-
随着人口的增长和经济的不断发展,我国环境污染问题日趋严重.据2014年全国土壤污染状况调查公报显示,我国污染土壤占比高达16.1%,推算污染面积近8400万公顷[1].2021年最新发布的中国生态环境状况公报中,全国地表水非优良水质断面(IV—劣V类)占15.1%,其中湖泊的污染情况尤其严峻,劣V类水质占比达到5.2%[2].镉、汞、砷、铜等重金属和多环芳烃、卤代烃、石油烃等有机污染物是导致土壤、水体遭受污染的主要物质.重金属和有机污染物通常具有较高的毒性和环境持久性,严重危害生态环境和人体健康,而且污染治理难度大,已成为当前突出的全球性挑战.因此,开发安全高效、功能多样、环境友好和成本低廉的污染修复材料及修复技术极具重要现实意义.
层状双氢氧化物(layered double hydroxides, LDHs)是一类天然矿物材料,由带正电荷的金属氢氧化物层板、具有电荷补偿作用的层间阴离子以及水分子组成[3].LDHs具有独特的超分子结构,其金属层板和层间阴离子易于调控,这为在原子尺度上分散和调控重金属和有机污染物反应活性位点提供了巨大的潜力[4].此外,LDHs的人工合成策略简便易行,易于实现修复材料的规模化制备[5].基于这样的特性,LDHs衍生出了众多功能多样的插层材料,在环境污染修复领域已经得到了广泛应用,特别是在污染物吸附固定、催化降解和油水分离等方面的应用研究日益增多[6 − 9].
近年来,LDHs材料在环境修复领域呈现出广阔的前景,然而真实环境复杂多变的污染状况使得其应用面临挑战.本文介绍了LDHs材料的结构、性质与合成方法,系统总结了LDHs材料在环境污染修复领域的应用现状,针对限制其广泛应用的问题,重点分析了LDHs材料的性能调控策略,以期为未来功能化LDHs材料的深入开发和应用提供新思路.发展和推广LDHs修复材料可以满足环境修复产业对高效、绿色、低碳解决方案的需求,并为生态环境保护技术的创新和产业的发展注入新活力.
-
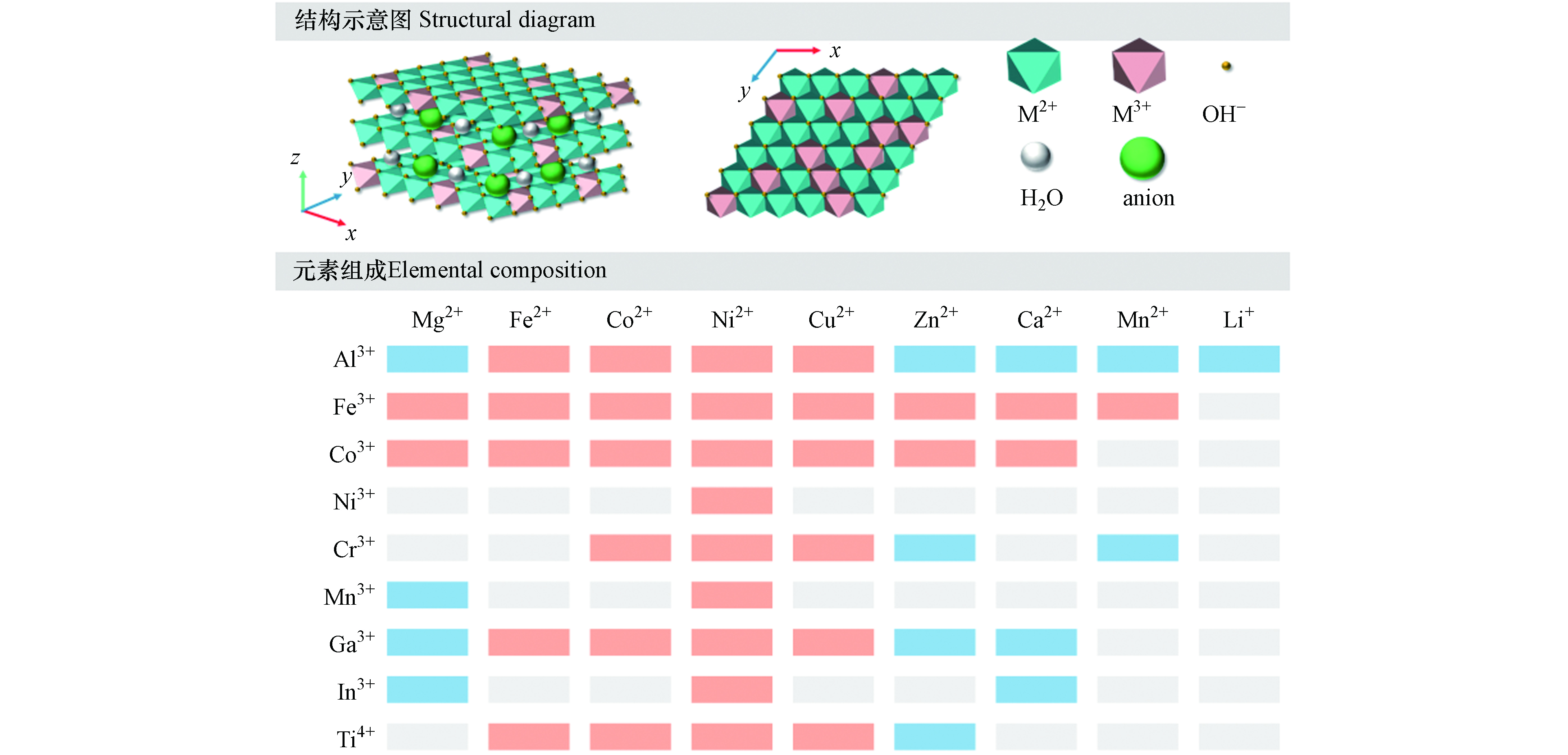
LDHs指的是一类由带正电荷的层板和层间阴离子有序组成的化合物(见图1).其中,层板包括由二价金属和三价金属氢氧化物(如Mg2+、Ca2+、Al3+和Fe3+等)构成,而层间则由可交换的的阴离子(如Cl−、
${\rm{NO}}_3^{-} $ 和${\rm{CO}}_3^{2-} $ 等)和水分子组成[3].部分过渡金属所构成的LDHs(例如Zn2+、Co2+和Ni3+等,见图1)具有催化反应活性[10].LDHs的化学通式可以描述为[M2+1-xM3+x (OH)2]x+(Am−)x/m·nH2O,其中x取值范围为0.2—0.4,即M2+和M3+的摩尔比为2—4[3, 5, 11].通过调整M2+/M3+的比值可以控制LDHs的电荷密度,使得层板化学性质和晶相比例发生变化[12].与广泛存在的阳离子黏土矿物(如蒙脱石、高岭石)不同,LDHs是一种阴离子交换材料.通过正电荷的金属层板与层间阴离子之间的静电引力、氢键和范德华力的相互作用,LDHs的整体结构呈现出电中性.这种结构具有相对较弱的层间结合力,因而对有机和无机阴离子表现出良好的捕获性能[13].而层内较强的电荷密度和层间的阴离子使LDHs层板堆叠成数层,其层间距的大小取决于阴离子的数量、体积、价态及其与层板羟基的结合强度. -
LDHs具有灵活的可调控性,其层板金属组成、摩尔比、层间离子、晶体尺寸以及微纳米尺度上的形貌/孔结构均易于调控,从而为化学反应过程的调整提供了可能.例如,通过掺杂过渡金属或还原性金属来制备LDHs,可以使其导电性增加或氧化还原性增强[14 − 16].另外,LDHs具有优异的阴离子交换能力,可以引入几乎所有种类的阴离子(包括无机离子和有机小分子)到其层间中[10].利用这种特性,LDHs适合吸附多种污染物,如氮、氟离子、重金属离子和有机污染物等.同时,LDHs上的各种结构单元(如阳离子或碱性位点)也为多种污染物提供了特定的活性位点.此外,LDHs表现出优异的热稳定性,在200 °C以内,仅损失表面水和孔隙水;在250—450 °C之间发生脱羟基反应;在450—500 °C的高温下层间碳酸根离子才会逐渐分解,进而转变成更大比表面积的层状双金属氧化物(LDO)[13].LDO在水中能够再水化和结构重建,从而恢复成原本的层板结构.利用这种“记忆效应”,可以将不同的阴离子和大分子物质引入层间,实现LDHs材料的改性和污染物的去除[10, 17].
-
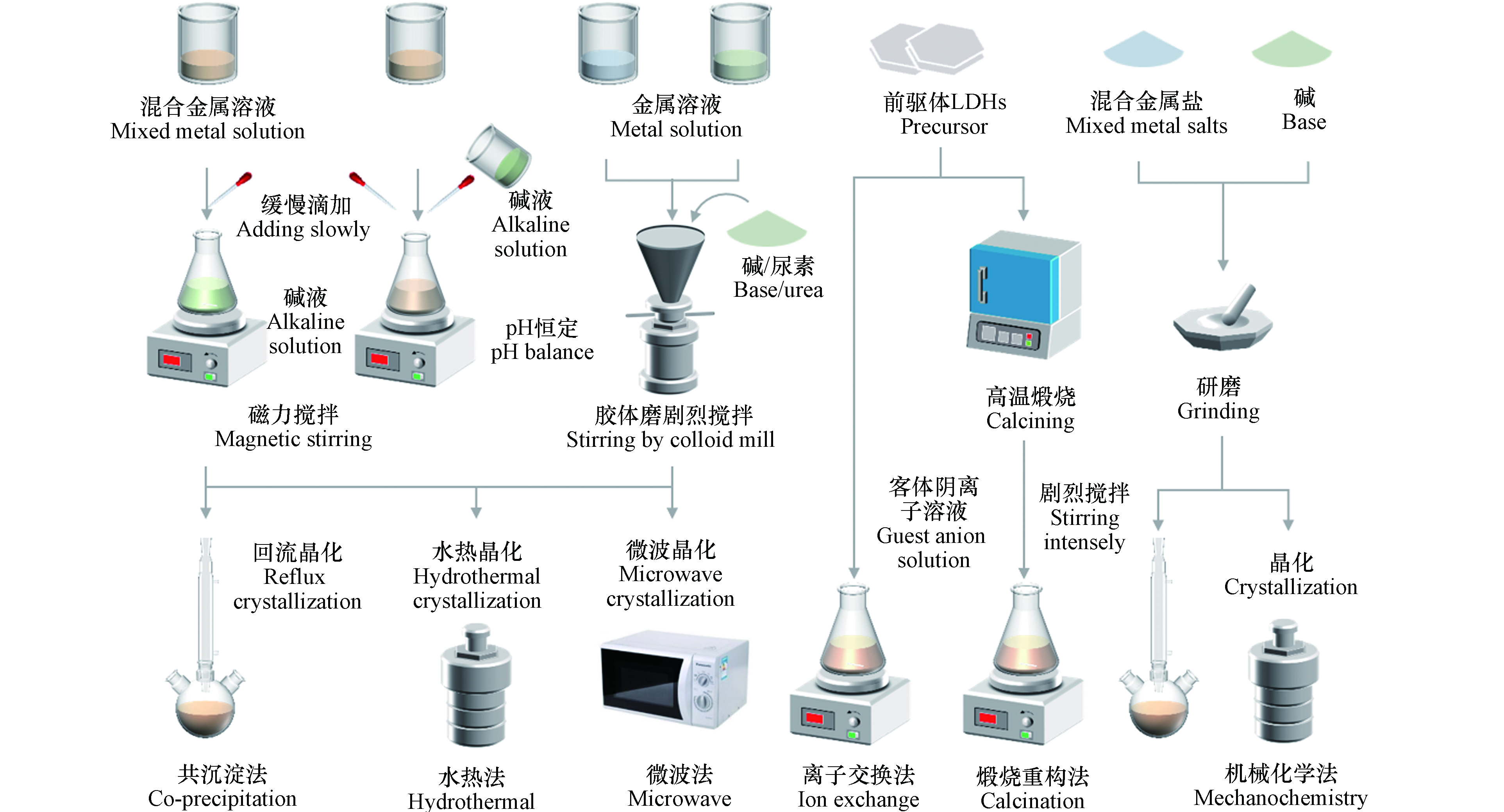
LDHs的合成涉及两个阶段,成核和结晶[18].在成核过程中,可以通过控制温度和浓度来调控.晶体的生长速度则可以通过改变晶化的温度、时间和方法来控制.目前常用的LDHs合成方法包括共沉淀法、水热法、微波法、离子交换法、煅烧重构法和机械化学法等,如图2所示.
(1)共沉淀法是一种简单有效的合成方法.该方法首先将金属离子溶液和碱液混合,然后在一定的温度和压力下进行晶化反应.变化pH法是将金属离子混合液缓慢滴加到碱液中,同时充分搅拌,随后混合液发生晶化.在这一过程中,pH不断变化,但金属离子始终过饱和.搅拌速度远慢于沉淀速度,因此该方法容易导致杂晶相的生成,如金属氢氧化物等.后续发展出了恒定pH法,通过将金属离子溶液和碱液共同滴定,可以控制成核过程,减少杂晶相的生成.保持pH恒定的沉淀过程会导致溶液饱和程度低、晶核数量少,进而产生大尺寸的结晶.共沉淀法难以控制LDHs的粒径和分布,因为成核和结晶过程同时发生.直到2002年,Zhao等[18]报道了成核晶化隔离法,该方法在胶体磨中进行非常快速的混合和成核过程,然后进行单独的老化过程,有利于得到尺寸均匀的晶体颗粒.该方法实现了LDHs的规模化生产[19],并形成了相应的行业标准(《合成水滑石吸附剂》,HG/T 5549—2019).
(2)水热法是以水为介质得到金属盐溶液,然后通过加热方式得到高温高压的晶化环境来制备LDHs[20].该方法宜于改变反应温度、压力、时间和原料比,可以控制不同结构和形态的LDHs.但该方法对高温高压反应容器的要求较高.
(3)与水热法相比,以微波为热源的方法极大地缩短了合成时间.研究表明,微波处理8 min所得LDHs的晶化度与水热处理24 h所得产物晶化度相似[21].
(4)离子交换法利用LDHs的离子交换特性可以将目标阴离子替换到LDHs层间.通常,离子的电荷密度越高,半径越小,交换能力越强[22].
(5)煅烧重构法利用了LDHs的“记忆效应”,先通过煅烧将LDHs转化为LDO,然后将产物置于特定阴离子溶液中水化复原,即可制备出新结构的LDHs[10, 17].这种方法可以将复杂的无机离子、有机分子和大分子物质引入到LDHs层间.
(6)机械化学法是将金属盐和氢氧化物共同研磨后进行水热处理的方法[23].与传统的合成方法相比,该方法具有污染小、能耗低和成本低等优点.该方法不需要溶液分散,只需要通过两步的研磨和晶化即可在短时间内得到高结晶度和形貌均匀的LDHs纳米片[24].
-
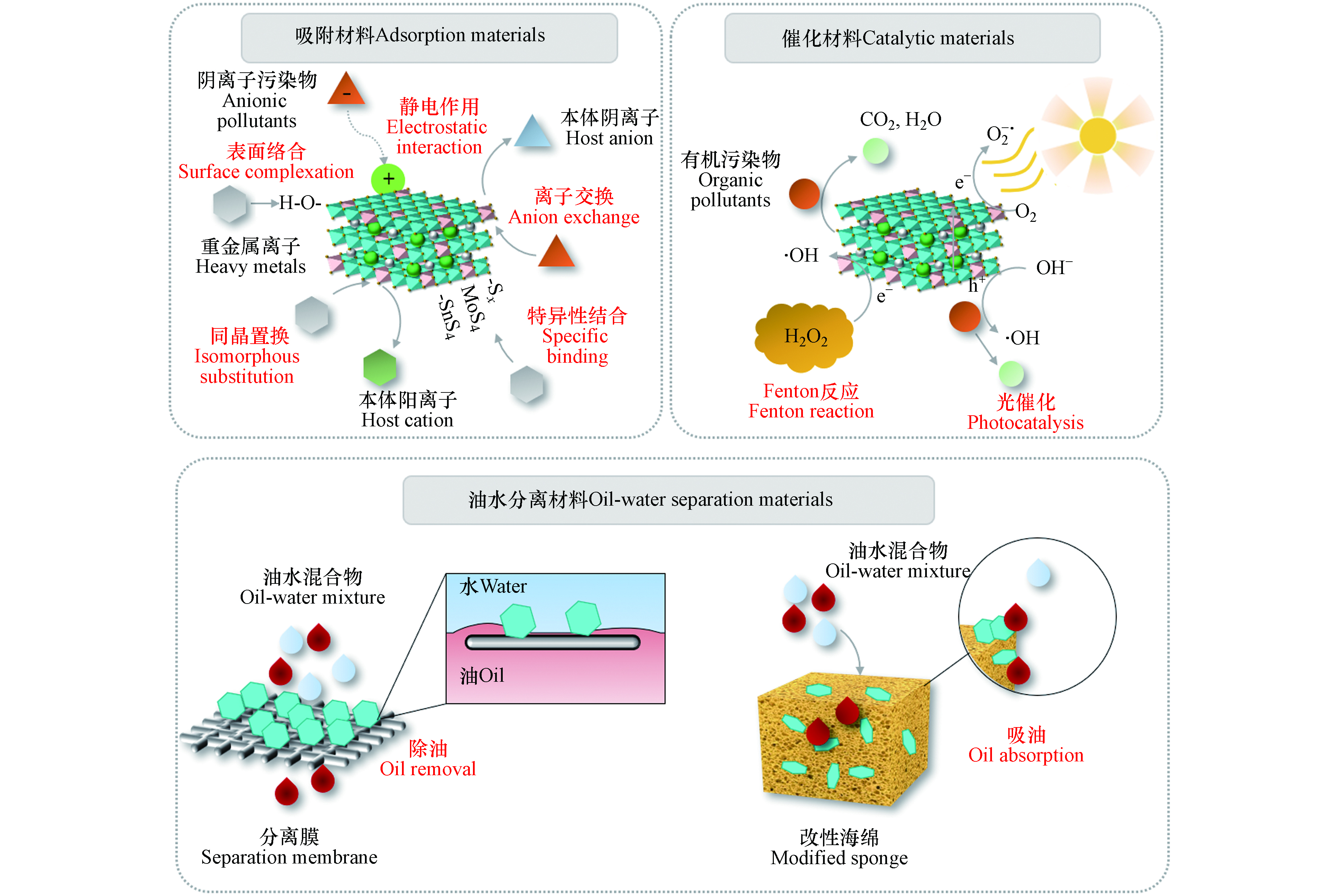
环境中广泛分布的污染物(如重金属离子、卤代烃、石油烃、油污等)在低浓度时即呈现出较高的毒性,严重危害生态安全和人类健康[25].LDHs具有非常灵活的可调控性能,易于在原子尺度上分散和调控污染物反应活性位点,被广泛应用于吸附剂、催化剂、油水分离材料等(图3)[4, 7, 11, 13, 26].在本节中,重点从除磷除氟、重金属离子去除、有机污染物去除、放射性核素去除和油水分离等方面阐述LDHs材料在环境污染修复中的应用.
-
随着氮磷等营养元素排放量的不断增加,水体富营养化问题日益凸显[27].此外,全球范围内的氟污染也危及到数亿人口的生活[28].但由于水体中磷和氟的浓度较低(通常为0.1 mg·L−1)、形态多样(有机和无机态),且环境条件复杂(如温度、pH、离子强度等),因此对于这类污染物的去除具有挑战性.
传统的除磷除氟方法包括沉淀、吸附、膜分离、电化学等方法,然而存在处理效率低、处理成本高、污泥产生量大等缺点.例如,活性炭、黏土等材料容易吸附饱和,需要定期更换或者再生.沉淀的方法处理效率低,且沉淀物难以回收.相比之下,LDHs材料在除磷和除氟方面备受关注[13, 29 − 31],原因在于:(1)LDHs拥有较大的比表面积,从几十到几百m2·g−1不等[13],这意味着它提供了大量的外表面结合位点;(2)LDHs层板上的三价阳离子对二价阳离子的同构取代会产生额外的正电荷,需要插入阴离子来平衡,使其成为吸附磷和氟离子的理想材料[32];(3)LDHs在高pH值下依然具有高稳定性,相较于传统铝基吸附剂克服了溶解失效的问题[33].
利用稀土元素对磷、氟的高亲和力,制备稀土改性的LDHs,可以显著提高除磷除氟能力.Chen等研究人员[34]直接通过共沉淀法制备了含镧(La)的三元LDHs,展现了优异的除氟性能.此外,将La引入LDHs层间也能够得到极佳的除磷效果,比商用除磷剂更具优势[35].层间空间允许LaPO4的形成,同时尺寸效应减少了大分子干扰对吸附反应的不利影响[36],这为LDHs在环境污染修复领域的应用提供了更多可能性.传统除磷剂基于沉淀作用除磷[37],但对于LDHs而言,除磷后其可作为缓释磷肥,用于改良缺乏磷素的土壤[30],具有社会经济效益.
-
重金属离子有毒且不可降解,长期存在于环境中,严重危及生态环境与人体健康.为去除重金属离子,化学沉淀、吸附、膜过滤、离子交换、电渗析等方法被应用,其中吸附法成本低、易操作,广受欢迎.开发低成本、高效率的吸附材料对于重金属离子的去除具有重要意义.
目前,传统吸附剂,如黏土、活性炭、沸石和离子交换树脂已被广泛用于重金属污染土壤和水体的修复.然而,由于这些吸附剂具有有限的吸附位点和单一的吸附机制,因此不能满足吸附速率、容量和选择性等要求.相比之下,LDHs则可通过多种机制有效地去除重金属离子(如图3所示)[38 − 41].例如,对于重金属阴离子,带有正电荷的LDHs可以通过静电吸引阴离子,进而通过阴离子交换去除Cr(Ⅵ)和As(Ⅲ)等.LDHs还可以利用煅烧后的“记忆效应”有效去除重金属阴离子[42].对于重金属阳离子,表面络合和化学沉淀是重要的吸附机制.由于LDHs中含有大量的羟基,因此易于与重金属离子形成化学键,从而形成金属络合物和氢氧化物沉淀[43].此外,LDHs中的缺陷位点和可变的层板组成能够实现对Cd(Ⅱ)、Ni(Ⅱ)、Pb(Ⅱ)等离子的选择性离子交换[43].由于晶格锁定,重金属离子不易再次释放,因此可以实现重金属污染土壤的原位修复[44].这种同晶置换作用被称为LDHs对重金属离子的“超稳矿化”.此外,LDHs可以利用阴离子交换特性将客体分子引入层间,利用客体分子与重金属的特异性结合实现重金属离子去除[45 − 49].
近年来,金属有机框架(MOFs)、共价有机框架(COFs)、氧化石墨烯(GO)和过渡金属氮/碳化合物(MXene)等新兴材料已被研究用于重金属离子去除.然而,有机框架材料的原料中含有机试剂,GO需要使用强酸氧化,制备MXene需要使用HF溶液.这些材料制备过程所需的试剂可能对人体和环境造成危害,并且它们的制备成本高、方法繁琐,暂时无法大规模生产.相比之下,LDHs具有制备方法简便、有利于规模化生产等优点.目前,段雪院士团队开发的成核晶化隔离法已实现了LDHs的产业化合成,并在江苏、山东等地建立了多条生产线[19].因此,LDHs吸附材料在水体重金属离子去除和土壤重金属污染治理方面具有广阔的应用前景.
-
在环境中,多环芳烃、卤代烃、石油烃、内分泌干扰物和抗生素等有机污染物难以分解,并在生态系统中容易积累,给人类健康和生态系统带来巨大威胁.吸附法或催化降解是常用的有机污染物去除技术.LDHs因其独特的结构和性质可以用作各种有机污染物的吸附剂[50 − 52].作为吸附剂,LDHs的吸附性能与反应条件密切相关,反应温度、体系pH、吸附剂用量、接触时间和初始浓度等都会对吸附性能产生影响.例如,LDHs对活性红和刚果红的吸附量随着温度升高而增加,是由于复合材料的孔径增大和表面活性变化所导致[53].体系pH通过改变LDHs和污染物的电荷属性进而影响二者之间的吸附作用,例如,在低pH值下,正电荷的LDHs与负电荷的双氯芬酸钠通过静电引力产生强烈的吸附作用[54].
除了作为吸附剂,LDHs还可以作为类芬顿催化剂和光催化剂用于有机污染物的降解和处理(图3).传统的类芬顿反应主要以铁、钴等活化H2O2,在酸性条件下才能发挥其催化活性,而过高或过低的pH值都会降低其催化效率,这限制了其在不同环境条件下的应用范围.同时金属离子在一定条件下可能会溶解在反应液中,从而造成二次污染.LDHs类芬顿催化剂具有诸多优点,例如,层状结构可使金属离子有序分布于层板之间,有效发挥催化作用[55].其pH缓冲能力有利于体系维持中性,产生各种活性氧[56].LDHs催化剂结构稳定,降低了金属浸出率[57].此外,LDHs易与其他材料(如碳基、半导体和磁性材料)构筑成复合材料,有利于提高稳定性、导电性和催化性能[57].
LDHs的光催化剂在有机污染物的光催化去除中已被广泛应用[58, 59],常见的有CuCr-LDH、ZnTi-LDH、ZnFe-LDH等.通过调控LDHs层板上金属离子成分和配比,使材料表面存在能带跃迁所需的电子能级区域,从而获得光催化性能.与其他光催化剂相比(金属硫化物、半导体氧化物、MOFs、COFs),LDHs光催化剂具有以下优势:制备工艺简便,成本低,大规模的合成操作也相对容易;通过改变合成条件、控制反应时间等方法来调节其形貌、结构和表面性质,能调控其光催化活性;具有坚固的层状结构和化学稳定性,能够承受高温、强酸、强碱及其他恶劣的环境条件,提高了光催化剂的使用寿命和稳定性.
总之,LDHs具有很高的实际应用价值,可以作为吸附剂、类芬顿催化剂和光催化剂用于有机污染物的吸附与降解.随着研究的不断深入,我们相信LDHs在环境有机污染修复领域的应用前景将会更加广阔.
-
随着核电工业的发展和应用,放射性废物的快速产生已经成为环境污染的严重问题.这些放射性核素可能随着降雨而迁移至周围的土壤和地表水中,给环境和人类健康带来长期放射性危害[11, 60].由于放射性核素存在多种氧化态并能形成不同的复杂物相,因此其在环境中的转化过程存在较大不确定性,为污染防治带来了巨大的挑战[60].在治理放射性污染问题上,吸附法因其简单易行、成本较低等优点成为了备受关注的技术手段.因此,研发低成本、高效率和抗干扰的吸附材料是目前吸附研究的热点和重点之一.
传统的吸附材料如黏土、活性炭等虽然也能起到一定的吸附作用,但效率低、容量小、选择性差,很难满足放射性污染治理的需求.近年来,GO、MOFs和COFs等热门吸附剂吸引了众多研究者对其在放射性核素去除方面的应用进行研究.然而,这些吸附剂存在一些关键限制,例如GO可能会对水体造成二次污染,并且存在一定的生物毒性.而MOFs和COFs的制备过程繁琐,且其较差的耐酸碱性制约了它们在极端环境中的应用.相较之下,LDHs作为一种绿色、高效且易制备的放射性核素去除材料备受关注.利用离子交换、静电引力、表面络合和配位作用等多种作用机制,LDHs可有效地捕获U(Ⅵ)、Tc(Ⅶ)和Re(Ⅶ)等放射性核素[38, 61 − 64].例如,Wang等[62]报道了碳球@LDH通过层板的金属氧基团和碳球上的含氧基团协同作用去除U(Ⅵ),饱和吸附量超过原始LDH吸附量2倍以上.Celik等[63]制备的LDH-Mo3S13通过还原沉淀、离子交换和表面吸附的多重机制作用去除Cr(Ⅵ)(Tc(Ⅶ)的替代物),饱和吸附量达到225 mg·g−1,远超过CoAl-LDH的93 mg·g−1.LDHs稳定的骨架结构还使其具备一定的耐酸碱性能,可用于污染土壤和地下水的原位修复.总之,LDHs作为放射性核素去除材料,具有十分广阔的应用前景.在今后的研究中,探索更多的改性技术和手段提高其污染物去除效率和选择性,将为更好地消除放射性核素污染提供坚实的技术基础.
-
目前,随着工业和社会经济的迅速发展,石油泄漏和工业有机溶剂排放越来越严重,给生态环境造成了严重的污染,成为全球亟待解决的问题.现今的处理技术包括过滤、浮选、絮凝、沉降等,使用的材料有聚合物纤维和吸油凝胶等,但它们存在分离效果不佳、操作成本较高等缺点.相比之下,LDHs材料具有高的吸附能力、重复使用性和对环境的友好等优点,同时不会对水质产生二次污染,使其在油水分离领域具有广阔的应用前景.此外,LDHs材料的制备成本相对较低,可以通过简单的合成方法进行大规模生产,对于解决油污污染问题具有重要意义.
超疏水/超亲油膜作为一种低成本、占地面积小、易于连续操作的分离纯化材料,受到了广泛的关注.利用有机离子插层改性LDHs可以显著提高膜疏水性[65 − 66].同时,LDHs的微纳米结构和丰富的表面羟基使其可以生长在基体表面,进一步提高材料表面粗糙度,获得超疏水表面[67, 68].例如,Liu等[67]利用原位生长方法将LDHs固定在商业织物的微纤维上,形成巢状微结构的LDHs改性的超疏水和超亲油织物(图3).LDHs的层级结构和低表面能分子的结合使纺织品具有超疏水性和超亲油性,不仅可以作为高效分离油水混合物的膜材料,还能够从水中选择性吸附油污.此外,制备的NiCo-LDH/PVDF复合膜具有超亲水性/水下超疏油性和较低的油粘附性能,主要归因于高的表面能和纳米NiCo-LDH的草状结构[68].复合膜可以处理多种油水乳液,分离效率在10次循环后仍高达99.5%以上,且膜形态未被破坏.
传统的二维膜普遍具有小孔径和短渗透通道的结构,容易受到表面活性剂吸附和孔堵塞等影响造成污染,然后导致通量和分离效率明显下降.LDHs材料可用于开发具有可调孔径和互连渗透路径的三维多孔结构.例如,Tian等[69]制备了超疏水的LDHs纳米片,将其负载到聚氨酯海绵上得到超疏水海绵,疏水的LDHs纳米片可以降低海绵表面能,显著提高表面粗糙度,使改性海绵具有超疏水性和亲油性(图3).改性海绵对各种有机溶剂和油污表现出优异的吸附能力,油水分离效率高达99.6%,其吸附容量达到其自身重量的31倍.改性海绵在10次吸收循环后对有机溶剂和油污的吸附能力几乎没有变化,显示了较高的重复使用能力.
-
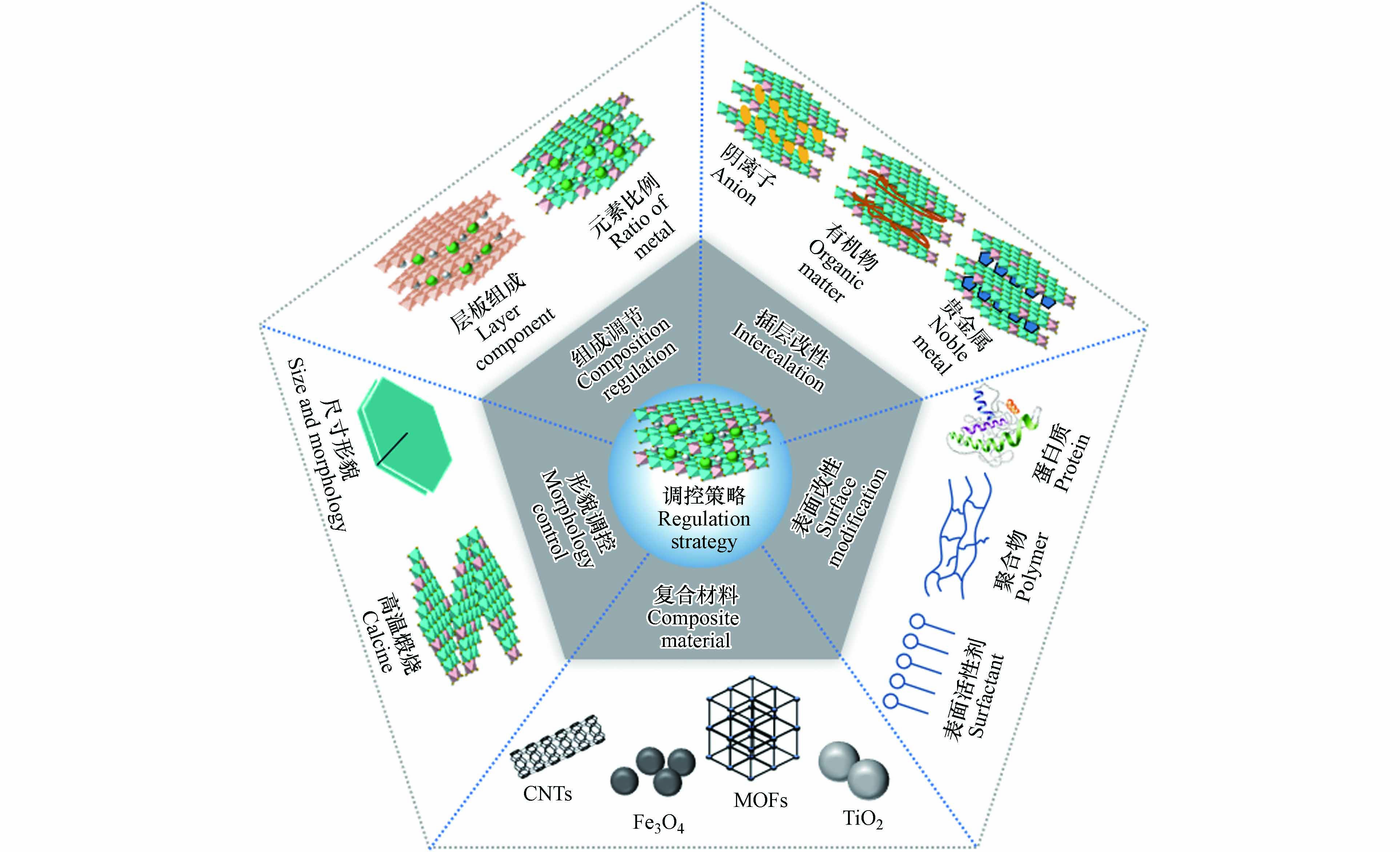
尽管已经有大量的研究报道了LDHs可用于环境污染修复,但其在实际应用中存在几个关键问题,例如:(1)LDHs在水中容易发生团聚,导致活性位点损失和处理效率下降[70];(2)原始的LDHs材料的污染物活性位点数量有限,限制了其污染物去除效率;(3)在复杂的环境介质中,存在多种有机物质和无机离子,它们会影响LDHs对目标污染物的选择性[71];(4)LDHs材料本身导电性较低,能带间隙较大,因此其在直接使用过程中表现出的催化效率通常较低[72];(5)由于LDHs材料粉状的特点,其难以应用于水处理后的分离和回收利用.因此,可以通过一系列的调控和改性策略来解决上述问题,并有效提高LDHs材料的污染物修复效率(图4).
-
LDHs材料凭借优良的化学稳定性、高比表面积以及特殊的阴阳离子组成结构,被广泛应用于环境污染修复领域.尽管其高Zeta电位能够带来静电斥力,提高分散性和稳定性,但是在一些特定的情况,如较高pH条件或阴离子吸附下,LDHs表面正电荷的减少会导致其颗粒团聚,降低其去除污染物的效率[70].因此,提升LDHs材料的分散性十分关键.常见的策略包括增强颗粒间的静电斥力、提高其与分散介质的亲和力以及引入空间位阻等[73 − 74].通过表面修饰材料(如表面活性剂、高分子聚合物和蛋白质等)包裹LDHs颗粒(图4),可以增加材料表面官能团,从而改变静电斥力和空间位阻,提高其稳定性[70, 75].
此外,将LDHs负载到其他基质材料(如TiO2、CNTs、Fe3O4、MOFs和黏土等)上也是提升LDHs分散性的有效方式(图4).例如,通过LDHs逐层沉积和原位生长技术,Shao等[76]成功合成了具有花状结构且分散均匀的LDHs微球.Wang等[77]的研究表明,将LDHs负载到凹凸棒石表面可以增加复合吸附剂对U(Ⅵ)的吸附效率,表面电荷亲和力可以增强材料结构稳定性,团聚现象也得以抑制.这些策略不仅可以有效提升LDHs材料的分散性,也可以提高其对目标污染物的去除效率,为LDHs的应用提供了更有力的支持.
-
材料反应活性位点数量直接影响其吸附容量和催化效率.因此,合理设计并增加LDHs材料的反应活性位点非常重要.一种简单有效的方法是形貌调控(图4),通过改进的方法可以得到形貌不同的LDHs,如纳米环、纳米卷、纳米棒和纳米线等[78 − 81].与传统的片状LDHs相比,这些三维结构的LDHs具有更大的比表面积和更多的活性位点.例如,LDHs纳米花的U(Ⅵ)吸附量是LDHs纳米片的2.5倍[82].另外一种策略是将LDHs煅烧成为LDO(图4),利用其在水中再水化、结构重建,实现高效捕获污染物[17, 83].研究表明,随着煅烧温度的升高,大量金属氧化物键在吸附剂的表面上出现,可以明显提高了LDO对U(Ⅵ)的吸附能力[84].另外,构建复合材料,如纤维素/LDH、LDH@碳纳米球、超顺磁微粒/LDH和LDH/GO气凝胶等,可以有效分散LDHs,创造更多的活性位点,大幅提高对污染物的去除效率.与原始的LDHs粉体相比,这些复合材料在操作可行性和稳定性方面具有更好的适用性[43, 85, 86].
除了形貌调控、煅烧和复合材料的方法外,还有其他方法可以增加LDHs的反应活性位点数量.例如,引入缺陷结构、掺杂离子和调节晶面等方法,可以增加LDHs表面的催化活性位点[59, 72].此外,调节LDHs的组成也可以影响其反应活性位点数量和催化效率.例如,Zn/Al比例为4的LDHs呈现柱状形貌,具有最大的比表面积,反应活性位点数量也最大[87].因此,在设计和合成LDHs材料时,需要考虑其组成、形貌、结构以及与其他材料的组合方式,以最大程度地提高其反应活性位点数量和催化效率.
-
环境中的各种污染物、无机离子与天然有机质之间经常存在着复杂的共存现象,这使得在复杂环境条件下,原始LDHs材料对于污染物的选择性去除变得困难.因此,设计和制备能够对目标污染物具有较高选择性去除能力且具有抗干扰性的LDHs材料,是一个十分严峻的挑战.为了解决这一问题,研究人员采用多种方法赋予LDHs更多表面官能团,以捕获目标金属离子.例如,Li等学者[88]报道了一种新型的多功能纳米材料,即磁性聚多巴胺-LDH,具备消除Cu(Ⅱ)和阴离子染料的双重功能.这是由于Cu(Ⅱ)与表面官能团(如羟基、酚羟基、亚胺和胺基)之间的相互作用实现的.另一项研究表明,尿素功能化的Fe3O4@LDH能够高效去除水中的有机磷阻燃剂,其中,尿素和污染物之间的π-π相互作用是关键的吸附机制[89].此外,无机物的官能团也可以对LDHs进行表面修饰.例如,研究人员用NaAlF6制备了氟化MgAl-LDH,引入氟离子可提高LDHs的碱性位点和铝密度,有利于对硝基甲烷的吸附[90].
除了使用有机或无机官能团改性外,插层改性也是一种常见的改性策略(图4).通过将具有特异性污染物结合能力的离子或大分子物质插入到LDHs层间,可以实现对目标污染物的选择性去除.插层改性可以通过离子交换法、煅烧重构法和共沉淀法来实现(图2).插层改性LDHs对污染物的去除机制包括两步反应:首先污染物扩散到LDHs层间,随后插层离子与污染物作用将其固定[91].Kanatzidis团队[38, 46, 63, 92]报道了一系列含硫化合物(如
${\rm{S}}_x^{2-} $ 、${\rm{SnS}}_4^{4-} $ 、${\rm{MoS}}_4^{2-} $ 和${\rm{Mo}}_3 {\rm{S}}_{13}^{2-} $ 等)插层改性的LDHs.插入的硫离子团簇增加了LDHs的层间间距,扩大比表面积并形成分级孔道,从而增强了对污染物的吸附能力.利用硫离子团簇与污染物的特异性结合实现了对重金属离子、金属含氧酸和放射性核素等污染物的高效和选择性吸附.值得注意的是,即使存在浓度比U(Ⅵ)高4个数量级的共存Na+和Ca2+,Sx-LDH仍然表现出对U(Ⅵ)的高选择性吸附[38]. -
LDHs光催化剂的电荷转移率较低,导致光诱导的电荷载流子重组较为严重.为提高LDHs的光诱导电子-空穴分离效率,需要对其进行调控.一种调控方法是通过引入过渡金属作为其层板组成元素来增强其电荷转移效率(图4).研究发现,双过渡金属的LDHs相比单过渡金属的类型具有更低的带隙能以及更高的电导率,这归因于不同跃迁原子三维轨道之间的杂化[93].另一种提高光吸收和电荷传输能力的方法是将贵金属元素(如Au、Ag、Pt、Pd和Ru等)或稀土元素(Ce、Tb和La等)锚定在层板或晶格中(图4)[94 − 96].这些元素本身可以传导电子,且具有优异的给电子的能力. 例如,在Au/ZnAl-LDH中,表面的Au(Ⅲ)能够很好地接受来自LDHs表面的电子,形成Au(0)纳米颗粒,从而增强光催化降解苯酚的效率[96].此外,贵金属和稀土元素的掺杂能使LDHs产生晶格畸变,有利于增强光诱导的电子-空穴对的分离[72].例如,掺杂Tb(Ⅲ)的ZnCr-LDH具有更好的光诱导载流子的分离能力和转移性能,其光催化效率可提高2倍[94].
此外,将半导体与LDHs复合可以提高其催化性能.由于复合过程会形成异质结,能提高受激电子和空穴的分离效率,从而抑制电荷载流子的重组,提高光催化活性[72].异质结结构的光催化剂在废水处理中具有巨大潜力.例如,BiOCl/NiFe-LDH相比原始LDHs具有更高的降解罗丹明B的光催化活性[97].这是因为LDHs的导带中的光诱导电子可以迁移到BiOCl的价带中,形成电子-空穴对.同时,分散良好的BiOCl颗粒能促进LDHs的羟基产生自由基来促进降解.另一项研究制备的ZnS/LDH光催化剂中,ZnS上的电子易于转移到LDHs上,从而促进了光生电子-空穴对的重组,增强了对罗丹明B的光降解活性[98].
-
环境污染修复中,一个普遍存在的问题是修复材料的成本较高,这激发了人们对修复材料分离和回收利用的兴趣.其中一个方法是制备LDHs复合材料.LDHs材料与载体组合使用时需要选择合适的载体,以提高LDHs材料的分离和回收效率.在选择载体时应考虑其物理化学性质,如比表面积、孔径分布和表面活性等.SiO2、TiO2、Fe3O4、MOFs、黏土和活性炭等被广泛应用于LDHs复合材料的制备(图4).载体的选择与LDHs材料的配合是需要考虑的重要因素.例如,当LDHs复合材料具有磁性时,可以在外加磁场下实现磁性分离,实现多次回收利用[76, 99].
目前,研究人员正在探索各种新型材料的设计和制备方法,包括结构合理化设计、新型多功能薄膜材料和多功能三维海绵等[67 − 69].这些新型材料不仅可以提高LDHs材料在环境修复应用后的分离和回收效率,同时可以改善LDHs材料的功能化,使之更适用于不同种污染物的修复,提高整体处理效果.
另外,可持续回收利用策略也应用于LDHs材料的回收中.这是指将LDHs材料从环境介质中分离出来,清洗、加工、转化等再利用.目前广泛采用的可持续回收利用策略包括:制备新型材料、制备肥料、制备功能性膜等[30, 44].例如,CaFe-LDH材料在高效处理含Ni(II)废水后,所得NiCaFe-LDH显示出增强的电催化析氧和光催化还原CO2的能力[44].
-
综上所述, LDHs材料在环境污染修复方面的应用潜力巨大,其结构稳定、可调控性优异和成本低廉,已广泛应用于污染物吸附、催化降解和油水分离等领域.尽管已取得了显著进展,但在实际应用中仍存在易团聚、低效率、易干扰和难分离等问题.因此,仍需进一步研究和开发各类性能调控策略,以有效提升LDHs的吸附和催化性能,推动其在环境修复中不断发展.为了进一步发挥LDHs作为环境污染修复材料的应用潜力,需要在以下几个方面加强研究:
(1)尽管LDHs材料的吸附和催化机理已得到广泛报道,但针对不同污染物和反应体系,仍然缺乏对LDHs结构性质变化的深入分析.未来的工作应进一步借助同步辐射、模型拟合、理论计算和机器学习等手段,以阐明LDHs材料的构效关系,为功能化LDHs材料的开发奠定基础.
(2)目前,LDHs材料的制备方法可能会引发二次污染问题.在LDHs材料制备和性能调控方面,有待充分遵循绿色化学品制备原则,采用无毒、无害原料,优化设计合成过程,减少副产物生成,实现LDHs材料制备和调控过程的绿色、低碳.
(3)目前,关于LDHs材料的环境污染修复研究大多集中在单一污染物的处理.然而,环境污染往往面临多污染物并存、污染转化过程叠加的问题.未来的研究需要加强LDHs材料在多金属复合污染、有机无机复合污染和有机复合污染的修复方面的应用研究.
(4)已有的实验室盆栽试验和短期田间试验证实了LDHs材料的土壤原位修复效果良好.然而,对于LDHs材料在真实污染修复中的信息仍然了解不足,亟待开展真实环境条件下LDHs修复效果的评估、LDHs修复后对土壤长期物化特性的影响以及修复后污染物长期固定的稳定性等方面的系统探究.
层状双氢氧化物在环境修复中的应用与调控策略
Application and regulation strategies of layered double hydroxides in environmental remediation
-
摘要: 环境污染问题已成为当前突出的全球性挑战,严重威胁人体健康和生态安全,迫切需要开发绿色、高效的修复材料去除环境污染物.层状双氢氧化物(LDHs)是一类天然矿物材料,具有良好的环境兼容性、低廉的成本和灵活的可调控性,而且制备方法简单,适宜于规模化生产,其在污染物的吸附固定、催化降解和相分离方面有着广阔的应用前景.本文总结了LDHs材料的结构、性质和主要的制备方法,综述了近年来LDHs材料在固定和消减环境中磷、氟、重金属、有机污染物和放射性核素等污染物的应用进展,针对LDHs应用中存在的问题,重点分析了材料的性能调控策略并对未来发展方向进行了展望.发展和推广LDHs材料可为环境修复功能材料的研发提供新的思路和方法,从而推动绿色、循环、低碳环境修复产业的持续发展.Abstract: Environmental pollution has become a prominent global challenge, which seriously threatens human health and ecological security. It is urgent to develop green and efficient remediation materials to remove environmental pollutants. Layered double hydroxides (LDHs) are a class of natural mineral materials with excellent environmental compatibility, low cost and flexible modifiability. They are simple to prepare and suitable for large-scale production. LDHs have broad application prospects in the adsorption and immobilization, catalytic degradation, and phase separation of contaminants. In this paper, the structure, properties, and main preparation methods of LDHs were summarized, and the application progresses of LDHs in immobilizing and reducing contaminants such as phosphorus, fluorine, heavy metals, organic pollutants, and radioactive nuclides in the environment in recent years were reviewed. In consideration of the problems encountered in the application of LDHs, the performance regulation strategies of materials were emphatically analyzed and the future development directions were prospected. The development and promotion of LDHs could provide new ideas and methods for the research and development of environmental remediation functional materials, thus promoting the sustainable development of the green, circular, and low-carbon environmental remediation industries.
-
Key words:
- layered double hydroxides /
- environmental remediation /
- adsorption /
- catalysis /
- regulation strategy.
-

-
[1] 中华人民共和国环境保护部, 国土资源部. 全国土壤污染状况调查公报 [EB/OL]. [2014-04-17]. http://www.gov.cn/foot/2014-04/17/content_2661768.htm. Ministry of Environmental Protection and Ministry of Land and Resources of the People's Republic of China. National soilpollution survey bulletin. [EB/OL]. [2014-04-17]. http://www.gov.cn/foot/2014-04/17/content_2661768.htm.
[2] 中华人民共和国生态环境部. 2021中国生态环境状况公报 [EB/OL]. [2022-05-27]. https://www.mee.gov.cn/hjzl/sthjzk/ Ministry of Ecology and Environment of the People's Republic of China. 2021 China's state of the environment report [EB/OL]. [2022-05-27]. https://www.mee.gov.cn/hjzl/sthjzk/
[3] WANG Q, O'HARE D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets [J]. Chemical Reviews, 2012, 112(7): 4124-4155. doi: 10.1021/cr200434v [4] LI C M, WEI M, EVANS D G, et al. Layered double hydroxide-based nanomaterials as highly efficient catalysts and adsorbents [J]. Small, 2014, 10(22): 4469-4486. doi: 10.1002/smll.201401464 [5] GU Z, ATHERTON J J, XU Z P. Hierarchical layered double hydroxide nanocomposites: structure, synthesis and applications [J]. Chemical Communications, 2015, 51(15): 3024-3036. doi: 10.1039/C4CC07715F [6] KONG X G, HAO P P, DUAN H H. Super-stable mineralization effect of layered double hydroxides for heavy metals: Application in soil remediation and perspective [J]. Exploration, 2021, 1(2): 20210052. doi: 10.1002/EXP.20210052 [7] ZUBAIR M, DAUD M, MCKAY G, et al. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation [J]. Applied Clay Science, 2017, 143: 279-292. doi: 10.1016/j.clay.2017.04.002 [8] PANG H W, WU Y H, WANG X X, et al. Recent advances in composites of graphene and layered double hydroxides for water remediation: A review [J]. Chemistry-an Asian Journal, 2019, 14(15): 2542-2552. doi: 10.1002/asia.201900493 [9] JIJOE P S, YASHAS S R, Shivaraju H P. Fundamentals, synthesis, characterization and environmental applications of layered double hydroxides: A review [J]. Environmental Chemistry Letters, 2021, 19(3): 2643-2661. doi: 10.1007/s10311-021-01200-3 [10] ZHAO M Q, ZHANG Q, HUANG J Q, et al. Hierarchical nanocomposites derived from nanocarbons and layered double hydroxides - properties, synthesis, and applications [J]. Advanced Functional Materials, 2012, 22(4): 675-694. doi: 10.1002/adfm.201102222 [11] GU P C, ZHANG S, LI X, et al. Recent advances in layered double hydroxide-based nanomaterials for the removal of radionuclides from aqueous solution [J]. Environmental Pollution, 2018, 240: 493-505. doi: 10.1016/j.envpol.2018.04.136 [12] JOBBAGY M, IYI N. Interplay of charge density and relative humidity on the structure of nitrate layered double hydroxides [J]. Journal of Physical Chemistry C, 2010, 114(42): 18153-18158. doi: 10.1021/jp1078778 [13] GOH K H, LIM T T, DONG Z L. Application of layered double hydroxides for removal of oxyanions: A review [J]. Water Research, 2008, 42(6-7): 1343-1368. doi: 10.1016/j.watres.2007.10.043 [14] ZHONG Y, YANG Q, LUO K, et al. Fe(II)-Al(Ⅲ) layered double hydroxides prepared by ultrasound-assisted co-precipitation method for the reduction of bromate[J]. [J]. Journal of Hazardous Materials, 2013, 250: 345-353. [15] GOMES SILVA C, BOUIZI Y, FORNES V, et al. Layered double hydroxides as highly efficient photocatalysts for visible light oxygen generation from water [J]. Journal of the American Chemical Society, 2009, 131(38): 13833-13839. doi: 10.1021/ja905467v [16] 廖玉梅, 余杰, 魏世强, 等. FeMnNi-LDHs对水中As(Ⅲ)的吸附性能与机制[J]. [J]. 环境科学, 2021, 42((1): ): 293-304. LIAO Y M, YU J, WEI S Q, et al. Adsorption effect and mechanism of aqueous arsenic on FeMnNi-LDHs[J]. [J]. Environmental Science, 2021, 42((1): ): 293-304 (in Chinese).
[17] EIBY S H J, TOBLER D J, NEDEL S, et al. Competition between chloride and sulphate during the reformation of calcined hydrotalcite [J]. Applied Clay Science, 2016, 132: 650-659. [18] ZHAO Y, LI F, ZHANG R, et al. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps [J]. Chemistry of Materials, 2002, 14(10): 4286-4291. doi: 10.1021/cm020370h [19] 李天, 郝晓杰, 白莎, 等. 单层类水滑石纳米片的可控合成及规模生产展望 [J]. 物理化学学报, 2020, 36(9): 71-87. LI T, HAO X J, BAI S, et al. Controllable synthesis and scale-up production prospect of monolayer layered double hydroxide nanosheets [J]. Acta Physico-Chimica Sinica. 2020, 36(9): 71-87 (in Chinese).
[20] OGAWA M, ASAI S. Hydrothermal synthesis of layered double hydroxide-deoxycholate intercalation compounds [J]. Chemistry of Materials, 2000, 12(11): 3253-3255. doi: 10.1021/cm000455n [21] FETTER G, HERNÁNDEZ F, MAUBERT A M, et al. Microwave irradiation effect on hydrotalcite synthesis [J]. Journal of Porous Materials, 1997, 4: 27-30. doi: 10.1023/A:1009619005529 [22] YAN K, LIU Y Q, LU Y R, et al. Catalytic application of layered double hydroxide-derived catalysts for the conversion of biomass-dderived molecules [J]. Catalysis Science & Technology, 2017, 7(8): 1622-1645. [23] QU J, ZHANG Q W, LI X W, et al. Mechanochemical approaches to synthesize layered double hydroxides: a review [J]. Applied Clay Science, 2016, 119: 185-192. doi: 10.1016/j.clay.2015.10.018 [24] MI J X, CHEN X P, ZHANG Q Y, et al. Mechanochemically synthesized MgAl layered double hydroxide nanosheets for efficient catalytic removal of carbonyl sulfide and H2S [J]. Chemical Communications, 2019, 55(63): 9375-9378. doi: 10.1039/C9CC03637G [25] SCHWARZENBACH R P, ESCHER B I, FENNER K, et al. The challenge of micropollutants in aquatic systems [J]. Science, 2006, 313(5790): 1072-1077. doi: 10.1126/science.1127291 [26] TRAN H N, NGUYEN D T, LE G T, et al. Adsorption mechanism of hexavalent chromium onto layered double hydroxides-based adsorbents: A systematic in-depth review [J]. Journal of Hazardous Materials, 2019, 373: 258-270. doi: 10.1016/j.jhazmat.2019.03.018 [27] MAYER B K, BAKER L A, BOYER T H, et al. Total value of phosphorus recovery [J]. Environmental Science & Technology, 2016, 50(13): 6606-6620. [28] YADAV K K, KUMAR S, QUOC BAO P, et al. Fluoride contamination, health problems and remediation methods in Asian groundwater: A comprehensive review [J]. Ecotoxicology and Environmental Safety, 2019, 182: 109362. doi: 10.1016/j.ecoenv.2019.06.045 [29] YAN H L, CHEN Q W, LIU J H, et al. Phosphorus recovery through adsorption by layered double hydroxide nano-composites and transfer into a struvite-like fertilizer [J]. Water Research, 2018, 145: 721-730. doi: 10.1016/j.watres.2018.09.005 [30] EVERAERT M, WARRINNIER R, BAKEN S, et al. Phosphate-exchanged Mg-Al layered double hydroxides: A new slow release phosphate fertilizer [J]. ACS Sustainable Chemistry & Engineering, 2016, 4(8): 4280-4287. [31] 吴俊麟, 林建伟, 詹艳慧, 等. 镁铁层状双金属氢氧化物对磷酸盐的吸附作用及对内源磷释放的控制效果及机制 [J]. 环境科学, 2020, 41(1): 273-284. doi: 10.13227/j.hjkx.201907174 WU J L, LIN J W, ZHAN Y H, et al. Adsorption of phosphate on Mg/Fe layered double hydroxides (Mg/Fe-LDH) and use of Mg/Fe-LDH as an amendment for controlling phosphorus release from sediments [J]. Environmental Science, 2020, 41(1): 273-283 (in Chinese). doi: 10.13227/j.hjkx.201907174
[32] REN C, ZHOU M Z, LIU Z M, et al. Enhanced fluoride uptake by layered double hydroxides under alkaline conditions: Solid-state NMR evidence of the role of surface >MgOH sites [J]. Environmental Science & Technology, 2021, 55(22): 15082-15089. [33] AYOOB S, GUPTA A K, BHAT V T. A conceptual overview on sustainable technologies for the defluoridation of drinking water [J]. Critical Reviews in Environmental Science and Technology, 2008, 38(6): 401-470. doi: 10.1080/10643380701413310 [34] CHEN P, WANG T, XIAO Y, et al. Efficient fluoride removal from aqueous solution by synthetic Fe-Mg-La tri-metal nanocomposite and the analysis of its adsorption mechanism [J]. Journal of Alloys and Compounds, 2018, 738: 118-129. doi: 10.1016/j.jallcom.2017.12.142 [35] ZHANG Y Y, KONG B, SHEN Z Y, et al. Phosphorus binding by lanthanum modified pyroaurite-like clay: Performance and mechanisms [J]. ACS ES&T Engineering, 2021, 1(11): 1565-1575. [36] ZHANG Y Y, WANG M L, GAO X, et al. Structural evolution of lanthanum hydroxides during long-term phosphate mitigation: Effect of nanoconfinement [J]. Environmental Science & Technology, 2021, 55(1): 665-676. [37] ASHEKUZZAMAN S M, JIANG J Q. Study on the sorption-desorption-regeneration performance of Ca-, Mg- and CaMg-based layered double hydroxides for removing phosphate from water [J]. Chemical Engineering Journal, 2014, 246: 97-105. doi: 10.1016/j.cej.2014.02.061 [38] MA S L, HUANG L, MA L, et al. Efficient uranium capture by polysulfide/layered double hydroxide composites [J]. Journal of the American Chemical Society, 2015, 137(10): 3670-3677. doi: 10.1021/jacs.5b00762 [39] ZHOU H G, TAN Y L, YANG Y M, et al. Application of FeMgMn layered double hydroxides for phosphate anions adsorptive removal from water [J]. Applied Clay Science, 2021, 200: 105903. doi: 10.1016/j.clay.2020.105903 [40] LIANG X F, ZANG Y B, XU Y M, et al. Sorption of metal cations on layered double hydroxides [J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2013, 433: 122-131. [41] GOH K-H, LIM T-T, DONG Z L. Enhanced arsenic removal by hydrothermally treated nanocrystalline Mg/Al layered double hydroxide with nitrate intercalation [J]. Environmental Science & Technology, 2009, 43(7): 2537-2543. [42] LV Z M, YANG S M, ZHU H S, et al. Highly efficient removal of As(V) by using NiAl layered double oxide composites [J]. Applied Surface Science, 2018, 448: 599-608. doi: 10.1016/j.apsusc.2018.04.162 [43] GONG J M, LIU T, WANG X Q, et al. Efficient removal of heavy metal ions from aqueous systems with the assembly of anisotropic layered double hydroxide nanocrystals@carbon nanosphere [J]. Environmental Science & Technology, 2011, 45(14): 6181-6187. [44] CHI H Y, WANG J K, WANG H J, et al. Super-stable mineralization of Ni2+ ions from wastewater using CaFe layered double hydroxide [J]. Advanced Functional Materials, 2022, 32(5): 2106645. doi: 10.1002/adfm.202106645 [45] MA S L, CHEN Q M, LI H, et al. Highly selective and efficient heavy metal capture with polysulfide intercalated layered double hydroxides [J]. Journal of Materials Chemistry A, 2014, 2(26): 10280-10289. doi: 10.1039/C4TA01203H [46] MA L J, WANG Q, ISLAM S M, et al. Highly selective and efficient removal of heavy metals by layered double hydroxide intercalated with the MoS42- ion [J]. Journal of the American Chemical Society, 2016, 138(8): 2858-2866. doi: 10.1021/jacs.6b00110 [47] YANG L X, XIE L X, CHU M L, et al. Mo3S132- intercalated layered double hydroxide: Highly selective removal of heavy metals and simultaneous reduction of Ag+ ions to metallic Ag0 ribbons [J]. Angewandte Chemie-International Edition, 2021, 61(1): e202112511. [48] CHEN H, LIN J H, ZHANG N, et al. Preparation of MgAl-EDTA-LDH based electrospun nanofiber membrane and its adsorption properties of copper(II) from wastewater [J]. Journal of Hazardous Materials, 2018, 345: 1-9. doi: 10.1016/j.jhazmat.2017.11.002 [49] CELIK A, BAKER D R, ARSLAN Z, et al. Highly efficient, rapid, and concurrent removal of toxic heavy metals by the novel 2D hybrid LDH-[Sn2S6] [J]. Chemical Engineering Journal, 2021, 426: 131696. doi: 10.1016/j.cej.2021.131696 [50] DAUD M, HAI A, BANAT F, et al. A review on the recent advances, challenges and future aspect of layered double hydroxides (LDH) - Containing hybrids as promising adsorbents for dyes removal [J]. Journal of Molecular Liquids, 2019, 288: 110989. doi: 10.1016/j.molliq.2019.110989 [51] ZHAO P W, LIU X H, TIAN W L, et al. Adsolubilization of 2, 4, 6-trichlorophenol from aqueous solution by surfactant intercalated ZnAl layered double hydroxides [J]. Chemical Engineering Journal, 2015, 279: 597-604. doi: 10.1016/j.cej.2015.05.037 [52] YANG X J, ZHANG P, LI P, et al. Layered double hydroxide/polyacrylamide nanocomposite hydrogels: Green preparation, rheology and application in methyl orange removal from aqueous solution [J]. Journal of Molecular Liquids, 2019, 280: 128-134. doi: 10.1016/j.molliq.2019.02.033 [53] SHAN R R, YAN L G, YANG K, et al. Magnetic Fe3O4/MgAl-LDH composite for effective removal of three red dyes from aqueous solution [J]. Chemical Engineering Journal, 2014, 252: 38-46. doi: 10.1016/j.cej.2014.04.105 [54] XIONG T, YUAN X Z, WANG H, et al. Highly efficient removal of diclofenac sodium from medical wastewater by Mg/Al layered double hydroxide-poly(m-phenylenediamine) composite [J]. Chemical Engineering Journal, 2019, 366: 83-91. doi: 10.1016/j.cej.2019.02.069 [55] XU Z P, ZHANG J, ADEBAJO M O, et al. Catalytic applications of layered double hydroxides and derivatives [J]. Applied Clay Science, 2011, 53(2): 139-150. doi: 10.1016/j.clay.2011.02.007 [56] WANG J, GONG Q, ALI J, et al. pH-dependent transformation products and residual toxicity evaluation of sulfamethoxazole degradation through non-radical oxygen species involved process [J]. Chemical Engineering Journal, 2020, 390: 124512. doi: 10.1016/j.cej.2020.124512 [57] FAN G L, LI F, EVANS D G, et al. Catalytic applications of layered double hydroxides: recent advances and perspectives [J]. Chemical Society Reviews, 2014, 43(20): 7040-7066. doi: 10.1039/C4CS00160E [58] HUANG D, MA J, FAN C, et al. Co-Mn-Fe complex oxide catalysts from layered double hydroxides for decomposition of methylene blue: Role of Mn [J]. Applied Clay Science, 2018, 152: 230-238. doi: 10.1016/j.clay.2017.11.018 [59] YANG F, CAO Z F, WANG J, et al. In situ self-assembly of molybdenum disulfide/Mg-Al layered double hydroxide composite for enhanced photocatalytic activity [J]. Journal of Alloys and Compounds, 2020, 817: 153308. doi: 10.1016/j.jallcom.2019.153308 [60] WANG L, LI Z, WU Q, et al. Layered structure-based materials: challenges and opportunities for radionuclide sequestration [J]. Environmental Science-Nano, 2020, 7(3): 724-752. doi: 10.1039/C9EN01429B [61] KANG M J, RHEE S W, MOON H. Sorption of MO4– (M = Tc, Re) on Mg/Al layered double hydroxide by anion exchange [J]. Radiochimica Acta, 1996, 75(3): 169-173. doi: 10.1524/ract.1996.75.3.169 [62] WANG X X, YU S Q, WU Y H, et al. The synergistic elimination of uranium (VI) species from aqueous solution using bi-functional nanocomposite of carbon sphere and layered double hydroxide [J]. Chemical Engineering Journal, 2018, 342: 321-330. doi: 10.1016/j.cej.2018.02.102 [63] CELIK A, LI D, QUINTERO M A, et al. Removal of CrO42–, a nonradioactive surrogate of 99TcO4– using LDH-Mo3S13 nanosheets [J]. Environmental Science & Technology, 2022, 56(12): 8590-8598. [64] LINGHU W S, YANG H, SUN Y X, et al. One-pot synthesis of LDH/GO composites as highly effective adsorbents for decontamination of U(VI) [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5608-5616. [65] ZHANG F Z, ZHAO L L, CHEN H Y, et al. Corrosion resistance of superhydrophobic layered double hydroxide films on aluminum [J]. Angewandte Chemie-International Edition, 2008, 47(13): 2466-2469. doi: 10.1002/anie.200704694 [66] DUTTA K, PRAMANIK A. Synthesis of a novel cone-shaped CaAl-layered double hydroxide (LDH): Its potential use as a reversible oil sorbent [J]. Chemical Communications, 2013, 49(57): 6427-6429. doi: 10.1039/c3cc42260g [67] LIU X J, GE L, LI W, et al. Layered double hydroxide functionalized textile for effective oil/water separation and selective oil adsorption [J]. ACS Applied Materials & Interfaces, 2015, 7(1): 791-800. [68] CUI J Y, ZHOU Z P, XIE A, et al. Facile preparation of grass-like structured NiCo-LDH/PVDF composite membrane for efficient oil-water emulsion separation [J]. Journal of Membrane Science, 2019, 573: 226-233. doi: 10.1016/j.memsci.2018.11.064 [69] TIAN Q Y, LIU Q Y, ZHOU J, et al. Superhydrophobic sponge containing silicone oil-modified layered double hydroxide sheets for rapid oil-water separations [J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2019, 570: 339-346. [70] PAVLOVIC M, ADOK-SIPICZKI M, NARDIN C, et al. Effect of macroraft copolymer adsorption on the colloidal stability of layered double hydroxide nanoparticles [J]. Langmuir, 2015, 31(46): 12609-12617. doi: 10.1021/acs.langmuir.5b03372 [71] KONG X R, CHEN J H, TANG Y J, et al. Enhanced removal of vanadium(V) from groundwater by layered double hydroxide-supported nanoscale zerovalent iron [J]. Journal of Hazardous Materials, 2020, 392: 122392. doi: 10.1016/j.jhazmat.2020.122392 [72] LAIPAN M W, YU J F, ZHU R L, et al. Functionalized layered double hydroxides for innovative applications [J]. Materials Horizons, 2020, 7(3): 715-745. doi: 10.1039/C9MH01494B [73] YU W, XIE H Q. A review on nanofluids: Preparation, stability mechanisms, and applications [J]. Journal of Nanomaterials, 2012: 435873. [74] VASTI C, GIACOMELLI C E, ROJAS R. Pros and cons of coating layered double hydroxide nanoparticles with polyacrylate [J]. Applied Clay Science, 2019, 172: 11-18. doi: 10.1016/j.clay.2019.02.016 [75] GU Z, ZUO H L, LI L, et al. Pre-coating layered double hydroxide nanoparticles with albumin to improve colloidal stability and cellular uptake [J]. Journal of Materials Chemistry B, 2015, 3(16): 3331-3339. doi: 10.1039/C5TB00248F [76] SHAO M F, NING F Y, ZHAO J W, et al. Preparation of Fe3O4@SiO2@layered double hydroxide core-shell microspheres for magnetic separation of proteins [J]. Journal of the American Chemical Society, 2012, 134(2): 1071-1077. doi: 10.1021/ja2086323 [77] WANG Q Q, HUANG J, MA C Y, et al. Highly efficient and reusable Mg-Fe layered double hydroxides anchored in attapulgite for uranium uptake from wastewater [J]. Chemosphere, 2023: 138055. [78] YANG M S, LIU J F, CHANG Z, et al. Mg/Al-CO3 layered double hydroxide nanorings [J]. Journal of Materials Chemistry, 2011, 21(38): 14741-14746. doi: 10.1039/c1jm12129d [79] LV W Y, DU M, YE W J, et al. The formation mechanism of layered double hydroxide nanoscrolls by facile trinal-phase hydrothermal treatment and their adsorption properties [J]. Journal of Materials Chemistry A, 2015, 3(46): 23395-23402. doi: 10.1039/C5TA05218A [80] WANG C J, O'HARE D. Topotactic synthesis of layered double hydroxide nanorods [J]. Journal of Materials Chemistry, 2012, 22(43): 23064-23070. doi: 10.1039/c2jm34670b [81] CHEN L B, LI C C, WEI Y H, et al. Hollow LDH nanowires as excellent adsorbents for organic dye [J]. Journal of Alloys and Compounds, 2016, 687: 499-505. doi: 10.1016/j.jallcom.2016.05.344 [82] WANG X, CAI Y W, HAN T H, et al. Phosphate functionalized layered double hydroxides (phos-LDH) for ultrafast and efficient U(VI) uptake from polluted solutions [J]. Journal of Hazardous Materials, 2020, 399: 123081. doi: 10.1016/j.jhazmat.2020.123081 [83] LEI C S, ZHU X F, ZHU B C, et al. Superb adsorption capacity of hierarchical calcined Ni/Mg/Al layered double hydroxides for Congo red and Cr(VI) ions [J]. Journal of Hazardous Materials, 2017, 321: 801-811. doi: 10.1016/j.jhazmat.2016.09.070 [84] ZOU Y D, WANG X X, WU F, et al. Controllable synthesis of Ca-Mg-Al layered double hydroxides and calcined layered double oxides for the efficient removal of U(VI) from wastewater solutions [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(1): 1173-1185. [85] MANDAL S, MAYADEVI S. Cellulose supported layered double hydroxides for the adsorption of fluoride from aqueous solution [J]. Chemosphere, 2008, 72(6): 995-998. doi: 10.1016/j.chemosphere.2008.03.053 [86] MANDEL K, DRENKOVA-TUHTAN A, HUTTER F, et al. Layered double hydroxide ion exchangers on superparamagnetic microparticles for recovery of phosphate from waste water [J]. Journal of Materials Chemistry A, 2013, 1(5): 1840-1848. doi: 10.1039/C2TA00571A [87] PATZKO A, KUN R, HORNOK V, et al. ZnAl-layer double hydroxides as photocatalysts for oxidation of phenol in aqueous solution [J]. Colloids and Surfaces a-Physicochemical and Engineering Aspects, 2005, 265(1-3): 64-72. [88] LI J, FAN Q H, WU Y J, et al. Magnetic polydopamine decorated with Mg-Al LDH nanoflakes as a novel bio-based adsorbent for simultaneous removal of potentially toxic metals and anionic dyes [J]. Journal of Materials Chemistry A, 2016, 4(5): 1737-1746. doi: 10.1039/C5TA09132B [89] HAO M J, GAO P, YANG D, et al. Highly efficient adsorption behavior and mechanism of Urea-Fe3O4@LDH for triphenyl phosphat [J]. Environmental Pollution, 2020, 267: 114142. doi: 10.1016/j.envpol.2020.114142 [90] LIMA E, DE JESUS MARTINEZ-ORTIZ M, GUTIERREZ REYES R I, et al. Fluorinated hydrotalcites: The addition of highly electronegative species in layered double hydroxides to tune basicity [J]. Inorganic Chemistry, 2012, 51(14): 7774-7781. doi: 10.1021/ic300799e [91] XU Y F, DAI Y C, ZHOU J Z, et al. Removal efficiency of arsenate and phosphate from aqueous solution using layered double hydroxide materials: intercalation vs. precipitation [J]. Journal of Materials Chemistry, 2010, 20(22): 4684-4691. doi: 10.1039/b926239c [92] CHEN L H, XU H M, XIE J K, et al. [SnS4]4- clusters modified MgAl-LDH composites for mercury ions removal from acid wastewater [J]. Environmental Pollution, 2019, 247: 146-154. doi: 10.1016/j.envpol.2018.12.009 [93] LI X J, XIN M Y, GUO S, et al. Insight of synergistic effect of different active metal ions in layered double hydroxides on their electrochemical behaviors [J]. Electrochimica Acta, 2017, 253: 302-310. doi: 10.1016/j.electacta.2017.09.075 [94] FU Y, NING F Y, XU S M, et al. Terbium doped ZnCr-layered double hydroxides with largely enhanced visible light photocatalytic performance [J]. Journal of Materials Chemistry A, 2016, 4(10): 3907-3913. doi: 10.1039/C5TA10093C [95] MORI K, TAGA T, YAMASHITA H. Isolated single-atomic Ru catalyst bound on a layered double hydroxide for hydrogenation of CO2 to formic acid [J]. ACS Catalysis, 2017, 7(5): 3147-3151. doi: 10.1021/acscatal.7b00312 [96] MIKAMI G, GROSU F, KAWAMURA S, et al. Harnessing self-supported Au nanoparticles on layered double hydroxides comprising Zn and Al for enhanced phenol decomposition under solar light [J]. Applied Catalysis B-Environmental, 2016, 199: 260-271. doi: 10.1016/j.apcatb.2016.06.031 [97] MA J F, DING J F, YU L M, et al. BiOCl dispersed on NiFe-LDH leads to enhanced photo-degradation of Rhodamine B dye [J]. Applied Clay Science, 2015, 109: 76-82. [98] LI Z, CHEN M, ZHANG Q W, et al. Mechanochemical synthesis of ultrafine ZnS/Zn-Al layered double hydroxide heterojunction and their photocatalytic activities in dye degradation [J]. Applied Clay Science, 2017, 144: 115-120. doi: 10.1016/j.clay.2017.05.015 [99] JUNG I K, JO Y, HAN S C, et al. Efficient removal of iodide anion from aqueous solution with recyclable core-shell magnetic Fe3O4@Mg/Al layered double hydroxide (LDH) [J]. Science of the Total Environment, 2020, 705: 135814. doi: 10.1016/j.scitotenv.2019.135814 -



 下载:
下载: