-
苯、甲苯、二甲苯等芳烃是重要的工业原料和挥发性溶剂[1-2],其具有强的血液毒性和致癌作用,均被列入美国EPA 的129 种优先污染物名单[3]. 美国职业苯暴露量为16 mg·m−3[4],而在发展中国家,职业性苯暴露量比美国还要高1—2个数量级[4-6]. 为此,许多研究人员进行了苯及苯系物暴露人群的代谢组学[7] 研究,并通过代谢组学软件筛选出内源性生物标志物与相应的代谢路径,这对于疾病诊断与监测、生物标志物鉴定和毒性机制探索等都能起到非常重要的作用[8-16]. 如:Sun [17-18]与Campo等[19]近几年开展了对苯及苯系物暴露的代谢组学研究. 已报道的苯及苯系物暴露的代谢组学方法是基于代谢物的非手性总量定性与定量分析,但对代谢产物的手性分析领域并没有涉及到. 代谢产物的手性分析在医学领域有重要的应用,如Kimura等[20]报道了手性氨基酸代谢物用于慢性肾炎诊断的新型的生物标志物监测. L-型氨基酸的异构体即D-型氨基酸越来越多地被认为是新型的生物标志物. 这项研究测试了多种手性氨基酸是否与肾功能、并发症以及慢性肾炎(CKD)的诊断有关并确认了手性氨基酸可作为肾炎疾病中的潜在生物标志物. 而人类机体的组成部分及其代谢产物一般也都具有手性[21-22]. 多糖和核酸中的糖是右旋的D-构型;除某些细菌以外,蛋白质都是由左旋的L-氨基酸组成;在机体的代谢过程中的细胞表面的受体和生物酶,一般也都具有手性. 当它们与环境中污染物相互作用时,通常要经过复杂的手性生物代谢过程[23-24]. 因此,从立体化学的角度研究代谢物的手性和含量变化对于深入评价污染物的药物功能和毒理学具有重要意义[25]. 将代谢组学技术与手性毒理学分析结合在疾病的病理、药理及食品、环境安全等领域的应用文献报道较少[26-33],在职业卫生领域的研究尚未见报道.
本研究借助自制的手性固定相,通过代谢组学技术和统计学软件筛查出职业场所苯及苯系物暴露人群尿液中有差异性的靶向手性代谢产物及相应的代谢途径,这对于揭示其体内代谢机理,评价职业场所相关从业人员的职业危害,以及职业卫生管理部门加强行业监管、职业性防护干预等都具有重要的现实意义.
-
QTRAP4500 LC-MS/MS系统(美国ABSCIEX),使用Analyst®1.6.3 工作站并采用3.0.2版本的MultiQuant定量分析软件,主要用于尿液代谢物定量分析;GC-2010 PLUS 气相色谱仪(日本SHIMADZU,配有FID检测器)主要用于工作场所中“三苯”的定量分析;Vario EL Ⅲ元素分析仪(德国Elementar);5700傅立叶变换红外光谱仪(美国Nicolet);AW-60柱填充机(美国Haskel公司);TGL-16C高速离心机(上海安亭科学仪器厂);KQ-100E超声波清洗装置(昆山超声仪器有限公司);MS-3微型涡旋振荡器(德国IKA公司);HSC-12A氮吹仪(南京科杰分析仪器有限公司);Milli-Q超纯水制备装置(美国Millipore公司).
球形硅胶(粒径5 μm,孔径12 nm,比表面积约为290 m2·g−1)由济南博纳生物技术有限公司提供;HLB和SAX固相萃取柱(美国Waters公司);FFAP毛细管柱(30 m×0.25 mm×0.25 μm)由美国Agilent公司提供;6-对甲苯磺酰化-β-环糊精(纯度 ≥99.5%,山东滨州智源生物科技有限公司);DL-苯巯基尿酸(简称PMA),DL-苄巯基尿酸(简称BMA)和同位素标记的PMA内标DL-d2-PMA,其纯度均大于99%,购自加拿大CDN公司;N-乙酰基-S-(3,4-二羟基丁基-L-半胱氨酸)(DHBMA),(R,S)-N-乙酰基-S-(1-羟基甲基-2-丙烯基)-L-半胱氨酸(MHBMA-1)+(R,S)-N-乙酰基-S-(2-羟基-3-丁烯基)-L-半胱氨酸(MHBMA-2) 1:1,纯度均大于95%,由加拿大Toronto Reasearch Chemicals公司提供;甲醇和乙腈均为色谱纯级(美国Honeywell Burdick&Jackson);153种常见的内源性氨基酸、脂肪酸和外源手性标志物等MRM负离子模式参数由美国AB SCIEX公司提供;异氰酸丙基三乙氧基硅烷(纯度 ≥95%,上海阿拉丁生化科技有限公司);6-对甲苯磺酰基-β-环糊精(纯度≥98%,武汉欣欣佳丽生物科技有限公司); 分析纯级别的乙二胺、丙酮、无水N,N-二甲基甲酰胺(DMF)等试剂均购自中国上海国药集团;甲酸为质谱纯级别(美国 AnAQUA公司);实验用水是由Milli-Q装置制备的超纯水(≥18.2 MΩ·cm);60名非吸烟工人(男40名,女20名,年龄21—53岁, P>0.05)的尿液样本是由景德镇市职业病防治所现场采集的班后尿.
-
手性固定相是参照实验室以前的报道[34-36]并进行了一些改进来制备的. 制备路线简要描述如下:将市售的6-对甲苯磺酰化-β-环糊精(A)用水多次重结晶,80 ℃下真空干燥10 h. 称取3.0 g(2.33 mmol) A于圆底烧瓶中, 慢慢加入30 mL乙二胺温升至80 ℃磁力搅拌反应8 h后减压蒸馏. 冷却至室温,加入丙酮-水溶液(10:1, V/V), 析出的固体重结晶2—3次,得到产物B. 产物通过ESI质谱来鉴定,[M + H]+ 峰(m/z) 1177.82(理论值1177.88),与理论值相符,产物为6-乙二胺单衍生化-β-环糊精,单步产率为62%.
称取1.0 g产物B,慢慢加入30 mL无水DMF使其溶解,在磁力搅拌下于冰浴中加入0.20 mL异氰酸丙基三乙氧基硅烷反应1 h,然后在氮气保护下85 ℃下反应6 h,得到产物C. 直接加入2.0 g实验室以前合成的SBA-15 (Santa Barbara Amorphous 15)升温至110 ℃反应12 h,过滤,然后将固体粗产品分别用DMF、丙酮和甲醇洗涤,用丙酮萃取16 h. 在60 ℃下减压干燥10 h后,得到乙二胺单衍生化-β-环糊精手性固定相(简称EACDP). EACDP的结构表征是通过元素分析仪和红外光谱仪获得的. 最后在恒定压力下将EACDP的匀浆填充不锈钢柱(4.6 mmI.D.×150 mm),并用溶剂依次冲洗此色谱柱.
-
以江西景德镇某汽车制造厂喷漆车间的工人及行政管理人员为研究对象,车间苯及苯系物暴露水平在 8 h轮班期间进行监测. 健康的没有职业苯暴露的受试者是办公室行政管理人员并与苯及苯系物暴露人群按照年龄、吸烟史和饮酒史(表1)进行了匹配.
表1中所有信息均来自问卷调查,包括人口学特征、工作史、吸烟史和酒精史、医疗病史、理化因素暴露、尿液疾病的用药史和家族病史等. 根据调查问卷,排除标准如下:(1)尿液病史,如尿毒症、尿道感染、前列腺炎等;(2) 其他已知的尿液毒性因素(如氯霉素使用和电离辐射); (3) 长期或目前用药或感染史. 本研究中涉及人类参与者的所有程序均符合世界医学协会“涉及人类受试者的医学研究伦理原则”中提到的伦理标准和人权准则. 该项目已经通过豫章师范学院审批,所有项目都给出了书面知情同意书,并在整个尿样采集等过程中都符合规范.
-
使用带有盖子的PVC塑料瓶收集班后尿(每份尿样约50 mL). 同时用6 mol·L−1盐酸酸化样品,尿液与酸的体积比为100:1(V/V),并于-20 ℃冷冻保存. 使用时将冷冻的人尿样品在室温下解冻并在使用前用水稀释10倍. 根据前人及本实验室报道的一些方法[35, 37-39]稍加改进进行样品的提取与净化.
将250 μL同位素内标D,L-d2-PMA储备液(2.0 μg·mL−1)加入到50 mL稀释的尿样中,混匀15 min. 将SAX SPE小柱分别用5.0 mL甲醇,5.0 mL 水,5.0 mL 1%的无水乙酸活化,然后向SAX小柱加入1.0 mL尿样. 接着萃取柱分别加入3.0 mL甲醇,3.0 mL 5mmol·L−1磷酸缓冲液(pH=7.0)洗涤,空气蒸干5 min,然后分别用3.0 mL 乙腈,3.0 mL 5%氨化乙酸乙酯洗脱. 用氮气将洗脱液缓慢吹至近干,并用0.1%甲酸-甲醇(50:50,V/V)定容至1.0 mL. 过滤后,进10 μL样液并通过LC-MS/MS进行分析.
-
将从工作场所收集“三苯”用的0.2 g活性炭放入溶剂解吸瓶中,然后加入1.0 mL二硫化碳解吸剂,最后将1.0 μL解吸液注入GC-2010 PLUS气相色谱仪. 气相色谱仪的氮气流速为40 mL·min−1,空气流速为400 mL·min−1,氢气流速为40 mL·min−1. 检测器为 FID 检测器,进样量为 1.0 μL,柱温为70 ℃. 进样口温度为150 ℃,检测器温度为 200 ℃,色谱柱为FFAP毛细管柱. 测量峰面积以计算苯、甲苯、邻二甲苯、间二甲苯和对二甲苯的相应浓度.
尿样通过ABSCIEX的LC-MS/MS以多反应监测(MRM)模式来检测4种生物标志物和内标(I.S.)的MS信号完成定量分析工作,5种内源性代谢物完成定性鉴定工作,离子对信息如表2所示,色谱柱为自制的EACDP.
色谱条件:25 ℃柱温,0.5 mL·min−1流速等度洗脱,流动相的构成为 5.25 mmol·L−1甲酸铵(pH=2.70)(A)与甲醇(B),V(A)/V(B)=10/90, 进样量为10 μL.
质谱条件:电喷雾离子源,负离子模式,雾化气的压力均为50 psi,气帘气的压力为35 psi,离子源温度为500 ℃,碰撞气为中等压力,电离电压为−4.5 kV.
-
本实验首先用液质分析得到MRM数据,然后用MultiQuant软件(3.0.2版本)积分得到153种靶向代谢物与4种外源性生物标志物的峰面积数据,接着导出存为CSV文件格式,将Sample ID和Sample type行删除,在第二行增加sample group行并在相应的组下面进行标识出control 与expose.
通过开放平台MetaboAnalyst 5.0 软件进行数据分析处理来找出差异性的化合物和差异通路,其网址为https://www.metaboanalyst.ca/MetaboAnalyst/faces/home.xhtml,接着选择statistical Analysis[one factor]进入界面后可以进行t值分析,PCA分析,PLS-DA分析,Heatmap,Correlation Heatmaps等找出有显著差异的内源性代谢物和外源性标志物进行统计学分析.
本文进行代谢路径分析是通过进入MetaboAnalyst 5.0 软件后选择The Pathway Analysis界面找出有显著差异的代谢路径.
-
具有脲键的环糊精三乙氧硅烷与SBA-15硅胶反应键合在硅胶上形成了衍生化环糊精固定相. 通过红外光谱、元素分析对新固定相进行了相应的结构表征.
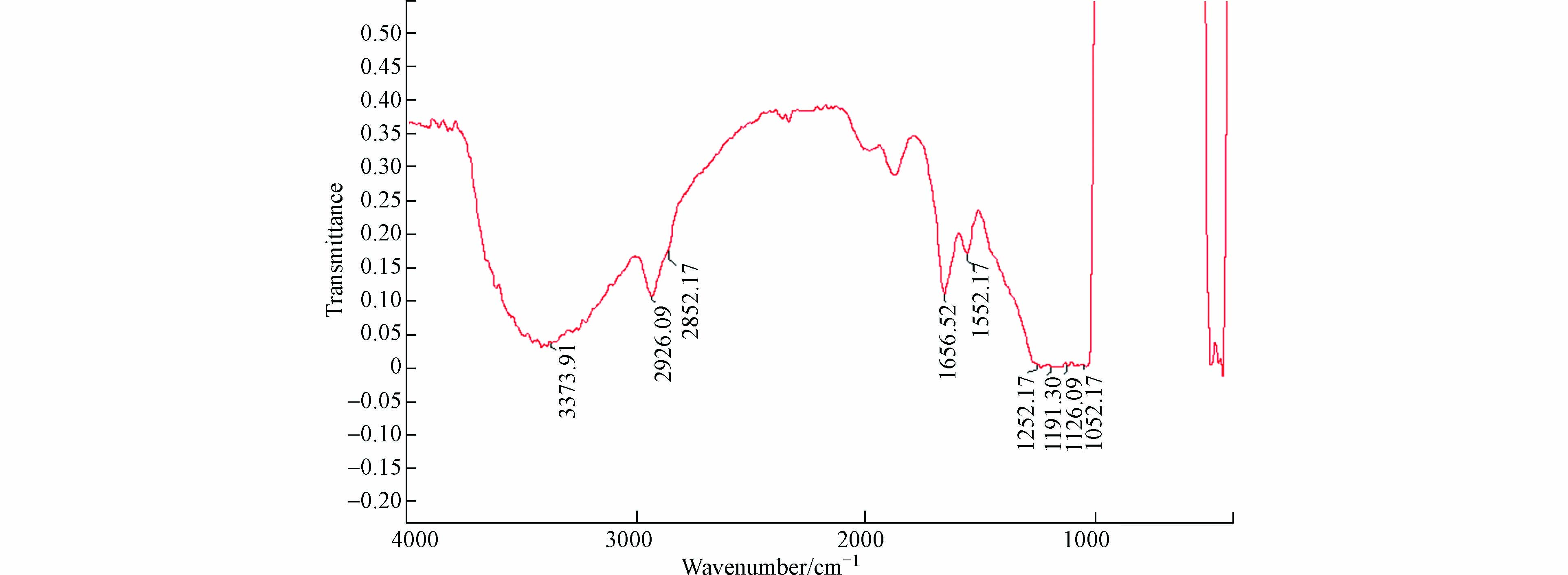
红外光谱如图1所示. 环糊精配体和残留的硅醇羟基的O—H吸收峰及胺基的N—H伸缩峰出现在约3474 cm−1处;有机配体在2926 cm−1和2852 cm−1处出现C—H伸缩振动峰;1656 cm−1为氨基甲酸酯的C=O伸缩振动峰;胺基的N—H面内弯曲峰位于1552 cm−1;环糊精配体和硅胶基质的C—O和Si—O—Si吸收峰是出现在1252、1191、1126、1052 cm−1处的重叠峰.
元素分析结果如下:C 3.67%,H 6.35%,N 0.23%. 根据碳含量计算,乙二胺-β-环糊精固定相EACDP上配体的键合量为0.102 μmol·m−2,这表明有一层有机配体键合在硅胶上.
-
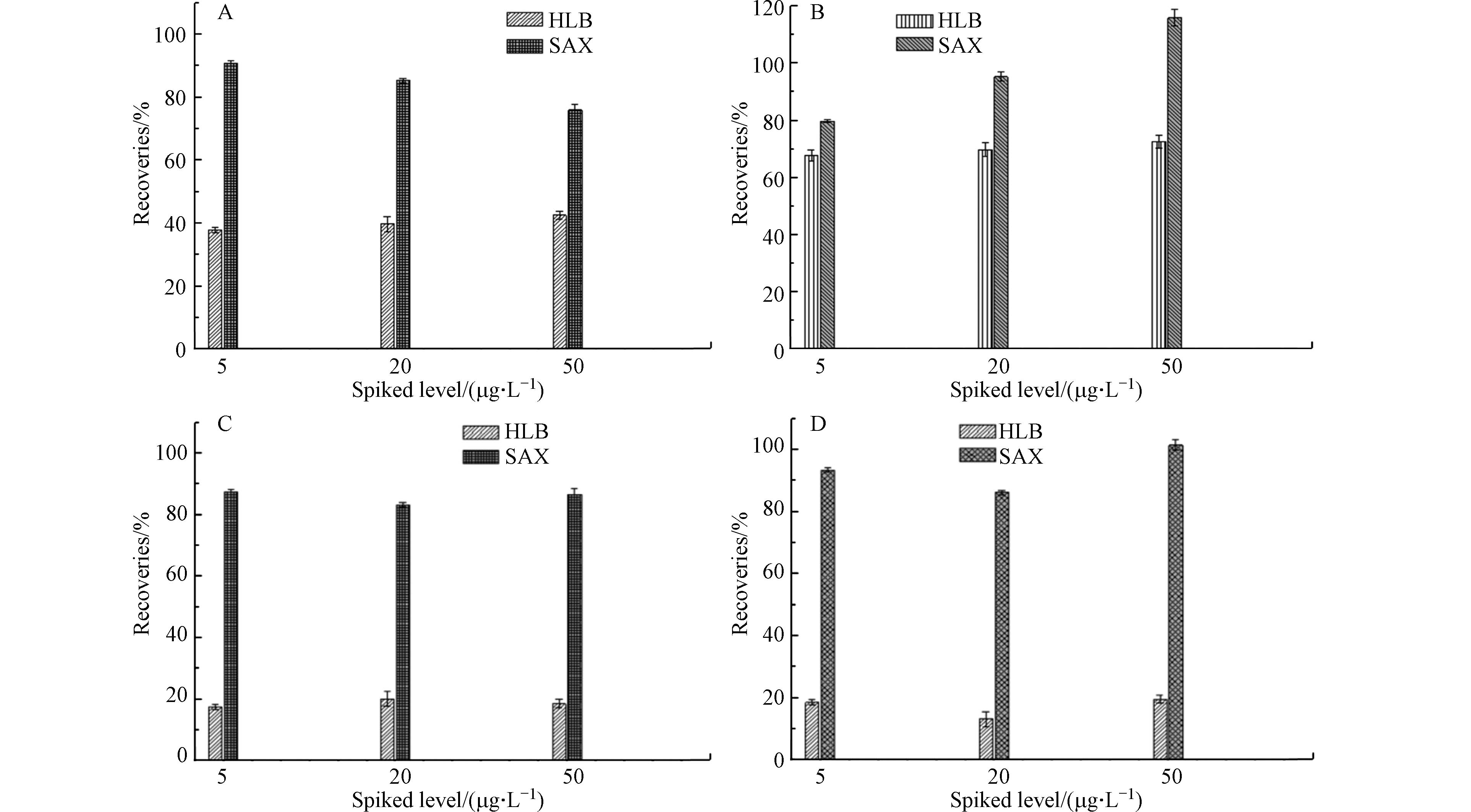
在已发表的文献中检测三苯(BETX)和1.3-丁二烯(BD)暴露的尿液代谢产物,应用固相萃取(SPE)方法更为方便有效[35, 40]. 在本研究中,评价了两种类型的SPE小柱,即OASIS®HLB和SAX小柱(6 cc/200 mg,美国Waters 公司). 目前,苯巯基尿酸(PMA)和苄巯基尿酸(BMA) [41-42]被认定为最可靠的苯系物职业危害经尿液排泄的生物标志物,是苯和甲苯在人体中具有较强特异性和较高敏感性的代谢产物. BD是一种大量生产的石化产品,也是一种致癌性空气污染物,其主要环境来源是生物质燃烧产生的废气以及汽车尾气,尤其是香烟烟雾. 丁二烯是人体致癌风险最高的环境污染物之一[43],其致癌作用的靶器官是淋巴-造血系统. 然而,丁二烯并不是直接致癌物,它的致癌作用源于其代谢产物[44],如:DHBMA与MHBMA等,而且研究发现MHBMA 被证明在监测近期的丁二烯暴露方面比 DHBMA 更敏感[45]. 为此通过分离和检测香烟中1,3-丁二烯成分所引起人体尿液代谢产物中DHBMA和MHBMA这两种手性生物标志物的含量变化及可能引起的手性转化来较为精准地预估非吸烟人群,这为后续的苯及苯系物暴露人群尿液的代谢组学筛查垫定了基础. 故尿液的前处理优化主要是针对PMA,BMA,MHBMA与DHBMA这四种代谢产物展开的.
SPE小柱的净化过程是使用不同的活化液和洗脱剂,比较OASIS®HLB和SAX固相萃取小柱(6 cc/200 mg,美国)的萃取效果(回收率比较见图2)[40]. 使用5.0 mL甲醇,5.0 mL 水和5.0 mL 1%的无水乙酸依次活化固相萃取柱. 加入1.0 mL尿样上样后,用3.0 mL甲醇,3.0 mL 5 mmol·L−1 磷酸缓冲液(pH=7.0)洗涤. 为了确认乙腈是否能较为有效洗脱出PMA、BMA、MHBMA与DHBMA,先将这两种固相萃取小柱分别用3.0 mL乙腈洗脱,然后用氮气吹干,加入1.0 mL甲醇溶液过0.45 µm有机相滤膜后进LC-MS/MS分析. 结果发现HLB小柱能将这4种物质洗脱出来,但对DHBMA与MHBMA的回收率却较低;SAX小柱中的洗脱产物中只有MHBMA与DHBMA,PMA与BMA却基本没有检出. 分析原因可能是PMA与BMA的酸性较MHBMA与DHBMA酸性强,故在SAX小柱中有较好的保留,用乙腈不能将其洗脱出来;而HLB小柱却是依据其极性大小进行吸附,MHBMA与DHBMA较PMA与BMA的极性小,故可能在前面的净化过程中已经被甲醇洗脱出来,这可能是造成MHBMA与DHBMA回收率不高的原因. 继续用3.0 mL 5% 氨化甲醇洗脱SAX小柱,合并乙腈洗脱液然后用氮气吹干,加入3.0 mL甲醇溶液过0.45 µm有机相滤膜后进LC-MS/MS分析,结果发现4种物质都能检出.
选择OASIS® SAX固相萃取小柱是由于其对这4种生物标志物较高的回收率、稳定性及较宽的pH适用范围. 在洗脱步骤中,发现PMA、BMA、MHBMA与DHBMA不能被3.0 mL乙腈完全洗脱,而后续加入3.0 mL 5% 氨化甲醇洗脱则能实现. 因此,选择乙腈与氨化甲醇可作为本实验中的最佳分步洗脱溶剂.
-
本研究共招募了60名参与者,包括30个暴露组人群和30个健康对照组人群. 暴露组与对照组平均年龄分别为(31.93±8.93)岁和(31.60±7.61)岁,男女比例为2:1(参见表1). 暴露组和对照组的性别、年龄、吸烟和饮酒的分布没有观察到统计学差异(P > 0.05).
苯及苯系物暴露强度测定结果分别为(4.94±0.0094)mg·m−3(甲苯)、(0.99±0.013)mg·m−3(邻二甲苯)、(1.44±0.036)mg·m−3(间二甲苯),平均暴露持续时间为(7.80±6.90)a. 暴露组和对照组的特点总结在表1中.
-
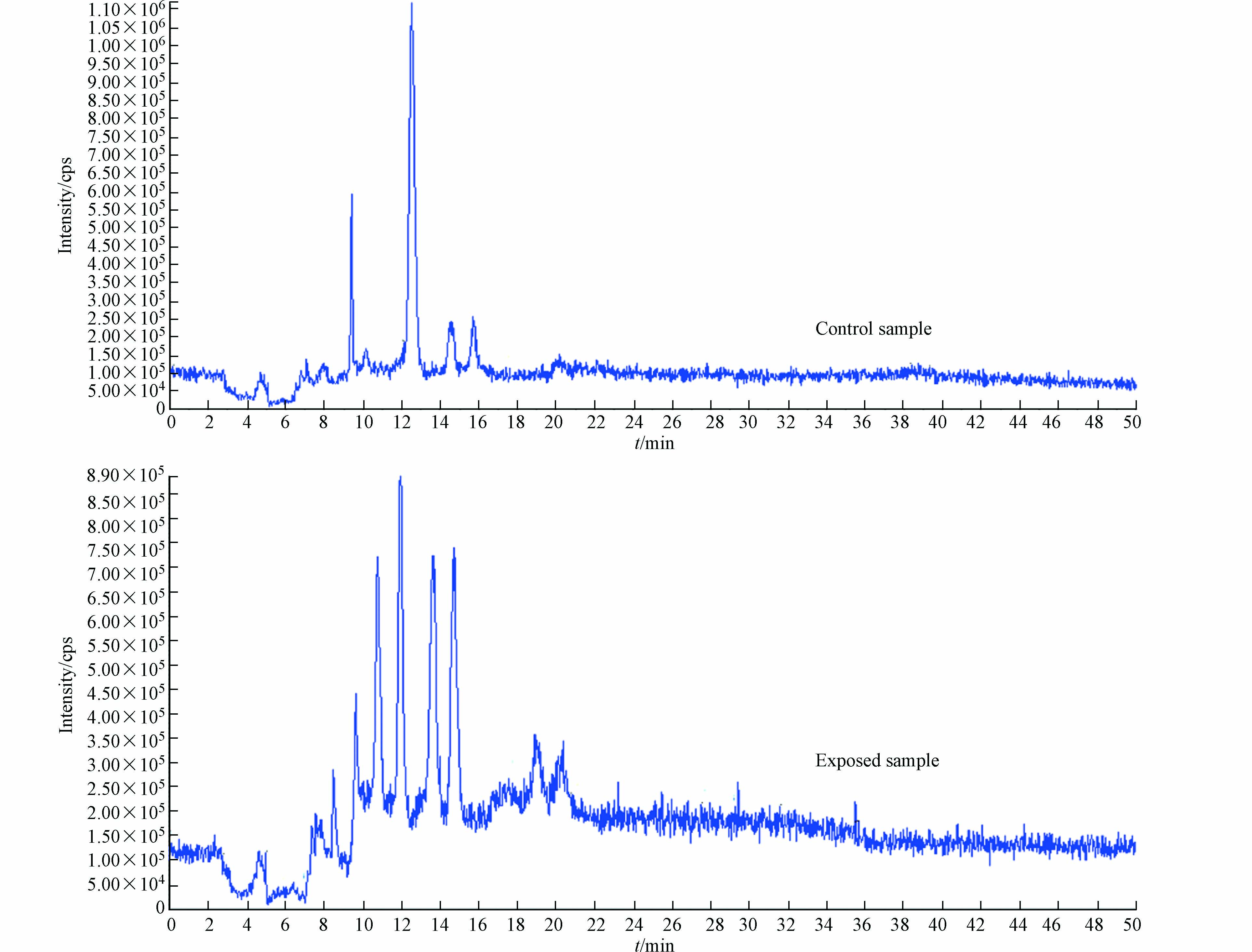
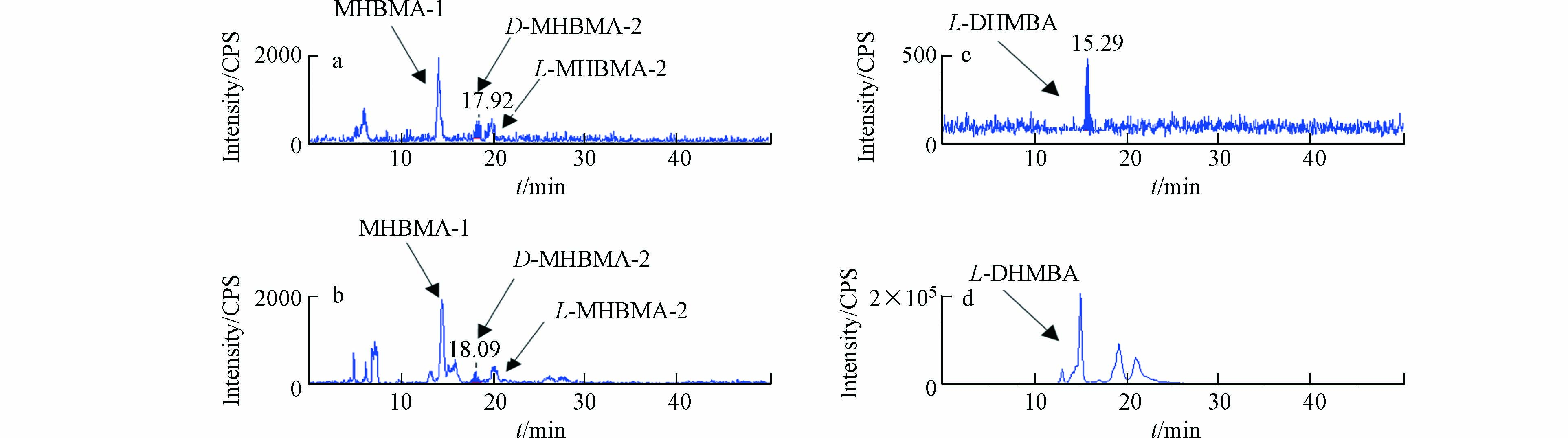
从m/z100到1000的负离子模式下的全扫描用于数据收集. LC-MS总离子流来自暴露组与对照组的尿液TIC色谱图,如图3所示. 观察到暴露组与对照组之间存在显著性差异的保留时间是在10—12 min和18—22 min,分别对应上述4种标志物中为DHBMA与MHBMA,谱图如图4所示).
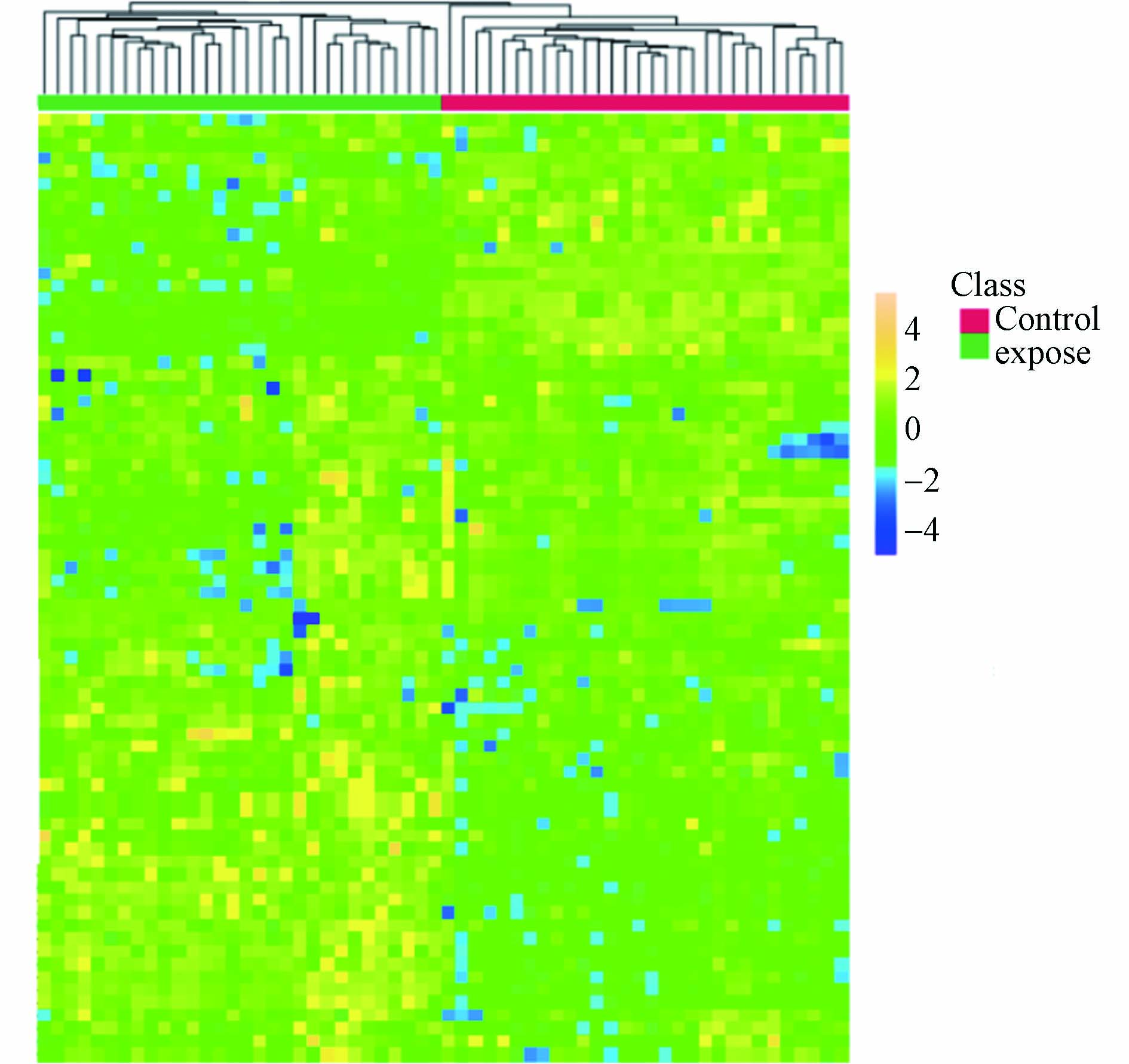
基于已知的尿样代谢物生成热图(图5),每行代表特定的代谢物的离子强度,每列代表30个对照组与30个暴露组工作场所人群的个体代谢特征. 红色块代表区域代表健康对照组,对应的每条线代表每个对照组工人;图中的绿色块代表区域代表暴露组,对应的每条线代表每个暴露组工人. 代谢水平的折叠变化是色标:黄色,上调;蓝色,下调;绿色,无显著性变化(见表3所示). 这揭示了暴露组和对照组之间的差异. 热图表明暴露组主要由于其代谢变化的内在差异与对照组能够分开聚集.
-
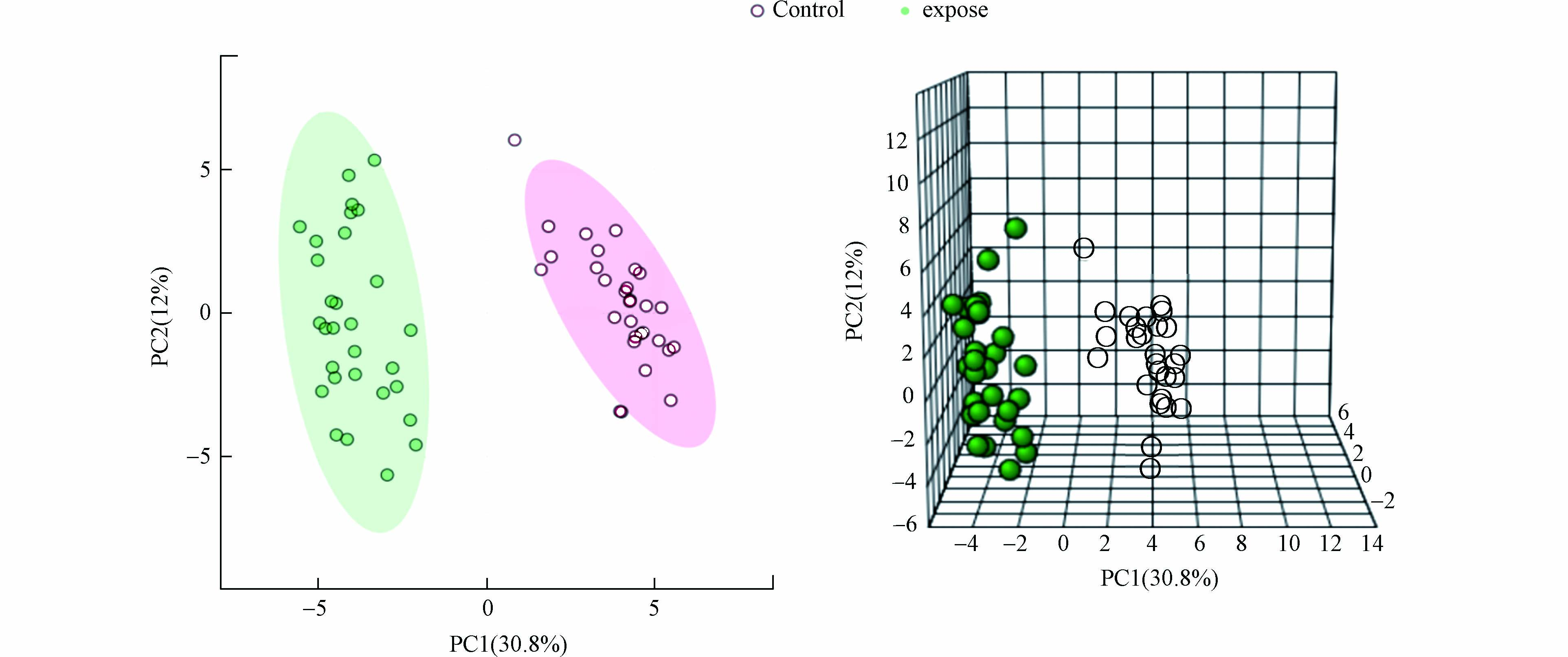
为了揭示暴露组的内源性与外源性的代谢变化,主成分分析(PCA)被选为无监督法. 如图6 所示,PCA 得分图显示来自不同样本的数据组倾向于聚集在一起,并且暴露组与健康对照组能够分开. 该分析表现出令人满意的性能,因为超60% 的变异性是通过使用5个组件来解释的.
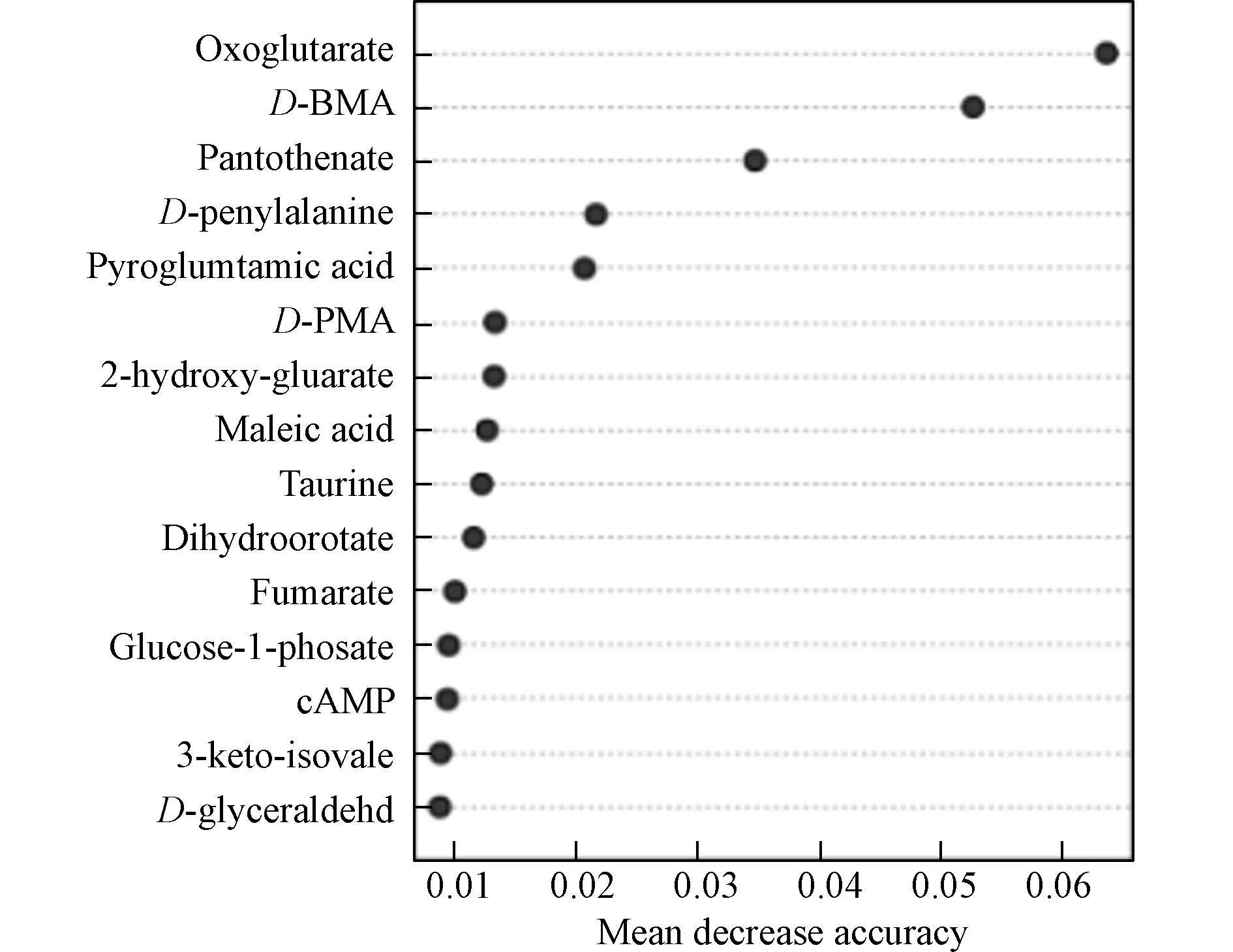
然后确定了哪些代谢物是造成上述差异的原因. 使用学生t检验法测量了暴露组和健康对照组之间具有相对较低的严格性(P < 0.05,倍数变化≥2)的显著差异的不同分子特征的数量. 结果表明,暴露组尿样与对照组尿样相比有50种代谢物发生显著变化. 通过随机森林识别(图7所示),这些特征在排列时按分类准确度的平均下降进行排序,最终识别出7种生物标志物(表3),有5种内源性的与2种外源性的. 即泛酸(pantothenate)、酮戊二酸(oxoglutarate)、D-苯丙氨酸(D-phenylalanine)、2-羟基-戊二酸(2-hydroxy-gluarate)、焦谷氨酸(pyroglutamic acid)、D-苯巯基尿酸(D-PMA)、D-苄巯基尿酸(D-BMA). D-PMA在对照组中均为阴性,暴露组在0—98.60 µg·L−1间变化;D-BMA在对照组中均为阴性,暴露组在0—11.04 µg·L−1间变化. 其他代谢物可通过峰面积变化得出含量变化. 人体的氨基酸一般为L型的,并且苯与苯系物暴露后生成的巯基尿酸生物标志物如PMA与BMA,正常的人群也应为L型的,这几种D型代谢物的出现可能意味着某种疾病的发生,应引起高度的重视[35].
-
可利用开放平台MetaboAnalyst 5.0 软件进行数据分析和处理来找出差异的代谢通路,其网址为https://www.metaboanalyst.ca/MetaboAnalyst/faces/home.xhtml. 统计学分析中选择Pathway Analysis进入界面后输入一列代谢物化合物列表名,得出化合物相应的KEGG代谢物通路(如表4所示),由于给定的化合物列表均为内源性化合物,而外源性化合物PMA与BMA的代谢通路只能通过文献报道[41-42]的方法进行分析. 内源性化合物有6组代谢通路有显著性差异(D-谷氨酰胺和D-谷氨酸代谢(D-glutamine and D-glutamate metabolism)、谷胱甘肽代谢(glutathione metabolism)、丁酸代谢(butanoate metabolism)、丙氨酸、天冬氨酸和谷氨酸代谢(alanine, aspartate and glutamate metabolism)、柠檬酸循环(citrate cycle (TCA cycle))、嘌呤代谢(purine metabolism)).
D-谷氨酰胺和D-谷氨酸代谢会生成L-谷氨酰胺与D-谷氨酰胺,而代谢过程形成的酮戊二酸(2-oxoglutarate)参与到柠檬酸循环中. 谷氨酰胺(glutamine)是肌肉和血液中数量最多的氨基酸. 人体不能自己生产必需氨基酸,只能通过饮食摄取,而谷氨酰胺却是一种有条件必需氨基酸. 这意味着健康和无压力的身体条件能自己产生足够的谷氨酰胺,但在人患病或遭受创伤时,对谷氨酰胺的需求量会超过供应量,就使这种氨基酸变成必需. 故在人体在有苯或苯系物暴露环境中,可能会通过谷氨酸代谢生成酮戊二酸(2-oxoglutarate). 同时代谢形成的酮戊二酸(2-oxoglutarate)也参与丁酸代谢(butanoate metabolism)过程中,从而生成2-羟基-戊二酸(2-hydroxy-gluarate). 杨臻峥[46] 曾报道了人体代谢产物2-羟基戊二酸(2-HG)可诱发神经胶质瘤和白血病, 尿液中2-羟基-戊二酸代谢产物的升高也能在一定程度上表明由于苯及苯系物暴露导致的血液毒性.
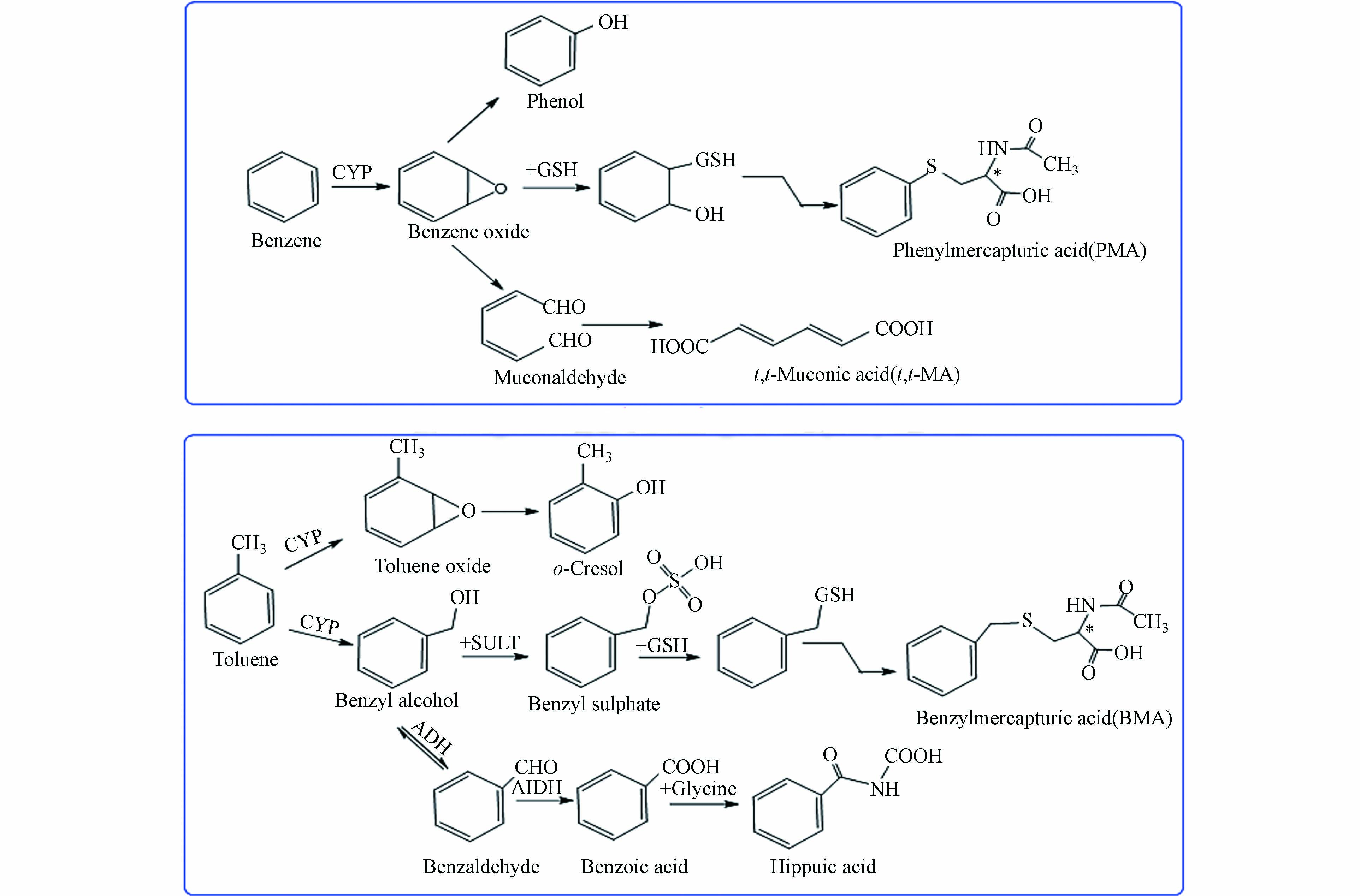
谷胱甘肽(GSH)是一种多功能三肽,它既是磷酸甘油醛脱氢酶的辅基,又是乙二醛酶和丙糖脱氢如酶,所以是细胞内重要的调节代谢物. 氢化酶的辅酶参与体内的三羧酸循环(TCA循环)和葡萄糖代谢,可以激活多种酶,巯基(SH)酶-辅酶等,从而促进碳水化合物、脂肪和蛋白质的代谢. GSH分子以活性巯基(—SH)为特征,这是最重要的官能团. 它可以参与体内多种重要的生化反应,保护体内的蛋白质和一些重要酶的巯基不被氧化和失活,保证能量代谢和细胞利用. 同时通过巯基与体内自由基结合,可直接将自由基还原为酸性物质如巯基尿酸,可以加速自由基的排泄,以抵抗对重要器官的损害. 参考相关文献[40-41, 47],外源性标志物PMA与BMA的代谢路径如图8 所示. 谷胱甘肽多为L型的,但是代谢过程中由于苯及苯系物毒性较大,可能会破坏谷胱甘肽-SH的活性进而造成构型的转化(如D-PMA与D-BMA的生成),从而影响人体的身体健康,这可为进一步研究苯及苯系物引起血液毒性的潜在机制奠定基础.
在谷胱甘肽循环中,焦谷氨酸也是一种重要的代谢物. 在某些多肽中谷氨酸的氨基与5-羧基脱水而成焦谷氨酸, 在一些活性肽类的N端的谷氨酸,其侧链羧基与N端的氨基脱水后形成的环化衍生物. 它也可通过5-氧代脯氨酸酶转化为谷氨酸. GSH循环是一种非酶促抗氧化防御,可防止在体内形成氧化剂并修复氧化损伤[48]. 文献报道在随访期间发现患有咽喉癌的吸烟者血清焦谷氨酸(GSH 代谢)水平升高[49]. 与上述文献报道的结果相一致,本研究在苯及苯系物暴露人群尿液中发现了焦谷氨酸水平的升高.
丙氨酸、天冬氨酸和谷氨酸代谢(alanine, aspartate and glutamate metabolism)生成L-丙氨酸参与到D-氨基酸的代谢(D-amino acid metabolism)中可以形成诸如D-苯丙氨酸(D-phenylalanine),而丙氨酸、天冬氨酸和谷氨酸代谢(alanine, aspartate and glutamate metabolism)形成L-谷氨酸再通过一系列代谢路径形成R-pantothenate(泛酸). Sun等[17]进行了苯暴露C3H/He小鼠骨髓细胞和血浆内源性代谢物变化的研究,在他们的研究中,发现可能是由于线粒体的功能障碍从而导致了骨髓细胞中苯丙氨酸水平的显著升高,这就需要苯丙氨酸、蛋白质、黑色素和酪氨酸的合成. 这两种氨基酸能够为糖异生和分解代谢为三羧酸循环(TCA)循环提供中间体. 本文的研究中也同样通过TCA循环出现了D-苯丙氨酸的升高,这可能是苯及苯系物暴露对细胞的毒性与TCA循环有关. 尿液中苯丙氨酸水平升高的相关的通路可能是苯及苯系物暴露导致血液毒性的重要机制.
嘌呤代谢(purine metabolism)会生成环状磷酸腺苷cAMP(cyclic adenosine monophosphate). 环磷酸腺苷(cAMP)是细胞内参与调节物质代谢和生物学功能的重要物质,是生命信息传递的“第二信使”. 此外,对核酸、蛋白质、糖、脂肪代谢的合成及代谢调节等方面起着重要的作用.
-
本研究基于自制的手性固定相EACDP来优化色谱分离与人尿的样品前处理条件,借助开放平台MetaboAnalyst 5.0 软件结合多元统计分析,结果显示苯及苯系物暴露人群尿液中检测到5种内源性的代谢物与2种外源性的代谢物. 6组代谢通路受到显著影响,包括D-谷氨酰胺和D-谷氨酸代谢、谷胱甘肽代谢、丁酸代谢、丙氨酸、天冬氨酸和谷氨酸代谢、柠檬酸循环和嘌呤代谢. 2-羟基-戊二酸、焦谷氨酸及D-苯丙氨酸会引起明显的升高,苯巯基尿酸与苄巯基尿酸也可能发生由L-型向D-型的手性转化,这为进一步研究苯及苯系物引起血液毒性的潜在机制奠定了基础,也为全面评估苯及苯系物暴露人群的健康风险提供了科学依据. 在后续研究中将进一步拓展代谢产物的研究范围,由靶向代谢物转向全面的非靶向代谢物,将具有显著性差异的代谢物进行动物学实验或建立代谢模型以进一步确认其危害性.
苯及苯系物暴露人群尿液的靶向代谢组学手性分析
Targeted metabolomics chirality analysis of human urine exposed to benzene and benzene series
-
摘要: “三苯”具有强的人体血液毒性和致癌作用,是一类重要的环境污染物. 以往常通过代谢组学软件筛查出工作场所中苯及苯系物暴露人群的内源性代谢物与相应的代谢路径,而未考虑机体的立体化学作用对代谢的影响. 基于自制的手性固定相,优化色谱分离与尿样的前处理条件后,借助开放平台MetaboAnalyst 5.0 软件结合多元统计分析,分析出有显著差异的代谢物和代谢通路的变化. 暴露组检测到5种内源性的代谢物即泛酸、酮戊二酸、D-苯丙氨酸、2-羟基-戊二酸、焦谷氨酸与2种外源性的代谢物即D-苯巯基尿酸与D-苄巯基尿酸. 6组代谢通路受到显著影响,包括D-谷氨酰胺和 D-谷氨酸代谢、谷胱甘肽代谢、丁酸代谢、丙氨酸、天冬氨酸和谷氨酸代谢、柠檬酸循环和嘌呤代谢. 结果表明,苯及苯系物暴露人群尿液代谢产物中2-羟基-戊二酸、焦谷氨酸及D-苯丙氨酸会引起明显的升高,苯巯基尿酸与苄巯基尿酸也可能发生由L-型向D-型的手性转化,这为进一步研究苯及苯系物引起血液毒性的潜在机制奠定了基础,也为全面评估苯及苯系物暴露人群的健康风险提供了科学依据.Abstract: "Triphenyl" has strong human blood toxicity and carcinogenic effects, which is an important environmental pollutants. As usual, metabolomics software was used to screen the endogenous metabolites and corresponding metabolic pathways of benzene and benzene series exposed people in the workplace, without considering the influence of stereochemistry on metabolism. Based on the home-made chiral stationary phase, after optimizing the chromatographic separation and pretreatment conditions of urine samples, the open platform MetaboAnalyst 5.0 software being combined with multivariate statistical analysis was used to analyze the changes of significantly different metabolites and metabolic pathways. Five endogenous metabolites (pantothenate, oxoglutarate, D-phenylalanine, 2-hydroxy-gluarate, pyroglutamic acid) and two exogenous metabolites (D-phenylmercapturic acid and D-benzylmercapturic acid) were detected in the exposed group. Six groups of metabolic pathways were significantly affected, including D-glutamine and D-glutamate metabolism, Glutathione metabolism, Butanoate metabolism, Alanine, aspartate and glutamate metabolism, Citrate cycle (TCA cycle) and Purine metabolism. Results show that 2-hydroxy-glutaric acid, glutamate and D-phenylalanine can cause obvious rise in human urine metabolites exposed to benzene and benzene series, the configurational transformation may also occur from L-type of D-type for phenylmercapturic acid and benzylmercapturic acid, which laid a foundation for further research on the potential mechanism of blood toxicity caused by benzene and benzene series, and also provides a scientific basis for comprehensive assessment of the health risk of benzene and benzene series exposed population.
-
Key words:
- workplace /
- benzene and benzene series /
- human urine /
- targeted metabolomics /
- chiral analysis.
-

-
表 1 不同组的相应参数表(n=30)
Table 1. Corresponding parameter table for different groups (n=30)
对照组
Control group暴露组
Exposure group行政人员 油漆工 * 年龄
Age31.93±8.93 31.60±7.61 性别
Gender男 19 21 女 11 9 吸烟
Smoke是 0 2 否 30 28 饮酒
Drink wine是 10 6 否 20 24 遗传病史
Whether there is a history of genetic disease是 0 0 否 30 30 暴露强度/(mg m−3)
Exposure intensity苯 — — 甲苯 — 4.94±0.0094 邻-二甲苯 — 0.99±0.013 间-二甲苯 — 1.44±0.036 对-二甲苯 — — 工作年限/a
Length of service8.43±7.05 7.80±6.90 *与对照组比较,P<0.05.吸烟:指吸烟超过1 d,烟龄超过1 a.饮酒:指饮酒超过1年至少1周1次. — 代表没有检出.
*Compared with the control group, P<0.05. Smoking: refers to smoking for more than 1 day and smoking for more than 1 year. Drinking: refers to drinking for more than 1 year at least once a week. — means no detection.表 2 PMA, BMA, d2-PMA, MHBMA-1+-2 与 DHBMA的多反应监测优化条件
Table 2. MRM optimization conditions for PMA, BMA, d2-PMA, MHBMA-1+-2 and DHBMA
化合物
Compounds母离子
Precusor
(m/z)子离子
Product
(m/z)驻留时间/
ms
Dwell time解簇电压/
eV
DP碰撞能量/
eV
CE(1) 苯巯基尿酸 (PMA)* 238.03 108.94 30 −16.91 −16.99 (2) 苄巯基尿酸 (BMA)* 252.07 122.99 30 −32.15 −24.22 (3) 同位素标记的苯巯基尿酸内标 (d2-PMA)* 241.10 109.93 30 −16.84 −17.14 (4) N-乙酰基-S-(1-羟基甲基-2-丙烯基)-L-半胱氨酸+N-乙酰基-S-(2-羟基-3-丁烯基)-L-半胱氨酸(MHBMA-1+-2)* 232.03 102.94 30 −19.90 −14.14 (5) N-乙酰基-S-(1-羟基甲基-2-丙烯基)-L-半胱氨酸+N-乙酰基-S-(2-羟基-3-丁烯基)-L-半胱氨酸(MHBMA-1+-2) 232.03 128.10 30 −33.14 −14.16 (6) N-乙酰基-S-(3,4-二羟基丁基-L-半胱氨酸) (DHBMA)* 250.05 120.95 30 −11.97 −18.96 (7) N-乙酰基-S-(3,4-二羟基丁基-L-半胱氨酸 )(DHBMA) 250.05 127.80 30 −14.00 −16.26 (8) 泛酸 Pantothenate 218.01 146.13 30 −25.00 −21.00 (9) 酮戊二酸 Oxoglutarate 145.10 101.12 30 −35.31 −13.00 (10) D-苯丙氨酸 D-phenylalanine 166.1 103.20 30 −16.93 −30.46 (11) 2-羟基-戊二酸 2-hydroxygluarate 147.12 128.74 30 −45.52 −17.12 (12) 焦谷氨酸 Pyroglutamic acid 128.08 82.13 30 −23.16 −19.15 *代表定量离子. *Represent quantitative ion. 表 3 尿液鉴定出的差异性代谢物
Table 3. Differential metabolites identified in urine
序号
NO.趋势(暴露组/对照组)
Tendency(exposure/control)代谢物
Metabolities相关路径
Related pathway1 ↑ D-苯巯基尿酸
D-PMA谷胱甘肽代谢 2 ↑ D-苄巯基尿酸
D-BMA谷胱甘肽代谢 3 — 泛酸Pantothenate 丙氨酸、天冬氨酸和谷氨酸代谢 4 ↓ 酮戊二酸Oxoglutarate D-谷氨酰胺和 D-谷氨酸代谢 5 ↑ D-苯丙氨酸
D-phenylalanine丙氨酸、天冬氨酸和谷氨酸代谢,柠檬酸循环 6 ↑ 2-羟基-戊二酸
2-hydroxy-gluarate柠檬酸循环 7 ↑ 焦谷氨酸
Pyroglutamic acidD-谷氨酰胺和 D-谷氨酸代谢,谷胱甘肽代谢 注:↑代表上调,↓代表下调,—代表不变.
Note:↑represent upward adjustment, ↓represent downward adjustment, and — represent unchanged.表 4 代谢路径P值表
Table 4. Metabolic pathway P values
路径名
Pathway NameP −lgP D-谷氨酰胺和 D-谷氨酸代谢
D-Glutamine and D-glutamate metabolism0.002683 2.57130 丁酸代谢
Butanoate metabolism0.006593 2.1578 柠檬酸循环
Citrate cycle (TCA cycle)0.008706 2.06020 谷胱甘肽代谢
Glutathione metabolism0.012002 1.92075 丙氨酸、天冬氨酸和谷氨酸代谢
Alanine, aspartate and glutamate metabolism0.012006 1.92060 嘌呤代谢
Purine metabolism0.025953 1.58620 -
[1] WALLACE L A. Major sources of benzene exposure [J]. Environmental Health Perspectives, 1989, 82: 165-169. doi: 10.1289/ehp.8982165 [2] OZKAYNAK H, RYAN P B, WALLACE L A, et al. Sources and emission rates of organic chemical vapors in homes and buildings//SEIFERT B, ESDORN H, FISCHER M, et al. Indoor Air ’87: Proceedings of the 4th International Conference on Indoor Air Quality and Climate, Institute for Water, Soil and Air Hygiene [C]. West Berlin, 1987, 1: 3–7. [3] GRIGORYAN H, EDMANDS W M B, LAN Q, et al. Adductomic signatures of benzene exposure provide insights into cancer induction [J]. Carcinogenesis, 2018, 39(5): 661-668. doi: 10.1093/carcin/bgy042 [4] MCCONNELL E E. Benzene. Environmental Health Criteria Series no. 150. International Program on Chemical Safety (IPCS) [M]. Geneva: World Health Organization, 1993: 14 and 42–45. [5] ROTHMAN N, LI G L, DOSEMECI M, et al. Hematotoxicity among Chinese workers heavily exposed to benzene [J]. American Journal of Industrial Medicine, 1996, 29(3): 236-246. doi: 10.1002/(SICI)1097-0274(199603)29:3<236::AID-AJIM3>3.0.CO;2-O [6] YIN S N, HAYES R B, LINET M S, et al. A cohort study of cancer among benzene-exposed workers in China: Overall results [J]. American Journal of Industrial Medicine, 1996, 29(3): 227-235. doi: 10.1002/(SICI)1097-0274(199603)29:3<227::AID-AJIM2>3.0.CO;2-N [7] RAAMSDONK L M, TEUSINK B, BROADHURST D, et al. A functional genomics strategy that uses metabolome data to reveal the phenotype of silent mutations [J]. Nature Biotechnology, 2001, 19(1): 45-50. doi: 10.1038/83496 [8] HALL R D, BROUWER I D, FITZGERALD M A. Plant metabolomics and its potential application for human nutrition [J]. Physiologia Plantarum, 2008, 132(2): 162-175. [9] WISHART D S. Applications of metabolomics in drug discovery and development [J]. Drugs in R & D, 2008, 9(5): 307-322. [10] KADDURAH-DAOUK R, KRISHNAN K R R. Metabolomics: A global biochemical approach to the study of central nervous system diseases [J]. Neuropsychopharmacology, 2009, 34(1): 173-186. doi: 10.1038/npp.2008.174 [11] 俞颖, 曹毅, 陈益民, 等. 基于液相色谱-质谱联用系统的系统性红斑狼疮患者血浆代谢组学分析 [J]. 色谱, 2010, 28(7): 644-648. doi: 10.3724/SP.J.1123.2010.00644 YU Y, CAO Y, CHEN Y M, et al. Plasma metabonomics study of systemic lupus erythematosus based on liquid chromatography-mass spectrometry [J]. Chinese Journal of Chromatography, 2010, 28(7): 644-648(in Chinese). doi: 10.3724/SP.J.1123.2010.00644
[12] 谷金宁, 牛俊, 皮子凤, 等. 尿液代谢组学方法研究人参总皂苷治疗糖尿病心肌病大鼠作用机制 [J]. 分析化学, 2013, 41(3): 371-376. GU J N, NIU J, PI Z F, et al. A urinary metabonomics research on total ginsenoside treated diabetes cardiomyopathy rats based on rapid resolution liquid chromatography/mass spectrometry [J]. Chinese Journal of Analytical Chemistry, 2013, 41(3): 371-376(in Chinese).
[13] 周红光, 陈海彬, 王瑞平, 等. 代谢组学在中药复方研究中的应用 [J]. 中国药理学通报, 2013, 29(2): 161-165. ZHOU H G, CHEN H B, WANG R P, et al. Metabonomics and its application in TCM formula study [J]. Chinese Pharmacological Bulletin, 2013, 29(2): 161-165(in Chinese).
[14] 张凤霞, 王国栋. 植物代谢组学应用研究: 现状与展望 [J]. 中国农业科技导报, 2013, 15(2): 28-32. ZHANG F X, WANG G D. The applications of metabolomics in plant biology—Current status and prospective [J]. Journal of Agricultural Science and Technology, 2013, 15(2): 28-32(in Chinese).
[15] 王伟华, 韩占江. 新疆慕萨莱思酒天然活性成分的代谢组学研究进展: 以原花青素为例 [J]. 食品安全质量检测学报, 2013, 4(6): 1810-1814. WANG W H, HAN Z J. Research progress on metabonomics method of natural active ingredients in Musalais wine in Xinjiang—Taking procyanidins for example [J]. Journal of Food Safety & Quality, 2013, 4(6): 1810-1814(in Chinese).
[16] 史怀, 刘波, 陈峥, 等. 基于LC/Q-TOF MS的芽胞杆菌代谢组学分析方法 [J]. 福建农业学报, 2012, 27(10): 1112-1119. SHI H, LIU B, CHEN Z, et al. Metabonomics analysis of Bacillus based on LC/Q-TOF MS [J]. Fujian Journal of Agricultural Sciences, 2012, 27(10): 1112-1119(in Chinese).
[17] SUN R L, ZHANG J, YIN L H, et al. Investigation into variation of endogenous metabolites in bone marrow cells and plasma in C3H/He mice exposed to benzene [J]. International Journal of Molecular Sciences, 2014, 15(3): 4994-5010. doi: 10.3390/ijms15034994 [18] SUN R L, XU K, ZHANG Q Y, et al. Plasma metabonomics investigation reveals involvement of fatty acid oxidation in hematotoxicity in Chinese benzene-exposed workers with low white blood cell count [J]. Environmental Science and Pollution Research, 2018, 25(32): 32506-32514. doi: 10.1007/s11356-018-3160-2 [19] CAMPO P, WANIUSIOW D, COSSEC B, et al. Toluene-induced hearing loss in phenobarbital treated rats [J]. Neurotoxicology and Teratology, 2008, 30(1): 46-54. doi: 10.1016/j.ntt.2007.10.001 [20] KIMURA T, HAMASE K, MIYOSHI Y, et al. Chiral amino acid metabolomics for novel biomarker screening in the prognosis of chronic kidney disease [J]. Scientific Reports, 2016, 6: 26137. doi: 10.1038/srep26137 [21] WAGNER A J, ZUBAREV D Y, ASPURU-GUZIK A, et al. Chiral sugars drive enantioenrichment in prebiotic amino acid synthesis [J]. ACS Central Science, 2017, 3(4): 322-328. doi: 10.1021/acscentsci.7b00085 [22] KNIGHT B J, STACHE E E, FERREIRA E M. An analysis of the complementary stereoselective alkylations of imidazolidinone derivatives toward α-quaternary proline-based amino amides [J]. Tetrahedron, 2015, 71(35): 5814-5823. doi: 10.1016/j.tet.2015.05.010 [23] CAMACHO-MUÑOZ D, KASPRZYK-HORDERN B. Multi-residue enantiomeric analysis of human and veterinary pharmaceuticals and their metabolites in environmental samples by chiral liquid chromatography coupled with tandem mass spectrometry detection [J]. Analytical and Bioanalytical Chemistry, 2015, 407(30): 9085-9104. doi: 10.1007/s00216-015-9075-6 [24] WANG X Y, LI Z, ZHANG H, et al. Environmental behavior of the chiral organophosphorus insecticide acephate and its chiral metabolite methamidophos: Enantioselective transformation and degradation in soils [J]. Environmental Science & Technology, 2013, 47(16): 9233-9240. [25] BARCLAY V K H, TYREFORS N L, JOHANSSON I M, et al. Trace analysis of fluoxetine and its metabolite norfluoxetine. Part I: Development of a chiral liquid chromatography-tandem mass spectrometry method for wastewater samples [J]. Journal of Chromatography, A, 2011, 1218(33): 5587-5596. doi: 10.1016/j.chroma.2011.06.024 [26] ZHANG P, ZHU W T, WANG D Z, et al. Enantioselective effects of metalaxyl enantiomers on breast cancer cells metabolic profiling using HPLC-QTOF-based metabolomics [J]. International Journal of Molecular Sciences, 2017, 18(1): 142. doi: 10.3390/ijms18010142 [27] TAKAYAMA T, MOCHIZUKI T, TODOROKI K, et al. A novel approach for LC-MS/MS-based chiral metabolomics fingerprinting and chiral metabolomics extraction using a pair of enantiomers of chiral derivatization reagents [J]. Analytica Chimica Acta, 2015, 898: 73-84. doi: 10.1016/j.aca.2015.10.010 [28] CHENG Q Y, XIONG J, HUANG W, et al. Sensitive determination of onco-metabolites of D- and L-2-hydroxyglutarate enantiomers by chiral derivatization combined with liquid chromatography/mass spectrometry analysis [J]. Scientific Reports, 2015, 5: 15217. doi: 10.1038/srep15217 [29] CHAI T T, CUI F, YIN Z Q, et al. Chiral PCB 91 and 149 toxicity testing in embryo and larvae (Danio rerio): Application of targeted metabolomics via UPLC-MS/MS [J]. Scientific Reports, 2016, 6: 33481. doi: 10.1038/srep33481 [30] HASAN M, HOFSTETTER R, FASSAUER G M, et al. Quantitative chiral and achiral determination of ketamine and its metabolites by LC-MS/MS in human serum, urine and fecal samples [J]. Journal of Pharmaceutical and Biomedical Analysis, 2017, 139: 87-97. doi: 10.1016/j.jpba.2017.02.035 [31] MASTERS A R, GUFFORD B T, LU J B L, et al. Chiral plasma pharmacokinetics and urinary excretion of bupropion and metabolites in healthy volunteers [J]. The Journal of Pharmacology and Experimental Therapeutics, 2016, 358(2): 230-238. doi: 10.1124/jpet.116.232876 [32] TEITELBAUM A M, FLAKER A M, KHARASCH E D. Development, validation and application of a comprehensive stereoselective LC/MS-MS assay for bupropion and oxidative, reductive, and glucuronide metabolites in human urine [J]. Journal of Chromatography, B, Analytical Technologies in the Biomedical and Life Sciences, 2016, 1027: 239-253. doi: 10.1016/j.jchromb.2016.05.036 [33] NAGAO R, TSUTSUI H, MOCHIZUKI T, et al. Novel chiral derivatization reagents possessing a pyridylthiourea structure for enantiospecific determination of amines and carboxylic acids in high-throughput liquid chromatography and electrospray-ionization mass spectrometry for chiral metabolomics identification [J]. Journal of Chromatography A, 2013, 1296: 111-118. doi: 10.1016/j.chroma.2013.03.019 [34] LI L, CHENG B P, ZHOU R D, et al. Preparation and evaluation of a novel N-benzyl-phenethylamino-β-cyclodextrin-bonded chiral stationary phase for HPLC [J]. Talanta, 2017, 174: 179-191. doi: 10.1016/j.talanta.2017.06.009 [35] LI L, WANG H, JIN Y J, et al. Preparation of a new benzylureido-β-cyclodextrin-based column and its application for the determination of phenylmercapturic acid and benzylmercapturic acid enantiomers in human urine by LC/MS/MS [J]. Analytical and Bioanalytical Chemistry, 2019, 411(21): 5465-5479. doi: 10.1007/s00216-019-01920-0 [36] 聂桂珍, 李来生, 程彪平, 等. 乙二胺β-环糊精键合SBA-15电色谱拆分β-受体阻滞剂的研究 [J]. 分析试验室, 2014, 33(7): 745-751. NIE G Z, LI L S, CHENG B P, et al. Study on mono-ethylenediamino-β-cyclodextrin-bonded SBA-15 capillary electrochromatography for enantioseparations of β-blockers [J]. Chinese Journal of Analysis Laboratory, 2014, 33(7): 745-751(in Chinese).
[37] PIERI M, MIRAGLIA N, ACAMPORA A, et al. Determination of urinary S-phenylmercapturic acid by liquid chromatography-tandem mass spectrometry [J]. Journal of Chromatography B, 2003, 795(2): 347-354. doi: 10.1016/S1570-0232(03)00602-0 [38] FAN R F, WANG D L, SHE J W. Method development for the simultaneous analysis of trans, trans-muconic acid, 1, 2-dihydroxybenzene, S-phenylmercapturic acid and S-benzylmercapturic acid in human urine by liquid chromatography/tandem mass spectrometry [J]. Analytical Methods, 2015, 7(2): 573-580. doi: 10.1039/C4AY02261K [39] MAESTRI L, NEGRI S, FERRARI M, et al. Determination of urinary S-phenylmercapturic acid, a specific metabolite of benzene, by liquid chromatography/single quadrupole mass spectrometry [J]. Rapid Communications in Mass Spectrometry:RCM, 2005, 19(9): 1139-1144. doi: 10.1002/rcm.1904 [40] SHI J W. Assessment of matrix effect in LC-MS/MS quantitative analysis with external standard method [J]. Physical Testing and Chemical Analysis, 2012, 48(11): 1261-1264. [41] KUO M L, SHIAH S G, WANG C J, et al. Suppression of apoptosis by Bcl-2 to enhance benzene metabolites-induced oxidative DNA damage and mutagenesis: A possible mechanism of carcinogenesis [J]. Molecular Pharmacology, 1999, 55(5): 894-901. [42] DOROSHYENKO O, FUHR U, KUNZ D, et al. In vivo role of cytochrome P450 2E1 and glutathione-S-transferase activity for acrylamide toxicokinetics in humans [J]. Cancer Epidemiology, Biomarkers & Prevention, 2009, 18(2): 433-443. [43] van SITTERT N J, MEGENS H J J J, WATSON W P, et al. Biomarkers of exposure to 1, 3-butadiene as a basis for cancer risk assessment [J]. Toxicological Sciences, 2000, 56(1): 189-202. doi: 10.1093/toxsci/56.1.189 [44] 刘楠, 程娟, 李斌, 等. 1, 3-丁二烯生物标志物的研究进展[J]. 国外医学(卫生学分册), 2007(6): 352-357. LIU N, CHENG J, LI B, et al. Research progress of biomarkers of 1, 3- butadiene[J]. Foreign Medical Sciences (Section Hygiene), 2007(6): 352-357 (in Chinese).
[45] BOOGAARD P, van SITTERT N J, MEGENS H. Urinary metabolites and haemoglobin adducts as biomarkers of exposure to 1, 3-butadiene: A basis for 1, 3-butadiene cancer risk assessment[J]. Chemico-Biological Interactions, 2001, 135: 695-701. [46] 杨臻峥. 科学家发现人体代谢产物2-羟基戊二酸致癌机制 [J]. 药学进展, 2011, 35(1): 35. YANG Z Z. Scientists discover the carcinogenic mechanism of human metabolite 2- hydroxyglutaric acid [J]. Progress in Pharmaceutical Sciences, 2011, 35(1): 35(in Chinese).
[47] SCHETTGEN T, MUSIOL A, KRAUS T. Fast determination of urinary S-phenylmercapturic acid (S-PMA) and S-benzylmercapturic acid (S-BMA) by column-switching liquid chromatography-tandem mass spectrometry [J]. Journal of Chromatography, B, 2008, 863(2): 283-292. doi: 10.1016/j.jchromb.2008.01.024 [48] ZHANG H Y, WANG X, WANG M Y, et al. Mammalian cells exhibit a range of sensitivities to silver nanoparticles that are partially explicable by variations in antioxidant defense and metallothionein expression [J]. Small (Weinheim an Der Bergstrasse, Germany), 2015, 11(31): 3797-3805. doi: 10.1002/smll.201500251 [49] JEE S H, KIM M, KIM M, et al. Clinical relevance of glycerophospholipid, sphingomyelin and glutathione metabolism in the pathogenesis of pharyngolaryngeal cancer in smokers: The Korean Cancer Prevention Study-II [J]. Metabolomics, 2016, 12(11): 164. doi: 10.1007/s11306-016-1114-6 -



 下载:
下载: