-
国内外的饮用水中均检测出医药品污染物,其浓度通常处于ng·L−1或更低水平. 风险评价研究显示,饮用水中微量的医药品污染物对于健康的成年人影响较小,但是敏感性人群特别是孕妇及新生儿暴露于环境中的风险需要加以考虑[1]. 布洛芬(IBU)属于抗炎药物,据估计全球年产量达数千吨,其在地表水和废水中检测到的浓度范围为ng·L−1至低μg·L−1水平[2];传统的水处理技术无法对持久性污染物IBU有效去除,研究发现IBU能对淡水环境中的无尾类动物的胚胎发育产生影响[3]. 非均相催化臭氧氧化技术是一种能够在室温和常压下,将那些难以通过臭氧单独氧化的有机污染物高效分解的方法[4-5]. 臭氧在催化剂表面有效分解产生羟基自由基等活性氧来降解水中有机污染物[6-7]. 由于不同催化剂的属性差异较大,其促进臭氧有效分解的机理还不清楚,需要深入研究催化臭氧氧化水中有机污染物的作用机制[8-9].
本文采用固体酸γ-Al2O3和多价态的钛活性组份负载法制备了TiO2/γ-Al2O3介孔催化剂. 由于TiO2的Lewis酸性强于Al2O3,通过调节钛的负载量来控制γ-Al2O3的酸量,从而更深入的研究Lewis酸性位对布洛芬去除的影响,并通过吡啶红外光谱、电子自旋共振和原位激光显微拉曼光谱等测试手段来探究催化反应机理.
-
布洛芬(IBU)从东京化成工业株式会社购买. 自由基捕捉剂BMPO从东仁化学科技(上海)有限公司购买. 实验用超纯水(电阻率为18.2 MΩ·cm)是用Milli-Q超纯水仪制备的. 异丙醇铝(Al[OCH(CH3)2]3)购买于Sigma-Aldrich公司. 葡萄糖、钛酸四正丁酯(TBOT)、盐酸(HCl)、硝酸(HNO3)和氢氧化钠(NaOH)在国药集团化学试剂北京有限公司采购. 全部化学试剂均是分析纯. 溶液pH由2 mol·L−1盐酸或者氢氧化钠溶液进行调节.
-
γ-Al2O3制备[10]. 称取16.8 g异丙醇铝和14.4 g葡萄糖溶于216 mL水中,在35 ℃恒温条件下搅拌6 h,用硝酸溶液(质量分数为10%)将pH调到5.5,继续搅拌24 h,将其在100 ℃下烘干,然后用马弗炉在600 ℃下煅烧6 h得到γ-Al2O3.
TiO2/γ-Al2O3制备. 称取6 g γ-Al2O3和适量TBOT(Al/Ti = 75)溶于200 mL水中,在35 ℃条件下搅拌24 h后在100 ℃烘干,然后用马弗炉600 ℃煅烧6 h得到TiO2/γ-Al2O3. 经前期实验优化,当Al/Ti = 75时,TiO2/γ-Al2O3催化臭氧氧化水中布洛芬具有最佳催化活性.
-
催化剂晶相结构由Scintag-XDS-2000型X射线衍射仪(XRD)测试分析,用Cu Kα(λ = 0.154 nm)辐射为激发源,操作电压为40 kV,操作电流为100 mA. 催化剂的比表面积、孔径和孔容采用美国Micromeritics公司的ASAP-2020比表面积仪测定. 孔隙度是基于氮气吸附-解吸等温线及Barrett-Joyner-Halenda(BJH)法获得的. 利用日本Hitachi公司 SU8020 FESEM电子扫描电镜对催化剂形貌和结构进行了观察. 用马尔文Nano-ZS90型Zeta电位计测定了催化剂的表面零电荷点(pHpzc). 利用Nicolet 6700红外光谱(Pyridine-FTIR)对吡啶吸附的催化剂酸性位进行了表征. 具体方法:压片后的催化剂放入红外的样品池中,将样品在常温真空处理30 min,获得样品背景谱图,该仪器的分辨率为4 cm−1. 再分别于20 ℃,150 ℃和350 ℃解吸得到样品的吡啶红外谱图. 因红外谱图峰高正比于催化剂的酸量. 依此,可对催化剂的表面酸性作定性或半定量的分析[11].
-
催化臭氧氧化试验是在一个1.2 L鼓泡式玻璃反应器内进行的. 利用高纯度氧气作为气源,通过臭氧发生器(购于北京同林科技有限公司)进行放电生成臭氧,流量计测量后送入玻璃反应器底部微孔砂心布气头,经过磁力搅拌器搅拌后臭氧可均匀的分布于水中. 具体实验参数:在20 ℃条件下,将1 L浓度为10 mg·L−1的布洛芬水溶液为模型化合物和前期优化确定的1.5 g催化剂加入到反应器中,利用盐酸或氢氧化钠调节pH到7.0,然后通入臭氧浓度为30 mg·L−1的O3/O2混合气体,气体的流速为200 mL·min−1. 反应后残余的臭氧用碘化钾溶液吸收. 每隔一段时间采样1次,所取样品立即加几滴0.1 mol·L−1硫代硫酸钠以终止溶液中残余臭氧,然后用0.45 μm醋酸纤维滤膜滤出样品待测.
-
水溶液中布洛芬的浓度采用高效液相色谱(1200 series;Agilent,Santa Clara,CA)测定,色谱柱为Eclipse XDB-C18 column(5 μm,4.6 mm×150 mm;Agilent). 分析条件为:流动相为60:40(V/V)乙腈:磷酸盐缓冲液(20 mmol·L−1,pH = 2.5)溶液,柱温40 ℃,进样量20 μL,流量为1 mL·min−1,紫外检测波长220 nm. 总有机碳分析(TOC)采用岛津TOC-VCPH型总有机碳分析仪测定. 利用电感耦合等离子体原子发射光谱分析(ICP-OES)分析溶液中金属离子浓度. 气相臭氧浓度采用IDEAL-2000型臭氧浓度检测仪进行检测. 用靛蓝法测定了水中溶解臭氧的浓度[12]. 利用BMPO作为自由基捕捉获剂,利用德国布鲁克公司ESP300E型电子顺磁共振波谱仪(EPR)对反应溶液中的羟基自由基及超氧自由基进行直接测定,微波频率为9.79 GHz,功率为5.05 mW. 原位激光显微拉曼光谱分析利用Lab RAM HR Evolution型共聚焦激光显微拉曼光谱仪进行测定. 激光激发波长633 nm、分辨率为1 cm−1、持续时间为100 s. 样品制备方法:将0.1 g催化剂与2 mL超纯水或者饱和臭氧水摇匀后,快速滴入载玻片,调整视野开始扫描测定.
-
图1是γ-Al2O3和TiO2/γ-Al2O3的小角和广角XRD图谱. 在小角范围,γ-Al2O3和TiO2/γ-Al2O3在1o附近都显示出一个很强的衍射峰,说明这两种催化剂都具有介孔结构;在广角范围,Al2O3显示出典型的γ相(JCPD no. 00-046-1131)[10, 13]. TiO2/γ-Al2O3样品除了出现γ-Al2O3的特征峰,同时在25.3o出现强的TiO2的特征峰,说明TiO2负载于γ-Al2O3表面.
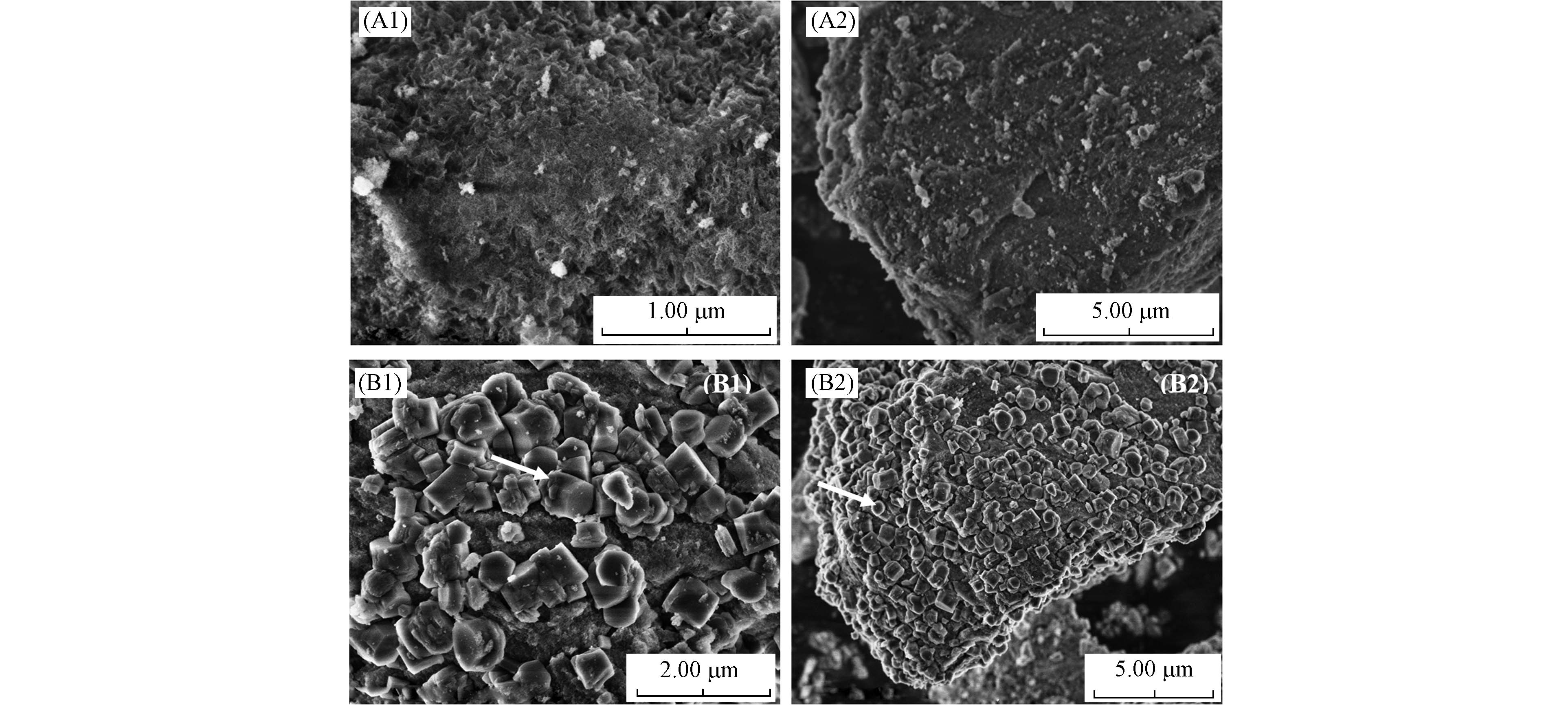
γ-Al2O3和TiO2/γ-Al2O3的扫描电镜照片见图2,γ-Al2O3显示出海绵结构,而对于TiO2/γ-Al2O3大的TiO2颗粒出现在γ-Al2O3表面,证实了TiO2负载于γ-Al2O3表面.
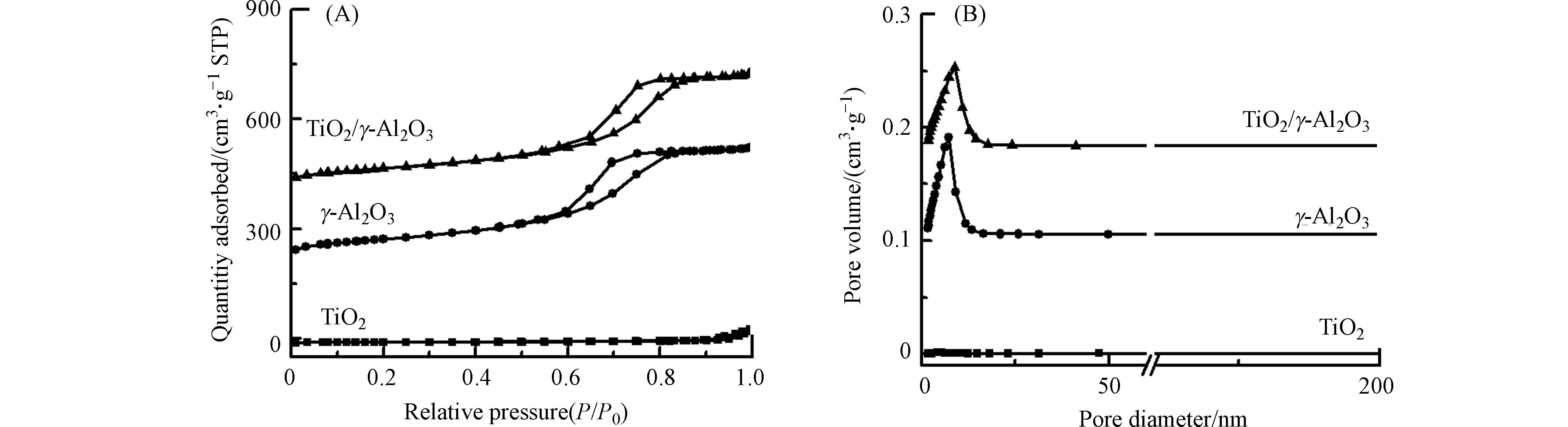
图3是γ-Al2O3和TiO2/γ-Al2O3的氮气吸附脱附等温线和孔径、孔容分布图. 所有样品都符合第Ⅳ类吸附等温线特征,说明这两种催化剂都有介孔结构,都表现出集中的孔径分布. 另外从表1可以看出,与γ-Al2O3相比,TiO2/γ-Al2O3的比表面积、孔径、孔容下降,这是由于TiO2负载于γ-Al2O3表面.
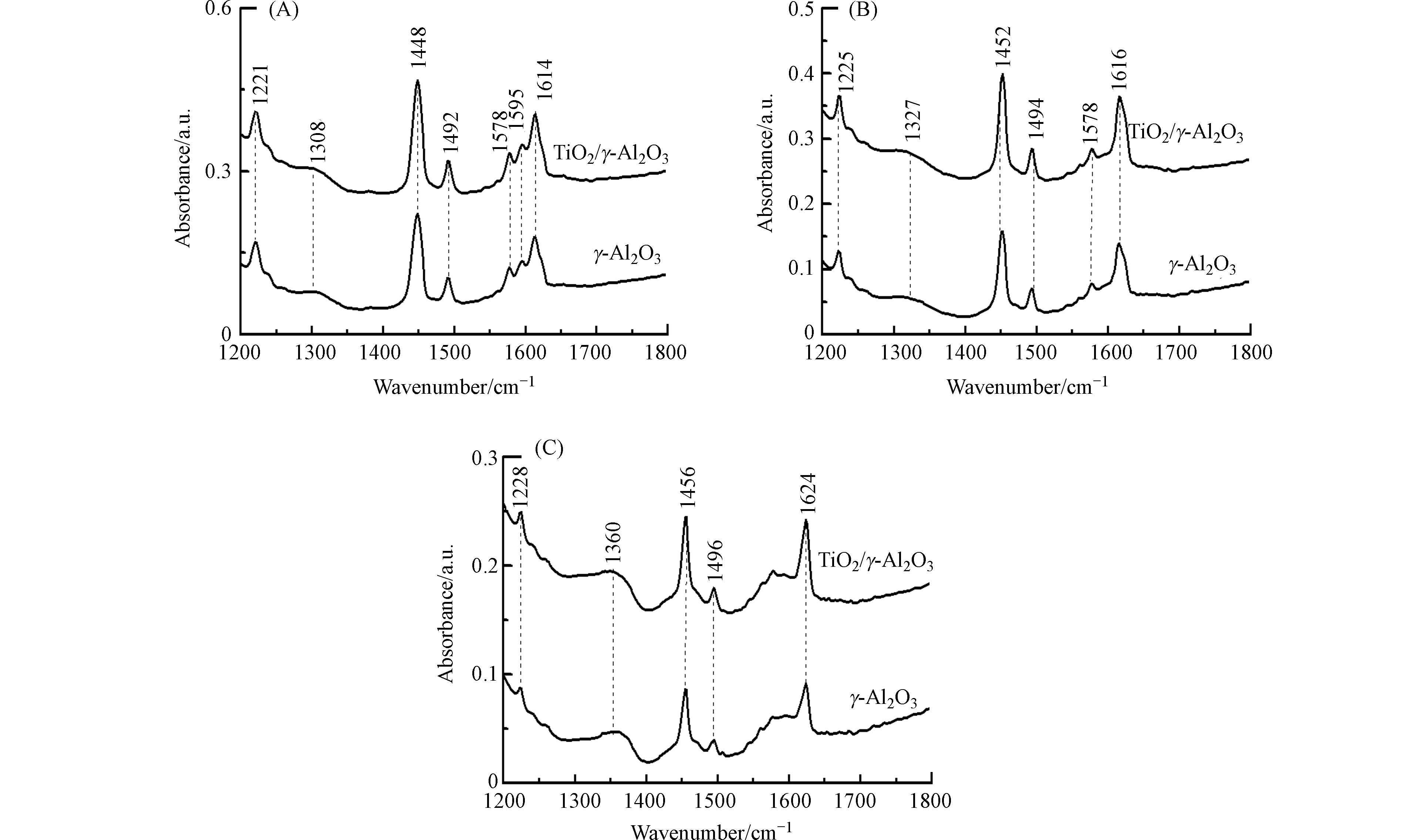
利用吡啶-红外光谱技术,对γ-Al2O3和TiO2/γ-Al2O3催化剂表面的酸性位进行了分析. 图4为催化剂在20 ℃、150 ℃和350 ℃脱附的吡啶-红外光谱. 在1200—1800 cm−1范围的吡啶-红外光谱代表吡啶环υ(C—N)和υ(C—C)振动[14]. 在20 ℃条件下脱附,γ-Al2O3和TiO2/γ-Al2O3催化剂在1221、1308、1448、1492、1578、1595、1614 cm−1位置都有吸收,TiO2/γ-Al2O3具有比γ-Al2O3更强的吸收强度. 吡啶-红外光谱在1221、1308、1448、1492、1614 cm−1处的吸收峰,代表吡啶被催化剂Lewis酸性位所吸附[15]. 150 ℃解吸时,1595 cm−1处的吸收峰消失表明吡啶和催化剂表面之间的弱相互作用,代表吡啶在催化剂表面上吸附氢键的位置. 350 ℃脱附过程中,1578 cm−1吸收峰逐渐消失,表明吡啶在中强的Lewis酸性位上发生了吸附. 随脱附温度升高,吡啶的吸收峰迁移到较高频位,表明吡啶在强Lewis酸性位发生了吸附. 基于不同温度条件下催化剂脱附曲线,进一步定量分析了γ-Al2O3和TiO2/γ-Al2O3表面的Lewis酸量[11]. 从表2可见,TiO2/γ-Al2O3与γ-Al2O3相比Lewis酸量略有增加. 这些结果说明钛负载于γ-Al2O3表面能够增大γ-Al2O3的Lewis酸量.
-
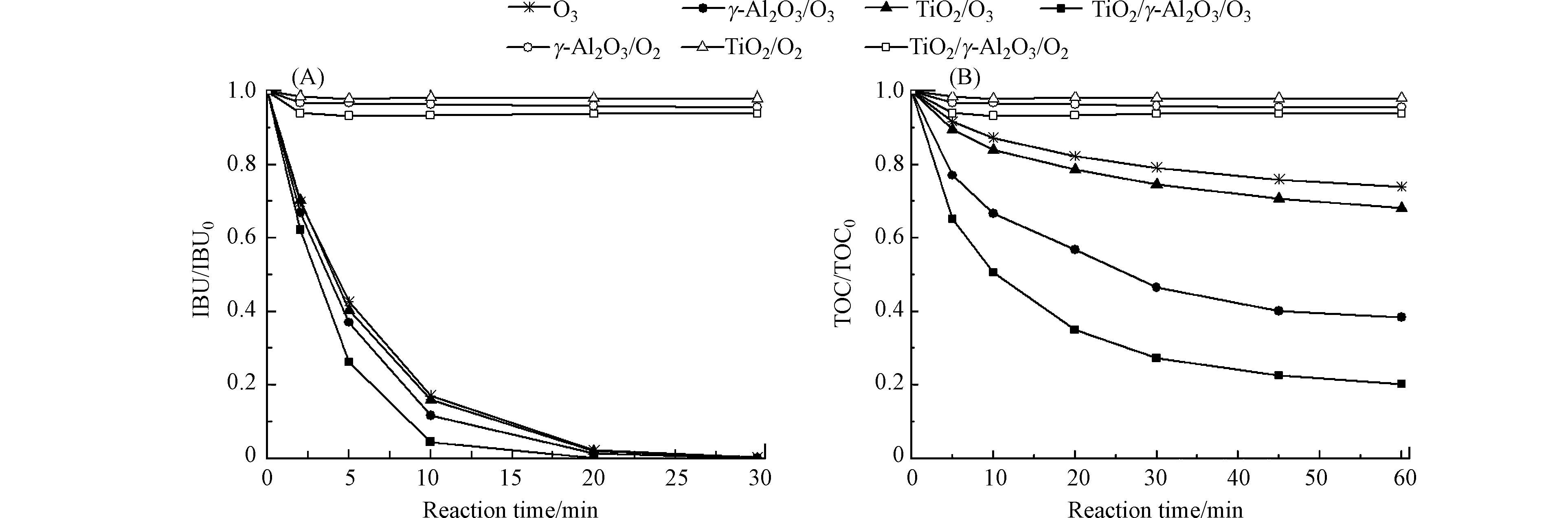
图5是不同工艺对布洛芬的催化性能. 由图5可知,TiO2/γ-Al2O3/O2、γ-Al2O3/O2和TiO2/O2过程对布洛芬的吸附去除率分别为6%、4%和2%;催化臭氧氧化比单独臭氧氧化更有利于布洛芬降解;TiO2/γ-Al2O3/O3工艺具有最高的催化活性. 反应30 min时,不同工艺过程均将布洛芬完全降解,催化臭氧氧化过程可极大提高布洛芬的矿化率. 反应60 min,TiO2/γ-Al2O3对布洛芬的TOC去除率达到80%,γ-Al2O3、TiO2及单独臭氧氧化过程中TOC去除率分别达62%、32%及26%. 由于布洛芬在TiO2/γ-Al2O3表面的吸附去除率只有6%,表明TiO2/γ-Al2O3与臭氧之间具有良好的协同作用. 通过对比表2和图5,可以发现催化剂表面的Lewis酸量与其催化活性呈正相关,TiO2/γ-Al2O3最大Lewis酸量使其具备最高的催化活性.
-
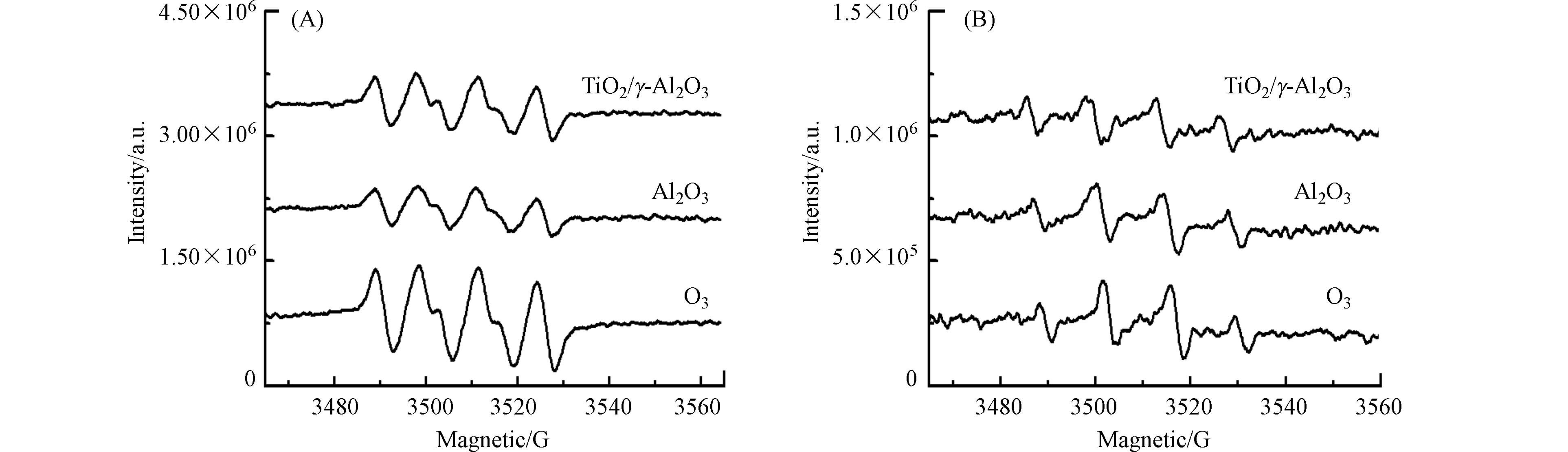
催化臭氧氧化过程,催化剂的表面属性与活性位共同决定了臭氧的吸附与高效分解. 文献报道臭氧可以在Lewis酸性位替代羟基基团与水分子结合,进行有效分解成活性氧物种[16]. 利用电子顺磁共振光谱对反应体系产生的羟基自由基及超氧自由基进行了考察. 由图6可见,在γ-Al2O3和TiO2/γ-Al2O3的臭氧悬浆,羟基自由基和超氧自由基的信号比单独臭氧溶液的要低,说明吸附于γ-Al2O3和TiO2/γ-Al2O3催化剂表面的臭氧没有分解产生羟基自由基和超氧自由基.
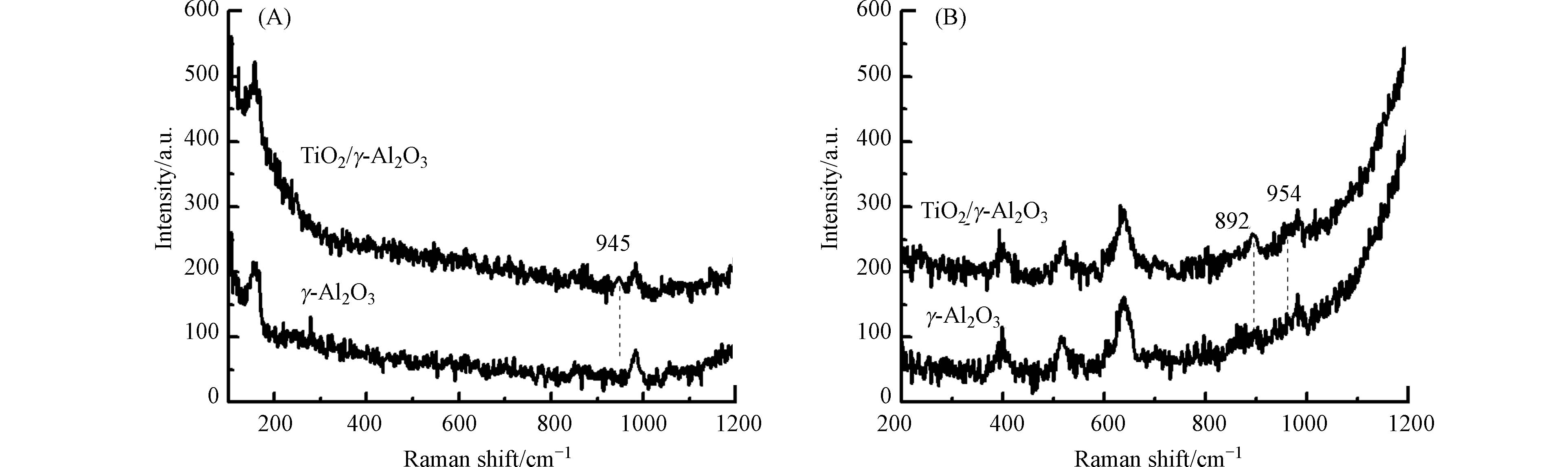
原位激光显微拉曼光谱用于研究γ-Al2O3及TiO2/γ-Al2O3催化剂上形成的活性氧物种(图7). γ-Al2O3的臭氧悬浆中,γ-Al2O3的拉曼光谱在945 cm−1有一个新的吸附峰,表明γ-Al2O3催化剂上有活性原子氧生成(≡Al—*O)[16-17];TiO2/γ-Al2O3的臭氧悬浆在892 cm−1和954 cm−1均出现了一个新吸收峰,表明TiO2/γ-Al2O3催化剂上既生成了过氧物种(≡Ti4+—*O2)又生成了活性原子氧(≡Al3+—*O). 通过与γ-Al2O3催化剂的原位激光显微拉曼光谱进行比较,发现TiO2/γ-Al2O3的拉曼光谱中活性氧物种吸收峰的位置有所偏移. 这是由于钛负载于γ-Al2O3表面改变了钛原子和铝原子对表面吸附的活性氧原子和过氧基团的键强度引起的[18-20]. 原位激光显微拉曼光谱证实了活性原子氧和过氧物种是TiO2/γ-Al2O3催化臭氧氧化反应的活性氧物种. 综合以上研究结果可得:TiO2/γ-Al2O3催化剂表面的Lewis酸性位为臭氧分解的活性位,当臭氧在γ-Ti-Al2O3催化剂表面分解时,会生成相对稳定的活性原子氧和过氧物种,这有助于有机物的吸附矿化.
-
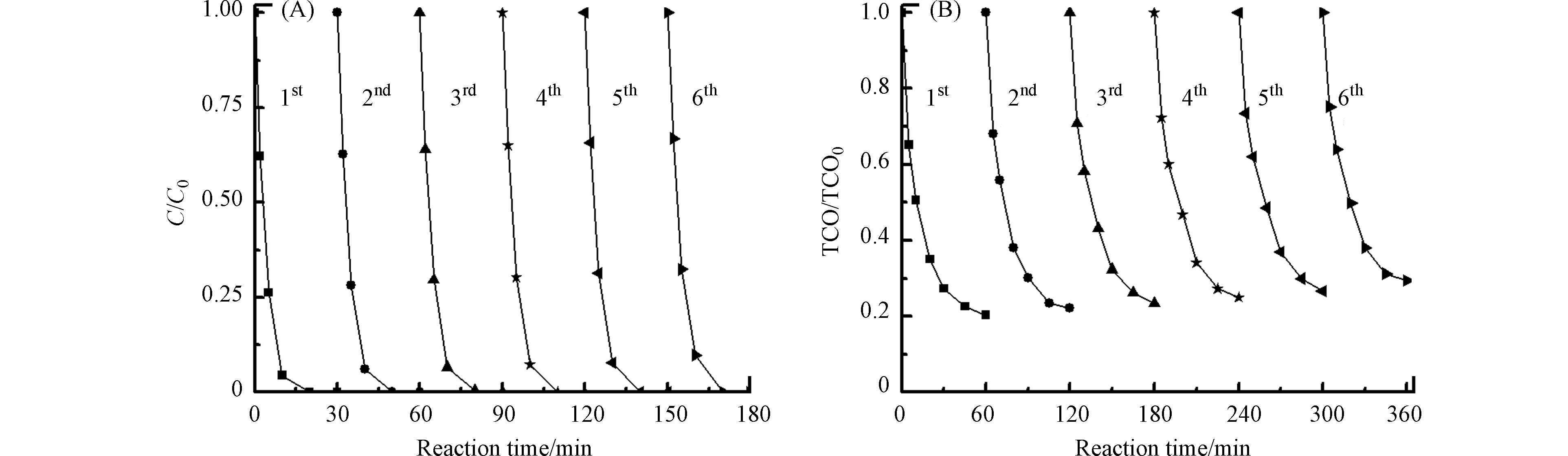
图8是TiO2/γ-Al2O3催化剂的活性与稳定性实验. 催化剂重复使用6次后,布洛芬的降解速率几乎不变,TOC的去除率重复3次后就基本稳定,仅下降6%,并且反应后溶液中未检测到Ti、Al的溶出,这说明TiO2/γ-Al2O3催化剂具有很好的活性与稳定性.
-
成功制备了钛负载γ-Al2O3介孔分子筛催化剂. XRD、SEM、氮气吸附脱附和吡啶红外光谱表征结果表明,TiO2/γ-Al2O3催化剂具有大的比表面积,更多的Lewis酸量,二氧化钛高度分散于γ-Al2O3表面. TiO2/γ-Al2O3催化臭氧氧化布洛芬结果表明,该催化剂具有良好的催化活性与稳定性,反应60 min时,TOC的去除率为80%. 单独臭氧氧化过程中TOC的去除率仅为26%. 电子顺磁共振和原位激光显微拉曼光谱实验结果表明,TiO2/γ-Al2O3催化剂表面的Lewis酸性位是催化臭氧氧化反应的活性位,臭氧分解产生活性原子氧和过氧物种有利于有机物的矿化,从而表现出最高的催化活性. TiO2/γ-Al2O3是一种有前景的臭氧化催化剂.
TiO2/γ-Al2O3催化臭氧氧化水中布洛芬
Catalytic ozonation of ibuprofen by TiO2/γ-Al2O3 in water
-
摘要: 以葡萄糖为模板,采用蒸发诱导自组装法合成了γ-Al2O3介孔催化剂,以钛酸四正丁酯(TBOT)为原料制备TiO2/γ-Al2O3催化剂,并将其用于催化臭氧氧化水中布洛芬(IBU). X射线衍射、扫描电子显微镜、氮气吸附-脱附和吡啶红外光谱表征结果表明,TiO2均匀负载于γ-Al2O3表面,保持γ-Al2O3有序的介孔结构,具有较大的比表面积和更多的Lewis酸性位. 不同催化剂活性评价结果表明,TiO2的负载显著提高γ-Al2O3催化臭氧氧化水中布洛芬的活性,反应60 min,TiO2/γ-Al2O3催化剂的TOC去除率达到80%,而γ-Al2O3、TiO2及单独臭氧氧化过程中TOC去除率则分别达到62%、32%及26%. 电子顺磁共振和原位激光显微拉曼光谱实验结果表明,Lewis酸性位为臭氧高效分解的活性位,活性原子氧和过氧物种是TiO2/γ-Al2O3催化臭氧氧化反应的活性氧物种,有利于有机物的矿化,从而表现出最高的催化活性. 重复利用实验结果表明,TiO2/γ-Al2O3催化剂具有较长的使用寿命.
-
关键词:
- TiO2/γ-Al2O3 /
- 催化臭氧氧化 /
- 活性原子氧 /
- 布洛芬.
Abstract: The γ-Al2O3 mesoporous catalyst was synthesized by evaporation-induced self-assembly method using glucose as template. TiO2/γ-Al2O3 catalyst was prepared from tetrabn-butyl titanate (TBOT) as raw material, and was used to catalytic ozonation of ibuprofen (IBU) in water. The results of X-ray diffraction, scanning electron microscopy, nitrogen adsorption-desorption and pyridine infrared spectroscopy showed that titanium dioxide was uniformly loaded on the surface of γ-Al2O3, maintaining the ordered mesoporous structure of γ-Al2O3, with larger specific surface area and more Lewis acidic sites. The evaluation results of different catalyst activities showed that TiO2 support significantly improved the activity of γ-Al2O3 catalytic ozonation of ibuprofen in water, and the TOC removal rate of TiO2/γ-Al2O3 catalyst reached 80% after 60 min reaction. The TOC removal rates of γ-Al2O3, TiO2 and ozonation alone reached 62%, 32% and 26%, respectively. The results of electron paramagnetic resonance (EPR) and in-situ laser micro-Raman spectroscopy showed that Lewis acid sites were the active site for effective decomposition of ozone, while active atomic oxygen and peroxy species were the active oxygen species of TiO2/γ-Al2O3 catalytic ozonation reaction, which was conducive to the mineralization of organic matter and showed the highest catalytic activity. The reuse experiment results showed that the TiO2/γ-Al2O3 catalyst had a long service life.-
Key words:
- TiO2/γ-Al2O3 /
- catalytic ozonation /
- active atomic oxygen /
- ibuprofen.
-

-
表 1 催化剂的比表面、孔径、孔容和孔壁
Table 1. Specific surface area, pore diameter, pore volume, and pore wall of catalysts
催化剂
Catalyst比表面积/ (m2·g−1)
S BET孔径/ nm
Pore diameter孔容/(cm3·g−1)
Pore volumeγ-Al2O3 252.81 0.50 6.13 TiO2/γ-Al2O3 221.97 0.47 5.86 表 2 吡啶-红外光谱测定的不同催化剂的Lewis酸量
Table 2. Amount of Lewis acid sites of different catalysts determined by Pyridine-FTIR
催化剂
Catalyst总酸量/(μmol·g−1)
Total acid中强酸量/(μmol·g−1)
Medium acid强酸量/(μmol·g−1)
Strong acidγ-Al2O3 540.1 197.4 61.1 TiO2/γ-Al2O3 653.8 222.9 85.4 -
[1] WEBB S, TERNES T, GIBERT M, et al. Indirect human exposure to pharmaceuticals via drinking water [J]. Toxicology Letters, 2003, 142(3): 157-167. doi: 10.1016/S0378-4274(03)00071-7 [2] NICA A V, OLARU E A, BRADU C, et al. Catalytic Ozonation of Ibuprofen in Aqueous Media over Polyaniline–Derived Nitrogen Containing Carbon Nanostructures [J]. Nanomaterials, 2022, 12(19): 3468. doi: 10.3390/nano12193468 [3] VELDHOEN N, SKIRROW R C, BROWN L L Y, et al. Effects of acute exposure to the non-steroidal anti-inflammatory drug ibuprofen on the developing North American Bullfrog (Rana catesbeiana) tadpole [J]. Environmental Science & Technology, 2014, 48(17): 10439-10447. [4] LEGUBE B, KARPEL VEL LEITNER N. Catalytic ozonation: A promising advanced oxidation technology for water treatment [J]. Catalysis Today, 1999, 53(1): 61-72. doi: 10.1016/S0920-5861(99)00103-0 [5] ISSAKA E, AMU-DARKO J N O, YAKUBU S, et al. Advanced catalytic ozonation for degradation of pharmaceutical pollutants―A review [J]. Chemosphere, 2022, 289: 133208. doi: 10.1016/j.chemosphere.2021.133208 [6] NAWROCKI J, KASPRZYK-HORDERN B. The efficiency and mechanisms of catalytic ozonation [J]. Applied Catalysis B:Environmental, 2010, 99(1/2): 27-42. [7] WANG Y, DUAN X, XIE Y, et al. Nanocarbon-Based Catalytic Ozonation for Aqueous Oxidation: Engineering Defects for Active Sites and Tunable Reaction Pathways [J]. ACS Catalysis, 2020, 10(22): 13383-13414. doi: 10.1021/acscatal.0c04232 [8] NAWROCKI J. Catalytic ozonation in water: Controversies and questions. discussion paper [J]. Applied Catalysis B:Environmental, 2013, 142/143: 465-471. doi: 10.1016/j.apcatb.2013.05.061 [9] WANG Y J, YU G. Challenges and pitfalls in the investigation of the catalytic ozonation mechanism: A critical review [J]. Journal of Hazardous Materials, 2022, 436: 129157. doi: 10.1016/j.jhazmat.2022.129157 [10] XU B J, XIAO T C, YAN Z F, et al. Synthesis of mesoporous alumina with highly thermal stability using glucose template in aqueous system [J]. Microporous and Mesoporous Materials, 2006, 91(1/2/3): 293-295. [11] EMEIS C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts [J]. Journal of Catalysis, 1993, 141(2): 347-354. doi: 10.1006/jcat.1993.1145 [12] BADER H, HOIGNÉ J. Determination of ozone in water by the indigo method [J]. Water Research, 1981, 15(4): 449-456. doi: 10.1016/0043-1354(81)90054-3 [13] OSTOVAR S, MOUSSAVI G, MOHAMMADI S, et al. Developing a novel Ti-doped γAl2O3 xerogel with high photocatalytic chemical and microbial removal performance: Characterization and mechanistic insights [J]. Chemical Engineering Journal, 2023, 464: 142545. doi: 10.1016/j.cej.2023.142545 [14] ANDONOVA S M, ŞENTÜRK G S, KAYHAN E, et al. Nature of the Ti−Ba Interactions on the BaO/TiO2/Al2O3 NOx Storage System [J]. The Journal of Physical Chemistry C, 2009, 113(25): 11014-11026. doi: 10.1021/jp9005026 [15] MORISHIGE K, KITTAKA S, KATSURAGI S, et al. Thermal desorption and infrared studies of ammonia, amines and pyridines chemisorbed on chromic oxide [J]. Journal of the Chemical Society, Faraday Transactions 1:Physical Chemistry in Condensed Phases, 1982, 78(10): 2947-2957. doi: 10.1039/f19827802947 [16] BING J, HU C, NIE Y, et al. Mechanism of Catalytic Ozonation in Fe2O3/Al2O3@SBA-15 Aqueous Suspension for Destruction of Ibuprofen [J]. Environmental Science & Technology, 2015, 49(3): 1690-1697. [17] YU G, WANG Y, CAO H, et al. Reactive oxygen species and catalytic active sites in heterogeneous catalytic ozonation for water purification [J]. Environmental Science & Technology, 2020, 54(10): 5931-5946. [18] RADHAKRISHNAN R, OYAMA S T. Ozone decomposition over Manganese oxide supported on ZrO2 and TiO2: A kinetic study using in situ laser Raman spectroscopy [J]. Journal of Catalysis, 2001, 199(2): 282-290. doi: 10.1006/jcat.2001.3167 [19] AND R R, OYAMA S, CHEN J G, et al. Electron transfer effects in ozone decomposition on supported Manganese oxide [J]. Journal of Physical Chemistry B, 2001, 105: 4245-4253. doi: 10.1021/jp003246z [20] ZHANG T, LI W W, CROUÉ J P. Catalytic ozonation of oxalate with a cerium supported palladium oxide: An efficient degradation not relying on hydroxyl radical oxidation [J]. Environmental Science & Technology, 2011, 45(21): 9339-9346. -




 下载:
下载: