-
因具有广谱、价廉和高效等优点,四环素(Tetracycline,TC)被广泛地应用于水产养殖和畜牧业[1]. 动物对TC的吸收利用率较低,超过75%的TC以活性物质形式排出体外,进而通过不同途径进入水环境,导致不同程度的水体污染问题[2]. TC在我国地表水中被广泛检出,其检出浓度为2.32 ng·L−1—100.75 μg·L−1[3 − 5]. 尽管浓度较低,但长期接触低剂量的抗生素,会破坏微生物生态,导致抗性基因的富集[6].
鉴于TC在水中赋存浓度极低且具有较大的溶解度,致使常规净水处理工艺(混凝、沉淀、过滤)难以将其有效去除[7]. 作为水处理中常见的氧化剂,KMnO4具有性质稳定、使用简单、成本低等优点[8],且处理过程中产生的水合MnO2,有利于氧化和吸附反应的进行[9]. 因此,KMnO4被广泛应用于降解包含TC在内的新污染物[10]. TC(解离常数为pKa1=3.3、pKa2=7.68和pKa3=9.3)为典型的酸碱两性化合物,随着溶液pH的升高,TC先后主要以TC+(pH<3.3)、TC0(3.3<pH<7.68)、TC−(7.68<pH<9.3)和TC2-(pH>9.3)形态存在. 有研究表明,KMnO4与TC反应遵循二级反应动力学,具有pH依赖性,pH为6—9时,kapp从10.12 mol·L−1·s−1增长到38.43 mol·L−1·s−1. 溶液pH增加,TC分子静电势负性加强,增强了化学结合能,是导致速率变化的主要原因[10]. 此外,不同的TC赋存形态会改变其分子表面的电子分布密度,可能会导致生成不同的降解产物,进而影响溶液的综合生物毒性,然而,相关研究尚未见报道.
为探究KMnO4对TC四种形态的氧化效率及机理,本研究系统探究了不同pH(2、6、9、11)条件下TC的降解动力学、矿化度、降解产物及路径,并选用表征急性毒性的费氏弧菌、湖泊水库中常见的斜生栅藻和传统好氧工艺中的活性污泥等受试生物,综合评估了降解产物的生物毒性. 此外,还采用ECOSAR软件对不同氧化产物单体的理论毒性进行评估,以期能够更好地揭示其在水环境中的转化和归宿.
-
四环素(分析纯)购自大连美仑生物试剂有限公司;甲醇(HPLC 级)购买于Sigma-Aldrich 公司(Bellefonte,PA,USA),磷酸氢二钠、磷酸二氢钠、抗坏血酸、氢氧化钠、硫代硫酸钠和高锰酸钾等试剂为优级纯或分析纯,购买于国药化学试剂有限公司;实验用水为Elga净水系统制备的超纯水(18.2 MΩ·cm),实验前,KMnO4浓度均用紫外可见分光光度计在525 nm波长下进行测定.
-
向含有2 mmol·L−1磷酸缓冲盐溶液(pH=2、6、9、11)中加入一定量TC溶液(15.75 µmol·L−1),然后加入157.5、236.3、315.0、393.7 µmol·L−1的KMnO4并摇匀. 在预设反应时间点(0、1、2、6、10、15 min)取出水样,加入过量抗坏血酸终止反应,并将溶液pH调至7. 水样经0.22 µm滤膜过滤后置于进样瓶中,测定TC浓度、矿化度、降解产物及生物毒性. 每组实验进行3次,取其平均值和标准偏差绘图.
-
TC定量分析采用超高效液相色谱-二极管阵列检测器(UPLC-DAD, Waters, Milford, MA, USA),配备ACQUITYTM UPLC BEH C18分离柱(2.1 mm×50 mm, 1.7 μm,Waters). 以甲醇和超纯水 (70:30, V/V)为流动相,流速为0.2 mL·min−1,采用等度洗脱模式进行分离. 柱温为35 ℃,进样体积为10 µL,DAD检测波长为355 nm. TC的矿化度采用总有机碳分析仪(TOC-LCPN, Shimadzu, Japan)进行测定.
-
采用超高效液相色谱-质谱联用仪(UPLC-MS/MS,Water,Milford, MA, USA),在全扫描模式下对TC的降解产物进行分析. 液相色谱为ACQUITYTM UPLC型,分离色谱柱使用ACQUITYTM UPLC BEH C8(2.1 mm×100 mm,1.7 μm,Waters);质谱为三重四极杆质谱仪,型号TQD,N2作为雾化和干燥气体,离子源温度为110 ℃,去溶剂气温度为350 ℃,去溶剂气(N2)流速为500 L·h−1,锥孔流速为30 L·h−1. 使用ESI+/ESI-模式同时扫描,扫描范围为100—600 m/z.
流动相使用超纯水(A2)和甲醇(B1);采用满环进样,进样体积为10 µL;流动相的流速为0.2 mL·min−1. 采用梯度法洗脱,首先用5%甲醇洗脱3 min,接着在9 min内将甲醇比例增至100%,并保持4 min,最后在1 min内将其降至5%,并保持3 min. 柱温为35 ℃,样品室温度为25 ℃.
-
参照BS EN ISO
11348 -3(ISO 2007)发光细菌急性毒性测试方法. 其中,测试溶液含有2%NaCl的400 μL样品溶液和100 μL稀释菌液,反应15 min,用生物化学发光仪(GloMaxTM)检测发光强度,每个样品设置1个空白样和3个平行样,并重复3次. 其中,急性毒性以实验组与空白组的发光细菌荧光的减少相对强度表示,其计算公式如下:式中,Lblank为空白组发光强度(RLU);Lsample为实验组发光强度值(RLU).
-
活性污泥生长抑制实验参照 ISO
15522 -1999进行,好氧活性污泥取自实验室SBR反应器,在其空闲期取活性污泥进行好氧曝气24 h,用去离子水将活性污泥洗涤3次后开始实验[11]. 将淬灭后的25 mL水样调整pH=7.6置于锥形瓶中,加入5 mL浓缩6倍的牛肉膏蛋白胨培养基(pH=7.6),再加入一定量处理后的活性污泥上清液,用无菌封口膜封住瓶口,置于空气摇床(25 ℃,140 r·min−1),待预定时间后,测其OD600值. 四环素氧化降解产物对活性污泥的抑制性计算公式如下:式中,S0为空白样,超纯水培养出培养基的OD600值;S为实验样,氧化实验淬灭后的水样培养出培养基的OD600值.
-
本实验选用BG-11培养基,取25 mL水样,调整pH至7.6,倒入含有5 mL浓缩6倍的BG-11培养基的锥形瓶内(pH=7.6),再加入藻液,使初始藻浓度为2×105 cell·mL−1. 使用无菌封口膜将其密封,摇匀,放置于光照培养箱内(温度25 ℃,光强为
5000 Lux,光照周期为白天:黑夜=12 h:12 h),每天3次晃动和移动锥形瓶位置,96 h后测其藻密度[12]. 其中,斜生栅藻的抑制性以实验样与空白样的藻密度减少相对强度表示,计算公式如下:式中,A——为空白样,超纯水培养的藻密度,cell·mL−1; B——为实验样,氧化实验淬灭后的水样培养出的藻密度,cell·mL−1;
-
基于定量构效关系(QSAR)模型,ECOSAR软件可估算以下几种毒性终点的值. 急性毒性:鱼(96 h-LC50)、水蚤(48 h-LC50)、藻(96 h-EC50);慢性毒性:鱼、水蚤、藻. 基于《全球化学品统一分类和标签制度》对预测结果进行分类,毒性判别标准如下:剧毒(very toxic)、有毒(toxic)、有害(harmful)和无害(not harmful)[13].
-
有文献表明,高锰酸钾氧化四环素的反应数遵循二级反应[10],其公式如下:
kapp为表观二级反应速率,是四环素浓度,[KMnO4]是高锰酸钾浓度. 如果[KMnO4]远大于[TC]的浓度([KMnO4]/[TC]>10),方程(4)可用准一级反应动力学模型表示:
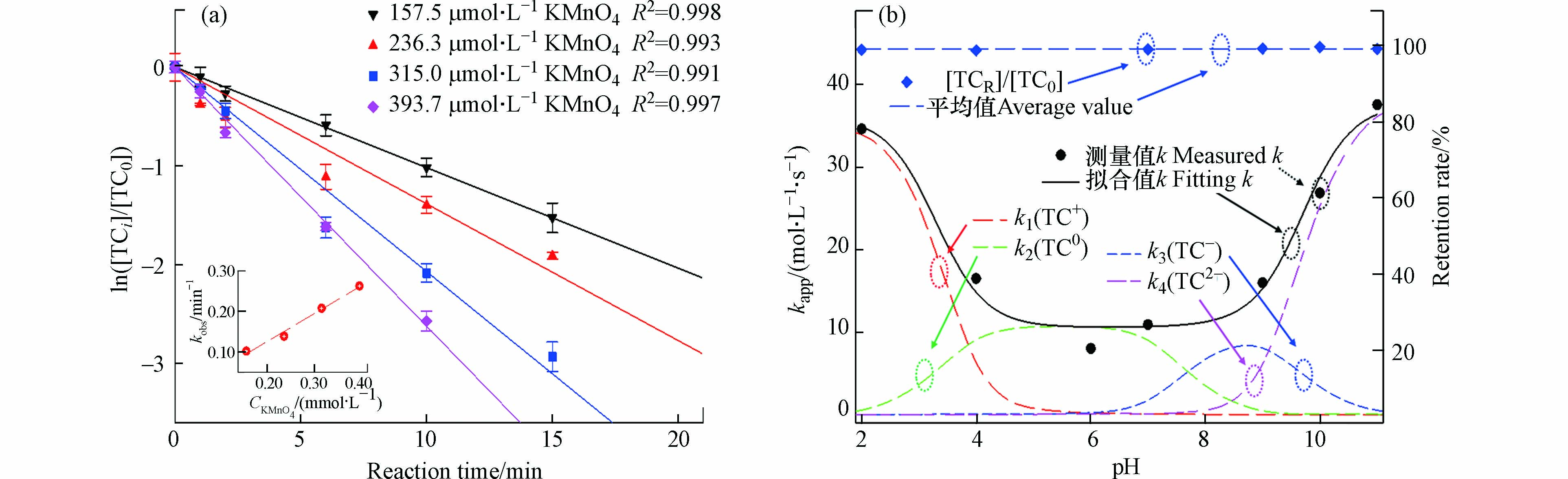
其中,[TC0]是TC的初始浓度,[TCi]是t时刻TC的浓度,kobs=kapp[KMnO4]是准一级速率常数. 由图1a可知,In([TCi]/[TC0])与反应时间t呈线性关系,表明TC随时间的降解符合准一级反应动力学模型. kobs与[KMnO4]呈零截距的线性关系(R2>0.997),表明KMnO4的反应符合一级反应. 因此,KMnO4对TC反应遵循二级反应动力学模型.
-
在不同溶液pH条件下,TC共有4种形态,解离状态如下:
在四环素的氧化实验中,kapp具有pH依赖性,其对应于两种反应物的具体存在形态. 由于高锰酸钾的pKa足够低(−2.25),因此忽略共轭酸HMnO4的影响,高锰酸根与TC+、TC0、TC−和TC2-的反应见公式(8)—(13):
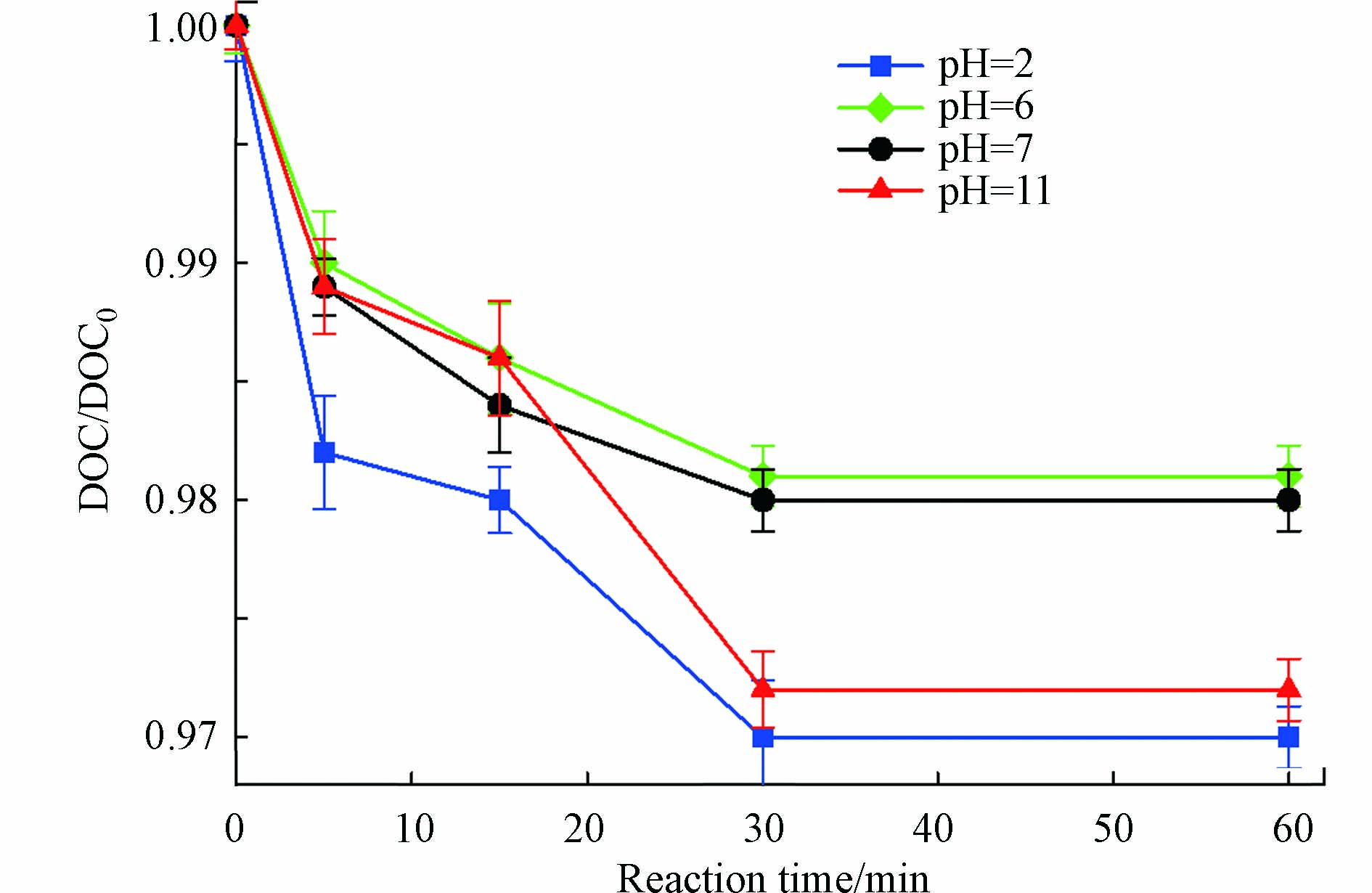
式中,ki(i=1、2、3、4)是高锰酸钾与相应TC物种形态反应的二级速率常数,αi(i=1、2、3、4)代表各物种的分布系数(通过三元酸的标准平衡分布方程进行计算[10]). 在不同pH值条件下(2—11),KMnO4与TC反应的二级反应速率以及空白样中2 h内TC的留存率见图1b,[TCR]/[TC0]为留存率,表示t时刻,空白样中留存的TC与零时刻TC的比值. 溶液pH值由2增至11时,TC在溶液中2 h的平均留存率高于99.1%(水解率低于0.9%),表明本研究中的pH值条件不会促使TC发生水解. kapp值呈现先减少,后增加的趋势. 基于kapp的实验数据,使用SigmaPlot 14.0中的非线性回归拟合计算了二级速率常数k1、k2、k3和k4,分别为36.19、10.57、11.04、37.78 mol·L−1·s−1(R2=0.993),呈现大小关系为k2< k3< k1< k4. 溶液pH影响TC降解速率的原因主要有以下两个:
第一,在pH为2—6时,pH值越低,物质的羟基质子化越严重,TC中的烯烃更加缺电子,促进高锰酸根的亲核攻击[14]. 此外,KMnO4反应过程中还伴随着MnO2的生成,其可作为催化剂,加速KMnO4与TC反应的进行. MnO2在水中溶解度很低,便以水合MnO2的形式从水中析出,亦具有一定的吸附能力[15].
第二,pH为6—11时,本研究的反应速率随溶液pH的增加呈非线性增加,这与先前研究结果一致[10]. 随着pH值增加,TC逐渐去质子化,反应所需活化能降低,有利于克服反应的能垒;且TC所带负电荷增多,使酚基成为更富电子的部分,更易受到亲电攻击[16],因而pH越高,TC的反应速率越大.
-
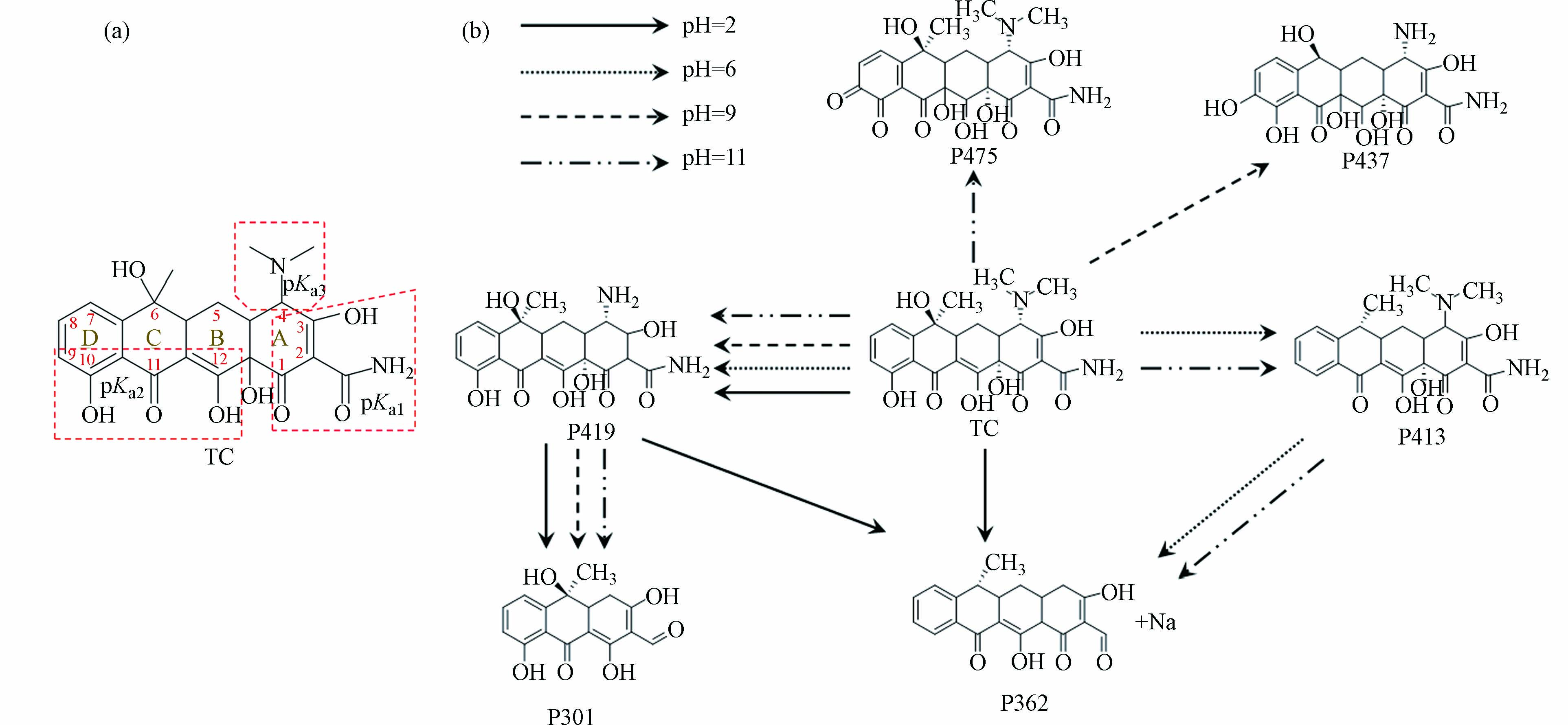
在4种溶液pH条件下,KMnO4降解TC的矿化情况如图2所示. 随着反应时间的增加,反应体系的DOC几乎没变化,反应时间为60 min时,矿化度均低于4%,表明降解的TC大部分转化成了其他有机降解产物,这与之前研究结果相同[10]. KMnO4氧化处理TC后的溶液中,共检出6种主要降解产物,分别将其记为P301、P413、P475、P437、P362和P419. 基于本研究得到的色谱质谱数据,并结合先前研究,对各产物的质谱图进行合理解析与产物鉴别推断,所得结果见表1.
结合图3a可知,TC脱去C环和D环上的羟基键后形成P413,先前有学者研究了TC在UV/TiO2工艺中的降解也得到此产物[17]. P362可能是P413脱去A环上的酰胺基、A2上的氨基及A1邻位的羟基所形成的,Shen等催化过硫酸盐氧化降解TC时亦得到此产物[18]. P419可能是由TC脱去二甲胺上的2个甲基,以及断裂A2-A3上的C=C而形成. Mei等采用芬顿法去除TC的研究也得到类似结构的产物,不过C=C断裂位置为B12处[19]. P301可能是P419脱去A4的氨基、A2上的酰胺基,在持续氧化下,A环断开,进行羟基氧化反应后所生成,Shen等研究光催化TC时也得到此产物[20].
P437是TC脱去二甲胺上的双甲基和C6上甲基,断掉敏感位置B12的C=C,B12邻位和D9发生了羟基氧化后所形成. P475是TC的酚羟基氧化形成,酚基通过电子转移与KMnO4反应形成羟基化产物[21],邻苯酚结构的羟基经历逐步夺氢的双电子氧化反应,形成邻苯醌结构[22],先前KMnO4和MnO2氧化降解TC也均得到了此产物[10, 22].
TC降解路径包括去羟基、去甲基、碳碳双键断裂、去酰胺基等,可能的降解路径推导见图3b. 其中,不同的pH条件下,TC的存在形态不同,导致TC的产物存在差异,故其降解路径也不同.
-
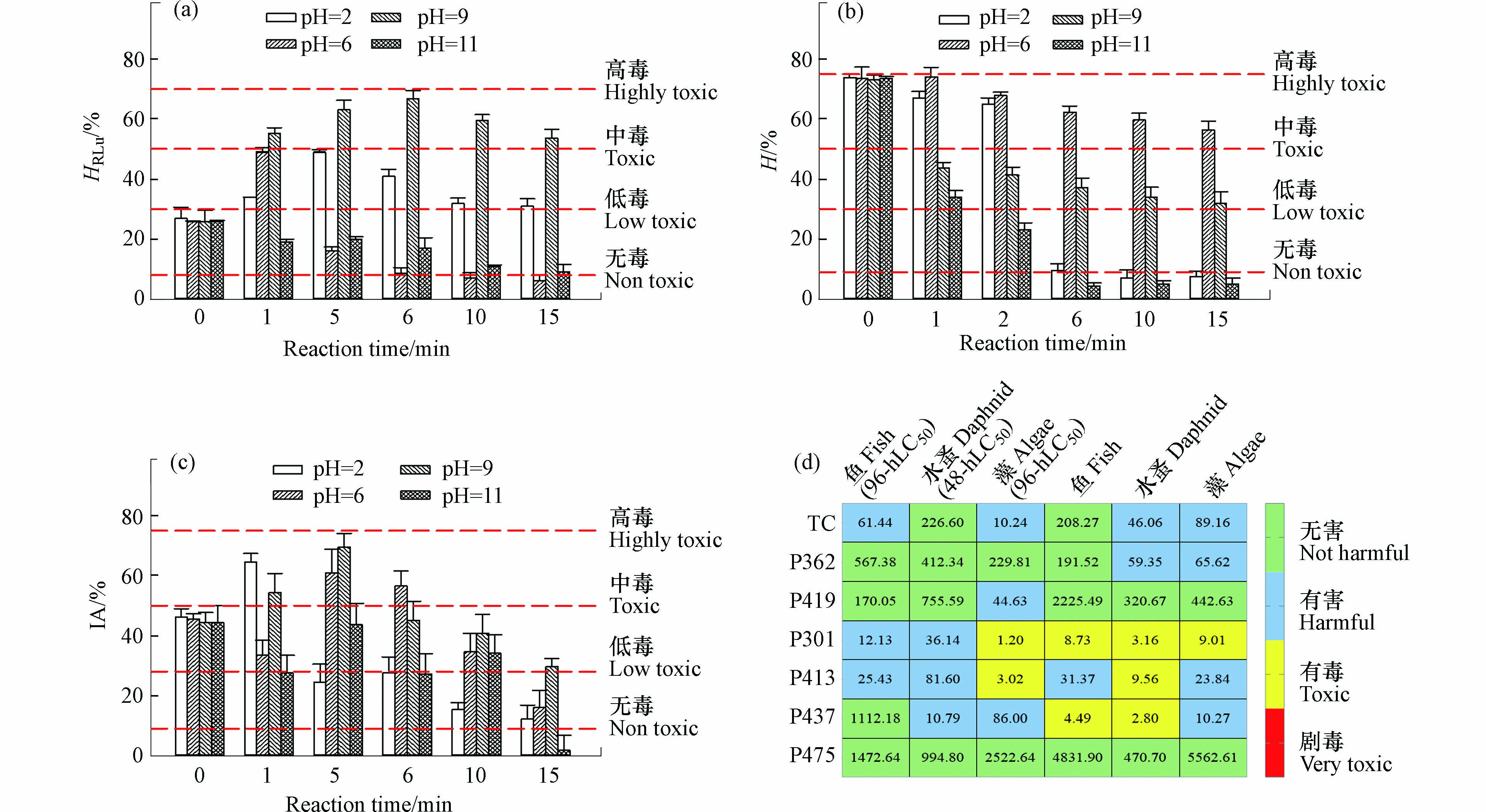
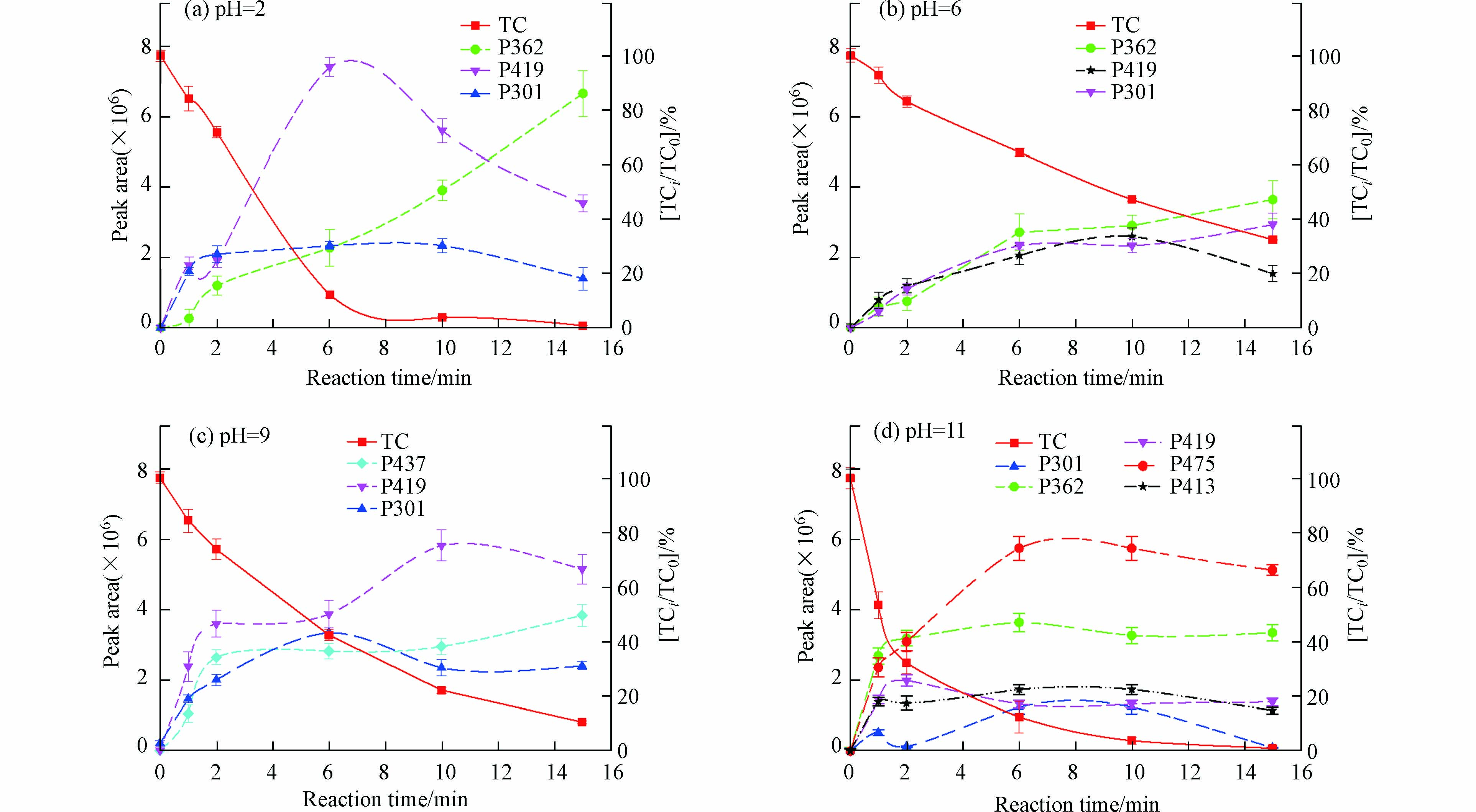
“无毒、低毒、中毒和高毒”是基于生物毒性等级分级方法,并结合实验数据进行分级,活性污泥和藻同理[23]. 由图4a可知,处理前,TC对费氏弧菌的平均抑制率(HRLu)为26.12%,pH=2和9时,在0—6 min时,HRLu随氧化的进行而急剧上升,分别达到了41.01%和66.82%,分别为低、中毒性,而后缓慢降低,但反应15 min时的HRLu分别为31.28%和53.67%,依然高于0 min的26.12%. pH=6时,反应时间为0—1 min时,HRLu急剧增加至49.11%,为低毒,而后缓慢降至6%,为无毒. 上述结果表明,pH为2、6和9时,部分降解产物的急性毒性比母体更强. 其中,P413主要涉及羟基取代,其生物毒性高于TC[24],ECOSAR模拟数据也印证该结论(见图4d). P301主要经过脱去羟基、氨基、二甲胺和开环后所生成,由ECOSAR模拟数值可知(见图4d),急性毒性较强. 图5为不同pH值下,TC的含量和降解产物含量随时间的变化关系. 由图5可知,随着氧化进行,P301和P413的峰面积均逐渐增加,而且含量相对较高. pH=2、6、9时,P301(P413)的峰面积最高占比为35.31%,43.54%和33.19%. 结合降解产物鉴别结果和ECOSAR数据可知(见图4d),P301和P413可能是TC溶液经KMnO4氧化处理后毒性增加的主要原因.
而pH=11时,随着氧化反应的进行,HRLu缓慢下降,15 min时为9.19%. 此条件下的主要产物P475为羟基持续氧化后所生成,生物毒性较低. P362涉及到TC的羟基去除,该反应降低物质的极性,能够影响氢键的成键能力,毒性较低[25]. P419是去甲基衍生物,主要活性基团破坏较少,急性毒性的鱼(96 h-LC50)和水蚤(48 h-LC50)毒性指标预测为“无害”物质,毒性变化幅度较小. 有毒产物P301和P413在产物中的占比较少(峰面积之和最高占比为23.19%),且峰面积增长速率缓慢,而P362、P475和P419含量相对较高(峰面积之和最高占比为88.21%),峰面积增长速率也较高,这些产物的生成决定了HRLu的变化规律.
-
由图4b可知,7 mg·L−1的TC对活性污泥的抑制性(H)高达73%,为中等毒性. 对于毒性评估效果而言,4种pH条件下的H值变化趋势相似,随氧化的进行,H值呈逐渐下降趋势. 当pH=2和11时,H值的降幅最大,反应15 min时,H值为7.54%和4.99%,较0 min时的毒性削减89.6%和93.2%. 这是由于反应15 min时,TC的降解率达99.8%以上,母体导致的抑制性显著降低,降解产物P301、P362的主要抗菌活性基团——酰胺基丢失[26],在这些共同作用下,H值就显著降低. pH=6和9时,H值减少幅度较小,反应15 min时的H值为59.65%和34.10%,较0 min的H值仅削减19.0%和46.6%,此时TC的降解率分别为67.6%和78.3%. 值得注意的是,4种pH条件下,H值降低趋势和TC剩余量存在相关性. 这是因为随着TC浓度的减低,TC对活性污泥的抑制性也逐渐减弱,而生成的降解产物若又以低毒或者无毒产物为主,产物也无明显抑制作用.
-
由图4c可知,pH=2、6和9时,随着氧化反应的进行,TC溶液的IA值先逐渐上升,后缓慢下降. 表明氧化过程中生成了毒性更强的降解产物,采用藻(96 h-EC50)指标预测P301和P413为“有毒”物质,P437和P419为“有害”物质,加上剩余TC的共同作用,导致IA值增加. 而pH=11,IA值呈现出不同程度的降低,此时的降解产物有P301和P413,但含量均较低,峰面积峰值仅为1.47×106和1.16×106,远远小于P475和P362的峰面积5.75×106和3.67×106. 表明除有毒降解产物的生成对IA值的影响外,其含量的多少也会显著影响IA值的变化.
-
1)KMnO4能有效降解TC,反应体系符合二级反应动力学模型. 不同pH值条件下,TC的降解速率受TC形态分布影响,随着溶液pH的增加,kapp呈现由34.68 mol·L−1·s−1降低至7.92 mol·L−1·s−1,后又增加至37.65 mol·L−1·s−1的变化趋势.
2)根据色谱质谱鉴定出6种主要氧化降解产物. pH=2、6、9和11时,分别生成了3、3、3和5种主要产物,主要降解反应包含去甲基、去羟基、羟基氧化、去氨基化和烯烃加成等. TC降解产物的生成及降解路径与溶液pH密切相关.
3)在急性毒性和藻毒性评估中,pH=2、6、9条件下,溶液的毒性随反应的进行有不同程度的增大,这是由于生成比TC生物毒性更强的产物所致;而活性污泥毒性评估中,毒性逐渐降低,说明相同降解产物对不同受试生物的毒性作用效果不一样. 对比三种毒性测试结果发现,溶液pH造成降解产物种类及生成量的差异,是导致毒性变化趋势的最主要原因.
溶液pH对高锰酸钾降解四环素动力学、产物和生物毒性的影响
Effect of solution pH on kinetics, product and biotoxicity of tetracycline degradation by potassium permanganate
-
摘要: 四环素(TC)是一种典型的酸碱两性化合物,其在水中的赋存形态受溶液pH影响. 为探究TC降解路径和溶液毒性是否会随pH产生规律性变化,本研究考察了KMnO4氧化过程中,溶液pH(2、6、9和11)对TC降解动力学、降解产物和溶液毒性的影响规律及机制. 结果表明,TC在KMnO4体系中的反应符合二级反应动力学模型,速率常数(kapp)为7.92 mol·L−1·s−1(pH=6)—37.74 mol·L−1·s−1(pH=11);使用超高效液相色谱-串联质谱法(UPLC-MS/MS)对经KMnO4处理后TC的降解产物进行定性分析,共有6种降解产物被检出,溶液pH为2、6、9和11时,分别有3、3、3和5种降解产物,表现出显著的pH依赖性. 采用费氏弧菌、活性污泥细菌和斜生栅藻对氧化处理后的TC溶液进行毒性评价,发现部分降解产物具有比母体更强的生物毒性,ECOSAR模拟毒性数值也能佐证.
-
关键词:
- 四环素 /
- 超高效液相色谱-串联质谱法(UPLC-MS/MS) /
- 化学氧化 /
- 降解产物 /
- 生物毒性.
Abstract: Tetracycline (TC) is an acid-base amphoteric compound that undergoes changes in its species within aqueous solutions due to solution pH. This study aimed to investigate the relationship between solution pH and the degradation pathways, solution toxicity, and degradation kinetics of TC during KMnO4 oxidation, as well as the underlying mechanism. The results indicated that the reaction of TC in the KMnO4 system followed a second-order rate law. The pH-dependent rate constants (kapp) ranged from 7.92 mol·L−1·s−1 (at pH 6) to 37.74 mol·L−1·s−1 (at pH 11). Using the ultra-performance liquid chromatography-mass spectrometry (UPLC-MS/MS) method, qualitative analysis revealed a total of six degradation products. The number of degradation products identified varied with pH, with three, three, three, and five degradation products observed at pH 2, 6, 9, and 11, respectively. The degradation products exhibited significant pH dependence. The toxicity of the oxidized TC solution was assessed using Vibrio fischeri, activated sludge bacteria, and Scenedesmus obliquus. The study discovered that some of the degradation products exhibited higher toxicity than TC itself, which was further supported by simulated toxicity values obtained from ECOSAR. -

-
表 1 TC及其降解产物的保留时间、分子式、MS质谱信息及分子结构
Table 1. Retention time, molecular formula, MS mass spectrum information and molecular structure of TC and its degradation products
pH 名称
Name保留时间/min
Retention time质荷比m/z 分子式
Molecular formula可能结构
Proposed structure理论值
Theoretical测量值
Measured2、6、11 P362 9.052 362.13 362.52 C20H18O5 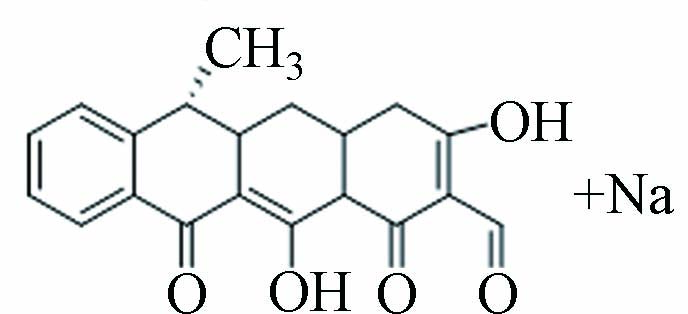
2、6、9、11 P419 9.263 419.1 419.07 C19H18N2O9 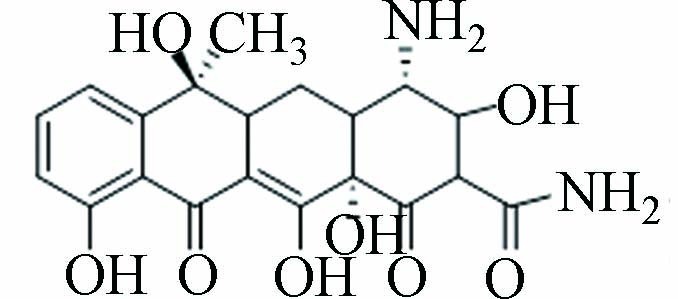
2、9、11 P301 11.904 302.08 301.36 C16H14O6 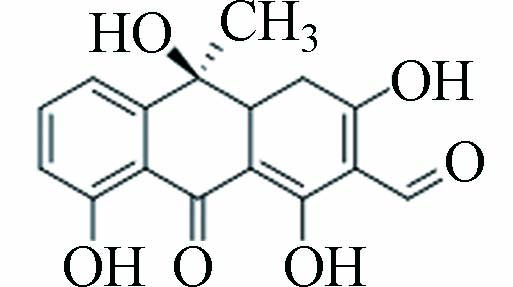
6、11 P413 13.418 413.16 413.54 C22H24N2O6 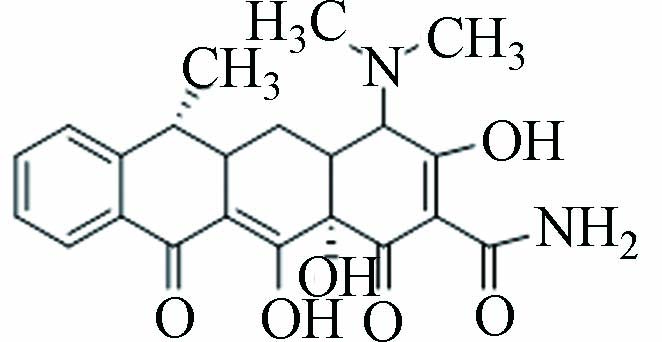
9 P437 11.202 437.15 437.46 C20H24N2O9 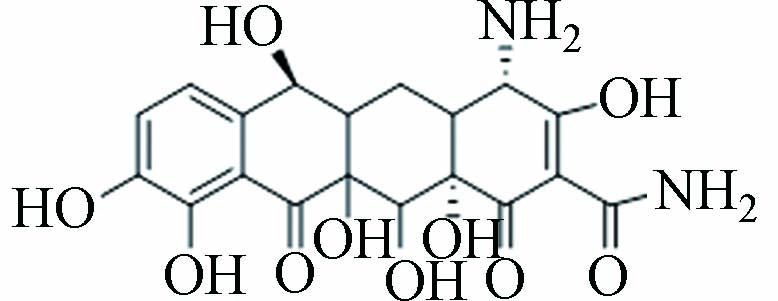
11 P475 9.547 476.14 475.49 C22H24N2O10 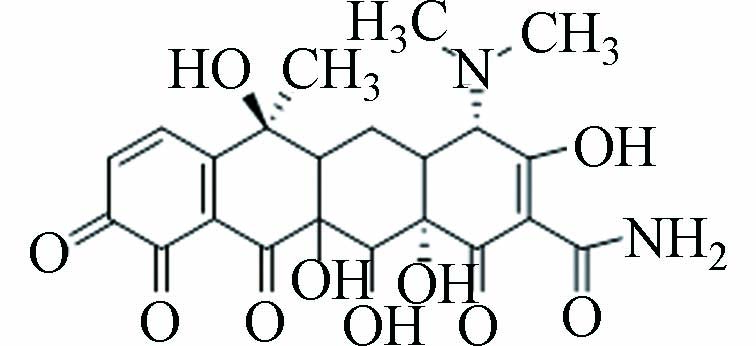
-
[1] 韩爽, 肖鹏飞. 过硫酸盐活化技术在四环素类抗生素降解中的应用进展[J]. 环境化学, 2021, 40(9): 2873-2883. doi: 10.7524/j.issn.0254-6108.2020052401 HAN S, XIAO P F. Application progress of persulfate activation technology in degradation of tetracycline antibiotics[J]. Environmental Chemistry, 2021, 40(9): 2873-2883 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020052401
[2] TRAN N H, CHEN H J, REINHARD M, et al. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes[J]. Water Research, 2016, 104: 461-472. doi: 10.1016/j.watres.2016.08.040 [3] WANG Z, DU Y, YANG C, et al. Occurrence and ecological hazard assessment of selected antibiotics in the surface waters in and around Lake Honghu, China[J]. Science of the Total Environment, 2017, 609: 1423-1432. doi: 10.1016/j.scitotenv.2017.08.009 [4] WEI R C, GE F, HUANG S Y, et al. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China[J]. Chemosphere, 2011, 82(10): 1408-1414. doi: 10.1016/j.chemosphere.2010.11.067 [5] LI Z, LI M, ZHANG Z Y, et al. Antibiotics in aquatic environments of China: A review and meta-analysis[J]. Ecotoxicology and Environmental Safety, 2020, 199: 110668. doi: 10.1016/j.ecoenv.2020.110668 [6] TOPAL M, USLU ŞENEL G, ÖBEK E, et al. Investigation of relationships between removals of tetracycline and degradation products and physicochemical parameters in municipal wastewater treatment plant[J]. Journal of Environmental Management, 2016, 173: 1-9. [7] SAITOH T, SHIBATA K, FUJIMORI K, et al. Rapid removal of tetracycline antibiotics from water by coagulation-flotation of sodium dodecyl sulfate and poly(allylamine hydrochloride) in the presence of Al(III) ions[J]. Separation and Purification Technology, 2017, 187: 76-83. doi: 10.1016/j.seppur.2017.06.036 [8] GUAN X H, HE D, MA J, et al. Application of permanganate in the oxidation of micropollutants: A mini review[J]. Frontiers of Environmental Science & Engineering in China, 2010, 4(4): 405-413. [9] JIANG J, PANG S Y, MA J. Oxidation of triclosan by permanganate (Mn(VII)) : Importance of ligands and in situ formed manganese oxides[J]. Environmental Science & Technology, 2009, 43(21): 8326-8331. [10] JIANG X H, JEFFERSON W A, SONG D A, et al. Regioselective oxidation of tetracycline by permanganate through alternating susceptible moiety and increasing electron donating ability[J]. Journal of Environmental Sciences (China), 2020, 87: 281-288. doi: 10.1016/j.jes.2019.07.004 [11] AL-AHMAD A, DASCHNER F D, KÜMMERER K. Biodegradability of cefotiam, ciprofloxacin, meropenem, penicillin G, and sulfamethoxazole and inhibition of waste water bacteria[J]. Archives of Environmental Contamination and Toxicology, 1999, 37(2): 158-163. doi: 10.1007/s002449900501 [12] KOVALAKOVA P, CIZMAS L, FENG M B, et al. Oxidation of antibiotics by ferrate(VI) in water: Evaluation of their removal efficiency and toxicity changes[J]. Chemosphere, 2021, 277: 130365. doi: 10.1016/j.chemosphere.2021.130365 [13] DELLARCO M, BANGS G. Update of the U. S. EPA guidelines for exposure assessment[J]. Epidemiology, 2006, 17(Suppl): S61. [14] KIM M S, LEE H J, LEE K M, et al. Oxidation of microcystins by permanganate: PH and temperature-dependent kinetics, effect of DOM characteristics, and oxidation mechanism revisited[J]. Environmental Science & Technology, 2018, 52(12): 7054-7063. [15] QIN Q D, ZHANG Y F, ZHANG H M, et al. Performance evaluation and mechanism analysis of potassium permanganate used for simultaneous removal of tetracycline and Cu(II)[J]. Journal of Water Process Engineering, 2022, 48: 102861. doi: 10.1016/j.jwpe.2022.102861 [16] 张静, 于金宝, 熊伶, 等. 高锰酸钾氧化及其联用技术在饮用水处理中的应用[J]. 给水排水, 2021, 57(8): 167-176. ZHANG J, YU J B, XIONG L, et al. Application of permanganate oxidation and its combined technologies in drinking water treatment[J]. Water & Wastewater Engineering, 2021, 57(8): 167-176 (in Chinese).
[17] ZHANG B, HE X, YU C Z, et al. Degradation of tetracycline hydrochloride by ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst: Influencing factors, products and mechanism insight[J]. Chinese Chemical Letters, 2022, 33(3): 1337-1342. doi: 10.1016/j.cclet.2021.08.008 [18] SHEN M X, HUANG Z J, LUO X W, et al. Activation of persulfate for tetracycline degradation using the catalyst regenerated from Fenton sludge containing heavy metal: Synergistic effect of Cu for catalysis[J]. Chemical Engineering Journal, 2020, 396: 125238. doi: 10.1016/j.cej.2020.125238 [19] MEI Y Q, ZHANG Y Q, LI J Q, et al. Synthesis of Co-doped CeO2 nanoflower: Enhanced adsorption and degradation performance toward tetracycline in Fenton-like reaction[J]. Journal of Alloys and Compounds, 2022, 904: 163879. doi: 10.1016/j.jallcom.2022.163879 [20] SHEN X F, ZHANG Y, SHI Z, et al. Construction of C3N4/CdS nanojunctions on carbon fiber cloth as a filter-membrane-shaped photocatalyst for degrading flowing wastewater[J]. Journal of Alloys and Compounds, 2021, 851: 156743. doi: 10.1016/j.jallcom.2020.156743 [21] ZHANG J, SUN B, GUAN X H. Oxidative removal of bisphenol A by permanganate: Kinetics, pathways and influences of co-existing chemicals[J]. Separation and Purification Technology, 2013, 107: 48-53. doi: 10.1016/j.seppur.2013.01.023 [22] CHEN W R, HUANG C H. Transformation kinetics and pathways of tetracycline antibiotics with manganese oxide[J]. Environmental Pollution, 2011, 159(5): 1092-1100. doi: 10.1016/j.envpol.2011.02.027 [23] PERSOONE G, MARSALEK B, BLINOVA I, et al. A practical and user-friendly toxicity classification system with microbiotests for natural waters and wastewaters[J]. Environmental Toxicology, 2003, 18(6): 395-402. doi: 10.1002/tox.10141 [24] dos SANTOS H F, de ALMEIDA W B, ZERNER M C. Conformational analysis of the anhydrotetracycline molecule: A toxic decomposition product of tetracycline[J]. Journal of Pharmaceutical Sciences, 1998, 87(2): 190-195. doi: 10.1021/js970275l [25] 董雪纯, 魏海峰, 刘长发. 4种多环芳烃对仿刺参(Apostichopus japonicus)原肠胚发育的急性毒性研究[J]. 生态毒理学报, 2019, 14(1): 161-167. doi: 10.7524/AJE.1673-5897.20181030001 DONG X C, WEI H F, LIU C F. Acute toxicity of four polycyclic aromatic hydrocarbons to the development of Apostichopus japonicus gastrointestinal embryo[J]. Asian Journal of Ecotoxicology, 2019, 14(1): 161-167 (in Chinese). doi: 10.7524/AJE.1673-5897.20181030001
[26] MITSCHER L A. The chemistry of the tetracycline antibiotics[M]. New York: M. Dekker, 1978. -



 下载:
下载: