-
粉煤灰是煤粉经炉膛高温燃烧后,烟气经除尘装置收集得到的粉状固体物质. 我国粉煤灰近年的产生量均在5亿t以上,2021年我国粉煤灰年产量达到7.9亿t,综合利用率在75%左右[1 − 4],主要用作建筑工程中,如生产水泥、混凝土和建筑材料等[2, 5];用作环保材料的粉煤灰陶粒也有大量研究并实现了工程应用. 然而,大量库存粉煤灰不仅存在占地面积大、长期管理成本高的问题,同时还有潜在环境污染风险.
从化学组成、矿物组成和反应性等方面了解粉煤灰性质,对发挥粉煤灰自身性质优势进行资源化有着不可忽视的作用[2]. 粉煤灰的主要化学成分为SiO2、Al2O3、Fe2O3和少量CaO、MgO等[2 − 4]. 粉煤灰的矿物组成主要包括铝硅酸盐玻璃体、晶体矿物(石英、石灰、磁铁矿、赤铁矿等)以及未燃尽的碳粒[2, 5]. 粉煤灰的化学组成和矿物组成赋予了粉煤灰一定的火山灰活性,即能够在水分存在的条件下与氢氧化钙等碱性金属氢氧化物发生反应,生成水硬胶凝性化合物[6 − 7]. 其次,粉煤灰具有疏松多孔、比表面积较大、表面能高、且存在着许多铝、硅等活性点以及具有较强的物理和化学吸附能力,因此将粉煤灰进行改性制备为吸附剂、絮凝剂等用于去除污水中重金属、COD、氨氮、磷酸盐等污染物也是粉煤灰资源化的研究热点[2, 5, 8].
由矿物相组成可知,硅铝玻璃体为粉煤灰的主要组成部分,硅铝玻璃体内含有大量的活性Al2O3和SiO2,但致密的结构抑制了活性成分发挥作用,导致粉煤灰对磷的吸附容量并不高[9 − 10],通过改性是提高粉煤灰吸附容量最为常用的办法,改性方式包括碱改性、酸改性和制成陶粒等.
碱改性可以通过OH-破坏粉煤灰玻璃体表面的Si—O和Al—O键而使其解构,增大比表面,释放玻璃体内部的活性Al2O3和活性SiO2,提升了除磷容量[11 − 12]. Pengthamkeerati利用氢氧化钠对粉煤灰改性后除磷能力大幅度提升,其主要原因是比表面积3.39 m2·g−1增加到35.38 m2·g−1,且玻璃体溶出的活性金属物质提升了粉煤灰絮凝沉淀除磷的能力[13]. 鉴于粉煤灰所具有的火山灰活性,在Ca2+等存在的条件下,粉煤灰可以发生水化反应产生水化硅酸钙、水合铝酸盐等凝胶态产物,提升粉煤灰颗粒的比表面积和吸附性能[14 − 15]. 但当前对于粉煤灰水化反应和除磷反应间的耦合反应机制还未见详细报道,两者之间的协同关系有待研究.
酸改性可以通过酸使粉煤灰玻璃体表面形成凹槽和孔洞,内部的Fe3+、Al3+溶出,提高了粉煤灰的粗糙度和比表面积,提升了对磷的去除效果[16]. 刘文辉等利用不同硫酸、盐酸、硫酸与盐酸混合酸对粉煤灰进行了改性处理,发现酸性改性剂可以激发粉煤灰活性,增加粉煤灰的比表面积,暴露大量的Al、Si等活性点,提高除磷容量. 但是随着酸改性剂浓度的增加,磷的去除率反而下降,可能是由于强酸改性剂的加入会使污水pH值急剧下降,影响了Al、Fe等絮凝性[17].
以黏土、页岩、污泥等为原材料制备的陶粒有较好的除磷效果,而粉煤灰的化学组成与上述材料的化学组成较为相似,因此利用粉煤灰制备陶粒是可行的[18]. Cheng等以粉煤灰、污泥和牡蛎壳为原材料在
1050 ℃下烧结8 min得到烧结陶粒作为湿地填料进行除磷,其最大吸附量可达到4.51 mg·g−1,化学吸附是其除磷的主要机制[19]. 常规粉煤灰陶粒制备方式主要有烧结法和免烧法两种,烧结陶粒和免烧颗粒孔隙较少且封闭,影响污染物去除容量,同时限制其内部有效成分的污染物去除效用[20 − 22].利用粉煤灰火山灰活性开发污染控制技术中的环保材料具有巨大资源化潜力,但目前国内外对于粉煤灰基除磷材料的研究依旧存在颗粒内部反应机制研究不充分、吸附容量小、能耗成本高等问题,限制了粉煤灰作为环保除磷材料的应用. 本研究利用生石灰作为弱碱激发剂激发粉煤灰活性,添加秸秆纤维煅烧造孔,开发了具备空间网络多孔结构的秸秆粉煤灰基除磷颗粒填料,并验证了除磷过程粉煤灰活性Al2O3激发水化与除磷反应相互耦合的机理.
-
粉煤灰、水泥、生石灰和硫酸钙均来自于巩义市元亨净水材料厂,秸秆选用破碎为纤维状的湖北鄂州水稻秸秆. 生石灰和硫酸钙纯度均大于90 %,粉煤灰以及水泥的化学组成见表1. 本实验所用含磷实验用水均采用磷酸二氢钾(AR,国药集团化学试剂有限公司)进行配置.
-
将粉煤灰、生石灰和水泥按质量比7:1:1混合,添加三者总质量3%的硫酸钙和1%的秸秆,再添加物料总质量20%的水混合均匀. 将混合物倒入圆盘造粒机,喷加物料总质量10%的雾状水进行造粒,取出直径为2—5 mm的颗粒在20 ℃下恒温保湿养护. 将养护5 d后的颗粒在马弗炉中于300 ℃下煅烧30 min使秸秆炭化形成网状多孔结构. 煅烧会导致水合硅酸钙胶凝结构脱水继而影响颗粒的机械强度,故须在保温(20 ℃)保湿的条件下对造孔后的颗粒进行二次养护,实现颗粒结构再水化,进一步提升颗粒的机械强度. 二次养护5 d后,即得到成品秸秆粉煤灰基颗粒填料. 参照《陶粒滤料》QB/T
4383 -2012测定其物理性质(表2). -
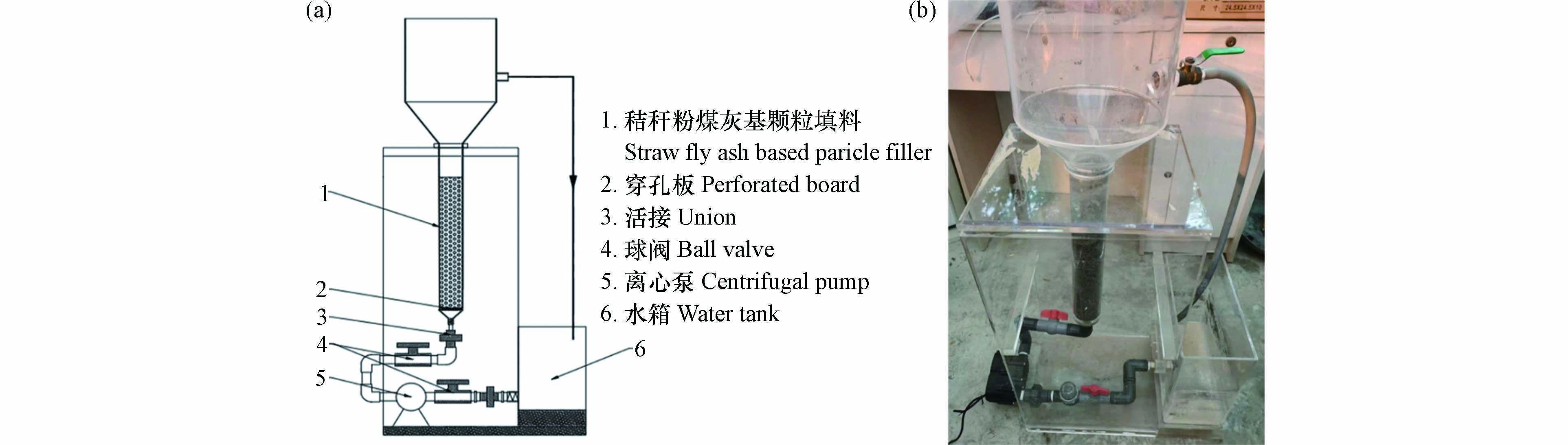
本研究设计间歇循环流态化完全混合反应器,在离心泵的作用下,水箱中的实验用水通过穿孔板从装置底部均匀分布至反应器中,颗粒填料在水力作用下呈流态化. 颗粒填料在反应器上部分离区与实验用水分离,沉降至反应器内,实验用水从溢流口排出至水箱进行循环(图1).
-
在间歇循环流态化完全混合反应器中装填500 g粒径为2—5 mm的秸秆粉煤灰基颗粒填料,加入5 L 实验用水(总磷TP为20 mg·L−1),对照组反应器中加入超纯水,启动循环水泵同步运行流态化反应器. 每批次实验循环周期为2 d,取反应结束后水样分析TP;每4天取出20 g填料测定相关指标. 通过比较除磷组和对照组颗粒填料的抗压强度、结合水含量以及粉煤灰反应程度,分析除磷过程对粉煤灰水化进程的影响;根据除磷组和对照组颗粒填料中反应产物(凝胶态)Al含量以及未反应粉煤灰(残余态)Al含量研究粉煤灰玻璃体中Al的激发规律;最后结合除磷组颗粒中磷形态及含量,分析除磷产物及秸秆粉煤灰基颗粒激发水化与除磷过程耦合机理.
-
(1)抗压强度
利用颗粒强度测定仪(型号JC-KQ-2),测定填料的抗压强度,计算公式如(1):
式中:
$ k $ 为平均抗压强度,N;$ {k}_{\mathrm{i}} $ 为每个颗粒的抗压强度,N;$ {S}_{\mathrm{i}} $ 为每个颗粒的接触面积,mm2;Z为颗粒的个数.参照《水泥化学分析方法》GB/T 176-2008中相关方法测定秸秆粉煤灰基颗粒填料内的结合水含量. 将填料放置无水乙醇中静置24 h后置于烘箱中,在105 ℃下烘干至恒重,记录其质量为m1,放入马弗炉在(950±25)℃下煅烧至恒重,记录其质量为m2. 化学结合计算公式如下:
式中:
$ {w}_{\mathrm{b}} $ 为试样化学结合水含量,%;m1为试样烘干后质量,g;m2为试样煅烧后质量,g;$ {w}_{\mathrm{F}\mathrm{A}} $ 、$ {w}_{\mathrm{F}\mathrm{A}. \mathrm{L}} $ 分别为粉煤灰的掺量和烧失量;$ {w}_{C} $ 、$ {w}_{\mathrm{C}. \mathrm{L}} $ 为秸秆粉煤灰基颗粒填料除去粉煤灰部分的质量分数和烧失量.$ {w}_{\mathrm{F}\mathrm{A}. \mathrm{C}} $ 为原料的平均烧失量.粉煤灰、水泥和生石灰的烧失量测定方法为:称取
1.0000 g样品,放入马弗炉中,在(950±25)℃下灼烧20 min,冷却后称量并反复灼烧,直至恒重. 烧失量的质量分数计算公式如下:式中:ω为烧失量的质量分数,%;m3为试样质量,g;m4为灼烧后试样质量,g.
根据《水泥化学分析方法》GB/T 176-2008,采用盐酸溶解法来测定秸秆粉煤灰基颗粒填料中粉煤灰的反应程度. 取
1.0000 g粉状样品加入100 mL盐酸(1+2)溶解液,在40 ℃下搅拌20 min. 抽滤后将滤纸及残余物在105 ℃下烘干至恒重. 粉煤灰反应程度计算公式如下:式中:
$ {\mathrm{\alpha }}_{\mathrm{F}\mathrm{A}} $ 为粉煤灰反应程度,%;$ {w}_{\mathrm{H}} $ 为粉煤灰水泥试样经盐酸溶解后残余的质量分数,%;$ {w}_{\mathrm{C}. \mathrm{H}} $ 为水泥试样(不掺粉煤灰)经盐酸溶解后的质量分数,%;$ {w}_{\mathrm{F}\mathrm{A}. \mathrm{H}} $ 为粉煤灰原灰经盐酸溶解后的质量分数,%;$ {w}_{\mathrm{b}} $ 为试样化学结合水含量,%;$ {w}_{\mathrm{F}\mathrm{A}} $ 为粉煤灰掺量,%;$ {w}_{\mathrm{C}} $ 为除去粉煤灰部分的质量分数,%.(4)残余态和凝胶态中钙和铝含量的测定[26]
利用盐酸将秸秆粉煤灰基颗粒填料内的水化产物和除磷产物(凝胶态)全部溶解,分离秸秆粉煤灰颗粒填料中未反应粉煤灰(残余态),分别测定两者之中的铝含量. 具体方法为:取
0.5000 g粉状样品加入40 mL盐酸(1+2)溶解液,在40 ℃下以160 r·min−1振荡20 min,并离心(3500 r·min−1,8 min)后分离上层清液待用. 将残余物在105 ℃下烘干至恒重,参照《固体废物 22种金属元素的测定电感耦合等离子体发射光谱法》(HJ 781)中的方法进行消解,消解液保存待用. 利用ICP-OES测定上清液、消解液中的Al含量可分别计算出溶解态和残余态中Al含量.(5)颗粒填料磷形态分级方法
参照鲍士旦《土壤农化分析》中石灰性土壤无机磷形态的分级测定方法测定秸秆粉煤灰基颗粒填料中的磷形态及含量[27]. 该提取过程将磷分为6种形态,分别为Ca2-P(磷酸二钙型磷)、Ca8-P(磷酸八钙型磷)、Al-P(铝结合态磷酸盐)、Fe-P(铁结合态磷酸盐)、O-P(闭蓄态磷)和Ca10-P(磷灰石型).
-
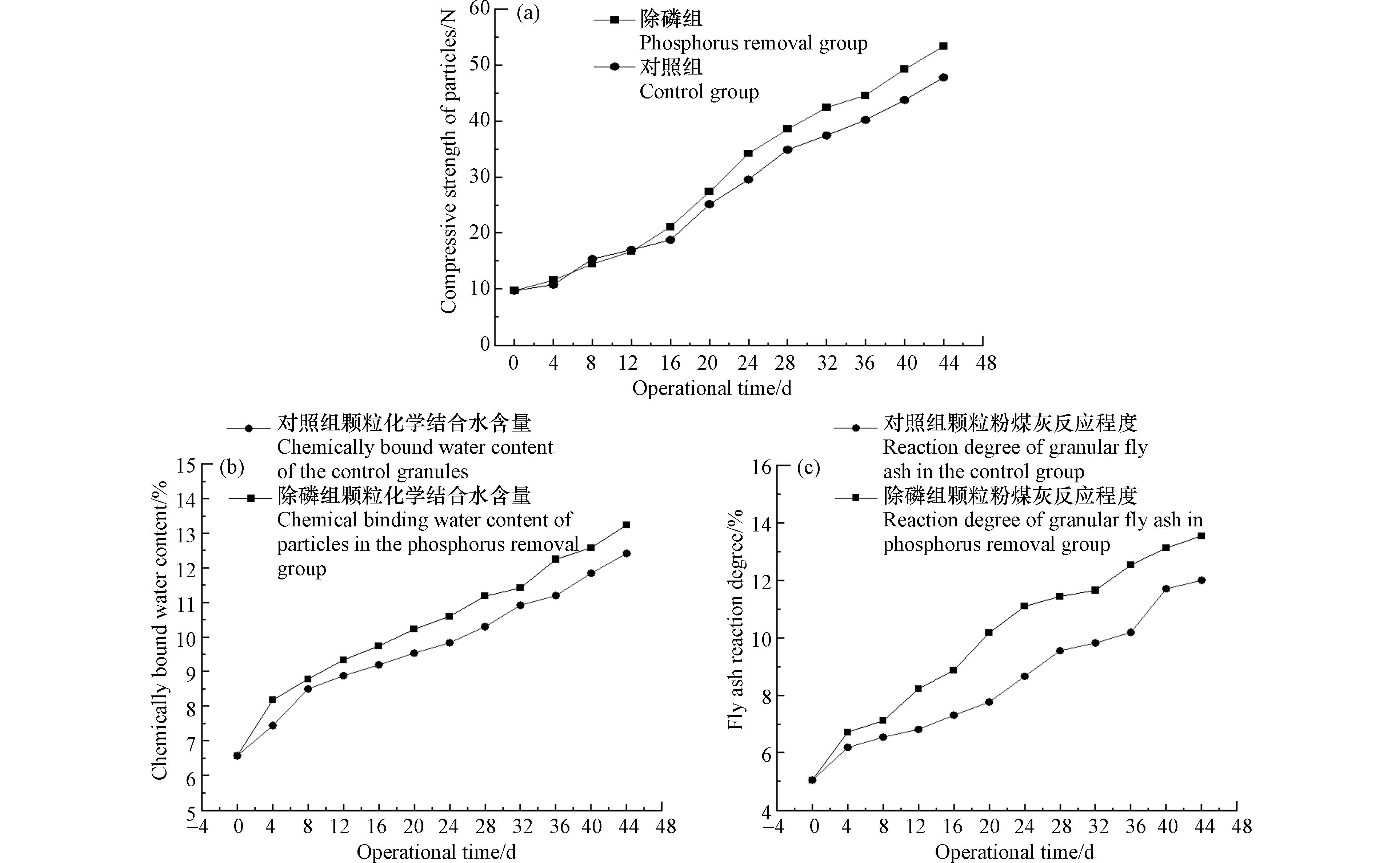
对照组和除磷组填料的颗粒强度、结合水含量和粉煤灰反应程度均随时间呈上升趋势(图2). 这说明填料内Ca(OH)2电离产生的OH-使粉煤灰玻璃体解构,释放了玻璃体内活性SiO2和活性Al2O3,发生火山灰反应生成水化硅酸钙(C—S—H)和水化铝酸钙(C—A—H)等凝胶态水化产物[28 − 29].
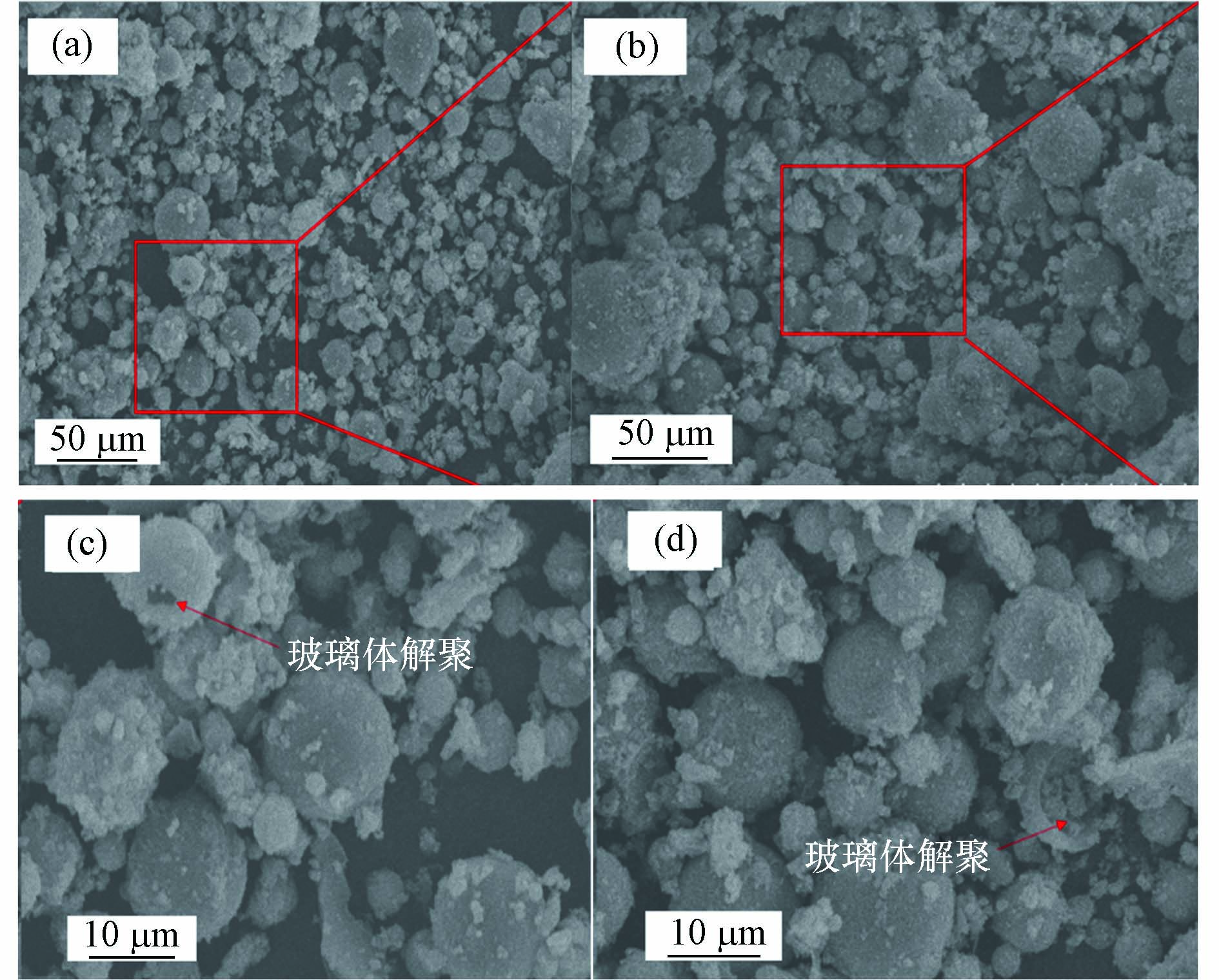
除磷组颗粒填料的颗粒强度、结合水含量和粉煤灰反应程度整体上均高于对照组,这表明除磷反应有促进水化反应进行的作用. 究其原因,颗粒填料中的钙大多以Ca(OH)2的形式存在,其溶解后的电离反应是一个动态平衡的过程(Ca(OH)2 → Ca2+ + 2OH-),在磷酸根存在的情况下,除磷组填料中Ca2+起到除磷的主导作用,因此会消耗更多的Ca2+,促使Ca(OH)2电解,提升填料孔隙内OH-浓度. 较高的OH-浓度有利于促进粉煤灰玻璃体的解构,释放更多的活性SiO2和活性Al2O3发生火山灰反应生成水化产物. 图3为对照组填料和除磷组填料在44 d时的扫描电镜图. 可以看出两个反应器的填料中均有大量凝胶态的水化产物生成,且除磷组填料中粉煤灰玻璃体解构程度更高,验证了除磷反应对粉煤灰玻璃解构的促进作用.
-
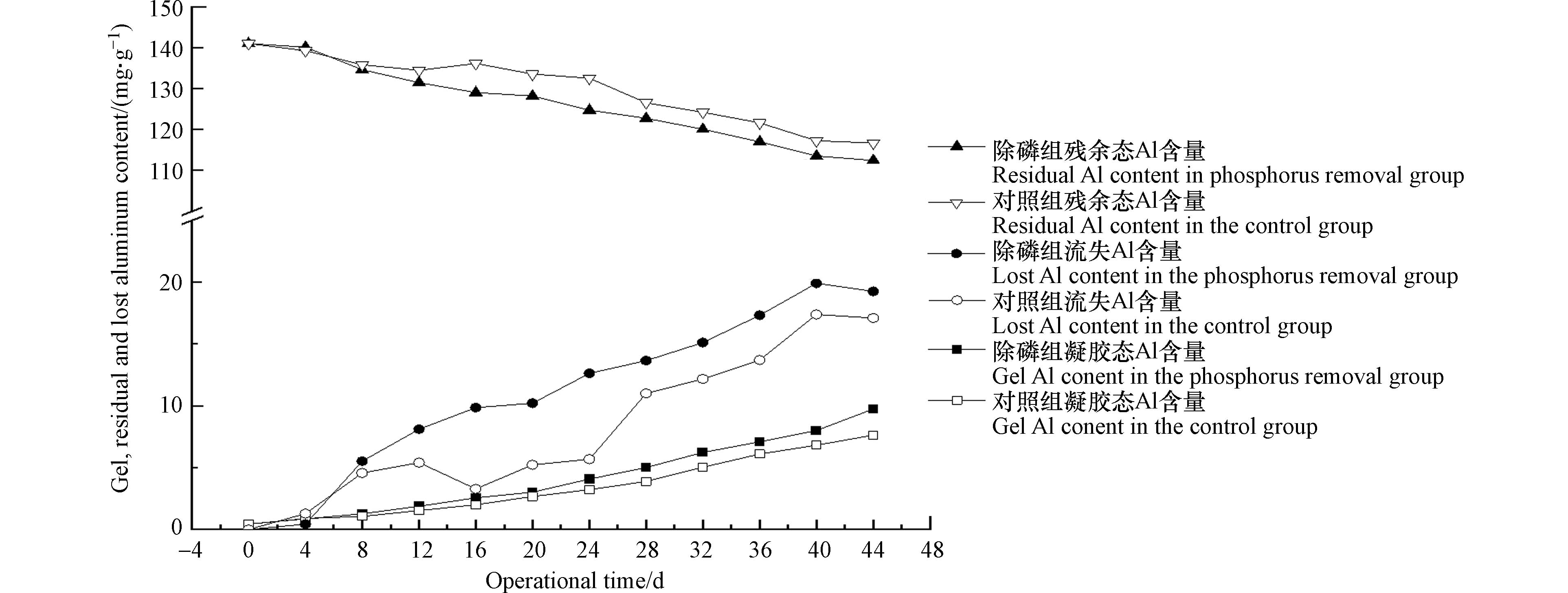
对照组和除磷组填料内凝胶态Al含量随时间稳步提升(图4),这表明粉煤灰玻璃体中的活性Al2O3得到了有效的释放,且发生水化反应或除磷反应生成相应产物. 粉煤灰玻璃体中残余态Al含量逐步降低且下降趋势较为平缓,这与冯春花等利用饱和氢氧化钙溶浸粉煤灰中活性Al2O3的结果较为类似[30]. 同时可以看出,除磷组填料内凝胶态Al含量高于对照组,这也说明除磷组填料的粉煤灰玻璃体解构程度更高,也进一步说明在部分Ca2+起到除磷作用后,填料体系内部环境有助于促进玻璃体的解构. 此外,对照组和除磷组填料激发出的Al均有较大的流失,这表明部分释放出的活性Al2O3未发生水化反应或除磷反应,在换水阶段随水流失. 在44 d时,除磷组填料中Al的流失量达到19.26 mg·g−1,约为发生凝胶态中Al含量(9.75 mg·g−1)的2倍,这说明粉煤灰激发出的Al含量远高于除磷需求量,可进一步控制粉煤灰释放活性Al2O3速率,提高活性Al2O3除磷的利用率.
-
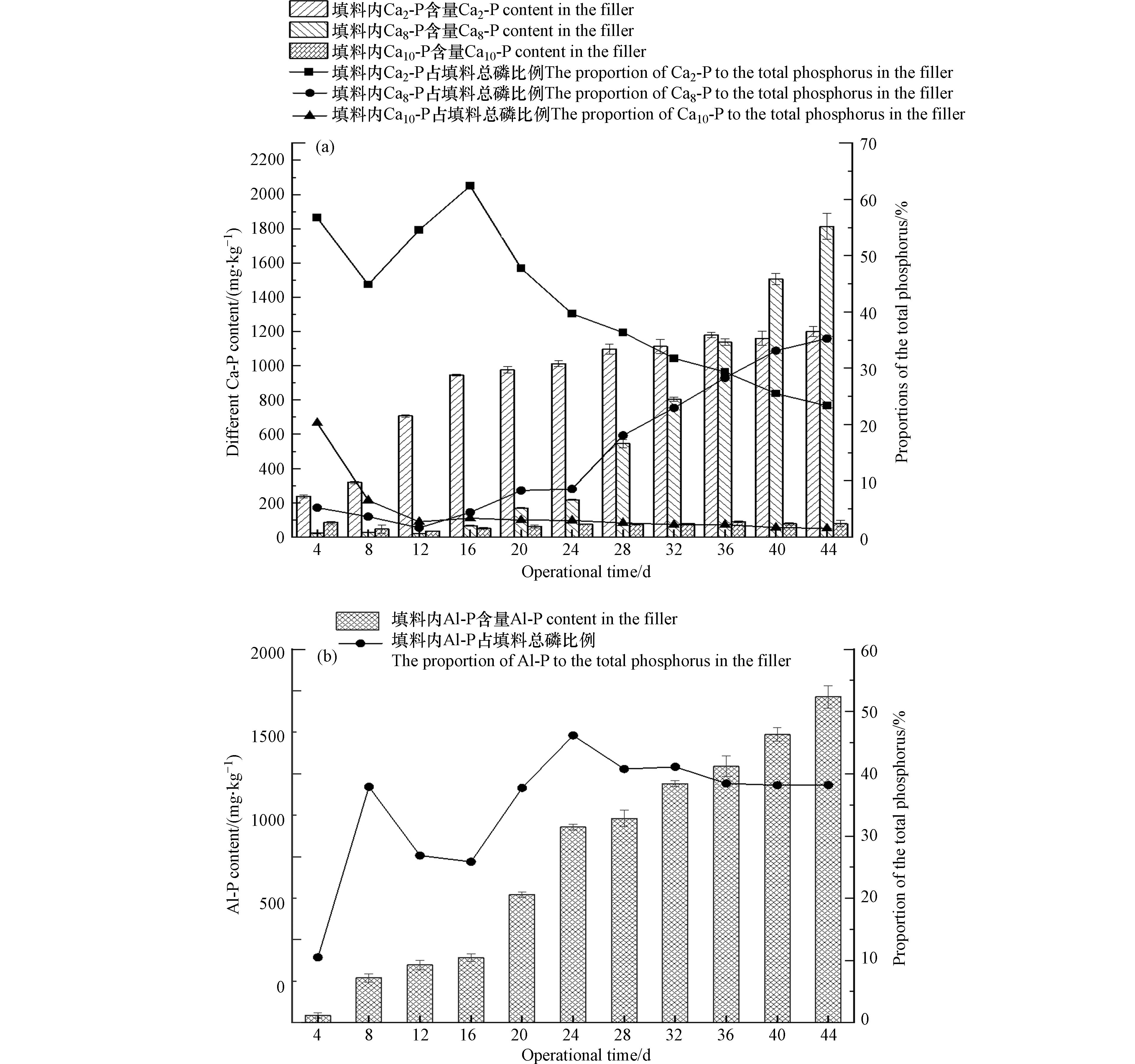
秸秆粉煤灰基颗粒填料中含有大量的Ca2+,可以与磷酸根结合形成不同形态的磷酸钙沉淀实现除磷. 根据不同时期除磷组填料内磷形态及含量可见,在0—16 d Ca2+与磷酸根结合生成Ca2-P沉淀起主导作用,占去除磷含量的44.9 %—62.4 %;在16 d后,Ca2-P含量增长速率减缓,Ca8-P含量大幅提升(图5a). 这一趋势与利用钙盐进行除磷的过程类似,即Ca2+与磷酸根先生成Ca2-P,经过再结晶后生成稳定的Ca8-P或Ca10-P[31]. 在第44 d时,填料内Ca2-P含量约占填料总磷的23 %,Ca8-P约占填料总磷的35 %,表明填料除磷产物的磷主要以钙结合态存在,Ca2+发挥较大的除磷作用.
除磷组填料中Al-P含量及占比随时间的变化趋势如图5(b)所示. 可以看出在0—8 d填料内Al-P含量经明显增长后,8—16 d填料内Al-P含量增长较为缓慢,一方面在除磷反应初期颗粒填料内Al2O3与磷反应生成Al-P后,颗粒填料内部主要发生粉煤灰玻璃体解构过程,活性Al2O3含量较低;另一方面填料内大量的Ca2+发挥主要的除磷作用. 在16 d后,除磷组填料中的Al-P含量开始显著增长,44 d后Al-P含量达到1900 mg·kg−1,在24 d后Al-P占比维持在40 %左右,在所有磷形态中占比最大. 这说明秸秆粉煤灰基颗粒填料除磷的过程中,粉煤灰玻璃体解构所释放的活性Al2O3可以发挥除磷作用. 此外,在碱激发剂的持续激发下,活性Al2O3的持续释放,可以对TP的长期有效去除.
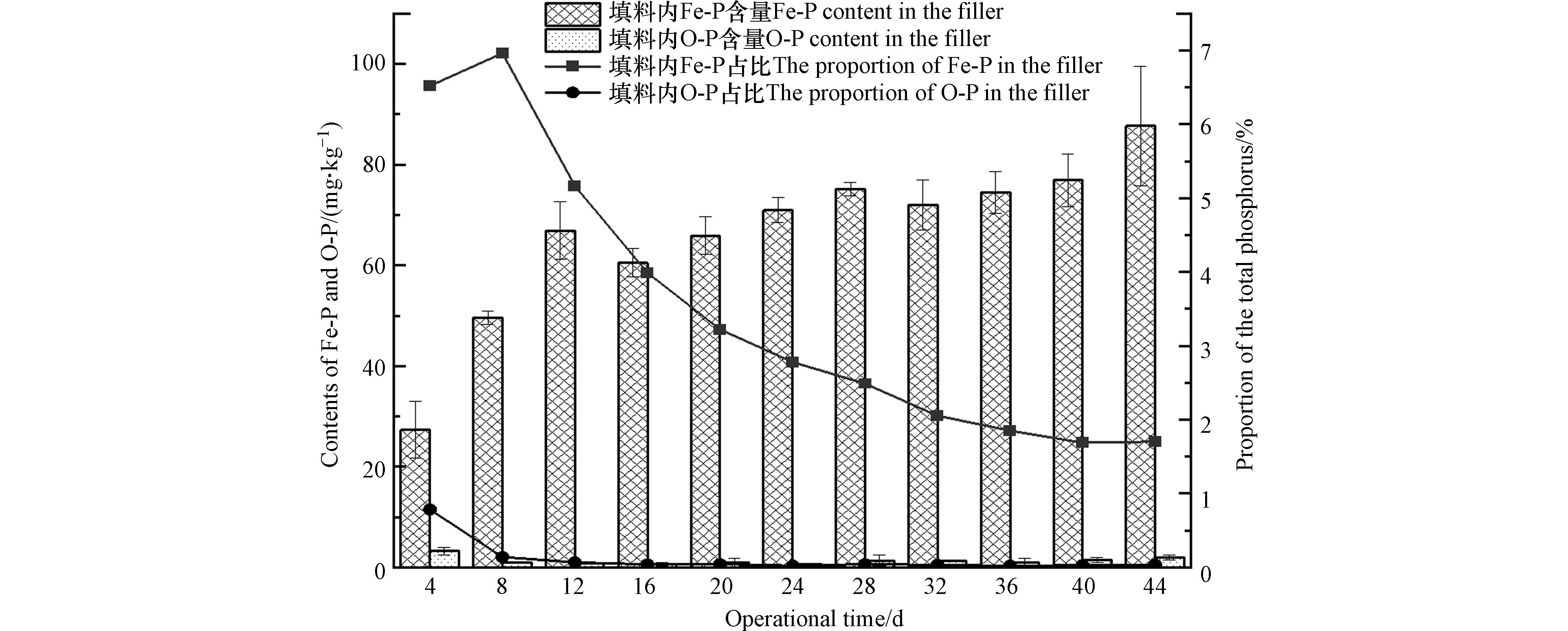
由图6可以看出在0—12 d Fe-P含量逐渐增长,在12 d时达到相对稳定,在后续运行过程中填料内Fe-P含量呈现出波动变化,在32 d后Fe-P含量缓慢增长,最终达到88 mg·kg−1,这可能是由于随着颗粒填料活性Al2O3的释放,填料颗粒内部的部分未反应的Fe参与除磷反应,使得Fe-P含量逐渐增长. 填料内Fe-P占比在0—8 d内迅速增长,8 d后逐渐下降,这是因为填料内的Fe含量有限,在0—8 d可以起到一定的化学除磷作用(占比5 %—8 %),在消耗殆尽后,主要由钙、铝发挥主要的化学除磷作用,随着Ca-P、Al-P含量的逐渐增加,Fe-P占比逐渐下降. Fe对磷的去除机理主要为体系中存在的部分表面带有正电荷的胶体状氢氧化物或氧化物吸附磷酸盐、多核氢氧化铁形成聚合物与磷酸盐发生络合反应形成共沉淀等[32 − 33]. 对于O-P而言,其定义为被氧化铁胶膜包蔽的磷酸盐. 在运行期间填料内部几乎没有闭蓄态磷的产生(图6).
综上所述,在利用秸秆粉煤灰基颗粒填料处理含磷污水时,除磷反应可以消耗更多Ca2+,从而提升填料OH-浓度,促进粉煤灰玻璃体解构,粉煤灰玻璃体解构释放的活性Al2O3可以起到较大的除磷作用. 粉煤灰激发水化和除磷反应之间的耦合作用机理为:当利用秸秆粉煤灰基颗粒填料去除污水中TP时,颗粒填料中的Ca2+与磷酸根结合占据化学除磷的主导地位. Ca2+的消耗促进了Ca(OH)2的电离,提升颗粒填料体系内的OH-浓度;OH-浓度的提升有助于促进粉煤灰玻璃体解构,释放活性Al2O3和活性SiO2. 此时,所释放的活性物质可以在Ca(OH)2存在的情况下发生火山灰反应,生成水化硅酸钙和水化铝酸钙等水化产物,而活性Al2O3同时可以发挥较大的除磷作用,进而实现长期有效除磷. 在此过程中,填料内发生的主要化学反应包括(M代表Al或Fe,与磷酸根形成络合物实现除磷):
(1)生石灰溶解和氢氧化钙电离
(2)火山灰反应[28]
-
1)利用秸秆粉煤灰基颗粒填料处理含磷污水时,Ca2+发挥除磷主导作用,前期除磷产物中磷的主要形态为Ca2-P,反应中存在Ca2-P向Ca8-P转化的过程.
2)除磷反应可以消耗更多Ca2+提升填料OH-浓度,促进粉煤灰玻璃体解构,释放更多的活性Al2O3,在Ca(OH)2存在的情况下发生火山灰反应,生成水化硅酸钙和水化铝酸钙等水化产物,可以实现长期有效除磷.
3)以生石灰作为碱激发剂可以在除磷过程中解构粉煤灰玻璃体,持续释放活性Al2O3,可实现对TP的持续去除,Al-P含量占填料内总磷含量的比例可达40%左右.
秸秆粉煤灰基颗粒填料激发水化与除磷耦合机理研究
Coupling mechanism of induced hydration and phosphorus removal with straw fly ash-based granular fillers
-
摘要: 粉煤灰添加水泥、生石灰和秸秆等辅料利用团粒、喷水养护并结合煅烧成孔的方式制备弱碱激发颗粒填料. 颗粒填料在间歇循环流态化完全混合反应器中进行除磷实验,通过比较除磷组和对照组(超纯水)中颗粒填料的抗压强度、结合水含量以及粉煤灰反应程度研究除磷过程对粉煤灰水化进程的影响,反应产物(凝胶态)Al含量、未反应粉煤灰(残余态)Al含量以及磷形态及含量研究粉煤灰玻璃体中Al的激发规律以及激发水化与除磷过程耦合机理. 研究结果表明,粉煤灰激发水化和除磷反应同步进行,难溶磷酸钙的形成有利于Ca(OH)2溶解释放OH-,促进粉煤灰玻璃体解构释放活性Al2O3和SiO2等除磷和水化活性物质,所释放的活性物质可以在Ca(OH)2存在的情况下发生火山灰反应,生成水化硅酸钙和水化铝酸钙等水化产物,进而实现长期有效除磷. 同时活性Al2O3可以发挥较大的除磷作用,Al-P含量占填料内总磷含量的比例可达40%左右.Abstract: The fly ash is added with auxiliary materials such as cement, quicklime and straw, and the weak alkali excited particle filler is prepared by agglomeration, water spray curing and combined with calcination to form pores. The granular packing is subjected to phosphorus removal experiments in a batch cyclic fluidized complete mixing reactor. The effect of phosphorus removal process on fly ash hydration process was studied by comparing the compressive strength, bound water content and fly ash reaction degree of granular fillers in phosphorus removal group and control group (ultrapure water). The activated law of Al in fly ash vitreous and the coupling mechanism of activated hydration and phosphorus removal processes were studied by comparing the Al content of reaction products (gel state), unreacted fly ash (residual state) Al content, and phosphorus speciation and content. The results show that the activated hydration and phosphorus removal of fly ash are synchronized. In this process, the formation of insoluble calcium phosphate is conducive to the electrolytic release of OH- from Ca(OH)2 ,then promote the release of active phosphorus removal and hydration active substances such as Al2O3 and SiO2 from fly ash glass phase, the released active material can undergo volcanic ash reaction in the presence of Ca(OH)2 to generate hydrated products such as calcium silicate hydrate and calcium aluminate hydrate, thereby achieving long-term effective phosphorus removal. The active Al2O3 can play a large phosphorus removal effect, and the proportion of Al-P content in the total phosphorus content in the filler can reach about 40%.
-
Key words:
- fly ash /
- hydration reaction /
- phosphorus removal /
- coupling mechanism.
-

-
表 1 粉煤灰和水泥的化学组成(%)
Table 1. Chemical composition of fly ash and cement
原料名称
Raw material nameSiO2 Al2O3 CaO MgO Fe2O3 粉煤灰 45.10 24.20 5.60 1.50 4.63 水泥 21.76 5.78 64.70 1.32 3.87 表 2 秸秆粉煤灰基颗粒填料物理性质
Table 2. Physical properties of straw fly ash based particle filler
粒径/mm Particle size 比表面积/(m2·g−1)
Specific surface area表观密度/(g·cm−3)
Apparent density堆积密度/(g·cm−3)
Bulk density孔隙率/%
Porosity破碎率与磨碎率之和/%
Sum of crushing rate and grinding rate2.00—5.00 16.25 2.10 0.74 64.80 2.30 -
[1] 邢静锴, 齐德娥, 秦身钧, 等. 粉煤灰中有价元素的高值化利用研究进展[J]. 现代化工, 2023, 43 (7): 39-43,49 . XING J K, QI D E, QIN S J, KANG S, WANG Q, LI S Y. Research progress on high-value utilization of valuable elements in fly ash[J]. Modern Chemical Industry, 2023, 43 (7): 39-43,49(in Chinese) .
[2] AHMARUZZAMAN M. A review on the utilization of fly ash[J]. Progress in Energy and Combustion Science, 2010, 36(3): 327-363. doi: 10.1016/j.pecs.2009.11.003 [3] YAO Z T, JI X S, SARKER P K, et al. A comprehensive review on the applications of coal fly ash[J]. Earth-Science Reviews, 2015, 141: 105-121. doi: 10.1016/j.earscirev.2014.11.016 [4] ZHUANG X Y, CHEN L, KOMARNENI S, et al. Fly ash-based geopolymer: clean production, properties and applications[J]. Journal of Cleaner Production, 2016, 125: 253-267. doi: 10.1016/j.jclepro.2016.03.019 [5] 王建新, 李晶, 赵仕宝, 等. 中国粉煤灰的资源化利用研究进展与前景[J]. 硅酸盐通报, 2018, 37(12): 3833-3841. WANG J X, LI J, ZHAO S B, et al. Research progress and prospect of resource utilization of fly ash in China[J]. Bulletin of the Chinese Ceramic Society, 2018, 37(12): 3833-3841(in Chinese).
[6] 邵宁宁. 碱激发粉煤灰过程机理及其发泡胶凝材料的高性能化[D]. 北京: 中国矿业大学(北京), 2018. SHAO N N. Process mechanism of alkali-activated fly ash and high performance of foamed cementitious materials[D]. Beijing: China University of Mining & Technology, Beijing, 2018 (in Chinese).
[7] 吴波波. 碱激发低活性粉煤灰复合胶凝材料制备与性能研究[D]. 重庆: 重庆大学, 2019. WU B B. Study on preparation and properties of alkali-activated low-activity fly ash composite cementitious materials[D]. Chongqing: Chongqing University, 2019(in Chinese).
[8] 雷瑞, 付东升, 李国法, 等. 粉煤灰综合利用研究进展[J]. 洁净煤技术, 2013, 19(3): 106-109. LEI R, FU D S, LI G F, et al. Research progress of fly ash comprehensive utilization[J]. Clean Coal Technology, 2013, 19(3): 106-109 (in Chinese).
[9] 刘学文. 改性粉煤灰除磷效果及其免烧结陶粒制备工艺研究[D]. 南昌: 南昌大学, 2019. LIU X W. Study on phosphorus removal effect of modified fly ash and preparation technology of sintering-free ceramsite[D]. Nanchang: Nanchang University, 2019 (in Chinese).
[10] 周光红, 项学敏, 李厚芬, 等. 粉煤灰对水溶液中磷的吸附性能及机理[J]. 环境工程学报, 2012, 6(8): 2600-2606. ZHOU G H, XIANG X M, LI H F, et al. Performance and mechanism of phosphorus adsorption in aqueous solution with fly ash[J]. Techniques and Equipment for Environmental Pollution Control, 2012, 6(8): 2600-2606 (in Chinese).
[11] 欧阳平, 范洪勇, 张贤明, 等. 基于吸附的粉煤灰改性机理研究进展[J]. 材料科学与工程学报, 2014, 32(4): 619-624. OUYANG P, FAN H Y, ZHANG X M, et al. Research progress of the modification mechanism of flyash baesd on adsorption[J]. Journal of Materials Science and Engineering, 2014, 32(4): 619-624 (in Chinese).
[12] 杨建林, 张宇鳌, 马淑花, 等. 不同粒径改性粉煤灰对磷酸根吸附性能的影响[J]. 过程工程学报, 2020, 20(11): 1281-1288. YANG J L, ZHANG Y A, MA S H, et al. Effect of different particle sizes of modified fly ash on phosphate adsorption performance[J]. The Chinese Journal of Process Engineering, 2020, 20(11): 1281-1288 (in Chinese).
[13] PENGTHAMKEERATI P, SATAPANAJARU T, CHULARUENGOAKSORN P. Chemical modification of coal fly ash for the removal of phosphate from aqueous solution[J]. Fuel, 2008, 87(12): 2469-2476. doi: 10.1016/j.fuel.2008.03.013 [14] 欧阳平, 杜杰. 粉煤灰吸附的碱改性研究进展[J]. 应用化工, 2021, 50(9)2559-2561, 2566. OUYANG P, DU J. Research advances in the adsorption of fly ash by alkali modification[J]. Applied Chemical Industry, 50(9)2559-2561, 2566 (in Chinese).
[15] 张宝平, 陈云琳, 魏琳, 等. 粉煤灰的改性及其吸附性能的研究[J]. 硅酸盐通报, 2012, 31(3): 675-678. ZHANG B P, CHEN Y L, WEI L, et al. Study on the modification and adsorption capacity of coal fly ash[J]. Bulletin of the Chinese Ceramic Society, 2012, 31(3): 675-678(in Chinese).
[16] 贾艳萍, 姜修平, 张兰河, 等. HCl/H2SO4改性粉煤灰的制备及其吸附性能[J]. 化工进展, 2017, 36(6)2331-2336. JIA Y P, JIANG X P, ZHANG L H , et al. Fly ash modified by HCl/H2SO4 and their adsorption capacity[J]. Chemical Industry and Engineering Progress, 2017, 36(6)2331-2336(in Chinese).
[17] 刘文辉, 刘恩同, 张治宏, 等. 酸改性粉煤灰对含磷污水处理的实验研究[J]. 环境科学与技术, 2011, 34(8): 164-168. LIU W H, LIU E T, ZHANG Z H, et al. Experimental study on phosphorus-containing wastewater treatment by acid modified fly ash[J]. Environmental Science & Technology, 2011, 34(8): 164-168(in Chinese).
[18] 柴春镜, 宋慧平, 冯政君, 等. 粉煤灰陶粒的研究进展[J]. 洁净煤技术, 2020, 26(6): 11-22. CHAI C J, SONG H P, FENG Z J, et al. Research progress on the fly ash ceramsite[J]. Clean Coal Technology, 2020, 26(6): 11-22(in Chinese).
[19] CHENG G, LI Q H, SU Z, et al. Preparation, optimization, and application of sustainable ceramsite substrate from coal fly ash/waterworks sludge/oyster shell for phosphorus immobilization in constructed wetlands[J]. Journal of Cleaner Production, 2018, 175: 572-581. doi: 10.1016/j.jclepro.2017.12.102 [20] HOSSAIN N, BHUIYAN M A, PRAMANIK B K, et al. Waste materials for wastewater treatment and waste adsorbents for biofuel and cement supplement applications: A critical review[J]. Journal of Cleaner Production, 2020, 255: 120261. doi: 10.1016/j.jclepro.2020.120261 [21] VOHLA C, KÕIV M, BAVOR H J, et al. Filter materials for phosphorus removal from wastewater in treatment wetlands—A review[J]. Ecological Engineering, 2011, 37(1): 70-89. doi: 10.1016/j.ecoleng.2009.08.003 [22] 许事成, 苏壮飞, 刘泽, 等. 硅灰掺量对免烧粉煤灰陶粒性能的影响[J]. 硅酸盐通报, 2022, 41(2): 506-512 XU S C, SU Z F, LIU Z, et al. Influence of silica fume content on performance of non-sintered fly ash ceramsite[J]. Bulletin of the Chinese Ceramic Society, 2022, 41(2): 506-512(in Chinese)
[23] 冯春花, 李东旭. 粉煤灰在水泥浆体中的反应程度研究[J]. 硅酸盐通报, 2015, 34(11): 3202-3208. FENG C, LI D X. Reaction degree of fly ash in the cement paste[J]. Bulletin of the Chinese Ceramic Society, 2015, 34(11): 3202-3208(in Chinese).
[24] 刘志辉, 邵伟, 兰祥辉, 等. 水泥-粉煤灰复合浆体中粉煤灰的火山灰反应程度[J]. 建筑材料学报, 2011, 14(2): 169-172. LIU Z H, SHAO W, LAN X H, et al. Hydration degree of fly ash in blended cement-fly ash paste[J]. Journal of Building Materials, 2011, 14(2): 169-172(in Chinese).
[25] BEN HAHA M, de WEERD K, LOTHENBACH B. Quantification of the degree of reaction of fly ash[J]. Cement and Concrete Research, 2010, 40(11): 1620-1629. doi: 10.1016/j.cemconres.2010.07.004 [26] HAN F H, LIU J H, YAN P Y. Comparative study of reaction degree of mineral admixture by selective dissolution and image analysis[J]. Construction and Building Materials, 2016, 114: 946-955. doi: 10.1016/j.conbuildmat.2016.03.221 [27] 鲍士旦. 土壤农化分析[M]. 北京: 中国农业出版社, 2000. BAO S D. Soil and agricultural chemistry analysis[M]. 3rd ed. Beijing: China Agriculture Press, 2000(in Chinese).
[28] 马鹏传, 李兴, 温振宇, 等. 粉煤灰的活性激发与机理研究进展[J]. 无机盐工业, 2021, 53(10): 28-35 MA P C, LI X, WEN Z Y, et al. Research progress on activation and mechanism of fly ash[J]. Inorganic Chemicals Industry, 2021, 53(10): 28-35(in Chinese)
[29] 阎培渝. 粉煤灰在复合胶凝材料水化过程中的作用机理[J]. 硅酸盐学报, 2007, 35(增刊1): 167-171. YAN P. Mechanism of fly ash's effects during hydration process of composite binder[J]. Journal of the Chinese Ceramic Society, 2007, 35(S1): 167-171(in Chinese).
[30] 冯春花, 沈振球, 李东旭. 粉煤灰在饱和Ca(OH)2溶液中的溶出行为[J]. 材料导报, 2014, 28(4): 130-133. FENG C H, SHEN Z Q, LI D X. Reaction behavior of fly ash in saturated calcium hydroxide solutions at room temperature[J]. Materials Reports, 2014, 28(4): 130-133(in Chinese).
[31] 陈瑶, 李小明, 曾光明, 等. 以磷酸钙盐的形式从污水处理厂回收磷的研究[J]. 环境科学与管理, 2006, 31(增刊4): 110-112. CHEN Y, LI X M, ZENG G M, et al. Study on phosphorus recovert as calcium phosphate from wastewater treatment plant[J]. Environmental Science and Management, 2006, 31(S4): 110-112(in Chinese).
[32] HERMASSI M, VALDERRAMA C, MORENO N, et al. Fly ash as reactive sorbent for phosphate removal from treated waste water as a potential slow release fertilizer[J]. Journal of Environmental Chemical Engineering, 2017, 5(1): 160-169. doi: 10.1016/j.jece.2016.11.027 [33] 唐代瑶, 刘文辉, 王东燕, 等. 粉煤灰改性造粒及对含磷污水处理的研究[J]. 环境与发展, 2020, 32(11): 101-102. TANG D Y, LIU W H, WANG D Y, et al. The research of phosphate-containing wastewater treatment by acid modified and grained fly ash[J]. Environment & Development, 2020, 32(11): 101-102(in Chinese).
[34] HERMASSI M, VALDERRAMA C, FONT O, et al. Phosphate recovery from aqueous solution by K-zeolite synthesized from fly ash for subsequent valorisation as slow release fertilizer[J]. Science of The Total Environment, 2020, 731: 139002. doi: 10.1016/j.scitotenv.2020.139002 [35] CHEN J G, KONG H N, WU D Y, et al. Removal of phosphate from aqueous solution by zeolite synthesized from fly ash[J]. Journal of Colloid and Interface Science, 2006, 300(2): 491-497. doi: 10.1016/j.jcis.2006.04.010 -



 下载:
下载: