-
近年来,基于过硫酸盐的高级氧化技术(persulfate−based advanced oxidation processes,PS−AOPs)作为去除水中难降解有机物的有效方法而受到广泛关注[1 − 3]. 研究表明,PS−AOPs主要依赖于体系中的硫酸根自由基(SO4·−)、羟基自由基(·OH)等作为活性氧化物质(reactive oxide species,ROS)发挥氧化作用,进而分解、矿化体系中的有机污染物. 含Co、Ag、Cu、Mn、Fe、Au、Ru等多种金属元素在内的多种非均相催化剂可有效激活过一硫酸盐(peroxymonosulfate,PMS)和过二硫酸盐(peroxydisulfate,PDS)去除体系中的有机污染物[4-5]. 非均相催化的显著优点在于固相催化剂可以通过过滤或离心等操作从反应后的体系中分离,能够有效控制产物中的金属残留、降低二次污染风险[6].
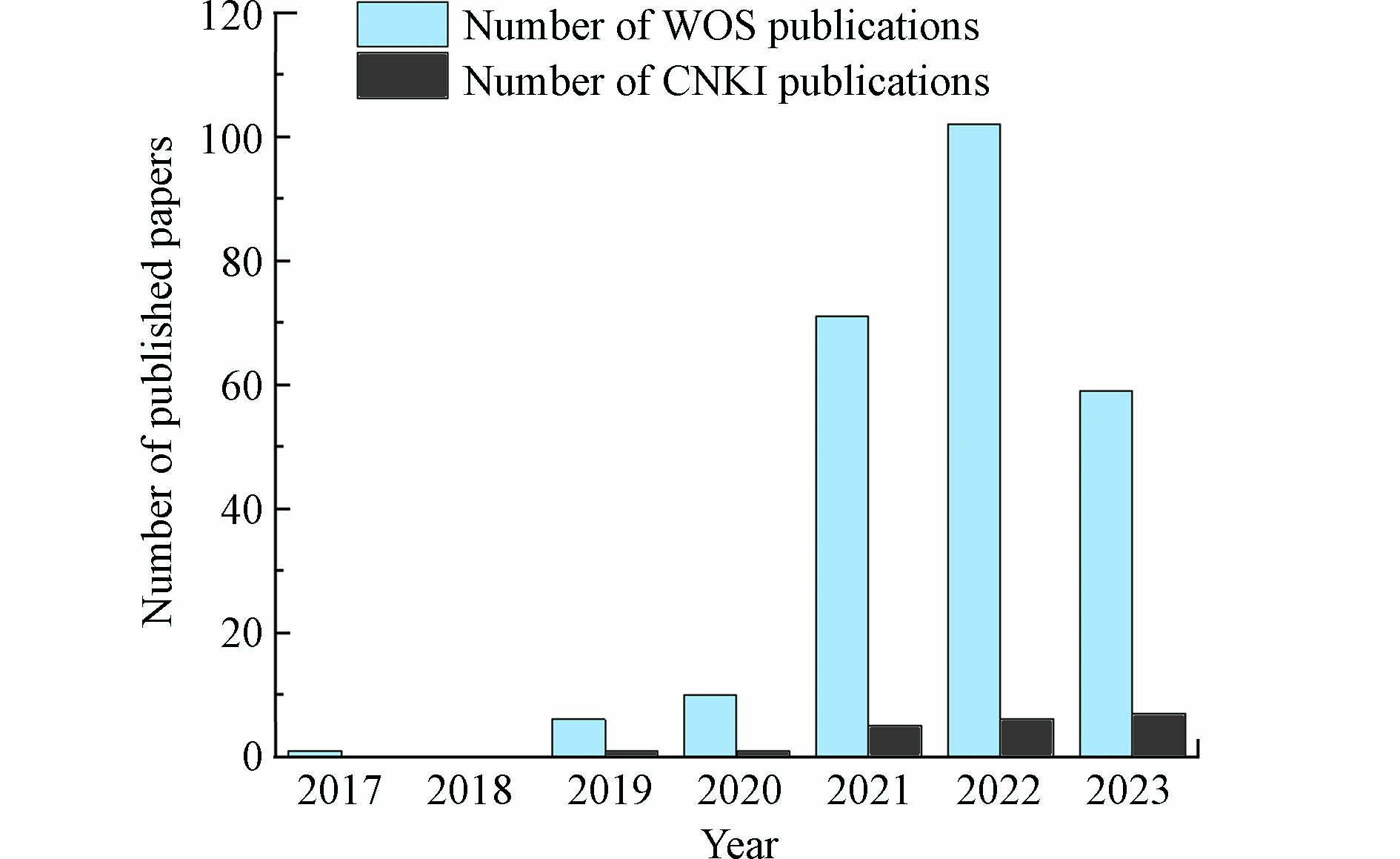
近年来,随着纳米催化科学的发展和表征技术的进步,金属催化剂表面锚定的活性粒子被缩小到原子水平,被定义为单原子催化剂(single−atom catalysts,SAC). SAC相较于常规纳米催化剂显示出以下几点突出优势[7 − 8]:(1)锚定在载体上的特定位点的金属单原子与载体阴离子之间将形成强化学键,将有效提高催化剂的反应活性和稳定性;(2)SAC表面金属原子理论分散度高达100%,具有理论上的最大原子利用效率;(3)活性位点结构及分散状态均一,可从原子甚至电子层次下研究催化体系中的构效关系. 基于以上显著优势,根据Web of Science(WOS)和China National Knowledge Infrastructure(CNKI)发文量统计数据(见图1)可以明显看出,SAC的开发和应用得到了越来越多研究者的关注,并逐渐成为非均相PS−AOPs技术研究的发展前沿[9 − 10]. 本文重点综述了SAC活化过硫酸盐产生氧化活性物质的驱动机制,对结构调控与催化性能的相关性进行了系统总结,深入了解结构−性能关系,指出了精确调整催化剂活性位点的策略,而后进一步讨论了当前的研究局限和未来的研究需求.
-
通过能量转移或还原电子转移过程均可分解PMS和PDS中过氧键(O—O)并产生SO4·−和·OH. 但由于PMS中的O—O键不对称,与氢相连的O—O带部分正电荷,而PDS中O—O键的电荷分布对称,因而二者具有不同的反应特性(表1)[11 − 12]. PMS更易受到各种亲核试剂的攻击,包括X−、CN−、N3−和HCO3−[13 − 15]. PMS与卤化物离子的反应尤其值得关注,其反应过程中有可能不涉及过硫酸盐活化(即SO4·−的形成)而是形成卤素自由基,但在PDS中未观察到此反应[14 − 15]. 此外,尽管PDS的氧化还原电位高于PMS(E0(HSO5−/HSO4−)=+1.82 vs. NHE;E0(S2O82−/HSO4−)=+2.08 vs. NHE),由于在过氧化物连接体的两侧存在两个SO3基团而产生的空间位阻,也使得PMS比PDS相对选定有机物的反应性更高,例如,PMS与芳香族和脂肪族醛的反应更快[11].
上述PMS和PDS的结构差异导致了具体活化过程的差异. 例如,由于离子结构不对称,PMS比PDS更容易被过渡金属(如Co2+、CuFe2O4、Fe2O3)激活生成SO4·−[16 − 17]. 在碳质材料和贵金属催化剂通过电子转移机制氧化有机物方面,PMS也比PDS更有效[18 − 19]. 相比于PMS,因为PDS键解离能较低,能量转移过程(即光解、热分解)激活PDS效果更明显[20 − 21]. 然而,相对处理效率取决于水基质. 由于水体背景成分的存在,如Cl−和HCO3−,使PMS氧化体系净水效果优于PDS. PMS与天然亲核试剂的反应提高了SO4·−的生成率,并产生二级非自由基氧化剂(如HOCl),而无机杂质在PDS过程中往往会淬灭自由基使其失去氧化活性[22 − 23]. 将pH值提高到PMS的pKa2以上(即9.3)会使摩尔吸收系数增加10倍(从13.8 (mol·L−1)−1·cm−1增加到149.5 (mol·L−1)−1·cm−1),从而提高UV/PMS过程的自由基生成效率[24]. 而在碱性条件下,UV/PDS体系中的主要物质从SO4·−转变为·OH,而摩尔吸收率和相关自由基生成率没有变化. 注意,由于pKa值极低,PDS在较宽的pH范围内不会发生质子化[25].
近年来,非自由基途径因其对复杂水体中痕量有机污染物的高效降解而在废水净化领域受到广泛关注. 具体来说,该反应途径对具有富电子基团的有机污染物具有更高的选择性,从而对多种无机离子和有机质等水环境背景物质具有更强的抵抗力[26]. 目前,对于非均相反应体系中的非自由基过程,提出了3种可能的反应途径:电子转移过程(固相催化剂作为电子传导媒介)、高价态金属氧化和单线态氧氧化[4]. 一般来说,过硫酸盐与催化剂表面金属物种之间的电子转移是ROS生成的主要过程. 因此,SAC中高度分散的单金属原子使催化中心数量和过硫酸盐活化的金属利用效率最大化[27 − 28],并且SAC中独特的电子结构使其表现出明显由于同类金属颗粒催化剂的过硫酸盐活化性能[29]. 与此同时,在有关于PS−AOPs的研究报道中,SAC中单金属原子的载体主要是各种碳材料,包括石墨烯[30]、还原氧化石墨烯[31]、碳纳米管[32]、非金属原子(如N、S、B等等)掺杂的石墨烯[33]、石墨氮[34]等等,其中往往含有丰富的潜在活性位点,如空穴结构、氧官能团和非金属原子等,可以作为过硫酸盐活化位点[35],或是作为污染物吸附位点促进体系中目标污染物的快速去除[36]. 其他载体材料,如金属氧化物(例如,MgO、FeOx、ZnO、WO3、CuO、Co3O4、Al2O3、CeO2)[37 − 38]和金属纳米颗粒(Pd、Pt、Rh、Ni、Ru、Cu、Ag、Au等)[39 − 40]也可作为SAC载体,此类SAC已被应用于多领域研究,而有关于PS−AOPs的研究报道有MXene[41]、Mn3O4[42]和In2O3[43]. 综上所述,SAC往往具有种类丰富、数量可观的活性位点,能够有效活化过硫酸盐产生自由基和非自由基氧化作用,实现目标污染物的高效去除.
-
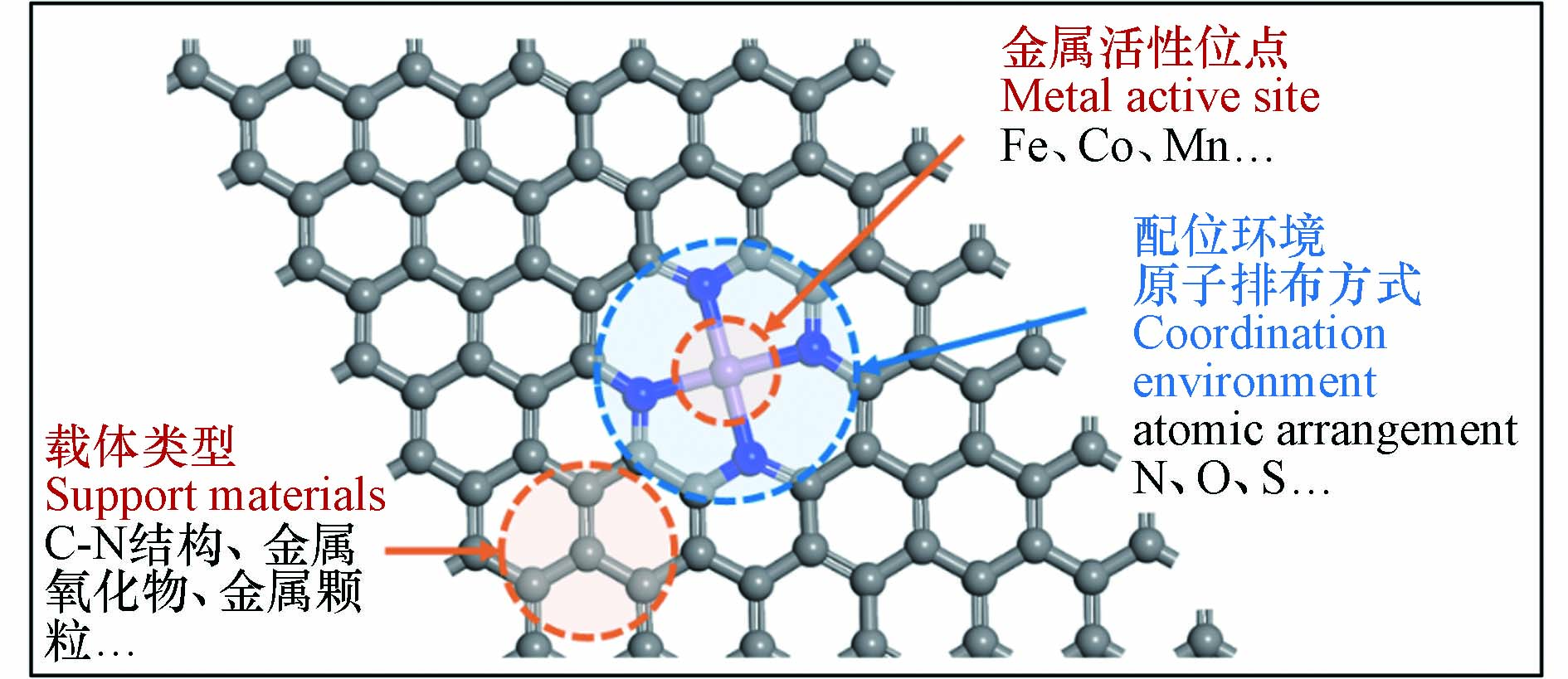
近年来,一系列SAC作为过硫酸盐活化剂被用于降解各种有机微污染物(表2),SAC均表现出远高于相应的具有纳米尺度金属位点的催化剂的催化性能[44]. 从催化机理看,纳米粒子结构复杂,其中组成金属原子可能位于不同的化学环境中发挥催化作用,因而活性位点种类繁杂,难以建立结构−性能关系. 一般而言,SAC的催化活性是由金属活性位点与载体类型、配位环境原子排布方式来协同决定的,如图2所示[45 − 46]. 相关研究从原子甚至电子层次下研究催化体系中的构效关系,揭示了过硫酸盐在催化剂的表面的反应机理、提出了相应的材料性能优化策略.
-
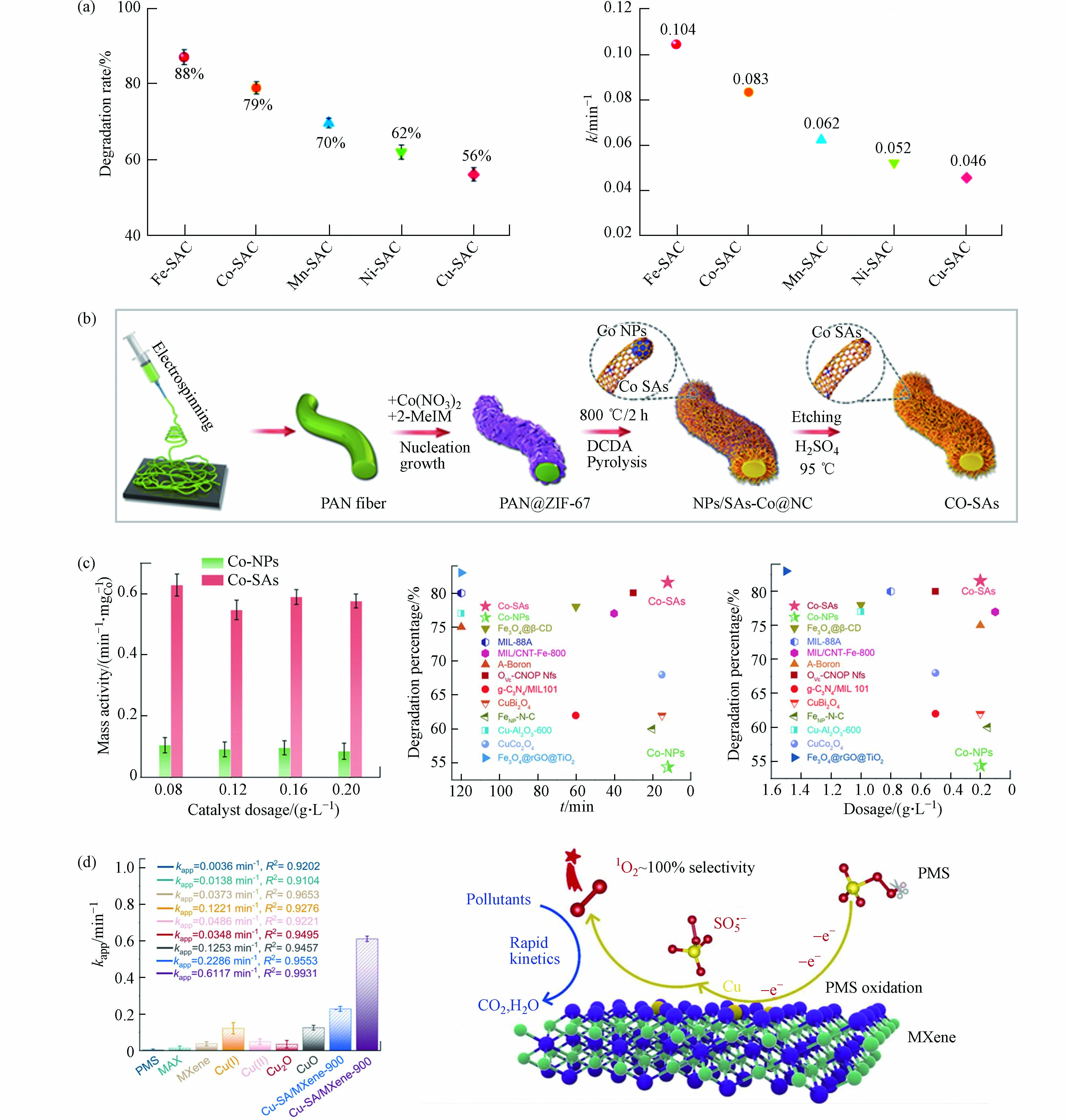
金属中心及其载体种类是影响氧化活性物质生成和氧化途径发生的关键性决定因素[46]. 相关研究表明,在保持金属原子负载量相近、配位环境相类似的情况下,具有不同的金属原子位点的SAC活化PMS将导致不同的ROS主导污染物的降解,污染物去除效果差异明显(图3a)[54]. Gao等[44]从Co−NPs中制备Co−SAC作为PMS催化剂,制备过程如图3b所示. Co−SAC比Co−NPs和其他Co基催化剂表现出显著提高的污染物分解活性(图3c). 研究者将Co−SAC的优异性能归因于单一金属原子的高密度和超细分散,进而最大限度地增加催化中心的数量和PMS活化反应中的金属利用效率,同时碳载体特性(石墨度、杂化和组成)也显著改变了SAC的电荷再分配,从而调节了催化剂对PMS的催化活性. 相似的结论在其他研究报告中也有提及[9, 55 − 56]. 目前大部分SAC是以具有C−N−金属配位的杂原子掺杂碳为载体,MXene的通式为Mn+1XnTx(M:过渡金属,X:C或N;Tx:表面官能团,如—OH、—O、—Cl或−F;n = 1、2或3),是一种过渡金属碳化物和碳氮化物,被广泛认定为一种优异的催化剂载体材料[57 − 59]. 将Cu单原子锚定在Ti3C2Tx MXene纳米片上的复合催化剂(Cu−SA/MXene−900)活化PMS降解污染物的效果明显优于其他含Cu催化剂,包括其同类型纳米催化剂(Cu−NP/MXene−900),如图3d中所示. 实验与密度泛函理论(density functional theory,DFT)计算结果表明,Cu−SA/MXene−900将PMS转化为1O2的效率高达99.71%[41],如图3d中所示,Cu−SA/MXene活化PMS时将选择性吸附PMS的末端O,促进SO5·−的产生进而转化为1O2. 因此,不同的金属活性位点和载体类型对于氧化活性物质和氧化途径的驱动机制有所差别.
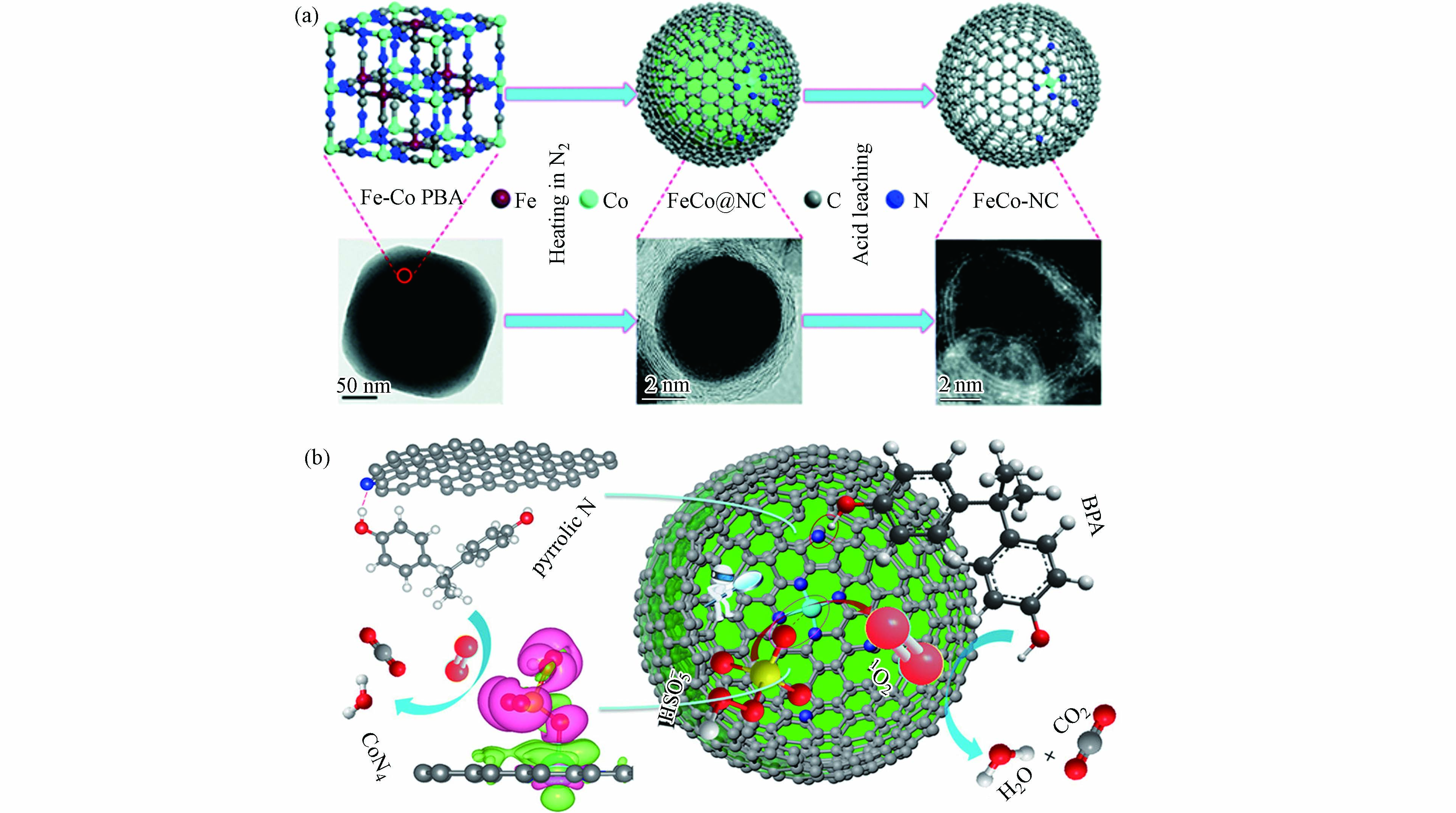
结合以上论述可知,合理的金属活性位点和载体类型组合有可能激发自由基和非自由基协同作用体系,有助于去除催化剂上的中间体,提高催化剂的稳定性和可重复使用性[60]. Li等[36]提出了Co−SAC激活PMS的双反应点催化机理,包括“供体−受体复合物(donor–acceptor complex)”机制和基于1O2的非自由基途径. Co−SAC由FeCo−PBA纳米球煅烧衍生制备(图4a),该催化剂活化PMS在6 min内对BPA的降解效率达100%,而不含钴的催化剂对BPA的去除率为22%,证明原子分散的Co对PMS的分解起着重要的催化作用. 通过理论计算验证了几种可能的活性位点与PMS之间的吸附能(Eads),结果表明Co−SAC中的吡咯N(CoN4)的吸附能最高. 因此,PMS更倾向与其结合并活化PMS分解生成1O2,与自由基淬灭和电子顺磁共振谱图(electron paramagnetic resonance,EPR)结果相一致. 同时,CoN4作为电子供体,而BPA中的−OH基团作为电子受体,两者之间遵循“供体−受体复合物”机制(图4b). 因此,基于CoN4位点上同步发生的PMS活化与污染物吸附过程,大大降低了ROS的迁移距离,原位生成的1O2与相邻的BPA在Co−SAC表面快速反应,进而大大提高了污染物降解速率. 马军院士课题组[61]近期研究报告中指出,在Co−SAC活化PMS体系中·OH和SO4·−主要来自催化剂表面CoNx位点与PMS之间的作用过程,与此同时,Co−SAC中N−C骨架将催化PMS诱导出现直接电子转移的非自由基氧化途径,而这种自由基氧化与非自由基氧化相结合的混合氧化途径使PMS氧化工艺在去除复杂水污染物方面具有广阔的应用前景. 相应地,实验结果证明Co−SAC活化PMS氧化体系在60 h的连续流反应中一直保持较高的污染物去除率. 因此,活性位点的催化机制及其周围ROS转化规律有所差异,明确的材料设计思路有助于开发自由基和非自由基协同作用体系.
除了直接改变位点的金属原子种类与载体类型外,通过调控SAC中原子排布也可以有效改善催化剂活性. 相关研究从原子尺度直观地揭示结构−活性和反应机理之间的关系,为定制具有高活性催化剂提供关键依据. Qi等[56]以木质素为碳源制备了N配位Co SAC用于活化PMS降解有机污染物,通过实验与DFT计算发现,Co单原子位点诱导的直接电子转移是体系中目标污染物降解的主要机制. 因为该催化剂在Co原子位点与配位N(C)处的电荷积累较多,此催化活性位点将作为高效电子转移渠道,使得污染物中的电子转移到过硫酸盐形成硫酸根离子进而得以分解. 对于N配位Fe SAC,相关研究指明PMS在Fe原子位点将首先被激活形成Fe−N−C−PMS*(*表示吸附位点),然后与邻近的Fe原子协调形成一个复合物,随后生成FeⅣ=O[62]. 该研究中确定了FeⅣ=O产生的浓度阈值,指明Fe1−Fe1距离为0.4 nm的铁原子是PMS活化反应生成FeⅣ=O的活性位点(图5a),通过PMS的双位点吸附形成络合物是关键步骤(图5b). 通过有机污染物降解实验证明,以FeⅣ=O为主要氧化活性物质的类Fenton体系具有优异的污染物去除能力、pH耐受性和对单电子氧化电位(one−electron oxidation potential)较高的污染物选择性降解. 除了金属原子位点间距以外,金属的平均氧化状态也会影响Fe SAC−PMS中自由基途径与高价物种途径的调控[63]. 处于高旋转状态的Fe2+−N4倾向于激活PMS形成SO4·−和·OH,而Fe3+−N4则容易生成Fe5+=O. 另外相关研究报告了通过将缺电子硼(B)或富电子磷(P)原子掺入碳基底的调控策略对Cu−N4电子结构的影响及其相应的PMS活化性能(图5c)[33]. 研究发现缺电子型Cu−N4/C−B催化剂表现出优异的PMS活化性能,而富电子型Cu−N4/C−P催化剂的性能明显低于Cu−N4/C催化剂,因为与B原子的长程相互作用降低了Cu活性位点的电子密度,从而优化了催化剂对于PMS的吸附能. 该研究提供了一种通过调节原子排布精细控制金属活性位点电子结构的方法. 类似地,通过将电子接收型氧化S基团(oxidized S groups)或电子供给型噻吩类S物种(thiophene−like S species)引入SACs的碳载体中,也可有效调整单个原子位点在氧还原反应(oxygen reduction reaction,ORR)过程中的动力学活性[64]. 然而,有关于S掺杂的SAC在过硫酸盐AOPs中的研究尚无报道. 除了碳基SAC,相关研究报道了在Mn3O4表面构建具有配位结构不对称的Co原子位点,探究金属原子配位环境对于催化剂性能的影响[42]. 该研究通过DFT计算揭示了N1O2配位结构中的N导致Co位点上发生了明显的电子离域现象,从而促进了催化剂表面对PMS的吸附、裂解和CoⅣ=O的形成.
由此可见,不同的金属活性位点与载体类型、配位环境原子排布方式对于氧化活性物质和氧化途径的驱动机制有所差别. 大部分文献中报道了不同的单原子金属位点被锚定在各种碳基质(例如,多孔碳、石墨烯和C3N4)上,从而形成用于PMS活化的碳基SAC,这些碳基SAC的制备方法主要有有机小分子、聚合物、MOFs和生物质的热解. 此外,在难降解有机污染物的降解过程中,通过单原子位点和碳载体中的非金属活性物质,触发了自由基和非自由基途径. 综上所述,对于PMS激活过程中无金属催化位点与SAC位点的协同作用及相互关系还有待进一步研究[10],金属载体SAC与非金属原子掺杂载体SAC的催化活性及其反应特性研究较少、缺乏相应案例说明.
-
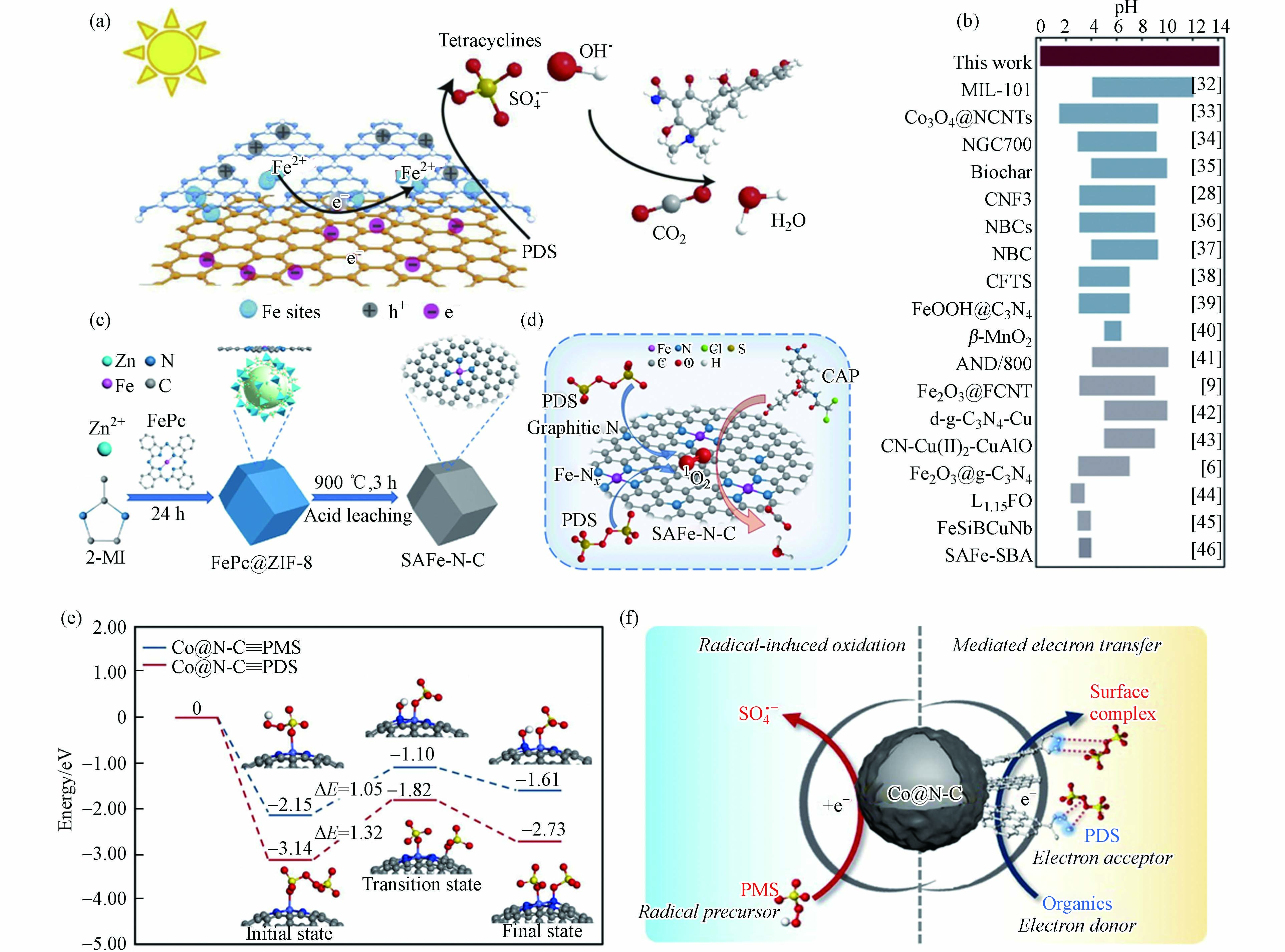
Zuo等[65]制备了锚定在双层C3N4−rGO载体中单原子Fe位点的催化剂(C3N4−Fe−rGO),表征结果证明Fe原子位于rGO和C3N4层之间,该碳基底具有优异的电子转移能力,有助于PDS被活化生成SO4·−(图6a). 特别地,双层结构显著抑制了铁原子的团聚和浸出,C3N4−Fe−rGO在0—14 pH范围内均表现出稳定的催化能力,这是目前非均相类Fenton体系所能达到的最宽pH范围(图6b). 然而,Jiang等[66]发现Fe单原子催化剂(Fe−N−C)活化PDS体系中没有自由基产生,而是生成了Fe(V),进而通过直接的双电子提取过程实现有机污染物的选择性降解. 此外,经热处理和酸浸处理后,酞菁铁和金属有机骨架合成了氮掺杂碳上的单原子铁(SAFe−N−C)(图6c)[67]. 原子分散的Fe−Nx位点的引入不仅提高了催化活性,而且调节了PDS活化的反应途径. 原子分散的Fe−Nx位点,产生更多的1O2,使反应途径以1O2为主(图6d). 因此,SAFe−N−C在较宽的pH范围内表现出较高的催化活性,并对无机阴离子具有良好的抗性. 基于以上分析可知,同种金属SAC活化PDS体系降解污染物反应过程中发挥主要作用的ROS不完全一致,说明活性位点ROS产生机制与其周围ROS转化规律有所差异,对此过程中发生的具体反应过程尚不明晰,影响污染物降解效果的关键因素有待阐明.
-
总而言之,SAC活化过硫酸盐的研究近年来取得了迅速的发展. 研究者致力于探索催化剂的活性位点,揭示其在催化氧化过程中的作用机制. 同时,一系列的原位分析方法被开发,用于深入研究SAC活化过硫酸盐体系中的ROS演化规律和污染物降解机制. 然而,对于SAC活化过硫酸盐体系的环境修复机制和实际应用,仍存在巨大的挑战. 未来的研究应集中在:
(1) 通过理论模拟和原位表征技术加强基础研究,填补过硫酸盐与SAC相互作用机制的研究空白——进一步确定反应过程中SAC表面活化的PDS/PMS的存在状况,揭示不同金属原子与其周围配位结构对氧化活性物质生成和转化的影响、明确单一反应物种对有机污染物整体降解的贡献及其协同效应. 与此同时,确定体系中氧化活性物质去除难降解有机污染物的效果及其造成有毒产物二次污染的风险.
(2) 以环境应用为基础进行材料设计与其反应器开发,积极应对与工程实践和复杂水质相关的挑战——之前综述了有关与PS−AOPs中纳米催化剂的固定化方法和反应器设计开发的最新研究进展,指明通过合理的高效催化剂设计,并结合膜技术、固定床反应器或其他集成设备或连续反应器中,是实现非均相PS−AOPs走向实际应用的必经之路. 此外,为了确认PS−AOPs的经济效益和环境后果,需要对反应系统的处理效果进行合理评估,涉及运行成本、可持续性和环境适用性.
单原子催化剂在过硫酸盐氧化体系中的研究进展
Research progress of single−atom catalysts in persulfate activation
-
摘要: 近年来,大量研究表明过硫酸盐高级氧化技术(PS−AOPs)是处理污水中难生物降解有机污染物的有效策略. 寻求高活性、高稳定性、低成本的催化剂是实现过硫酸盐高效活化降解新污染物的关键. 相比于传统金属纳米颗粒催化剂,单原子催化剂(SAC)在PS−AOPs中表现出超快的反应速率、较低的金属负载量以及强金属与载体相互作用等优点. 更重要的是,SAC具有原子级分布的活性中心,以便于从原子甚至电子层次下研究非均相催化体系中的构效关系和多种反应机制. 然而,目前SAC在PS−AOPs中的研究尚处于起步阶段. 本文重点综述了SAC活化过硫酸盐产生氧化活性物质的驱动机制,对结构调控与催化性能的相关性进行了系统总结,深入了解结构−性能关系,指出了精确调整催化剂活性位点的策略,并对相关研究领域未来的机遇和挑战提出了展望.Abstract: In recent years, persulfate−based advanced oxidation processes (PS−AOPs) have emerged as effective strategies for treating refractory organic pollutants in sewage. The key to achieving efficient persulfate activation for degrading such pollutants lies in the search for high-performance, highly stable, and cost−effective catalysts. In comparison to traditional metal nanoparticle catalysts, single−atom catalysts (SAC) used in PS−AOPs offer several advantages, including ultra−fast reaction rate, low metal loading capacity, and strong metal−carrier interaction. SAC exhibit atomic−level distribution of active centers, enabling the study of structure-activity relationships and multiple reaction mechanisms in heterogeneous catalytic systems at the atomic or even electronic level. However, research on SAC in PS−AOPs is still in its infancy. The review focuses on the activation mechanism of SAC in PS−AOPs to produce oxidation active species. The correlation between structural regulation and catalytic performance was systematically summarized, the structure−performance relationship was deeply understood, and the strategy of precise adjustment of catalyst active site was pointed out. Finally, the review provides insights into the opportunities and challenges in related research fields.
-
Key words:
- persulfate /
- advanced oxidation processes /
- single−atom catalyst /
- reaction mechanism.
-

-
图 3 (a)M−SACs在30min内对BPA的降解曲线及降解速率;(b)由IF−67包覆PAN纳米纤维制备Co−NPs和Co−SAC的方案;(c)不同体系中按Co重量归一化的反应速率常数,该体系和其他研究中提及的催化剂对BPA降解百分比(%)(反应时间和催化剂用量)[44];(d)双酚A在不同催化剂活化PMS作用下的速率常数,Cu−SA/MXene活化PMS高效转化生成1O2过程示意图[41]
Figure 3. (a) Degradation curves and degradation rate of BPA catalyzed by M−SACs within 30 min; (b) Preparation of Co−NPs and Co−SAC by IF−67 coated PAN nanofibers; (c) Reaction rate constants normalized by Co weight in different systems, and the percentage (%) of BPA degradation by catalysts mentioned in this system and other studies (reaction time and catalyst dosage)[44]; (d) Apparent rate constant of BPA in the presence of various catalysts/PMS and the schematic diagram of the efficient conversion process of Cu−SA/MXene activated PMS to generate 1O2 [41]
图 4 (a)Co−SAC的制备路线及相关的电镜图像,(b)Co−SAC活化PMS的双反应点机理示意图(紫色和绿色分别代表电子积累和电子消耗)[36]
Figure 4. (a) Preparation of Co−SAC and related electron microscope images. (b) Schematic diagram of the mechanism of the double reaction sites of Co−SAC activation of PMS (purple and green represent electron accumulation and electron consumption, respectively) [36]
图 5 (a)2Fe−N4的计算结构模型(粉色、蓝色和灰色的球分别代表Fe、N和C原子),(b)PMS在2Fe−N4位点上的吸附结构和相应的吸附能[62]; (c) Cu−N4/C−B和Cu−N4/C−P的制备策略示意图,色条表示Cu−N4位点的电子密度,富电子(蓝色)和缺电子(红色)[33]
Figure 5. (a) The computational structure model of 2Fe−N4 (the pink, blue and gray spheres represent Fe, N and C atoms, respectively), (b) the adsorption structure and corresponding adsorption energy of PMS at the 2Fe−N4 site[62]; Schematic of the preparation strategy for Cu−N4/C−B and Cu−N4/C−P. The color bar indicates the electronic density of Cu−N4 site, electro−rich (blue) and electro−poor (red)[33]
图 6 (a)C3N4−Fe−rGO活化PDS的反应机理示意图,(b)类Fenton反应催化剂的典型工作pH范围[65];(c)SAFe−N−C制备示意图,(d)PDS和SAFe−75−N−C体系的反应机理[67];(e)Co@N−C上PMS和PDS解离过程计算的势能分布和(f)催化剂表面反应机理示意图(浅蓝色、蓝色、灰色、红色、黄色和白色球体分别代表Co、N、C、O、S和H原子)[68]
Figure 6. (a) Schematic diagram of reaction mechanism of C3N4−Fe−rGO activation of PDS, (b) Typical working pH range of Fenton−like catalysts[65]; (c) Schematic diagram of preparation of SAFe−N−C, (d) Reaction mechanism of PDS and SAFe−75−N−C system[67]; (e) Potential energy distribution calculated during the dissociation of PMS and PDS at Co@N−C and (f) Schematic diagram of catalyst surface reaction mechanism (light blue, blue, grey, red, yellow and white spheres represent Co, N, C, O, S and H atoms, respectively)[68]
表 1 PMS和PDS理化性质、反应性和主要氧化剂对比
Table 1. Comparison of Physical–Chemical Properties, Reactivity, and Main Oxidants for PMS and PDS
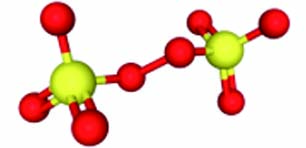
PMS PDS 离子式 HSO5− S2O82− 结构示意图 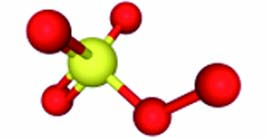
标准还原电位(E0 vs NHE.) 1.82 V 2.08 V 双氧键解离能 377 kJ·mol−1 92 kJ·mol−1 248 nm处的摩尔吸收系数 19.1 L·mol−1·cm−1 27.5 L·mol−1·cm−1 解离常数(pKa) 9.3 −3.5 与亲核试剂的反应性 对亲核试剂(如X−和HCO3−)的有效氧原子转移反应
将导致二级氧化剂的形成可忽略(在过量背景阴离子下稳定) 与自由基的
反应性与pH相关
pH < pKa2 = 9.3 (HSO5−)
k(SO4·−) < 105 ( mol·L−1·s)−1
k(·OH) = 1.7 × 107 (mol·L−1·s)−1
pH > pKa2 = 9.3 (SO52−)
k(SO4·−) < 105 (mol·L−1·s)−1
k(·OH) = 2.1 × 109 (mol·L−1·s)−1k(SO4·−) = 1.2 ×
106 (mol·L−1·s)−1首选活化方法 基于非均相催化剂的电子转移活化 基于能量转移的活化 表 2 单原子催化剂/过硫酸盐降解有机污染物概况
Table 2. Summary of degradation of organic pollutants by single atom catalyst/persulfate
催化剂
Catalyst单原子位点
Single atomic site反应条件
Reaction condition催化性能
Catalytic performance金属离子溶出
Metal ion leaching concentrationFe−g−C3N4 [47] Fe [亚甲基蓝]0 = 10 mg·L−1
[PMS]0 = 0.5 mmol·L−1
[催化剂]0 = 50 mg·L−1
反应时间 = 30 min100%(第一次循环)
~85%(第四次循环)
100%(再生处理后第五次循环)[Fe] = 0.022 mg·L−1(第1次循环)
[Fe] = 0.017 mg·L−1(第2次循环)
[Fe] = 0.011 mg·L−1(第3次循环)Fe−NC[28] Fe [污染物]0 = 22 mg·L−1
[PMS]0 = 0.5 mmol·L−1
[催化剂]0 = 100 mg·L−1
反应时间 = 160 s100%(双酚A)
~80%(磺胺甲恶唑)
100%(2−氯苯酚)
~35%(马卡西平)
100%(4−氯苯酚)[Fe] = 0.391 mg·L−1 Co−NC[48] Co [污染物]0 = 50 µmol·L−1
[PMS]0 = 0.24 mmol·L−1
[催化剂]0 = 20 mg·L−1
反应时间 = 15 min100%(苯甲酸)
100%(布洛芬)
100%(对羟基苯甲酸)
100%(双酚A)
100%(对氯苯酚)[Co] = 0.048 mg·L−1(第1次循环)
[Co] = 0.039 mg·L−1(第5次循环)Co−g−C3N4[34] Co [污染物]0 = 5 mg·L−1
[PMS]0 = 1.0 mmol·L−1
[催化剂]0 = 100 mg·L−1
反应时间 = 20 min100%(环丙沙星)
100%(磺胺甲恶唑)
100%(四环素)
100%(苯酚)— Mn−NC[49] Mn [磷酸氯喹]0 = 25 mg·L−1
[PMS]0 = 2.0 mmol·L−1
[催化剂]0 = 1000 mg·L−1
反应时间 = 30 min~90%(第一次循环)
~55%(第二次循环)
~55%(第三次循环)[Mn] = 0.018 mg·L−1 Mn−NC[50] Mn [污染物]0 = 20 mg·L−1
[PMS]0 = 0.2 g·L−1
[催化剂]0 = 200 mg·L−1
反应时间 = 6 min100%(双酚A)
100%(亚甲基橙)
100%(苯酚)
97.8%(环丙沙星)
100%(氧氟沙星)[Mn] = 2.097 mg·L−1 CuSA−NC[51] Cu [2,4 −二氯苯酚]0 = 100 µmol·L−1
[PDS]0 = 0.5 mmol·L−1
[催化剂]0 = 40 mg·L−1
反应时间 = 30 min~95% [Cu] = 0.013 mg·L−1 Cu−N4/C−B[33] Cu [双酚A]0 = 20 mg·L−1
[PMS]0 = 0.2 g·L−1
[催化剂]0 = 100 mg·L−1
反应时间 = 5 min~95% — Ni−NC[52] Ni [苯酚]0 = 10 mg·L−1
[PMS]0 = 0.1 g·L−1
[催化剂]0 = 100 mg·L−1
反应时间 = 60 min94.8%(第1次循环)
86.8%(第2次循环)
81.9%(第3次循环)[Ni] = 0.0291 mg·L−1(第1次循环)
[Ni] = 0.0111 mg·L−1(第2次循环)
[Ni] = 0.0058 mg·L−1(第3次循环)Fe/Cu–N–C[53] Fe和Cu [氯霉素]0 = 20 mg·L−1
[PDS]0 =5.0 mmol·L−1
[催化剂]0 = 100 mg·L−1
反应时间 = 60 min82.4%(第1次循环)
~70%(第5次循环)— Cu−SA/MXene[41] Cu [双酚A]0 = 10 mg·L−1
[PMS]0 = 2.0 mmol·L−1
[催化剂]0 = 500 mg·L−1
反应时间 = 10 min>90% (5次循环降解) [Cu] = 6.8×10−4 mg·L−1 pH=3.0
[Cu] = 4.5×10−4 mg·L−1 pH=5.0
[Cu] = 2.1×10−4 mg·L−1 pH=7.0
[Cu] = 1.6×10−4 mg·L−1 pH=9.0
[Cu] = 0.8×10−4 mg·L−1 pH=11.0Co SAC/Mn3O4[42] Co [磺胺甲恶唑]0 = 2 mg·L−1
[PMS]0 = 0.2 mmol·L−1
[催化剂]0 = 200 mg·L−1
反应时间 = 30 min>90% (6次循环降解) [Co] = ~0.055 mg·L−1(第1次循环)
[Co] = ~0 mg·L−1(第6次循环)Cu−In2O3/Ov Cu [四环素]0 = 20 mg·L−1
[PMS]0 = 1 mmol·L−1
[催化剂]0 = 500 mg·L−1
反应时间 = 20 min>90% (5次循环降解) [Cu] = 0.032 mg·L−1 -
[1] CHEN C B, FENG H, DENG Y. Re-evaluation of sulfate radical based-advanced oxidation processes (SR-AOPs) for treatment of raw municipal landfill leachate[J]. Water Research, 2019, 153: 100-107. doi: 10.1016/j.watres.2019.01.013 [2] HODGES B C, CATES E L, KIM J H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials[J]. Nature Nanotechnology, 2018, 13(8): 642-650. doi: 10.1038/s41565-018-0216-x [3] 钱林波, 李航宇, 陈梦舫. 高级氧化技术修复苯并[a]芘污染土壤研究进展[J]. 环境化学, 2022, 41(10): 3205-3213. doi: 10.7524/j.issn.0254-6108.2021110203 QIAN L B, LI H Y, CHEN M F. Research progress on the remediation of benzo[a]pyrene contaminated soil by advanced oxidation technology[J]. Environmental Chemistry, 2022, 41(10): 3205-3213 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021110203
[4] GUO R N, XI B D, GUO C S, et al. Comprehensive insight into heterogeneous persulfate activation for environmental pollutants degradation: Approaches and mechanism[J]. Environmental Functional Materials, 2022, 1(3): 2773-0581. [5] 徐梓淞, 宋雄伟, 黄闻宇, 等. 不同活化过硫酸盐体系的机理分析及不同无机阴离子的作用: 以两种有机染料为例[J]. 环境化学, 2022, 41(4): 1412-1424. doi: 10.7524/j.issn.0254-6108.2020122103 XU Z S, SONG X W, HUANG W Y, et al. Mechanism analysis of different activated persulfate systems and effects of different inorganic anions: A case study of two organic dyes[J]. Environmental Chemistry, 2022, 41(4): 1412-1424 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020122103
[6] MOLNÁR Á, PAPP A. Catalyst recycling—a survey of recent progress and current status[J]. Coordination Chemistry Reviews, 2017, 349: 1-65. doi: 10.1016/j.ccr.2017.08.011 [7] XIA J X, WANG B, DI J, et al. Construction of single-atom catalysts for electro-, photo- and photoelectro-catalytic applications: State-of-the-art, opportunities, and challenges[J]. Materials Today, 2022, 53: 217-237. doi: 10.1016/j.mattod.2021.11.022 [8] YANG J R, LI W H, WANG D S, et al. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis[J]. Advanced Materials, 2020, 32(49): e2003300. doi: 10.1002/adma.202003300 [9] LIU D B, HE Q, DING S Q, et al. Structural regulation and support coupling effect of single-atom catalysts for heterogeneous catalysis[J]. Advanced Energy Materials, 2020, 10(32): 2001482. doi: 10.1002/aenm.202001482 [10] SHANG Y N, XU X, GAO B Y, et al. Single-atom catalysis in advanced oxidation processes for environmental remediation[J]. Chemical Society Reviews, 2021, 50(8): 5281-5322. doi: 10.1039/D0CS01032D [11] LEE J, von GUNTEN U, KIM J H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks[J]. Environmental Science & Technology, 2020, 54(6): 3064-3081. [12] PENG Y T, TANG H M, YAO B, et al. Activation of peroxymonosulfate (PMS) by spinel ferrite and their composites in degradation of organic pollutants: A Review[J]. Chemical Engineering Journal, 2021, 414: 128800. [13] LENTE G, KALMÁR J, BARANYAI Z, et al. One- versus two-electron oxidation with peroxomonosulfate ion: Reactions with iron(Ⅱ), vanadium(Ⅳ), halide ions, and photoreaction with cerium(Ⅲ)[J]. Inorganic Chemistry, 2009, 48(4): 1763-1773. doi: 10.1021/ic801569k [14] LI J A, JIANG J, ZHOU Y, et al. Kinetics of oxidation of iodide (I–) and hypoiodous acid (HOI) by peroxymonosulfate (PMS) and formation of iodinated products in the PMS/I–/NOM system[J]. Environmental Science & Technology Letters, 2017, 4(2): 76-82. [15] RIVAS F J, SOLÍS R R. Chloride promoted oxidation of tritosulfuron by peroxymonosulfate[J]. Chemical Engineering Journal, 2018, 349: 728-736. doi: 10.1016/j.cej.2018.05.117 [16] JAAFARZADEH N, GHANBARI F, AHMADI M. Catalytic degradation of 2, 4-dichlorophenoxyacetic acid (2,4-D) by nano-Fe2O3 activated peroxymonosulfate: Influential factors and mechanism determination[J]. Chemosphere, 2017, 169: 568-576. doi: 10.1016/j.chemosphere.2016.11.038 [17] GUAN Y H, MA J, REN Y M, et al. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals[J]. Water Research, 2013, 47(14): 5431-5438. doi: 10.1016/j.watres.2013.06.023 [18] LEE H, LEE H J, JEONG J, et al. Activation of persulfates by carbon nanotubes: Oxidation of organic compounds by nonradical mechanism[J]. Chemical Engineering Journal, 2015, 266: 28-33. doi: 10.1016/j.cej.2014.12.065 [19] YUN E T, MOON G H, LEE H, et al. Oxidation of organic pollutants by peroxymonosulfate activated with low-temperature-modified nanodiamonds: Understanding the reaction kinetics and mechanism[J]. Applied Catalysis B:Environmental, 2018, 237: 432-441. doi: 10.1016/j.apcatb.2018.04.067 [20] HE X X, deLa CRUZ A A, DIONYSIOU D D. Destruction of cyanobacterial toxin cylindrospermopsin by hydroxyl radicals and sulfate radicals using UV-254 nm activation of hydrogen peroxide, persulfate and peroxymonosulfate[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2013, 251: 160-166. doi: 10.1016/j.jphotochem.2012.09.017 [21] LUO C W, MA J, JIANG J, et al. Simulation and comparative study on the oxidation kinetics of atrazine by UV/H2O2, UV/HSO5− and UV/S2O82−[J]. Water Research, 2015, 80: 99-108. doi: 10.1016/j.watres.2015.05.019 [22] AO X W, LIU W J. Degradation of sulfamethoxazole by medium pressure UV and oxidants: Peroxymonosulfate, persulfate, and hydrogen peroxide[J]. Chemical Engineering Journal, 2017, 313: 629-637. doi: 10.1016/j.cej.2016.12.089 [23] MAHDI-AHMED M, CHIRON S. Ciprofloxacin oxidation by UV-C activated peroxymonosulfate in wastewater[J]. Journal of Hazardous Materials, 2014, 265: 41-46. doi: 10.1016/j.jhazmat.2013.11.034 [24] GUAN Y H, MA J, LI X C, et al. Influence of pH on the formation of sulfate and hydroxyl radicals in the UV/peroxymonosulfate system[J]. Environmental Science & Technology, 2011, 45(21): 9308-9314. [25] KOLTHOFF I M, MILLER I K. The chemistry of persulfate. I. the kinetics and mechanism of the decomposition of the persulfate ion in aqueous Medium1[J]. Journal of the American Chemical Society, 1951, 73(7): 3055-3059. doi: 10.1021/ja01151a024 [26] JAWAD A, ZHAN K, WANG H B, et al. Tuning of persulfate activation from a free radical to a nonradical pathway through the incorporation of non-redox magnesium oxide[J]. Environmental Science & Technology, 2020, 54(4): 2476-2488. [27] YANG M, WU K Y, SUN S D, et al. Unprecedented relay catalysis of curved Fe1–N4 single-atom site for remarkably efficient 1O2 generation[J]. ACS Catalysis, 2023, 13(1): 681-691. doi: 10.1021/acscatal.2c05409 [28] SHAO S T, CUI J H, WANG K, et al. Efficient and durable single-atom Fe catalyst for fenton-like reaction via mediated electron-transfer mechanism[J]. ACS ES& T Engineering, 2023, 3(1): 36-44. [29] ZHANG L S, JIANG X H, ZHONG Z A, et al. Carbon nitride supported high-loading Fe single-atom catalyst for activation of peroxymonosulfate to generate 1O2 with 100% selectivity[J]. Angewandte Chemie (International Ed. in English), 2021, 60(40): 21751-21755. doi: 10.1002/anie.202109488 [30] GAO Y W, ZHU Y, LI T, et al. Unraveling the high-activity origin of single-atom iron catalysts for organic pollutant oxidation via peroxymonosulfate activation[J]. Environmental Science & Technology, 2021, 55(12): 8318-8328. [31] CHEN F, WU X L, YANG L, et al. Efficient degradation and mineralization of antibiotics via heterogeneous activation of peroxymonosulfate by using graphene supported single-atom Cu catalyst[J]. Chemical Engineering Journal, 2020, 394: 124904. doi: 10.1016/j.cej.2020.124904 [32] QIAN K, CHEN H, LI W L, et al. Single-atom Fe catalyst outperforms its homogeneous counterpart for activating peroxymonosulfate to achieve effective degradation of organic contaminants[J]. Environmental Science & Technology, 2021, 55(10): 7034-7043. [33] ZHOU X, KE M K, HUANG G X, et al. Identification of Fenton-like active Cu sites by heteroatom modulation of electronic density[J]. Proceedings of the National Academy of Sciences of the United States of America, 2022, 119(8): e2119492119. [34] WANG Z W, ALMATRAFI E, WANG H, et al. Cobalt single atoms anchored on oxygen-doped tubular carbon nitride for efficient peroxymonosulfate activation: Simultaneous coordination structure and morphology modulation[J]. Angewandte Chemie International Edition, 2022, 61(29): e202202338. doi: 10.1002/anie.202202338 [35] HAN B, LUO Y, LIN Y F, et al. Microenvironment engineering of single-atom catalysts for persulfate-based advanced oxidation processes[J]. Chemical Engineering Journal, 2022, 447: 137551. doi: 10.1016/j.cej.2022.137551 [36] LI X N, HUANG X, XI S B, et al. Single cobalt atoms anchored on porous N-doped graphene with dual reaction sites for efficient fenton-like catalysis[J]. Journal of the American Chemical Society, 2018, 140(39): 12469-12475. doi: 10.1021/jacs.8b05992 [37] LANG R, DU X R, HUANG Y K, et al. Single-atom catalysts based on the metal-oxide interaction[J]. Chemical Reviews, 2020, 120(21): 11986-12043. doi: 10.1021/acs.chemrev.0c00797 [38] CHOI C H, KIM M, KWON H C, et al. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst[J]. Nature Communications, 2016, 7: 10922. doi: 10.1038/ncomms10922 [39] HANNAGAN R T, GIANNAKAKIS G, FLYTZANI-STEPHANOPOULOS M, et al. Single-atom alloy catalysis[J]. Chemical Reviews, 2020, 120(21): 12044-12088. doi: 10.1021/acs.chemrev.0c00078 [40] QIAO B T, WANG A Q, YANG X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeOx[J]. Nature Chemistry, 2011, 3(8): 634-641. doi: 10.1038/nchem.1095 [41] YANG P Z, LONG Y H, HUANG W L, et al. Single-atom copper embedded in two-dimensional MXene toward peroxymonosulfate activation to generate singlet oxygen with nearly 100% selectivity for enhanced Fenton-like reactions[J]. Applied Catalysis B:Environmental, 2023, 324: 122245. doi: 10.1016/j.apcatb.2022.122245 [42] LI X, WEN X, LANG J Y, et al. CoN1O2 single-atom catalyst for efficient peroxymonosulfate activation and selective cobalt(Ⅳ)=O generation[J]. Angewandte Chemie International Edition, 2023, 62(27): e202303267. doi: 10.1002/anie.202303267 [43] ZHAO Z Y, WANG P F, SONG C L, et al. Enhanced interfacial electron transfer by asymmetric Cu-Ov-In sites on In2O3 for efficient peroxymonosulfate activation[J]. Angewandte Chemie International Edition, 2023, 62(11): e202216403. doi: 10.1002/anie.202216403 [44] GAO Y, YANG C D, ZHOU M, et al. Transition metal and metal-Nx codoped MOF-derived fenton-like catalysts: A comparative study on single atoms and nanoparticles[J]. Small, 2020, 16(50): e2005060. doi: 10.1002/smll.202005060 [45] BENIYA A, HIGASHI S. Towards dense single-atom catalysts for future automotive applications[J]. Nature Catalysis, 2019, 2(7): 590-602. doi: 10.1038/s41929-019-0282-y [46] LI X N, HUANG Y Q, LIU B. Catalyst: Single-atom catalysis: Directing the way toward the nature of catalysis[J]. Chem, 2019, 5(11): 2733-2735. doi: 10.1016/j.chempr.2019.10.004 [47] 彭小明, 吴健群, 戴红玲, 等. Fe、N共掺杂原子级分散Fe-g-C3N4催化剂活化过硫酸盐降解亚甲基蓝的机制[J]. 环境科学学报, 2022, 42(5): 225-236. doi: 10.13671/j.hjkxxb.2021.0342 PENG X M, WU J Q, DAI H L, et al. Degradation mechanism of methylene blue by Fe-g-C3N4 catalyst activation peroxymonosulfate[J]. Acta Scientiae Circumstantiae, 2022, 42(5): 225-236 (in Chinese). doi: 10.13671/j.hjkxxb.2021.0342
[48] LI H C, QIAN J S, PAN B C. N-coordinated Co containing porous carbon as catalyst with improved dispersity and stability to activate peroxymonosulfate for degradation of organic pollutants[J]. Chemical Engineering Journal, 2021, 403: 126395. doi: 10.1016/j.cej.2020.126395 [49] YANG Q A, WANG W X, ZHOU Y Y, et al. Facile pyrolysis treatment for the synthesis of single-atom Mn catalysts derived from a hyperaccumulator[J]. ACS ES& T Engineering, 2023, 3(5): 616-626. [50] YANG J R, ZENG D Q, ZHANG Q G, et al. Single Mn atom anchored on N-doped porous carbon as highly efficient Fenton-like catalyst for the degradation of organic contaminants[J]. Applied Catalysis B:Environmental, 2020, 279: 119363. doi: 10.1016/j.apcatb.2020.119363 [51] LI F, LU Z C, LI T, et al. Origin of the excellent activity and selectivity of a single-atom copper catalyst with unsaturated Cu-N2 sites via peroxydisulfate activation: Cu(III) as a dominant oxidizing species[J]. Environmental Science & Technology, 2022, 56(12): 8765-8775. [52] 彭小明, 吴健群, 戴红玲, 等. Ni-N-C单原子催化剂活化过硫酸盐降解苯酚[J]. 高等学校化学学报, 2021, 42(8): 2581-2591. PENG X M, WU J Q, DAI H L, et al. Activation of peroxymonosulfate by single atom catalysts Ni-N-C for high efficiency degradation of phenol[J]. Chemical Journal of Chinese Universities, 2021, 42(8): 2581-2591 (in Chinese).
[53] WU H H, YAN J J, XU X, et al. Synergistic effects for boosted persulfate activation in a designed Fe-Cu dual-atom site catalyst[J]. Chemical Engineering Journal, 2022, 428: 132611. doi: 10.1016/j.cej.2021.132611 [54] GAO Y, WU T W, YANG C D, et al. Activity trends and mechanisms in peroxymonosulfate-assisted catalytic production of singlet oxygen over atomic metal-N-C catalysts[J]. Angewandte Chemie International Edition, 2021, 60(41): 22513-22521. doi: 10.1002/anie.202109530 [55] PENG L, DUAN X G, SHANG Y N, et al. Engineered carbon supported single iron atom sites and iron clusters from Fe-rich Enteromorpha for Fenton-like reactions via nonradical pathways[J]. Applied Catalysis B:Environmental, 2021, 287: 119963. doi: 10.1016/j.apcatb.2021.119963 [56] QI Y F, LI J, ZHANG Y Q, et al. Novel lignin-based single atom catalysts as peroxymonosulfate activator for pollutants degradation: Role of single cobalt and electron transfer pathway[J]. Applied Catalysis B:Environmental, 2021, 286: 119910. doi: 10.1016/j.apcatb.2021.119910 [57] JIN L M, YOU S J, YAO Y, et al. An electroactive single-atom copper anchored MXene nanohybrid filter for ultrafast water decontamination[J]. Journal of Materials Chemistry A, 2021, 9(46): 25964-25973. doi: 10.1039/D1TA07396F [58] DING L, WEI Y Y, LI L B, et al. MXene molecular sieving membranes for highly efficient gas separation[J]. Nature Communications, 2018, 9: 155. doi: 10.1038/s41467-017-02529-6 [59] ZHANG M M, LAI C, LI B S, et al. MXenes as superexcellent support for confining single atom: Properties, synthesis, and electrocatalytic applications[J]. Small, 2021, 17(29): e2007113. doi: 10.1002/smll.202007113 [60] REN W, CHENG C, SHAO P H, et al. Origins of electron-transfer regime in persulfate-based nonradical oxidation processes[J]. Environmental Science & Technology, 2022, 56(1): 78-97. [61] XU H D, JIANG N, WANG D, et al. Improving PMS oxidation of organic pollutants by single cobalt atom catalyst through hybrid radical and non-radical pathways[J]. Applied Catalysis B:Environmental, 2020, 263: 118350. doi: 10.1016/j.apcatb.2019.118350 [62] CHENG C, REN W, MIAO F, et al. Generation of FeIV=O and its contribution to fenton-like reactions on a single-atom iron-N-C catalyst[J]. Angewandte Chemie International Edition, 2023, 62(10): e202218510. doi: 10.1002/anie.202218510 [63] ZHANG B F, LI X Q, AKIYAMA K, et al. Elucidating the mechanistic origin of a spin state-dependent FeNx-C catalyst toward organic contaminant oxidation via peroxymonosulfate activation[J]. Environmental Science & Technology, 2022, 56(2): 1321-1330. [64] MUN Y, LEE S, KIM K, et al. Versatile strategy for tuning ORR activity of a single Fe-N4 site by controlling electron-withdrawing/donating properties of a carbon plane[J]. Journal of the American Chemical Society, 2019, 141(15): 6254-6262. doi: 10.1021/jacs.8b13543 [65] ZUO S J, JIN X M, WANG X W, et al. Sandwich structure stabilized atomic Fe catalyst for highly efficient Fenton-like reaction at all pH values[J]. Applied Catalysis B:Environmental, 2021, 282: 119551. doi: 10.1016/j.apcatb.2020.119551 [66] JIANG N, XU H D, WANG L H, et al. Nonradical oxidation of pollutants with single-atom-Fe(III)-activated persulfate: Fe(V) being the possible intermediate oxidant[J]. Environmental Science & Technology, 2020, 54(21): 14057-14065. [67] DU N J, LIU Y, LI Q J, et al. Peroxydisulfate activation by atomically-dispersed Fe-Nx on N-doped carbon: Mechanism of singlet oxygen evolution for nonradical degradation of aqueous contaminants[J]. Chemical Engineering Journal, 2021, 413: 127545. doi: 10.1016/j.cej.2020.127545 [68] XUE Y D, PHAM N N T, NAM G, et al. Persulfate activation by ZIF-67-derived cobalt/nitrogen-doped carbon composites: Kinetics and mechanisms dependent on persulfate precursor[J]. Chemical Engineering Journal, 2021, 408: 127305. doi: 10.1016/j.cej.2020.127305 [69] YANG G, DONG J W, XING B, et al. Ni, Fe, and N-tridoped activated carbon as a highly active heterogeneous persulfate catalyst toward the degradation of organic pollutant in water[J]. Separation and Purification Technology, 2020, 252: 117440. doi: 10.1016/j.seppur.2020.117440 [70] WANG C, YANG Q Q, LI Z H, et al. A novel carbon-coated Fe-C/N composite as a highly active heterogeneous catalyst for the degradation of Acid Red 73 by persulfate[J]. Separation and Purification Technology, 2019, 213: 447-455. doi: 10.1016/j.seppur.2018.12.072 -



 下载:
下载: