-
四环素类抗生素有良好的预防和治疗疾病的作用,占兽用抗生素总使用量的57%[1],但其给药量的60%—90%会以动物排泄物的形式进入环境[2],此外在人尿中检出浓度为0.19—7.69 μg·L−1[3]. 因此,四环素类抗生素的检出率和含量在环境中居于榜首[4 − 5],在表层土壤中四环素类抗生素的平均检出率最高为76%[6],浓度范围为μg·kg−1至mg·kg−1[7]. 天津城郊土壤中四环素类抗生素含量高达1.34×103 μg·kg−1,公园土壤中四环素的检出率为85.60%[8]. 因中水灌溉北京市公园土壤中四环素类抗生素浓度为145.2 μg·kg−1[9].
厨余垃圾的资源化技术通常包括堆肥、厌氧发酵、焚烧和生产生物柴油等[10],但由于厨余垃圾高含水量、高盐份、高含油量的特点导致这些处理技术在应用中存在难点. 堆肥和厌氧发酵虽可实现厨余垃圾的资源化,但高油高盐不利于微生物生长;焚烧在脱水环节就消耗大量能量而限制了该技术的应用;厨余垃圾的复杂组成难以保证生物柴油的转化率和纯度[11]. 随着城市生活垃圾的分类和资源化需求,亟需探讨生活垃圾资源化的新技术,如将生活垃圾制备成生物炭.
生物炭是在低氧环境下热解生成的固体产物,比表面积大、孔隙结构发达且表面官能团丰富[12]. 生物炭加入土壤中后,影响土壤的孔隙结构和离子交换作用,从而影响抗生素的环境行为[13],是控制抗生素污染的一种理想吸附材料. 草原土壤加入生物炭后,增加了四环素的有效性而加速了微生物对四环素的降解,四环素及其中间体的去除率增加了10%左右[14]. 在土壤表面施加改性的生物炭后,仅在0—30 cm的表层土壤中检出四环素,有效抑制了四环素向深层土壤进行迁移[15]. 溶解性有机质(dissolved organic matter,DOM)是一类组成复杂、物化性质活跃的非均相混合物[16 − 17],既可能含有多种游离氨基酸、糖等低分子量物质,又含有各种类型的大分子组分,如酶、氨基糖、腐殖酸、多酚等,因而具有疏水芳香性和亲水性两种性质[18 − 19]. 因含有丰富的苯酚、羟基等有机官能团和高芳香族化合物[20],DOM可以直接参与污染物的络合. 因此,生物炭源DOM在抗生素的环境行为方面的作用值得探究.
四环素类抗生素属于两性化合物,含有酚羟基和烯醇式羟基而显弱酸性,含有二甲氨基而显弱碱性. 因此DOM的疏水性和亲水性,可能会影响DOM与四环素类抗生素的结合能力. 分馏技术可以用于表征DOM的亲水性和疏水性. 具有离子交换吸附能力和反相保留的固相萃取柱,因含有聚合物而对疏水性和部分透明性化合物均有较好的吸附作用,能够吸附树脂无法吸附的弱酸性和弱碱性化合物,具有更高的分馏效应. 利用固相萃取柱成功地将DOM分馏成4个成分并研究了与Cu的络合作用[21]. 然而,生物炭DOM及其馏分对抗生素络合方面的研究鲜见报道,本研究将城市生活垃圾制备成生物炭,探究DOM不同萃取组分对四环素的络合作用. 三维荧光光谱结合平行因子分析(three-dimensional excitation emission matrix with parallel factor analysis, 3DEEM-PARAFAC)和同步荧光光谱(synchronous fluorescence, SF)是阐明荧光团与金属离子相互作用机制的高效、灵敏且快速的方法[22],此外,二维相关光谱(two-dimensional correlation spectroscopy, 2D-COS)分析进一步提供了分子结构随猝灭剂添加的变化信息和顺序. 因此,本研究拟借助这些光谱技术进行多光谱研究,可以多角度探究DOM中不同分馏组分络合四环素的顺序和能力.
-
参照吴文雨等[21]的方法制备生活垃圾生物炭:厨余米饭、菜叶、骨头和果皮(比例为4:4:1:1)混合均匀后,烘干,升温速率10 ℃·min−1,终温800 ℃. 生物炭的基本性质:溶解性有机碳(dissolved organic carbon, DOC)浓度为
2320 mg·kg−1,pH值为10.6,水溶性盐分为6.15 mS·cm−1.混合型阴离子交换反相吸附剂(mixed anion exchange reversed-phase adsorbent, Waters Oasis MAX, 200 mg)和混合型阳离子交换反相吸附剂(mixed cation exchange reversed-phase adsorbent, Waters Oasis MCX, 200 mg)购自美国Waters公司. 乙腈(LC-MS级)购自美国Fisher Scientific 公司. 盐酸、甲酸、氢氧化铵、氢氧化钠和盐酸等分析级试剂购自国药集团. 四环素(tetracycline, TC, 分析纯)购自上海源叶公司,基本理化性质见表1。
-
DOM的浸提:将生活垃圾生物炭用研钵研磨后过100目,按照10∶1的液固比加入超纯水,在25 ℃下以250 r·min−1振荡24 h,以
3000 r·min−1离心20 min,上清液过0.45 μm玻璃纤维滤膜,得到的滤液即为DOM[23].两种固相萃取柱(MAX和MCX)将DOM分馏成4个馏分:疏水酸性组分(hydrophobic-acidic component, HOA)、疏水中性组分(hydrophobic-neutral component, HON)、疏水碱性组分(hydrophobic-base component, HOB)和亲水性组分(hydrophilic substance, HIS). 4个馏分的分馏步骤参考Fang等[24]和吴文雨等[21,23].
-
将生活垃圾生物炭DOM(以下简称Raw-DOM)和分馏后的三馏分(HON、HOB和HIS)按DOC稀释到10 mg·L−1,分别取2.475 mL HON、2.475 mL HOB、4.950 mL HIS和4.950 mL Raw-DOM加入离心管,然后添加不同体积的TC储备液(6.0 mmol·L−1,溶于甲醇),使TC在离心管中的浓度分别为0、10、20、30、40、50、60 μmol·L−1(离心管中甲醇的体积不超过总体积的1%),每个浓度设置2个重复. 然后将离心管放置在25 ℃恒温振荡器上以250 r·min−1避光振荡24 h. 反应平衡后进行紫外光谱、同步荧光光谱和EEM光谱测定.
-
DOC、紫外光谱、同步荧光光谱和EEM光谱测定参照吴文雨等[23]:总有机碳分析仪(TOC V-CPH, Elementar)测定DOC浓度;荧光分光光度计(Lumina, Thermo)测定同步荧光光谱,激发波长200—450 nm,发射波长和激发波长差值固定为60 nm,扫描速度300 nm·min−1,波长间隔0.1 nm;紫外可见分光光度计(Specord 250 plus, 德国)测定紫外可见光谱,波长间隔1 nm.
荧光分光光度计(F-
4600 , 日立)测定EEM光谱:激发波长范围220—450 nm,间隔5 nm;发射波长范围为260—600 nm,间隔1 nm;扫描速度12000 nm·min−1. 利用Matlab 2016b中的DOMfluor工具箱进行瑞丽散射和拉曼散射的修正,通过平行因子分析对光谱数据进行定量分析,并用Matlab 2016b绘制三维荧光图. -
分别利用Stern—Volmer模型(式1)和修正的Stern—Volmer模型(式2)对猝灭过程进行拟合[25]. Stern—Volmer模型可用于判断猝灭类型,修正的Stern—Volmer模型假设引起荧光猝灭反应的结合点位与猝灭剂为1:1结合的静态猝灭.
式中,
$ {F}_{0} $ 和F分别代表未加入TC的初始的荧光峰强度和加入不同TC浓度下的荧光峰强度;Ksv为Stern-Volmer方程的猝灭常数(L·mol−1),当猝灭类型为静态猝灭时,其值即为结合常数;kq为双分子猝灭常数(L·mol−1·s−1),当kq值大于最大散射猝灭常数2×1010 L·mol−1·s−1时,可认为是静态猝灭,反之即为动态猝灭;$ {\tau }_{0} $ 为生物大分子荧光寿命,为10−8 s;$ {f} $ 为可猝灭的荧光团比例,$ \left[\mathrm{T}\mathrm{C}\right] $ 为加入的TC浓度(μmol·L−1),$ {K}_{\mathrm{a}} $ 是有效猝灭常数(L·mol−1).为进一步探究TC-DOM体系间的作用情况,继续应用位点结合方程进行拟合[26]. 该模型假设猝灭剂与DOM以1:n结合,如式3所示:
式中n为结合位点数,Kb是结合常数(L·mol−1),其余参数与式1相同. 运用Origin 2021软件对3个公式分别进行拟合.
-
DOM中可溶性有机碳的含量可以用DOC的浓度来表征. 生活垃圾浸提的DOM中,DOC浓度为174.21 mg·L−1,分馏后将4个馏分(HOA、HON、HOB和HIS)氮吹干燥后,用超纯水定容至10 mL,DOC的浓度分别为8.89、50.88、29.02、68.41 mg·L−1,按DOC计算回收率约90.24%. 4个馏分以DOC值计算分别占5.66%、18.46%、32.37%和43.52%,其中疏水性组分(HOA、HON、HOB)为56.48%,略低于吴文雨等[21]和Fang等[23]用同样的方法分别对生活垃圾生物炭和炼油厂废水DOM的分馏结果.
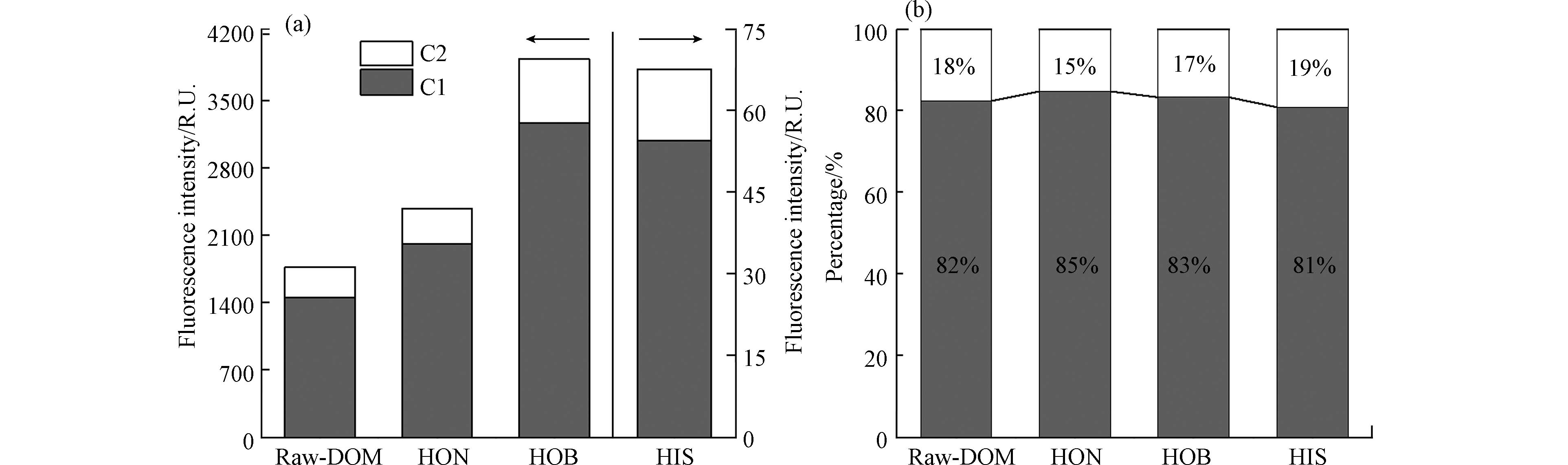
为避免内滤效应,将DOM原液(以下简称Raw-DOM)和3个馏分(HON、HOB和HIS)按DOC稀释到10 mg·L−1,用荧光分光光度计测定3DEEM光谱(图1). Em<380 nm的荧光峰通常认为含有羟基和氨基等电子供体基团的苯环,被鉴定为类蛋白质样;而Em>380 nm的荧光峰涉及多环芳族结构,被鉴定为类腐殖质样[27]. 从EEM图可看出,Raw-DOM、HON和HOB主要为类腐殖质样的荧光峰特征,HIS的荧光峰数值较低,主要分布在类蛋白质区域. 3DEEM-PARAFAC可准确识别独立荧光组分,根据OpenFluor数据库,共鉴定了1种微生物源DOM成分(C1)和1种腐殖质成分(C2);C1在Ex/Em=255(305)/420 nm处达到峰值,被认为是微生物源腐殖质[28];C2被归类为陆源类腐殖质成分,最大Ex/Em在265(380)/474 nm处[29]. 由于HIS荧光强度较低,与Raw-DOM、HON和HOB一起进行平行因子分析时信号被覆盖,因此并未鉴定出类蛋白质样.
2个荧光组分(C1和C2)在DOM原液和3个馏分中的分布(图2)可以看出,2个组分总的荧光值按照由高到低的顺序排列为:HOB(
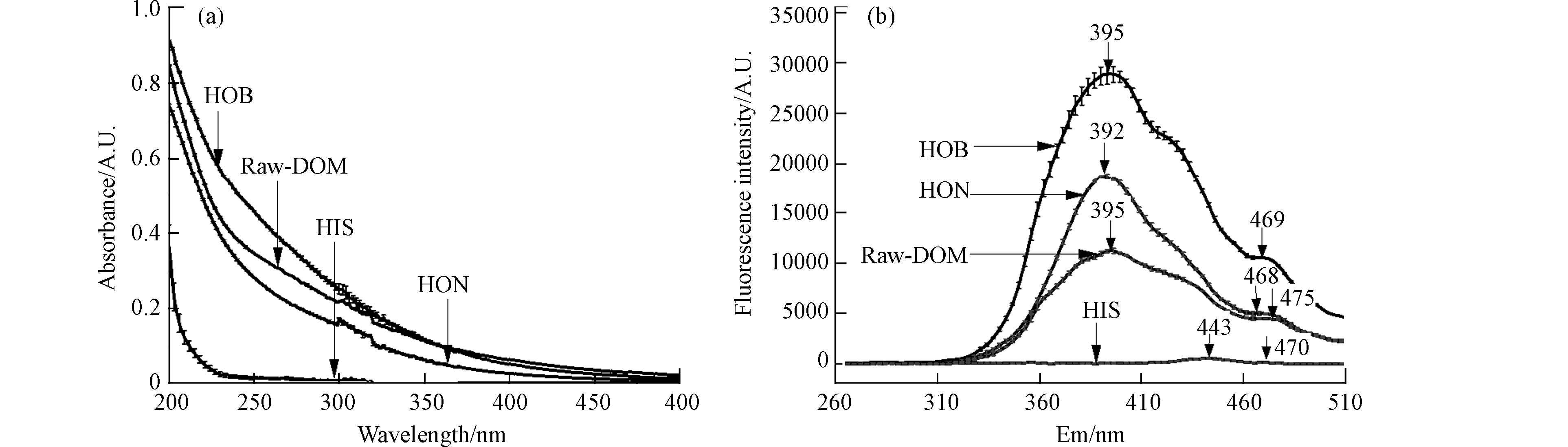
3930.8 R.U.)>HON(2376.9 R.U.)>Raw-DOM(1766.3 R.U.)>HIS(67.5 R.U.)(图2a),HON和HOB的荧光强度分别是Raw-DOM的1.2倍和2.2倍,而HIS的荧光强度仅为Raw-DOM的4%. 2个荧光组分的荧光强度由高到低排序为HOB-C1>HON-C1>Raw-DOM-C1>HOB-C2>HON-C2>Raw-DOM-C2>HIS-C1>HIS-C2(图2a),因此C1在DOM原液和3个馏分中占比80.7%—84.6%,C2占比15.4%—19.3%(图2b). DOM分馏显著增加了HOB和HON馏分的荧光值,显著降低了HIS的荧光值,但是并没有显著影响C1和C2组分在各馏分中的分布.紫外可见吸收光谱通常用于提供环境中DOM浓度和组成的信息. SUVA254为254 nm处UV的吸收系数与DOC浓度之比,可表示DOM的芳香性和亲水性,该值越大,表明芳香化程度越高,DOM越稳定[30]. 通常SUVA254>4 L·mg−1·m−1表明DOM中主要是疏水性组分尤其是芳香结构物质含量占优势;而SUVA254<3 L·mg−1·m−1代表DOM中亲水性组分含量较高[31]. SUVA260为260 nm处UV的吸收系数与DOC浓度比值,用来表示DOM中疏水性组分的含量[32]. 按DOC稀释到10 mg·L−1,分别测定DOM原液和3个馏分的紫外吸收光谱,吸光度由大到小排序为HOB>Raw-DOM>HON>HIS(图3a). DOM原液的SUVA254和SUVA260分别为7.60 L·mg−1·m−1和7.37 L·mg−1·m−1,这说明原液中疏水性组分尤其是芳香结构物质含量占优势,HON的SUVA254和SUVA260分别为6.22 L·mg−1·m−1和5.76 L·mg−1·m−1,疏水性组分含量和芳香化程度比原液低;而HOB的SUVA254和SUVA260分别为9.90 L·mg−1·m−1和9.44 L·mg−1·m−1,疏水性组分含量和芳香化程度都比DOM原液高. 此外,HIS的SUVA254和SUVA260均为0.46 L·mg−1·m−1,亲水性组分含量比DOM原液高很多. 因此,根据紫外可见吸收光谱的数据分析可知,固相萃取技术成功地对DOM原液进行了分馏,HIS中以亲水性组分为主,而HON和HOB中以疏水性组分为主,芳香化程度较高.
DOM原液和3个馏分的同步荧光光谱可以看出,Raw-DOM的主峰在Em=395 nm处,最大荧光值为
11231 A.U.;肩峰在Em 475 nm处,最大荧光值为4438 A.U.(图3b). DOM分馏之后,荧光强度和峰位发生变化. 与DOM原液相比,HON的主峰荧光强度增加了0.67倍,并蓝移至Em=392 nm处,肩峰荧光强度增加了0.13倍,并蓝移到Em=468 nm处;HOB主峰的荧光强度增加了1.58倍,主峰位置未变,肩峰荧光强度增加了1.37倍,并蓝移到Em=469 nm处. 相反的是,HIS的主峰和肩峰的最大荧光强度分别是原液的5%和4%,主峰红移到Em=443 nm,而肩峰蓝移到Em=470 nm. -
用不同浓度四环素滴定DOM原液和馏分后测定紫外吸收光谱(图4),DOM原液在271—363 nm范围内出现了明显的吸收峰;HON在271—357 nm范围内出现明显吸收峰,并略微红移;HOB和HIS的紫外吸收峰分别在271—356 nm和271—371 nm范围. 随着TC浓度升高,DOM原液和3个馏分的吸光度和光谱形状均发生改变,TC改变了DOM的微环境(大分子构象), 属于静态猝灭. 因为动态猝灭(如分子间碰撞)仅可影响激发态分子,而吸收光谱并不会改变[33 − 34]. DOM原液和3个馏分均在275 nm和356 nm附近有吸收峰,这应该属于四环素的特征峰. Cheng等[35]扫描了四环素溶液的紫外光谱,在276和355 nm处出现吸收峰;Bi等[36]研究发现356 nm附近的吸收峰是TC的特征峰;用四环素猝灭湖泊大型植物DOM和藻源DOM的紫外图谱与本文类似,亦有TC的特征峰[33, 37].
-
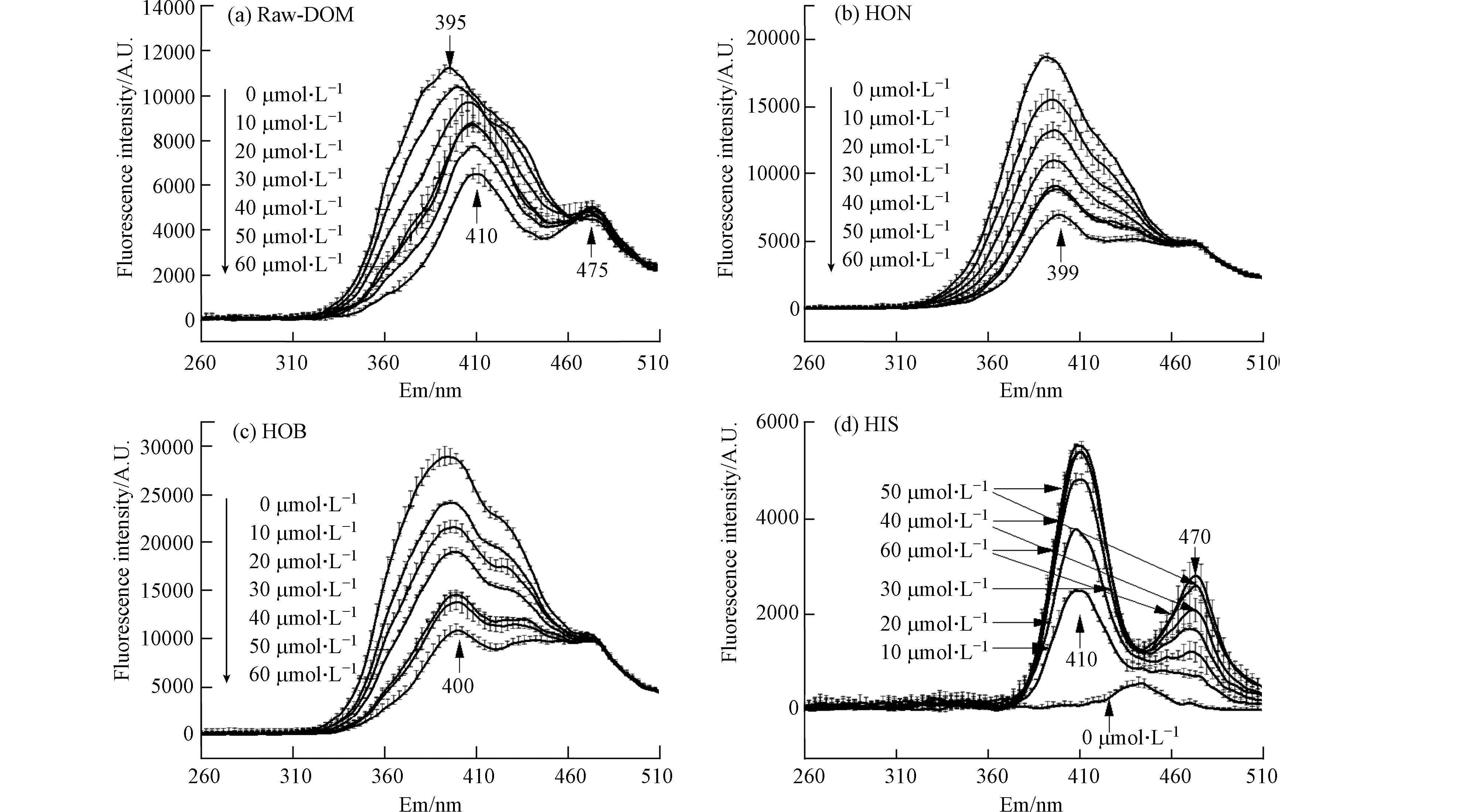
DOM原液和3个馏分用不同浓度TC滴定后,测定同步荧光光谱(图5). Raw-DOM的荧光强度在320—460 nm范围内,随TC浓度升高而不断降低,主峰395 nm处的荧光值显著降低,并持续红移至410 nm处;肩峰475 nm附近的荧光强度随TC浓度升高而略微升高. 馏分HON和HOB与Raw-DOM的变化规律类似,在320—475 nm范围内,随TC浓度升高而不断降低,但肩峰基本无变化;HON和HOB的主峰分别红移到399 nm和400 nm处. 加入60 μmol·L−1 TC后,Raw-DOM、HON和HOB在主峰处分别猝灭58.09%、65.77%和63.92%. 然而,HIS在410 nm和470 nm处,随TC浓度的升高荧光强度却不断增加,加入60 μmol·L−1 TC后,荧光强度分别增加了52倍和
2715 倍. 滴定实验中出现位移情况,可能是形成配合物时,类腐殖酸物质构象发生了变化[38]. 因此,TC与DOM的络合反应主要发生在微生物源腐殖质(C1)上,而陆源类腐殖质(C2)因为相对含量低而络合不明显. -
对同步荧光图谱数据进行2D-COS分析以揭示TC与DOM原液和馏分中的荧光组分的络合顺序(图6). Raw-DOM同步图(图6a)中,沿对角线381、418、432 nm处各存在一个正自相关峰,说明该区域对应的类腐殖酸物质光谱变化显著. 同时,对角线下方432/381 nm处的正交叉峰证实381 nm和432 nm处的类腐殖质光谱变化方向一致,随TC浓度增加而降低. Raw-DOM异步图(图6b)中,对角线下方408/381 nm处观察到1个负交叉峰,436/408 nm存在1个正交叉峰. 基于Noda规则[39],如果同步图中λ1/λ2处的交叉峰为正,异步图中也为正,表明λ1处的强度变化优于λ2处发生;若异步图中为负,则表明λ2处的强度变化优于λ1处发生. 对2D-COS图的分析可知,TC的络合顺序为381、436>408 nm. 沿着HON同步图的对角线仅在发射波长387 nm处观察到1个正自相关峰,说明该峰相对应的类腐殖酸物质荧光强度变化最为显著(图6c);在HON异步图中,对角线下方387—425/374 nm处有1个负交叉峰(图6d);基于Noda规则,HON与TC的络合顺序为374>387—425 nm. 同步荧光光谱按照发射波长分为3个区域,分别为类蛋白质物质区域(250—300 nm)、类富里酸物质区域(300—380 nm)和类腐殖酸物质区域(380—600 nm)[40-41];因此类富里酸物质在TC与HON的络合反应中占主导地位. HOB同步图(图6e)中,沿对角线382 nm处观察到1个正自相关峰,说明该峰对应的类腐殖质对TC最敏感;HOB异步图对角线下方390—425/382 nm有1个负交叉峰(图6f),因此HOB中络合顺序为382>390—425 nm.
-
加入TC后不同组分的猝灭程度不一样,加入60 μmol·L−1 TC后,组分C1猝灭效果更明显,HOB、HON和Raw-DOM分别猝灭68.6%、67.4%和73.5%;而组分C2猝灭效果不如C1明显,HOB、HON和Raw-DOM分别猝灭34.7%、33.3%和8.1%. 各荧光组分的荧光强度和TC的剂量-效应关系(图7a)可以看出,TC猝灭属于非线性猝灭,因此分别用Stern-Volmer方程、修正的Stern-Volmer方程和结合位点方程进行拟合(图7b和表2). kq值(0.90—3.89×1012 L·mol−1·s−1)远远大于最大散射碰撞猝灭常数(2×1010 L·mol−1·s−1),因此属于静态猝灭,与紫外光谱图谱结果一致(图4). 修正的Stern-Volmer方程仅对Raw-DOM-C1和HON-C1模拟较好,lgKa分别为3.77和4.05(表2,P<0.001),TC与HON-C1的络合能力大于Raw-DOM-C1;其余组分拟合不佳,这可能是因为引起荧光猝灭反应的结合点位与猝灭剂不是1:1结合. 结合位点方程有助于进一步了解TC与DOM中不同位点的络合作用. n值(1.22—2.06)表明C1和C2组分中有多个结合位点与TC发生了络合作用(图7b和表2,P<0.01),lgKb范围为5.43—8.47,结合稳定性由高到低为:HON-C2>HOB-C1>Raw-DOM-C1>HOB-C2>HON-C1. HON-C1中n=1.22为最低,而lgKb=5.43最低,表明HON-C1与TC因结合位点少而导致结合并不稳定;HON-C2中n=2.06为最高,而lgKb=8.47最高,表明HON-C2与TC因结合位点多导致结合很稳定. HOB的两个组分C1和C2的n值分别为1.52和1.50,差别不大,而HOB-C1的lgKb为6.75,略大于HOB-C2的6.12. DOM原液中C1的n值和lgKb分别为1.46和6.58,结合稳定性介于HOB-C1和HOB-C2之间. Raw-DOM-C2因猝灭不明显,Stern-Volmer方程、修正的Stern-Volmer方程和结合位点方程模拟均欠佳. 藻源和植物源DOM中的类腐殖质与TC络合系数分别为3.84—6.85和4.22—5.12[33],沉积物DOM中腐殖酸与TC的络合系数为6.00[42],土壤DOM中腐殖酸与TC络合系数为2.82—3.30[35],略低于我们的研究结果,这些差异主要由DOM的组成和络合模型不同引起的.
-
利用多光谱技术,并结合二维相关分析和模型拟合,探究了DOM原液和3个馏分与TC的络合能力和顺序,主要结论如下:
1)生活垃圾生物炭DOM分馏后得到4个馏分:HOA、HON、HOB和HIS,按DOC计算分别占5.66%、18.46%、32.37%和43.52%.
2)DOM原液和3个馏分中,平行因子分析共解析出2个荧光组分,分别是微生物源腐殖质C1(Ex/Em=255(305)/420 nm)和陆源类腐殖质成分C2(Ex/Em在265(380)/474 nm). DOM分馏增加了馏分HOB和HON的荧光值,显著降低了馏分HIS的荧光值,但是并没有影响C1和C2组分在各馏分中的分布.
3)固相萃取成功地对DOM原液进行了分馏,HON和HOB中以疏水性组分为主,芳香化程度较高,SUVA254和SUVA260分别为6.22—9.90 L·mg−1·m−1和5.76—9.44 L·mg−1·m−1;而HIS则以亲水性组分为主,SUVA254和SUVA260均为0.46 L·mg−1·m−1.
4)DOM和馏分的吸收光谱随着TC浓度升高而升高,Stern-Volmer方程中kq值(0.90—3.89×1012 L·mol−1·s−1)远远大于最大散射碰撞猝灭常数(2×1010 L·mol−1·s−1),因此属于静态猝灭.
5)TC与DOM的络合反应主要发生在微生物源腐殖质(C1)上,而陆源类腐殖质(C2)因为相对含量低而络合不明显.
6)HON-C1与TC因结合位点少(n=1.22)而导致结合不稳定(lgKb=5.43),HON-C2与TC因结合位点多(n=2.06)而导致结合很稳定(lgKb=8.47);DOM-C1、HOB-C1和HOB-C2的结合位点数和结合稳定性处于HON-C1和HON-C2之间.
溶解性有机质疏水性馏分的四环素络合机制
Complexation mechanism of tetracycline with fractionated hydrophobic components from dissolved organic matter
-
摘要: 四环素属于两性化合物,含有显弱酸性的酚羟基和烯醇式羟基和显弱碱性的二甲氨基,因此溶解性有机质(DOM)的疏水性和亲水性,可能会影响它与四环素的络合能力. 本文采用两种固相萃取柱对生活垃圾生物炭的DOM进行萃取分馏,得到以下4个馏分:疏水酸性组分(HOA)、疏水中性组分(HON)、疏水碱性组分(HOB)和亲水性组分(HIS). 紫外光谱数据表明HON和HOB以疏水性组分为主,芳香化程度较高,它们的SUVA254、SUVA260分别为6.22—9.90、5.76—9.44 L·mg−1·m−1;而HIS则以亲水性组分为主,其SUVA254和SUVA260均为0.46 L·mg−1·m−1. DOM分馏增加了HOB和HON的荧光值,显著降低了HIS的荧光值,但是并没有影响微生物源腐殖质C1(80.7%—84.6%)和陆源类腐殖质C2(15.4%—19.3%)在各馏分中的分布. DOM和3个馏分(HON、HOB和HIS)的吸收光谱随着四环素浓度增大而升高,Stern-Volmer方程中的kq值(0.90×1012—3.89×1012 L·mol−1·s−1)远远大于最大散射碰撞猝灭常数(2×1010 L·mol−1·s−1),因此属于静态猝灭. 四环素与DOM的络合反应主要发生在微生物源腐殖质(C1)上,而陆源类腐殖质(C2)因为相对含量低而不呈现明显的络合作用. HON-C1与TC因结合位点少(n=1.22)而导致结合不稳定(lgKb=5.43),HON-C2与TC则因结合位点多(n=2.06)而引起结合很稳定(lgKb=8.47);DOM-C1、HOB-C1和HOB-C2的结合位点数和结合稳定性处于HON-C1和HON-C2之间. DOM因存在疏水性和亲水性组分对四环素的结合能力存在显著差异,从而影响了四环素在环境中的生态毒性和迁移转化能力.Abstract: Tetracycline belongs to amphoteric compounds, containing phenolic hydroxyl groups and enol hydroxyl groups with a weak acidity as well as dimethylamino groups with a weak alkalinity. Therefore, the hydrophobicity and hydrophilicity of dissolved organic matter (DOM) may affect its chelating abilities with tetracycline. Two types of solid-phase extraction columns were used to extract and fractionate DOM from domestic waste biochar, resulting in following four fractions: hydrophobic acidic component (HOA), hydrophobic neutral component (HON), hydrophobic alkaline component (HOB), and hydrophilic component (HIS). The UV spectral data showed that HON and HOB were mainly of hydrophobic components, with a higher degree of aromatization, and their values of SUVA254 and SUVA260 are 6.22—9.90 L·mg−1·m−1 and 5.76—9.44 L·mg−1·m−1. However, HIS was mainly composed of hydrophilic components, with SUVA254 and SUVA260 both being 0.46 L·mg−1·m−1. DOM fractionation increased the fluorescence values of HOB and HON while significantly reduced the fluorescence values of HIS, but did not affect the distribution of microbial humus C1 (80.7%—84.6%) and terrestrial humus C2 (15.4%—19.3%) in each fraction. The absorption spectra of DOM and three fractions (HON, HOB, and HIS) increased with increasing tetracycline concentrations, and the kq values (0.90 × 1012—3.89 × 1012 L·mol−1·s−1) in the Stern Volmer Equation were much greater than the maximum scattering collision quenching constant (2 × 1010 L·mol−1·s−1); therefore, TC-DOM belonged to static quenching. On the contrary, the absorption spectra of DOM and three fractions (HON, HOB, and HIS) increased with increasing tetracycline concentrations, belonging to static quenching. The absorption peaks near 275 and 356 nm were linked to the characteristic peaks of tetracycline. The complexation reaction of tetracycline with DOM mainly occurred on microbial humus (C1), while the complexation of terrestrial humus (C2) was not obvious due to its low relative content. In addition, HON-C1 complexation with TC was unstable (lgKb=5.43) due to limited binding sites (n=1.22), while HON-C2 complexation with TC was stable (lgKb=8.47) because of multiple binding sites (n=2.06). The number of binding sites and binding stabilities of DOM-C1, HOB-C1, and HOB-C2 were between HON-C1 and HON-C2. In summary, DOM has significant differences in the binding capacity of hydrophobic and hydrophilic components to tetracycline, which may affect the ecotoxicity, migration and transformation capacity of tetracycline in the environment.
-

-
表 1 抗生素的基本理化性质
Table 1. Basic physicochemical properties of antibiotics
抗生素
Antibiotic分子式和相对分子质量
Molecular formula and
relative molecular mass分子结构
Molecular溶解度/(mg·L−1)
SolubilitypKa lg Kow 四环素
(TC)C22H24N2O8
444.43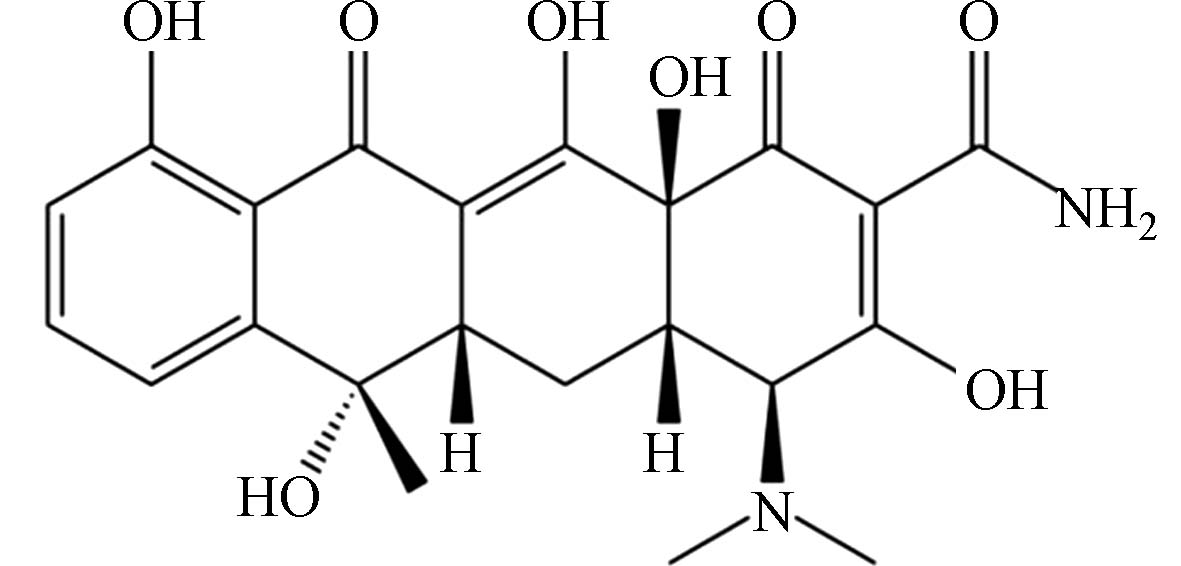
0.041 3.30; 7.68; 9.68 −1.25 表 2 DOM荧光组分与TC络合作用的拟合参数
Table 2. Fitting parameters for the complexation of DOM fluorescence components with TC
DOM荧光组分
DOM fluorescence
componentsStern-Volmer模型
Stern-Volmer model修正的Stern-Volmer模型
Modified Stern-Volmer model结合位点方程
Binding site equationkq Ksv lgK R2a Pd lgKa R2b Pe lgKb n R2c Pf Raw-DOM C1 3.89 3.89 4.59 0.91 <0.001 3.77 0.9978 <0.001 6.58±0.32 1.46±0.07 0.9907 <0.001 HON C1 2.94 2.94 4.47 0.94 <0.001 4.05 0.9945 <0.001 5.43±0.33 1.22±0.07 0.9860 <0.001 C2 0.72 0.72 3.86 0.94 <0.001 — — — 8.47±1.61 2.06±0.35 0.8935 <0.01 HOB C1 3.10 3.10 4.49 0.92 <0.001 — — — 6.75±0.45 1.52±0.10 0.9835 <0.001 C2 0.90 0.90 3.95 0.93 <0.001 — — — 6.12±0.60 1.50±0.13 0.9695 <0.001 注:a、b和c是式(1)、(2)和(3)的回归系数;d、e和f是式(1)、(2)和(3)的显著性检验值. kq/(×1012 L·mol−1·s−1),Ksv/(×104 L·mol−1).
Notes: a, b, and c are the regression coefficients of equations (1), (2), and (3); d, e, and f are the significance values for equations (1), (2) and (3). -
[1] 白金顺, 王伟红, 李艳丽, 等. 我国猪粪中四环素类抗生素残留及好氧堆肥消减研究进展[J]. 中国土壤与肥料, 2022(3): 231-238. BAI J S, WANG W H, LI Y L, et al. Advances in residues of tetracyclines and its degradation by aerobic composting in pig manure in China[J]. Soil and Fertilizer Sciences in China, 2022(3): 231-238 (in Chinese).
[2] 王卫中, 迟荪琳, 徐卫红. 四环素类抗生素对土壤-生菜系统的生物效应及其迁移降解特性[J]. 环境科学, 2021, 42(3): 1545-1558. WANG W Z, CHI S L, XU W H. Biological effect of tetracycline antibiotics on a soil-lettuce system and its migration degradation characteristics[J]. Environmental Science, 2021, 42(3): 1545-1558 (in Chinese).
[3] ZHOU X Q, CUASQUER G J P, LI Z F, et al. Occurrence of typical antibiotics, representative antibiotic-resistant bacteria, and genes in fresh and stored source-separated human urine[J]. Environment International, 2021, 146: 106280. doi: 10.1016/j.envint.2020.106280 [4] TANG J F, SUN J, WANG W D, et al. Pharmaceuticals in two watersheds in Eastern China and their ecological risks[J]. Environmental Pollution, 2021, 277: 116773. doi: 10.1016/j.envpol.2021.116773 [5] 纵亚男, 邵美玲, 梁梦琦, 等. 长三角某城镇典型小流域水体抗生素的污染分布特征[J]. 农业环境科学学报, 2018, 37(5): 965-973. ZONG Y N, SHAO M L, LIANG M Q, et al. Occurrence and distribution of antibiotics in the surface water of a typical urban river in the Yangtze River Delta[J]. Journal of Agro-Environment Science, 2018, 37(5): 965-973 (in Chinese).
[6] 赵晓东, 乔青青, 秦宵睿, 等. 近15年我国土壤抗生素污染特征与生物修复研究进展[J]. 环境科学, 2023, 44(7): 4059-4076. ZHAO X D, QIAO Q Q, QIN X R, et al. Characteristics of antibiotic contamination of soil in China in past fifteen years and the bioremediation technology: A review[J]. Environmental Science, 2023, 44(7): 4059-4076 (in Chinese).
[7] CHANG D H, MAO Y Z, QIU W, et al. The source and distribution of tetracycline antibiotics in China: A review[J]. Toxics, 2023, 11(3): 214. doi: 10.3390/toxics11030214 [8] 裴浩鹏, 徐艳, 陈蕊, 等. 天津市城郊不同土地利用类型土壤中抗生素分布特征及影响因素分析[J]. 环境工程, 2021, 39(1): 166-173. PEI H P, XU Y, CHEN R, et al. Distribution characteristics and influencing factors of antibiotics in soils of different land use types in suburbs of Tianjin[J]. Environmental Engineering, 2021, 39(1): 166-173 (in Chinese).
[9] WANG F H, QIAO M, LV Z E, et al. Impact of reclaimed water irrigation on antibiotic resistance in public parks, Beijing, China[J]. Environmental Pollution, 2014, 184: 247-253. doi: 10.1016/j.envpol.2013.08.038 [10] GAO A Q, TIAN Z Y, WANG Z Y, et al. Comparison between the technologies for food waste treatment[J]. Energy Procedia, 2017, 105: 3915-3921. doi: 10.1016/j.egypro.2017.03.811 [11] 徐栋, 沈东升, 冯华军. 厨余垃圾的特性及处理技术研究进展[J]. 科技通报, 2011, 27(1): 130-135. XU D, SHEN D S, FENG H J. Discussion on characteristics and resource recycling technology of food residue[J]. Bulletin of Science and Technology, 2011, 27(1): 130-135(in Chinese).
[12] BIELSKÁ L, ŠKULCOVÁ L, NEUWIRTHOVÁ N, et al. Sorption, bioavailability and ecotoxic effects of hydrophobic organic compounds in biochar amended soils[J]. Science of the Total Environment, 2018, 624: 78-86. doi: 10.1016/j.scitotenv.2017.12.098 [13] 李经涵, 张建强, 夏丽琼, 等. 生物炭影响抗生素在土壤中环境行为的Meta分析[J]. 环境科学, 2023, 44(1): 531-539. LI J H, ZHANG J Q, XIA L Q, et al. Effects of biochar on antibiotic environmental behaviors in soil: A meta-analysis[J]. Environmental Science, 2023, 44(1): 531-539(in Chinese).
[14] YUE Y, SHEN C C, GE Y. Biochar accelerates the removal of tetracyclines and their intermediates by altering soil properties[J]. Journal of Hazardous Materials, 2019, 380: 120821. doi: 10.1016/j.jhazmat.2019.120821 [15] PAN M. Biochar adsorption of antibiotics and its implications to remediation of contaminated soil[J]. Water, Air & Soil Pollution, 2020, 231(5): 221. [16] LI G, KHAN S, IBRAHIM M, et al. Biochars induced modification of dissolved organic matter (DOM) in soil and its impact on mobility and bioaccumulation of arsenic and cadmium[J]. Journal of Hazardous Materials, 2018, 348: 100-108. doi: 10.1016/j.jhazmat.2018.01.031 [17] LI M, ZHANG A F, WU H M, et al. Predicting potential release of dissolved organic matter from biochars derived from agricultural residues using fluorescence and ultraviolet absorbance[J]. Journal of Hazardous Materials, 2017, 334: 86-92. doi: 10.1016/j.jhazmat.2017.03.064 [18] SUN J L, DROSOS M, MAZZEI P, et al. The molecular properties of biochar carbon released in dilute acidic solution and its effects on maize seed germination[J]. Science of the Total Environment, 2017, 576: 858-867. doi: 10.1016/j.scitotenv.2016.10.095 [19] DONG X L, MA L Q, GRESS J, et al. Enhanced Cr(VI) reduction and As(III) oxidation in ice phase: Important role of dissolved organic matter from biochar[J]. Journal of Hazardous Materials, 2014, 267: 62-70. doi: 10.1016/j.jhazmat.2013.12.027 [20] SOJA G, WIMMER B, ROSNER F, et al. Compost and biochar interactions with copper immobilisation in copper-enriched vineyard soils[J]. Applied Geochemistry, 2018, 88: 40-48. doi: 10.1016/j.apgeochem.2017.06.004 [21] 吴文雨, 唐剑锋, 郑思俊, 等. 生活垃圾生物炭源溶解性有机质分馏和络合重金属的作用[J]. 环境化学, 2023, 42(7): 2382-2391. doi: 10.7524/j.issn.0254-6108.2022022203 WU W Y, TANG J F, ZHENG S J, et al. Fractionation of dissolved organic matter from domestic waste biochar and its complexation with heavy metals[J]. Environmental Chemistry, 2023, 42(7): 2382-2391 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022022203
[22] AFTAB B, SHIN H S, HUR J. Exploring the fate and oxidation behaviors of different organic constituents in landfill leachate upon Fenton oxidation processes using EEM-PARAFAC and 2D-COS-FTIR[J]. Journal of Hazardous Materials, 2018, 354: 33-41. doi: 10.1016/j.jhazmat.2018.04.059 [23] 吴文雨. 生物炭对土壤中溶解性有机质的影响及其与重金属污染的阻控作用机制研究[D]. 上海: 上海应用技术大学, 2022. WU W Y. Effect of Biochar on dissolved organic matter in soil and its mechanism of inhibiting and controlling heavy metal pollution[D]. Shanghai: Shanghai Institute of Technology, 2022(in Chinese).
[24] FANG Z, HE C, LI Y Y, et al. Fractionation and characterization of dissolved organic matter (DOM) in refinery wastewater by revised phase retention and ion-exchange adsorption solid phase extraction followed by ESI FT-ICR MS[J]. Talanta, 2017, 162: 466-473. doi: 10.1016/j.talanta.2016.10.064 [25] DING F, ZHAO G Y, CHEN S C, et al. Chloramphenicol binding to human serum albumin: Determination of binding constants and binding sites by steady-state fluorescence[J]. Journal of Molecular Structure, 2009, 929(1/2/3): 159-166. [26] SONG W, MU G, ZHANG D, et al. Interaction of acetamiprid with extracellular polymeric substances (EPS) from activated sludge: A fluorescence study[J]. African Journal of Biotechnology, 2010, 9(45): 7667-7673. [27] ISHII S K L, BOYER T H. Behavior of reoccurring PARAFAC components in fluorescent dissolved organic matter in natural and engineered systems: A critical review[J]. Environmental Science & Technology, 2012, 46(4): 2006-2017. [28] AMARAL V, ORTEGA T, ROMERA-CASTILLO C, et al. Linkages between greenhouse gases (CO2, CH4, and N2O) and dissolved organic matter composition in a shallow estuary[J]. The Science of the Total Environment, 2021, 788: 147863. doi: 10.1016/j.scitotenv.2021.147863 [29] PELEATO N M, SIDHU B S, LEGGE R L, et al. Investigation of ozone and peroxone impacts on natural organic matter character and biofiltration performance using fluorescence spectroscopy[J]. Chemosphere, 2017, 172: 225-233. doi: 10.1016/j.chemosphere.2016.12.118 [30] YAN L H, SU R G, ZHANG C S, et al. Assessing the dynamics of chromophoric dissolved organic matter(CDOM) in the Yellow Sea and the East China Sea in autumn by EEMs-PARAFAC[J]. Science China Chemistry, 2012, 55(12): 2595-2609. doi: 10.1007/s11426-012-4617-7 [31] YEH Y L, YEH K J, HSU L F, et al. Use of fluorescence quenching method to measure sorption constants of phenolic xenoestrogens onto humic fractions from sediment[J]. Journal of Hazardous Materials, 2014, 277: 27-33. doi: 10.1016/j.jhazmat.2014.03.057 [32] 陈昭宇, 李思悦. 三峡库区城镇化影响下河流DOM光谱特征季节变化[J]. 环境科学, 2021, 42(1): 195-203. CHEN Z Y, LI S Y. Seasonal variation of DOM spectral characteristics of rivers with different urbanization levels in the Three Gorges Reservoir Area[J]. Environmental Science, 2021, 42(1): 195-203(in Chinese).
[33] BAI L L, ZHAO Z, WANG C L, et al. Multi-spectroscopic investigation on the complexation of tetracycline with dissolved organic matter derived from algae and macrophyte[J]. Chemosphere, 2017, 187: 421-429. doi: 10.1016/j.chemosphere.2017.08.112 [34] XU J, SHENG G P, MA Y, et al. Roles of extracellular polymeric substances (EPS) in the migration and removal of sulfamethazine in activated sludge system[J]. Water Research, 2013, 47(14): 5298-5306. doi: 10.1016/j.watres.2013.06.009 [35] CHENG X X, HOU H B, LI R S, et al. Adsorption behavior of tetracycline on the soil and molecular insight into the effect of dissolved organic matter on the adsorption[J]. Journal of Soils and Sediments, 2020, 20(4): 1846-1857. doi: 10.1007/s11368-019-02553-7 [36] BI H N, TANG L, GAO X, et al. Spectroscopic analysis on the binding interaction between tetracycline hydrochloride and bovine proteins β-casein, α-lactalbumin[J]. Journal of Luminescence, 2016, 178: 72-83. doi: 10.1016/j.jlumin.2016.05.048 [37] 曹驰程, 王友权, 章奇, 等. 典型湖泊天然有机质与四环素的相互作用[J]. 湖泊科学, 2018, 30(4): 1004-1011. CAO C C, WANG Y Q, ZHANG Q, et al. Interactions of tetracycline and dissolved organic matter from freshwater lakes. [J]. Journal of Lake Sciences. 2018, 30(4): 1004-1011(in Chinese).
[38] DAWOUD BANI-YASEEN A. Spectrofluorimetric study on the interaction between antimicrobial drug sulfamethazine and bovine serum albumin[J]. Journal of Luminescence, 2011, 131(5): 1042-1047. doi: 10.1016/j.jlumin.2011.01.019 [39] ISAO N O D A. Two-dimensional correlation analysis useful for spectroscopy, chromatography, and other analytical Measurements;Reviews[J]. Analytical Sciences, 2007, 23(2): 139-146. doi: 10.2116/analsci.23.139 [40] HUR J, KIM G. Comparison of the heterogeneity within bulk sediment humic substances from a stream and reservoir via selected operational descriptors[J]. Chemosphere, 2009, 75(4): 483-490. doi: 10.1016/j.chemosphere.2008.12.056 [41] NGUYEN H V M, HUR J, SHIN H S. Changes in spectroscopic and molecular weight characteristics of dissolved organic matter in a river during a storm event[J]. Water, Air, & Soil Pollution, 2010, 212(1): 395-406. [42] WU C Y, TANG R, LI H Q, et al. Interaction between organic matter and tetracycline in river sediments in cold regions[J]. Environmental Science and Pollution Research, 2022, 29(17): 24941-24950. doi: 10.1007/s11356-021-17682-1 -




 下载:
下载: