-
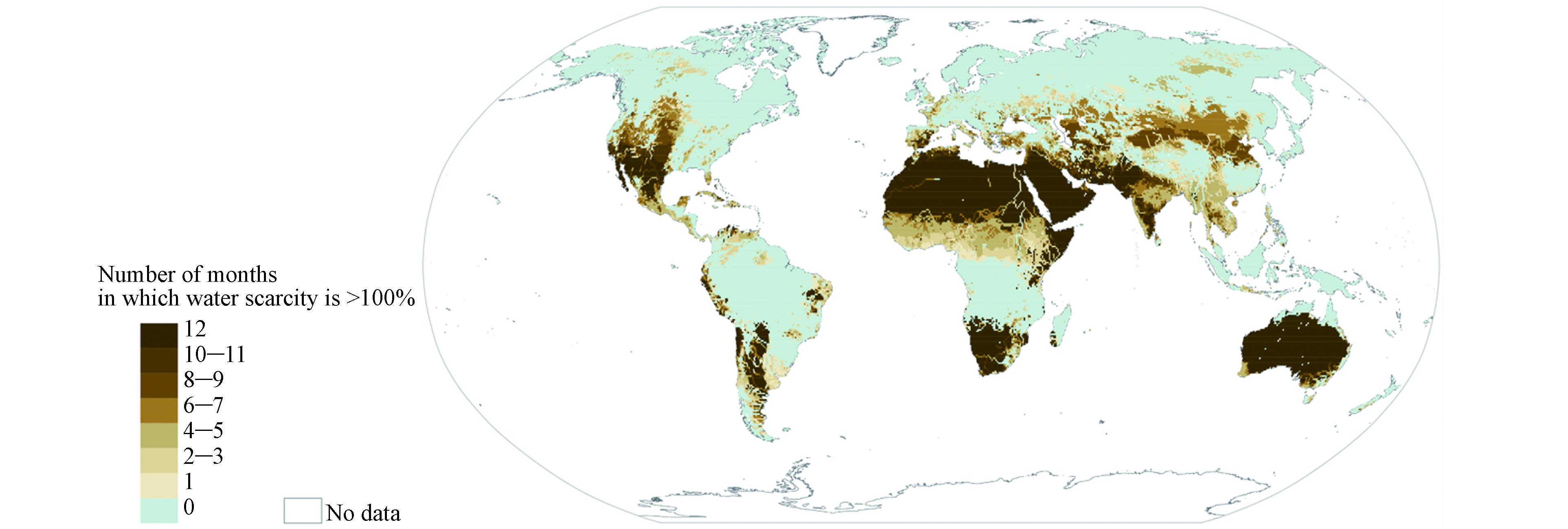
水资源对于人类的生存和发展至关重要. 然而由于人口增长、城市化、气候变化、污染和淡水资源过度开发等问题,世界正面临严重的水危机. 据统计,世界上约有三分之二的人口每年至少有一个月处于中度缺水,并且有超过5亿人在一年中经历极端缺水[1]. 在我国,西北和华北地区是主要的缺水区域(图1),受影响的人口约占我国总人口的24.5%[2]. 联合国于2015年制定了可持续发展目标6(SDG6, 清洁饮水和卫生设施),旨在到2030年为所有人提供水和环境卫生并对其进行可持续管理[3].
据估算,大气中水资源超过1.29×104 km3,其中绝大部分以水蒸气形式存在,相当于江河湖泊淡水资源总量的八分之一[4],且可通过自然水体的蒸发不断得到更新. 然而,由于技术、经济和环境等因素的限制,此项非常规且可持续性的水资源当前利用率较低. 从大气中获取水通常有3种方式:(1)雾收集[5](2)露水收集[6]和(3)基于吸附剂的大气集水(atmospheric water harvest,AWH). 其中,雾收集不适用于干旱和半干旱地区. 相较于露水收集,基于吸附剂的AWH关键优势在于,解吸过程中释放的水蒸气可在局部形成一个高相对湿度(relative humidity,RH)的环境,有效提高露点,从而提高在干旱条件下的产水量.
尽管很多吸附材料都可用于基于吸附剂的AWH,但早期研究较多的水吸附剂(如硅胶、沸石)的性能并不尽如人意[7]. 硅胶近似线性的水吸附等温线导致其在低RH条件下吸附容量较低[8];虽然沸石具有高亲水性,但其再生温度过高(>127 ℃)限制了实际应用[9];金属盐(如CaCl2、LiCl)虽然廉价易得,但经过多次循环后形成钝化层,降低其长期性能[10]. 因此,设计和合成具有理想的水吸附和解吸特性的新型AWH吸附材料仍具有重要意义.
金属有机框架(Metal-organic Framework,MOF)是一类由金属离子或簇与有机配体通过配位键形成的材料,具有坚固、结晶和永久多孔的框架结构[11]. 由于MOF中金属和有机配体的多样性,其结构可设计,化学性质高度可调,能够针对特定的应用场景进行定制. 在AWH领域,部分MOF作为一种高效的吸附剂,具有超高的水蒸汽吸附容量和阶梯形的水吸附等温线,可以在较低的温度和压力变化下实现水的吸收和释放,从而降低AWH过程的能耗[12 − 13]. 利用Web of Science核心数据库检索近5年以Metal-Organic Framework和atmospheric water harvest为主题词的文献,并对其进行分析. 结果显示,该主题的发文量呈现递增趋势(图2a),并且MOF及其复合材料在低RH条件下产水能力成为近期研究的热点(图2b).
本文综述了近年在AWH领域应用的MOF材料和相关AWH装置,分析了它们的工作原理和性能优劣,并讨论了MOF在AWH商业化应用中面临的挑战,旨在为缓解水资源短缺问题提供一种新的技术方案.
-
MOF对水的吸附通常涉及3种机制:化学吸附、孔隙填充和毛细管冷凝[14],其中后两者属于物理吸附.
化学吸附通常发生在MOF的开放金属位点上,具有高度的热力学稳定性. Fjellvag等[15]利用原位X射线衍射(X-Ray diffraction,XRD)揭示了Zn-MOF-74对水的不同结合位点(图3a):位于框架中心和靠近二级构建单元(secondary building unit, SBU)的水分子,在100 ℃和108 ℃下完全脱附;而结合在金属位点的水分子,需要195 ℃才能完全脱附.
孔隙填充是指水分子以水层或水簇的形式占据MOF的微孔孔道. Yaghi等[13]通过XRD和中子衍射证实,水分子优先吸附在靠近MOF-801 SBU的μ3-OH基团. 随着水相对分压(P/P0)的增加,更多的水分子与已吸附在μ3-OH基团的水分子结合,在MOF孔隙内形成立方水簇. Yaghi等[16]还结合单晶XRD和密度泛函理论计算研究了水分子在MOF-303上的孔隙填充过程(图3b),结果与MOF-801类似:水分子首先与有机配体发生强烈的相互作用,然后与其他水分子形成孤立的簇、簇链,并最终形成水分子网络.
毛细管冷凝发生在MOF材料孔径尺寸大于水临界直径(2.076 nm[17])的条件下(图3c),主要发生在介孔MOF中(如MIL-100、MIL-101和NU-1000). 与孔隙填充不同,毛细管冷凝是一种不可逆的水吸附机制,通常表现为MOF水吸附等温线的滞后环[18 − 20].
-
由于水分子的氧亲核,而MOF的金属中心亲电,二者的长期接触会导致MOF晶格的破坏,因此适用于AWH的MOF必须具有良好的水稳定性. MOF自身的水稳定性可以从热力学和动力学两方面进行评价[22]. 根据硬软酸碱理论,高电荷的金属阳离子(硬酸),例如Zr4+、Al3+和Cr3+倾向于和羧酸盐配体(硬碱)的氧原子形成更强的配位键[12 − 13, 23];而二价金属阳离子(软酸),例如Zn2+和Cu2+倾向于和唑类配体(软碱)的氮原子形成更强的配位键[24 − 25],从而形成热力学更稳定的MOF. 而由高配位数SBU,例如Zr6O4(OH)4(—COO)12和(Al(OH)(—COO)2)∞构建的MOF,可通过空间位阻抵抗水分子对配位键的进攻,从而表现出良好的动力学稳定性[13, 26 − 27]. 配体的刚性对于MOF的动力学水稳定性也至关重要,例如UiO-67虽然具有和UiO-66相似的配位键键能和相同的拓扑结构[26],但由于UiO-67配体的柔性更强,其水稳定性显著低于UiO-66[28]. MOF水稳定性可通过XRD进行评估[29 − 30]. 由MOF水解导致的结晶度下降往往表现为XRD衍射峰变宽或强度减弱,因此通过比较MOF在水吸附前后的XRD图谱,可以有效地判断MOF的水稳定性,并给出定性的信息. 同时,XRD对MOF水解产生的少量无定形相不敏感,因此需要结合比表面积测试来补充分析[29 − 30]. 应用于AWH的MOF还应尽量满足以下条件:(1)较高的水饱和吸附量(2)较高的亲水性和(3)较低的再生温度[31 − 32]. 此外,近年来水在MOF上的吸附和解吸动力学也逐渐成为评价其在AWH领域商业化应用前景的重要指标[33].
-
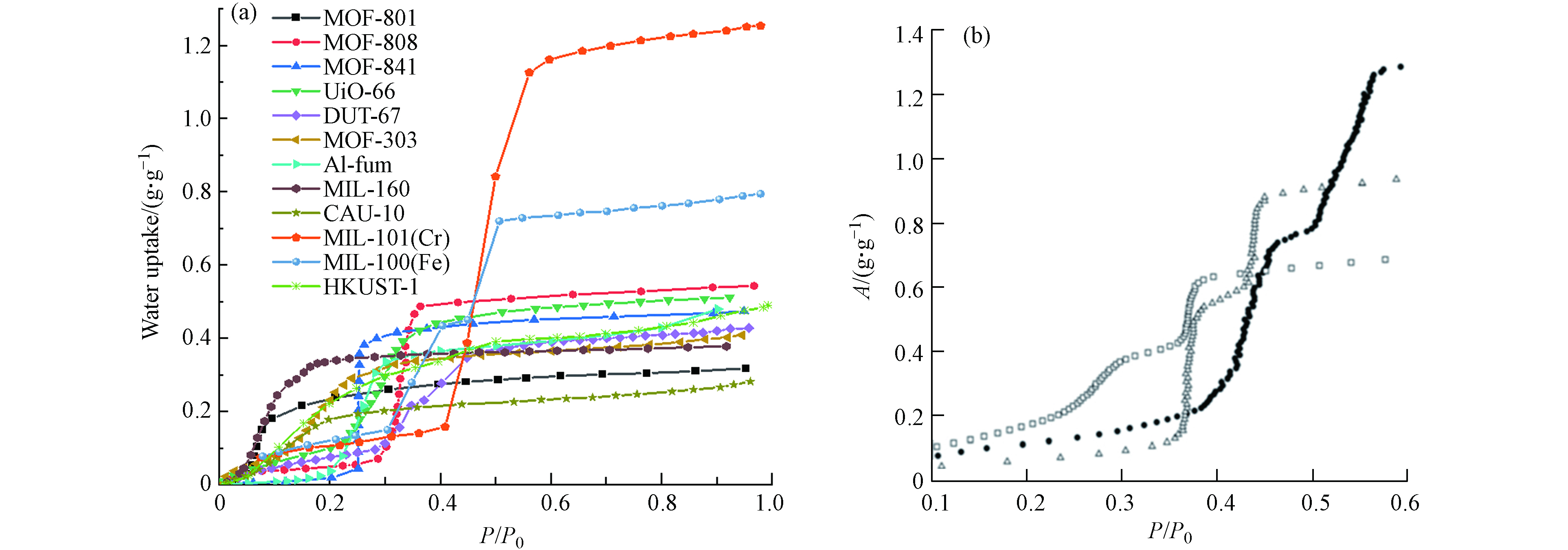
表1 列出了近10年AWH领域研究较为广泛的MOF,图4a展示了这些MOF的水吸附等温线.
这些MOF根据孔径可分为两类:主要以毛细管冷凝为水吸附机制,水饱和吸附量较高的介孔MOF(例如MIL-101(Cr)和MIL-100(Fe))和主要以孔隙填充为水吸附机制,拐点较低的微孔MOF(例如MOF-801、UiO-66和MOF-303等).
Chang等[12]于2012年报道了介孔MOF MIL-100(Fe)和MIL-101(Cr)用于高效空气除湿的研究,发现这两种材料在60% RH条件下吸附水蒸气达到饱和后,可在70 ℃干燥N2流中10 min内全部解吸. 基此,他们首次提出了应用这两种MOF进行AWH的可能性. Eddaoudi等[38]合成了一种新型介孔MOF材料Cr-soc-MOF-1,其水饱和吸附量高达1.95 g·g−1,是目前所有MOF、碳和无机材料中的最高值. 然而介孔MOF在低RH下的水吸附能力较弱,不利于在干旱地区进行AWH. 此外,由于介孔MOF孔径较大,水分子在孔道内的毛细管冷凝使得解吸需要更多的能量,且可能导致结构不稳定. 为了解决这些问题,Lu等[32]提出了一种针对介孔MOF的改性方法,通过引入卤离子替代MOF-808中节点缺陷处的甲酸根离子,增强了MOF-808的水稳定性,实现了其在低RH条件下的高水吸附容量(25 ℃,30% RH, 0.65 g·g−1).
微孔MOF由于其在低RH下的高吸附性能和低再生温度,被认为是AWH的理想材料. MOF-801的水吸附拐点位于0.08,在30% RH时其水吸附能力已达到饱和吸附量(0.33 g·g−1)的88%,且表现出良好的循环稳定性[13, 39]. Yaghi等[27]开发了一种新型铝基MOF,即MOF-303. 该材料相较于MOF-801具有更快的水吸附/解吸速率(完整的集水循环仅需要10 min),和更高的水饱和吸附量(0.48 g·g−1),在相同的条件下其产水量较MOF-801增加114%. 但是,相较于介孔MOF,微孔MOF的孔容积较小,其水饱和吸附量通常较低.
UiO-66和MOF-801具有相同的fcu拓扑结构,但二者的配体长度不同. 具有较长配体的UiO-66结构更疏水,其拐点较MOF-801向更高的P/P0方向移动. 但由于UiO-66的孔容积更大,因此其水饱和吸附量比MOF-801高约54%. 并非所有具有相同拓扑结构的MOF都遵循这一规律. 例如以4,4’-联苯二甲酸为配体的UiO-67虽然其孔容积高达0.99 cm3·g−1,但水饱和吸附量仅有0.29 g·g-1[40],主要是由于配体疏水性较对苯甲酸增加所致 [40 − 41] .
MOF的AWH应用前景需要综合考虑材料的亲水性,水吸附/解吸动力学和水稳定性等指标. 在表1总结的MOF材料中,MOF-303可能具有最佳的AWH应用前景. 它具有以下优点:低拐点(0.15)有利于实现干旱条件下AWH应用[27];适中的吸附热(52 kJ·mol−1)有利于在温和条件下水解吸[42];水吸附动力学快:30 ℃,40% RH条件下80 min吸附饱和[42];循环稳定性高:150次水吸附解吸循环后,材料结构和吸水容量仍保持稳定[27];已实现原料商业化供应的千克级水相绿色合成[43]. 其他一些MOF,如MOF-801、MOF-841、MIL-160和CAU-10,也具有较好的AWH应用前景,因为它们都表现出高水稳定性,具有低拐点或狭窄的吸附/解吸区间,但水解吸温度较高或水吸附/解吸动力学较慢[27, 37, 44]. 再次,MOF-808、Al-fum,UiO-66、DUT-67、MIL-101(Cr)和MIL-100(Fe)由于拐点较高,在低RH条件下难以实现有效水吸附,故AWH应用前景有待进一步开发. 最后,HKUST-1的水稳定性较差(在25 ℃,90% RH条件下3 d后结构发生崩解[30]),无法单独应用于AWH,需要通过材料复合等方式改进水稳定性.
-
向MOF材料的配体上引入亲水性官能团,例如—OH、—NH2和—SO3H等,可以有效增强MOF在低P/P0区间的水吸附,使得一些拐点较高的MOF材料更适合在低RH条件下中开展AWH工作. Kitagawa等[19]对MIL-101(Cr)进行了亲水基团(—SO3H、—NH2)的修饰,发现修饰后的MIL-101(Cr)具有约0.8—1.2 g·g−1的水饱和吸附量和降低至0.34—0.42的拐点(图4b);Walton等[45]报道了UiO-66-NH2的拐点为0.15,显著低于UiO-66的0.28;UiO-67-(NH2)2拐点(0.22)较UiO-67(0.57)显著降低[40]. 另一方面,疏水性官能团,例如—CH3、—OCH3和—CF3等,则可以阻碍水分子攻击配位键而提高MOF的水稳定性,同时MOF孔道仍可吸附水分子[34, 46]. Zhong等[47]对UiO-67进行了邻位—CF3修饰,发现修饰后的UiO-67在沸水中处理1 d和常温水中处理60 d均未损失结晶度和比表面积(UiO-67在常温水中仅1 d就降解为ZrO2[28]). 然而,引入的官能团会占用部分孔容积,降低水饱和吸附量. 并且MOF工业化生产的总成本主要由配体和金属盐构成[48],使用成本更高的官能团修饰的配体可能不利于提高商业竞争力.
-
相同晶胞周期性无限排列的完美MOF晶体并不存在,与理想有序MOF晶体结构的偏差被定义为MOF的缺陷[49]. 不同文献报道的UiO-66的水饱和吸附量数据间差异可达28%[13, 34];MOF-801单晶样品的水饱和吸附量仅为粉末样品的78%[13],这可能都源于不同合成策略导致MOF结构上的缺陷差异. Snurr等[50]利用蒙特卡罗模拟方法研究了这一问题,通过羟基替换对苯二甲酸配体构建了缺陷的UiO-66,模拟结果如图5所示:理想的无缺陷UiO-66在低P/P0下几乎不发生水吸附;缺陷使MOF的亲水性增加,从而使模拟水吸附曲线更贴近实验曲线. Grenev等[51]对CAU-10也进行了模拟缺陷计算得出了相同的结论. 缺陷可以增加MOF材料的比表面积和孔容量,提供更多的活性位点和吸附空间,促进孔结构中团簇水和冷凝水的形成,从而增强其对水分子的吸附能力,但这也会降低MOF的水稳定性和结构强度[49, 52]. 因此在引入缺陷时需要权衡其数量和类型,在不影响稳定性的前提下调控MOF的水吸附性能.
-
MOF材料虽然在低RH吸水和低再生温度上相较于传统材料表现出更大的优势,但其较低的热转化以及热传导效率限制了实际的AWH应用[53]. 此外, MOF材料一般是粉末形态,而实际应用则需要整体材料和足够的机械强度[54 − 55]. 将MOF与其他材料通过化学键合或物理混合的方式制备复合和整体材料以提升AWH性能是近年来领域新兴的研究方向之一. 表2总结了一些应用于AWH的MOF复合材料.
碳基材料,例如碳纳米管、氧化石墨烯和Ti3C2等,具有优异的光热转化能力和导热性能,常用于制备MOF复合材料以提高热传导和水解吸效率. 例如,Maji等[56]制备了HKUST-1/氧化石墨烯/氨基黏土复合材料,结果表明,与未掺杂氧化石墨烯的材料相比,氧化石墨烯掺杂量为9% wt的复合材料从室温升高到103 ℃所需时间(8.5 min)缩短了37%;Li等[59]将Ti3C2与UiO-66-NH2复合,并使用海藻酸钠成型造粒. 在103 mW·cm−2的模拟光照下,Ti3C2含量分别为4.3%wt、8.5%wt、12.8% wt的复合材料在5 min内可迅速升温至86.6、100.2、106.8 ℃,而未掺杂Ti3C2的复合材料温度在5 min内仅能升至74.9 ℃.
MOF与金属盐的复合是一种改善其低RH条件下水吸附性能的有效策略. Wang等[66]制备了LiCl@MIL-101(Cr)复合材料(图6a). 结果表明,30 ℃,30% RH的条件下,该复合材料在160 min即达到饱和吸附量(0.77 g·g−1)的88%. 与纯LiCl相比,复合材料具有更快的水解吸速率,纯LiCl的脱水过程从表面开始,形成的致密表面晶体层阻碍了内部水分子的扩散;而复合材料中LiCl倾向于在MOF的孔道内结晶,从而促进了水分子的迅速释放,加快了水分子从MOF复合材料中解吸. 类似的,Zhao等[65]将LiCl引入HKUST-1中,在25 ℃,50% RH和30% RH的条件下,该复合材料水吸附量分别达到1.09 g·g−1 和0.50 g·g−1,显著高于纯HKUST-1. MOF与金属盐复合不仅能够避免金属盐自身的潮解和团聚现象,还可提升MOF的水吸附动力学和热力学性能[65 − 67]. 但需要注意高RH条件下MOF中过量金属盐导致的潮解渗漏问题,因此应适当控制金属盐与MOF的复合比例.
将MOF与聚合物构成的复合材料在AWH领域也取得了一些研究进展. Li等[60]利用一种类似于汤圆制作的方法,实现了MOF的可塑成型(图6b). 作者采用高孔隙率的聚乙烯醇将UiO-66和Fe3O4包裹在内,材料具有宏观整体形态和良好的机械稳定性,同时保持了MOF原有的高比表面积和孔隙率.
Ho等[62]利用原位自由基聚合交联制备了多孔MOF-聚合物三维网状材料(图6c),可在无需外部加热/冷凝的条件下自发集水,并在实际环境下实现了4.16 g水·g吸附剂−1·d−1的水收集效率. Zhao等[63]在MIL-101(Cr)的介孔内原位聚合N-异丙基丙烯酰胺(图6d):当聚合物含量为 38% wt 时,该复合材料通过表面冷凝和孔隙储水的方式,在98% RH下实现高达4.4 g·g−1的吸水量,显著高于MIL-101(Cr)(1.1 g·g−1)和聚合物(0.74 g·g−1). 复合材料亲水性可由该聚合物在最低临界溶解温度(lower critical solution temperature, LCST)发生的亲水-疏水构象转变来调节, 可在40 ℃,40% RH条件下自主解吸98%的吸附水.
-
MOF和MOF复合材料在大气集水方面具有巨大的潜力,但目前大多数研究只关注了它们的水吸附性能,而AWH应用需要将水吸附、解吸和冷凝模块集成于装置中以实现连续产水. AWH装置可以分为被动式和主动式两种类型:被动式AWH装置利用太阳能和自然冷却来驱动水的解吸和冷凝,一天内只进行一次循环,其性能主要取决于MOF在环境RH下的吸水能力[68];主动式AWH装置使用电能来加速水的解吸和冷凝,一天内可以进行多次循环,其性能主要取决于MOF的解吸速率和冷凝效率[67]. 表3 总结了一系列基于MOF材料的AWH装置.
Yaghi等[39]在2017年设计了一种基于MOF-801的被动式AWH装置,以多孔泡沫铜为载体提高结构刚性和热传导性能,其中装填1.34 g的MOF-801粉末. 该装置在室外测试条件下(10%—65% RH)的产水效率为0.3 L水·kg吸附剂·d−1. 随后,Yaghi等[27]将装置规模扩大(图7a),MOF-801粉末装填量提高至1.2 kg,并混合0.45 kg无孔石墨以提高光热水解吸效率. 该装置在美国亚利桑那州的沙漠(5%—40% RH)中产水效率为0.1 L水·kg吸附剂·d−1. 在此基础上,Yaghi等[42]进一步开发了一种主动式AWH装置(图7b),由太阳能电池驱动,并使用MOF-303作为水吸附剂(装填量:0.433 kg). 该装置通过风扇加强空气流动,促进水吸附解吸,并使用压缩机冷凝收集. 该装置在美国莫哈韦沙漠的极端干旱条件下(12—27 ℃,最低10% RH)仍实现了0.7 L水·kg吸附剂·d−1的产水效率.
在实际AWH过程中,环境温度和RH随时间变化. 而特定吸附材料的水吸附特性往往是固定的,倘若采用固定周期的集水模式,势必造成产水效率的损失. 因此,近年在主动式AWH的基础上发展的自适应AWH策略可以有效解决这一问题. Wang等[69]利用MOF在狭窄RH范围内吸附的特性,通过冷却器调整,以保持装置内RH在MOF(MIL-101(Cr))最适宜的工作范围内. 该装置在25 ℃,30% RH的环境条件下,水吸附量可达1.05 g·g−1. Cordova等[68]设计一种基于环境实时温度和RH自动调整循环时间的AWH装置(图7c),以MOF-801为吸附剂. 该装置在约旦安曼的沙漠中(17%—32% RH)产水效率为3.5 L水·kg吸附剂-1·d−1,相较于先前的主动式装置[42]提高了169%.
目前评价AWH装置的产水效率均采用归一化的指标(L水·kg吸附剂-1·d−1). 然而,AWH装置中吸附剂的装填用量和形状不尽相同(从克到千克,粉末和块状). 有研究表明,这些因素会显著改变水在吸附剂上的吸附热力学和动力学特性[71]. 例如,在25 ℃,40% RH条件下,随着粉末MOF-303用量从10 mg增加到5 g,其水吸附速率常数从0.0017 s−1下降到了0.00027 s−1;机械压缩成型的MOF-303相较于粉末MOF-303的水吸附速率常数和吸附量分别下降了89.9 %和42.5%. 因此,在设计AWH装置时需要充分考虑吸附剂装填量和装填形式对产水效率的影响.
-
AWH是缓解水资源短缺问题的有效策略. 自2012年首次报道MOF在AWH的应用以来,该领域的研究已取得了显著进展. 本文系统概述了MOF在AWH领域的研究现状,涵盖了MOF水吸附机制、AWH对MOF水稳定性的要求,以及MOF、MOF复合材料、AWH装置和它们的工作原理与性能评价等内容. MOF作为一种具有高吸水能力、可调节孔隙率和功能性以及潜在环境友好性的AWH材料,已经在实验室规模下得到了广泛研究,通过选择金属和配体调节孔径和孔容积、引入官能团和缺陷以及材料复合的方式,可以提高其水吸附性能,展现出巨大的应用潜力. 然而,要实现基于MOF的AWH系统的商业化应用,目前仍需要在以下材料开发和装置设计等方面完善:
(1)提高装置产水量. 《中国居民膳食指南(2022)》建议,温和气候、低身体活动水平的成年男性每天饮水量为1.7 L,女性为1.5 L[72]. 如果以家庭为单位,仅饮用水就需每天4 L以上,干旱地区则更多. 目前,基于MOF的AWH装置虽然已有显著进步[14, 68],但仍难以满足这一需求. 简单地增加装置数量或规模会占用更多空间和浪费更多材料,因此还需材料和工程学科的交叉合作,从材料复合、装置传质传热和集水模式等角度提升产水效率,契合实际需求.
(2)因地制宜设计AWH装置. 当前的研究主要集中于低RH条件下MOF吸水和装置AWH性能上[31, 67]. 但并非所有地区的缺水都是由于气候干旱,社会经济因素也会导致水资源短缺. 例如撒哈拉以南非洲地区的气候跨度从热带雨林到干旱沙漠,但总体基础设施薄弱无法有效利用水资源[73];印度和墨西哥等一些发展中国家由于人口快速增长和城市化致使人均水资源量紧缺和供水能力不足[74]. 因此根据当地气候条件选择和设计AWH装置有利于缓解更多地区的缺水问题.
(3)利用计算化学设计合成MOF. 已有的研究利用模拟计算揭示了MOF的缺陷对其水吸附性能具有正向作用[50 − 51],但尚未构建合成方法-缺陷-MOF特性之间的定量关联[75]. 未来,机器学习或许是解决此问题的有力手段[52, 76],通过算法和建模预测不同溶剂、调节剂,以及后修饰等合成条件对MOF材料水吸附性能和水稳定性的影响,以期在不影响AWH过程中MOF长期稳定的前提下,有针对性地合成水吸附性能更佳的MOF.
(4)开发MOF材料的绿色可持续合成方法. MOF的制备需要消耗大量的原材料(金属盐、配体和不可回收的有机溶剂)和加工资源[48, 54]. 尽管一些MOF具有出色的AWH性能,但它们高昂的配体成本和不可持续的合成方法限制了它们的商业化应用[34, 77-78]. 使用小分子简单配体是降低MOF合成成本和构建适合AWH的微孔MOF的有效策略[54, 79];水热法、机械研磨法和电化学合成法等方法是实现MOF环境友好和良好成本效益生产的优选方案[80 − 81]. 未来需要针对特定MOF开发可持续合成方法,以取代传统方法并降低生产成本.
(5)评估AWH装置的长期运行稳定性和经济适用性. AWH作为一种不受时空限制获取淡水的方式,为缺水地区提供了一种新的解决方案. 然而,海水/盐碱水淡化、污水回用、淡水进口、跨区域调水、开采地下水等方法也有其优势,且技术更加成熟[82 − 83]. 当前的研究主要集中在设计和开发功能化和高产水能力MOF复合材料和AWH装置上[7, 67]. 但实际运行中,长期的光照会导致材料老化,产生微塑料,内分泌干扰物和重金属等污染[84 − 85];大气颗粒物沉积在装置中,降低吸附剂吸附能力,并引入新的化学和微生物污染[86 − 88],这些将影响AWH装置的寿命、效率和水质安全. 此外,MOF复合材料的原料和生产工艺以及AWH装置的复杂性将影响最终的生产成本[68]. 因此设计基于MOF的AWH装置需要对其经济适用性和生命周期内运行状态进行评估,以达到实际应用的稳定性要求,并具备良好的市场竞争力.
基于金属有机框架材料大气集水的研究进展
Research progress of atmospheric water harvest based on metal-organic framework materials
-
摘要: 大气集水(atmospheric water harvest,AWH)是一种从空气获取淡水的技术,对于缓解水资源危机具有潜在的应用前景. 金属有机框架(metal-organic framework,MOF)是一种具有高比表面积、可调孔道结构的晶态多孔材料. MOF材料对水蒸气的逐级吸附和适中的脱附温度使得其在AWH领域具有较好的适用性. 本文对近年来MOF材料在AWH领域的研究进展进行了系统综述,总结了MOF水吸附机理,梳理了用于AWH的MOF材料和装置及其集水性能. 本文最后还从AWH装置产水效率、运行稳定性和MOF的可持续设计与合成等方面进行了展望,旨在为MOF材料在AWH领域的应用发展提供参考.Abstract: To overcome the challenges of water scarcity for humans, especially in arid regions, atmospheric water harvest (AWH) is a promising technology for obtaining fresh water from the air. Metal-organic Framework (MOF) is a kind of crystalline porous material with high specific surface area and tunable pore structure, and its stepwise adsorption of water vapor and moderate desorption temperature make it a promising candidate for AWH. This review paper critically and systematically analyzes recent research progress of MOF materials in AWH fields, summarizes the mechanism of MOF on water adsorption, and combs out the MOF materials/devices for AWH and their water harvesting performances. The challenges and outlook on the MOF materials for AWH and freshwater production efficiency, operational stability of AWH devices, and sustainable MOF design and synthesis are also included. Therefore, this review could benefit researchers aiming to design MOF materials in the AWH field.
-
Key words:
- MOF /
- atmospheric water harvest /
- water adsorption /
- composite /
- device.
-

-
图 2 MOF在AWH领域应用的文献计量分析
Figure 2. Bibliometric analysis of MOF applications in AWH (a) Annual number of publications in the Web of Science core collection on the topics of Metal-Organic Framework and atmospheric water harvest in the last five years (b) VOSviewer word frequency and relevance analysis of the search results above
图 3 水分子在MOF上的吸附机制
Figure 3. Adsorption mechanisms of water molecules on MOF (a) Water molecules at Zn-MOF-74 binding sites: the smaller the ellipsoid, the higher the binding strength[21] (b) Evolution of water molecule structures in MOF-303 at different water adsorption quantities[16] (c) Condensation of water molecules in MIL-101(Cr), red spheres indicate oxygen atoms of water molecules, red and green backgrounds indicate pore channels of 3.4 nm and 2.9 nm diameter, respectively[20]
图 4 MOF在25 ℃的水吸附等温线的比较
Figure 4. Comparison of water adsorption isotherms of MOF at 25 ℃ (a) Water sorption isotherms of the MOF described in Table 1 ;(b) Water adsorption isotherms of MIL-101 (●), MIL-101-NH2 (△), and MIL-101-SO3H (□)[19]
图 6 用于AWH的MOF复合材料(a)LiCl@MIL-101(Cr)的多步骤水吸附/解吸过程示意图[66];(b)包状磁性MOF的制备及其大气水分收集循环的示意图[60];(c)自主渗水复合材料的设计[62];(d)MOF-聚合物复合材料的制备及温度触发的水吸附和解吸过程[63]
Figure 6. MOF composites for AWH (a) Schematic of the multi-step water adsorption/desorption process by LiCl@MIL-101(Cr) [66] ;(b) Schematic of the preparation of encapsulated magnetic MOF and its atmosphere water collection cycles[60] ;(c) Design of autonomous water permeation composites[62] (d) Preparation of MOF-polymer composites and temperature-triggered water adsorption and desorption processes[63]
图 7 基于MOF材料的AWH装置设计(a)由吸水装置和外壳组成的被动式AWH装置的示意图[27];(b)在莫哈韦沙漠测试的主动式AWH装置[42];(c)自适应式AWH装置的示意图[68]
Figure 7. Designs of AWH devices based on MOF materials (a) Schematic diagram of a passive AWH device consisting of an absorbing device and a housing[27] ;(b) Active AWH device tested in the Mojave Desert[42] ;(c) Schematic diagram of an adaptive AWH device[68]
表 1 可用于AWH的MOF及其性质
Table 1. MOF used for AWH and their properties
MOF 金属
Metal配体
LigandBET比表面积/(m2·g−1)
BET specific surface area孔容积/(cm3·g−1)
Pore volume拐点*
Inflection point水饱和吸附量/(g·g−1)
Water saturated
adsorption quantityMOF-801[13] Zr 富马酸 990 0.45 0.08 0.33 MOF-808[13] 均苯三甲酸 2060 0.84 0.36 0.54 MOF-841[13] 4,4’,4”,4”’-甲烷四基四苯甲酸酯 1390 0.53 0.24 0.47 UiO-66[34] 对苯二甲酸 1421 0.58 0.28 0.51 DUT-67[35] 2,5-噻吩二羧酸 — 0.47 0.34 0.42 MOF-303[27] Al 3,5-吡唑二羧酸 989 0.54 0.15 0.48 Al-fum[36] 富马酸 792 0.93 0.26 0.48 MIL-160[37] 2,5-呋喃二羧酸 1070 0.40 0.11 0.37 CAU-10[13] 1,3-苯二羧酸 600 0.26 0.15 0.27 MIL-101(Cr)[18] Cr 对苯二甲酸 3017 1.61 0.47 1.28 MIL-100(Fe)[18] Fe 均苯三甲酸 1549 0.82 0.33 0.81 HKUST-1[18] Cu 均苯三甲酸 1340 0.72 0.16 0.55 *达到水饱和吸附量50%的P/P0. *P/P0 that reaches 50% of the water saturated adsorption quantity. 表 2 用于AWH的MOF复合材料及其水吸附性能
Table 2. MOF composites for AWH and their water adsorption properties
MOF复合材料
MOF composites吸附条件
Adsorption conditions水吸附量/(g·g−1)
Water adsorption quantityHKUST-1/氧化石墨烯/氨基粘土[56] 25 ℃,90% RH 0.67 空心MIL-101(Cr)/Fc(COOH)2[57] 25 ℃,90% RH 1.20 MIL-101(Cr)/纳米纤维[58] 25 ℃,50%、68%和98% RH 0.26、0.56和1.04 UiO-66-NH2/Ti3C2/海藻酸钠[59] 25 ℃,20% RH 0.20 UiO-66/Fe3O4/聚乙烯醇[60] 25 ℃,40% RH 0.20 MIL-101(Cr)/镍金属泡沐/海藻酸钠[53] 25 ℃,60% RH 0.81 Al-Fum/多壁碳纳米管/碳纤维/海藻酸钠[61] 25 ℃,35% RH 0.34 MIL-101(Cr)/聚(N-异丙基丙烯酰胺)[62] 25 ℃,90% RH 3.01 聚(N-异丙基丙烯酰胺)@MIL-101(Cr)[63] 25 ℃,98% RH 4.40 MIL-100(Fe)/硅胶[64] 25 ℃,90% RH 0.57 HKUST-1/LiCl[65] 25 ℃,30%和50% RH 0.50和1.09 LiCl@MIL-101(Cr)[66] 30 ℃,30% RH 0.77 表 3 基于MOF的AWH装置和它们的产水效率
Table 3. MOF-based AWH devices and their water production efficiency
吸附剂
Adsorbent装置类型
Device types操作条件
Operation conditions产水效率/
(L水·kg吸附剂·d−1)
Water production efficiencyMOF-801[68] 自适应 25 ℃,17%—32% RH 3.5 MIL-101(Cr)[69] 自适应 — — Al-Fum/多壁碳纳米管/交联海藻酸钠/碳纤维[61] 主动、被动 23—26 ℃,33%—43% RH 1.4 MIL-101(Cr)/镍金属泡沫/海藻酸钠[53] 主动 25 ℃,60% RH 2.2 MOF-303[42] 主动 27 ℃,32% RH和12—27 ℃,10%—72% RH 1.3和0.7 UiO-66-NH2/Ti3C2/海藻酸钠[59] 被动 25 ℃,20% RH 1.4 MOF-801[70] 被动 22—35 ℃,15%—36% RH 0.25 MOF-801/无孔石墨[27] 被动 10—45 ℃,5%—40% RH 0.1 MOF-801[39] 被动 26—31 ℃,10%—65% RH 0.3 -
[1] IPCC CLIMATE. In climate change 2022: Impacts, adaptation and vulnerability. Contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change [M]. Cambridge, UK and New York, NY, USA: Cambridge University Press, 2022. [2] 国务院第七次全国人口普查领导小组办公室. 第七次全国人口普查公报(第三号) [R]. 北京: 国家统计局, 2021. Office of the Leading Group for the Seventh National Population Census of the State Council. Announcement of the Seventh National Population Census (No. 3) [R]. Beijing: National Bureau of Statistics, 2021 (in Chinese).
[3] UNITED NATIONS. Transforming our world: the 2030 agenda for sustainable development [R]. United Nations: New York, NY, USA, 2015. [4] BEYSENS D. Estimating dew yield worldwide from a few meteo data[J]. Atmospheric Research, 2016, 167: 146-155. doi: 10.1016/j.atmosres.2015.07.018 [5] LIU Y F, YANG N, GAO C L, et al. Bioinspired nanofibril-humped fibers with strong capillary channels for fog capture[J]. ACS Applied Materials & Interfaces, 2020, 12(25): 28876-28884. [6] 赵鹏, 徐先英, 李广宇, 等. 民勤沙区凝结水收集产量与微气象因子的关系[J]. 环境生态学, 2021(10): 81-88. ZHAO P, XU X Y, LI G Y, et al. Relationship between collected condensed water yield and micrometeorological factors in Minqin sand area[J]. Environmental Ecology, 2021(10): 81-88 (in Chinese).
[7] GORDEEVA L G, TU Y D, PAN Q W, et al. Metal-organic frameworks for energy conversion and water harvesting: A bridge between thermal engineering and material science[J]. Nano Energy, 2021, 84: 105946. doi: 10.1016/j.nanoen.2021.105946 [8] ALCAÑIZ-MONGE J, PÉREZ-CADENAS M, LOZANO-CASTELLÓ D. Influence of pore size distribution on water adsorption on silica gels[J]. Journal of Porous Materials, 2010, 17(4): 409-416. doi: 10.1007/s10934-009-9317-0 [9] HUNGER B, MATYSIK S, HEUCHEL M, et al. Adsorption of water on zeolites of different types[J]. Journal of Thermal Analysis, 1997, 49(1): 553-565. doi: 10.1007/BF01987483 [10] WANG P, ZHANG D, LU Z. Advantage of super-hydrophobic surface as a barrier against atmospheric corrosion induced by salt deliquescence[J]. Corrosion Science, 2015, 90: 23-32. doi: 10.1016/j.corsci.2014.09.001 [11] BATTEN S R, CHAMPNESS N R, CHEN X M, et al. Terminology of metal-organic frameworks and coordination polymers (IUPAC Recommendations 2013)[J]. Pure and Applied Chemistry, 2013, 85(8): 1715-1724. doi: 10.1351/PAC-REC-12-11-20 [12] SEO Y K, YOON J W, LEE J S, et al. Energy-efficient dehumidification over hierachically porous metal–organic frameworks as advanced water adsorbents[J]. Advanced Materials, 2012, 24(6): 806-810. doi: 10.1002/adma.201104084 [13] FURUKAWA H, GÁNDARA F, ZHANG Y B, et al. Water adsorption in porous metal-organic frameworks and related materials[J]. Journal of the American Chemical Society, 2014, 136(11): 4369-4381. doi: 10.1021/ja500330a [14] XU W T, YAGHI O M. Metal-organic frameworks for water harvesting from air, anywhere, anytime[J]. ACS Central Science, 2020, 6(8): 1348-1354. doi: 10.1021/acscentsci.0c00678 [15] DIETZEL P , JOHNSEN R, BLOM R, et al. Structural changes and coordinatively unsaturated metal atoms on dehydration of honeycomb analogous microporous metal–organic frameworks[J]. Chemistry – A European Journal, 2008, 14(8): 2389-2397. doi: 10.1002/chem.200701370 [16] HANIKEL N, PEI X K, CHHEDA S, et al. Evolution of water structures in metal-organic frameworks for improved atmospheric water harvesting[J]. Science, 2021, 374(6566): 454-459. doi: 10.1126/science.abj0890 [17] RIETH A J, YANG S, WANG E N, et al. Record atmospheric fresh water capture and heat transfer with a material operating at the water uptake reversibility limit[J]. ACS Central Science, 2017, 3(6): 668-672. doi: 10.1021/acscentsci.7b00186 [18] KÜSGENS P, ROSE M, SENKOVSKA I, et al. Characterization of metal-organic frameworks by water adsorption[J]. Microporous and Mesoporous Materials, 2009, 120(3): 325-330. doi: 10.1016/j.micromeso.2008.11.020 [19] AKIYAMA G, MATSUDA R, SATO H, et al. Effect of functional groups in MIL-101 on water sorption behavior[J]. Microporous and Mesoporous Materials, 2012, 157: 89-93. doi: 10.1016/j.micromeso.2012.01.015 [20] FEI S B, GAO J, MATSUDA R, et al. Temperature effect on water adsorption and desorption processes in the mesoporous metal-organic framework MIL-101(Cr)[J]. The Journal of Physical Chemistry C, 2022, 126(36): 15538-15546. doi: 10.1021/acs.jpcc.2c05603 [21] KALMUTZKI M J, DIERCKS C S, YAGHI O M. Metal–organic frameworks for water harvesting from air[J]. Advanced Materials, 2018, 30(37): 1704304. doi: 10.1002/adma.201704304 [22] BURTCH N C, JASUJA H, WALTON K S. Water stability and adsorption in metal-organic frameworks[J]. Chemical Reviews, 2014, 114(20): 10575-10612. doi: 10.1021/cr5002589 [23] 曹小聪, 熊曾恒, 张鸣珊, 等. 锆基金属有机框架材料对酸性水中甲萘威的吸附[J]. 环境化学, 2021, 40(11): 3627-3630. doi: 10.7524/j.issn.0254-6108.2021.11.hjhx202111037 CAO X C, XIONG Z H, ZHANG M S, et al. Generated zirconium based metal organic framework materials for carbaryl adsorption in acidic aqueous solutions[J]. Environmental Chemistry, 2021, 40(11): 3627-3630 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021.11.hjhx202111037
[24] CHOI H J, DINCÄ M, DAILLY A, et al. Hydrogen storage in water-stable metal-organic frameworks incorporating 1, 3- and 1, 4-benzenedipyrazolate[J]. Energy & Environmental Science, 2010, 3(1): 117-123. [25] ZHANG K, LIVELY R P, DOSE M E, et al. Alcohol and water adsorption in zeolitic imidazolate frameworks[J]. Chemical Communications, 2013, 49(31): 3245-3247. doi: 10.1039/c3cc39116g [26] CAVKA J H, JAKOBSEN S, OLSBYE U, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. doi: 10.1021/ja8057953 [27] FATHIEH F, KALMUTZKI M J, KAPUSTIN E A, et al. Practical water production from desert air[J]. Science Advances, 2018, 4(6): eaat3198. doi: 10.1126/sciadv.aat3198 [28] DeCOSTE J B, PETERSON G W, JASUJA H, et al. Stability and degradation mechanisms of metal-organic frameworks containing the Zr6O4(OH)4 secondary building unit[J]. Journal of Materials Chemistry A, 2013, 1(18): 5642-5650. doi: 10.1039/c3ta10662d [29] 武恩宇, 钱国栋, 李斌. 铝基金属-有机框架材料的水吸附性能与大气集水应用[J]. 浙江大学学报(工学版), 2022, 56(1): 186-192. doi: 10.3785/j.issn.1008-973X.2022.01.021 WU E Y, QIAN G D, LI B. Water adsorption in aluminum-based metal-organic framework for atmospheric water harvesting[J]. Journal of Zhejiang University (Engineering Science), 2022, 56(1): 186-192(in Chinese). doi: 10.3785/j.issn.1008-973X.2022.01.021
[30] DeCOSTE J B, PETERSON G W, SCHINDLER B J, et al. The effect of water adsorption on the structure of the carboxylate containing metal–organic frameworks Cu-BTC, Mg-MOF-74, and UiO-66[J]. Journal of Materials Chemistry A, 2013, 1(38): 11922-11932. doi: 10.1039/c3ta12497e [31] HANIKEL N, PRÉVOT M S, YAGHI O M. MOF water harvesters[J]. Nature Nanotechnology, 2020, 15(5): 348-355. doi: 10.1038/s41565-020-0673-x [32] LU Z Y, DUAN J X, DU L T, et al. Incorporation of free halide ions stabilizes metal–organic frameworks (MOFs) against pore collapse and renders large-pore Zr-MOFs functional for water harvesting[J]. Journal of Materials Chemistry A, 2022, 10(12): 6442-6447. doi: 10.1039/D1TA10217F [33] WANG J Y, HUA L J, LI C F, et al. Atmospheric water harvesting: Critical metrics and challenges[J]. Energy & Environmental Science, 2022, 15(12): 4867-4871. [34] LU F F, GU X W, WU E Y, et al. Systematic evaluation of water adsorption in isoreticular UiO-type metal-organic frameworks[J]. Journal of Materials Chemistry A, 2023, 11(3): 1246-1255. doi: 10.1039/D2TA07392G [35] BON V, SENKOVSKA I, EVANS J D, et al. Insights into the water adsorption mechanism in the chemically stable zirconium-based MOF DUT-67-a prospective material for adsorption-driven heat transformations[J]. Journal of Materials Chemistry A, 2019, 7(20): 12681-12690. doi: 10.1039/C9TA00825J [36] TEO H W B, CHAKRABORTY A, KITAGAWA Y, et al. Experimental study of isotherms and kinetics for adsorption of water on Aluminium Fumarate[J]. International Journal of Heat and Mass Transfer, 2017, 114: 621-627. doi: 10.1016/j.ijheatmasstransfer.2017.06.086 [37] CADIAU A, LEE J S, DAMASCENO BORGES D, et al. Design of hydrophilic metal organic framework water adsorbents for heat reallocation[J]. Advanced Materials, 2015, 27(32): 4775-4780. doi: 10.1002/adma.201502418 [38] TOWSIF ABTAB S M, ALEZI D, BHATT P M, et al. Reticular chemistry in action: A hydrolytically stable MOF capturing twice its weight in adsorbed water[J]. Chem, 2018, 4(1): 94-105. doi: 10.1016/j.chempr.2017.11.005 [39] KIM H, YANG S, RAO S R, et al. Water harvesting from air with metal-organic frameworks powered by natural sunlight[J]. Science, 2017, 356(6336): 430-434. doi: 10.1126/science.aam8743 [40] KO N, HONG J S, SUNG S, et al. A significant enhancement of water vapour uptake at low pressure by amine-functionalization of UiO-67[J]. Dalton Transactions, 2015, 44(5): 2047-2051. doi: 10.1039/C4DT02582B [41] MONDLOCH J E, KATZ M J, PLANAS N, et al. Are Zr6-based MOFs water stable?Linker hydrolysis vs. capillary-force-driven channel collapse[J]. Chemical Communications, 2014, 50(64): 8944-8946. doi: 10.1039/C4CC02401J [42] HANIKEL N, PRÉVOT M S, FATHIEH F, et al. Rapid cycling and exceptional yield in a metal-organic framework water harvester[J]. ACS Central Science, 2019, 5(10): 1699-1706. doi: 10.1021/acscentsci.9b00745 [43] ZHENG Z L, NGUYEN H L, HANIKEL N, et al. High-yield, green and scalable methods for producing MOF-303 for water harvesting from desert air[J]. Nature Protocols, 2023, 18(1): 136-156. doi: 10.1038/s41596-022-00756-w [44] LOGAN M W, LANGEVIN S, XIA Z Y. Reversible atmospheric water harvesting using metal-organic frameworks[J]. Scientific Reports, 2020, 10(1): 1-11. doi: 10.1038/s41598-019-56847-4 [45] CMARIK G E, KIM M, COHEN S M, et al. Tuning the adsorption properties of UiO-66 via ligand functionalization[J]. Langmuir, 2012, 28(44): 15606-15613. doi: 10.1021/la3035352 [46] WANG C H, LIU X L, KESER DEMIR N, et al. Applications of water stable metal–organic frameworks[J]. Chemical Society Reviews, 2016, 45(18): 5107-5134. doi: 10.1039/C6CS00362A [47] WANG K K, HUANG H L, ZHOU X C, et al. Highly chemically stable MOFs with trifluoromethyl groups: Effect of position of trifluoromethyl groups on chemical stability[J]. Inorganic Chemistry, 2019, 58(9): 5725-5732. doi: 10.1021/acs.inorgchem.9b00088 [48] SEVERINO M I, GKANIATSOU E, NOUAR F, et al. MOFs industrialization: A complete assessment of production costs[J]. Faraday Discussions, 2021, 231(0): 326-341. [49] SHOLL D S, LIVELY R P. Defects in metal–organic frameworks: Challenge or opportunity?[J]. The Journal of Physical Chemistry Letters, 2015, 6(17): 3437-3444. doi: 10.1021/acs.jpclett.5b01135 [50] GHOSH P, COLÓN Y J, SNURR R Q. Water adsorption in UiO-66: The importance of defects[J]. Chemical Communications, 2014, 50(77): 11329-11331. doi: 10.1039/C4CC04945D [51] GRENEV I V, SHUBIN A A, SOLOVYEVA M V, et al. The impact of framework flexibility and defects on the water adsorption in CAU-10-H[J]. Physical Chemistry Chemical Physics, 2021, 23(37): 21329-21337. doi: 10.1039/D1CP03242A [52] REN J W, LEDWABA M, MUSYOKA N M, et al. Structural defects in metal-organic frameworks (MOFs): Formation, detection and control towards practices of interests[J]. Coordination Chemistry Reviews, 2017, 349: 169-197. doi: 10.1016/j.ccr.2017.08.017 [53] TAO Y L, LI Q Q, WU Q N, et al. Embedding metal foam into metal-organic framework monoliths for triggering a highly efficient release of adsorbed atmospheric water by localized eddy current heating[J]. Materials Horizons, 2021, 8(5): 1439-1445. doi: 10.1039/D1MH00306B [54] REN J W, DYOSIBA X, MUSYOKA N M, et al. Review on the current practices and efforts towards pilot-scale production of metal-organic frameworks (MOFs)[J]. Coordination Chemistry Reviews, 2017, 352: 187-219. doi: 10.1016/j.ccr.2017.09.005 [55] FONSECA J, GONG T H. Fabrication of metal-organic framework architectures with macroscopic size: A review[J]. Coordination Chemistry Reviews, 2022, 462: 214520. doi: 10.1016/j.ccr.2022.214520 [56] LAHA S, MAJI T K. Binary/ternary MOF nanocomposites for multi-environment indoor atmospheric water harvesting[J]. Advanced Functional Materials, 2022, 32(34): 2203093. doi: 10.1002/adfm.202203093 [57] HU Y, FANG Z, WAN X Y, et al. Ferrocene dicarboxylic acid ligand-exchanged hollow MIL-101(Cr) nanospheres for solar-driven atmospheric water harvesting[J]. ACS Sustainable Chemistry & Engineering, 2022, 10(19): 6446-6455. [58] LI A L, XIONG J, LIU Y, et al. A rapid-ab/desorption and portable photothermal MIL-101(Cr) nanofibrous composite membrane fabricated by spray-electrospinning for atmosphere water harvesting[J]. Energy & Environmental Materials, 2023, 6(1): e12254. [59] WU Q N, SU W, LI Q Q, et al. Enabling continuous and improved solar-driven atmospheric water harvesting with Ti3C2-incorporated metal–organic framework monoliths[J]. ACS Applied Materials & Interfaces, 2021, 13(32): 38906-38915. [60] SU W, TAO Y L, WU Q N, et al. Magnetic stuffed bun-structured metal-organic framework monoliths with noncompromised accessible pores and highly efficient recycling capability[J]. ACS Applied Materials & Interfaces, 2022, 14(34): 39637-39645. [61] TAO Y L, WU Q N, HUANG C, et al. Sandwich-structured carbon paper/metal-organic framework monoliths for flexible solar-powered atmospheric water harvesting on demand[J]. ACS Applied Materials & Interfaces, 2022, 14(8): 10966-10975. [62] YILMAZ G, MENG F L, LU W, et al. Autonomous atmospheric water seeping MOF matrix[J]. Science Advances, 2020, 6(42): eabc8605. doi: 10.1126/sciadv.abc8605 [63] KARMAKAR A, MILEO P G M, BOK I, et al. Thermo-responsive MOF/polymer composites for temperature-mediated water capture and release[J]. Angewandte Chemie International Edition, 2020, 59(27): 11003-11009. doi: 10.1002/anie.202002384 [64] MAHER H, RUPAM T H, ROCKY K A, et al. Silica gel-MIL 100(Fe) composite adsorbents for ultra-low heat-driven atmospheric water harvester[J]. Energy, 2022, 238: 121741. doi: 10.1016/j.energy.2021.121741 [65] ZHAO H Z, LEI M, LIU T, et al. Synthesis of composite material HKUST-1/LiCl with high water uptake for water extraction from atmospheric air[J]. Inorganica Chimica Acta, 2020, 511: 119842. doi: 10.1016/j.ica.2020.119842 [66] XU J X, LI T X, CHAO J W, et al. Efficient solar-driven water harvesting from arid air with metal-organic frameworks modified by hygroscopic salt[J]. Angewandte Chemie International Edition, 2020, 59(13): 5202-5210. doi: 10.1002/anie.201915170 [67] POREDOŠ P, SHAN H, WANG C X, et al. Sustainable water generation: Grand challenges in continuous atmospheric water harvesting[J]. Energy & Environmental Science, 2022, 15(8): 3223-3235. [68] ALMASSAD H A, ABAZA R I, SIWWAN L, et al. Environmentally adaptive MOF-based device enables continuous self-optimizing atmospheric water harvesting[J]. Nature Communications, 2022, 13(1): 1-10. doi: 10.1038/s41467-021-27699-2 [69] FENG Y H, GE T S, CHEN B, et al. A regulation strategy of sorbent stepwise position for boosting atmospheric water harvesting in arid area[J]. Cell Reports Physical Science, 2021, 2(9): 100561. doi: 10.1016/j.xcrp.2021.100561 [70] KIM H, RAO S R, KAPUSTIN E A, et al. Adsorption-based atmospheric water harvesting device for arid climates[J]. Nature Communications, 2018, 9(1): 1-8. doi: 10.1038/s41467-017-02088-w [71] CHEN Z H, SHAO Z, TANG Y C, et al. Study of the scale-up effect on the water sorption performance of MOF materials[J]. ACS Materials Au, 2023, 3(1): 43-54. doi: 10.1021/acsmaterialsau.2c00052 [72] 中国营养学会. 中国居民膳食指南-2022[M]. 北京: 人民卫生出版社, 2022. Chinese Nutrition Society. Dietary guidelines for Chinese residents: 2022[M]. Beijing: People's Medical Publishing House, 2022(in Chinese).
[73] SONO D, WEI Y, JIN Y. Assessing the climate resilience of sub-Saharan Africa (SSA): A metric-based approach[J]. Land, 2021, 10(11): 1205. doi: 10.3390/land10111205 [74] SALEHI M. Global water shortage and potable water safety;Today’s concern and tomorrow’s crisis[J]. Environment International, 2022, 158: 106936. doi: 10.1016/j.envint.2021.106936 [75] XIANG W L, ZHANG Y P, CHEN Y F, et al. Synthesis, characterization and application of defective metal–organic frameworks: Current status and perspectives[J]. Journal of Materials Chemistry A, 2020, 8(41): 21526-21546. doi: 10.1039/D0TA08009H [76] WU Y, DUAN H P, XI H X. Machine learning-driven insights into defects of zirconium metal-organic frameworks for enhanced ethane–ethylene separation[J]. Chemistry of Materials, 2020, 32(7): 2986-2997. doi: 10.1021/acs.chemmater.9b05322 [77] XU H S, WU Y C, YANG L Y, et al. Water-harvesting metal-organic frameworks with gigantic Al24 units and their deconstruction into molecular clusters[J]. Angewandte Chemie International Edition, 2023, 62(6): e202217864. doi: 10.1002/anie.202217864 [78] YOUSSEF P G, DAKKAMA H, MAHMOUD S M, et al. Experimental investigation of adsorption water desalination/cooling system using CPO-27Ni MOF[J]. Desalination, 2017, 404: 192-199. doi: 10.1016/j.desal.2016.11.008 [79] DOU H Z, XU M, WANG B Y, et al. Microporous framework membranes for precise molecule/ion separations[J]. Chemical Society Reviews, 2021, 50(2): 986-1029. doi: 10.1039/D0CS00552E [80] FU J R, WU Y N. A showcase of green chemistry: Sustainable synthetic approach of zirconium-based MOF materials[J]. Chemistry - A European Journal, 2021, 27(39): 9967-9987. doi: 10.1002/chem.202005151 [81] JULIEN P A, MOTTILLO C, FRIŠČIĆ T. Metal-organic frameworks meet scalable and sustainable synthesis[J]. Green Chemistry, 2017, 19(12): 2729-2747. doi: 10.1039/C7GC01078H [82] HE C Y, LIU Z F, WU J G, et al. Future global urban water scarcity and potential solutions[J]. Nature Communications, 2021, 12(1): 1-11. doi: 10.1038/s41467-020-20314-w [83] HADADIN N, QAQISH M, AKAWWI E, et al. Water shortage in Jordan-Sustainable solutions[J]. Desalination, 2010, 250(1): 197-202. doi: 10.1016/j.desal.2009.01.026 [84] KANNAN K, VIMALKUMAR K. A review of human exposure to microplastics and insights into microplastics as obesogens[J]. Frontiers in Endocrinology, 2021, 12: 724989. doi: 10.3389/fendo.2021.724989 [85] CHOWDHURY S, JAFAR MAZUMDER M A, AL-ATTAS O, et al. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries[J]. Science of the Total Environment, 2016, 569/570: 476-488. doi: 10.1016/j.scitotenv.2016.06.166 [86] 陶红, 张小红, 王亚娟, 等. 银川市城区地表灰尘重金属污染分布特征及健康风险评价[J]. 环境化学, 2022, 41(8): 2573-2585. doi: 10.7524/j.issn.0254-6108.2021042501 TAO H, ZHANG X H, WANG Y J, et al. Pollution characteristics and health risk assessment of heavy metals of surface dust in urban areas of Yinchuan[J]. Environmental Chemistry, 2022, 41(8): 2573-2585 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021042501
[87] CHEN P, GUO X Y, LI F X. Antibiotic resistance genes in bioaerosols: Emerging, non-ignorable and pernicious pollutants[J]. Journal of Cleaner Production, 2022, 348: 131094. doi: 10.1016/j.jclepro.2022.131094 [88] SHABBAJ I, ALGHAMDI M, SHAMY M, et al. Risk assessment and implication of human exposure to road dust heavy metals in Jeddah, Saudi Arabia [J]. International Journal of Environmental Research and Public Health, 2018, 15(1): 36. -



 下载:
下载: