-
砷是地球上最丰富的元素之一,具有金属和非金属特性,普遍存在于水体、土壤和沉积物颗粒中,可作为水环境中暴露生物体的介质[1]. 近年来,砷污染对人类和动物健康构成了巨大威胁[2],并成为全球主要的环境问题[3]. 人类接触砷的主要来源是饮食[4],具有高毒性和高致癌性的砷会通过食物链摄取和转移进入人体[5]. 淡水生物能有效代谢砷,并调节砷的生物地球化学循环[6]. 克氏原螯虾(Procambarusclarkii)俗称小龙虾,是一种广泛分布于欧亚大陆的底栖动物[7],因其美味可口、富含蛋白质、脂肪酸、维生素D、碘和矿物质而深受我国人民喜爱[8]. 但是,小龙虾易从水环境中积累高浓度的砷(As)[9],对人类饮食安全存在很大的隐患[10]. 底栖生物吸收砷的途径主要有两种[11]:一种是水和沉积物中的砷暴露,另一种是食物中的砷暴露.
一些底栖生物,如虾、螺类、牡蛎等,因其营养丰富、易于捕捞而成为人们喜爱的食物. 但由于沉积环境比其他环境介质积累更多的污染物,底栖生物可能比水环境中的其他生物更能积累有毒物质[12]. 砷的形态影响其流动性和生物利用度,从而影响其进入食物链[13]. 在所有的虾消化过程中,研究发现底栖生物体内有机砷的毒性较小,砷甜菜碱(AsB)是主要的形态[14]. 目前,关于砷在淡水水生生物中的积累和转化,只能通过观察生物的肌肉组织来实现. 这可能导致低估了砷化合物在非肌肉组织中的重要性,从而忽视了砷的不同形态在某些底栖生物或其他鱼类生物可食用组织中的积累[15]. 因此,研究底栖生物不同组织中砷的生物积累和转化机制具有重要意义.
矿山开采、有色金属冶炼、电镀等人类活动使得大量As(V)进入环境中[16]. Chételat等[17]对加拿大金矿开采附近的耶洛奈夫湾沉积物孔隙水进行了砷剖面分析,发现孔隙水剖面最大砷含量为3220 μg·L−1,主要为As(Ⅲ). 陈明[18]对贵州独山县瑞丰采矿公司下游麻球河不同河段进行采样测定与分析,发现As(V)含量最高为14200 μg·L−1. 砷在天然水中的浓度范围为0.5 μg·L−1至10000 μg·L-1[19],天然水体底部及沉积物中的砷浓度相较水体其他部分更高[20],且现有对小龙虾及轮叶黑藻(Hydrilla verticillata)的砷的积累转化研究实验的砷浓度设置通常为500、1000、5000 μg·L−1等,超高浓度的累积实验较少,且高浓度的累积能更好地对比不同培养条件下克氏原螯虾组织中砷的累积与转化规律,为小龙虾的食用安全提供理论依据. 本研究的目的是探讨不同暴露条件下,砷在小龙虾主要组织中的积累和生物转化,以及在受As(V)污染的水环境中,小龙虾在水-食物两相暴露体系下和水相、食物相单相相加积累的砷的情况进行对比分析.
-
本实验中使用的克氏原螯虾(下文简称小龙虾)从当地水产市场购买,选择体重在6.48—9.35 g之间,体长在8.75—11.5 cm之间和表观颜色(红色外壳)相似的成虾. 在60 cm×40 cm×40 cm的鱼缸中培养驯化7 d,驯化过程中投喂小龙虾专用饵料(在当地水产市场购买),投喂量为水箱内虾体总重量的3%—4%. 实验期间,每天观察小龙虾的生长状况,有蜕皮或由于打斗所致受伤现象的虾立刻取出,死亡率<2%. 经检测得:曝气后的自来水中As(T)含量为(4.86±0.03)μg·g−1,饵料中As(T)的含量为(0.11±0.06)mg·kg−1,由于背景水样和小龙虾饵料中的砷含量相对较低,所以它们对小龙虾体内砷的累积将忽略不计. 驯化后的小龙虾被分到4个鱼缸中,每个鱼缸含有20 L自来水和55只小龙虾,鱼缸上覆盖着网眼,防止小龙虾爬出来,其中,溶解氧浓度大致维持在(4.35±0.05) mg·L−1,为小龙虾的日常生活提供充足的氧气. 实验养殖水是干净的自来水,并在饲养小龙虾之前曝气超过24 h,以去除实验水中的余氯. 实验过程中,当鱼缸透明度降到15—20 cm时进行换水,前期从底部抽取1/2的水来进行更换,后期抽取1/4的水进行更换(实验过程中,鱼缸透明度均大于20 cm,未换水). 使用泵在水箱中建立水循环系统,并用砾石来过滤虾的排泄物以保持水的清洁. 在本研究中,轮叶黑藻被用作小龙虾的植物诱饵. 实验挑选长势良好的株体用淡水培养液培养于培养箱中,进行7 d的驯化,而后挑选形态完整,大小均匀的株体进行实验. 实验中使用的人工淡水培养液比例见表1[21].
-
本研究采用室内模拟实验探讨不同暴露条件下小龙虾对无机砷的吸收、积累和转化规律. 暴露实验分别为水相砷暴露、食物相砷暴露、水相和食物相砷暴露. 在本研究中,通过添加Na2HAsO4调整鱼缸中As(V)的暴露浓度为10000 μg·L−1,该浓度的设定是参考湖南省砷矿附近受砷严重污染的黄水溪河流中的砷浓度[22],该河流曾受到无机砷的严重污染,最大As(V)浓度高达10400 μg·L−1.
将实验分为3组(每组设3个平行):(1)水相暴露实验组,用未受砷污染的淡水中培养的轮叶黑藻喂养暴露于10000 μg·L−1As(V)环境中的小龙虾,1天1次. 每次轮叶黑藻重量约为100 g(占虾体总重量的3%—4%),2 h后将剩余轮叶黑藻取出;(2)食物相暴露实验组,用暴露于10000 μg·L−1As(V)的环境中6 d的轮叶黑藻(浓度为(45±0.23) mg·kg−1)喂养小龙虾,1天1次. 每次喂食的质量约为100 g,2 h后将剩余轮叶黑藻取出;(3)水相与食物相暴露实验组,共55只生长条件相似的小龙虾和10株在培养箱中培养了7 d的生长良好的轮叶黑藻暴露于10000 μg·L−1As(V)的环境中. 通过恒温鱼缸将水体温度控制在28 ℃左右,并于第0、2、6、10、14、18、22、26 d取样(每个样设3个平行),将小龙虾解剖,分别采集鳃、腹部外骨骼和尾部肌肉组织,测定各组织中总砷含量,并同步测定第0、2、6、10、14、18 d对应的组织中各砷形态含量.
-
取6只小龙虾,用清水洗干净,放置去离子水中1.5h,清洗来自虾体腮室内的含砷水[23]. 将其按不同部位解剖,采集鳃、腹部外骨骼和尾部肌肉组织,并进行标记. 冷冻干燥48 h后,储存于4 ℃的冰箱中待测.
将适量的轮叶黑藻浸泡在冰川磷酸盐溶液中除去植物表面吸附的砷,用去离子水反复冲洗植物表面3次. 拭干后称取0.5 g(FW)轮叶黑藻,用冷冻干燥机(Scientz-N)将其冷冻干燥24 h后研磨成粉末,移至10 mL离心管中,密封置于4 ℃的冰箱中待测.
-
准确称取处理好的小龙虾和轮叶黑藻样品各0.05 g至25 mL比色管中(每个样设3个平行),依次加入10 mL硝酸、2 mL硫酸、0.65 mL高氯酸,混合均匀后放置过夜. 次日,将已溶于酸的样品溶液用纯水多次润洗转移至小烧杯中,置于电热板上消解(160—170 ℃),消解至冒大量白烟且消化液只剩2—3 mL时转移至25 mL规格的容量瓶中,加入10 mL 5% 硫脲+5%抗坏血酸混合液,再用5% HCl定容,室温静置30 min后待测.
-
准确称取处理好的小龙虾和轮叶黑藻样品各0.1 g至50 mL的离心管中,加入15 mL 50%甲醇-水溶液,在振荡速度为200 r·min−1的恒温振荡器(SHZ-C)上振荡10 min,室温下用超声波清洗仪(SB-100D)超声提取30 min,再用高速冷冻离心机(TGL-16M)8000 r·min−1离心10 min,转移上清液于洁净的100 mL离心管中,继续往原样品离心管中加入15 mL 50%甲醇-水溶液,重复提取3—5次,合并上清液,在40 ℃下旋转蒸发浓缩至1—2 mL,转移至10 mL离心管中,用超纯水稀释定容至10 mL,测试前经0.45 μm滤膜过滤,在3 d内测试分析完毕.
-
总砷和砷形态采用高效液相色谱-质谱联用仪(HPLC-ICP-MS; Agilent 1100,USA,detection limit = 0.01 μg·L−1)联用系统进行测定,测定总砷含量使用1000 mg·L−1 As的标准溶液(GBW08611). 实验过程中使用的As(Ⅲ)溶液标准物质(GBW08666)、As(Ⅴ)溶液标准物质(GBW08667)、MMA溶液标准物质(GBW08668)、DMA溶液标准物质(GBW08669)、AsB溶液标准物质(GBW08670)均来自于中国国家标准物质研究中心. 总砷的加标回收率为88.1%—105.3%,砷形态的加标回收率为85%—105%.
-
载气:高纯氩气(纯度>99.99%);载气流量:300 mL·min−1;屏蔽气流量:900 mL·min−1;蠕动泵转速:60 r·min−1;负高压:300 V;灯电流:50 mA;读数时间:10 s;延迟时间:1 s.
-
实验获得的数据用Microsoft®Excel 17.0和IBM SPSS Statistics 25.0软件(IBM Corp.,Armonk,NY,USA)进行整理与分析,利用单因素方差分析(ANOVA)评估不同实验组的平均值之间的差异显著性,当概率水平P<0.05时被认为具有统计学意义. 使用Origin2020(OriginLab Corporation, Northampton, MA, USA)绘图.
-
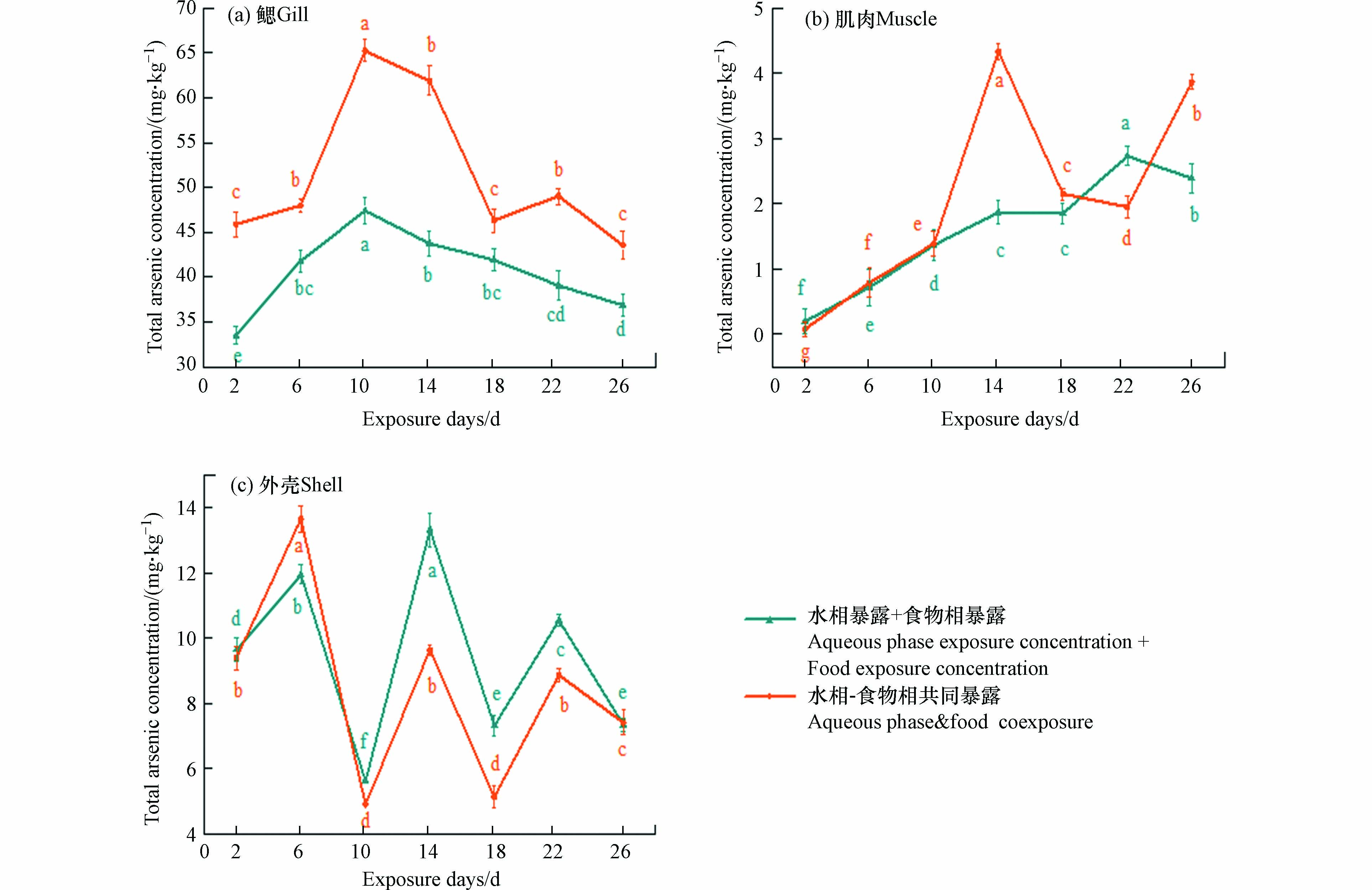
在水相暴露的初始阶段,小龙虾鳃组织中As(T)的积累显著增加(P<0.05),第10天时达到峰值(42.91±1.91) mg·kg−1. 随后,鳃中的As(T)浓度逐渐降低并于第22天趋于稳定. 鳃中的As(T)含量接近外壳中的4倍,肌肉中的As(T)含量显著低于鳃中的As(T)含量(图1a). 与水相暴露体系不同,食物相暴露条件下,小龙虾鳃和肌肉组织中As(T)的浓度显著降低,且鳃中As(T)在第6天时达到较高浓度,6—22 d一直维持在4.65 mg·kg−1左右,第22—26天,小龙虾鳃组织中的As(T)浓度明显下降(P<0.05),其在体内的积累也随之减少(图1b). 在水-食物两相共暴露条件下,轮叶黑藻体内砷积累量在前6 天急剧增加,而后增幅减缓. 小龙虾鳃组织中As(T)含量显著高于水相和食物相. 在最初的12 d里,鳃组织累积浓度迅速增加,峰值为(65.21±1.11) mg·kg−1,第12—18 天时逐渐下降. 其外壳中As(T)累积浓度与水相暴露体系接近,均在(4.92—13.64) mg·kg−1之间波动. 肌肉组织中As(T)累积浓度相较于其他两个暴露体系有显著增加(P<0.05),峰值为4.33 mg·kg−1(图1c).
本研究发现暴露于食物相的小龙虾组织中As(T)浓度远低于其他两种暴露条件,说明小龙虾在水相暴露中对As(V)的生物积累程度较高. 即食物相砷对食物的毒性远低于水相砷对食物的毒性,这也使得食物相暴露条件下的小龙虾比其他两相暴露条件下的生长更活跃. Kolts等[24]将食物暴露于有毒的水环境中,然后投喂浮游动物. 实验结果表明,浮游动物的存活率不受影响. 还有研究发现[25],当生物体暴露于某些金属时,其食物相的积累大于水相的积累,有些金属通过食物相的积累破坏生物体的繁殖能力,甚至导致死亡. 该结果说明水相暴露的毒性远小于食物相. 因此,水相与食物相的比较结果取决于食物的来源,金属或污染物的种类、产生毒物的机理以及生物的种类. 在本实验中,水相与食物相中砷的积累差异是由于两种不同暴露方式对砷的富集和食物进入小龙虾体内后砷的分布不同造成的. 同时,食物相摄取的砷不易吸收,部分通过肠道后在短时间排出. 水相摄取的砷可以通过磷酸盐进入小龙虾体内,并扩散到全身. 以前的研究还发现[26],一些底栖动物在摄食过程中的主动吸收效率是随机被动吸收的300多倍,导致砷在食物相暴露中的积累浓度远远小于水相暴露. 对3种暴露条件下的As(T)积累浓度进行比较,可得As(T)在小龙虾各组织中的富集率和最高富集浓度均为:鳃>外壳>肌肉(图1).
-
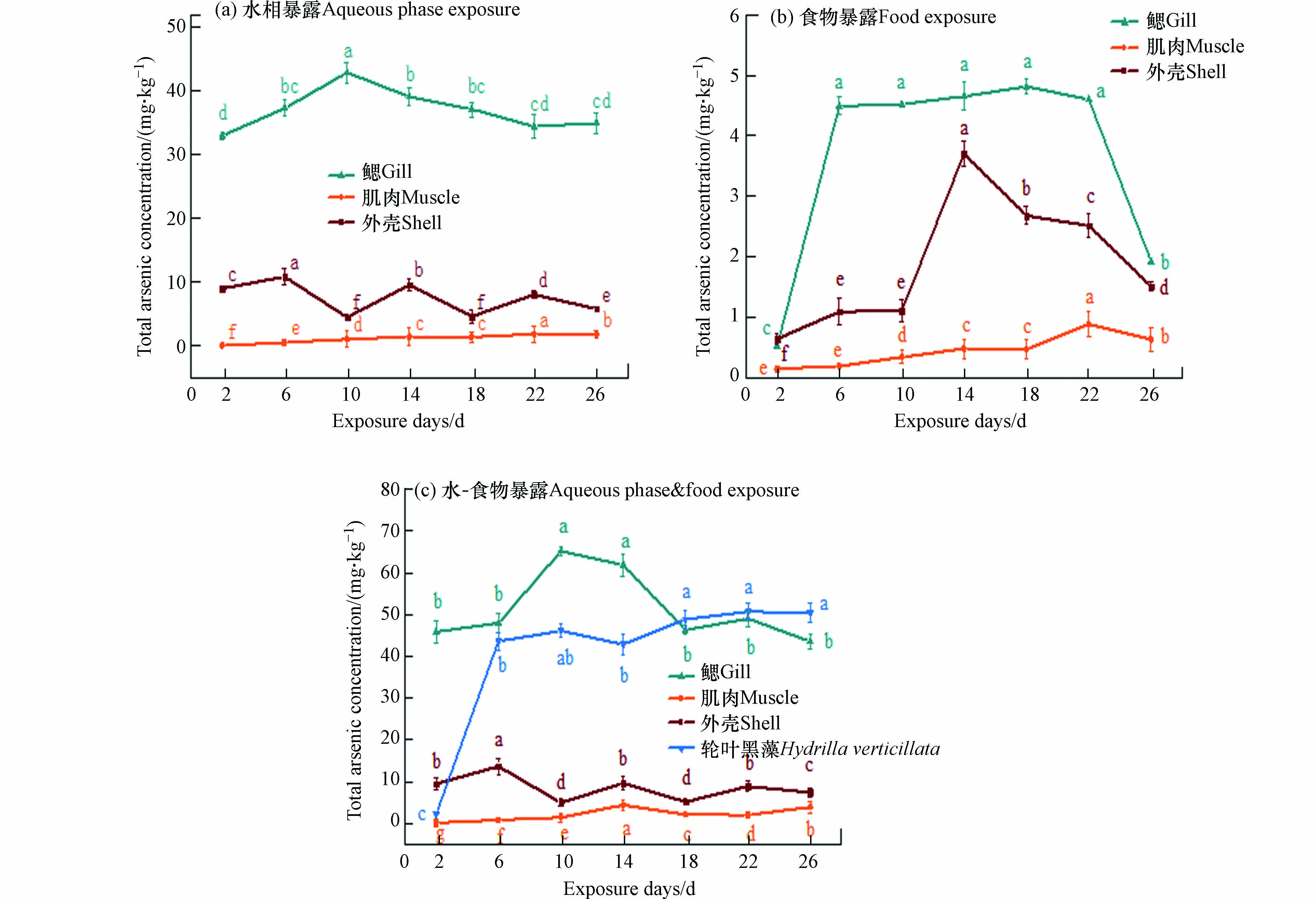
比较不同暴露条件下小龙虾体内As(T)的积累情况,结果表明,水相暴露体系和食物相暴露体系中小龙虾体内砷的富集量较大. 为了进一步阐明水相和食物相共同作用下的砷积累变化与水相和食物相单独作用下的砷积累的相关性. 加入单次水相暴露和食物相暴露的As(T)积累浓度记为“水相暴露+食物暴露”,并与水-食物相共暴露组的积累浓度按时间序列进行比较(图2). 可知,鳃中砷浓度变化相似,在第10天左右达到峰值,但水-食物相共暴露组的As(T)浓度始终较高(图2a). 两组中的总砷在肌肉部位前10 d的积累浓度基本一致,而水-食物相共暴露组的As(T)在第10—14天出现了显著的增加(P<0.05),第14—18天又显著下降(图2b). 外壳中砷浓度在两组中的变化幅度都较大,但都在第10天时达到最低(图2c). 结果表明,第10天的暴露时间是一个转折点,砷在小龙虾不同组织中的积累在实验前10 d和后10 d存在明显差异.
水-食物两相暴露体系下的小龙虾鳃组织中的As(T)浓度明显高于水相和食物相单独作用下的As(T)浓度,肌肉组织和外壳组织则没有明显差别,这可能是因为单相系统获取As(T)的来源单一,而两相系统中的小龙虾可以通过水和轮叶黑藻来获取As(T). 同时可以推断,As(T)在水-食物两相系统中的积累比在水相或食物相单相系统中更复杂.
-
对3种暴露条件下小龙虾的不同组织和轮叶黑藻中的砷形态进行测定,得到As(V)的生物转化结果如图3所示. 在水相暴露体系下,小龙虾鳃组织中As(V)含量占比最大,其次是As(Ⅲ),还有少量的AsB. 肌肉组织中,除了As(V)、As(Ⅲ)和AsB,后期还出现了少量的DMA. 外壳组织中,主要是As(V)、As(Ⅲ)和AsB,其中,As(V)、As(Ⅲ)的占比均较大. 食物相暴露体系下,小龙虾肌肉组织中砷形态主要是MMA和AsB,鳃组织中As(V)含量占比最大,由于砷在鳃组织中发生了甲基化作用,也出现了少量的As(Ⅲ)和AsB. 外壳组织与鳃组织相比,在第6—18天时出现了少量的DMA. 只有在食物相暴露体系下,小龙虾肌肉组织中的无机砷含量满足《食品安全国家标准食品中污染物限量》(GB 2762—2022)中规定的限值(≤0.5 mg·kg−1),食物相暴露体系的鳃和外壳组织以及水相和水-食物相体系的鳃、外壳和肌肉组织中的无机砷含量都超过了食品安全国家标准. 相比之下,砷在水-食物两相暴露体系的鳃组织中的形态分布和变化趋势与其他两个暴露体系明显不同,但3组鳃组织中砷的形态主要由As(V)和As(Ⅲ)组成,其中As(V)的积累量要明显多于As(Ⅲ). 其原因是小龙虾鳃组织可以通过直接接触水相的被动扩散进行累积,该途径使更多的As(V)在鳃组织中积累而不发生转化. 与小龙虾相比,在食物相、水-食物相体系中,轮叶黑藻能将砷转化为更丰富的砷形态,包括As(V)、As(Ⅲ)、MMA和DMA,且各砷形态浓度依次为As(V)>As(Ⅲ)>DMA>MMA.
小龙虾同期鳃组织中As(V)和As(Ⅲ)的累积比例高于肌肉组织. 水相、食物相、水-食物相中As(Ⅲ)的累积转化率分别达到36%、22%和54%,As(V)的累积转化率分别达到96%、78%和97%. 同时,肌肉组织对有机砷形态(MMA、DMA和AsB)的转化比例高于鳃组织. 且在水相、食物相和水-食物两相暴露条件下,肌肉组织中均出现AsB形态,峰值比例分别为56%、36%和63%. 食物相和水-食物相中As(Ⅲ)的累积比例分别为36%和39%,As(V)的累积比例分别为71%和84%. 与此同时,暴露于10000 μg·L−1的As(V)6 d后,小龙虾的砷形态基本稳定,主要为As(V)、As(Ⅲ)、MMA和DMA. 其中,无机砷的积累量为84%—89%,As(V)的积累最多,而甲基化As(MMA和DMA)的比例相对较低(11%—16%).
在砷形态转化方面,也表现出组织特异性. 暴露于As(V)后,小龙虾发生了砷形态的生物转化,其中,无机砷形态(As(V)、As(Ⅲ))占主要比例,有机砷形态(MMA、DMA、AsB)所占比例较小. 砷As(V)的甲基化作用是水生生物中As解毒的重要途径[27]. Suhendrayatna等[28]通过鉴别砷暴露后小球藻体内的形态得到As(Ⅲ)(55.4%—75%)、As(V)(24%—44.5%)和痕量DMA(0.13%—5.4%). Caumette等[29]还研究了从砷富集的湖泊中采集的水溞体内检测到56% As(V),10% As(Ⅲ) 和34%有机砷(MMA、DMA,etc). 结合相关研究结果,本研究进一步证明了小龙虾能将水环境中毒性较强的As(V)通过解毒机制转化为AsB等有机砷,并且相同的暴露条件下,小龙虾肌肉组织将无机砷转化为有机砷的能力高于鳃组织. 其中一个重要原因可能是,砷需要通过小龙虾的口腔、肠道和肝脏等途径才能到达肌肉组织[30],因此,砷的甲基化作用增强. 两相的As(V)暴露条件下,随着时间的延长,小龙虾鳃组织中出现了一定量的As(V)、As(Ⅲ)和少量的AsB,未检出MMA或DMA. 且As(V)在鳃组织中的积累呈现先增加后减少的趋势. 结果表明,两相培养的小龙虾鳃组织虽然具有将As(V)还原为As(Ⅲ)的能力,但其甲基化作用较弱. 这与淡水鱼肌肉组织更有能力使砷甲基化并将无机砷转化为AsB的研究结果相似[31].
As(V)也可被藻类等水生植物通过生化转化和甲基化的方式转化为低毒的有机砷形态[32 − 33]. 在本研究中,砷在轮叶黑藻体内主要以毒性较高的无机砷形态存在,但仍能检测到MMA、DMA和AsB,说明轮叶黑藻对As(V)有解毒作用. 已有研究发现,水生植物对砷的解毒过程为:首先,As(V)被还原为As(Ⅲ),然后甲基化生成MMA、DMA等有机砷形态[34],这与轮叶黑藻能通过细胞内巯基肽的络合作用降低砷酸盐的毒性研究结果相似.
研究发现砷在水生动物中的积累途径有两种:一种是通过鳃膜扩散积累[35], 另一种是直接与水体接触[36 − 37]. 砷通过小龙虾的食物摄取进入体内,扩散到各种组织,并产生积累效应[38]. 这种积累途径在本研究的小龙虾的3个目标组织中都存在. 鳃组织具有较大的接触面积,因此,砷在鳃组织中的积累速率和容量均较高. 而肌肉组织只能通过摄食,经肠壁吸收和释放,并经肝脏吸收[39 − 40].
综上所述,小龙虾肌肉组织和鳃组织中砷形态的转化具有组织特异性,而且,不同时期生物转化砷形态的结果也不一致. 同时,与鳃组织相比,小龙虾肌肉组织对As(V)的甲基化能力更强,表明肌肉组织会降低砷的毒性. 虽然小龙虾在生物转化过程中能观察到更多的形态,但其肌肉组织和鳃组织中的比例与轮叶黑藻的生物转化结果关系并不密切.
-
(1)水相是砷积累的主要途径. 小龙虾在水相中的生物积累量明显高于食物相,两相培养中的砷积累也是以水相暴露为主的,可推测小龙虾体内的砷积累主要来源于含砷水环境.
(2)小龙虾不同组织中砷的积累和生物转化具有明显的特异性,砷的累积浓度和累积速率如下:鳃>外壳>肌肉.
(3)砷在小龙虾体内通过甲基化作用降低毒性,且不同组织中的甲基化能力为肌肉>鳃>外壳,从食物安全角度考虑,只食用小龙虾肌肉组织可以降低砷中毒的风险.
不同暴露条件下克氏原螯虾组织中砷的积累与转化
Accumulation and transformation of arsenic in the tissues of Procambarusclarkii under different exposure conditions
-
摘要: 砷(As)可在水生食物链中积累转化,并通过食物链影响人体健康. 克氏原螯虾(Procambarusclarkii)是一种生活在淡水底层的杂食性动物,能够从水和食物中吸收和转化无机砷. 本研究探究了砷酸盐As(V)在3种不同的暴露条件下(水相、食物相、水相与食物相),克氏原螯虾不同组织中As(V)的生物积累和转化. 结果表明,其在水-食物两相体系中的砷积累浓度较高,而且水相暴露体系对总砷积累速率的贡献大于食物相暴露体系. 砷在克氏原螯虾不同组织中的积累和转化具有显著的特异性,其富集浓度和积累速率如下:鳃>外壳>肌肉. 克氏原螯虾对无机砷的生物转化途径是将部分As(V)还原为As(Ⅲ),部分转化为一甲基砷酸(MMA)、二甲基砷酸(DMA)和砷甜菜碱(AsB). 本文对了解底栖生物链中砷的积累和转化以及小龙虾的食用安全具有重要意义.Abstract: Arsenic (As) can accumulate and transform in the aquatic food chain and affect human health through the food chain. The crayfish (Procambarusclarkii), an omnivore living in freshwater substrates, is capable of absorbing and transforming inorganic arsenic from water and food. In this study, we investigated the bioaccumulation and transformation of arsenate As(V) in different tissues of Procambarusclarkii under three different exposure conditions (aqueous phase, food phase, aqueous phase&food phase). The results showed that the arsenic accumulation concentration was higher in aqueous phase&food phase system, and the contribution of the aqueous phase exposure system to the total arsenic accumulation rate was greater than that of the food phase exposure system. The accumulation and transformation of arsenic in different tissues of Procambarusclarkii were significantly specific, with the following enrichment concentrations and accumulation rates: gill > shell > muscle. The biotransformation pathway of inorganic arsenic in Procambarusclarkii was the partial reduction of As(V) to As(Ⅲ) and partial conversion to monomethylarsenic acid (MMA), dimethylarsenic acid (DMA) and arsenic betaine (AsB). This is important for understanding the accumulation and transformation of arsenic in benthic biota and the food safety of crayfish.
-
Key words:
- Procambarusclarkii /
- Hydrilla verticillata /
- As /
- bioaccumulation /
- biotransformation.
-

-
表 1 人工淡水培养液配比表
Table 1. Artificial freshwater nutrient solution ration table
化合物
Compound浓度
Concentration化合物
Compound浓度
ConcentrationMgSO4·7H2O 22.7 mg·L−1 FeCl3·6H2O 1.41 mg·L−1 MgCl2·2H2O 30.7 mg·L−1 Al2(SO4)3·18H2O 0.97 mg·L−1 CaCl2·2H2O 20.4 mg·L−1 MnCl2·4H2O 0.19 mg·L−1 NaHCO3 26.0 mg·L−1 ZnSO4·7H2O 3.86 μg·L−1 KCl 3.61 mg·L−1 CuCl2·2H2O 2.17 μg·L−1 NaCl 45.7 mg·L−1 注:pH用1 mol·L−1的NaOH和HCl调至7.0. Note: The pH is adjusted to 7.0 with 1 mol·L−1 NaOH and HCl. -
[1] SHARMA V K, SOHN M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation[J]. Environment International, 2009, 35(4): 743-759. doi: 10.1016/j.envint.2009.01.005 [2] ZHANG H, SELIM H M. Reaction and transport of arsenic in soils: Equilibrium and kinetic modeling[J]. Advances in Agronomy, 2008, 98: 45-115. [3] IRSHAD S, XIE Z M, NAWAZ A, et al. Influence of Aquatic pH on chemical speciation, phytochelation and vacuolar compartmentalization of arsenic in Vallisneria denseserrulata (Makino)[J]. International Journal of Phytoremediation, 2020, 22(11): 1147-1155. doi: 10.1080/15226514.2020.1741507 [4] CHÁVEZ-CAPILLA T. The need to unravel arsenolipid transformations in humans[J]. DNA and Cell Biology, 2022, 41(1): 64-70. doi: 10.1089/dna.2021.0476 [5] SURUCU O. Electrochemical removal of arsenic and remediation of drinking water quality[J]. Desalination and Water Treatment, 2021, 216: 246-251. doi: 10.5004/dwt.2021.26808 [6] MAMUN M A A, RAHMAN I M M, DATTA R R, et al. Arsenic speciation and biotransformation by the marine macroalga Undaria pinnatifida in seawater: A culture medium study[J]. Chemosphere, 2019, 222: 705-713. doi: 10.1016/j.chemosphere.2019.01.185 [7] FAN H L, FAN D M, HUANG J L, et al. Cooking evaluation of crayfish (Procambarus clarkia) subjected to microwave and conduction heating: A visualized strategy to understand the heat-induced quality changes of food[J]. Innovative Food Science & Emerging Technologies, 2020, 62: 102368. [8] CHAI L Q, LI W W, WANG X W. Identification and characterization of two arasin-like peptides in red swamp crayfish Procambarus clarkii[J]. Fish & Shellfish Immunology, 2017, 70: 673-681. [9] 李思佳, 沈俊毅, 韩若冰. 上海市虹口区市售小龙虾中汞和砷的污染水平分析[J]. 现代食品, 2022, 28(19): 109-113. doi: 10.16736/j.cnki.cn41-1434/ts.2022.19.032 LI S J, SHEN J Y, HAN R B. Analysis of the contamination levels of mercury and arsenic in commercially available crayfish in Hongkou district, Shanghai[J]. Modern Food, 2022, 28(19): 109-113 (in Chinese). doi: 10.16736/j.cnki.cn41-1434/ts.2022.19.032
[10] HAN R, KHAN A, LING Z M, et al. Feed-additive Limosilactobacillus fermentum GR-3 reduces arsenic accumulation in Procambarus clarkii[J]. Ecotoxicology and Environmental Safety, 2022, 231: 113216. doi: 10.1016/j.ecoenv.2022.113216 [11] ANANDKUMAR A, LI J, PRABAKARAN K, et al. Accumulation of toxic elements in an invasive crayfish species (Procambarus clarkii) and its health risk assessment to humans[J]. Journal of Food Composition and Analysis, 2020, 88: 103449. doi: 10.1016/j.jfca.2020.103449 [12] ZHANG W, GUO Z Q, SONG D D, et al. Arsenic speciation in wild marine organisms and a health risk assessment in a subtropical bay of China[J]. Science of the Total Environment, 2018, 626: 621-629. doi: 10.1016/j.scitotenv.2018.01.108 [13] WANG J J, KERL C F, HU P J, et al. Thiolated arsenic species observed in rice paddy pore waters[J]. Nature Geoscience, 2020, 13(4): 282-287. doi: 10.1038/s41561-020-0533-1 [14] CHI H F, ZHANG Y C, WILLIAMS P N, et al. In vitro model to assess arsenic bioaccessibility and speciation in cooked shrimp[J]. Journal of Agricultural and Food Chemistry, 2018, 66(18): 4710-4715. doi: 10.1021/acs.jafc.7b06149 [15] VAHTER M. Mechanisms of arsenic biotransformation[J]. Toxicology, 2002, 181/182: 211-217. doi: 10.1016/S0300-483X(02)00285-8 [16] ZHENG L L, ZHOU Z K, RAO M M, et al. Assessment of heavy metals and arsenic pollution in surface sediments from rivers around a uranium mining area in East China[J]. Environmental Geochemistry and Health, 2020, 42(5): 1401-1413. doi: 10.1007/s10653-019-00428-x [17] CHÉTELAT J, PALMER M J, PAUDYN K, et al. Remobilization of legacy arsenic from sediment in a large subarctic waterbody impacted by gold mining[J]. Journal of Hazardous Materials, 2023, 452: 131230. doi: 10.1016/j.jhazmat.2023.131230 [18] 陈明. 采取合理措施 避免跨界污染: 贵州独山瑞丰矿业砷污染事件解析[J]. 环境保护, 2009, 37(3): 63-64. CHEN M. Take reasonable measures to avoid cross-border pollution—Analysis of arsenic pollution in Ruifeng mining, Dushan, Guizhou Province[J]. Environmental Protection, 2009, 37(3): 63-64 (in Chinese).
[19] LI Y Z, MA L, ABUDUWAILI J, et al. Spatiotemporal distributions of fluoride and arsenic in rivers with the role of mining industry and related human health risk assessments in Kyrgyzstan[J]. Exposure and Health, 2022, 14(1): 49-62. doi: 10.1007/s12403-021-00417-5 [20] WANG C, WANG K, ZHOU W Q, et al. Occurrence, risk, and source of heavy metals in lake water columns and sediment cores in Jianghan Plain, central China[J]. International Journal of Environmental Research and Public Health, 2023, 20(4): 3676. doi: 10.3390/ijerph20043676 [21] XUE P Y, YAN C Z. Arsenic accumulation and translocation in the submerged macrophyte Hydrilla verticillata (L. f. ) Royle[J]. Chemosphere, 2011, 85(7): 1176-1181. doi: 10.1016/j.chemosphere.2011.09.051 [22] LI W X, LIU J, HUDSON-EDWARDS K A. Seasonal variations in arsenic mobility and bacterial diversity: The case study of Huangshui Creek, Shimen Realgar Mine, Hunan Province, China[J]. The Science of the Total Environment, 2020, 749: 142353. doi: 10.1016/j.scitotenv.2020.142353 [23] NAKAYA M, TAKATSU T, NAKAGAMI M, et al. Spatial distribution and feeding habits of the shrimp Crangon uritai as a predator on larval and juvenile marbled sole Pleuronectes yokohamae[J]. Fisheries Science, 2004, 70(3): 445-455. doi: 10.1111/j.1444-2906.2004.00824.x [24] KOLTS J M, BOESE C J, MEYER J S. Acute toxicity of copper and silver to Ceriodaphnia dubia in the presence of food[J]. Environmental Toxicology and Chemistry, 2006, 25(7): 1831-1835. doi: 10.1897/05-501R.1 [25] HOOK S E, FISHER N S. Sublethal effects of silver in zooplankton: Importance of exposure pathways and implications for toxicity testing[J]. Environmental Toxicology and Chemistry, 2001, 20(3): 568-574. doi: 10.1002/etc.5620200316 [26] ROSENKRANZ P, CHAUDHRY Q, STONE V, et al. A comparison of nanoparticle and fine particle uptake by Daphnia magna[J]. Environmental Toxicology and Chemistry, 2009, 28(10): 2142-2149. doi: 10.1897/08-559.1 [27] 王雨璇, 陈冠虹, 喻敏, 等. 淡水硅藻的砷甲基化和砷氧化代谢机制[J]. 环境工程学报, 2023, 17(5): 1620-1630. WANG Y X, CHEN G H, YU M, et al. Metabolic mechanisms of arsenic methylation and oxidation in freshwater diatoms[J]. Chinese Journal of Environmental Engineering, 2023, 17(5): 1620-1630 (in Chinese).
[28] SUHENDRAYATNA, OHKI A, KUROIWA T, et al. Arsenic compounds in the freshwater green microalga Chlorella vulgaris after exposure to arsenite[J]. Applied Organometallic Chemistry, 1999, 13(2): 127-133. doi: 10.1002/(SICI)1099-0739(199902)13:2<127::AID-AOC810>3.0.CO;2-K [29] CAUMETTE G, KOCH I, MORIARTY M, et al. Arsenic distribution and speciation in Daphnia pulex[J]. Science of the Total Environment, 2012, 432: 243-250. doi: 10.1016/j.scitotenv.2012.05.050 [30] GEDIK K, KONGCHUM M, DeLAUNE R D, et al. Distribution of arsenic and other metals in crayfish tissues (Procambarus clarkii) under different production practices[J]. Science of the Total Environment, 2017, 574: 322-331. doi: 10.1016/j.scitotenv.2016.09.060 [31] CIARDULLO S, AURELI F, RAGGI A, et al. Arsenic speciation in freshwater fish: Focus on extraction and mass balance[J]. Talanta, 2010, 81(1/2): 213-221. [32] 周志豪, 黄振华, 周朝生, 等. 振荡提取-高效液相色谱-电感耦合等离子体质谱法测定藻类中6种形态砷化合物[J]. 山东化工, 2018, 47(21): 71-73, 76. doi: 10.19319/j.cnki.issn.1008-021x.2018.21.027 ZHOU Z H, HUANG Z H, ZHOU C S, et al. Arsenic speciation analysis of algae by using vibrated extraction high performance liquid chromatography-inductively coupled plasma mass spectrometry[J]. Shandong Chemical Industry, 2018, 47(21): 71-73, 76 (in Chinese). doi: 10.19319/j.cnki.issn.1008-021x.2018.21.027
[33] 宋梦萍, 杨常亮, 张璟, 等. 食物相暴露条件下尼罗罗非鱼对砷的累积与转化[J]. 环境化学, 2022, 41(6): 1897-1904. doi: 10.7524/j.issn.0254-6108.2021113002 SONG M P, YANG C L, ZHANG J, et al. Accumulation and transformation of arsenic in Oreochromis niloticus under food phase exposure[J]. Environmental Chemistry, 2022, 41(6): 1897-1904 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021113002
[34] PAPRY R I, FUJISAWA S, ZAI Y H, et al. Freshwater phytoplankton: Salinity stress on arsenic biotransformation[J]. Environmental Pollution, 2021, 270: 116090. doi: 10.1016/j.envpol.2020.116090 [35] WEBSTER L, RUSSELL M, WALSHAM P, et al. Halogenated persistent organic pollutants in relation to trophic level in deep sea fish[J]. Marine Pollution Bulletin, 2014, 88(1/2): 14-27. [36] ZHANG J Q, HU X L, ZHANG K J, et al. Desorption of calcium-rich crayfish shell biochar for the removal of lead from aqueous solutions[J]. Journal of Colloid and Interface Science, 2019, 554: 417-423. doi: 10.1016/j.jcis.2019.06.096 [37] XIONG B, XU T, LI R P, et al. Heavy metal accumulation and health risk assessment of crayfish collected from cultivated and uncultivated ponds in the Middle Reach of Yangtze River[J]. Science of the Total Environment, 2020, 739: 139963. doi: 10.1016/j.scitotenv.2020.139963 [38] PALOMA A, ANGEL B. The trophic ecology of the red swamp crayfish (Procambarus clarkii) in Mediterranean aquatic ecosystems: A stable isotope study[J]. Limnetica, 2013(32): 121-138. doi: 10.23818/limn.32.12 [39] SCHAEFFER R, FRANCESCONI K A, KIENZL N, et al. Arsenic speciation in freshwater organisms from the River Danube in Hungary[J]. Talanta, 2006, 69(4): 856-865. doi: 10.1016/j.talanta.2005.11.025 [40] SOEROES C, GOESSLER W, FRANCESCONI K A, et al. Arsenic speciation in farmed Hungarian freshwater fish[J]. Journal of Agricultural and Food Chemistry, 2005, 53(23): 9238-9243. doi: 10.1021/jf0516639 -



 下载:
下载: