-
塑料作为日常生产生活中常见的材料,据估计,1950年至2019年塑料产量达到100亿吨,产生约76亿吨塑料废物[1],塑料分布广泛,不仅在土壤中,而且在饮用水以及海鲜产品中也发现了大量塑料[2 − 4]. 通常认为微塑料的粒径小于5 mm[5],与大中型塑料相比,微塑料具有粒径小、比表面积大、疏水性强等特点[6-7]. 这些特性使得微塑料更易于吸附污染物质[8-9]. 微塑料本身作为一种新兴污染物,由于其会对生物体造成影响以及本身不可降解的特性而备受关注[10-11]. 微塑料会在食物链中不断传递[12],并在动植物中积累[13],最终进入人体,危害人体健康[14].
微塑料进入自然环境中,受到各种自然条件的影响而逐步老化,导致其表面形态特征、理化性质等发生变化,从而影响其对污染物的吸附性能[15]. Song等[15 − 17]分别使用UV灯,氙灯和金卤灯模拟老化条件,结果表明在老化过程中微塑料产生了含氧官能团,增加了对污染物的吸附能力. Muller等[18]利用紫外光对PS和聚丙烯(PP)进行老化,发现其老化后生成了羰基等含氧基团. LV等[19]对自然老化2年的微塑料采用FTIR分析,结果表明其生成了羰基产物,相应特征峰随着老化时间的增加不断增多. 微塑料在自然环境中经历自然老化、紫外线老化、过氧化氢老化等老化过程[20]. 模拟自然条件下的老化方式,分析自然环境中老化微塑料和有机污染物的相互作用. 如Lang等 [21]采用芬顿法和过氧化氢分别对PS进行老化处理,分析得出经芬顿法老化后的PS具有更多的吸附位点和更强的吸附能力. Mao等[22]采用紫外辐射(UV)在不同环境条件下(空气、纯水和海水)对PS进行老化,显著增加了PS的吸附能力. Zhang等[23]发现在自然条件下,PS老化后表面粗糙程度增加、比表面积也增加.
薛彬等[24]利用臭氧对PS进行老化,老化后的PS表面产生丰富的裂缝、沟壑,表面粗糙程度增加. Miranda等[25]采用臭氧处理不同种类的微塑料样品,一些区域会剥落,造成样品表面粗糙程度均发生了变化,同时也增加了其吸附能力. Luo等[26]将微塑料经臭氧、Fenton、热活化过硫酸盐处理后,微塑料表面可见明显的碎裂和裂纹. 但臭氧老化对不同微塑料吸附有机物的影响相关研究较少. 且目前,O3已经成为首要的大气污染物[27],且在大气中,臭氧的浓度逐渐升高,会与环境中的微塑料发生老化反应,老化后的微塑料成为有机污染物的载体,加速有机污染物在环境中的迁移转换. 因此本研究使用O3老化PE和PS两种生活中较为常见的微塑料,研究老化PE和PS与有机污染物之间的相互作用,以及不同老化程度的微塑料吸附量的变化,为研究微塑料对有机污染物的富集及迁移转化提供理论依据.
-
联苯胺(C12H12N2,分析纯95%,国药化学试剂有限公司);PS、PE(粒径为300 μm,科信达高分子材料有限公司).
-
分别称取0.5 g PS和PE,放入不同烧杯中,分别加入500 mL去离子水后,加热搅拌,通入流量为5 L·min−1的O3. 分别按照0 h、1 h、2 h、3 h、6 h进行老化处理,其余步骤均相同.
-
实验样品为PS和PE,采用扫描电子显微镜(SEM)观察老化前后微塑料颗粒表面形貌以及其表面结构变化;采用傅里叶变换红外光谱仪(FTIR)分析微塑料表面官能团的变化;采用X射线衍射仪(XRD)分析微塑料的结晶度.
-
分别配制5、10、15、20、25 mg·L−1的联苯胺溶液,使用280 nm波长测定不同浓度联苯胺溶液的吸光度,换算吸附前联苯胺的浓度.
按照老化时间将2种微塑料样品分成不同的实验组,每组取0.02 g老化后的微塑料,加入20 mL浓度为10 mg·L−1的联苯胺标准溶液,并恒温振荡,振速为200 r·min−1,温度设置为(25±2) ℃. 在不同时间取样,测定吸光度. 样品平均吸附量由式(1)计算得出. 每个样品实验重复3次,取其平均值,并设置空白对照组.
式中,qe为微塑料样品吸附联苯胺的含量(μg·g−1);m为微塑料PS和PE的质量(mg);V为联苯胺溶液的体积(L);C0和Ce分别为吸附前后联苯胺溶液的浓度(μg·L−1).
吸附等温线实验:取0.02 g不同老化时间的PS和PE加入到不同浓度的联苯胺溶液中,溶液量均为20 mL. 达到吸附平衡,其余实验条件与吸附动力学实验相同,进行空白对照,每个实验重复3次,取其平均值. 采用式(1)计算吸附量.
吸附热力学实验:取0.02 g不同老化时间的PS和PE加入到不同浓度的联苯胺溶液中,溶液量均为20 mL. 分别在恒温25、35、45 ℃条件下进行振荡平衡,测定其吸光度,计算吸附量.
-
使用O3对不同粒径PS和PE老化1 h和6 h. 将0.02 g老化后的微塑料样品加入到20 mL浓度为10 mg·L−1的联苯胺溶液. 在温度为(25±2)℃,振荡速度为200 r·min−1时恒温振荡,达到吸附平衡,测定平衡吸附量.
-
采用excel 2010和SPSS 27对数据进行统计分析. 利用Origin 2022作图,图表中数据均为平均值±标准差.
-
图1为老化前后PE和PS的表面形貌. PS属于玻璃态塑料,未老化前表面较光滑平整,经不同老化时间处理后PS表面呈现出了不同程度的刻蚀,随着老化时间的增加,其表面的裂纹不断加深,呈现出了明显的鳞片结构;而PE属于橡胶态塑料,原始表面较为粗糙. O3老化后,其表面发生了部分颗粒的氧化和剥落[28],与原始样品相比,其粗糙程度变化较小.
-
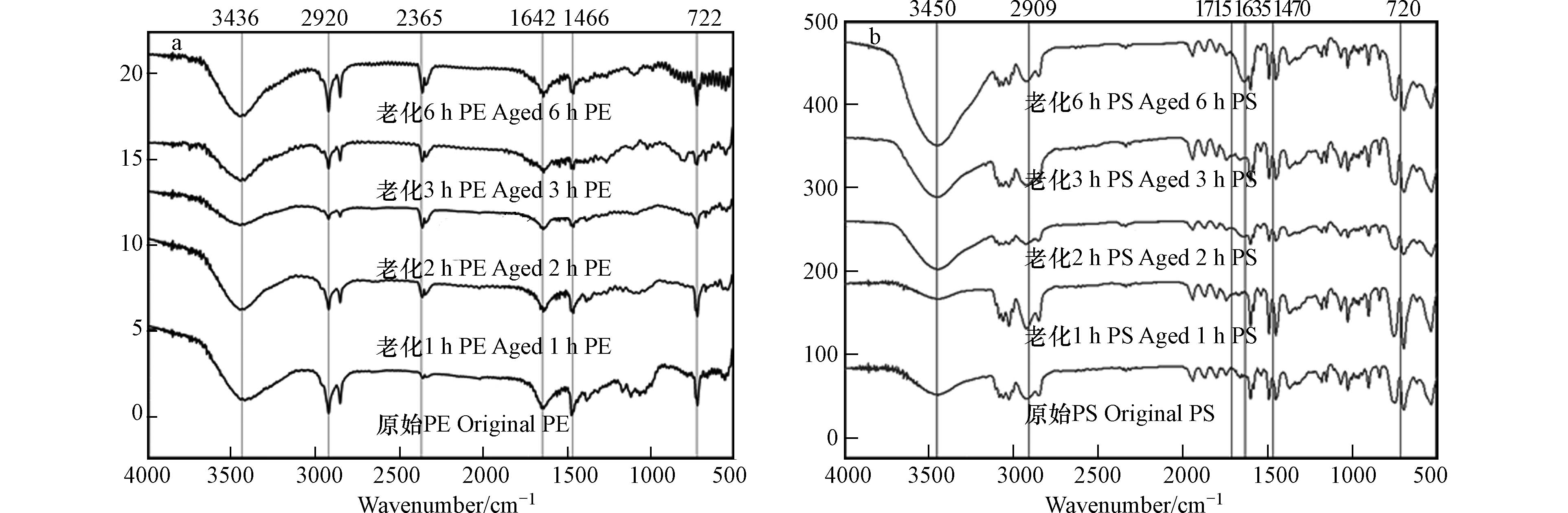
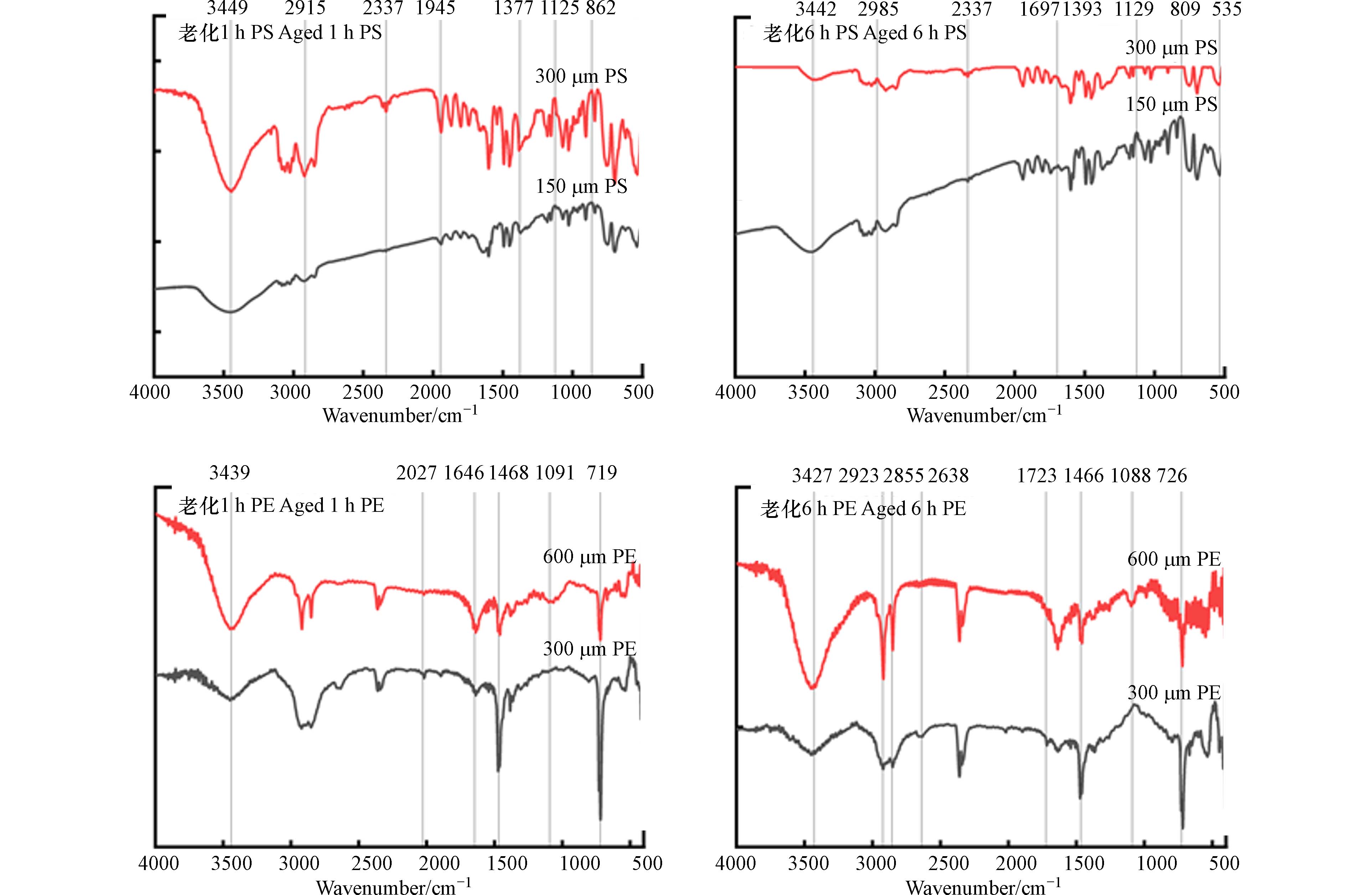
如图2所示, O3老化后的PS样品主要在
3450 、1715 、1635 、1470 、720 cm−1处发生伸缩振动,表明PS发生了表面的结构变化. 随着老化时间的增加,3450 cm−1处的吸收强度显著增加,即有苯环=CH的生成和断裂[21];1715 cm−1处的伸缩振动峰代表有新的羰基产生,1635 cm−1处的特征峰对应苯环C=C发生了弯曲振动[29];1470 cm−1处特征峰对应—C=C—振动[30];720 cm−1处的特征峰对应C—H变行和骨架振动[31].PE原始和老化后的样品均出现了以下几种振动峰:主要是730—650 cm−1、
1500 —1400 cm−1、2851 cm−1和2919 cm−1.2851 cm−1和2919 cm−1处的吸收峰强度显著降低,说明发生了C—H键的断裂和对称及不对称的伸缩振动[32 − 34],在1640 cm−1左右处出现了双键伸缩振动区,也可能是苯环的骨架振动所形成[35]. 在1466 cm−1处吸收峰的强度发生变化,可能是CH2的平面弯曲震动或新键的生成以及旧键的断裂[32]. 此外,老化的PE在2357 cm−1处出现了新的吸收峰,可能是三键和双键累积区发生了断裂[36]. 不同老化时间下,PE均未显示出在1700 —1800 cm−1范围内的羰基吸收带,与已有的研究结果不一致[37-38]. 可能是由于老化的时间不足或者是老化方式的不同. -
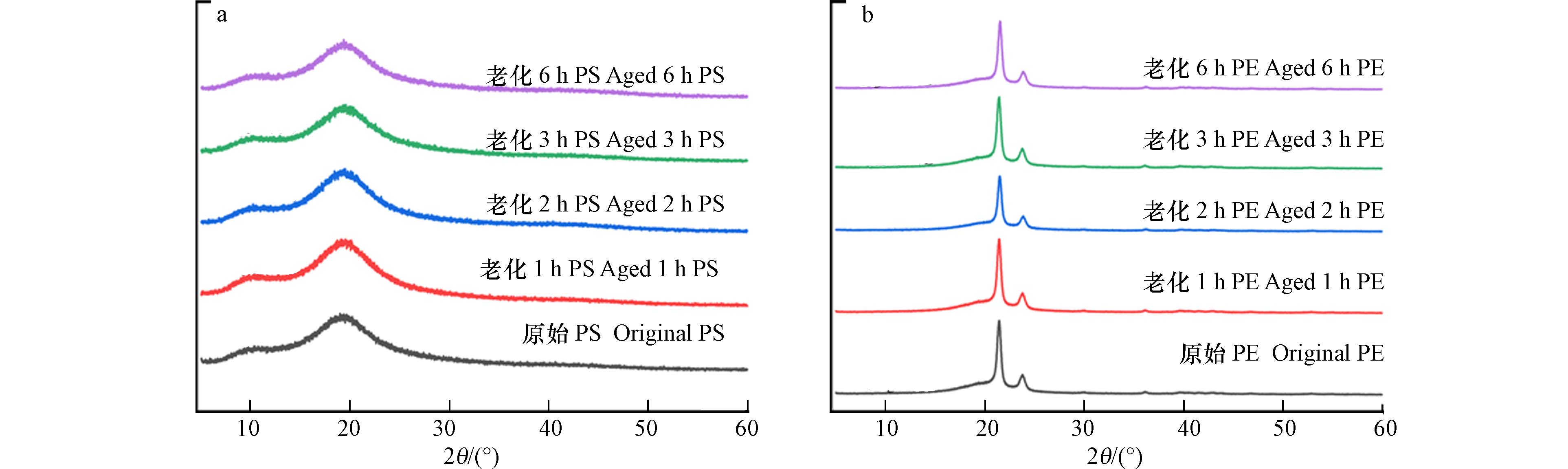
如图3所示. PE的XRD图谱中既存在细而尖的结晶相衍射峰,又有弥散的无定形相衍射峰,说明PE为部分结晶态聚合物. 但PS的XRD图谱并没有形成尖锐的衍射峰,主要是弥散的无定形衍射峰,说明PS是非结晶体[39],结晶度较低. 原始样品与不同老化时间的两种样品的突出特征峰基本吻合,并无太大差异,说明老化后的样品与原始样品的晶体结构基本一致,两种微塑料的老化过程对其结晶度的影响较小.
-
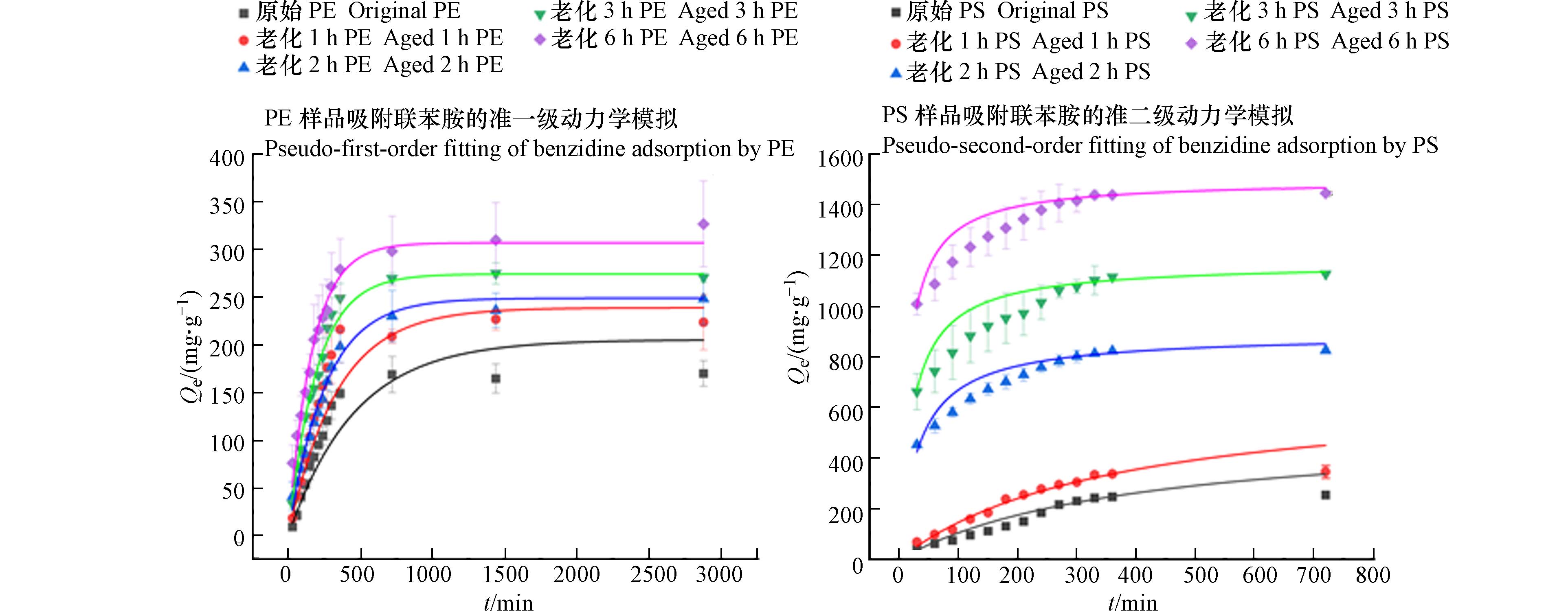
老化PS微塑料样品吸附动力学拟合结果如表1所示. 准一级动力学模型对原始PS的吸附动力学具有较好的拟合效果(R2>0.96);而对于老化后的PS而言,准二级吸附动力学模型拟合效果更好. 可能是由于PS对联苯胺的吸附不仅仅是单一吸附,而是多种吸附作用共同影响的结果,如物理化学联合吸附作用,吸附过程更为复杂,涉及到了表面吸附和内部扩散等过程. 吸附量随老化时间增多,但吸附速率却随之减小. 可能是由于O3老化改变微塑料表面结构,又由于颗粒扩散是一个缓慢的过程[40],短时间内吸附量增加较少,因此吸附速率下降.
老化PE样品的吸附动力学的拟合结果如表2所示. 表明联苯胺在老化前后的PE上的吸附过程均可以用二级模型来描述. 作为芳香胺类有机污染物,联苯胺在PE上的吸附可能是由于PE老化后能够吸附亲水性和极性化合物[41- 42]. 与PS对联苯胺的吸附量相比,PE吸附量增加相对较少. 可能是由于老化前后的PE表面粗糙程度变化较小,但也在一定程度上增加了比表面积和一些吸附位点. 同时PS经老化后生成了更多的含氧官能团,其与联苯胺之间存在化学吸附作用,而PE表面含氧官能团的变化量较少. 可能也存在其他影响因素,例如疏水作用、氢键等.
吸附动力学结果如图4所示. 整个吸附阶段大致可以分成三个阶段,分别为快速吸附阶段、缓慢吸附阶段和吸附平衡阶段. 在吸附前期,微塑料具有较大的比表面积,其表面存在大量未被占据的吸附位点,联苯胺能迅速占据这些吸附位点. 随着吸附过程的进行,微塑料表明的吸附位点逐渐被占据,导致吸附减弱[43]. 但两种微塑料达到吸附平衡所需的时间不同,PS样品仅需要6 h达到了吸附平衡,而PE则需要48 h.
两种微塑料样品对联苯胺的吸附能力均随老化时间的增加而增加,这与前人的研究相同,即老化过程提高了微塑料的亲水性,进而强化了对亲水有机物的吸附能力[44]. 与原始吸附量相比,O3老化1 h时,PS对联苯胺吸附量由254.8 μg·g−1增长到了356.1 μg·g−1,增长了1.4倍;PE对联苯胺的吸附量由170 μg·g−1增长为223 μg·g−1,增长了1.3倍. 老化2 h后,PS的吸附量由254.8 μg·g−1增长到826.1μg·g−1,增长了3.2倍;PE的吸附量增长了1.5倍(由170 μg·g−1增长到248 μg·g−1). 老化3 h后,PS的吸附量增长了4.4倍(由254.8 μg·g−1增长到
1127.7 μg·g−1);PE的吸附量增长了1.6倍(由170 μg·g−1增长到270 μg·g−1). 老化6 h后,PS对联苯胺的吸附能力增长了4.5倍(由254.8 μg·g−1增长到1146.5 μg·g−1);PE对联苯胺的吸附能力增长了1.9倍(由170 μg·g−1增长到326 μg·g−1). 可以看出,在相同的老化时间下,O3老化后的PE对联苯胺的吸附能力显著小于PS的吸附能力. 这可能是由于两种微塑料样品均为非极性的化合物,但PS结构中的苯环可能会增强其极性,而联苯胺也具有极性,因此,极性作用也可能是微塑料吸附性能的影响因素. -
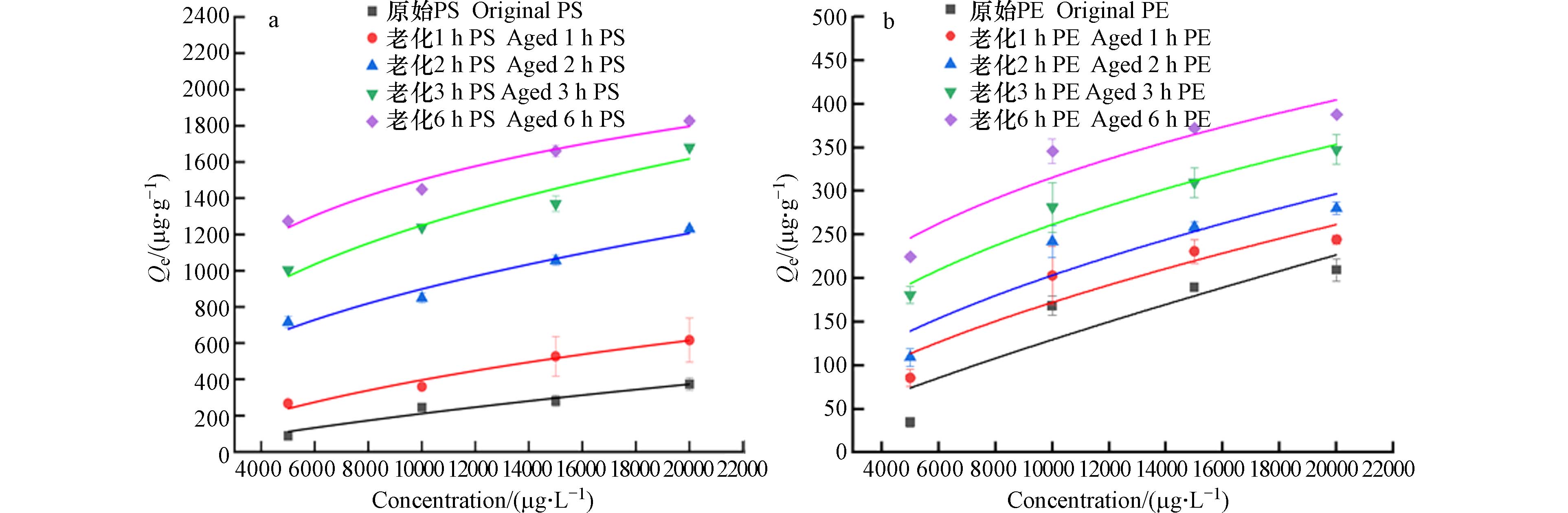
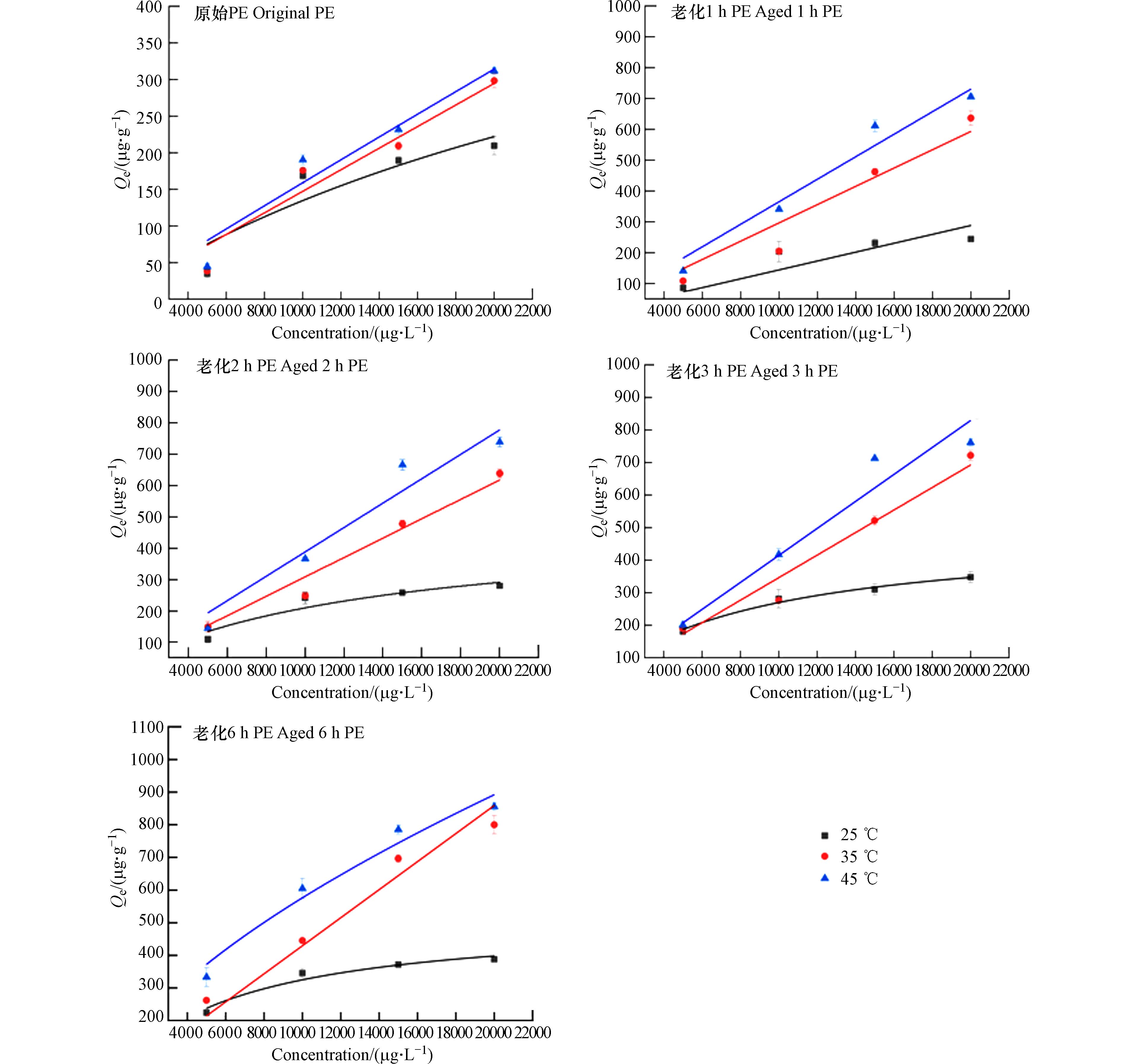
PS、PE对联苯胺的吸附等温线实验结果如图5所示,拟合参数见表3. 与Henry 模型相比,Langmuir和Freundlich模型对两种微塑料吸附拟合效果较好,表明吸附过程中除分配作用以外,还存在一定的疏水作用、静电作用、氢键作用[45].
原始PS对联苯胺的吸附过程既符合Langmuir吸附模型,也符合Freundlich吸附模型(R2>0.94),说明该吸附过程既符合非线性吸附中的单层吸附也符合多层吸附. 原始PE样品吸附过程只与Langmuir模型高度拟合(R2>0.9),符合非线性吸附的单层吸附. Freundlich模型对老化后PS和PE的吸附拟合度更高,老化后两种微塑料样品的吸附过程均属于非线性吸附的多层吸附. 1/n均小于1,老化前后PS对联苯胺的吸附性能越好,联苯胺易吸附在PS表面上属于优惠吸附过程. 原始PE样品对联苯胺的吸附过程以单层吸附为主,老化后PE对联苯胺的吸附以多层吸附为主. Freundlich模型中1/n逐渐降低,表明反应自发进行,疏水分配作用可能是影响吸附过程的一个重要机制[46].
-
本研究模拟25、35、45 ℃时的吸附等温线并进行吸附热力学计算,拟合数据见表4和表5,结果如图6、图7所示. 在本次实验条件下,△G0为负值,即两种样品对联苯胺的吸附过程均为自发反应. △G0的绝对值随温度升高而增大,表明高温利于该吸附的进行,随着温度的升高,两种微塑料对联苯胺的吸附性能均增强. 随着老化时间的增加,两种微塑料样品的∆S0随之增加,有利于该过程的进行.
-
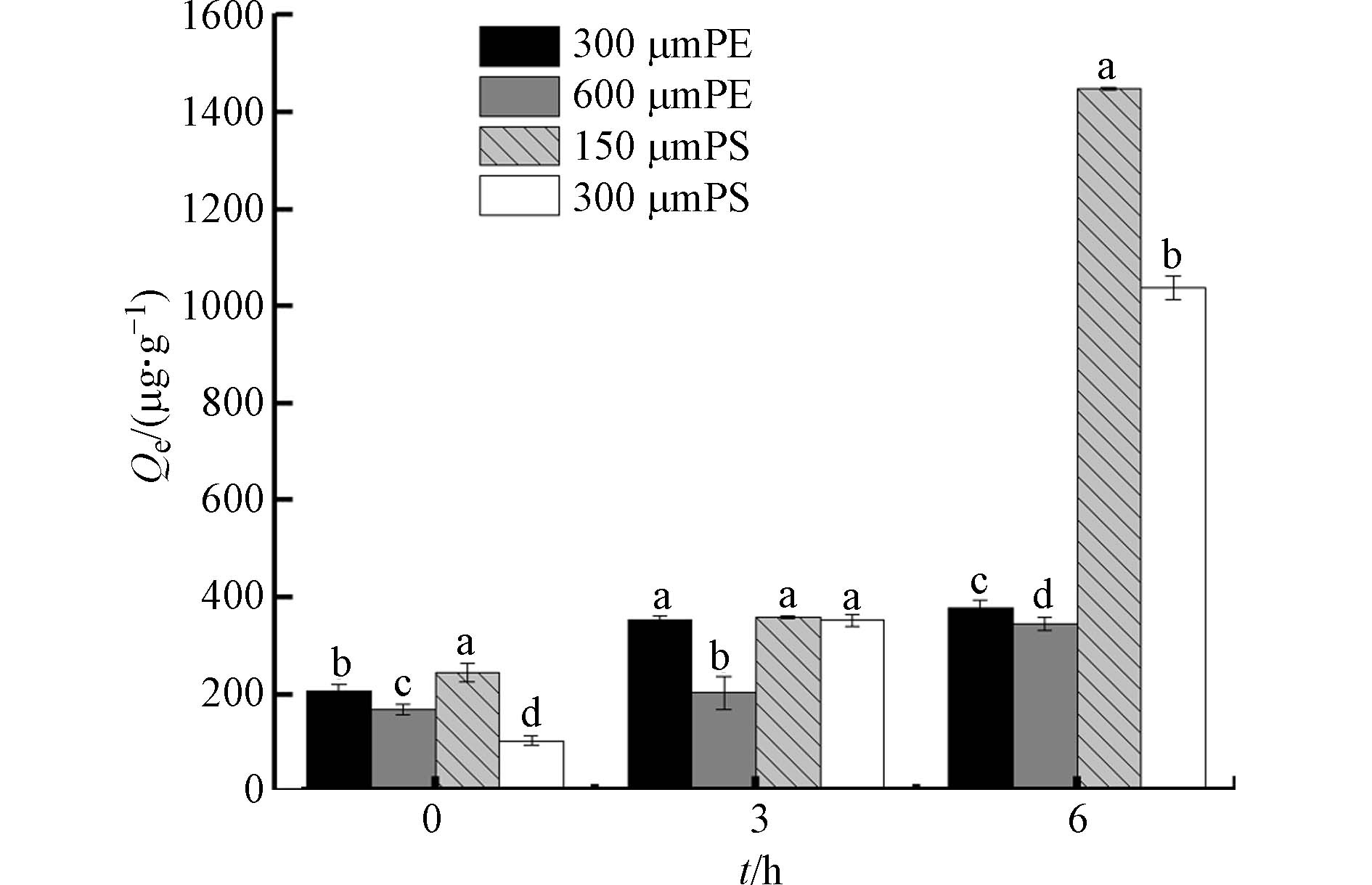
结果如图8所示. 本研究中,微塑料的粒径也会影响吸附实验,小粒径的两种微塑料的吸附效果均更好,如老化6 h时粒径为150 μm的PS吸附量是300 μm的1.4倍;老化1 h时,两种粒径PS的吸附量大致相同,则可能是由于大粒径的PS在短时间内,其表明粗糙程度较大. 同样,老化6 h后300 μm PE样品的吸附量是600 μm PE样品的1.1倍.
针对不同粒径的两种微塑料样品绘制红外光谱图,结果如图9所示. 在相同的老化情况下,不同粒径大小的两种微塑料样品的特征峰基本相同,但吸收强度变化较大,增加了微塑料的亲水性[47]. 也可能是随着微塑料粒径的减小,微塑料的比表面积更大,微塑料表面产生更多的吸附位点[48],则吸附量随之增加.
-
本文研究了O3老化对PS和PE两种微塑料理化性质的影响及其与环境中有机污染物之间的相互作用.
(1)理化性质:在相同老化时间下,相较于PE,PS的官能团和表面形貌变化较大.
(2)两种微塑料对联苯胺的吸附量随着老化时间增加而增加. 老化6 h后,两种微塑料对联苯胺的吸附量最大,其中,PS的吸附量是PE吸附量的3.5倍. 此外,不同粒径样品在相同的老化时间下,粒径较小的PS和PE的吸附量更大.
(3)对老化前后的两种微塑料样品进行拟合,原始PS对联苯胺的吸附过程既符合Langmuir吸附模型,也符合Freundlich吸附模型(R2>0.9);原始PE样品对联苯胺的等温吸附过程只与Langmuir模型高度拟合(R2>0.9),Freundlich模型对老化后的PS和PE样品的拟合效果均更好.
(4)本文中仅对两种微塑料进行了臭氧老化,对PE的影响较小,与前人的研究结果略有不同,后续研究可针对PE老化时间的阈值进行研究. 并探究其机理,也可针对微塑料种类和老化方式和时间进行补充实验.
O3促进聚乙烯(PE)和聚苯乙烯(PS)与联苯胺的相互作用
Enhanced the interaction of polyethylene and polystyrene with benzidine Via O3 treatment
-
摘要: 微塑料作为一种新兴污染物,广泛存在于土壤和水体环境中. 为模拟老化微塑料与有机污染物的相互作用,本研究选用聚乙烯(PE)和聚苯乙烯(PS)进行O3老化,采用X射线衍射(XRD)、扫描电子显微镜(SEM)、傅里叶红外光谱仪(FTIR)手段对微塑料进行表征并探究其老化后对联苯胺的吸附行为. 结果表明:老化后的2种微塑料表面的粗糙程度均发生不同程度的变化,其含氧官能团数量和强度增加、亲水性增强. 两种微塑料对联苯胺的吸附量均随着老化时间的增加而增加,其中在老化6 h后,吸附量最大,PS对联苯胺的吸附量增长了4.5倍;PE对联苯胺的吸附量增长了1.9倍. 此外,微塑料粒径的增加会减少微塑料对联苯胺的吸附.Abstract: As an emerging pollutant, microplastics are widely present in soil and water environment. In order to study the reaction of aging microplastics with organic pollutants, ozone aging was performed on polyethylene (PE) and polystyrene (PS), X-ray diffraction (XRD), scanning electron microscopy (SEM) and Fourier infrared spectroscopy (FTIR) were used as characterized methods to explore the adsorption behavior of benzidine after aging. The results show that: after aging, the roughness of the surface of the two microplastics has changed to different degrees, and the number and strength of oxygen-containing functional groups was increased, and the hydrophilicity was enhanced. The adsorption capacity of both microplastics for benzidine increased with the increase of aging time. After 6 h of aging, The two microplastics have the largest adsorption capacity for benzidine, the adsorption capacity of PS p-benzidine increased by 4.5 times; the adsorption capacity of PE p-benzidine increased by 1.9 times. Furthermore, particle size also has a certain influence on the adsorption of benzidine by microplastics, and the increase of particle size will weaken the adsorption of benzidine by microplastics.
-
Key words:
- ozone aging /
- polyethylene microplastics /
- polystyrene microplastics /
- benzidine /
- adsorption
-

-
表 1 不同老化时间PS对联苯胺的吸附动力学参数
Table 1. Kinetic parameters of adsorption of Benzidine by PS at different aging time
准一级动力学
Pseudo-first-order准二级动力学
Pseudo-second-orderK1/min−1 Qe/(μg·g−1) R2 K2/(μg·g−1·min−1) Qe/(μg·g−1) R2 原始PS
Original PS0.0035 346 0.97 4.85×10−2 522 0.96 老化1 h PS
Aged 1 h PS0.0041 439 0.98 4.03×10−2 680 0.98 老化2 h PS
Aged 2 h PS0.0235 811 0.83 3.84×10−2 883 0.95 老化3 h PS
Aged 3 h PS0.0159 1121 0.75 3.84×10−2 1170 0.84 老化6 h PS
Aged 6 h PS0.0329 1445 0.76 3.54×10−2 1503 0.88 表 2 不同老化时间PE对联苯胺的吸附动力学参数
Table 2. Kinetic parameters of adsorption of benzidine by PE at different aging time
准一级动力学
Pseudo-first-order准二级动力学
Pseudo-second-orderK1/min−1 Qe/(μg·g−1) R2 K2/(g·μg−1·min−1) Qe/(μg·g−1) R2 原始PE
Original PE0.0021 205 0.90 5.85×10−2 278 0.85 老化1 h PE
Aged1 h PE0.0029 239 0.97 6.89×10−2 325 0.93 老化2 h PE
Aged2 h PE0.0038 249 0.98 1.38×10−2 300 0.95 老化3 h PE
Aged3 h PE0.0051 274 0.99 1.98×10−2 304 0.91 老化6 h PE
Aged6 h PE0.0060 307 0.97 1.93×10−2 356 0.90 表 3 原始和老化PS和PE对联苯胺的吸附等温线常数
Table 3. Adsorption isotherm constants for Benzidine adsorbed by original and aged PS and PE
Langmuir模型
Langmuir modelFreundlich模型
Freundlich modelHenry模型
Henry modelQm/(μg·g−1) KL/(L·μg−1) R2 KF/(μg·g−1) 1/n/(L·g−1) R2 Kd/(L·μg−1) R2 原始PS
Original PS203 1.61×10−5 0.95 28.066 0.0534 0.95 0.0203 0.88 老化1 h PS
Aged1 h PS207 1.32×10−5 0.96 29.591 0.0617 0.99 0.0349 0.89 老化2 h PS
Aged2 h PS215 1.36×10−5 0.91 31.481 0.0629 0.92 0.6361 0.88 老化3 h PS
Aged3 h PS383 1.72×10−5 0.90 32.517 0.0633 0.94 0.1119 0.89 老化6 h PS
Aged6 h PS416 2.88× 10−5 0.91 36.666 0.0635 0.97 0.1646 0.92 原始PE
Original PE630 0.0272 0.87 20.158 0.8079 0.90 12.144 0.84 老化1 h PE
Aged1 h PE460 0.0632 0.91 31.359 0.6895 0.96 12.592 0.89 老化2 h PE
Aged2 h PE474 0.0795 0.89 57.931 0.5454 0.91 15.809 0.88 老化3 h PE
Aged3 h PE486 0.1246 0.96 96.243 0.4342 0.98 20.955 0.82 老化6 h PE
Aged6 h PE513 0.1727 0.95 127.844 0.3761 0.96 21.026 0.86 表 4 老化PS对联苯胺的吸附热力学参数
Table 4. Thermodynamic parameters of benzidine adsorption by PS
微塑料
MPs温度/℃
T∆G0/(kJ·mol−1) ∆H0/(kJ·mol−1) ∆S0/J·(mol·K)−1 R2 原始PS
Original PS25 −7.98 19.27 86.33 0.86 35 −8.51 0.88 45 −8.73 0.99 老化1 h PS
Aged1 h PS25 −8.11 0.97 35 −8.69 19.56 87.36 0.96 45 −8.78 0.99 老化2 h PS
Aged2 h PS25 −8.26 0.98 35 −8.68 19.88 87.66 0.98 45 −8.81 0.95 老化3 h PS
Aged3 h PS25 −8.34 0.94 35 −8.82 19.89 87.95 0.98 45 −9.39 0.99 老化6 h PS
Aged6 h PS25 −8.62 0.97 35 −9.13 20.11 88.68 0.99 45 −9.68 0.95 表 5 老化PE对联苯胺的吸附热力学参数
Table 5. Thermodynamic parameters of benzidine adsorption by PE
微塑料
MPsT/℃ ∆G0/(kJ·mol−1) ∆H0/(kJ·mol−1) ∆S0/J·(mol·K)−1 R2 原始PE
Original PE25 −6.39 0.84 35 −6.85 11.93 55.69 0.93 45 −7.22 0.93 老化1 h PE
Aged1 h PE25 −6.51 0.90 35 −6.99 18.56 77.96 0.92 45 −7.35 0.96 老化2 h PE
Aged2 h PE25 −6.72 0.89 35 −7.12 23.98 81.63 0.97 45 −7.47 0.94 老化3 h PE
Aged3 h PE25 −6.89 0.98 35 −7.29 27.66 88.97 0.96 45 −7.99 0.93 老化6 h PE
Aged6 h PE25 −7.02 0.95 35 −7.53 32.41 96.31 0.95 45 −8.36 0.96 -
[1] 张晨曦, 孙景春, 林承刚. 海洋微塑料对重金属的吸附行为及其复合毒性研究进展[J]. 海洋科学, 2022, 46(8): 155-170. ZHANG C X, SUN J C, LIN C G. Research progress on the adsorption behavior of heavy metals on microplastics in the ocean and their combined toxicity[J]. Marine Sciences, 2022, 46(8): 155-170(in Chinese).
[2] FERRAZ M, BAUER A L, VALIATI V H, et al. Microplastic concentrations in raw and drinking water in the sinos river, southern Brazil[J]. Water, 2020, 12(11): 3115. doi: 10.3390/w12113115 [3] McCOY K A, HODGSON D J, CLARK P F, et al. The effects of wet wipe pollution on the Asian clam, Corbicula fluminea (Mollusca: Bivalvia) in the River Thames, London[J]. Environmental Pollution, 2020, 264: 114577. doi: 10.1016/j.envpol.2020.114577 [4] RENZI M, GRAZIOLI E, BERTACCHINI E, et al. Microparticles in table salt: Levels and chemical composition of the smallest dimensional fraction[J]. Journal of Marine Science and Engineering, 2019, 7(9): 310. doi: 10.3390/jmse7090310 [5] THOMPSON R C, OLSEN Y, MITCHELL R P, et al. Lost at sea: Where is all the plastic?[J]. Science, 2004, 304(5672): 838. doi: 10.1126/science.1094559 [6] GODOY V, BLÁZQUEZ G, CALERO M, et al. The potential of microplastics as carriers of metals[J]. Environmental Pollution, 2019, 255: 113363. doi: 10.1016/j.envpol.2019.113363 [7] HOLMES L A, TURNER A, THOMPSON R C. Interactions between trace metals and plastic production pellets under estuarine conditions[J]. Marine Chemistry, 2014, 167: 25-32. doi: 10.1016/j.marchem.2014.06.001 [8] TOURINHO P S, KOČÍV, LOUREIRO S, et al. Partitioning of chemical contaminants to microplastics: Sorption mechanisms, environmental distribution and effects on toxicity and bioaccumulation[J]. Environmental Pollution, 2019, 252: 1246-1256. doi: 10.1016/j.envpol.2019.06.030 [9] WANG F, SHIH K M, LI X Y. The partition behavior of perfluorooctanesulfonate (PFOS) and perfluorooctanesulfonamide (FOSA) on microplastics[J]. Chemosphere, 2015, 119: 841-847. doi: 10.1016/j.chemosphere.2014.08.047 [10] BHATTACHARYA P, LIN S J, TURNER J P, et al. Physical adsorption of charged plastic nanoparticles affects algal photosynthesis[J]. The Journal of Physical Chemistry C, 2010, 114(39): 16556-16561. doi: 10.1021/jp1054759 [11] YANG Y Y, LIU G H, SONG W J, et al. Plastics in the marine environment are reservoirs for antibiotic and metal resistance genes[J]. Environment International, 2019, 123: 79-86. doi: 10.1016/j.envint.2018.11.061 [12] D’SOUZA J M, WINDSOR F M, SANTILLO D, et al. Food web transfer of plastics to an apex riverine predator[J]. Global Change Biology, 2020, 26(7): 3846-3857. doi: 10.1111/gcb.15139 [13] SANTANA M F M, MOREIRA F T, TURRA A. Trophic transference of microplastics under a low exposure scenario: Insights on the likelihood of particle cascading along marine food-webs[J]. Marine Pollution Bulletin, 2017, 121(1/2): 154-159. [14] CARBERY M, O’CONNOR W, PALANISAMI T. Trophic transfer of microplastics and mixed contaminants in the marine food web and implications for human health[J]. Environment International, 2018, 115: 400-409. doi: 10.1016/j.envint.2018.03.007 [15] SONG Y K, HONG S H, JANG M, et al. Combined effects of UV exposure duration and mechanical abrasion on microplastic fragmentation by polymer type[J]. Environmental Science & Technology, 2017, 51(8): 4368-4376. [16] CHEN C Z, CHEN L, YAO Y, et al. Organotin release from polyvinyl chloride microplastics and concurrent photodegradation in water: Impacts from salinity, dissolved organic matter, and light exposure[J]. Environmental Science & Technology, 2019, 53(18): 10741-10752. [17] ZHU K C, JIA H Z, ZHAO S, et al. Formation of environmentally persistent free radicals on microplastics under light irradiation[J]. Environmental Science & Technology, 2019, 53(14): 8177-8186. [18] MÜLLER A, BECKER R, DORGERLOH U, et al. The effect of polymer aging on the uptake of fuel aromatics and ethers by microplastics[J]. Environmental Pollution, 2018, 240: 639-646. doi: 10.1016/j.envpol.2018.04.127 [19] LV Y D, HUANG Y J, YANG J L, et al. Outdoor and accelerated laboratory weathering of polypropylene: A comparison and correlation study[J]. Polymer Degradation and Stability, 2015, 112: 145-159. doi: 10.1016/j.polymdegradstab.2014.12.023 [20] 姜晶, 阮呈杰, 陈霄宇, 等. 微塑料模拟老化及其对污染物吸附行为影响研究进展[J]. 生态环境学报, 2022, 31(11): 2263-2274. JIANG J, RUAN C J, CHEN X Y, et al. Research progress on simulated aging of microplastics and its effects on pollutant adsorption[J]. Ecology and Environmental Sciences, 2022, 31(11): 2263-2274(in Chinese).
[21] LANG M F, YU X Q, LIU J H, et al. Fenton aging significantly affects the heavy metal adsorption capacity of polystyrene microplastics[J]. Science of the Total Environment, 2020, 722: 137762. doi: 10.1016/j.scitotenv.2020.137762 [22] MAO R F, LANG M F, YU X Q, et al. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals[J]. Journal of Hazardous Materials, 2020, 393: 122515. doi: 10.1016/j.jhazmat.2020.122515 [23] ZHANG H B, WANG J Q, ZHOU B Y, et al. Enhanced adsorption of oxytetracycline to weathered microplastic polystyrene: Kinetics, isotherms and influencing factors[J]. Environmental Pollution, 2018, 243: 1550-1557. doi: 10.1016/j.envpol.2018.09.122 [24] 张鸿宇, 王媛, 卢亚灵, 等. 我国臭氧污染控制分区及其控制类型识别[J]. 中国环境科学, 2021, 41(9): 4051-4059. doi: 10.3969/j.issn.1000-6923.2021.09.010 ZHANG H Y, WANG Y, LU Y L, et al. Identification of ozone pollution control zones and types in China[J]. China Environmental Science, 2021, 41(9): 4051-4059(in Chinese). doi: 10.3969/j.issn.1000-6923.2021.09.010
[25] 薛彬, 蓝文陆, 林海英, 等. 老化微塑料对Hg(Ⅱ)的吸附解吸行为及机理研究[J]. 环境科学与技术, 2022, 45(8): 31-37. XUE B, LAN W L, LIN H Y, et al. Studies of behavior and mechanism of Hg(Ⅱ) adsorption-desorption onto aged polystyrene[J]. Environmental Science & Technology, 2022, 45(8): 31-37(in Chinese).
[26] MIRANDA M N, SAMPAIO M J, TAVARES P B, et al. Aging assessment of microplastics (LDPE, PET and uPVC) under urban environment stressors[J]. Science of the Total Environment, 2021, 796: 148914. doi: 10.1016/j.scitotenv.2021.148914 [27] LUO H W, ZENG Y F, ZHAO Y Y, et al. Effects of advanced oxidation processes on leachates and properties of microplastics[J]. Journal of Hazardous Materials, 2021, 413: 125342. doi: 10.1016/j.jhazmat.2021.125342 [28] CAI L Q, WANG J D, PENG J P, et al. Observation of the degradation of three types of plastic pellets exposed to UV irradiation in three different environments[J]. Science of the Total Environment, 2018, 628/629: 740-747. doi: 10.1016/j.scitotenv.2018.02.079 [29] 孙璇, 俞安琪, 王学松, 等. 富里酸在聚苯乙烯微塑料上的吸附行为[J]. 中国环境科学, 2022, 42(1): 285-292. doi: 10.3969/j.issn.1000-6923.2022.01.031 SUN X, YU A Q, WANG X S, et al. Adsorption behaviors of fulvic acid onto polystyrene microplastics[J]. China Environmental Science, 2022, 42(1): 285-292(in Chinese). doi: 10.3969/j.issn.1000-6923.2022.01.031
[30] GUO X, WANG J L. Sorption of antibiotics onto aged microplastics in freshwater and seawater[J]. Marine Pollution Bulletin, 2019, 149: 110511. doi: 10.1016/j.marpolbul.2019.110511 [31] LIU J, ZHANG T, TIAN L L, et al. Aging significantly affects mobility and contaminant-mobilizing ability of nanoplastics in saturated loamy sand[J]. Environmental Science & Technology, 2019, 53(10): 5805-5815. [32] WANG F Y, YANG W W, CHENG P, et al. Adsorption characteristics of cadmium onto microplastics from aqueous solutions[J]. Chemosphere, 2019, 235: 1073-1080. doi: 10.1016/j.chemosphere.2019.06.196 [33] ZHANG W, ZHANG L Y, HUA T, et al. The mechanism for adsorption of Cr(VI) ions by PE microplastics in ternary system of natural water environment[J]. Environmental Pollution, 2020, 257: 113440. doi: 10.1016/j.envpol.2019.113440 [34] RANJAN V P, GOEL S. Degradation of low-density polyethylene film exposed to UV radiation in four environments[J]. Journal of Hazardous, Toxic, and Radioactive Waste, 2019, 23(4): 000453. [35] LUO H W, XIANG Y H, LI Y, et al. Weathering alters surface characteristic of TiO2-pigmented microplastics and particle size distribution of TiO2 released into water[J]. Science of the Total Environment, 2020, 729: 139083. doi: 10.1016/j.scitotenv.2020.139083 [36] HUANG Y J, DING J N, ZHANG G S, et al. Interactive effects of microplastics and selected pharmaceuticals on red tilapia: Role of microplastic aging[J]. Science of the Total Environment, 2021, 752: 142256. doi: 10.1016/j.scitotenv.2020.142256 [37] 刘鹏. 微塑料的加速老化过程及其与药物和铁红颜料的相互作用研究[D]. 南京: 南京大学, 2020. LIU P. Study on the accelerated aging of microplastics and the interaction of aged microplastics with pharmaceuticals and iron red pigment[D]. Nanjing: Nanjing University, 2020(in Chinese).
[38] 陈博. 老化EVA鞋底微塑料与PE微塑料对菲的吸附—解吸行为[D]. 兰州: 兰州大学, 2022. CHEN B. Adsorption-desorption behavior of phenanthrene by aged EVA shoe sole microplastics and PE microplastics[D]. Lanzhou: Lanzhou University, 2022(in Chinese).
[39] FU L N, LI J, WANG G Y, et al. Adsorption behavior of organic pollutants on microplastics[J]. Ecotoxicology and Environmental Safety, 2021, 217: 112207. doi: 10.1016/j.ecoenv.2021.112207 [40] 张凯娜, 李嘉, 李晓强, 等. 微塑料表面土霉素的吸附-解吸机制与动力学过程[J]. 环境化学, 2017, 36(12): 2531-2540. doi: 10.7524/j.issn.0254-6108.2017032703 ZHANG K N, LI J, LI X Q, et al. Mechanisms and kinetics of oxytetracycline adsorption-desorption onto microplastics[J]. Environmental Chemistry, 2017, 36(12): 2531-2540(in Chinese). doi: 10.7524/j.issn.0254-6108.2017032703
[41] GOMES de ARAGÃO BELÉ T, NEVES T F, CRISTALE J, et al. Oxidation of microplastics by O3 and O3/H2O2: Surface modification and adsorption capacity[J]. Journal of Water Process Engineering, 2021, 41: 102072. doi: 10.1016/j.jwpe.2021.102072 [42] ZHANG H Y, PAP S, TAGGART M A, et al. A review of the potential utilisation of plastic waste as adsorbent for removal of hazardous priority contaminants from aqueous environments[J]. Environmental Pollution, 2020, 258: 113698. doi: 10.1016/j.envpol.2019.113698 [43] FAN C Z, LI K, LI J X, et al. Comparative and competitive adsorption of Pb(II) and Cu(II) using tetraethylenepentamine modified chitosan/ CoFe2O4 particles[J]. Journal of Hazardous Materials, 2017, 326: 211-220. doi: 10.1016/j.jhazmat.2016.12.036 [44] 宋亚丽, 赵健淇, 朱文芳, 等. 老化前后聚苯乙烯微塑料对天然有机物的吸附[J]. 环境科学学报, 2023, 43(2): 181-191. SONG Y L, ZHAO J Q, ZHU W F, et al. Adsorption of natural organic matter on original and aged polystyrene microplastics[J]. Acta Scientiae Circumstantiae, 2023, 43(2): 181-191(in Chinese).
[45] 程新峰, 留芳芳, 潘玲, 等. 微塑料老化对其理化性质和盐酸四环素吸附行为的影响研究[J]. 环境科学学报, 2023, 43(3): 150-161. CHENG X F, LIU F F, PAN L, et al. Study on the effect of aging of microplastics on their physicochemical properties and adsorption behavior of tetracycline hydrochloride[J]. Acta Scientiae Circumstantiae, 2023, 43(3): 150-161(in Chinese).
[46] 林陆健, 汤帅, 孙璇, 等. 铅离子和四环素在微塑料表面的吸附机理与协同效应[J]. 环境科学学报, 2021, 41(10): 4022-4031. LIN L J, TANG S, SUN X, et al. Adsorption of Pb(Ⅱ) ions and tetracycline onto microplastics: Interaction mechanisms and synergistic effects[J]. Acta Scientiae Circumstantiae, 2021, 41(10): 4022-4031(in Chinese).
[47] 范秀磊, 常卓恒, 邹晔锋, 等. 可降解微塑料对铜和锌离子的吸附解吸特性[J]. 中国环境科学, 2021, 41(5): 2141-2150. FAN X L, CHANG Z H, ZOU Y F, et al. Adsorption and desorption properties of degradable microplastic for Cu2+ and Zn2+[J]. China Environmental Science, 2021, 41(5): 2141-2150(in Chinese).
[48] 付东东, 张琼洁, 范正权, 等. 微米级聚苯乙烯对铜的吸附特性[J]. 中国环境科学, 2019, 39(11): 4769-4775. doi: 10.3969/j.issn.1000-6923.2019.11.036 FU D D, ZHANG Q J, FAN Z Q, et al. Adsorption characteristics of copper ions on polystyrene microplastics[J]. China Environmental Science, 2019, 39(11): 4769-4775(in Chinese). doi: 10.3969/j.issn.1000-6923.2019.11.036
-




 下载:
下载: