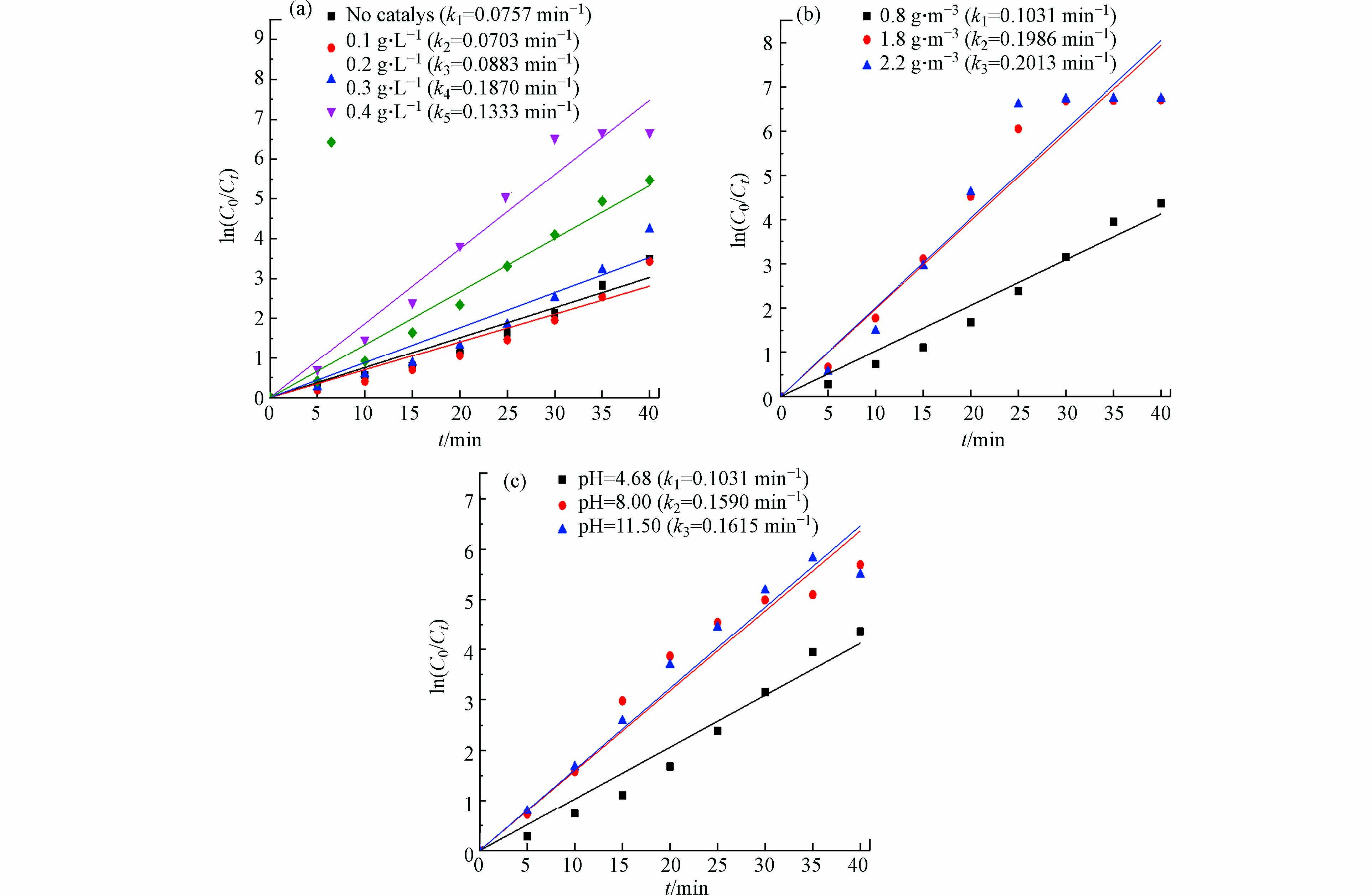
图6分别为TiO2/MnO2催化剂改变催化剂浓度、臭氧浓度和初始pH的降解速率图. 为了探究上述效果较好催化剂浓度对该实验降解速率的影响,加入不同含量的催化剂,从图6(a)中可得出在0.3 g·L−1浓度下处理效果极佳,反应速率常数分别在0.1870 min−1,高于无催化剂时的反应速率常数0.0757 min−1的. 加入催化剂能提高溶液的降解效率,是因为催化剂促进了反应过程中·OH的产生,使溶液降解速率加快. 随着浓度的增高呈现出了先增大再减少,存在的原因可能是一定浓度的MnO2能抑制TiO2催化热力学活性,发生了催化剂中毒现象,使得降解速率相应有所减低[36].
图6(b)为改变臭氧浓度处理RhB的速率常数图. 在该实验中,臭氧浓度绝对是影响RhB降解速率的重要因素[37],根据臭氧发生器的浓度范围设置了臭氧浓度为0.80 g·m−3、1.80 g·m−3、2.20 g·m−3的变量实验. 当浓度为2.20 g·m−3时处理效果显著提高,臭氧浓度在0.80 g·m−3和1.80 g·m−3条件下,气液传质效率受到限制,没有明显提高,但在臭氧浓度为2.20 g·m−3时,气液传质效率明显提高,臭氧在溶液中的溶解度上升,引发降解速率突变[17]. 但是存在经济的问题也会越发明显.
图6(c)为改变RhB初始pH对RhB降解效率图. 为了研究不同pH环境下的催化臭氧氧化对RhB降解结果的影响,可以通过滴加盐酸或氢氧化钠来调节溶液pH. TiO2/MnO2催化剂的反应速率分别在0.103 min−1、0.159 min−1和0.162 min−1,pH在弱碱性条件下,降解染料废水较为显著,在此条件下能明显促进自由基反应,以此提高RhB的降解效率[38].
-
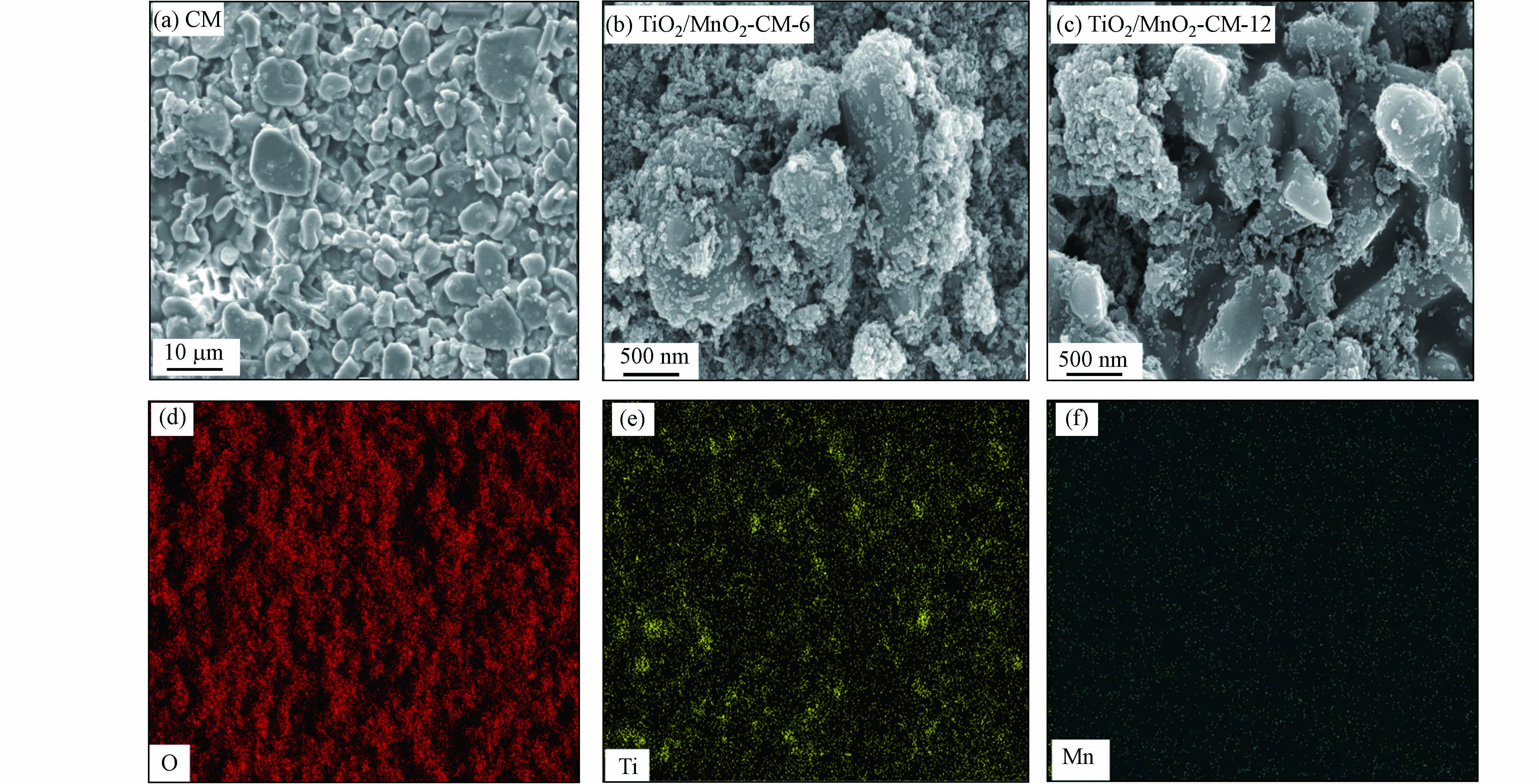
采用全自动喷涂成膜技术将TiO2/MnO2催化剂喷涂至陶瓷膜表面. 通过图7(a)清晰的显示TiO2/MnO2催化剂能喷涂至陶瓷膜表面,附着均匀、不易脱落,随着喷涂层数的增加表面催化剂颗粒增大,严密附和,从而提高了膜的抗污能力和催化效率,确保后续降解实验完整进行. 通过图7(b) EDS面扫,绿色为Mn,黄色为Ti,红色为O,结果表明TiO2/MnO2催化剂成功负载至陶瓷膜表面.
-
在陶瓷膜表面均匀喷涂TiO2/MnO2催化剂,分别喷涂不同层数含量的催化剂,研究不同层数对RhB的去除以及陶瓷膜的膜通量和抗污能力的影响,结果如图8所示. 由图8(a)和(b)可以看出,对比空白膜,随着喷涂层数的增加,其催化O3氧化降解RhB性能不断提高,喷涂12层为最优,当负载层数增至15层其性能反而降低. 对比空白陶瓷膜的纯水通量228.69 kg·m−2·h−1,喷涂12层的反应性陶瓷膜膜纯水膜通量为172.40 kg·m−2·h−1,仅下降24.61%. 图8(c)在喷涂不同层数下处理2 L初始浓度为20 mg·L−1的RhB,臭氧浓度为2.5 g·m−3,40 min的去除率均在85%以上,其中喷涂12层催化剂的陶瓷膜去除RhB效率在100%,空白陶瓷膜的反应速率常数在0.0499 min−1,12层的反应速率常数为0.104 min−1,是空白陶瓷膜反应速率的2倍,较其他不同层数的陶瓷膜也明显提升. 催化剂含量在30 mg·L−1,相比初始浓度为20 mg·L−1 300 mL的RhB用催化剂含量为300 mg·L−1臭氧浓度为0.8 g·m−3时效率有显著提高. 在压力为-0.030 kPa时,喷涂催化剂的陶瓷膜抗污能力明显高于未喷涂催化剂的陶瓷膜,延长了陶瓷膜的使用寿命.
采用喷涂12层催化剂的陶瓷膜,分别加入0.5 mol·L−1 SO42-、NO3-、Mg2+、Cu2+干扰离子及不同浓度腐殖酸,对模拟工业废水对RhB去除效果的影响进行研究,如图9(a)和(b)结果表明,当加入SO42-时去除率高于模拟废水,膜通量也会提高,SO42-的存在促进与水溶液反应生成·OH;反而加入重金属离子Cu2+较未加离子的废水通量下降了22.51%,其中Cu2+会与废水中的其他离子如SO42-发生反应抑制·OH的生成,因此减缓RhB的去除[39]. 以腐殖酸代表腐殖物质,在喷涂12层催化剂的陶瓷膜,臭氧浓度为2.5 g·m−3的条件下,分别加入不同浓度的腐殖酸溶液[40],图9(c)和(d)表明,加入不同浓度的腐殖酸对去除率影响不显著,并未对催化臭氧氧化造成淬灭作用. 但对膜的污染程度较为明显,随着浓度的升高,在陶瓷膜表面附着了黑色物质,废水通量显著降低,这种降低可能是由于陶瓷膜表面被污染.
-
图10为TiO2/MnO2催化臭氧氧化耦合陶瓷膜机理研究图,其反应机理图如图11所示. 通过从水中溶解臭氧浓度及·OH产生量两方面考察对RhB去除的效率,加入催化剂后,反应40 min水中溶解臭氧及·OH含量分别为0.7327 mg·L−1、68.16 μmol·L−1,去除效果得到了一定程度提升,也证明催化剂的加入促进了强氧化自由基·OH自由基的生成,提高了RhB的去除效率[41]. 该作用下产生的反应机理如式(4)—(8)[42]。
-
本研究通过水热法制备了TiO2/MnO2催化剂,将其通过喷涂法负载于陶瓷膜表面(TiO2/MnO2-CM),对模拟染料废水进行了降解处理研究,并对粉体催化剂和反应性陶瓷膜材料进行了XRD、SEM、XPS等表征,主要得出以下结论:
(1)成功制备TiO2/MnO2催化剂,从XRD结果表明峰值与标准比对卡重合,证明主要物质为TiO2/MnO2,从SEM中可以看出TiO2/MnO2催化剂为颗粒状和棒状物质,XPS分析表明锰以Mn3+和Mn4+形式存在. TiO2/MnO2催化臭氧氧化处理RhB的适宜条件为催化剂浓度为0.3 g·L−1,臭氧浓度0.8 g·m−3,pH调至弱碱性时,处理20 mg·L−1的RhB,反应35 min即可完全去除RhB.
(2)通过简单的喷涂-低温烧结,成功将TiO2/MnO2催化剂负载于陶瓷膜表面,与空白膜相比,TiO2/MnO2-CM催化O3性能显著提升,且随着喷涂层数的增加,其催化O3氧化降解RhB性能不断提高,喷涂12层为最优,当负载层数增至15层其性能反而降低. 对比空白陶瓷膜的纯水通量228.69 kg·m−2·h−1,喷涂12层的反应性陶瓷膜膜纯水膜通量为172.40 kg·m−2·h−1,仅下降24.61%.
(3)构建了TiO2/MnO2-CM水处理系统,其对RhB处理的适宜操作参数为:TiO2/MnO2喷涂12层,臭氧浓度在2.5 g·m−3,2 L的20 mg·L−1的RhB,反应40 min,去除率可达100%. 陶瓷膜表面负载适量的TiO2/MnO2能提高O3在水中溶解度,提升O3利用率并加速生成·OH,降低陶瓷膜污染.
喷涂法负载TiO2/MnO2陶瓷膜催化臭氧氧化降解染料废水
Efficient degradation of dye wastewater by catalytic ozone reactive ceramic membrane of TiO2/MnO2 by a facile spraying method
-
摘要: 染料废水存在排放量大、色度高、COD大、可生化性差、难降解等特点,其处理存在低效高耗的问题. 本研究首先通过水热法制备了TiO2/MnO2催化剂,对催化剂进行了XRD、XPS、SEM/EDS表征,以罗丹明B(RhB)溶液为模拟染料废水,进行了催化臭氧氧化降解RhB的性能对比研究. 再通过喷涂成膜技术将TiO2/MnO2催化剂负载于平板式陶瓷膜表面,研制成反应性陶瓷膜(TiO2/MnO2-CM),自制了配套膜反应器,研究了TiO2/MnO2-CM水处理系统对RhB降解去除效果和水通量变化规律. 结果表明,本工作成功合成了棒状和不规整球状结合的TiO2/MnO2催化剂,XPS结果表明存在Mn4+活性中心,促进了催化臭氧活化能力. 喷涂TiO2/MnO2催化剂后,陶瓷膜的纯水通量略有下降,12层为适宜的喷涂层数,采用TiO2/MnO2-CM对2 L初始浓度为20 mg·L−1的RhB在臭氧浓度为2.5 g·m−3条件下,反应40 min去除率可达100%,去除效率远高于空白膜,陶瓷膜负载TiO2/MnO2有助于提高O3溶解性、加速生成·OH. 本工作可为染料废水等难降解有机废水的高效低耗处理,提供新技术思路.
-
关键词:
- 染料废水 /
- TiO2/MnO2催化剂 /
- 催化臭氧氧化 /
- 反应性陶瓷膜 /
- 作用机理.
Abstract: Dye wastewater exhibits characteristics of large production, high color, high COD, low biodegradability, and refractory. In this work, TiO2/MnO2 hybrid catalyst was prepared by a hydrothermal method, and was characterized by XRD, XPS, and SEM / EDS. Its catalytic ozonation performance was evaluated to degrade a simulated dye wastewater of Rhodamine B (RhB). Afterwards, the TiO2/MnO2 catalyst was loaded onto a planar ceramic membrane (CM) by a facile spraying method to prepare a reactive ceramic membrane (TiO2/MnO2-CM). Besides, a new TiO2/MnO2-CM fixed bed water treatment system was proposed, and its degradation performnce of RhB and permeate flux was comprehensively investigated. The results showed that rod-shaped and irregular spherical combined TiO2/MnO2 catalysts were successfully synthesized, and the XPS results showed that the existence of Mn4+ provided active centers for the O3 reaction and promoted the ozone activation ability. In addition, the TiO2/MnO2-CM with twelve spraying layers revealed better degradation performance of the dye solution. As for 2 L of RhB with an initial concentration of 20 mg·L−1, under the condition of ozone concentration of 2.5 g·m−3, a reaction time of 40 min, the removal efficiency of RhB by TiO2/MnO2-CM could reach 100%. Besides, the up-mentioned removal rate is much higher than that of blank CM, which was mainly attributed to a increase of ozone solubility in water and promoting the production of ·OH. This work could provide an alternative solution for the treatment of refractory organic wastewater with lower energy comsuption but higher efficiency. -

-
-
[1] ABDI J, VOSSOUGHI M, MAHMOODI N M, et al. Synthesis of metal-organic framework hybrid nanocomposites based on GO and CNT with high adsorption capacity for dye removal[J]. Chemical Engineering Journal, 2017, 326: 1145-1158. doi: 10.1016/j.cej.2017.06.054 [2] BILIŃSKA L, BLUS K, GMUREK M, et al. Coupling of electrocoagulation and ozone treatment for textile wastewater reuse[J]. Chemical Engineering Journal, 2019, 358: 992-1001. doi: 10.1016/j.cej.2018.10.093 [3] YASEEN D A, SCHOLZ M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review[J]. International Journal of Environmental Science and Technology, 2019, 16(2): 1193-1226. doi: 10.1007/s13762-018-2130-z [4] DE GISI S, LOFRANO G, GRASSI M, et al. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review[J]. Sustainable Materials and Technologies, 2016, 9: 10-40. doi: 10.1016/j.susmat.2016.06.002 [5] HOLKAR C R, JADHAV A J, PINJARI D V, et al. A critical review on textile wastewater treatments: Possible approaches[J]. Journal of Environmental Management, 2016, 182: 351-366. [6] DONKADOKULA N Y, KOLA A K, NAZ I, et al. A review on advanced physico-chemical and biological textile dye wastewater treatment techniques[J]. Reviews in Environmental Science and Bio/Technology, 2020, 19(3): 543-560. doi: 10.1007/s11157-020-09543-z [7] IWUOZOR K O. Prospects and Challenges of using coagulation-flocculation method in the treatment of Effluents[J]. Advanced Journal of Chemistry-Section A, 2019: 105-127. [8] IKEDA K, KAWAMURA Y, YAMAMOTO T, et al. Effectiveness of the template-ion exchange method for appearance of catalytic activity of Ni-MCM-41 for the ethene to propene reaction[J]. Catalysis Communications, 2008, 9(1): 106-110. doi: 10.1016/j.catcom.2007.05.032 [9] ALSAMMAN M T, SÁNCHEZ J. Recent advances on hydrogels based on chitosan and alginate for the adsorption of dyes and metal ions from water[J]. Arabian Journal of Chemistry, 2021, 14(12): 103455. doi: 10.1016/j.arabjc.2021.103455 [10] SINGHA I, KUMAR MISHRAB P. Nano-membrane filtration a novel application of nanotechnology for waste water treatment[J]. Materials Today: Proceedings, 2020, 29: 327-332. doi: 10.1016/j.matpr.2020.07.284 [11] ZHANG H, LUO Z, ZHANG X, et al. Electro-flocculation pretreatment experiments of shale gas drilling wastewater[J]. Natural Gas Industry B, 2020, 7(4): 309-316. doi: 10.1016/j.ngib.2019.12.001 [12] GIWA A, YUSUF A, BALOGUN H A, et al. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review[J]. Process Safety and Environmental Protection, 2021, 146: 220-256. doi: 10.1016/j.psep.2020.08.015 [13] GIANNAKIS S. A review of the concepts, recent advances and niche applications of the (photo) Fenton process, beyond water/wastewater treatment: Surface functionalization, biomass treatment, combatting cancer and other medical uses[J]. Applied Catalysis B: Environmental, 2019, 248: 309-319. doi: 10.1016/j.apcatb.2019.02.025 [14] HUANG X, ZHU T, DUAN W, et al. Comparative studies on catalytic mechanisms for natural chalcopyrite-induced Fenton oxidation: Effect of chalcopyrite type[J]. Journal of Hazardous Materials, 2020, 381: 120998. doi: 10.1016/j.jhazmat.2019.120998 [15] WANG F F, YU X L, GE M F, et al. One-step synthesis of TiO2/ γ-Fe2O3/GO nanocomposites for visible light-driven degradation of ciprofloxacin[J]. Chemical Engineering Journal, 2020, 384: 123381. doi: 10.1016/j.cej.2019.123381 [16] 刘莹, 吴德礼, 何宏平, 等. 颗粒活性炭催化臭氧氧化降解活性黑5[J]. 环境化学, 2016, 35(5): 990-997. doi: 10.7524/j.issn.0254-6108.2016.05.2016011001 LIU Y, WU D L, HE H P, et al. Catalytic ozonation of aqueous reactive black 5 with granular activated carbon[J]. Environmental Chemistry, 2016, 35(5): 990-997 (in Chinese). doi: 10.7524/j.issn.0254-6108.2016.05.2016011001
[17] 彭澍晗, 吴德礼. 催化臭氧氧化深度处理工业废水的研究及应用[J]. 工业水处理, 2019, 39(1): 1-7. doi: 10.11894/1005-829x.2019.39(1).001 PENG S H, WU D L. Research on catalytic ozonation and its application to the advanced treatment of industrial wastewater[J]. Industrial Water Treatment, 2019, 39(1): 1-7 (in Chinese). doi: 10.11894/1005-829x.2019.39(1).001
[18] 周谨. 无机陶瓷膜在印染废水处理中的应用[J]. 膜科学与技术, 2010, 30(3): 116-119. doi: 10.3969/j.issn.1007-8924.2010.03.023 ZHOU J. Application of inorganic ceramic membrane in the treatment of dyeing and printing wastewater[J]. Membrane Science and Technology, 2010, 30(3): 116-119 (in Chinese). doi: 10.3969/j.issn.1007-8924.2010.03.023
[19] ASIF M B, ZHANG Z H. Ceramic membrane technology for water and wastewater treatment: A critical review of performance, full-scale applications, membrane fouling and prospects[J]. Chemical Engineering Journal, 2021, 418: 129481. doi: 10.1016/j.cej.2021.129481 [20] PARK K H, SUN P F, KANG E H, et al. Photocatalytic anti-biofouling performance of nanoporous ceramic membranes treated by atomic layer deposited ZnO[J]. Separation and Purification Technology, 2021, 272: 118935. doi: 10.1016/j.seppur.2021.118935 [21] SUN S B, YAO H, FU W Y, et al. Enhanced degradation of antibiotics by photo-Fenton reactive membrane filtration[J]. Journal of Hazardous Materials, 2020, 386: 121955. doi: 10.1016/j.jhazmat.2019.121955 [22] LI M M, YANG K L, HUANG X, et al. Efficient degradation of trimethoprim by catalytic ozonation coupled with Mn/FeO x -functionalized ceramic membrane: Synergic catalytic effect and enhanced anti-fouling performance[J]. Journal of Colloid and Interface Science, 2022, 616: 440-452. doi: 10.1016/j.jcis.2022.02.061 [23] MANSAS C, MENDRET J, BROSILLON S, et al. Coupling catalytic ozonation and membrane separation: A review[J]. Separation and Purification Technology, 2020, 236: 116221. doi: 10.1016/j.seppur.2019.116221 [24] 张昌远, 黄祥平, 王昭, 等. TiO2/γ-MnO2纳米复合物的制备及其在可见光下的催化性能[J]. 三峡大学学报(自然科学版), 2010, 32(3): 105-109. ZHANG C Y, HUANG X P, WANG Z, et al. Preparation of TiO2/ γ-MnO2 nanocomposite and photocatalytic activity in visible light[J]. Journal of China Three Gorges University (Natural Sciences), 2010, 32(3): 105-109 (in Chinese).
[25] YU C L, FAN C F, MENG X J, et al. A novel Ag/BiOBr nanoplate catalyst with high photocatalytic activity in the decomposition of dyes[J]. Reaction Kinetics, Mechanisms and Catalysis, 2011, 103(1): 141-151. doi: 10.1007/s11144-011-0291-6 [26] YAN C Q, CHENG Z L, WEI J, et al. Efficient degradation of antibiotics by photo-Fenton reactive ceramic membrane with high flux by a facile spraying method under visible LED light[J]. Journal of Cleaner Production, 2022, 366: 132849. doi: 10.1016/j.jclepro.2022.132849 [27] ZHU S, CHENG G, QUAN X J, et al. Intensification of ozone mass transfer and generation of hydroxyl radicals by ceramic membrane[J]. Ozone: Science & Engineering, 2022, 44(4): 372-383. [28] HUANG X X, QUAN X J, CHENG W, et al. Enhancement of ozone mass transfer by stainless steel wire mesh and its effect on hydroxyl radical generation[J]. Ozone: Science & Engineering, 2020, 42(4): 347-356. [29] ZULFIQAR S, LIU S, RAHMAN N, et al. Construction of S-scheme MnO2@CdS heterojunction with core-shell structure as H2-production photocatalyst[J]. Rare Metals, 2021, 40(9): 2381-2391. doi: 10.1007/s12598-020-01616-w [30] FU P F, ZHANG P Y, LI J. Photocatalytic degradation of low concentration formaldehyde and simultaneous elimination of ozone by-product using palladium modified TiO2 films under UV254+185nm irradiation[J]. Applied Catalysis B: Environmental, 2011, 105(1/2): 220-228. [31] CHEN J Y, WU X F, GONG Y, et al. Synthesis of Mn3O4/N-doped graphene hybrid and its improved electrochemical performance for lithium-ion batteries[J]. Ceramics International, 2017, 43(5): 4655-4662. doi: 10.1016/j.ceramint.2016.12.138 [32] PERRON H, VANDENBORRE J, DOMAIN C, et al. Combined investigation of water sorption on TiO2 rutile (110) single crystal face: XPS vs. periodic DFT[J]. Surface Science, 2007, 601(2): 518-527. doi: 10.1016/j.susc.2006.10.015 [33] CHOU S L, CHENG F Y, CHEN J. Electrodeposition synthesis and electrochemical properties of nanostructured γ-MnO2 films[J]. Journal of Power Sources, 2006, 162(1): 727-734. doi: 10.1016/j.jpowsour.2006.06.033 [34] GOPI T, SWETHA G, CHANDRA SHEKAR S, et al. Catalytic decomposition of ozone on nanostructured potassium and proton containing δ-MnO2 catalysts[J]. Catalysis Communications, 2017, 92: 51-55. doi: 10.1016/j.catcom.2017.01.002 [35] GUO H Z, WANG J O, HE X, et al. The origin of oxygen vacancies controlling La2/3Sr1/3MnO3 electronic and magnetic properties[J]. Advanced Materials Interfaces, 2016, 3(5): 1500753. doi: 10.1002/admi.201500753 [36] LI S J, MA Z C, WANG L, et al. Influence of MnO2 on the photocatalytic activity of P-25 TiO2 in the degradation of methyl orange[J]. Science in China Series B: Chemistry, 2008, 51(2): 179-185. [37] BAI C P, XIONG X F, GONG W Q, et al. Removal of rhodamine B by ozone-based advanced oxidation process[J]. Desalination, 2011, 278(1/2/3): 84-90. [38] 程雯. 催化臭氧氧化处理难降解有机废水及其机理研究[D]. 重庆: 重庆理工大学, 2019. CHENG W. Catalytic ozonation for the treatment of refractory organic wastewaters and its mechanism[D]. Chongqing: Chongqing University of Technology, 2019 (in Chinese).
[39] PAN Y W, ZHOU M H, ZHANG Y, et al. Enhanced degradation of Rhodamine B by pre-magnetized Fe0/PS process: Parameters optimization, mechanism and interferences of ions[J]. Separation and Purification Technology, 2018, 203: 66-74. doi: 10.1016/j.seppur.2018.03.039 [40] LIU W, ZHANG Y Q, ZHANG L F, et al. Polysulfone ultrafiltration membrane promoted by brownmillerite SrCu x Co1- x O3–λ-deposited MCM-41 for industrial wastewater decontamination: Catalytic oxidation and antifouling properties[J]. Industrial & Engineering Chemistry Research, 2020, 59(16): 7805-7815. [41] 黄小雪. 催化臭氧化处理垃圾渗滤液生化出水的研究[D]. 重庆: 重庆理工大学, 2020. HUANG X X. Catalytic ozonation for the treatment of biologically-treated leachate[D]. Chongqing: Chongqing University of Technology, 2020 (in Chinese).
[42] WANG Y, YANG W Z, YIN X S, et al. The role of Mn-doping for catalytic ozonation of phenol using Mn/γ-Al2O3 nanocatalyst: Performance and mechanism[J]. Journal of Environmental Chemical Engineering, 2016, 4(3): 3415-3425. doi: 10.1016/j.jece.2016.07.016 -




 下载:
下载: