-
近年来,随着化石燃料的储量骤减,以及化石燃料的使用给环境带来了严重的污染,促使人们对可再生和可持续能源的研究和开发[1]. 生物质作为一种来源广泛、储备量大、可再生的清洁能源受到了广泛的关注[2 − 3]. 与化石燃料相比,生物质经资源化利用后SOx、NOx和灰尘排放量较小,是理想的清洁燃料之一[4]. 我国每年稻草秸秆产量占全国农作物秸秆总产量的1/5以上,达到2亿多吨,为第二大农作物残留物[5]. 稻草秸秆作为生物质能的重要来源,其在热解资源化利用方面存在巨大潜力. 热解是在惰性气氛下或者无氧条件下将生物质热解成生物质气(bio-gas)、生物质油(bio-oil)和生物质炭(biochar)[6 − 8]. 生物质气一般是由H2、CO和CH4等组成的混合气体[4],可以直接用作燃料;生物质油可以经过加氢使其拥有更高的热值[9 − 11];生物质炭可以用作土壤改良剂,增加农作物的收成,此外,生物质炭还可以有效去除土壤和水环境中的污染物[12 − 15].
生物质的热解产物具有较大的经济价值,因此,很多研究者对生物质的热解机理进行了大量的研究. 目前,用于分析非等温固相反应动力学数据的方法有很多,一般可分为模型法和等转化率法[16 − 17]. 由于动力学参数测定的固有不确定性,国际热分析及量热学联合会(International Confederation for Thermal Analysis, ICTAC)动力学委员会不建议采用模型法[18]. 在使用等转化率法时,不受固定模型带来的约束,同时也能避免估算动力学参数的不确定性. 例如,Wu等[19]采用Friedman和Cai-Chen等转化率法揭示了烟草废弃物的热解机理,根据活化能的变化趋势,推断不同转化率区间下的热解反应. Ceylan等[20]采用KAS、FWO和Coats-Redfern方法计算废榛子壳生物质的活化能、指前因子和反应级数等热解动力学参数. Ma等[21]采用DAEM方法计算得出污泥热解活化能分布在 70—180 kJ·mol−1 之间,且随转化率升高,活化能与指前因子均呈先增加再降低的趋势. 然而,这些研究均通过对TGA数据的分析获得热解动力学参数,进而推断生物质的热解机理,并没有根据热解过程中生物炭的官能团、元素含量、自由基等理化性质的变化来进一步揭示反应机理. 而且,忽略了纤维素、半纤维素和木质素等组分对热解过程中生物质理化性质的影响. 电子顺磁共振(electron paramagnetic resonance, EPR),也称为电子自旋共振(electron spin resonance, ESR),目前研究人员大都利用EPR技术检测生物质热解过程中自由基的产生及变化过程,进而探究生物质热解机理. Ma等[22]采用EPR检测法探讨了纤维素与木质素共热解过程中SFRs的演变规律,分析了纤维素和木质素衍生自由基的相互作用以及对热解过程中的焦产率、焦结构和SFRs演变的影响. Tao等[23]发现,稻草和木屑热解挥发份自由基的类型为苯氧基自由基和碳中心自由基,其中碳上单电子与含氧官能团相邻. 木质素的热解是可凝挥发物产生 ESR 信号的原因,且其有效的结构单元为 2-甲氧基酚.
本文采用无模型的等转化率方法—Friedman和分布式活化能(distributed activation energy model, DAEM)法,计算不同转化率下的热解活化能. 等转化率法也叫多重扫描速率法,该方法对多条不同β测得的TGA曲线在同一转化率(α)处的温度数据进行分析. Friedman法能够在不涉及动力学反应机理函数的条件下获得可靠的E,从而避免误差. DAEM法假设热解过程中发生的是许多平行和独立的反应,所以可以通过热重分析数据得到物质热解的活化能分布情况,并且在描述复杂组分的热解过程时更为准确. 本文还通过对活化能转折点处的生物炭进行元素分析和EPR表征,揭示纤维素、半纤维素和木质素对热解生物炭理化性质的影响,从而指导生物质的热解应用和生物炭的制备.
-
以云南当地稻草秸秆为原料,经自然晾干后,粉碎至100目以下. 纤维素、半纤维素和木质素均购买于aladdin, CAS:
9004 -34-6,利用热重分析仪STA6000(PE,美国)对其进行热失重实验,载气为高纯氮气(99.999%),流速为40 mL·min−1,升温速率为20、30、40、50 ℃·min−1,加热至1000 K. 实验样品的质量控制在(10 ± 0.5) mg,每次实验重复3次. -
热解稻草秸秆的转化率(α)可以用式(1)来描述:
式中:m0为反应物初始质量(mg);mt为t时刻物质质量(mg);mf为物质反应终止后的质量(mg)
热解反应的速率方程可以用式(2)来描述:
式中:f(α)为α的函数的反应模型;κ(T)是以Arrhenius方程表示的速率常数:
式中 :A –指前因子(s−1);E –反应活化能(kJ·mol−1) ;R —气体常数 (8.314 J· (mol−1K)−1);T –温度(K)
将式(3)带入式(2),可以得到:
升温速率(β)为:
将式(5)带入式(4),可以得到:
式(6)的积分形式为:
式中的G(α)是ƒ(α)的积分式,P(u)为温度的近似函数,其中.
根据TG和DTG数据,用Friedman(式8)和DAEM(式9)方法计算生物质热解的活化能.
根据不同升温速率下,ln(dα/dt)与ln(β/T2)对1/T作图,斜率可求得活化能E.
-
焓(ΔH)、吉布斯自由能(ΔG)和熵(ΔS)等热力学参数的计算公式如下:
其中,KB,h,和Tm分别是Boltzmann常数(1.381×10−23 J·K−1)、Plank常数(6.626×10−34 J·s)和DTG曲线上的峰值温度(K).
-
稻草秸秆及其生物炭中元素C、H、O、N和S的含量均采用元素分析仪测定(MicroCube, Elememtar, Germany). 测定C、H、N和S的含量时,燃烧管温度为850 ℃,测定O含量时,温度设置为
1150 ℃. 每批实验设置3个平行样. -
稻草秸秆及其生物炭的自由基特征通过电子顺磁共振波谱仪(A300-6/1, Bruker, Germany)测定. 取20 mg左右的样品于顺磁管中,将顺磁管放入EPR的共振腔,室温(20 ± 0.5) ℃下测量,参数设置:100 kHz调制,微波频率9.2—9.9 GHz,扫描宽度1.00 G,X轴的分辨率为
1024 点,微波功率31 dB,扫描时间为81.92 ms. -
实验数据采用平均值±标准偏差(Mean ± SEM)表述,利用Minitab 17进行单因素方差分析,Tukey检验差异的显著性(P < 0.05).
-
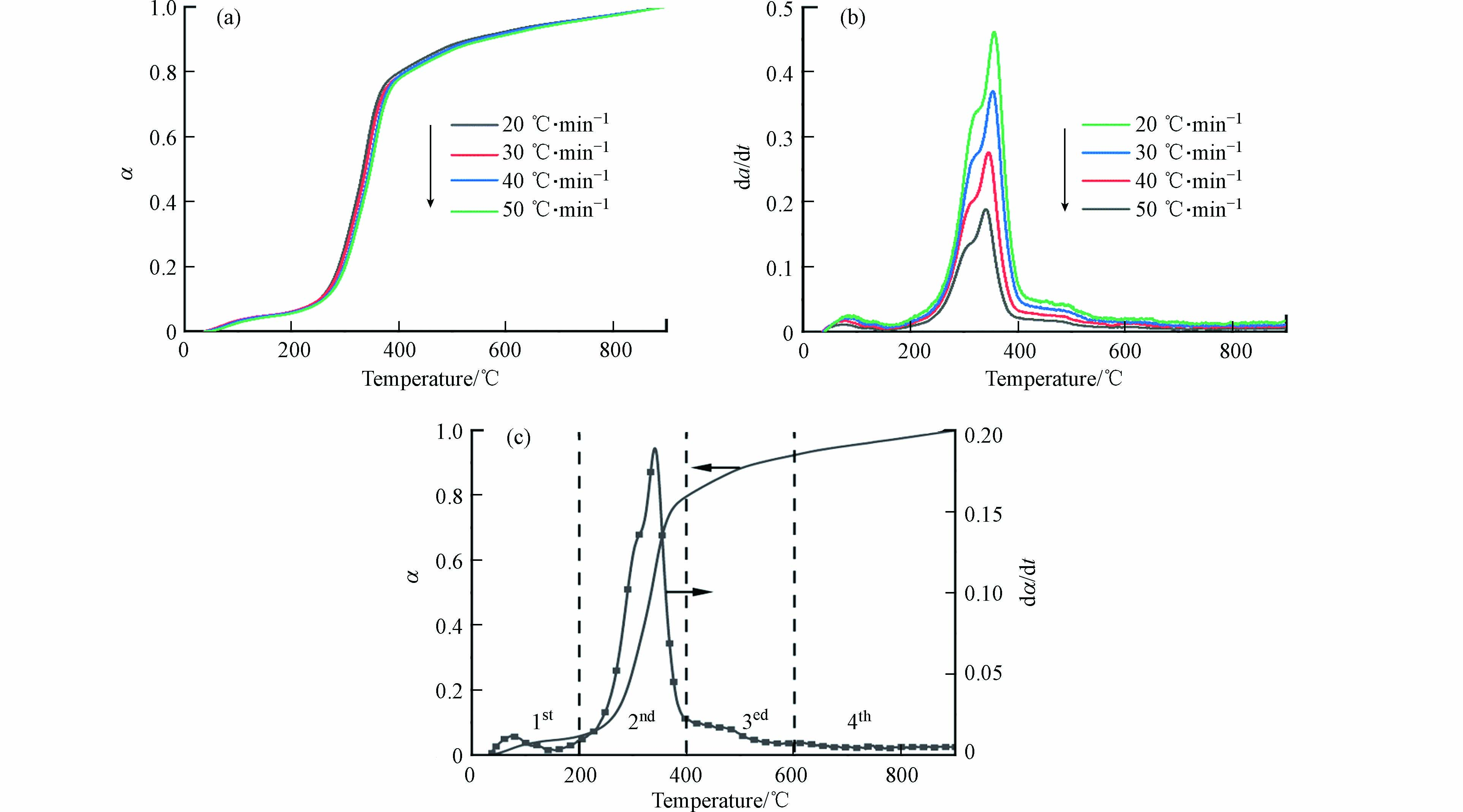
稻草秸秆在20、30、40、50 ℃·min−1的4个升温速率下的转化率和其微分曲线如图1a和1b所示. 随着升温速率的升高,曲线均稍微向高温侧移动,这是由于升温速率提高产生的热滞后[24 − 26]. 据文献[23]报道,半纤维素、纤维素和木质素分别在180—340 ℃、230—450 ℃和大于500 ℃下热分解. 因此,图1b中的肩峰是半纤维素的热解,最大热转化率的峰是纤维素的热解.
根据转化率与其微分曲线的变化趋势,将稻草秸秆的热解分为4个阶段,如图1c所示. 第一阶段(20—200 ℃)是水分蒸发和易挥发有机物的挥发,第二阶段(200—400 ℃)的转化率为80%,是热失重的主要阶段,主要是半纤维素和纤维素的热解,这一阶段的反应速率较快. 第三阶段(400—600 ℃)主要是木质素的热解,由于木质素芳香环结构的稳定性,这一阶段的反应速率远远小于第二阶段. 第四阶段(600—800 ℃)是剩余物继续热解成炭.
-
转化率(α)选取范围为0.1—0.9,以0.05为间隔,ln(dα/dt)-1/T与ln(β/T2) -1/T的拟合线如图2a和2b所示. α在低于0.15和高于0.75时拟合曲线的可决系数Radj2均低于0.90,拟合效果较差,其余各转化率下均显示出良好的拟合效果,这表明α在0.15到0.75范围内计算活化能具有较高的可信度[27 − 28]. 根据方程式8和9,计算了稻草秸秆在不同转化率下的活化能,结果如图2c所示. 虽然Friedman方法得到的E值略高于DAEM计算的E值,但两种方法计算的E值在各阶段的波动趋势一致,差异百分比为0.89%—9.23%,其E值的差异可以忽略不计.
根据E值的波动规律,将转化率分为3个相段,即0.10—0.30,0.30—0.75,0.75—0.90. 结合稻草秸秆的热解区间,可以得到,对于第一相段,主要是半纤维素的分解;第二相段,主要是纤维素的分解和木质素的解聚;第三相段主要是木质素的分解. 由此推断出,纤维素、半纤维素和木质素主体热解区间的活化能表现出以下规律:纤维素 ≈ 半纤维素 > 木质素,这与我们前期的研究结果一致[29].
根据方程式10、11和12,计算不同α的∆H、∆G和∆S值(表1). ∆H反映了活化络合物和试剂之间的能量差异,因此它可以作为一个指标来解释热解过程的本质. ∆H越小,反应发生所需要的热量愈小,热裂解反应越容易发生[30]. 计算的∆H均大于零,说明不同阶段的热解反应均是吸热反应. ∆H的两个峰值分别出现在转化率为0.3和0.75,与E值的变化规律相似,再次说明了对于稻草秸秆的热解可以将转化率分为3个相段. E值和∆H值之间的差值4.37—6.72,它们之间的差异微小,说明热解过程中能垒较低,所以样品容易形成活性络合物,这对热解产物的生成是有利的[31]. 反应体系的总能量增加由ΔG表示. ∆G > 0,说明热解反应是非自发的. ΔG值随着转化率的升高而增加,这是由于反应速率逐渐减缓,导致可用自由能增加[32] . 转化率为0.85和0.90时,∆G值较高,表明木质素分解需要更高的热量. 此外,∆S是反应体系混乱度的指标,∆S > 0,表示反应从有序状态转移到无序状态. 转化率小于0.75时,其∆S > 0,表明纤维素和半纤维素的热分解反应远离热平衡[33]. ∆S < 0,表明反应活化过渡态比反应物具有更有序的分子结构[34]. 转化率为0.10到0.75时,∆S值逐渐减小,说明热分解反应逐渐达到了新的热力学平衡. 当转化率大于0.80时,较高的∆G值和负的∆S值反映木质素的热降解反应速率缓慢[35].
-
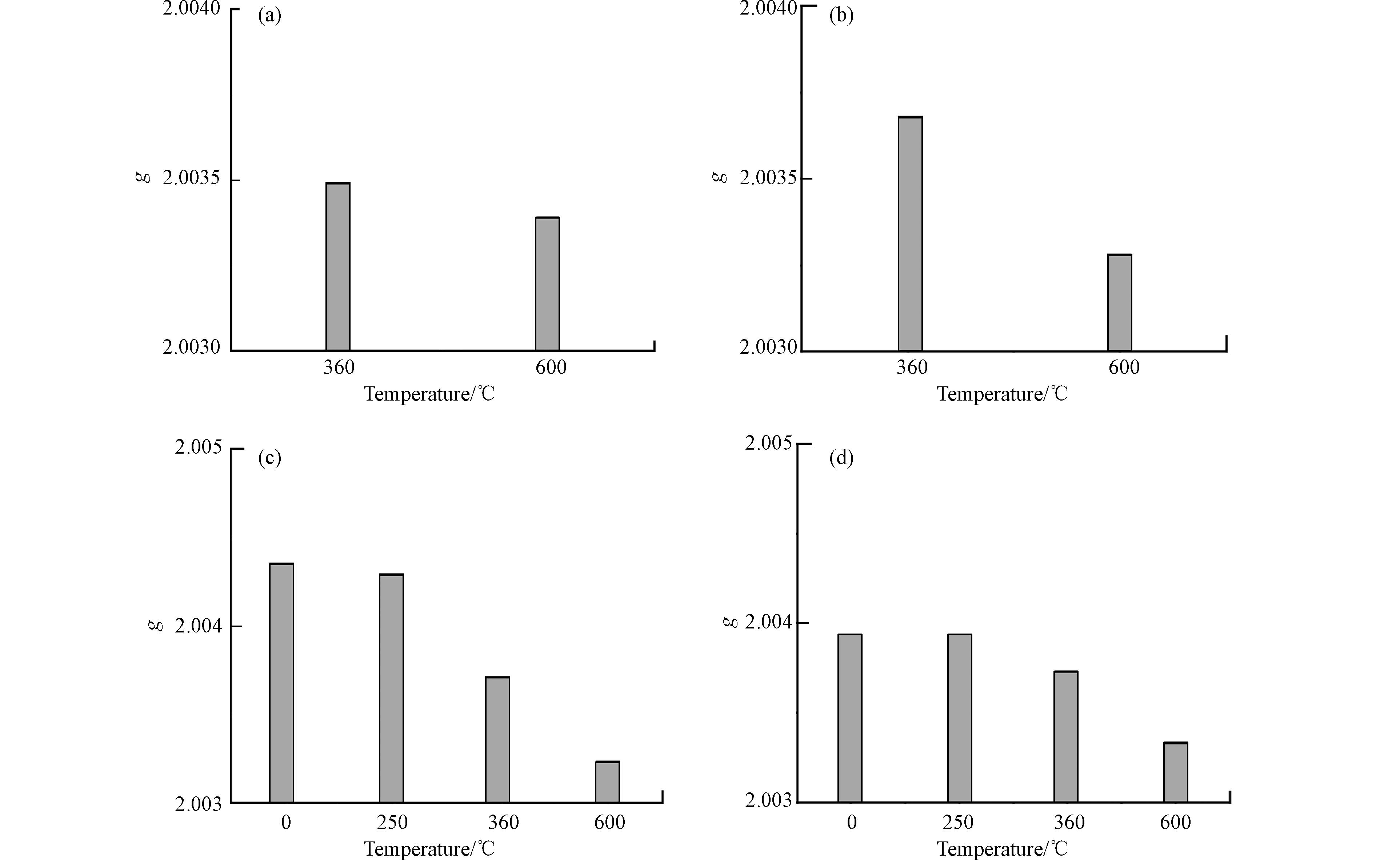
根据热解区间,选取转化率为0、0.1、0.7、0.9和1所对应温度(0、250、360、600 、900 ℃)的生物炭进行元素分析,其结果见表2. 由表2可以看出,C含量随热解温度的升高而增大,而H和O的含量降低,这是由于在热解过程中,生物质发生脱水、脱羰和脱羧等反应造成的[36]. 此外,O/C和H/C随着热解温度的升高而降低,说明稻草秸秆中的饱和脂肪烃在向芳香烃和不饱和脂肪烃转化,生物炭的极性降低,而芳香性增加,其结构也由松散的“软质碳”转变为致密的“硬质碳”[37].
除此之外,与原生质相比,250 ℃的生物炭在C、H和H/C均没有明显的差异,仅在O和O/C上具有差异性;与250 ℃生物炭相比,360 ℃生物炭中元素的含量、O/C和H/C均具有明显的差异,说明在此温度区间,稻草秸秆发生剧烈的脱氧和脱氢等热解反应,与前文热解区间划分的结果一致. 温度升高至600 ℃和900 ℃时,C含量的变化较小,而O和H的含量存在明显差异,即在该阶段继续发生脱水和还原的反应,实现碳基结构的进一步浓缩[38].
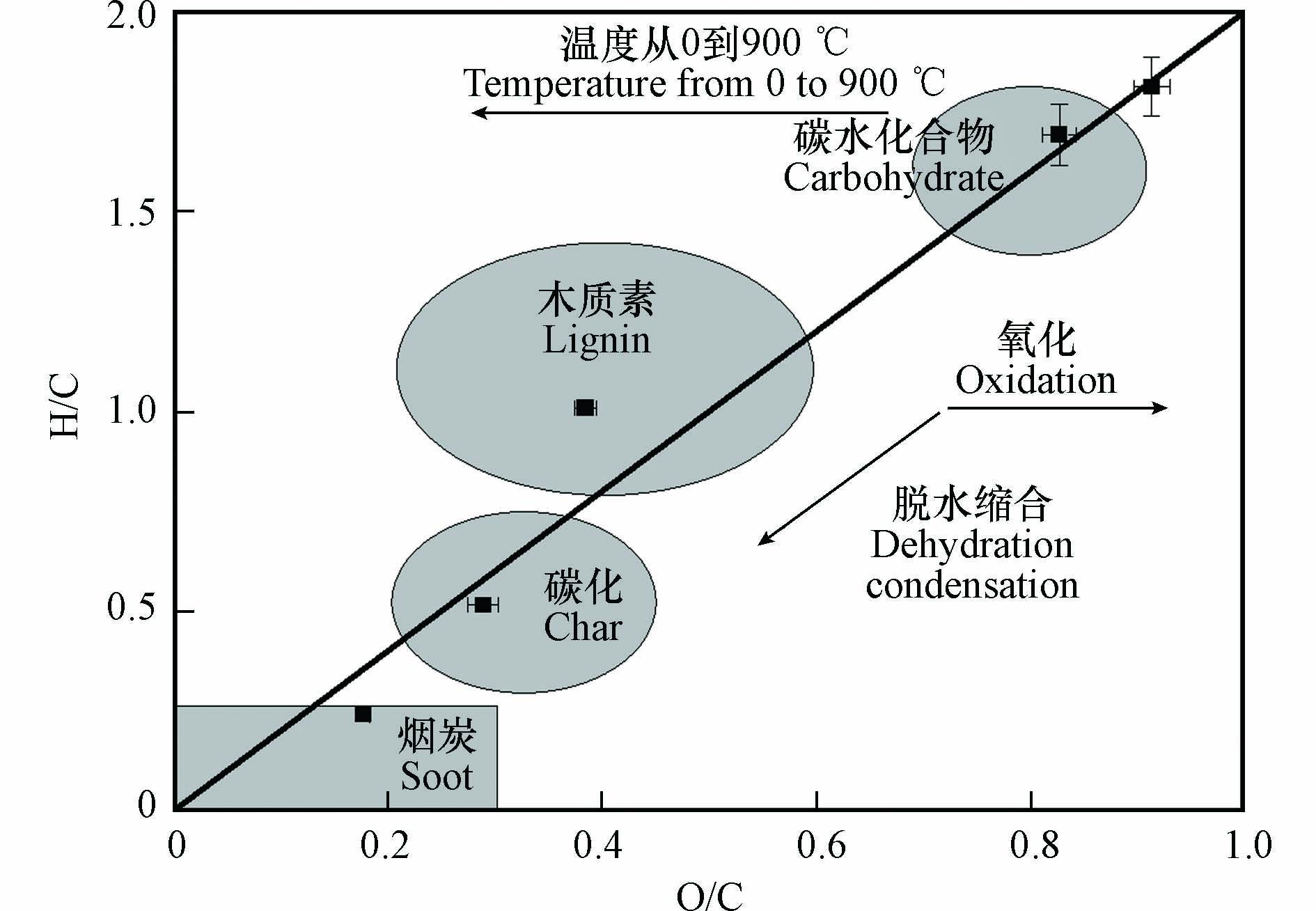
为清晰地反应热解过程中生物质发生的反应,以O/C为横坐标、H/C为纵坐标绘制了不同热解温度生物炭的范氏图(图3). 根据范氏图的分类,热解过程可以分为四个阶段,在0 ℃到250 ℃温度区间,稻草秸秆主要发生的是水分和易挥发有机物的挥发,纤维素和半纤维素发生解聚反应,但其结构变化很小;温度升高至360 ℃,纤维素和半纤维素发生剧烈的脱水和还原反应,同时炭的芳香性增加;继续升高温度到600 ℃,主要是木质素的热解,炭的芳香性进一步增加;升高到900℃,剩余物质继续热解成炭,生物炭高度碳化,这与热解区间的划分结果一致.
-
对于上述生物炭进行了EPR的表征,同时将纤维素(简写为CL)、半纤维素(HC)和木质素(LG)加热到相同的温度,以考察CL、HC和LG对稻草秸秆(RS)生物炭理化性质的影响. 当热解温度为900 ℃时,所有生物炭均没有EPR信号,因此图4和图5均没有显示.
热解温度为0 ℃和250 ℃时,CL和HC系列样品没有自由基信号,因此,低于250 ℃稻草秸秆生物炭的自由基是由其木质素决定的. 朗德g因子是一个重要的ESR参数,简称为g因子,每种自由基的g因子值均为特定值,根据文献报道,g因子值大于
2.0040 时,以氧为中心的自由基(g 值>2.0045 时为半醌自由基);g因子值小于2.0030 时,以碳为中心的自由基;而当g因子值介于2.0030 —2.0040 时,以氧为中心的自由基和以碳为中心的自由基的复合体系[39-40].由图4可以发现,g值随热解温度的升高而降低,CL-360表示CL热解温度为360 ℃的生物炭. 样品LG-0、LG-250、RS-0和RS-250的g值均大于2.004,均是以氧为中心的自由基,这可能来自于原生质和大分子样品发生脱自由水的反应以及木质素侧支链的脱甲氧基[23]. 当热解温度为360 ℃和600 ℃时,生物炭的g值均位于2.003—2.004之间,表明以氧为中心的自由基和以碳为中心的自由基存在的复合体系. 这可能来自于各组分的脱水、脱羟基、脱羰基和脱羧基的化学反应,并且在热解过程中发生了烷氧基向烷基和酰基的转变[41]. 但360 ℃生物炭的g值大于600 ℃,表明随着温度升高,生物炭逐渐向以C为中心的自由基过渡,这可能是由于在360 ℃与600 ℃温度区间,生物炭继续脱氧,同时碳结构浓缩引起的,与范氏图(图3)的结果分析一致.
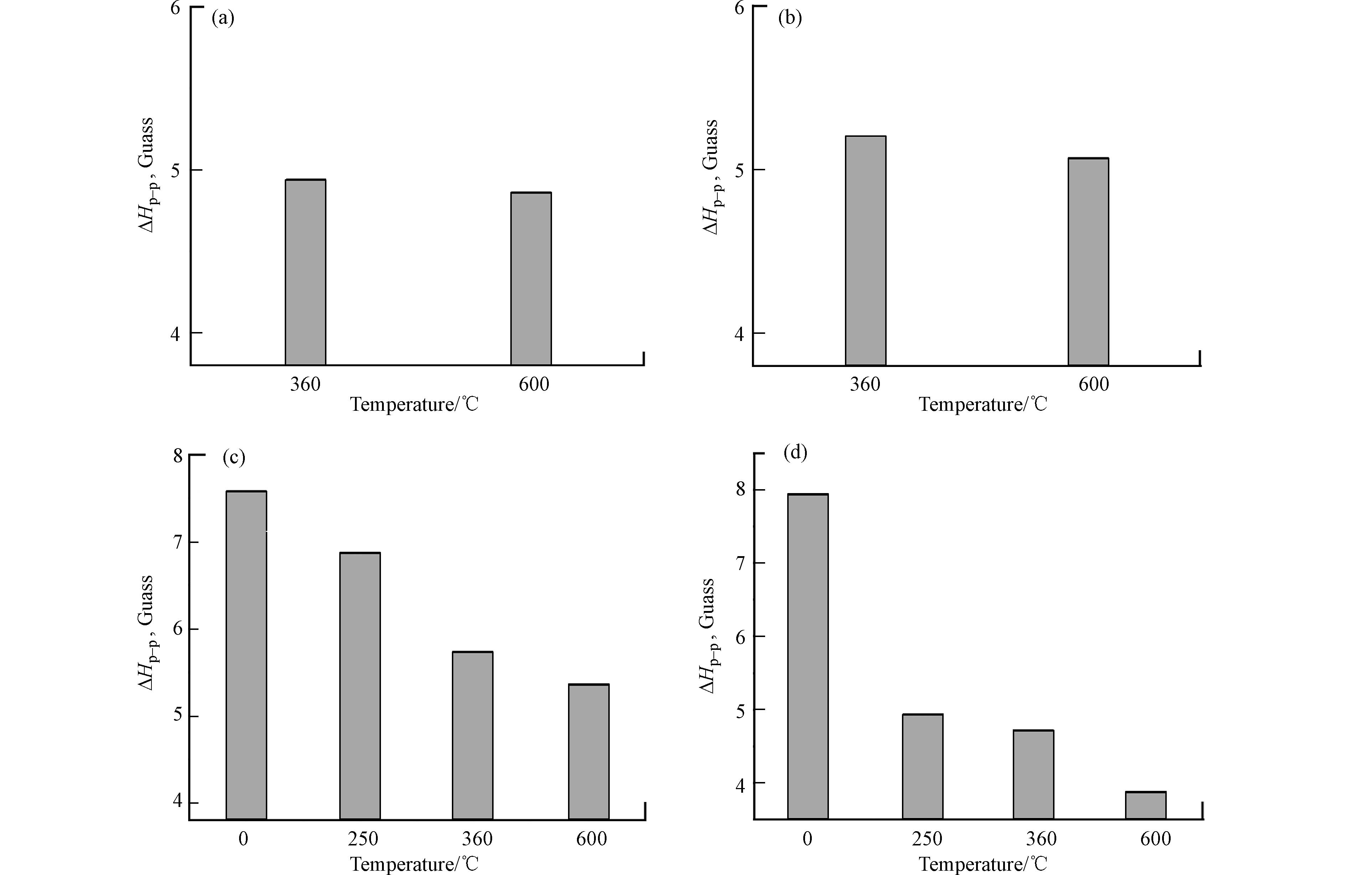
纤维素(a)、半纤维素(b)、木质素(c)和稻草秸秆(d)不同温度样品的EPR半峰宽如图5所示. 生物炭上的持久性自由基的种类可以用ΔH表示. 由图5可以看出,样品的半峰宽随热解温度的升高而降低,说明随着温度的升高,生物炭上持久性自由基的种类也逐渐减少,结合g值的分析,更进一步说明生物炭的自由基类型向以碳为中心的自由基转变. 此外,随着碳含量的增加和氧含量的降低,生物炭上的持久性自由基从以氧为中心的自由基向以碳为中心的自由基转变,所获得的生物炭上的持久性自由基种类逐渐减少[42].
-
1)根据转化率的变化趋势、元素分析和范氏图得出,稻草秸秆的热解分为四个阶段,第一阶段是水分和易挥发有机物的挥发,第二阶段是热失重的主要阶段,主要是半纤维素和纤维素的热解,转化率为80%,第三阶段主要是木质素的热解,第四阶段是剩余物继续热解成炭.
2)通过热解活化能的分析得出,木质素主体热解区间的活化能小于纤维素和半纤维素;表现出以下规律:纤维素 ≈ 半纤维素 > 木质素;较高的∆G值和负的∆S值显示木质素的热解反应是缓慢的.
3)低温下,稻草秸秆生物炭的自由基类型和种类主要是由木质素决定的;低于250 ℃,以氧为中心的自由基为主,360 ℃到600 ℃之间,以氧为中心的自由基和以碳为中心的自由基存在的复合体系,但逐渐向以碳为中心的自由基过渡.
4)随着热解温度的升高,生物炭的碳含量增加,氧含量降低,所获得的生物炭上的持久性自由基种类逐渐减少.
基于等转化率法和生物炭性质揭示稻草秸秆的热解特性
Pyrolytic behavior of rice straw based on the detailed analysis of iso-conversional method and biochar characterization
-
摘要: 对稻草秸秆在氮气气氛下的热解进行热重分析. 基于等转化率方法和生物炭表征,揭示稻草秸秆的热解机理. 结果表明:(1)热解区间划分为4个阶段,第一阶段是水分蒸发和易挥发有机物的挥发,纤维素和半纤维素的解聚;第二阶段为主热解阶段,纤维素和半纤维素发生剧烈的脱水和还原反应;第三阶段主要是木质素的热解,炭的芳香性进一步增加;第四阶段为剩余物质继续热解成炭,生物炭高度碳化;(2)在主热解阶段,∆H、∆G和∆S均大于0,表明纤维素和半纤维素的热分解反应是吸热、非自发的、熵增大的反应;(3)热解温度低于250 ℃时,木质素是稻草秸秆生物炭 PFR 生成的主要贡献者,g值大于2.004,是以氧为中心的自由基;随着热解温度的升高,g值有所降低但始终位于2.003—2.004之间,稻草秸秆生物炭的自由基类型由以氧为中心的自由基为主逐渐转变为以碳为中心的自由基为主,且自由基的种类减少. 本研究为稻草秸秆生物质的热解机制和生物炭的制备提供一定的理论支撑.Abstract: Abstracts: Rice straw was pyrolyzed by thermogravimetric analysis under nitrogen atmosphere. Based on the, The pyrolysis mechanism was elucidated with the analysis of activation energy obtain by Friedman, DAEM method and biochar characterization. Results showed that: (1) the pyrolysis process of rice straw can be divided into four stages. In the first stage, the reaction was mainly composed by the losing moisture and depolymerization of cellulose and hemicellulose. Subsequently, the dehydration and reduction reaction of cellulose and hemicellulose was mostly occurred in the second stage, which is the main pyrolysis stage of rice straw. The main pyrolysis reaction of lignin was mainly located in the third stage. In the last stage, the biochar was highly carbonized. (2) the positive ΔH and ΔG values indicated that the pyrolysis reactions for the second stage were endothermic and nonspontaneous. (3) when the pyrolysis temperature is lower than 250 ℃, lignin is the main contributor to the formation of persistent free radical in rice straw biochar. Additionally, the g value is greater than 2.004, which is the oxygen-centered free radical on. With the increase of pyrolysis temperature, g was decreasing but was still between 2.003 and 2.004, indicating that the free radical type was gradually changing from oxygen-centered free radicals to carbon-centered free radicals. This study provides useful information to understand the thermal pyrolysis process of rice straw and the application of the derived biochar.
-
Key words:
- friedman method /
- activation energy /
- lignin /
- free radicals.
-

-
表 1 Friedman和DAEM方法计算的热力学参数
Table 1. Thermodynamic parameters derived from Friedman and DAEM methods
α ΔH (kJ·mol−1) ΔG (kJ·mol−1) ΔS (J·mol−1·K−1) Friedman DAEM Friedman DAEM Friedman DAEM 0.10 178.83 173.72 129.60 124.49 93.56 93.56 0.15 178.40 173.38 138.68 133.67 72.38 72.38 0.20 183.89 173.56 148.82 138.49 62.46 62.46 0.25 188.49 174.60 155.74 141.85 57.37 57.37 0.30 193.22 179.05 158.56 144.39 59.85 59.85 0.35 190.53 179.86 157.71 147.04 55.94 55.94 0.40 185.19 179.68 155.14 149.62 50.60 50.60 0.45 179.79 177.65 154.19 152.05 42.60 42.60 0.50 178.61 175.82 156.98 154.18 35.65 35.65 0.55 180.46 175.24 161.21 155.99 31.42 31.42 0.60 185.04 175.41 167.36 157.73 28.60 28.60 0.65 192.38 177.00 174.97 159.58 27.91 27.91 0.70 198.08 180.14 179.88 161.94 28.82 28.82 0.75 204.69 188.57 181.98 165.86 35.27 35.27 0.80 168.92 170.48 172.74 174.30 -5.65 -5.65 0.85 175.44 159.38 202.00 185.94 -36.35 -36.35 0.90 115.43 107.45 198.40 190.42 -102.59 -102.59 表 2 不同热解温度生物炭元素质量分数及原子比
Table 2. Mass percentage of C, H,O and atom ratioof RS-derived biochar at different pyrolytic temperature
温度/℃
TemperatureC /% H /% O /% O/C H/C 0 39.06 ± 0.79c 5.99 ± 0.09a 47.60 ± 0.14a 0.91 ± 0.02a 1.81 ± 0.07a 250 40.98 ± 1.20c 5.77 ± 0.15a 45.22 ± 0.49b 0.83 ± 0.02b 1.69 ± 0.08a 360 50.03 ± 1.01b 4.20 ± 0.09b 25.65 ± 0.40c 0.38 ± 0.01c 1.01 ± 0.01b 600 53.26 ± 0.95a 2.29 ± 0.06c 20.52 ± 0.86d 0.29 ± 0.01d 0.51 ± 0.01c 900 55.64 ± 1.34a 1.12 ± 0.08d 13.15 ± 0.21e 0.18 ± 0.01e 0.24 ± 0.01d 注:同一列中的不同字母a、b、c 和 d表示统计差异.
Note: different letters a, b, c, and d in the same column show statistical difference (P < 0.05). -
[1] LOPEZ-VELAZQUEZ M A, SANTES V, BALMASEDA J, et al. Pyrolysis of orange waste: A thermo-kinetic study[J]. Journal of Analytical and Applied Pyrolysis, 2013, 99: 170-177. doi: 10.1016/j.jaap.2012.09.016 [2] CHEN J B, FAN X T, JIANG B, et al. Pyrolysis of oil-plant wastes in a TGA and a fixed-bed reactor: Thermochemical behaviors, kinetics, and products characterization[J]. Bioresource Technology, 2015, 192: 592-602. doi: 10.1016/j.biortech.2015.05.108 [3] ERTAŞ M, HAKKı ALMA M. Pyrolysis of laurel (Laurus nobilis L. ) extraction residues in a fixed-bed reactor: Characterization of bio-oil and bio-char[J]. Journal of Analytical and Applied Pyrolysis, 2010, 88(1): 22-29. doi: 10.1016/j.jaap.2010.02.006 [4] 郑赟. 基于组分分析的生物质热裂解动力学机理研究[D]. 杭州: 浙江大学, 2006. ZHENG (B/Y). Mechanism research of biomass pyrolysis on component analysis and kinetic study[D]. Hangzhou: Zhejiang University, 2006 (in Chinese).
[5] JI L Q. An assessment of agricultural residue resources for liquid biofuel production in China[J]. Renewable and Sustainable Energy Reviews, 2015, 44: 561-575. doi: 10.1016/j.rser.2015.01.011 [6] PANG S S. Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals[J]. Biotechnology Advances, 2019, 37(4): 589-597. doi: 10.1016/j.biotechadv.2018.11.004 [7] AHMED A, ABU BAKAR M S, AZAD A K, et al. Intermediate pyrolysis of Acacia cincinnata and Acacia holosericea species for bio-oil and biochar production[J]. Energy Conversion and Management, 2018, 176: 393-408. doi: 10.1016/j.enconman.2018.09.041 [8] BRIDGWATER A V. Review of fast pyrolysis of biomass and product upgrading[J]. Biomass and Bioenergy, 2012, 38: 68-94. doi: 10.1016/j.biombioe.2011.01.048 [9] GRILC M, LIKOZAR B, LEVEC J. Hydrodeoxygenation and hydrocracking of solvolysed lignocellulosic biomass by oxide, reduced and sulphide form of NiMo, Ni, Mo and Pd catalysts[J]. Applied Catalysis B:Environmental, 2014, 150/151: 275-287. doi: 10.1016/j.apcatb.2013.12.030 [10] GRILC M, LIKOZAR B, LEVEC J. Hydrotreatment of solvolytically liquefied lignocellulosic biomass over NiMo/Al2O3 catalyst: Reaction mechanism, hydrodeoxygenation kinetics and mass transfer model based on FTIR[J]. Biomass and Bioenergy, 2014, 63: 300-312. doi: 10.1016/j.biombioe.2014.02.014 [11] CHEN D Y, ZHENG Y, ZHU X F. Determination of effective moisture diffusivity and drying kinetics for poplar sawdust by thermogravimetric analysis under isothermal condition[J]. Bioresource Technology, 2012, 107: 451-455. doi: 10.1016/j.biortech.2011.12.032 [12] ZWIETEN L V, KIMBER S, MORRIS S, et al. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility[J]. Plant and Soil, 2010, 327(1): 235-246. [13] 安增莉, 侯艳伟, 蔡超, 等. 水稻秸秆生物炭对Pb(Ⅱ)的吸附特性[J]. 环境化学, 2011, 30(11): 1851-1857. AN Z L, HOU Y W, CAI C, et al. Lead(ⅱ) adsorption characteristics on different biochars derived from rice straw[J]. Environmental Chemistry, 2011, 30(11): 1851-1857 (in Chinese).
[14] 孔露露, 周启星. 新制备生物炭的特性表征及其对石油烃污染土壤的吸附效果[J]. 环境工程学报, 2015, 9(5): 2462-2468. KONG L L, ZHOU Q X. Characterization of new-prepared biochars and their adsorption effectiveness on petroleum hydrocarbon contaminated soil[J]. Chinese Journal of Environmental Engineering, 2015, 9(5): 2462-2468 (in Chinese).
[15] 王萌萌, 周启星. 生物炭的土壤环境效应及其机制研究[J]. 环境化学, 2013, 32(5): 768-780. doi: 10.7524/j.issn.0254-6108.2013.05.008 WANG M M, ZHOU Q X. Environmental effects and their mechanisms of biochar applied to soils[J]. Environmental Chemistry, 2013, 32(5): 768-780 (in Chinese). doi: 10.7524/j.issn.0254-6108.2013.05.008
[16] KHAWAM A, FLANAGAN D R. Complementary use of model-free and modelistic methods in the analysis of solid-state kinetics[J]. The Journal of Physical Chemistry. B, 2005, 109(20): 10073-10080. doi: 10.1021/jp050589u [17] VYAZOVKIN S, WIGHT C A. Model-free and model-fitting approaches to kinetic analysis of isothermal and nonisothermal data[J]. Thermochimica Acta, 1999, 340/341: 53-68. doi: 10.1016/S0040-6031(99)00253-1 [18] VYAZOVKIN S, BURNHAM A K, CRIADO J M, et al. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data[J]. Thermochimica Acta, 2011, 520(1/2): 1-19. [19] WU W X, MEI Y F, ZHANG L, et al. Kinetics and reaction chemistry of pyrolysis and combustion of tobacco waste[J]. Fuel, 2015, 156: 71-80. doi: 10.1016/j.fuel.2015.04.016 [20] CEYLAN S, TOPÇU Y. Pyrolysis kinetics of hazelnut husk using thermogravimetric analysis[J]. Bioresource Technology, 2014, 156: 182-188. doi: 10.1016/j.biortech.2014.01.040 [21] 马秋娜, 姬爱民, 李海英. 基于分布式活化能模型的污泥热解特性研究[J]. 节能, 2017, 36(9): 10-13. doi: 10.3969/j.issn.1004-7948.2017.09.003 MA Q N, JI A M, LI H Y. Study of sludge pyrolysis characteristics based on the distributed activation energy model[J]. Energy Conservation, 2017, 36(9): 10-13 (in Chinese). doi: 10.3969/j.issn.1004-7948.2017.09.003
[22] MA L Q, SYED-HASSAN S S A, TONG Y X, et al. Interactions of cellulose- and lignin-derived radicals during pyrolysis: An in situ Electron Paramagnetic Resonance (EPR) study[J]. Fuel Processing Technology, 2023, 239: 107536. doi: 10.1016/j.fuproc.2022.107536 [23] TAO W M, YANG X W, LI Y, et al. Components and persistent free radicals in the volatiles during pyrolysis of lignocellulose biomass[J]. Environmental Science & Technology, 2020, 54(20): 13274-13281. [24] MISHRA R K, MOHANTY K. Pyrolysis kinetics and thermal behavior of waste sawdust biomass using thermogravimetric analysis[J]. Bioresource Technology, 2018, 251: 63-74. doi: 10.1016/j.biortech.2017.12.029 [25] CHANDRASEKARAN A, RAMACHANDRAN S, SUBBIAH S. Determination of kinetic parameters in the pyrolysis operation and thermal behavior of Prosopis juliflora using thermogravimetric analysis[J]. Bioresource Technology, 2017, 233: 413-422. doi: 10.1016/j.biortech.2017.02.119 [26] SAMUELSSON L N, BABLER M U, MORIANA R. A single model-free rate expression describing both non-isothermal and isothermal pyrolysis of Norway Spruce[J]. Fuel, 2015, 161: 59-67. doi: 10.1016/j.fuel.2015.08.019 [27] DAMARTZIS T, VAMVUKA D, SFAKIOTAKIS S, et al. Thermal degradation studies and kinetic modeling of cardoon (Cynara cardunculus) pyrolysis using thermogravimetric analysis (TGA)[J]. Bioresource Technology, 2011, 102(10): 6230-6238. doi: 10.1016/j.biortech.2011.02.060 [28] ALSULAMI R A, EL-SAYED S A, E LTAHER M A, et al. Thermal decomposition characterization and kinetic parameters estimation for date palm wastes and their blends using TGA[J]. Fuel, 2023, 334(P1). [29] TAO W M, DUAN W Y, LIU C B, et al. Formation of persistent free radicals in biochar derived from rice straw based on a detailed analysis of pyrolysis kinetics[J]. The Science of the Total Environment, 2020, 715: 136575. doi: 10.1016/j.scitotenv.2020.136575 [30] ÖZSIN G, PÜTÜN A E. Co-pyrolytic behaviors of biomass and polystyrene: Kinetics, thermodynamics and evolved gas analysis[J]. Korean Journal of Chemical Engineering, 2018, 35(2): 428-437. doi: 10.1007/s11814-017-0308-6 [31] MISHRA A, KUMARI U, TURLAPATI V Y, et al. Extensive thermogravimetric and thermo-kinetic study of waste motor oil based on iso-conversional methods[J]. Energy Conversion and Management, 2020, 221: 113194. doi: 10.1016/j.enconman.2020.113194 [32] TABAL A, BARAKAT A, ABOULKAS A, et al. Pyrolysis of ficus nitida wood: Determination of kinetic and thermodynamic parameters[J]. Fuel, 2021, 283: 119253. doi: 10.1016/j.fuel.2020.119253 [33] SONG F H, LI T T, ZHANG J, et al. Novel insights into the kinetics, evolved gases, and mechanisms for biomass (sugar cane residue) pyrolysis[J]. Environmental Science & Technology, 2019, 53(22): 13495-13505. [34] RESHAD A S, TIWARI P, GOUD V V. Thermal degradation kinetic study of rubber seed oil and its methyl esters under inert atmosphere[J]. Energy & Fuels, 2017, 31(9): 9642-9651. [35] TAO W M, ZHANG P, YANG X W, et al. An integrated study on the pyrolysis mecanism of peanut shell based on the kinetic analysis and solid/gas characterization[J]. Bioresource Technology, 2021, 329: 124860. doi: 10.1016/j.biortech.2021.124860 [36] KEILUWEIT M, NICO P S, JOHNSON M G, et al. Dynamic molecular structure of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2010, 44(4): 1247-1253. [37] GUNASEKARA A S, SIMPSON M I, XING B S. Identification and characterization of sorption domains in soil organic matter using strucuturally modified humic acids[J]. Environmental Science & Technology, 2003, 37(5): 852-858. [38] 杨芳. 生物质热解过程中持久性自由基的产生过程及机理[D]. 昆明: 昆明理工大学, 2016. YANG F. Environmentally persistent free radical generation and mechanism during the pyrolysis of biomasses[D]. Kunming: Kunming University of Science and Technology, 2016 (in Chinese).
[39] DELLINGER B, LOMNICKI S, KHACHATRYAN L, et al. Formation and stabilization of persistent free radicals[J]. Proceedings of the Combustion Institute, 2007, 31(1): 521-528. doi: 10.1016/j.proci.2006.07.172 [40] DUAN S Y, ZHANG M H, SUN Y Q, et al. Mechanism of PM2.5-induced human bronchial epithelial cell toxicity in central China[J]. Journal of Hazardous Materials, 2020, 396: 122747. doi: 10.1016/j.jhazmat.2020.122747 [41] TAO W M, ZHANG P, LI H, et al. Generation mechanism of persistent free radicals in lignocellulose-derived biochar: Roles of reducible carbonyls[J]. Environmental Science & Technology, 2022, 56(15): 10638-10645. [42] KHACHATRYAN L, BAREKATI-GOUDARZI M, ASATRYAN R, et al. Metal-free biomass-derived environmentally persistent free radicals (bio-EPFRs) from lignin pyrolysis[J]. ACS Omega, 2022, 7(34): 30241-30249. doi: 10.1021/acsomega.2c03381 -



 下载:
下载: