-
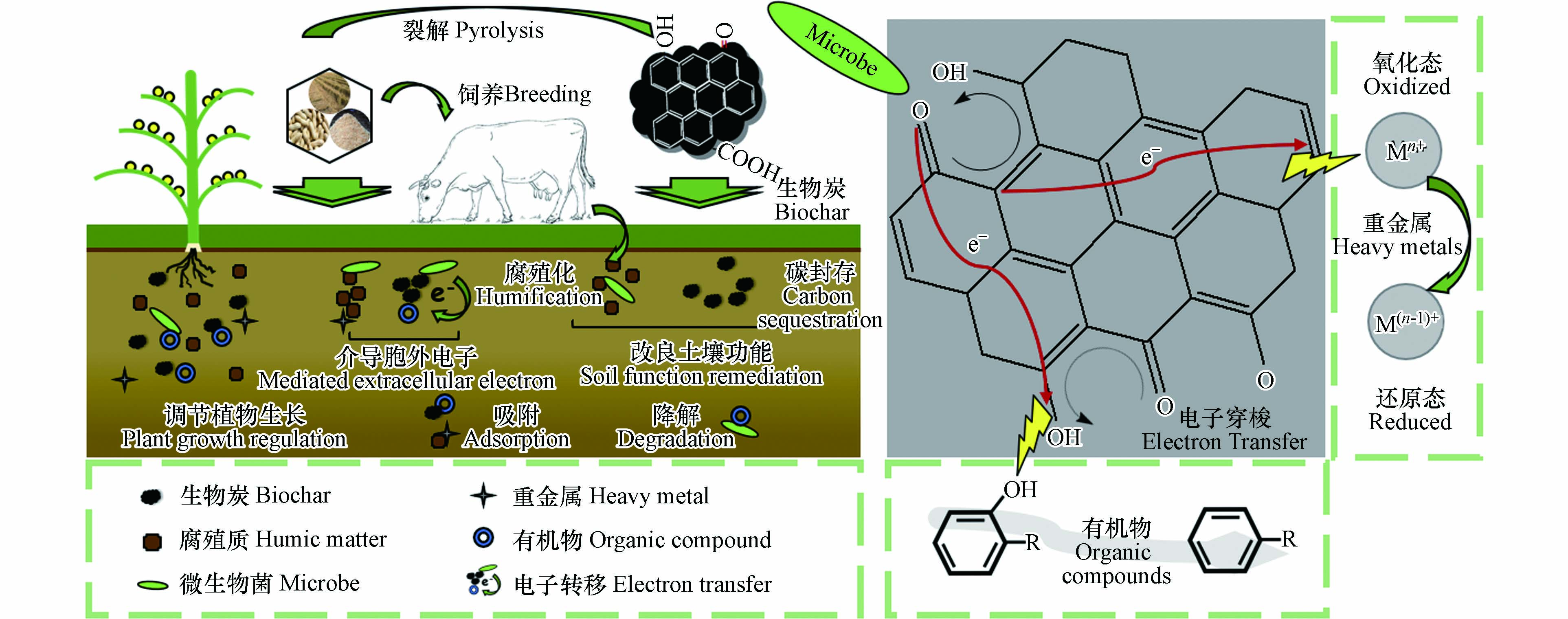
电子转移过程在水、土壤和沉积物圈层中普遍发生,它是生物地球化学循环的基础. 在氧化还原反应体系,电子穿梭过程参与和控制诸多环境行为,如温室气体的排放、土壤矿物的还原、污染物的转化等[1 − 2]. 自然界中,微生物的胞外呼吸过程引起的电子传递是促进生物地球化学循环的关键驱动力[3]. 据报道,氢氧化细菌(Hydrogen-oxidizing bacteria)是一类化能自养型细菌,该细菌氧化无机电子供体H2获得能量,将捕获大气中CO2作为生长所需碳源,达到固碳减排效果[4]. Rotaru 等[5]发现,产甲烷菌(Methanosaeta harundinacea)能够直接接受来自金属还原地杆菌(Geobacter metalreducens)释放的电子,并利用得到的电子还原CO2生成甲烷. 氧化亚铁硫杆菌(Thiobacillus ferrooxidans)和硫氧化细菌(Azospirillum thiophilum)属于化能自养微生物,也可利用氧化还原过程产生的能量参与到氮素循环[6]. Myers 和 Nealson[7]研究发现,微生物菌Alteromnas putrefaciens MR-1 能够将电子传递至锰氧化物从而实现生长代谢. Lovely 和 Phillips [8]曾报道由淡水沉积物分离获得一种金属异化还原菌,命名为Geobacter metallireducens GS-15,它在厌氧环境下能够还原铁(Ⅲ)和锰(IV)的金属氧化物,并实现有机物的污染降解.
胞外电子传递涉及微生物利用电子供体进行自身代谢,或微生物通过胞内代谢将产生的电子导至胞外的受体的过程[9]. 微生物进行胞外呼吸作用产生的电子会依次经过细胞质膜-周质-细胞外膜,之后将电子供给胞外受体利用,外膜结构上存在的一系列功能蛋白细胞色素c(c-Cyts)是实现电子从胞内传输到胞外受体的关键部分. 例如,Shewanella oneidensis MR-1质子泵内产生的电子在脱氢酶的作用下传递给醌类介体后,经由胞内电子传递链传导至外膜,外膜上的MtrC和OmcA是与胞外受体作用的重要蛋白[10]. 而Geobacter sulfurreducens利用的外膜蛋白主要包括OmcB、OmcE、OmcS[11 − 12]. 直接和间接电子传递是微生物胞外电子传递过程的两种形式. 直接电子传递过程中,微生物菌和胞外电子受体可以直接接触,通过外膜上的功能蛋白c-Cyts与末端受体结合. 例如Shewanella菌属,合成的功能蛋白复合物MtrCAB直接负责电子传递至膜外的受体 [13]. 此外,当微生物的电子传递结构与胞外电子受体的距离在纳米范围内即无法直接接触时,“纳米导线”机制则成为可利用的电子传导的直接方式. 此方式类似于金属导电过程或者依托c-Cyts之间的电子跃迁,实现与远端受体的电子传递. Reguera 等首次发现Geobacter sulfurreducens的菌毛具有导电能力,将此结构命名为“纳米导线”[14]. 随后,Gorby 等在Shewanella oneidensis、蓝细菌等微生物中也发现类似现象[15]. 间接电子传递是利用由微生物自身分泌的或者外源环境提供的电子穿梭体来实现长距离的电子传递过程. 电子穿梭体通常是一类具有氧化还原活性的、可在环境体系中扩散迁移的化学物质. 某些微生物可以分泌核黄素、吩嗪类色素等小分子物质可以进入细胞内部,与内膜上的c-Cyts反应,将电子带出细胞外[13]. 像腐殖质这类外源性电子穿梭体能与外膜上的c-Cyts结合反应,在微生物和胞外受体间不断的扩散往返,达到电子传递的目的[16].
目前,研究较多的两类胞外呼吸微生物是Geobacter属和Shewanella菌属,两种菌属在胞外电子传递方式上存在差异. 例如,Geobacter菌属的“纳米导线”结构与金属导电体相似,具有较强的导电性能. 但Geobacter菌属无法自身分泌穿梭介体,在无外源穿梭介质的情况下,主要依靠微生物具有的“纳米导线”来传导电子. 而Shewanella菌属的“纳米导线”结构并非纤毛类物质,导电性能远不如Geobacter菌属,但由于表面具有丰富的c-Cyts也使得电子沿着导线传递过程顺利进行. 而且,Shewanella属具备分泌醌类介质的能力,常通过电子穿梭机制来完成胞外电子传递过程[15]. 电子穿梭机制能帮助突破微生物和电子受体间电子传递距离的限制,在氧化还原反应中起到电子载体的作用,实现电子迁移和转运,同时还克服了微生物自身合成附属物“纳米导线”所需能量的投入[9]. 电子穿梭体在微生物的胞外呼吸过程中发挥着重要作用,调控电子转移过程能够影响环境污染物(如重金属和有机物)的迁移和转化行为[17]. 例如,电子穿梭体通过介导微生物和非金属元素砷之间的电子传递,加速了厌氧环境下砷(V)转化砷(Ⅲ)的还原过程,增加了砷在环境介质中的迁移能力[18]. 电子穿梭体也可直接作用于五氯苯酚(Pentachlorophenol,PCP)的降解过程,促进其脱卤反应,完成对有机物的污染降解[17,19].
环境炭质按来源的不同可以将其分为天然有机质和人工有机炭,其中天然有机质(腐殖酸、富里酸、胡敏素)是土壤中的关键组分,在调控土壤的结构发育过程、保障土壤功能、土壤水分蓄持和减碳减排等方面起到了重要作用[19]. 而人工有机炭(生物炭、石墨、碳纳米管)具有的多孔结构、较大比表面积以及丰富的功能基团,导致其具有较高的反应活性. 这些炭质材料在结构和性质等方面呈现出多样性,发挥着不同的环境效应. 例如,利用稻杆制备的生物炭对过硫酸盐进行活化后可以有效降解苯胺[20],碳纳米管在降解染料、废水处理方面也有重要应用[21 − 22]. 研究发现,分散在土壤、沉积物、水体的腐殖酸、富里酸、生物炭、石墨等均可作为典型的电子穿梭体,参与到调控微生物胞外电子转运的过程中[23]. 本文对天然有机质和人工有机炭的代表性电子穿梭体进行类比,系统概述两类电子介质在自然环境中介导微生物胞外电子转移而引起的氧化还原反应、以及典型环境污染物(重金属、有机物)的行为和归驱,从而为研究介导电子转移参与的生物地球化学过程提供新思路.
-
腐殖质是一类重要的天然有机质,它是由动、植物和微生物残体经过长时间腐殖化作用转化的大分子聚合物,其结构复杂多样,且广泛分布在土壤、沉积物、水体环境中. 据研究,土壤腐殖质占土壤有机质的比例高达85%—90%[24]. 腐殖质属于典型的电子穿梭体,能以电子受体的形式直接参与微生物的厌氧呼吸(即“腐殖质呼吸”),也可以通过运移电子还原污染物的途径参与微生物和污染物之间的反应 (见表1). 根据其在酸碱条件下的溶解度,腐殖质可分为腐殖酸、富里酸以及胡敏素等3大组分[25]. Lovley曾发现,Geobacter metallireducens菌能以乙酸作为碳源,通过厌氧呼吸来还原腐殖酸[26]. 李东阳等[27]发现,在微生物还原六氯苯的反应体系内加入腐殖酸后,六氯苯的去除效率有所提升,有利于脱氯反应进行. 刘思佳等[28]研究了富里酸的电子转移能力,其羟基为供电子基团,羧基为接受电子基团. 当加入富里酸后,Shewanella oneidensis MR-1还原PCP的降解被显著提高(>80%),这有力地说明富里酸在微生物胞外降解PCP过程中起到的介导作用. 这两类溶解性腐殖质,能够在液相环境中扩散迁移,可自由往返于菌体和环境污染物之间,有序地实现氧化还原过程[9,17]. 对于不溶性的胡敏素而言,它亦可作为电子介质在微生物和环境污染物之间进行电子的传递运移,加速污染物质的还原转化. 根据Zhang 等[19]研究,发现从水稻土和底泥中提取的胡敏素,能够参与到微生物胞外呼吸,也能够耦合微生物实现PCP的还原脱氯. 可见,腐殖质在介导微生物胞外电子传递、以及污染物转化过程中发挥不可忽视的作用.
腐殖质的氧化还原特性受醌类、酚类等表面官能团的影响,其中醌类基团是腐殖质具有电化学活性的重要功能基团. Scott 等[41]研究了金属还原地杆菌Geobacter metallireducens与腐殖质作用过程. 研究发现,醌类基团接收微生物胞外电子后,由氧化态转变成还原态,生成对苯二酚. 他们运用电子自旋共振(ESR)的方法检测到此过程伴随含有未配对的自由基产生,且发现半醌自由基含量增加,半醌自由基和对苯二酚均是醌基的还原产物,证明醌类基团参与了电子转移过程. Nurmi和Tratnyek结合6种模型醌类化合物 (如5-羟基-1,4 -萘醌、泛醌),使用循环伏安法表征了腐殖酸和富里酸的氧化还原特性,发现醌类化合物的氧化还原电位与腐殖质相似,表明了腐殖酸和富里酸同样具有参与电子运输和传递的氧化还原活性,且醌类基团是腐殖质发挥介导作用的关键位点[26,42]. 而且,腐殖质的醌类基团并不是唯一能介导电子转移的官能团. Yang 等[43]认为,腐殖质的电子穿梭容量(EEC)与其含有的醌类结构含量存在直接关系,醌类组分的增加会引起腐殖质EEC水平增加,同时还肯定了非醌类组分对腐殖质电子穿梭能力的贡献. Ratasuak 曾将腐殖质的氧化还原基团分为3个组分,包括2种不同类型的醌类基团和1种非醌类基团. 不同类型的醌类基团的主要区别在于取代基: 一类(Q1)是在醌类基团附近存有吸电子基团,而另一类(Q2)在醌类基团附近没有取代基,或者存有供电子基团. 而对于非醌类基团,它的电子转移能力亦是不可忽视的,估计约占总电子转移能力的21%—56%[44]. 此外,结构位点的差异也会影响醌类基团的氧化还原活性. 例如,在偶氮染料的微生物还原过程中,携有不同取代基的醌类物质导致偶氮染料还原的相对速率有所差异[45].
-
随着大量炭质材料的工程应用,诸如生物炭、石墨烯、碳纳米管、活性炭等富碳物质不断地在环境中被释放和积累. 在生物地球化学循环过程中,它们也作为一类重要的电子穿梭体,参与了微生物的呼吸代谢过程. 生物炭是利用生物质废弃物厌氧裂解而成的富碳产物,主要由碳、氢、氧等元素组成[46 − 48]. 以微观分子层面来看,生物炭结构是碳骨架与多重元素紧密结合、由芳香稠环片层堆叠构成[49]. 它含有丰富的酚羟基、醌基、以及羧基等官能团和刚性稠环芳香结构[50]. 生物炭的理化性质与其热解温度有密切的关系[51 − 52]. 在低温条件下(300—400 ℃),O―烷基碳(O―alkyl C)和羧基碳(carboxyl C)两种结构逐渐消失. 而在较高的温度(如500 ℃)下,生物炭的功能基团和烷基碳结构会被破坏,产生更多的芳香碳结构,从而电导率急剧提升[52 − 54]. 研究发现,生物炭不仅可以通过表面官能团的氧化还原来实现电子充-放电过程,即接收和释放电子[1,55 − 56],而且能够通过稠环芳香结构储存、介导胞外电子的长距离运移[52]. Klüpfel 提出生物炭的供电子基团很可能是酚类,而受电子基团可能是醌类和缩聚的稠环芳香结构[52]. 醌类结构可以加速醌呼吸菌和硝酸盐之间的电子传递,促进微生物反硝化过程 (见表1). Sathishkumar 等[34]观察6 d微生物在400、500、600 ℃生物炭的反硝化反应体系中,初始浓度为100 mg·L−1硝酸盐分别降至11.3、26.3、56.3 mg·L−1,而对照组硝酸盐的降低率仅为20%左右. 研究表明,生物炭显著提高了微生物菌对硝酸盐还原速率. Yan 等[57]发现,生物炭表面含氧基团—COOH和—OH作为电子介质有效加速了三氯乙烯的降解反应,而且含氧基团与过硫酸盐作用,成功激活过硫酸盐产生强氧化性的硫酸根自由基,从而实现对有机污染物的降解. Xu 等[58]指出红橡木裂解的生物炭能够刺激硫化物对六氢-1,3,5-三硝基-1,3,5-三嗪(hexahydro-1,3,5-trinitro-1,3,5-triazine,RDX)进行转化,电子通过生物炭含有的导电石墨结构,从电子供体转移至有机污染物,他们发现RDX的转化速率和生物炭的电导率呈正相关,证实了生物炭的石墨化区域是参与电子转移的重要结构之一. Lu 等[59]发现,Geobacter sulfurreducens降解硝基苯时,污泥制备的生物炭的醌类结构和石墨化区域可以促进该反应的胞外电子传递,增强硝基苯的微生物还原. Yu 等[36]观察了不同温度生物炭介导Geobacter sulfate reducens菌对PCP的降解行为. 研究发现,与400 ℃原始生物炭相比,添加富醌处理的生物炭,介导PCP降解率由(22.1%±0.9%)增加到(34.6%±1.5%). 这表明醌类结构能显著促进PCP的生物降解过程. 而且,他们定量出900 ℃生物炭的表面氧化还原活性组分和稠环石墨化结构对于介导PCP生物降解的贡献分别占56%和41%. 这说明生物炭的表面含氧活性组分和导电石墨区域均为介导微生物胞外电子转移的重要结构.
石墨烯是由碳原子sp2杂环的连接方式构成的单原子层,苯环结构是其稳定的基本结构单元[60]. 它具有的高热导性[61]、高机械强度[62]、以及强导电性而在工程应用上备受青睐. 石墨烯分散到环境后,它具有的共轭π体系结构将构成电子通道促进电子从电子供体转移至电子受体,从而对氧化还原过程中电子转移行为产生影响[63]. 例如,还原氧化石墨烯(rGO)相较于氧化石墨烯,含氧官能团数量减少,但因电导率提升,rGO对介导污染物的生物还原却有更高的促进效率[64 − 65]. Zhang 等[66]指出rGO为电子供体,提供电子分解高锰酸钾,生成高氧化性的中间锰物种(RMNs),增强对有机污染物的降解. 此外,rGO也可利用空位和边缘缺陷作为反应的活性中心,增大反应发生的能隙,提升电子迁移速率,促进双氯芬酸钠的转化[67]. Jiang 等[68]对石墨烯参与生物反硝化过程展开研究,发现石墨烯能刺激碳源乙酸盐的代谢从而产生更多的电子,结果表明在石墨烯存在下,电子从电子供体(乙酸盐)流向电子受体(NOx还原酶)的速率更快. 而且,起到电子消耗作用的NOx还原酶的活性也得到提升. 可见,石墨烯促进了电子的产生、运移和消耗. 此外,石墨烯还可以加速反硝化细菌获得铁,提高微生物胞内铁浓度,通过对电子传递的介导作用促进了硝酸盐的还原. Igarashi 等[69]报道在乙醇氧化菌Geobacter metallireducens和产甲烷菌Methanosarcina barkeri的共培养体系中加入氧化石墨烯,可有效促进其种间电子传递和甲烷气体的产生,而且材料表面的亲水性和促进种间直接电子转移效率呈正相关. 碳纳米管是一种由呈六边形排列的碳原子构成单层到数十层的同轴圆管,可分为单壁、多壁碳纳米管[70]. 不同于生物炭、氧化石墨烯等炭质材料,碳纳米管石墨化程度高,形成空间拓扑结构,导电性能优良. 碳纳米管中碳原子具有sp2和sp3杂化态,结构中含有的p轨道彼此重叠形成高度离域化的大π键,使得π-电子结构沿石墨烯层自由移动,形成微生物和胞外受体之间的电子通道,加速胞外电子传递过程[71]. Lu 等[72]利用碳纳米管介导Geobacter sulfurreducens PCA微生物还原硝基苯. 在Geobacter sulfurreducens PCA与碳纳米管复合反应体系内,9 h硝基苯被完全去除,而在无碳纳米管的对照组中,硝基苯在90 h 内仅降解了(16.5%±3.2%). 碳纳米管与Geobacter sulfurreducens PCA紧密结合在一起,通过碳纳米管的石墨化结构,电子沿着石墨区域片层运移转移至末端受体. 同时,碳纳米管也能吸附硝基苯从而缩短了电子传递到受体污染物的距离,从而微生物的胞外电子传递速率得到提升. Yan 等[73]利用碳纳米管改变了海藻酸盐固定化菌Shewanella oneidensis MR-1电子传递路线,介导菌胞外膜表面存在的c型细胞色素OmcA和 MtrC,将电子传递到胞外末端受体硝基苯还原为苯胺的过程. 碳纳米管介导电子传递行为将原本依靠NnfB 硝基还原酶的硝基苯的胞内还原反应阻隔. 此过程实现了微生物菌长距离的电子运移,提高电子利用率,使硝基苯还原效率提高了74%,进一步促进了硝基苯的降解[74].
-
分散在自然环境的环境炭质(如腐殖酸和生物炭)以介导微生物电子向胞外受体传递的方式连接生物和非生物过程. 在生物-地化循环过程,重金属的赋存状态受到环境炭质的调节. 天然有机质和人工有机炭的电子穿梭能力协助重金属转化,影响它的环境行为. 而环境有机污染物诸如染料、硝基芳香族污染物、卤代有机污染物等,会与环境炭质发生吸附、络合、光催化氧化作用,例如腐殖质在光作用下可产生大量活性氧(ROS). 这些ROS自由基会加速有机污染物的降解[75]. 生物炭含碳丰富、芳香度高、离子交换能力强,且有着较高的孔隙率和比表面积,对有机物污染的吸附有着重要作用 [76]. 此外,有机污染物还会与环境炭质发生氧化还原反应,环境炭质介导微生物胞外呼吸的过程,能够促进微生物参与代谢有机物的活动. 例如,Wang 等[77]研究了Desulfuromonas和Geobacter菌以挥发性脂肪酸作为电子供体,构建了微生物燃料电池. 他们发现生物炭悬浮液能通过胞外电子传递机制促进微生物燃料电池的发电,而且挥发性脂肪酸发生显著氧化. 微生物可以通过醌呼吸的方式促进有机污染物的降解转化. 炭质结构的醌类基团转化成还原态物质(氢醌),利用氢醌将介导的胞外电子传递给有机污染物. 可见,电子穿梭体介导污染物的转化在环境污染调控方面发挥着不可忽视的作用 (如图1所示).
-
铬(Cr)元素在大气、土壤、水体中常常可以被检测到,它在环境中占主导地位的形态是Cr(Ⅵ)和Cr(Ⅲ). 通常,Cr(Ⅵ)毒性远高于Cr(Ⅲ),具有生物累积性,人体一旦摄入,它会对呼吸道、消化道产生刺激、诱变甚至致癌作用. 而Cr(Ⅲ)在低浓度下可以作为一种微量营养元素被生物利用. 工业生产如电镀、制革、印染是Cr污染的主要来源,工艺过程产生的大量Cr废水会对环境造成巨大危害[78]. Cr (Ⅵ)还原成 Cr (Ⅲ)是降低环境中Cr (Ⅵ)污染的有效方法. 研究发现,Cr(Ⅵ)与腐殖质不仅可以吸附、络合,而且在腐殖质参与下可以促进微生物对重金属的转化. 在Shewanella putrefaciens CN32生物还原Cr(Ⅵ)的体系中加入腐殖质,Cr (Ⅵ)的还原速率得到明显提升. 经过25 h 反应后,约50%的Cr(Ⅵ)被还原成Cr(Ⅲ) [79]. Zhou 等[80]同样研究了Cr(Ⅵ)的微生物还原,分别加入15 mg·L−1 C和50 mg·L−1 C腐殖酸,Shewanella oneidensis MR-1去除Cr(Ⅵ)量由4.07%分别提升到35.74%和46.11%. Yu 等[81]发现,添加生物炭后,Shewanella oneidensis MR-1对Cr(Ⅵ)的还原从1.8倍(无炭组)大幅增高到33.7倍(有炭组).
-
过渡金属元素铁(Fe)在地表环境中是最为丰富的,主要存在形态是还原态的Fe(Ⅱ)和氧化态的Fe(Ⅲ). 铁(Fe)在自然环境下多以不溶性的氧化物和矿物形式存在,例如水铁矿、赤铁矿、针铁矿以及磁铁矿等. 这类物质很难进入微生物细胞内反应,环境炭质介导电子传递过程促进微生物还原铁氧矿物渐渐受到关注 [23]. Wu 等[33]证实了腐殖质能极大地加速嗜碱菌Corynebacterium humireducens MFC-5还原针铁矿. 他们几乎没有观察到Corynebacterium humireducens MFC-5菌还原Fe(Ⅲ)氧化物,生成Fe(Ⅱ)的现象,但是加入腐殖酸和富里酸后,Fe(Ⅱ)浓度由 0.03 mmol·L−1分别增加到0.51 mmol·L−1和0.71 mmol·L−1. Wu 等[38]报道了通过活性炭表面的氧化还原活性基团加速Shewanella oneidensis MR-1还原水铁矿的反应,水铁矿的还原速率提高了1.7—8.2倍. Kappler 等[35]研究了生物炭介导Shewanella oneidensis MR-1还原水铁矿过程. 在5 g·L−1和10 g·L−1生物炭的介导作用下,Fe(Ⅲ)还原速率由(0.54±015) mmol·h−1 增加到(0.87±0.19) mmol·h−1和(1.49±0.23) mmol·h−1.
-
砷(As)作为一种类金属元素,其毒性与重金属相似,会导致细胞出现癌变和功能上的诱变反应. 采矿、冶金、农业生产以及化石燃料的燃烧等人类活动均是造成水环境和土壤中As污染的重要原因. As(V)和As(Ⅲ)是As元素最常见的存在形态,As的流动性、毒性、以及生物可利用度受其化学形态支配. As元素与环境炭质发生氧化还原反应会改变其赋存状态[82,83]. 例如Jiang 等[84]利用天然有机质中醌类模型化合物蒽醌-2,6-二磺酸(AQDS)量化As的转化过程. AQDS在Geobacter sulfurreducens微生物还原过程中所产生的半醌自由基可以作为强氧化剂,将As(Ⅲ)氧化成As(V),从而实现As(Ⅲ)毒性和迁移率的降低. Chen等[37]也观察到生物炭可以促进As(V)的还原转化,添加生物炭49 d后,沉积物中As(Ⅲ)的释放量达到(656.35±89.25) μg·L−1,这显著高于未加生物炭时的As(Ⅲ)的释放量(98.06±19.38) μg·L−1. 其中,87%—90%的As(V)的转化归因于生物炭介导微生物还原作用,仅10%—13%的As(V)的转化是由于非生物过程引起的还原贡献. 这表明生物炭介导的胞外电子传递对As(V)形态转化占据主导地位.
-
染料母体主要是芳烃和杂环类化合物,携带显色基团和极性基团. 染料结构复杂,性能稳定,其废水颜色深,含大量的有机物质和盐分,这些特点使得染料废水的处理变得困难[85]. 例如,偶氮染料是生产中最常见的着色剂,分子中含有特殊的偶氮键(—N=N—),它广泛应用于纺织、人造皮革和纤维等生产中. 环境炭质可以参与到微生物氧化还原染料的反应中,例如,Zee 等[86]发现,在偶氮染料废水中加入AQDS后,可以使染料活性红 2 (RR2) 氮键的断裂过程变得更加迅速,提高染料去除效率. Colunga 等[87]研究rGO介导微生物还原厌氧污泥中RR2染料,结果表明rGO的介导促进染料去除的效果. Tan 等[88]针对生物炭对Shewanella oneidensis MR-1菌还原橙G为苯胺的过程的影响展开研究. Shewanella oneidensis MR-1对橙G有微弱的降解作用,当加入生物炭后,橙G的去除速率显著加快. 生物炭的氧化还原活性官能团和石墨导电结构对微生物还原染料的过程有着重要作用.
-
硝基芳香族有机物具有高毒性且结构复杂,被广泛用于农业化学品、纺织染料、炸药、医药等生产中. 随着它们分散进入环境,硝基芳香族有机物易被土壤吸附固定. 土壤微生物对硝基芳香烃可以实现有效的降解,而且通过环境炭质介导能提升硝基芳香烃的降解效能. Osmar 等[89]提出Geobacter anodireducens菌通过呼吸代谢腐殖质或AQDS来间接还原硝基芳烃类化合物. Bhushan 等[90]发现,腐殖酸参与微生物Clostridium sp. EDB2还原RDX的过程,在腐殖酸存在的情况下,体系内的RDX在5 d内被完全去除,他们认为腐殖酸加速了RDX的微生物降解过程. Pan 等[39]合成了一种Shewanella@石墨烯核壳材料,他们采用rGO包裹Shewanella putrefaciens CN-32菌,促进介导微生物胞外电子. 而且rGO有助于增强硝基苯的缺电子苯基与 rGO 的富电子基质形成 π—π EDA作用,有利于吸附更多的硝基苯分子,进一步缩短了电子穿梭体与电子受体之间的距离. 该种材料降解硝基苯的效果比单独依靠Shewanella putrefaciens CN-32菌生物还原硝基苯高出30%,表现出优良的介导循环性能.
-
卤代污染物是指分子结构中氢被卤素所取代的一类有机化合物,具有高毒性、高持久性、半挥发性,在环境中有较强的蓄积潜力,广泛存在于全球大气圈层、水体环境及土壤沉积物中[91]. 环境炭质可以显著提升微生物去除卤代有机污染物的过程. Zhang 等[29]评估了4种不同来源的胡敏素对2,2',4,4',5,5'-六氯联苯的介导降解效能. 研究发现,加入4种胡敏素后,多氯联苯的脱氯产物产量由0.02 mg·L−1分别增加为0.15、0.23、0.25、0.31 mg·L−1,这表明胡敏素能够介导电子转移提高多氯联苯的脱氯效率. Li 等[40]报道在Shewanella putrefaciens CN32还原纳米针铁矿(α-FeOOH)过程中,碳纳米管能有效促进四溴化酚A (TBBPA)的降解. 在厌氧环境下,加入碳纳米管,Fe(Ⅱ)浓度急剧增加,TBBPA的去除率由20.5%提升到87.1%. 碳纳米管极大地提高 Shewanella putrefaciens CN32与α-FeOOH 之间的电子转移效率,导致产生更多的 Fe(Ⅱ). 而且,形成的Fe(Ⅱ)亦可作为TBBPA的还原剂,从而间接促进有机污染物的降解.
-
天然或人工的环境炭质(如腐殖质、生物炭)在土壤、沉积物或水体系统里借助炭质的氧化还原活性基团或石墨化结构参与微生物胞外电子的接收,传递和释放过程. 本文归纳了典型的电子穿梭体介导胞外电子的行为、以及电子传递机制对环境污染物(重金属和有机物)归驱的影响. 介导电子传递效应为拓展环境生物修复治理提供新的途经,具有重要的技术应用前景. 但是,关于介导胞外电子传递的研究仍面临许多挑战: (1)在复杂环境体系下(如稻田、湿地、河流等),各类电子穿梭介质的作用范围和限制因素仍有待探索. (2)介导胞外电子传递效应的贡献尚需准确评估,它将为研发高性能介电材料在环境修复方面的应用提供基础支撑. (3)环境炭质通过介导微生物胞外电子功能对新型污染物或多重复合污染物行为的影响亟待解决.
环境炭质介导胞外电子传递转化污染物的机制
The mechanism of mediating extracellular electron transfer by environmental carbonaceous matter to contaminant transformation
-
摘要: 环境炭质可作为电子穿梭体,介导微生物胞外电子传递行为,调控着生物地球化学循环过程. 直接和间接电子传递是微生物胞外电子传递过程的两种形式. 在间接电子传递中,微生物利用电子穿梭体实现电子从胞内到胞外的迁移,除了微生物自身分泌的穿梭体之外,外源性电子穿梭体也被发现可以促进胞内外的电子转移过程,其中天然和人工的电子穿梭体(如腐殖质、生物炭等)在微生物胞外电子传递过程中均发挥着重要的介导作用,改变了胞外电子转移途径. 环境炭质的微尺度结构决定了其介导电子转移的功能. 于此,本文以两种外源性电子穿梭体的介导表现为研究主体,系统归纳了以腐殖质、生物炭为代表的环境炭质具有的氧化还原活性基团(如醌/酚类基团)和石墨化芳香炭结构(稠环芳香区域)、以及富碳介质的电子穿梭性能和机制;详述了环境炭质介导胞外电子转化典型的污染物(重金属和有机物)的效果;最后,总结了环境炭质(天然有机质和人工有机炭)在土壤、沉积物或水体系统参与介导胞外电子转移过程,并展望了当前研究所面临的挑战,这为新型电子穿梭介质的设计研发和工程应用提供技术支撑.Abstract: Environmental carbonaceous matter as an electron shuttle, can mediate electron transferthat regulates biogeochemical cycling processes. Microbial extracellular electron processes are of two types; direct and indirect electron transfers. In indirect electron transfer, microorganisms use electron shuttles to migrate electrons from intracellular to extracellular. In addition to microorganisms' own secreted shuttles, exogenous electron shuttles have also been found facilitating the intra- and extracellular electron transfer processes. Both natural and artificial electron shuttles (e.g., humic substance, biochar, etc.) play an important mediating role in extracellular electron transfer process by changing the pathway of electron transfer. The microscale structure of environmental carbonaceous matter determines its function in mediating electron transfer. Herein, the main subject of this review is to highlight the mediating behavior of two exogenous electron shuttles along with systematical summarization of the redox-active groups (e.g., quinone/phenol groups) and graphitized aromatic carbon (e.g., polycyclic aromatic regions), as well as the electron transfer properties and mechanisms of environmental carbonaceous matter. The effect of mediating extracellular electron transfer on the transformation of representative contaminants (e.g., heavy metals and organic pollutants) by environmental carbonaceous matter was described in detail. Conclusively, we included the mediated extracellular electron transfer by natural and engineered carbons in soil, sediment, or water systems and came up with the emerging future research challenges. The aim of this review is at providing a technical support for the design development and engineered application of novel electron shuttle materials.
-

-
表 1 天然有机质和人工有机炭介导胞外电子结构和污染物转化效果
Table 1. Natural and artificial carbon structure of mediating extracellular electron to pollutant transformation
电子穿梭介质
Electronic shuttle medium介导结构
Mediated structures微生物菌
Microorganisms环境污染物
Environmental pollutants介导降解效果
Mediated degradation effect文献
Literature胡敏素
(沉积物中提取)羧基、酚基官能团 未知菌(HQ688519)
Clostridium sp.
(AB275141)
Bacillus sp.(JX434141)
(甲酸盐20 mmol·L−1)2,2′,4,4′,5,5′-六氯联苯
(PCB153:
100 mg·L−1)胡敏素可以明显提高介导还原PCB153的脱氯,生成
2,2',5,5'-四氯联苯(PCB52)和
2,2',4,5,5'-五氯联(PCB101)[29] 胡敏素
(土壤和沉积物中
提取)醌类基团 Shewanella putrefaciens strain CN-32
(甲酸盐10 mmol·L−1)五氯苯酚
(PCP: 20 μmol·L−1)胡敏素促进微生物对五氯苯酚的还原脱氯,脱氯速率在0.99—7.63 [30] 胡敏素
(土壤和沉积物中
提取)醌类基团 Shewanella putrefaciens strain CN-32
(甲酸盐4 mmol·L−1)五氯苯酚
(PCP: 20 μmol·L−1)胡敏素能介导维持五氯苯酚的脱氯活性 [19] 富里酸
(填埋垃圾中提取)醌基、羧基、酚基官能团 Shewanella oneidensis MR-1
(乳酸钠5 mmol·L−1)五氯苯酚
(PCP: 6 mg·L−1)加入富里酸,Shewanella oneidensis MR-1 还原PCP降解率由 40% 显著提高到 80% 以上 [28] 土壤腐殖酸
(土壤中提取)含氧官能团(醌基) Geobacter sulfurreducens
(乙酸盐2 mmol·L−1)四氯化碳
(CT: 5 μmol )土壤腐殖酸提高了 CT 的一级转化率6.1倍 [31] 胡敏酸、富里酸
(土壤中提取)醌类基团 Shewanella putrefaciens CN32
(乳酸钠 10 mmol·L−1)铀 U(Ⅵ)
(初始浓度:
0.4 mmol·L−1)腐殖酸和富里酸的存在提高了U(Ⅵ)的还原率(高达10倍)
U(Ⅵ)转化为U(IV)[32] 腐殖质(腐殖酸、
富里酸)醌类基团 Geobacter metallireducens
(乙酸盐 10 mmol·L−1)铁氧化物 Fe(Ⅲ)
(初始浓度:
6 mmol·L−1)加入腐殖酸后,Fe(Ⅲ)还原量由0.25 mmol·L−1增加到
2.75 mmol·L−1以上[26] 腐殖质(腐殖酸、
富里酸)醌类基团 嗜碱菌Corynebacterium humireducens MFC-5
(蔗糖 5 mmol·L−1)针铁矿
(α-FeOOH:
5 mmol·L−1)
2,4-二氯苯氧乙酸
(2,4-D:
180 μmol ·L−1)A.富里酸加速针铁矿的生物还原 Fe(Ⅲ)转化为Fe(Ⅱ),Fe(Ⅱ)量由0.03 mmol·L−1 增加到
0.71 mmol·L−1,2,4-二氯苯氧乙酸降解率提高到38.7%;
B.腐殖酸加速针铁矿的生物还原 Fe(Ⅲ)转化为Fe(Ⅱ),Fe(Ⅱ)量由0.03 mmol·L−1 增加到
0.51 mmol·L−1; 2,4-二氯苯氧乙酸降解率提高到26.4%[33] 生物炭
(白杨木)醌类基团 反硝化微生物 硝酸盐
(初始浓度:
100 mg·L−1)BC400,BC500,和BC600生物炭介导硝酸盐还原,浓度分别降至
11.3 mg·L−1,26.3 mg·L−1和
56.3 mg·L−1[34] 生物炭
(木屑)醌类基团和芳香炭结构 Shewanella oneidensis MR-1
(乳酸钠 30 mmol·L−1)水铁矿
(初始浓度:
15 mmol·L−1)5g·L−1和10 g·L−1生物炭均加速了水铁矿还原,还原速率由(0.54±
015) mmol·h−1增加到(0.87±
0.19 )mmol·h−1 和(1.49±
0.23) mmol·h−1[35] 生物炭
(秸秆)醌基、酚基官能团
石墨结构Geobacter sulfurreducens
(乙酸盐 15 mmol·L−1)五氯苯酚
(PCP: 20 mg·L−1)生物炭显著加速了五氯苯酚的降解; 900°C生物炭表面氧化还原活性部分和电导率分别占PCP生物降解率的56%和41% [36] 生物炭
(秸秆、猪粪)醌类基团 金属还原菌
(Geobacter,Anaeromyxobacter,Desulfosporosinus,Pedobacter)
(乙酸盐 50 mmol·L−1)Fe(Ⅲ)
(初始浓度:
36.13 g·kg−1)
As(V)
(初始浓度:
244.25 mg·kg−1)
生物炭促进As(V)的生物还原转化As(Ⅲ),As(Ⅲ)浓度由(98.06±19.38) μg·L−1增加到(656.35±89.25) μg·L−1[37] 活性炭(AC) 含氧官能团 Shewanella oneidensis
MR-1
(乳酸钠30 mmol·L−1)水铁矿
(初始浓度:
30 mmol·L−1)活性炭加速了微生物还原铁氧矿物,其还原速率提高了1.7 — 8.2倍 [38] 还原氧化石墨烯(rGO)
(Shewanella@石墨烯核壳材料)石墨结构 Shewanella putrefaciens CN-32
(乳酸钠20 mmol·L−1)硝基苯
(NB: 0.8 mmol·L−1)提高了介导硝基苯的还原速率 [39] 碳纳米管(CNT) 半醌自由基 Shewanella putrefaciens CN32
(乳酸钠20 mmol·L−1)纳米针铁矿
(α-FeOOH: 2 g·L−1)
四溴双酚A
(TBBPA:
20 mg· L−1)CNT加入促使Fe(Ⅲ)转化为Fe(Ⅱ),增加了Fe(Ⅱ)生成,提高了四溴双酚A的去除(由20.5%提高至87.1%) [40] -
[1] SUN T R, LEVIN B D A, GUZMAN J J L, et al. Rapid electron transfer by the carbon matrix in natural pyrogenic carbon[J]. Nature Communications, 2017, 8(1): 14873. doi: 10.1038/ncomms14873 [2] 吴云当, 李芳柏, 刘同旭. 土壤微生物—腐殖质—矿物间的胞外电子传递机制研究进展[J]. 土壤学报, 2016, 53(2): 277-291. WU Y D, LI F B, LIU T X. Mechanism of extracellular electron transfer among microbe—humus—mineral in soil: A review[J]. Acta Pedologica Sinica, 2016, 53(2): 277-291(in Chinese).
[3] 田晓春. 微生物电化学技术研究希瓦氏菌的胞外电子传递机制[D]. 厦门: 厦门大学, 2017. TIAN X C. Research on mechanisms of extracellular electron transfer for Shewanella strains using microbial electrochemical technologies[D]. Xiamen: Xiamen University, 2017(in Chinese).
[4] 史涵, 王亚楠, 王清照, 等. 典型氢氧化细菌固碳特性种间差异及胞外有机物对固碳过程影响[J]. 环境科学学报, 2022, 42(11): 455-463. SHI H, WANG Y N, WANG Q Z, et al. Interspecific differences of carbon fixation characteristics of typical hydrogenoxidizing bacteria and effects of extracellular organic compounds on carbon fixation process[J]. Acta Scientiae Circumstantiae, 2022, 42(11): 455-463(in Chinese).
[5] ROTARU AE, SHRESTHA P M, LIU F H, et al. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane[J]. Energy & Environmental Science, 2014, 7(1): 408-415. [6] 王加龙, 刘驰, 雷丽, 等. 非共生固氮菌及其固氮作用[J]. 微生物学报, 2022, 62(8): 2861-2878. WANG J L, LIU C, LEI L, et al. Asymbiotic nitrogen-fixing bacteria and their nitrogen fixation potential[J]. Acta Microbiologica Sinica, 2022, 62(8): 2861-2878(in Chinese).
[7] MYERS C, NEALSON K. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor[J]. Science, 1988, 240(4857): 1319-1321. doi: 10.1126/science.240.4857.1319 [8] LOVLEY D R, PHILLIPS E J. Novel mode of microbial energy metabolism: Organic carbon oxidation coupled to dissimilatory reduction of iron or manganese[J]. Applied and Environmental Microbiology, 1988, 54(6): 1472-1480. doi: 10.1128/aem.54.6.1472-1480.1988 [9] 林霄涵, 杨帆, 赵峰. 微生物的胞外电子传递界面[J]. 环境化学, 2021, 40(11): 3283-3296. doi: 10.7524/j.issn.0254-6108.2021033106 LIN X H, YANG F, ZHAO F. The interface of microbial extracellular electron transfer[J]. Environmental Chemistry, 2021, 40(11): 3283-3296(in Chinese). doi: 10.7524/j.issn.0254-6108.2021033106
[10] COURSOLLE D, GRALNICK J A. Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1[J]. Molecular Microbiology, 2010, 77(4): 995-1008. doi: 10.1111/j.1365-2958.2010.07266.x [11] LEANG C, COPPI M V, LOVLEY D R. OmcB, a c-type polyheme cytochrome, involved in Fe(Ⅲ) reduction in Geobacter sulfurreducens[J]. Journal of Bacteriology, 2003, 185(7): 2096-2103. doi: 10.1128/JB.185.7.2096-2103.2003 [12] MEHTA T, COPPI M V, CHILDERS S E, et al. Outer membrane c-type cytochromes required for Fe(Ⅲ) and Mn(IV) oxide reduction in Geobacter sulfurreducens[J]. Applied and Environmental Microbiology, 2005, 71(12): 8634-8641. doi: 10.1128/AEM.71.12.8634-8641.2005 [13] WHITE G F, SHI Z, SHI L, et al. Rapid electron exchange between surface-exposed bacterial cytochromes and Fe(Ⅲ) minerals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(16): 6346-6351. [14] REGUERA G, MCCARTHY K D, MEHTA T, et al. Extracellular electron transfer via microbial nanowires[J]. Nature, 2005, 435(7045): 1098-1101. doi: 10.1038/nature03661 [15] GORBY Y A, YANINA S, MCLEAN J S, et al. Electrically conductive bacterial nanowires produced by Shewanella oneidensis strain MR-1 and other microorganisms[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(30): 11358-11363. [16] GRALNICK J A, NEWMAN D K. Extracellular respiration[J]. Molecular Microbiology, 2007, 65(1): 1-11. doi: 10.1111/j.1365-2958.2007.05778.x [17] 张玉龙, 陈雪丽, 吴云当. 电子穿梭体及其介导的环境与地球化学过程研究进展[J]. 生态环境学报, 2021, 30(1): 213-222. ZHANG Y L, CHEN X L, WU Y D. Electron shuttle-mediated microbial extracellular electron transfer: Mechanisms and geochemical implications[J]. Ecology and Environmental Sciences, 2021, 30(1): 213-222(in Chinese).
[18] QIAO J T, LI X M, LI F B, et al. Humic substances facilitate arsenic reduction and release in flooded paddy soil[J]. Environmental Science & Technology, 2019, 53(9): 5034-5042. [19] ZHANG C F, KATAYAMA A. Humin as an electron mediator for microbial reductive dehalogenation[J]. Environmental Science & Technology, 2012, 46(12): 6575-6583. [20] WU Y, GUO J, HAN Y J, et al. Insights into the mechanism of persulfate activated by rice straw biochar for the degradation of aniline[J]. Chemosphere, 2018, 200: 373-379. doi: 10.1016/j.chemosphere.2018.02.110 [21] 焦新亭, 李晓东, 刘国文, 等. 碳纳米管对亚甲基蓝的吸附性能研究[J]. 安全与环境学报, 2007, 7(3): 44-47. doi: 10.3969/j.issn.1009-6094.2007.03.011 JIAO X T, LI X D, LIU G W, et al. Study on adsorption of methylene blue by carbon nanotubes[J]. Journal of Safety and Environment, 2007, 7(3): 44-47 (in Chinese). doi: 10.3969/j.issn.1009-6094.2007.03.011
[22] 王昕, 张春丽, 任广军, 等. 碳纳米管吸附染料甲基橙的性能研究[J]. 当代化工, 2008, 37(4): 375-377,381. WANG X, ZHANG C L, REN G J, et al. Study on the adsorption of methyl orange from aqueous solution by carbon nanotubes[J]. Contemporary Chemical Industry, 2008, 37(4): 375-377,381(in Chinese).
[23] 柳广飞, 朱佳琪, 于华莉, 等. 电子穿梭体介导微生物还原铁氧化物的研究进展[J]. 地球科学, 2018, 43(增刊1): 157-170. LIU G F, ZHU J Q, YU H L, et al. Review on electron-shuttle-mediated microbial reduction of iron oxides minerals[J]. Earth Science, 2018, 43(Sup 1): 157-170(in Chinese).
[24] 崔恒钊, 王育来, 邹丽敏, 等. 河岸带土壤溶解有机质垂直分布及其影响因素研究[J]. 环境科学与技术, 2015, 38(6): 8-13. CUI H Z, WANG Y L, ZOU L M, et al. Vertical distribution and impact factors of soil dissolved organic matter in riparian zones[J]. Environmental Science & Technology, 2015, 38(6): 8-13(in Chinese).
[25] PETTIT R E. Organic matter, humus, humate, humic acid, fulvic acid and humin: their importance in soil fertility and plant health[J]. CTI Research, 2004, 10: 1-7. [26] LOVLEY D R, COATES J D, BLUNT-HARRIS E L, et al. Humic substances as electron acceptors for microbial respiration[J]. Nature, 1996, 382(6590): 445-448. doi: 10.1038/382445a0 [27] 李东阳, 杨天学, 吴明红, 等. 腐殖酸强化六氯苯厌氧降解规律及其中间产物[J]. 环境科学研究, 2016, 29(6): 870-876. LI D Y, YANG T X, WU M H, et al. Anaerobic degradation regulation of hexachlorobenzene and degradation products enhanced by humic acid[J]. Research of Environmental Sciences, 2016, 29(6): 870-876(in Chinese).
[28] 刘思佳, 何小松, 张慧, 等. 生活垃圾不同填埋阶段的富里酸对五氯苯酚的降解[J]. 环境科学, 2018, 39(12): 5699-5707. LIU S J, HE X S, ZHANG H, et al. Degradation of pentachlorophenol by fulvic acid in a municipal solid waste landfill[J]. Environmental Science, 2018, 39(12): 5699-5707(in Chinese).
[29] ZHANG D D, ZHANG N, YU X W, et al. Effect of humins from different sediments on microbial degradation of 2, 2’, 4, 4’, 5, 5’-hexachlorobiphenyl (PCB153), and their polyphasic characterization[J]. RSC Advances, 2017, 7(12): 6849-6855. doi: 10.1039/C6RA25934K [30] ZHANG C F, ZHANG D D, XIAO Z X, et al. Characterization of humins from different natural sources and the effect on microbial reductive dechlorination of pentachlorophenol[J]. Chemosphere, 2015, 131: 110-116. doi: 10.1016/j.chemosphere.2015.02.043 [31] CERVANTES F J, VU-THI-THU L, LETTINGA G, et al. Quinone-respiration improves dechlorination of carbon tetrachloride by anaerobic sludge[J]. Applied Microbiology and Biotechnology, 2004, 64(5): 702-711. doi: 10.1007/s00253-004-1564-z [32] GU B H, YAN H, ZHOU P, et al. Natural humics impact uranium bioreduction and oxidation[J]. Environmental Science & Technology, 2005, 39(14): 5268-5275. [33] WU C Y, ZHUANG L, ZHOU S G, et al. Humic substance-mediated reduction of iron(Ⅲ) oxides and degradation of 2, 4-D by an alkaliphilic bacterium, Corynebacterium humireducens MFC-5[J]. Microbial Biotechnology, 2013, 6(2): 141-149. doi: 10.1111/1751-7915.12003 [34] SATHISHKUMAR K, LI Y, SANGANYADO E. Electrochemical behavior of biochar and its effects on microbial nitrate reduction: Role of extracellular polymeric substances in extracellular electron transfer[J]. Chemical Engineering Journal, 2020, 395: 125077. doi: 10.1016/j.cej.2020.125077 [35] KAPPLER A, WUESTNER M L, RUECKER A, et al. Biochar as an electron shuttle between bacteria and Fe(Ⅲ) minerals[J]. Environmental Science & Technology Letters, 2014, 1(8): 339-344. [36] YU L P, YUAN Y, TANG J, et al. Biochar as an electron shuttle for reductive dechlorination of pentachlorophenol by Geobacter sulfurreducens[J]. Scientific Reports, 2015, 5: 16221. doi: 10.1038/srep16221 [37] CHEN Z, WANG Y P, XIA D, et al. Enhanced bioreduction of iron and arsenic in sediment by biochar amendment influencing microbial community composition and dissolved organic matter content and composition[J]. Journal of Hazardous Materials, 2016, 311: 20-29. doi: 10.1016/j.jhazmat.2016.02.069 [38] WU S, FANG G D, WANG Y J, et al. Redox-active oxygen-containing functional groups in activated carbon facilitate microbial reduction of ferrihydrite[J]. Environmental Science & Technology, 2017, 51(17): 9709-9717. [39] PAN T T, CHEN B L. Facile fabrication of Shewanella@graphene core-shell material and its enhanced performance in nitrobenzene reduction[J]. Science of the Total Environment, 2019, 658: 324-332. doi: 10.1016/j.scitotenv.2018.12.028 [40] LI H, CAO W, WANG W B, et al. Carbon nanotubes mediating nano α-FeOOH reduction by Shewanella putrefaciens CN32 to enhance tetrabromobisphenol A removal[J]. Science of the Total Environment, 2021, 777: 146183. doi: 10.1016/j.scitotenv.2021.146183 [41] SCOTT D T, MCKNIGHT D M, BLUNT-HARRIS E L, et al. Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms[J]. Environmental Science & Technology, 1998, 32(19): 2984-2989. [42] NURMI J T, TRATNYEK P G. Electrochemical properties of natural organic matter (NOM), fractions of NOM, and model biogeochemical electron shuttles[J]. Environmental Science & Technology, 2002, 36(4): 617-624. [43] YANG P J, JIANG T, CONG Z Y, et al. Loss and increase of the electron exchange capacity of natural organic matter during its reduction and reoxidation: The role of quinone and nonquinone moieties[J]. Environmental Science & Technology, 2022, 56(10): 6744-6753. [44] RATASUK N, NANNY M A. Characterization and quantification of reversible redox sites in humic substances[J]. Environmental Science & Technology, 2007, 41(22): 7844-7850. [45] RAU J, KNACKMUSS H J, STOLZ A. Effects of different quinoid redox mediators on the anaerobic reduction of azo dyes by bacteria[J]. Environmental Science & Technology, 2002, 36(7): 1497-1504. [46] GOLDBERG E D. Black carbon in the environment: properties and distribution[M]. New York: J. Wiley, 1985. [47] LEHMANN J, RONDON M. Bio-char soil management on highly weathered soils in the humid tropics[J]. Biological Approaches to Sustainable Soil Systems, 2006, 113(517): e530. [48] UPHOFF N T, Biological approaches to sustainable soil systems[M]. Boca Raton: CRC/Taylor & Francis, 2006. [49] LEHMANN J, JOSEPH S. Biochar for environmental management: science, technology and implementation[M]. Second Edition. [50] 吕鹏, 李莲芳, 黄晓雅. 改性生物炭修复砷镉复合污染土壤研究进展[J]. 环境科学,2023, 44(7): 4077-4090. LYU P, LI L F, HUANG X Y. Modified Biochar for Remediation of Soil Contaminated with Arsenic and Cadmium: A Review[J]. Environmental Science,2023, 44(7): 4077-4090(in Chinese).
[51] LIU W J, JIANG H, YU H Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material[J]. Chemical Reviews, 2015, 115(22): 12251-12285. doi: 10.1021/acs.chemrev.5b00195 [52] KLÜPFEL L, KEILUWEIT M, KLEBER M, et al. Redox properties of plant biomass-derived black carbon (biochar)[J]. Environmental Science & Technology, 2014, 48(10): 5601-5611. [53] TANG Y, ALAM M S, KONHAUSER K O, et al. Influence of pyrolysis temperature on production of digested sludge biochar and its application for ammonium removal from municipal wastewater[J]. Journal of Cleaner Production, 2019, 209: 927-936. doi: 10.1016/j.jclepro.2018.10.268 [54] CHEN B L, ZHOU D D, ZHU L Z. Transitional adsorption and partition of nonpolar and polar aromatic contaminants by biochars of pine needles with different pyrolytic temperatures[J]. Environmental Science & Technology, 2008, 42(14): 5137-5143. [55] SUN T R, LEVIN B D A, SCHMIDT M P, et al. Simultaneous quantification of electron transfer by carbon matrices and functional groups in pyrogenic carbon[J]. Environmental Science & Technology, 2018, 52(15): 8538-8547. [56] JIANG D Q, LI B K. Granular activated carbon single-chamber microbial fuel cells (GAC-SCMFCs): A design suitable for large-scale wastewater treatment processes[J]. Biochemical Engineering Journal, 2009, 47(1/2/3): 31-37. [57] YAN J C, HAN L, GAO W G, et al. Biochar supported nanoscale zerovalent iron composite used as persulfate activator for removing trichloroethylene[J]. Bioresource Technology, 2015, 175: 269-274. doi: 10.1016/j.biortech.2014.10.103 [58] XU W Q, PIGNATELLO J J, MITCH W A. Role of black carbon electrical conductivity in mediating hexahydro-1, 3, 5-trinitro-1, 3, 5-triazine (RDX) transformation on carbon surfaces by sulfides[J]. Environmental Science & Technology, 2013, 47(13): 7129-7136. [59] LU Y, XIE Q Q, TANG L, et al. The reduction of nitrobenzene by extracellular electron transfer facilitated by Fe-bearing biochar derived from sewage sludge[J]. Journal of Hazardous Materials, 2021, 403: 123682. doi: 10.1016/j.jhazmat.2020.123682 [60] NOVOSELOV K S, GEIM A K, MOROZOV S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669. doi: 10.1126/science.1102896 [61] BALANDIN A A, GHOSH S, BAO W Z, et al. Superior thermal conductivity of single-layer graphene[J]. Nano Letters, 2008, 8(3): 902-907. doi: 10.1021/nl0731872 [62] LEE C G, WEI X D, KYSAR J W, et al. Measurement of the elastic properties and intrinsic strength of monolayer graphene[J]. Science, 2008, 321(5887): 385-388. doi: 10.1126/science.1157996 [63] JIANG Y F, YANG L J, SUN T, et al. Significant contribution of intrinsic carbon defects to oxygen reduction activity[J]. ACS Catalysis, 2015, 5(11): 6707-6712. doi: 10.1021/acscatal.5b01835 [64] LIU G F, DONG B, ZHOU J T, et al. Enhanced bioreduction of nitrobenzene by reduced graphene oxide materials: Effects of surface modification and coexisting soluble electron shuttles[J]. Environmental Science and Pollution Research, 2017, 24(34): 26874-26880. doi: 10.1007/s11356-017-0673-z [65] TORAL-SÁNCHEZ E, RANGEL-MENDEZ J R, ASCACIO VALDÉS J A, et al. Tailoring partially reduced graphene oxide as redox mediator for enhanced biotransformation of iopromide under methanogenic and sulfate-reducing conditions[J]. Bioresource Technology, 2017, 223: 269-276. doi: 10.1016/j.biortech.2016.10.062 [66] ZHANG P, ZHOU P, PENG J L, et al. Insight into metal-free carbon catalysis in enhanced permanganate oxidation: Changeover from electron donor to electron mediator[J]. Water Research, 2022, 219: 118626. doi: 10.1016/j.watres.2022.118626 [67] TIAN S Q, WANG L, LIU Y L, et al. Enhanced permanganate oxidation of sulfamethoxazole and removal of dissolved organics with biochar: Formation of highly oxidative manganese intermediate species and in situ activation of biochar[J]. Environmental Science & Technology, 2019, 53(9): 5282-5291. [68] JIANG M, FENG L Y, ZHENG X, et al. Bio-denitrification performance enhanced by graphene-facilitated iron acquisition[J]. Water Research, 2020, 180: 115916. doi: 10.1016/j.watres.2020.115916 [69] IGARASHI K, MIYAKO E, KATO S. Direct interspecies electron transfer mediated by graphene oxide-based materials[J]. Frontiers in Microbiology, 2020, 10: 3068. doi: 10.3389/fmicb.2019.03068 [70] 邹龙. 基于纳米结构阳极的腐败希瓦氏菌胞外电子传递机理研究[D]. 重庆: 西南大学, 2016. ZOU L. Extracellular electron transfer mechanism of Shewanella putrefaciens on nanostructured anodes[D]. Chongqing: Southwest University, 2016(in Chinese).
[71] SHI L J, LI X, TUO Y X, et al. Microwave-assisted hydrogen releasing from liquid organic hydride over Pt/CNT catalyst: Effects of oxidation treatment of CNTs[J]. Catalysis Today, 2016, 276: 121-127. doi: 10.1016/j.cattod.2015.12.024 [72] LU Y, ZHANG S J, LIU Q, et al. Nitrobenzene reduction promoted by the integration of carbon nanotubes and Geobacter sulfurreducens[J]. Environmental Pollution, 2023, 325: 121444. doi: 10.1016/j.envpol.2023.121444 [73] YAN F F, HE Y R, WU C, et al. Carbon nanotubes alter the electron flow route and enhance nitrobenzene reduction by Shewanella oneidensis MR-1[J]. Environmental Science & Technology Letters, 2014, 1(1): 128-132. [74] 王贺飞. 生物炭强化希瓦氏菌还原转化硝基苯类化合物的机制[D]. 杭州: 浙江大学, 2020. WANG H F. Mechanisms for pyrogenic carbon-enhanced bioreduction of nitroaromatic compounds by Shewanella[D]. Hangzhou: Zhejiang University, 2020(in Chinese).
[75] SHI Y F, DAI Y C, LIU Z W, et al. Light-induced variation in environmentally persistent free radicals and the generation of reactive radical species in humic substances[J]. Frontiers of Environmental Science & Engineering, 2020, 14(6): 1-10. [76] YUAN Y, BOLAN N, PRÉVOTEAU A, et al. Applications of biochar in redox-mediated reactions[J]. Bioresource Technology, 2017, 246: 271-281. doi: 10.1016/j.biortech.2017.06.154 [77] WANG G J, XING Y, LIU G H, et al. Poorly conductive biochar boosting extracellular electron transfer for efficient volatile fatty acids oxidation via redox-mediated mechanism[J]. Science of the Total Environment, 2022, 809: 151113. doi: 10.1016/j.scitotenv.2021.151113 [78] 李爱琴, 唐宏建, 王阳峰. 环境中铬污染的生态效应及其防治[J]. 中国环境管理干部学院学报, 2006, 16(1): 74-77. doi: 10.13358/j.issn.1008-813x.2006.01.023 LI A Q, TANG H J, WANG Y F. Ecological effect and prevention of chromium pollution in the environment[J]. Journal of Environmental Management College of China, 2006, 16(1): 74-77(in Chinese). doi: 10.13358/j.issn.1008-813x.2006.01.023
[79] GU B H, CHEN J. Enhanced microbial reduction of Cr(VI) and U(VI) by different natural organic matter fractions[J]. Geochimica et Cosmochimica Acta, 2003, 67(19): 3575-3582. doi: 10.1016/S0016-7037(03)00162-5 [80] ZHOU Y, DUAN J T, JIANG J, et al. Effect of TOC concentration of humic substances as an electron shuttle on redox functional groups stimulating microbial Cr(VI) reduction[J]. International Journal of Environmental Research and Public Health, 2022, 19(5): 2600. doi: 10.3390/ijerph19052600 [81] YU C, ZHU X X, MOHAMED A, et al. Enhanced Cr(VI) bioreduction by biochar: Insight into the persistent free radicals mediated extracellular electron transfer[J]. Journal of Hazardous Materials, 2023, 442: 129927. doi: 10.1016/j.jhazmat.2022.129927 [82] SMEDLEY P L, KINNIBURGH D G. A review of the source, behaviour and distribution of arsenic in natural waters[J]. Applied Geochemistry, 2002, 17(5): 517-568. doi: 10.1016/S0883-2927(02)00018-5 [83] 于云江, 王菲菲, 房吉敦, 等. 环境砷污染对人体健康影响的研究进展[J]. 环境与健康杂志, 2007, 24(3): 181-183. doi: 10.3969/j.issn.1001-5914.2007.03.026 YU Y J, WANG F F, FANG J D, et al. Advance in research on environmental arsenic pollution to human health[J]. Journal of Environment and Health, 2007, 24(3): 181-183(in Chinese). doi: 10.3969/j.issn.1001-5914.2007.03.026
[84] JIANG J, BAUER I, PAUL A, et al. Arsenic redox changes by microbially and chemically formed semiquinone radicals and hydroquinones in a humic substance model quinone[J]. Environmental Science & Technology, 2009, 43(10): 3639-3645. [85] ZHANG W X, YANG Q, LUO Q H, et al. Laccase-Carbon nanotube nanocomposites for enhancing dyes removal[J]. Journal of Cleaner Production, 2020, 242: 118425. doi: 10.1016/j.jclepro.2019.118425 [86] VAN DER ZEE F P, BOUWMAN R H M, STRIK D P B T B, et al. Application of redox mediators to accelerate the transformation of reactive azo dyes in anaerobic bioreactors[J]. Biotechnology and Bioengineering, 2001, 75(6): 691-701. doi: 10.1002/bit.10073 [87] COLUNGA A, RANGEL-MENDEZ J R, CELIS L B, et al. Graphene oxide as electron shuttle for increased redox conversion of contaminants under methanogenic and sulfate-reducing conditions[J]. Bioresource Technology, 2015, 175: 309-314. doi: 10.1016/j.biortech.2014.10.101 [88] TAN W B, WANG L, YU H X, et al. Accelerated microbial reduction of azo dye by using biochar from iron-rich-biomass pyrolysis[J]. Materials, 2019, 12(7): 1079 doi: 10.3390/ma12071079 [89] MENEZES O, KOCAMAN K, WONG S, et al. Quinone moieties link the microbial respiration of natural organic matter to the chemical reduction of diverse nitroaromatic compounds[J]. Environmental Science & Technology, 2022, 56(13): 9387-9397. [90] BHUSHAN B, HALASZ A, HAWARI J. Effect of iron(III), humic acids and anthraquinone-2, 6-disulfonate on biodegradation of cyclic nitramines by Clostridium sp. EDB2[J]. Journal of Applied Microbiology, 2006, 100(3): 555-563. doi: 10.1111/j.1365-2672.2005.02819.x [91] 王楠, 周宇齐, 姜子叶, 等. 还原-氧化协同降解全/多卤代有机污染物[J]. 化学进展, 2022, 34(12): 2667-2685. WANG N, ZHOU Y Q, JIANG Z Y, et al. Synergistically consecutive reduction and oxidation of per-and poly-halogenated organic pollutants[J]. Progress in Chemistry, 2022, 34(12): 2667-2685(in Chinese).
-



 下载:
下载:

