-
近年来,随着化工工业的快速发展,催化、吸附、电化学等过程的研究与应用也愈发广泛,其中,催化剂、吸附剂、电极等关键材料的制备及性能研究更是受到了研究者的重视. 以碳为主要构成元素的碳材料,因碳元素具有多样的电子轨道特性杂化,尤其是sp2的异向性导致晶体和排列的各向异性,使其具有各式各样的性质,如较大的比表面积、较强的吸附能力、丰富的表面官能团等。且价格低廉,来源广泛,因此可作为催化剂、催化剂载体、吸附剂、电极等,在催化、吸附、电化学等领域得到广泛应用[1 − 2].
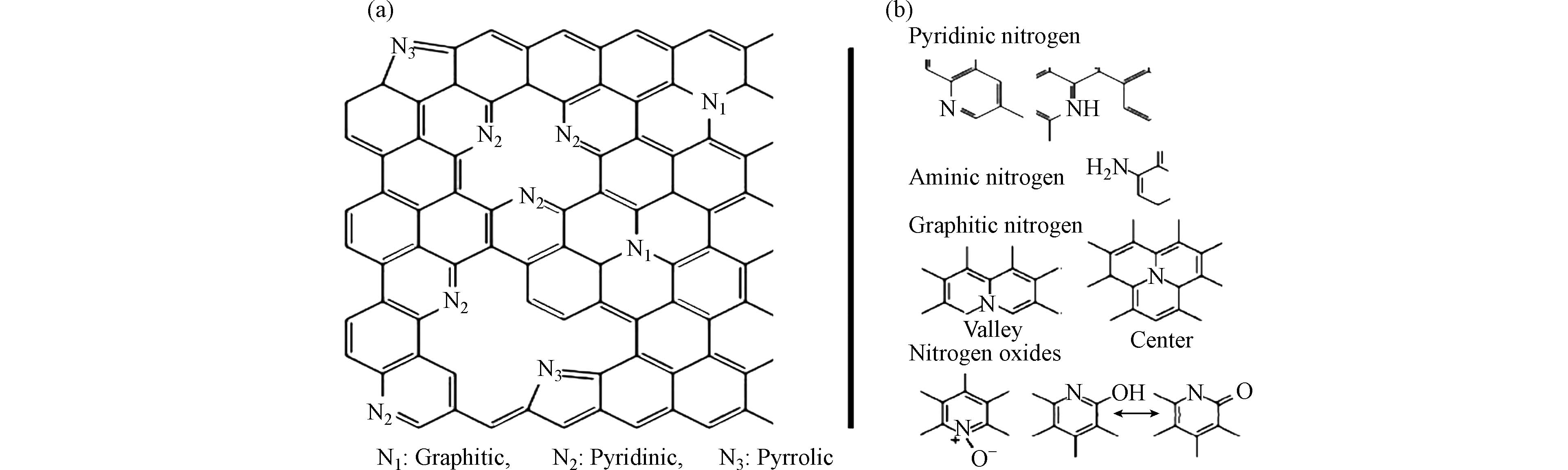
尽管碳材料本身具有较好的性能,但在一些特殊的反应或应用过程中仍需要对其性能进行改善. 杂原子掺杂(N、B、S、P等) 可以进一步改善碳材料的物理化学性能,以优化碳材料在上述领域的应用[3 − 7]. 而在这些杂原子中,氮元素的原子序数与 碳 原子非常接近,但具有不同于碳的电子构型和电负性,使得它可作为碳材料的给电子掺杂原子,以增强碳材料表面极性、提高碳材料表面润湿性、修饰碳材料的电子结构、降低离子吸脱附时的能垒,从而提升碳材料催化和吸附等方面的性能[8 − 10]. 掺杂在碳材料中的氮元素可分为化学氮和结构氮两类,其中化学氮在碳材料表面主要以氨基、亚硝基等官能团形式存在,可以增强多孔材料的 Bronsted 碱性,能有效改变碳材料表面理化性质,提高碳材料的催化活性,但其化学性质活泼,高温条件下易分解,热稳定性较差;结构氮中N原子则直接嵌入碳骨架中,增强了碳材料的Lewis碱性,如石墨氮、吡啶氮、吡咯氮等[11 − 14],如图1所示. 依据掺杂氮类型和碳材料种类的不同特性将氮掺杂碳材料分别应用于各个领域,并通过改善其制备方法和工艺以获得更廉价、性能更优异的氮掺杂碳材料,或针对不同氮掺杂碳材料对各过程的影响机理开展研究.
本文主要针对N原子和多原子共掺杂碳材料的制备及其在催化、吸附、电化学等领域的作用机理及应用现状进行综述,并对未来可能开展的研究方向进行展望和分析,以期为氮掺杂碳材料的新型制备和工业化发展提供思路和借鉴.
-
氮掺杂碳材料的制备过程是影响其物理化学性质的关键因素. 氮掺杂碳材料的制备方法依据掺氮过程中引入氮源的顺序主要分为原位合成法和后处理法两大类. 前者以富氮物质为前驱体直接碳化然后再进一步通过活化造孔而制得,可以在碳材料制备的过程中直接将N原子引入到碳骨架中,形成的含氮官能团以石墨型氮为主. 后者则先合成碳材料前体,再通过含氮原子的试剂对碳前体进行表面改性处理,所制备氮掺杂碳材料中的含氮官能团以吡啶型为主[15]. 表1是几种常见N原子和多原子共掺杂碳材料的制备方法及应用.
-
原位合成法是指在制备氮掺杂碳材料的过程中同步引入氮源(三聚氰胺、苯甲胺、胺基糖等)到制备碳材料的前驱体中,一步制备出氮掺杂碳材料. 乔文强等[16]以多聚甲醛、1,3,5-三甲基苯和对苯二胺为C源和N源聚合制得CNy前体,在N2氛围中不同温度下制得催化性能良好的氮掺杂碳基催化剂. Xie等[17]以储量丰富且亟待解决的大豆渣为前体水热碳化后使用KOH活化,制备出一系列具有高比表面积和孔体积的生物质基富氮多孔炭材料. Zhu等[18]以生物炼油残渣为原料,将精炼残渣与C7H5KO2和二苯基硫脲混合后采用一步碳化活化工艺,得到了高比表面积与高孔隙率的氮掺杂多孔生物炭. 原位合成法流程简便,能够将杂原子均匀高效地掺杂于材料表面及内部[19 − 20],包含液相模板剂法、气相沉积法、水热法、直接热解法和化学活化法等方法[21 − 23]. 但该方法需要使用模板剂、合成温度较高、合成的碳材料孔径分布较宽、会产生废气,而且反应活化剂受温度影响较大,因此,要根据不同的需求选择合适的原位合成条件.
-
与原位合成法不同,后处理法是指先合成碳材料前体,后采用NH3、尿素、硝酸、单氰胺和双氰胺等氮含量较高的试剂进行掺氮处理[24 − 25]. Shaku等[26]将马鲁拉坚果废料通过KOH活化和碳化制备活性炭(AC),后与三聚氰胺混合高温制备出具有高比表面积且氮掺杂量高达5.1%的异位氮掺杂活性炭(N-ACs). Niu等[27]通过氮离子注入氧化石墨烯薄膜的表层,然后在氮气气氛下退火,成功制备出具有高电化学性能的氮掺杂石墨烯电极材料. Song等[28]以甲烷为碳前体,在氩气环境中点燃电弧等离子体,并且将N2注入以取代原来的氩气作为缓冲气体和氮源,制备出氮掺杂水平和形态可调的氮掺杂石墨烯. 显然,相比于原位合成法,后处理法合成过程相对复杂,常使用的气体氮源如NH3,具有一定毒性,掺杂的氮元素分布不均、含量较低,通常仅仅导致表面功能化而不会改变整体的性质,因此在实际应用中较为受限. 无论是原位合成法还是后处理法,均是要针对氮掺杂碳的应用领域及所需性能进行合理选择.
-
新型的碳纳米材料和杂原子掺杂碳基材料因其比表面积高、活性位点多、稳定性好等特点已在催化领域广泛应用. 其中氮掺杂碳材料在氮掺杂过程中引入了缺陷位及氮物种,一方面,可利用这些缺陷位及氮物种作为活性位点而将其直接作为催化剂;另一方面,氮的引入也使碳材料具有较大的比表面积、较强的吸附能力,有利于金属活性组分的分散,因此也常被用作催化剂载体.
-
氮掺杂碳材料表面的含氮基团可以生成活性位点,充当一些催化反应的催化剂,如氧化还原、催化加氢、催化重整、生物质热解等反应. Teng等[43]以氮掺杂多孔碳为催化剂,将5-羟基甲酰基呋喃(HMF)选择性催化氧化合成2,5-二甲酰基呋喃(DFF), 结果表明在700 ℃下K2CO3活化的N掺杂多孔碳在最佳反应条件下将HMF转化为DFF的催化活性最高可达95.3%,且DFF的选择性达到94.6%. 这是由于氮掺杂催化剂表面的石墨氮在活化O2形成氧自由基中起了关键作用,促进了HMF的氧化脱氢. Lei等[44]以乙二胺、二甲基甲酰胺、均苯四甲酸二酐等为原料制备了3D团簇状氮掺杂介孔碳用于选择性催化氧化H2S,其中催化剂HNMC-800效果最佳,在120 ℃时使H2S转化率达到100%,具有高硫选择性(98%),是使用普通活性炭的7.9倍. Gao等[38]以聚苯胺(PA)为碳前驱体,采用碳化和磺化过程制备了氮掺杂碳固酸催化剂PAC-S,在木糖生产糠醛中表现出良好的催化能力,使糠醛收率高达88.2%. 这是由于掺杂的N原子能与磺酸基团形成氢键,增加酸度,且氢键可以将质子限制在催化剂表面周围,限制质子迁移,有效抑制了糠醛与游离质子的相互作用并抑制糠醛的再水化,从而提高糠醛产率. 马晓等[45]以木屑为碳源、尿素为氮源、KOH为活化剂,制备了一系列氮掺杂生物炭CH4-CO2重整催化剂. 通过表征分析发现,氮掺杂可以显著提升生物炭材料中氮及碱性位含量,从而为重整反应提供更多的活性位点. Wang等[31]以玉米芯为碳源、三聚氰胺为氮源通过简单的一步热共聚方法实现了聚合氮化碳(PCN)与石墨碳(BC)的非共价掺杂. 当玉米芯和三聚氰胺以1%的质量比混合,在550 ℃下进行热聚合处理时,得到的PCN/BC复合材料(CNBC)具有插层结构. 结果表明,CNBC3 / PMS系统对双酚A的去除达到了96.6%,具有优异的催化耐受性. 在生物质热解方面,Chen等[46]通过将竹废料在NH3气氛中富氮热解生物质制备N-掺杂碳基催化剂用以催化竹子热解生产酚类油品,发现氮掺杂碳基催化剂能显著促进酚类物质的生成,含量达到82%,特别是高附加值的4-乙烯基酚和4-乙基酚含量分别可达31%和16%,产率可达6.65%wt和3.04 %wt. 他们研究了其催化热解机理,认为N-掺杂碳基催化剂中氮的主要存在形式为吡啶氮、吡咯氮和季氮,这些碱性含氮基团能有效吸附酸性气体或挥发分并与竹子热解产生的大量酸性中间体及羰基结合,从而促进催化反应. 氮掺杂碳基催化剂在催化热解过程中主要起到吸附剂、催化剂和反应物的作用,其热解过程中酚类物质的形成途径如图2所示.
为了进一步提升催化剂的催化性能,有些学者在氮掺杂的基础上引入了其他的杂原子以强化氮原子或其他杂原子的作用. 叶雨阳[40]将生物质废弃物锯末通过低温限氧热处理后得到的生物炭(BC)作为前体,然后将BC与CoCl2混合后烘干,最后在NH3气氛下得到氮掺杂多孔生物炭Co@NCBC,表现出了比商用铂碳电催化剂更好的稳定性和甲醇耐受性,经过
10000 s反应后依然保留了98.1%的电流值,具有更高的ORR活性. 他认为低温限氧热处理提高了碳材料表面的含氧官能团数量,其中一部分含氧官能团和NH3发生反应,O被N取代形成组成C—N(主要是石墨N和吡啶N),产生了更多的ORR活性位点. 夏营港等[41]以ZIF-8为模板进行高温碳化,通过吸附三苯基膦、硫脲分子制备出含 N、S、P 单独或共掺杂的三维纳米多孔碳材料,相比于单掺杂和双掺杂,三元掺杂碳表现出更优异的电催化活性和稳定性. 这可能是N、P、S原位共掺杂产生了协同效应,提高了催化剂材料的导电性和电化学性能,但多原子掺杂间的协调机理尚不明确.氮掺杂通过增强碳基催化剂碱性位点、强化活性组分、吸附和活化酸性气体反应物等作用提高碳催化剂的催化性能,但目前氮掺杂类型与碳催化剂催化活性之间的准确关系尚未完全认识,其催化机理也存在争议,有待进一步深入研究.
-
氮掺杂碳材料不仅能充当催化剂,还因其较大的比表面积、较强的吸附能力常被用作催化剂载体,在生物质催化热解方面也得到深入应用. Pham等[47]将三聚氰胺掺杂后的活性炭负载Ni2P制备Ni2P/CN催化剂,用其催化热解棕榈核壳,发现Ni2P/CN催化剂对生物油的组成有显著影响,尤其是Ni2P/CN50使棕榈核壳热解过程中烷基酚选择性达到63%,且液体产物的脱氧率提升至56%. 这是由于石墨氮改善了碳结构中的电子结构,并影响了镍络合物的形成,使Ni2P颗粒沿催化剂碳表面分布均匀,且石墨氮和吡咯氮增加了碳材料吸附位点的数量,促进了烷基苯酚生成. Xu等[48]以尿素为氮源,通过后处理法得到氮掺杂活性炭作为金属Ni的载体,发现催化剂Ni/AC-N在高浓度乙炔的选择性加氢反应中表现出良好的活性,使乙炔转化率最高达到约96%,乙烯的选择性达到约46%. 这是由于N和Ni之间的电子转移增强了金属与载体之间的相互作用,促进了催化剂的稳定性. Zhang等[49]开发了一种具有独特核壳结构的新型氮掺杂碳负载钴催化剂,用其催化乙二醇生产1,3,5-三嗪,由于吡啶氮有很好的兼容性,Co嵌入氮掺杂碳基质中为乙二醇中C—C键的选择性氧化裂解提供了稳定的中间体,使1,3,5-三嗪最高产率达52%. Wu等[50]利用氮掺杂多孔碳材料对Pt进行负载,与非掺氮碳材料相比,掺氮多孔碳材料具有更多的缺陷位点与电子密度,且Pt颗粒的尺寸与分布更加均匀,表明氮掺杂多孔碳材料是Pt催化剂的优良载体. 氮掺杂碳材料做催化剂载体时,主要通过N原子对金属的锚定作用、与金属件的电子转移等增强了金属活性组分和载体间的相互作用,从而促进了催化剂的稳定性、分散性或活性.
综上所述,氮掺杂有利于提高碳基催化剂性能,但不论作为催化剂还是催化剂载体,氮掺杂碳材料都存在氮源中的氮含量远远高于得到的氮掺杂碳材料中的氮含量,掺入率低,反应活性低、选择性差等问题. 寻找本身含氮量高的生物质炭作催化剂或可解决这一问题.
-
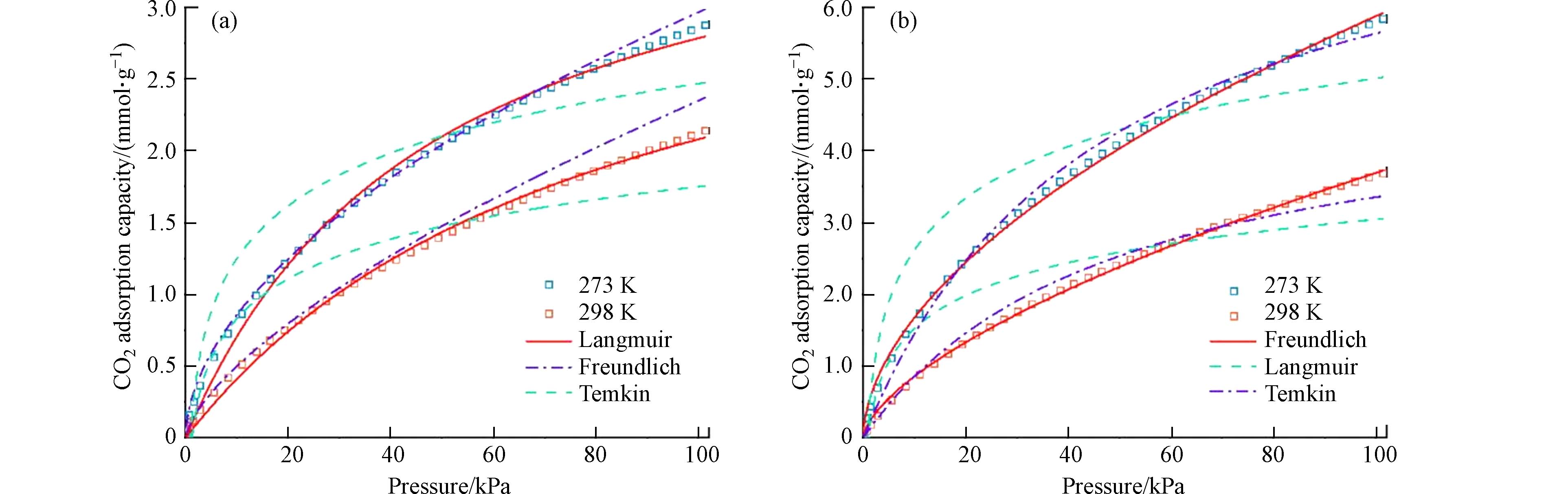
除了在催化领域的应用,氮掺杂碳材料还被广泛用于CO2、SO2等温室气体和有害气体的吸附研究. 目前,世界上90%的能源仍然由化石燃料提供,产生了大量以CO2为代表的温室气体,导致全球变暖和环境污染. 由于短期内无法改变化石燃料在能源结构中的主体地位,碳的捕集与封存(CCS)成为近年来的研究热点,而具有优良CO2吸附性能的吸附剂开发是其中的关键[51 − 53]. 在多种吸附剂中,氮掺杂碳材料一方面由于表面存在丰富的碱基可以作为捕获弱酸性CO2分子的锚,另一方面具有碳材料高比表面积和发达孔隙结构的特点,因此被广泛用于CO2吸附[54 − 55]. Yuan等[56]采用一釜法成功地将废旧PET塑料经氮掺杂处理后制备成微孔碳,在700 ℃下所制备微孔碳在0 ℃和25 ℃(1 atm)的CO2吸附量分别为6.23 mmol·g−1和4.58 mmol·g−1,且极易实现CO2解吸,具有良好的再生性和循环稳定性. Yu等[57]通过一步水热法合成含氮介孔碳材料,当介孔碳的表面和骨架中加入了丰富的氮元素会具有更强的亲水性,从而显著提高了CO2的吸附和选择性. He等[35]以稻壳为原料、壳聚糖作为氮源通过碳化和KOH活化制备的氮掺杂活性炭比普通活性炭(AC-5)具有更好的CO2吸附性能(图3),在273 K和1 bar条件下达到5.83 mmol·g−1,这可能是由于改性AC-5在AC表面形成了亲CO2活性位点. Xie等[17]也发现氮掺杂生物炭中的超微孔结构和多种含氮物种提供了较高的CO2吸附能力. 这是由于以吡啶氮、吡咯氮等主要形式存在含氮基团以具有捕集与活化CO2的能力,并与超微孔协同作用使材料表现出优异的CO2吸附容量. 氮掺杂对碳材料吸附CO2能力和选择性的改善更多的是通过碳表面形成的含氮碱性基团与CO2形成化学键实现对CO2的强化学吸附.
此外,N掺杂碳材料对其他气体也有良好的吸附作用,孙飞等[58]以三聚氰胺为氮源,苯酚为碳源,通过改变二者的比例,制备了一系列孔隙结构基本相同、氮掺杂量梯级变化的多孔碳材料(NPC). 由于含氮官能团的引入增强了NPC表面与SO2分子的相互作用,从而使单位面积碳材料表面能够吸附更多的SO2分子,SO2饱和吸附容量与多孔碳的氮掺杂量呈现正相关关系,高氮掺杂量(10.2%)多孔碳NPC-3的SO2吸附容量约是无氮掺杂多孔碳的4倍. Zhang等[36]也得出了相似的结论,他们提出了一种高温下通入CO2-NH3的改性方法,获得了具有高比表面积和氮含量的氮掺杂碳材料. 结果表明,在800 ℃的高温下获得的改性生物炭BCAN-800的微孔比表面积和氮含量分别达到最高值563.83 m2·g−1和6.63%wt,具有良好的SO2吸附性能.
-
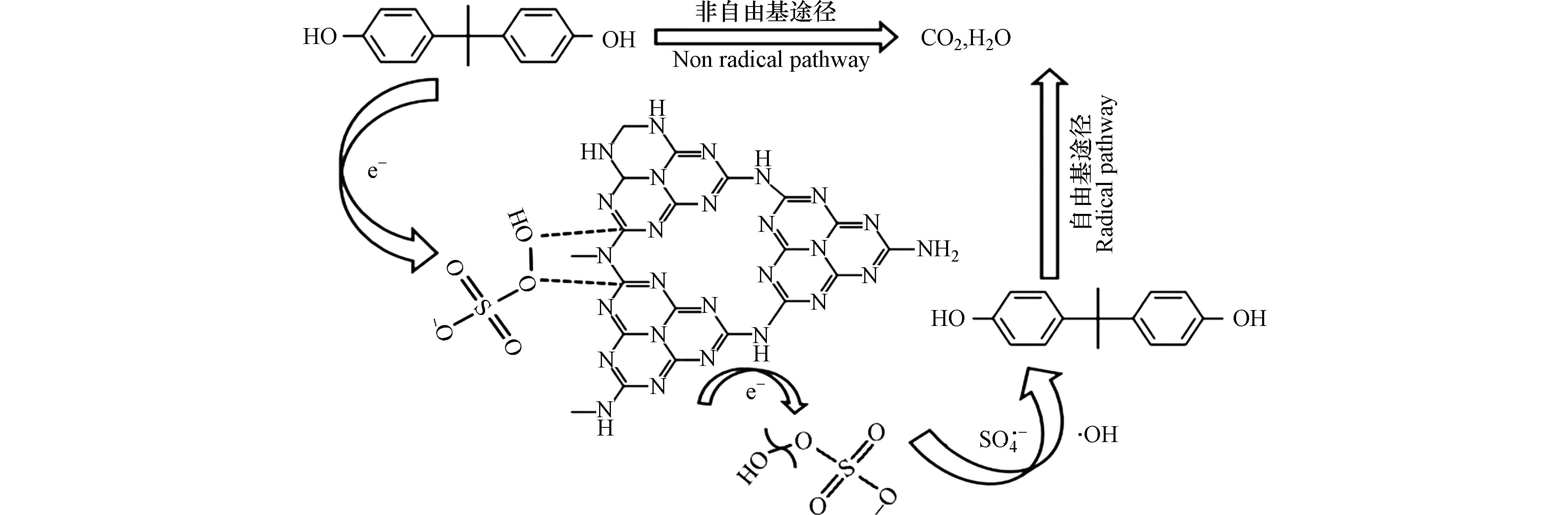
氮掺杂多孔碳材料不仅对气体有良好的吸附性,对水体中残留的有机物、无机盐和重金属等污染物也有很强的吸附性. 左盼星[59]发现氮掺杂碳材料可作为吸附质头孢氨苄的吸附剂,由于氮掺杂改善了碳材料的表面润湿性、增大了比表面积、增加了吸附位点,使其对头孢氨苄的吸附量高达353.1 mg·g−1. 王亮等[60]认为氮掺杂增大了碳材料的比表面积阻止了多孔碳海绵中石墨片层的团聚和堆叠,进而提高了共晶盐的吸附量. 如图4所示,吴尚迪等[61]通过一步热聚合法制备出氮掺杂碳催化剂(NCN-x),提高了碳材料中石墨氮的含量,有效破坏了sp2杂化碳层的化学惰性并产生新的活性位点,有效活化 PMS 降解双酚A. Peng等[62]认为氮掺杂多孔碳NC-750对罗沙胂(ROX)和对氨基苯胂酸(PASA)两种典型的苯胂酸表现出优异的吸附性能,这是由于NC-750的多孔结构和含氮基团为PASA和ROX提供了充足的吸附空间、丰富的缺陷结构和高亲和力. Chen等[63]和Zhu等[64]也得到了相似的结论.
总的来说,氮掺杂碳的吸附作用主要是通过物理孔隙结构和含氮官能团进行物理和化学吸附,其中氮掺杂主要是增强了吸附物与碳材料间的化学键合作用,从而提高了碳材料的吸附性能[65]. 目前,研究中大多数的高吸附性能氮掺杂碳材料均为粉末状,在工业应用时相比于粉末状的吸附剂颗粒状碳吸附剂具有更易回收、床层压降较小等优势,但目前研究较少,因此如何借助现有粉末碳的生产工艺来实现高性能颗粒碳的制备值得深入研究.
-
储能技术是通过储能电池将电能与化学能进行存储转化以解决能量储存问题的技术,可突破太阳能和风能发电的间歇性问题,提高可再生能源发电的可靠性与稳定性,降低油气燃料消耗量[66 − 68]. 而储能技术的关键是电催化剂和电极材料,目前以石墨为主的碳材料因电容大、循环稳定性强而受到了广泛的关注和研究[69]. Shi等[70]通过在NH3中简单地高温处理稻壳基多孔碳(RHPC),制备了一种氮掺杂稻壳基多孔碳N-RHPC电催化剂,用作阴极反应(ORR)催化剂. N-RHPC不仅在碱性溶液中具有与商业Pt/C电催化剂相似的ORR活性,而且具有良好的稳定性和甲醇耐受性. 这是由于高温NH3处理一方面可以增加RHPC的介孔体积,为离子转移提供更多的通道,另一方面,它可以提高石墨化程度,并在碳骨架中引入N原子,提供更多活性位点,从而提高N-RHPC的催化活性. 刘磊等[71]以纤维素为前驱体结合高温热解和NH3处理的方法合成氮掺杂缺陷类石墨烯碳纳米材料(ND-GLC)催化剂,大量的缺陷位点、类石墨烯的薄层碳纳米结构使得ND-GLC催化剂具有优于商用材料的电催化性能.
此外,在N原子单掺杂的基础上,多原子掺杂碳材料在电化学性能方面明显更优[72]. 武鲁明等[73]采用一步磷化法以三聚氰胺和磷酸氢二铵为原料制备出N/P共掺杂的纳米空心碳壳(PNC),及其与磷化铁纳米颗粒复合材料(FeP@PNC),表现了出优异的电催化活性. 他们认为这是因为N-P之间的协同作用提供了更多的活性位点,有利于制备更多的金属磷化物碳材料以提升电催化反应效率. 罗明洪等[32]以尿素为原料通过高温煅烧得到氮掺杂碳纳米片(NCN),将其浸渍还原制备了氮掺杂碳纳米片载钴催化剂(Co/NCN)表现出了比石墨烯载钴更高的电流值. Lu等[74]将大豆秸秆用KOH活化后制备成多孔生物质炭为载体,再加入CoCl2 混合热解得到Co /N共掺杂多孔生物炭电催化剂CoNASS,如图5所示,其在KOH电解质溶液中的ORR性能媲美商用铀碳. Liu等[75]也发现N,B共掺杂碳点(BN-CDs) 具有高ORR活性. 其他一些学者[76 − 78]以氮掺杂碳为载体或催化剂进行电催化时也获得了相似的结论,如氮掺杂多孔碳催化剂的硝基苯反应速率和产苯胺电流效率分别是商业石墨电极的4.2倍和6.4倍.
-
Jian 等[79]证明了NH3活化制备木质素衍生氮掺杂多孔碳(NLPC)具有较大的电容值(181.0 Fg−1)和极高的循环稳定性. 且与电催化剂一样,多原子共掺杂被广泛应用在电极材料的制备中. Yeon等[80]先以木质素为前驱体,添加尿素为氮源,制得类似蜂窝结构的多孔N掺杂碳材料,后添加硫代硫酸钠与盐酸在高温条件下得到了循环稳定性更好的N/S共掺杂碳材料. Wan等[81]以大麦为原料同样制得了N/S共掺杂的多孔碳材料,其比表面积可达
2139.6 m2·g−1,表现出了极其优异的电容性及循环稳定性. 王心如等[82]也发现在N-Fe的协同作用下,可有效提高产物收率,加速包覆反应的进程,同时增加产物的比电容,在双电容的基础上形成赝电容,提高了碳材料的电化学性质.虽然大量研究表明氮掺杂可以在一定程度上提高碳材料的电化学性能,但掺杂氮过多会导致碳基基底材料结构的破坏,降低碳材料导电性与反应性能[83],因此如何平衡氮掺杂量和掺杂氮材料的电化学性能还需要更深入的研究.
-
氮掺杂碳材料在生物医药、传感器、药物负载、隐形眼睛等领域的运用也很常见,例如介孔碳、碳量子点等微粒的巧妙使用[34]. 余星等[84]通过拓展水溶液法,提出碱催化双模板协同构筑法,成功得到了具有高氮含量和均一单分散的氮掺杂介孔碳纳米球. 这种掺氮介孔碳作为药物载体时具有良好的药物运载能力. 针对口服吸收差的药物,Lu等[85]开发了一种聚合物功能化介孔碳材料,该材料表现出了高载药量和良好的控释能力. 在探针方面,王领[86]以玉米秸秆为碳源,乙二胺为氮源,利用水热法制备氮掺杂的碳量子点(N-CQDs),借助其表面丰富的氨基官能团,与生物大分子水溶两性霉素B缩合形成一种复合探针(N-CQDs@Amp B),表现出了对白色念珠菌有很强的特异性与荧光性,定量检测效果很好.
-
目前,氮掺杂碳材料在各大领域已经得到长足发展,氮掺杂使碳材料生成了碱性活性位点,不仅改善了碳材料的电子结构与表面化学性质,还有助于孔结构和比表面积的提高,在一些应用领域表现出了良好的催化性能、负载性能、吸附性能和电化学性能. 尽管如此,氮掺杂碳材料的研究仍存在一些问题值得进一步研究,以推动其工业化应用进程.
(1)如大豆、畜禽废弃物等特定农林废弃物中富含丰富的氮元素和碳基质,如何一步利用这些碳基质和氮源实现高性能N掺杂碳材料的可控制备值得深入思考.
(2)各种氮掺杂过程中,所制备碳材料往往具有各种含氮官能团,如何实现特定含氮官能团的精准引入仍需探究.
(3)研究中大多数的氮掺杂碳材料均为粉末状,在一些特定领域如吸附、催化等,如何制备性质同样优异的颗粒成型碳材料需进一步开发.
(4)对多原子共掺杂的协同机理、氮元素在反应中的存在形式及转变机理、掺杂原子性质与其性能间的构效关系,尤其是氮掺杂碳材料催化剂中氮基团在催化反应过程中的演变机制与催化性能间的关系,仍不明确.
氮掺杂技术在碳基材料中的应用进展
Application Progress of nitrogen doping technology in carbon-based materials
-
摘要: 碳材料具有较大的比表面积、较强的吸附能力、丰富的表面官能团等理化性质,在催化、吸附、电化学等领域广泛应用. 氮掺杂可以增强碳材料表面极性、提高表面润湿性、降低离子吸脱附时的能垒、修饰碳原子表面电子结构等,是改善碳材料理化性能的有效手段. 目前,已有较多文献对氮掺杂碳材料在催化、电化学领域的应用进行了广泛综述,但均是针对某一具体领域的应用,而针对氮掺杂技术尤其是多原子共掺杂在碳基材料中各个领域应用的整体性综述还较少. 因此,本文从N原子和多原子共掺杂碳材料的制备方法及性能出发,对其在催化、吸附、电化学等领域的作用机理及应用现状进行综述,并对未来可能开展的研究方向进行展望和分析,以期为氮掺杂碳材料的新型制备和工业化发展提供思路和借鉴.Abstract: Carbon materials have large specific surface area, strong adsorption capacity, rich surface functional groups and other physicochemical properties, and are widely used in catalysis, adsorption, electrochemistry and other fields. Nitrogen doping can enhance the surface polarity of carbon materials, improve the surface wettability, reduce the energy barrier during ion adsorption and desorption, modify the surface electronic structure of carbon atoms and so on, which is an effective mean to improve the physical and chemical properties of carbon materials. At present, there are many reviews on the application of N-doped carbon materials in the fields of catalysis and electrochemistry for a specific field, but few comprehensive reviews on the application of nitrogen doping technology, especially multi atom co-doping technology in various fields of carbon based materials. Therefore, based on the preparation method and performance of N atom and multi atom co-doping carbon materials, this paper summarizes their action mechanism and application status in catalysis, adsorption, electrochemistry and other fields, and prospects their future possible research direction, to provide ideas and reference for the new preparation and industrial development of nitrogen doped carbon materials.
-
Key words:
- N doping carbon material /
- co-doping /
- catalysis /
- adsorption /
- electrochemistry.
-

-
表 1 N原子和多原子共掺杂碳材料的制备方法及应用
Table 1. Preparation method and application of N atom and multi atom codoped carbon materials
掺杂元素
Dopingelement前躯体
Precursor制备方法
Preparation Method应用
Application数据来源
ReferencesN 三聚氰胺、琼脂 原位合成法 光催化 [29] N 三聚氰胺、吐司 原位合成法 微生物燃料电池阳极 [30] N 三聚氰胺、玉米芯 原位合成法 降解双酚A [31] N 尿素、醋酸 原位合成法 金属催化剂载体 [32] N 氨水、鳞片石墨 原位合成法 吸附和降解4-氯苯酚 [33] N 乙二胺、抗坏血酸 原位合成法 Hg2+的荧光探针 [34] N 壳聚糖、稻壳 后处理法 吸附CO2 [35] N NH3、大豆秸秆 后处理法 吸附SO2 [36] N、B 三聚氰胺、硼酸 后处理法 降解四环素 [37] N、S 聚苯胺、硫酸 原位合成法 催化木糖生产糠醛 [38] N、P 六氟磷酸铵、硫氰酸铵 原位合成法 光催化 [39] N、Co NH3、锯末、氯化钴 后处理法 电催化 [40] N、S、P 2-甲基咪唑、三苯基膦、硫脲 原位合成法 电催化 [41] N、B、Co 2-甲基咪唑、6水合硝酸钴、硼酸 原位合成法 降解罗丹明B [42] -
[1] ZHANG X D, ZHANG G J, QIN X W, et al. Catalytic performance of CH4-CO2 reforming over metal free nitrogen-doped biomass carbon catalysts: Effect of different preparation methods[J]. International Journal of Hydrogen Energy, 2021, 46(62): 31586-31597. doi: 10.1016/j.ijhydene.2021.07.043 [2] 杨慧聪, 梁骥, 王振兴, 等. 多孔碳质材料在氧还原电催化中的应用[J]. 新型炭材料, 2016, 31(3): 243-263. YANG H C, LIANG J, WANG Z X, et al. Applications of porous carbon materials in the electrocatalysis of the oxygen reduction reaction[J]. New Carbon Materials, 2016, 31(3): 243-263 (in Chinese).
[3] YANG S L, PENG L, HUANG P P, et al. Nitrogen, phosphorus, and sulfur co-doped hollow carbon shell as superior metal-free catalyst for selective oxidation of aromatic alkanes[J]. Angewandte Chemie, 2016, 55(12): 4016-4020. doi: 10.1002/anie.201600455 [4] ZHOU Q, ZHAO Z K. Sulfate surfactant assisted approach to fabricate sulphur-doped supported nanodiamond catalyst on carbon nanotube with unprecedented catalysis for ethylbenzene dehydrogenation[J]. ChemCatChem, 2020, 12(1): 342-349. doi: 10.1002/cctc.201901267 [5] PARK J E, JANG Y J, KIM Y J, et al. Sulfur-doped graphene as a potential alternative metal-free electrocatalyst and Pt-catalyst supporting material for oxygen reduction reaction[J]. Physical Chemistry Chemical Physics:PCCP, 2014, 16(1): 103-109. doi: 10.1039/C3CP54311K [6] LIU Z W, PENG F, WANG H J, et al. Novel phosphorus-doped multiwalled nanotubes with high electrocatalytic activity for O2 reduction in alkaline medium[J]. Catalysis Communications, 2011, 16(1): 35-38. doi: 10.1016/j.catcom.2011.08.038 [7] FULVIO P F, LEE J S, MAYES R T, et al. Boron and nitrogen-rich carbons from ionic liquid precursors with tailorable surface properties[J]. Physical Chemistry Chemical Physics:PCCP, 2011, 13(30): 13486-13491. doi: 10.1039/c1cp20631a [8] PAN G X, CAO F, ZHANG Y J, et al. N-doped carbon nanofibers arrays as advanced electrodes for supercapacitors[J]. Journal of Materials Science & Technology, 2020, 55: 144-151. [9] GUO F Q, PENG K Y, LIANG S, et al. Evaluation of the catalytic performance of different activated biochar catalysts for removal of tar from biomass pyrolysis[J]. Fuel, 2019, 258: 116204. doi: 10.1016/j.fuel.2019.116204 [10] 陈倍宁, 王恩语, 杨正爽, 等. 基于氮掺杂碳量子点的水体氟离子选择性荧光开启检测[J]. 环境化学, 2024, 43(3): 875-884. doi: 10.7524/j.issn.0254-6108.2022090802 CHEN B N, WANG E Y, YANG Z S, et al. Rapid and selective “turn-on ” fluorescent detection of fluoride ion in aqueous solution using nitrogen-doped carbon quantum dots[J]. Environmental Chemistry, 2024, 43(3): 875-884 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022090802
[11] ZHAO P, SHEN B X, YANG M T, et al. Effect of nitrogen species on electrochemical properties of N-doped carbon nanotubes derived from co-pyrolysis of low-density polyethylene and melamine[J]. Journal of Energy Storage, 2023, 67: 107569. doi: 10.1016/j.est.2023.107569 [12] JANA D, SUN C L, CHEN L C, et al. Effect of chemical doping of boron and nitrogen on the electronic, optical, and electrochemical properties of carbon nanotubes[J]. Progress in Materials Science, 2013, 58(5): 565-635. doi: 10.1016/j.pmatsci.2013.01.003 [13] LIU L, LU J, ZHANG Y X, et al. Synthesis of nitrogen-doped graphitic carbon nanocapsules from a poly(ionic liquid) for CO2 capture[J]. New Carbon Materials, 2017, 32(4): 380-384. doi: 10.1016/S1872-5805(17)60129-X [14] INAGAKI M, TOYODA M, SONEDA Y, et al. Nitrogen-doped carbon materials[J]. Carbon, 2018, 132: 104-140. doi: 10.1016/j.carbon.2018.02.024 [15] XIE C, YANG S H, XU X Q, et al. Core-shell structured carbon nanotubes/N-doped carbon layer nanocomposites for supercapacitor electrodes[J]. Journal of Nanoparticle Research, 2020, 22(1): 1-7. doi: 10.1007/s11051-019-4718-8 [16] 乔文强, 孙玺, 王连杰, 等. 含氮聚合物催化剂的制备及乙炔氢氯化性能研究[J]. 石油化工高等学校学报, 2022, 35(3): 30-35. QIAO W Q, SUN X, WANG L J, et al. Preparation of N-containing polymer catalyst and its performance in acetylene hydrochlorination[J]. Journal of Petrochemical Universities, 2022, 35(3): 30-35 (in Chinese).
[17] XIE W H, YAO X Y, LI H, et al. Biomass-based N-rich porous carbon materials for CO2 capture and in situ conversion[J]. ChemSusChem, 2022, 15(18): e202201004. doi: 10.1002/cssc.202201004 [18] ZHU X F, LUO Z J, GUO W W, et al. Reutilization of biomass pyrolysis waste: Tailoring dual-doped biochar from refining residue of bio-oil through one-step self-assembly[J]. Journal of Cleaner Production, 2022, 343: 131046. doi: 10.1016/j.jclepro.2022.131046 [19] MITOME T, HIROTA Y, UCHIDA Y, et al. Porous structure and pore size control of mesoporous carbons using a combination of a soft-templating method and a solvent evaporation technique[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2016, 494: 180-185. [20] CHEN C, YU D F, ZHAO G Y, et al. Three-dimensional scaffolding framework of porous carbon nanosheets derived from plant wastes for high-performance supercapacitors[J]. Nano Energy, 2016, 27: 377-389. doi: 10.1016/j.nanoen.2016.07.020 [21] ZHAO L, FAN L Z, ZHOU M Q, et al. Nitrogen-containing hydrothermal carbons with superior performance in supercapacitors[J]. Advanced Materials, 2010, 22(45): 5202-5206. doi: 10.1002/adma.201002647 [22] CANDELARIA S L, GARCIA B B, LIU D W, et al. Nitrogen modification of highly porous carbon for improved supercapacitor performance[J]. Journal of Materials Chemistry, 2012, 22(19): 9884-9889. doi: 10.1039/c2jm30923h [23] PENG H, MA G F, SUN K J, et al. Facile synthesis of poly(p-phenylenediamine)-derived three-dimensional porous nitrogen-doped carbon networks for high performance supercapacitors[J]. The Journal of Physical Chemistry C, 2014, 118(51): 29507-29516. doi: 10.1021/jp508684t [24] LIU L, DENG Q F, MA T Y, et al. Ordered mesoporous carbons: Citric acid-catalyzed synthesis, nitrogen doping and CO2 capture[J]. Journal of Materials Chemistry, 2011, 21(40): 16001-16009. doi: 10.1039/c1jm12887f [25] HORIKAWA T, SAKAO N, SEKIDA T, et al. Preparation of nitrogen-doped porous carbon by ammonia gas treatment and the effects of N-doping on water adsorption[J]. Carbon, 2012, 50(5): 1833-1842. doi: 10.1016/j.carbon.2011.12.033 [26] SHAKU B, MOFOKENG T P, COVILLE N J, et al. Biomass valorisation of marula nutshell waste into nitrogen-doped activated carbon for use in high performance supercapacitors[J]. Electrochimica Acta, 2023, 442: 141828. doi: 10.1016/j.electacta.2023.141828 [27] NIU L, YANG Q, WANG W, et al. Creation of nanopores and nitrogen doping in the surface layers of reduced graphene oxide electrode via ions implantation resulting in enhanced electrochemical performance for supercapacitor[J]. J Energy Storage, 2023, 58: 106453. doi: 10.1016/j.est.2022.106453 [28] SONG M, WANG C, ZHU C, et al. An effective fabrication and highly tunable microwave absorption of nitrogen-doped graphene[J]. Diamond and Related Materials, 2022, 129: 109348. doi: 10.1016/j.diamond.2022.109348 [29] WANG Y M, CAI H Y, QIAN F F, et al. Facile one-step synthesis of onion-like carbon modified ultrathin g-C3N4 2D nanosheets with enhanced visible-light photocatalytic performance[J]. Journal of Colloid and Interface Science, 2019, 533: 47-58. doi: 10.1016/j.jcis.2018.08.039 [30] 王紫嫙, 王婕, 王兴源, 等. 氮掺杂多孔碳材料阳极制备及其在微生物燃料电池上的应用[J]. 环境化学, 2024, 43(2): 614-622. doi: 10.7524/j.issn.0254-6108.2022071204 WANG Z X, WANG J, WANG X Y, et al. Preparation of nitrogen-doped porous carbon anode and its application in microbial fuel cells[J]. Environmental Chemistry, 2024, 43(2): 614-622 (in Chinese). doi: 10.7524/j.issn.0254-6108.2022071204
[31] WANG H Z, GUO W Q, SI Q S, et al. Non-covalent doping of carbon nitride with biochar: Boosted peroxymonosulfate activation performance and unexpected singlet oxygen evolution mechanism[J]. Chemical Engineering Journal, 2021, 418: 129504. doi: 10.1016/j.cej.2021.129504 [32] 罗明洪, 刘卫红. 氮掺杂碳纳米片载钴催化剂的制备及其对硼氢化钠的电催化氧化[J]. 化工新型材料, 2022, 50(8): 282-286. LUO M H, LIU W H. Preparation of Co/NCN catalyst and its electro-oxidation of NaBH4[J]. New Chemical Materials, 2022, 50(8): 282-286 (in Chinese).
[33] 王小波, 唐鹏, 丁聪, 等. 氮掺杂石墨烯高效移除水中4-氯苯酚[J]. 环境化学, 2017, 36(12): 2641-2649. doi: 10.7524/j.issn.0254-6108.2017051402 WANG X B, TANG P, DING C, et al. Efficient removal of 4-chlorophenol in water by nitrogen doped reduced graphene oxide[J]. Environmental Chemistry, 2017, 36(12): 2641-2649 (in Chinese). doi: 10.7524/j.issn.0254-6108.2017051402
[34] 孙雪花, 张锦婷, 赵李艳, 等. 基于氮掺杂碳量子点的制备及其对Hg2+的响应[J]. 环境化学, 2021, 40(1): 321-326. doi: 10.7524/j.issn.0254-6108.2020061504 SUN X H, ZHANG J T, ZHAO L Y, et al. Preparation of nitrogen-doped carbon quantum dots and its response to Hg2+[J]. Environmental Chemistry, 2021, 40(1): 321-326 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020061504
[35] HE S, CHEN G Y, XIAO H, et al. Facile preparation of N-doped activated carbon produced from rice husk for CO2 capture[J]. Journal of Colloid and Interface Science, 2021, 582: 90-101. doi: 10.1016/j.jcis.2020.08.021 [36] ZHANG X, ZHANG S B, ZHANG J J, et al. Enhanced SO2 adsorption performance on nitrogen-doped biochar: Insights from generalized two-dimensional correlation infrared spectroscopy[J]. Fuel, 2023, 354: 129266. doi: 10.1016/j.fuel.2023.129266 [37] PREEYANGHAA M, VINESH V, SABARIKIRISHWARAN P, et al. Investigating the role of ultrasound in improving the photocatalytic ability of CQD decorated boron-doped g-C3N4 for tetracycline degradation and first-principles study of nitrogen-vacancy formation[J]. Carbon, 2022, 192: 405-417. doi: 10.1016/j.carbon.2022.03.011 [38] GAO J H, WANG H, CAO X M, et al. Nitrogen doped carbon solid acid for improving its catalytic transformation of xylose and agricultural biomass residues to furfural[J]. Molecular Catalysis, 2023, 535: 112890. doi: 10.1016/j.mcat.2022.112890 [39] CHAI B, YAN J T, WANG C L, et al. Enhanced visible light photocatalytic degradation of Rhodamine B over phosphorus doped graphitic carbon nitride[J]. Applied Surface Science, 2017, 391: 376-383. doi: 10.1016/j.apsusc.2016.06.180 [40] 叶雨阳. 生物质热解氮掺杂碳基催化剂的制备及其电催化氧还原性能研究[D]. 合肥: 中国科学技术大学, 2020. YE Y Y. Synthesis of nitrogen doped carbon-based catalyst derived from biomass pyrolysis and study on their catalytic performance for oxygen reduction reaction[D]. Hefei: University of Science and Technology of China, 2020 (in Chinese).
[41] 夏营港, 王聪, 刘冬澳, 等. N、S、P共掺杂沸石咪唑酯骨架(ZIF)多孔碳材料制备和电化学性能[J]. 广州化学, 2022, 47(5): 26-34. XIA Y G, WANG C, LIU D A, et al. Preparation and electrochemical properties of N, S, P co-doped zeolitic imidazolate frameworks(ZIF) porous carbon materials[J]. Guangzhou Chemistry, 2022, 47(5): 26-34 (in Chinese).
[42] 罗漩, 易子铭, 谢金伶, 等. 硼氮共掺杂多孔碳活化过硫酸盐降解罗丹明 B 性能和机制[J]. 环境化学, 2023, 42(11): 3849-3860. LUO X, YI Z M, XIE J L, et al. Performance and mechanism of boron-nitrogen co-doped porous carbon as permonosulfate activator for Rhodamine B degradation[J]. Environmental Chemistry,2023, 42(11): 3849-3860 (in Chinese).
[43] TENG N, LI J L, LU B Q, et al. The selective aerobic oxidation of 5-hydroxymethylfurfural to produce 2, 5-diformylfuran using Nitrogen-doped porous carbons as catalysts[J]. New Carbon Materials, 2019, 34(6): 593-599. doi: 10.1016/S1872-5805(19)60034-X [44] LEI G C, FAN Z J, HOU Y F, et al. Facile template-free synthesis of 3D cluster-like nitrogen-doped mesoporous carbon as metal-free catalyst for selective oxidation of H2S[J]. Journal of Environmental Chemical Engineering, 2023, 11(1): 109095. doi: 10.1016/j.jece.2022.109095 [45] 马晓, 秦晓伟, 张晓娣, 等. 氮掺杂生物质碳材料催化剂的制备及其催化CH4-CO2重整性能[J]. 洁净煤技术, 2022, 28(5): 59-70. MA X, QIN X W, ZHANG X D, et al. Preparation of biomass nitrogen-doped carbon material catalyst and its catalytic performance for CO2 reforming of CH4[J]. Clean Coal Technology, 2022, 28(5): 59-70 (in Chinese).
[46] CHEN W, FANG Y, LI K X, et al. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products[J]. Applied Energy, 2020, 260: 114242. doi: 10.1016/j.apenergy.2019.114242 [47] PHAM L K H, KONGPARAKUL S, DING M Y, et al. High-efficiency catalytic pyrolysis of palm kernel shells over Ni2P/nitrogen-doped activated carbon catalysts[J]. Biomass and Bioenergy, 2023, 174: 106836. doi: 10.1016/j.biombioe.2023.106836 [48] XU Z, ZHOU S Z, ZHU M Y. Ni catalyst supported on nitrogen-doped activated carbon for selective hydrogenation of acetylene with high concentration[J]. Catalysis Communications, 2021, 149: 106241. doi: 10.1016/j.catcom.2020.106241 [49] ZHANG X Y, LIU H B, SHENG X, et al. Nitrogen-doped carbon supported nanocobalt for the synthesis of functionalized triazines via oxidative cleavage of biomass derived vicinal diols as carbon synthons[J]. Journal of Catalysis, 2022, 408: 227-235. doi: 10.1016/j.jcat.2022.03.013 [50] WU G, LI D Y, DAI C S, et al. Well-dispersed high-loading pt nanoparticles supported by shell-core nanostructured carbon for methanol electrooxidation[J]. Langmuir:the ACS Journal of Surfaces and Colloids, 2008, 24(7): 3566-3575. doi: 10.1021/la7029278 [51] SOLANGI N H, HUSSIN F, ANJUM A, et al. A review of encapsulated ionic liquids for CO2 capture[J]. Journal of Molecular Liquids, 2023, 374: 121266. doi: 10.1016/j.molliq.2023.121266 [52] DING S, LIU Y X. Adsorption of CO2 from flue gas by novel seaweed-based KOH-activated porous biochars[J]. Fuel, 2020, 260: 116382. doi: 10.1016/j.fuel.2019.116382 [53] CHIANG Y C, JUANG R S. Surface modifications of carbonaceous materials for carbon dioxide adsorption: A review[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 214-234. doi: 10.1016/j.jtice.2016.12.014 [54] SHAO L S, WAN H A, WANG L Z, et al. N-doped highly microporous carbon derived from the self-assembled lignin/chitosan composites beads for selective CO2 capture and efficient p-nitrophenol adsorption[J]. Separation and Purification Technology, 2023, 313: 123440. doi: 10.1016/j.seppur.2023.123440 [55] GE K, HE Y T, CAI W Y, et al. Nitrogen-doped microporous carbon materials derived from DBU-modified carboxylic acid polymers for CO2 capture[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107849. doi: 10.1016/j.jece.2022.107849 [56] YUAN X Z, LI S J, JEON S, et al. Valorization of waste polyethylene terephthalate plastic into N-doped microporous carbon for CO2 capture through a one-pot synthesis[J]. Journal of Hazardous Materials, 2020, 399: 123010. doi: 10.1016/j.jhazmat.2020.123010 [57] YU J, SO J. Synthesis and characterization of nitrogen-containing hydrothermal carbon with ordered mesostructure[J]. Chemical Physics Letters, 2019, 716: 237-246. doi: 10.1016/j.cplett.2018.12.014 [58] 孙飞, 高继慧, 曲智斌, 等. 碳基材料纳孔空间内SO2吸附转化机理[J]. 中国电机工程学报, 2019, 39(20): 5979-5988,6178. SUN F, GAO J H, QU Z B, et al. Mechanism of SO2 adsorption and conversion in nanopores of carbon-based materials[J]. Proceedings of the CSEE, 2019, 39(20): 5979-5988,6178 (in Chinese).
[59] 左盼星. 氮掺杂碳基吸附药物的研制与性能研究[D]. 石家庄: 河北科技大学, 2021. ZUO P X. Preparation and properties of nitrogen-doped carbon-based adsorbent drugs[D]. Shijiazhuang: Hebei University of Science and Technology, 2021 (in Chinese).
[60] 王亮, 柳馨, 汪长安, 等. N掺杂多孔碳海绵定形芒硝基复合相变材料的制备及其热性能[J]. 储能科学与技术, 2023, 12(1): 79-85. WANG L, LIU X, WANG C A, et al. Preparation and thermal performance of nitrogen-doped porous carbon sponge-type mirabilite-based composite phase-change material[J]. Energy Storage Science and Technology, 2023, 12(1): 79-85 (in Chinese).
[61] 吴尚迪, 葛夏菁, 余雅琳, 等. 基于席夫碱反应合成氮掺杂的碳活化PMS降解双酚A[J]. 环境化学, 2022, 41(1): 340-351. doi: 10.7524/j.issn.0254-6108.2020082601 WU S D, GE X J, YU Y L, et al. Degradation of BPA by activating PMS over N-doped carbon synthesized based on Schiff base reaction[J]. Environmental Chemistry, 2022, 41(1): 340-351 (in Chinese). doi: 10.7524/j.issn.0254-6108.2020082601
[62] PENG X, LUO Z J, XIE H M, et al. Removal of phenylarsonic acid compounds by porous nitrogen doped carbon: Experimental and DFT study[J]. Applied Surface Science, 2022, 606: 154859. doi: 10.1016/j.apsusc.2022.154859 [63] CHEN W J, HE H M, LEI L L, et al. Green synthesis of novel Fe nanoparticles embedded in N-doped biochar composites derived from bagasse for sulfadiazine degradation via peroxymonosulfate activator: Mechanism insight and performance assessment[J]. Journal of Water Process Engineering, 2022, 49: 103131. doi: 10.1016/j.jwpe.2022.103131 [64] ZHU K, BIN Q, SHEN Y Q, et al. In-situ formed N-doped bamboo-like carbon nanotubes encapsulated with Fe nanoparticles supported by biochar as highly efficient catalyst for activation of persulfate (PS) toward degradation of organic pollutants[J]. Chemical Engineering Journal, 2020, 402: 126090. doi: 10.1016/j.cej.2020.126090 [65] ZOU Y B, LI W T, YANG L, et al. Activation of peroxymonosulfate by sp2-hybridized microalgae-derived carbon for ciprofloxacin degradation: Importance of pyrolysis temperature[J]. Chemical Engineering Journal, 2019, 370: 1286-1297. doi: 10.1016/j.cej.2019.04.002 [66] 余本善, 孙乃达, 焦姣. 储能技术与产业现状及发展趋势[J]. 石油科技论坛, 2017, 36(1): 57-61,67. YU B S, SUN N D, JIAO J. Current conditions and development trend of energy-storing technology and industry[J]. Oil Forum, 2017, 36(1): 57-61,67 (in Chinese).
[67] SHARMILI N, NAGI R, WANG P F. A review of research in the Li-ion battery production and reverse supply chains[J]. Journal of Energy Storage, 2023, 68: 107622. doi: 10.1016/j.est.2023.107622 [68] MAGETO T, BHOYATE S D, de SOUZA F M, et al. Developing practical solid-state rechargeable Li-ion batteries: Concepts, challenges, and improvement strategies[J]. Journal of Energy Storage, 2022, 55: 105688. doi: 10.1016/j.est.2022.105688 [69] WANG B L, JIAO R T, SHI F, et al. Novel strategy for efficient conversion of biomass into N-doped graphitized carbon nanosheets as high-performance electrode material for supercapacitor[J]. Journal of Physics and Chemistry of Solids, 2023, 181: 111509. doi: 10.1016/j.jpcs.2023.111509 [70] SHI J, LIN N, LIN H B, et al. A N-doped rice husk-based porous carbon as an electrocatalyst for the oxygen reduction reaction[J]. New Carbon Materials, 2020, 35(4): 401-409. doi: 10.1016/S1872-5805(20)60497-8 [71] 刘磊, 安升辉, 张建. 氮掺杂缺陷类石墨烯碳纳米材料的制备及电催化氧还原性能研究[J]. 当代化工研究, 2020(14): 34-35. LIU L, AN S H, ZHANG J. Preparation and electrocatalytic oxygen reduction study of N-doped defective graphene-like carbon nanomaterial[J]. Modern Chemical Research, 2020(14): 34-35 (in Chinese).
[72] JIN J T, JIANG P, QIAO X C, et al. Highly doped N, S-Codoped carbon nanomeshes for excellent electrocapacitive performance[J]. Journal of Alloys and Compounds, 2019, 803: 704-710. doi: 10.1016/j.jallcom.2019.06.295 [73] 武鲁明, 于海斌, 王亚权. 多孔碳材料的制备及其金属磷化物氧还原性能研究[J]. 无机盐工业, 2023, 55(4): 104-110. WU L M, YU H B, WANG Y Q. Study on preparation of porous carbon materials and oxygen reduction properties of their metal phosphide[J]. Inorganic Chemicals Industry, 2023, 55(4): 104-110 (in Chinese).
[74] LU G L, LI Z Y, FAN W X, et al. Sponge-like N-doped carbon materials with Co-based nanoparticles derived from biomass as highly efficient electrocatalysts for the oxygen reduction reaction in alkaline media[J]. RSC Advances, 2019, 9: 4843-4848. doi: 10.1039/C8RA10462J [75] LIU H, LIU Z H, ZHANG J Q, et al. Boron and nitrogen co-doped carbon dots for boosting electrocatalytic oxygen reduction[J]. New Carbon Materials, 2021, 36(3): 585-593. doi: 10.1016/S1872-5805(21)60043-4 [76] WANG X D, FANG J J, LIU X R, et al. Nitrogen-doped carbon as selectively permeable layer to enhance the anti-poisoning ability of hydrogen oxidation reaction catalysts for hydroxide exchange membrane fuel cells[J]. Applied Catalysis B:Environmental, 2023, 327: 122442. doi: 10.1016/j.apcatb.2023.122442 [77] 赵学洋. 氮掺杂多孔碳基材料电催化还原性能调控及其污染物转化与CO2还原研究[D]. 大连: 大连理工大学, 2021. ZHAO X Y. Regulation of electrocatalytic reduction performance of nitrogen-doped porous carbon-based materials and the electrocatalysis in pollutants conversion and CO2 reduction[D]. Dalian: Dalian University of Technology, 2021 (in Chinese).
[78] SUN Y, XUE S, SUN J H, et al. Silk-derived nitrogen-doped porous carbon electrodes with enhanced ionic conductivity for high-performance supercapacitors[J]. Journal of Colloid and Interface Science, 2023, 645: 297-305. doi: 10.1016/j.jcis.2023.04.130 [79] JIAN W B, ZHANG W L, WU B C, et al. Enzymatic hydrolysis lignin-derived porous carbons through ammonia activation: Activation mechanism and charge storage mechanism[J]. ACS Applied Materials & Interfaces, 2022, 14(4): 5425-5438. [80] YEON J S, PARK S H, SUK J, et al. Confinement of sulfur in the micropores of honeycomb-like carbon derived from lignin for lithium-sulfur battery cathode[J]. Chemical Engineering Journal, 2020, 382: 122946. doi: 10.1016/j.cej.2019.122946 [81] WAN L, XIAO R, LIU J X, et al. A novel strategy to prepare N, S-codoped porous carbons derived from barley with high surface area for supercapacitors[J]. Applied Surface Science, 2020, 518: 146265. doi: 10.1016/j.apsusc.2020.146265 [82] 王心如, 刘嘉怡, 丁晗, 等. 超级电容器碳材料的制备及性能测试[J]. 广州化工, 2022, 50(13): 76-80. WANG X R, LIU J Y, DING H, et al. Preparation and electrochemical performance testing of carbon materials for supercapacitors[J]. Guangzhou Chemical Industry, 2022, 50(13): 76-80 (in Chinese).
[83] WU Z Y, IQBAL Z, WANG X Q. Metal-free, carbon-based catalysts for oxygen reduction reactions[J]. Frontiers of Chemical Science and Engineering, 2015, 9(3): 280-294. doi: 10.1007/s11705-015-1524-4 [84] 余星. 氮掺杂介孔碳纳米球的构筑及其作为药物载体的初步研究[D]. 广州: 广东药科大学, 2021. YU X. Fabrication of nitrogen doped mesoporous carbon nanospheres and their preliminary study as drug carriers[D]. Guangzhou: Guangdong Pharmaceutical University, 2021 (in Chinese).
[85] LU H Y, YANG G Z, RAN F, et al. Polymer-functionalized mesoporous carbon nanoparticles on overcoming multiple barriers and improving oral bioavailability of Probucol[J]. Carbohydrate Polymers, 2020, 229: 115508. doi: 10.1016/j.carbpol.2019.115508 [86] 王领. 玉米秸秆制备氮掺杂碳量子点应用于检测白色念珠菌[D]. 吉林: 东北电力大学, 2019. WANG L. Synthesis of nitrogen-doped carbon quantum dots from cornstalk for detection of Candida albicans[D]. Jilin: Northeast Electric Power University, 2019 (in Chinese).
-



 下载:
下载: