-
赤泥是以铝土矿为原料,在氧化铝冶炼工业生产过程中排出的固体粉状废弃物, 具有强碱性,富含钙、铝和铁等氧化物,颗粒极细,按氧化铝的生产工艺可将赤泥分为烧结法赤泥、拜耳法赤泥及联合法赤泥3种[1-2]。中国是氧化铝生产大国,截至2016年,我国的赤泥累积堆存量超5.0×108 t,赤泥大量堆存,既占用土地,浪费资源,又会对自然环境和人身健康都产生重要影响[3-4]。因此,如何妥善处理和合理利用这些固体废弃物,已成为社会关注的热点。赤泥中富含铝、铁等氧化物且具有孔状的骨架结构,比表面积大,这些特点使赤泥具有较好的吸附性能,能有效吸附水溶液中的有机物和重金属物质[5-6]。
含铅废水主要来自电池、涂料、五金和印刷等行业[7],大量排放含铅废水会造成湖泊、河流、海洋、土壤等的污染[8]。化学沉淀法和吸附法是处理含铅废水最常见的技术方法。国内外已有学者对赤泥进行改性后,用于处理Pb(Ⅱ)废水,SAHU等[9] 使用盐酸酸化赤泥,经过中和、沉淀和煅烧后得到改性赤泥,对Pb(Ⅱ)的最大吸附容量为6.027 3 mg·g−1。
赤泥中的Na+、Ca2+和Mg2+等阳离子可与Pb(Ⅱ)发生交换,促进Pb(Ⅱ)的稳定化。此外,赤泥中含有的碱性物质(如OH−、
$\text{CO}_{3}^{2-}$ 等)也可与Pb(Ⅱ)发生沉淀反应,生成Pb(OH)2和PbCO3沉淀物,促进Pb(Ⅱ)的去除。本研究利用XRF、XRD、粒度分析和SEM-EDS等手段对赤泥样品进行特性分析,保留和利用了赤泥的碱性,碱性和吸附性能共同作用,应用于水溶液中Pb(Ⅱ)的去除实验研究中,探究其去除效果,考察了Pb(Ⅱ)初始浓度和pH对去除效果的影响,分析其动力学过程与去除机理。
全文HTML
-
实验用赤泥样品采用重庆某氧化铝生产企业排放的联合法赤泥。赤泥先被破碎至直径小于5 cm,在通风橱中自然风干后,使用粉碎机粉碎、研磨并过200目筛(过筛率>98%),于105 ℃烘箱中烘干过夜,即得到实验用赤泥粉末。
实验用试剂包括硝酸铅(Pb(NO3)2)、氢氧化钠(NaOH)、硝酸(HNO3)、冰乙酸(CH3COOH)、MES等,药剂均为分析纯。实验用水为去离子水(电阻率ρ>18.2 MΩ·cm)。
-
采用X射线荧光光谱仪(XRF-1800,日本岛津公司)对赤泥样品的化学元素组成进行分析;采用场发射扫描电镜(JSM-7800F,JEOL公司)对赤泥样品进行表观形态分析;采用X射线衍射仪(XRD-7000S/L,日本岛津公司),Cu Ka靶(40 kV,40 mA),在扫描速度为2 (°)·min−1,扫描角度5 °~80 °的条件下进行矿物组成定性分析;采用激光衍射粒度分析仪(Mastersizer 200,马尔文仪器有限公司)对赤泥样品的比表面积和平均粒度进行分析,分散剂为水,测定粒径为0.02~2 000.00 μm。采用pH计(FE20,梅特勒-托利多仪器有限公司),检测溶液的pH。
-
1)酸中和能力实验。为探讨赤泥的酸碱性,设计定pH酸滴定实验,使用500 mL锥形瓶,加入300 mL去离子水,并利用HNO3将pH调节为7.0,加入0.6 g赤泥粉末,使赤泥投加量达到2.0 g·L−1。采用磁力搅拌混合,转速为1 000 r·min−1,温度为25 ℃。不断加入HNO3,使得pH保持在为7.0±0.2,通过不同时刻赤泥对HNO3的累积消耗量,研究赤泥与酸溶液的反应能力,分析赤泥的耗酸特性。
2) Pb(Ⅱ)的去除实验。在500 mL锥形瓶中开展实验,向体积为300 mL、pH=4的Pb(Ⅱ)水溶液中加入0.6 g赤泥,使赤泥投加量达到2.0 g·L−1,采用磁力搅拌混合,转速为1 000 r·min−1,温度为25 ℃。Pb(Ⅱ)初始浓度分别为4.96、10.42和51.81 mg·L−1,反应过程中不对溶液的pH进行调节,只记录pH随着时间的变化。在一定时间内取样,用0.45 μm的滤膜过滤,并测定滤液中Pb(Ⅱ)浓度。每组实验开展3个重复实验。
在化学动力学实验中,采用乙酸/乙酸钠缓冲体系,将pH控制为4.0±0.2,向初始浓度为5.37、10.69和52.08 mg·L−1的Pb(Ⅱ)水溶液中加入2 g·L−1赤泥,采用磁力搅拌混合,转速为1 000 r·min−1,温度为25 ℃。在一定时间内取样,用0.45 μm的滤膜过滤,并测定滤液中Pb(Ⅱ)浓度。每组实验开展3个重复实验。
3)初始浓度和pH对去除效果的影响实验。在250 mL锥形瓶中开展实验,分别配制100 mL初始浓度为0.86、4.96、10.42、51.81和101.09 mg·L−1的Pb(Ⅱ)水溶液,调节至pH=4,再加入0.2 g赤泥,使赤泥投加量达到2.0 g·L−1,研究初始浓度对Pb(Ⅱ)去除效果的影响。
分别配置初始浓度为5.0 mg·L−1,pH为4、7和10的Pb(Ⅱ)水溶液,在反应过程中,利用乙酸/乙酸钠缓冲体系和硝酸、氢氧化钠分别将Pb(Ⅱ)溶液的pH维持在4.0±0.2、7.0±0.2和10.0±0.2,研究pH对Pb(Ⅱ)去除效果的影响。
4)分析测试方法。根据《水质 铜、锌、铅、镉的测定 原子吸收分光光度法》(GB 7475-1987)的标准方法,使用原子吸收分光光度计(AA-6300C,岛津企业管理有限公司)对Pb(Ⅱ)进行测试。
1.1. 实验材料
1.2. 赤泥的特性表征
1.3. 实验方法
-
如表1所示,供试赤泥样品主要化学组分包括CaO(25.50%)、SiO2(24.44%)、Al2O3(21.68%)、Fe2O3(8.53%)和Na2O(6.93%),占总量的87%。与顾汉念等[10]对贵州某铝厂烧结法赤泥进行化学组成CaO(34.29%)、SiO2(20.41%)、Al2O3(10.84%)、Fe2O3(9.06%)类似,具有高钙低铁的特点,只是供试赤泥的铝含量更高。
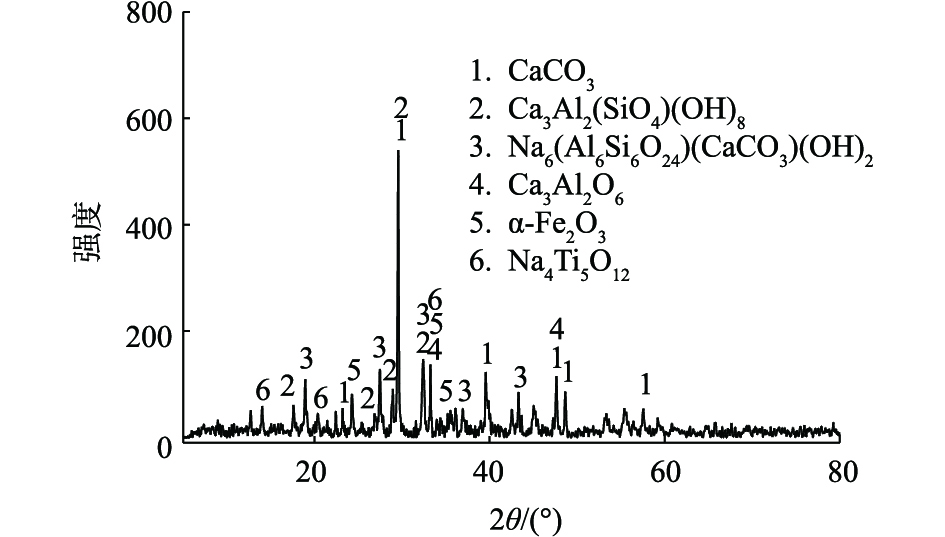
如图1所示,赤泥样品的物相组成主要包括方解石(CaCO3)、水钙铝榴石 (Ca3Al2(SiO4)(OH)8)、钙霞石(Na6(Al6Si6O24)(CaCO3)(OH)2)、铝酸钙(Ca3Al2O6)、赤铁矿(α-Fe2O3)。与拜耳法赤泥的物相中,赤铁矿(α-Fe2O3)、水化石榴石(Ca3Al2(SiO4)(OH)8·H2O)、方解石(CaCO3)成分相似[11-12]。薛生国等[13]对赤泥中可能存在的碱性物质进行了分析,结果表明,赤泥中的主要碱性矿物质包括钙霞石、水化石榴石、方解石、氢氧化钠、铝酸钠和碳酸盐等矿物。其中方解石、钙霞石、石榴石和铝酸盐这一结果与本研究相符合。但是,由于氧化铝工艺和堆存时间的差别,不同堆场赤泥矿物相组分存在显著差异,山西河津联合法赤泥中的矿物相与本研究就较为不同,其物相组成主要包括包括钙铝黄长石、石英、钠长石、钙铁榴石和钙钛榴石等[14]。
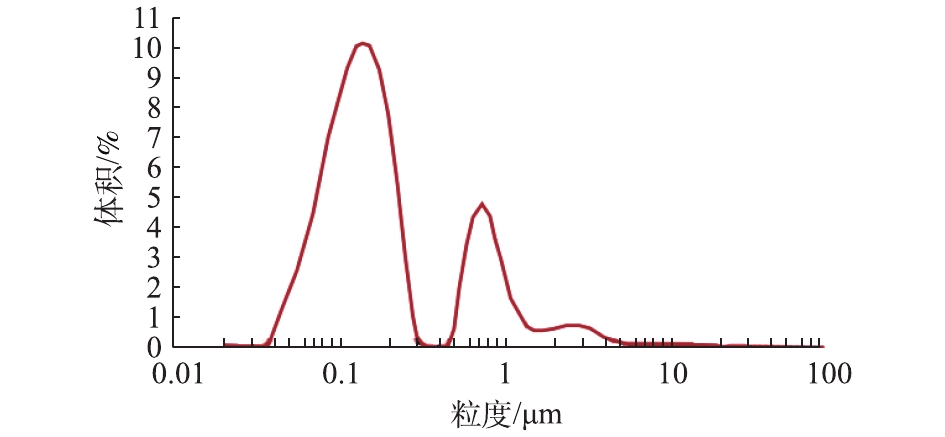
如图2所示,赤泥颗粒的粒径主要集中分布在0.1 μm和0.8 μm左右,平均比表面积为43.8 m2·g−1,表面积平均粒径D[3,2]为0.137 μm,体积平均粒径D[4,3]为0.365 μm。10%的赤泥颗粒粒径小于0.073 μm,50%小于0.151 μm,90%小于0.863 μm。与顾汉念等[10]的研究中赤泥的比表面积(0.26 m2·g−1)和表面积平均粒径(16.486 μm)相比,供试赤泥的比表面积更大,平均粒径更小。通常赤泥的比表面积越大,孔隙结构越丰富,则具有更好的吸附性能,可使其作为吸附剂去除水环境和土壤中的污染物[13-15]。
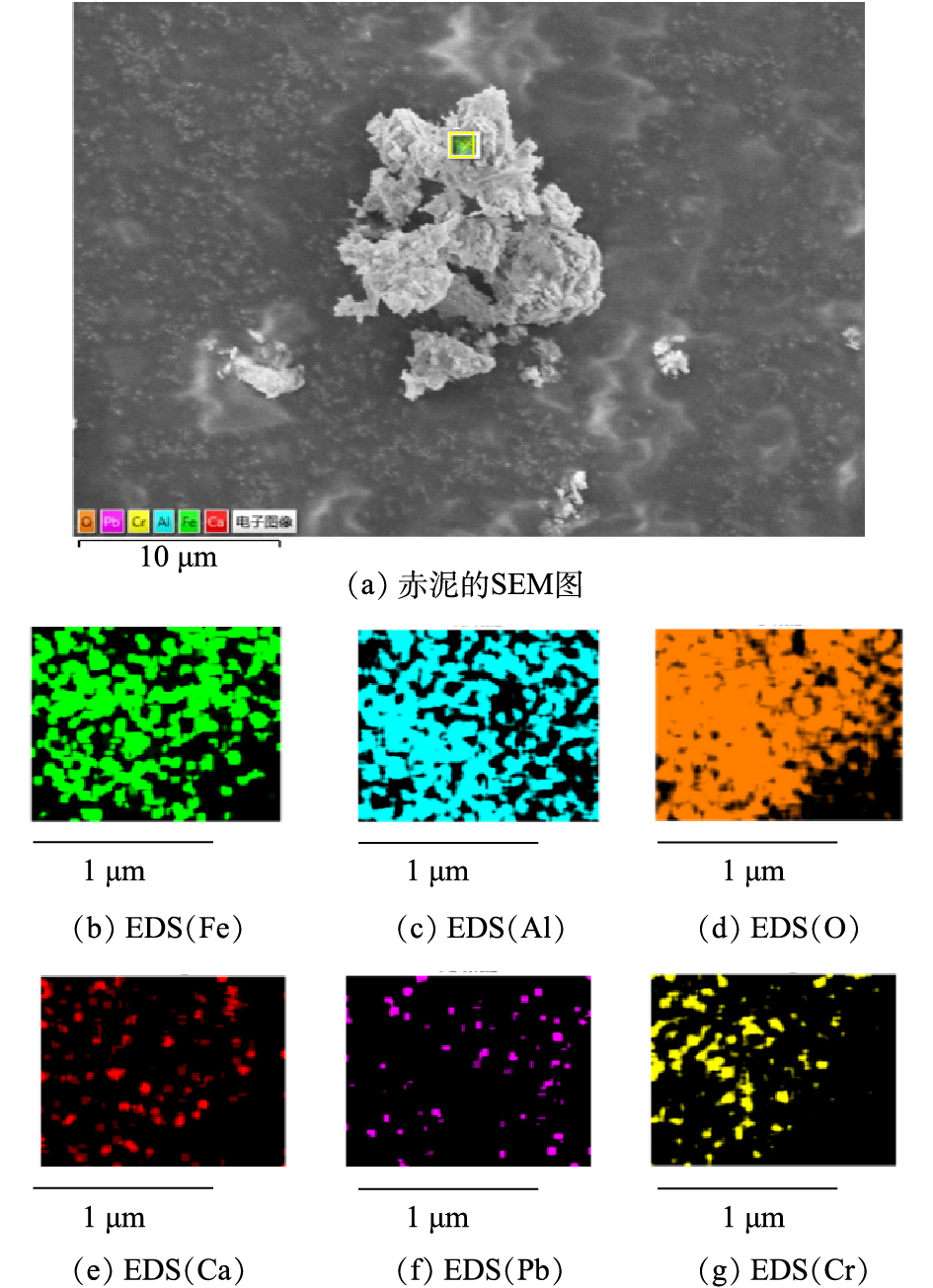
供试赤泥样品颗粒细小,颜色偏黄棕色。由图3可见,赤泥颗粒形状不规则,表面凹凸不平,存在空隙,有部分赤泥颗粒相互黏结,形成了较大的聚集体。通过EDS扫描观测到赤泥样品表面含O、Ca、Al、Fe、Cr、Pb 6种元素的分布,其中O、Ca、Al、Fe 4种元素含量较高,且分布均匀,还存在少量Cr、Pb等元素,含量较低。
氧化铝企业采用的生产工艺,通常会导致赤泥呈现明显的碱性特征。联合法是将拜耳法工艺产生的赤泥与低品质铝土矿配合碳酸钠一起,混合进行高温煅烧、溶出、分离结晶,在此过程中分离的废渣即为联合法赤泥,烧结后赤泥会产生大量碳酸盐矿物。在拜耳法工艺过程中,会添加石灰进行预脱硅处理,此时,铝土矿将会与氢氧化钙反应生成钙霞石和水化石榴石进入赤泥中,再向脱硅后的铝土矿中加入苛性碱,溶出铝土矿中的氧化铝,经过矿浆稀释和沉降分离后的杂质包括碳酸钠、铝酸钠、方解石、铝酸钙、磷灰石等固体废物,这些固体废物与钙霞石和石榴石一起,组成了拜耳法赤泥的碱性物质[13]。由于联合法赤泥的原料是拜耳法赤泥,这使联合法赤泥的碱性组成中不仅包括碳酸盐矿物,还包括拜耳法赤泥在经过烧结之后残留下来的碱性物质,正是这些物质,造就了赤泥的高碱度特性。
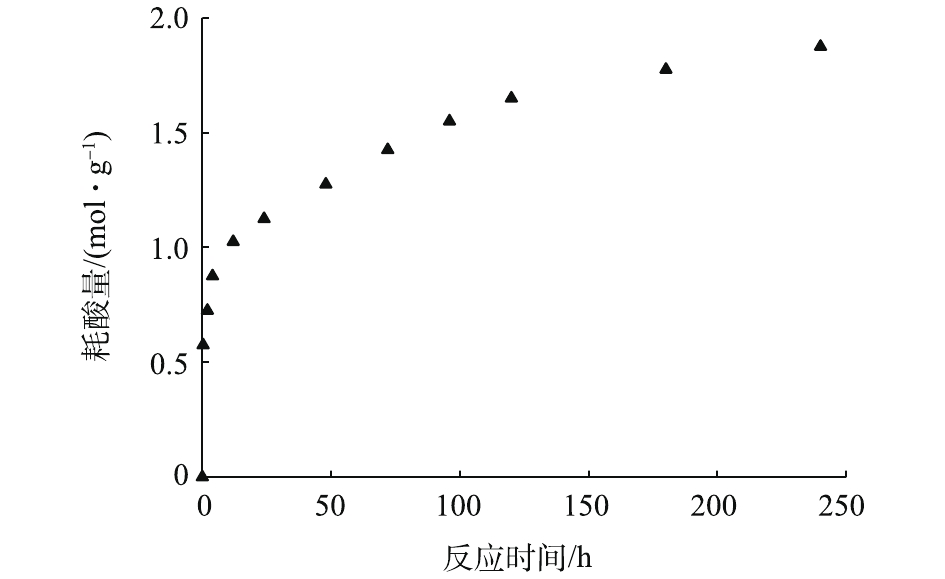
如图4所示,在T=25 ℃,转速为1 000 min−1的条件下,向pH=7.0的水中,投加2.0 g·L−1的赤泥。在240 h内,供试赤泥消耗硝酸的能力为 1.875 mol·kg−1;在反应初始8 h内,赤泥表面的可溶盐和碱性氧化物(如苛性碱、碳酸钠、铝酸钠等)快速溶解,并与水溶液中的H+发生中和反应,使得硝酸的消耗速率较快;随着反应的进行,碱性物质浓度降低,耗酸速率开始下降。因而,赤泥不仅具有较高的酸中和能力,还具有较强的碱性缓慢释放性。
-
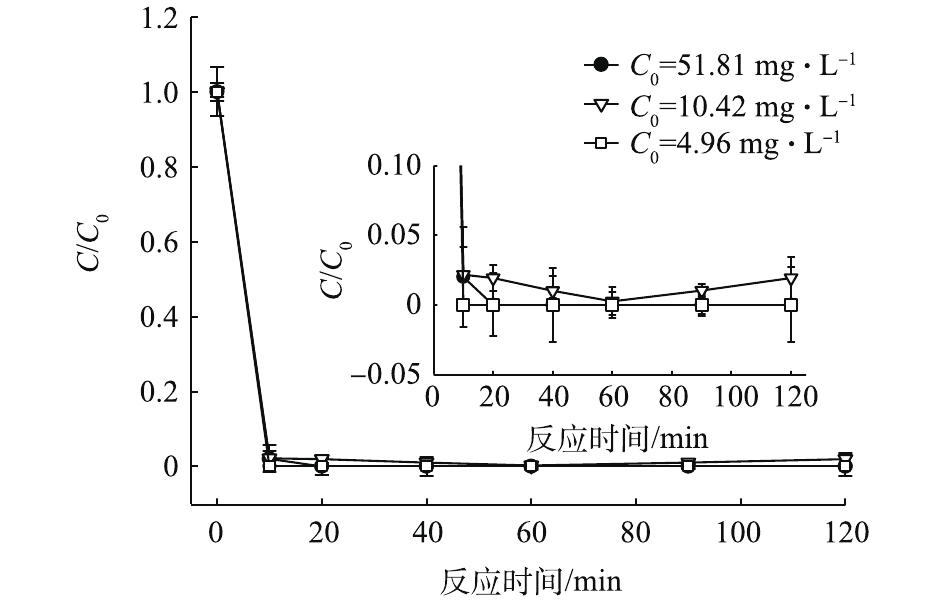
赤泥中含有一定量的碱性物质,在向Pb(Ⅱ)水溶液中投加赤泥后,水溶液的pH产生明显影响。如图5所示,当T=25 ℃,磁力搅拌转速为1 000 r·min−1时,向初始pH为4.0,初始浓度为4.96、10.42和51.81 mg·L−1的Pb(Ⅱ)水溶液中加入赤泥粉末后,监测水溶液中pH随时间的变动情况,发现pH有了明显上升,溶液的pH由4.0上升至9.8~10.10。如图6所示,赤泥对Pb(Ⅱ)的去除反应迅速发生,在10 min内,去除率分别达到了100.0%、98.8%和100.0%;对Pb(Ⅱ)的去除能力分别达到2.48、5.20和25.90 mg·g−1。反应后,水溶液中Pb(Ⅱ)浓度分别为0、0.12和0 mg·L−1,均能较好达到污水综合排放标准中第一类污染物最高允许排放浓度1.0 mg·L−1。
SASH等[9]使用盐酸加热制备的改性赤泥,在pH=4时的最大吸附容量为6.027 3 mg·g−1。李明等[16]使用木质素制备改性活性炭,在pH=5且初始浓度为100 mg·L−1的条件下,去除能力可达到36 mg·g−1。对比而言,联合法赤泥粉末具有制备方式简单且成本低廉的优势,同时对Pb(Ⅱ)的也有较好的去除效果。
-
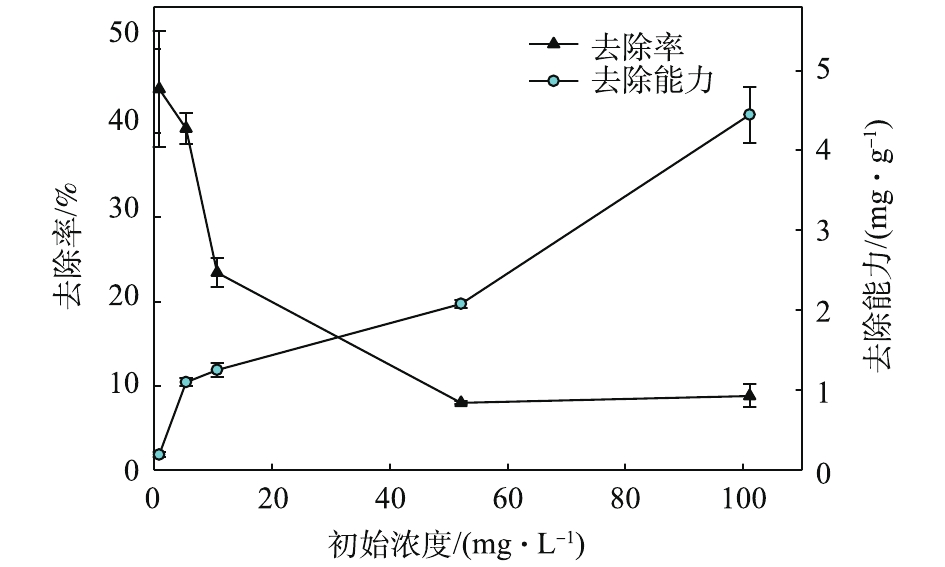
为了观测Pb(Ⅱ)的初始浓度对Pb(Ⅱ)去除效果的影响,保持pH为4.0±0.2,在1~100 mg·L−1内调整Pb(Ⅱ)的初始浓度,并开展去除实验。如图7所示,赤泥对Pb(Ⅱ)的去除能力随着溶液中初始浓度的提高而增大;去除率随着Pb(Ⅱ)初始浓度的增加而降低。在低浓度Pb(Ⅱ)溶液中,Pb(Ⅱ)去除效果明显,当Pb(Ⅱ)初始浓度为0.86 mg·L−1和4.96 mg·L−1时去除率分别为45.2%和40.5%,去除能力为0.19 mg·g−1和1.10 mg·g−1。初始浓度较高时,去除率不理想,但是去除能力较高,当初始浓度为100 mg·L−1时,去除率为8.8%,去除能力可达4.45 mg·g−1。
-
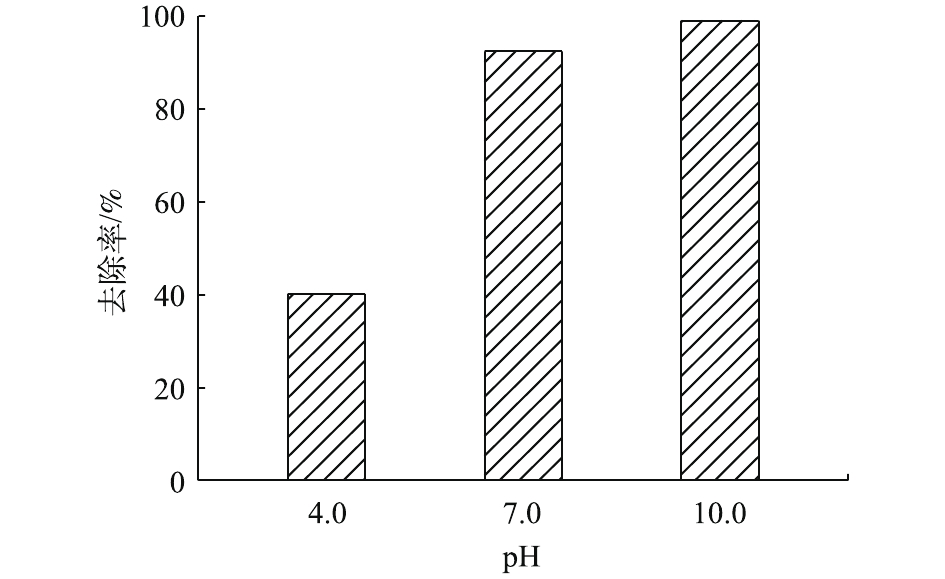
pH是赤泥去除水溶液中Pb(Ⅱ)的重要影响因素。由图8可知,在典型的pH水平下,赤泥对水溶液中Pb(Ⅱ)都具有较好的去除效果,其中在中性和碱性的条件下,对Pb(Ⅱ)的去除率可以达到90%以上。研究表明,使用碱性氢氧化物对水溶液中Pb(Ⅱ)进行化学沉淀时,最佳pH为7.5~11.5[17]。理论上,由于铅离子的水解反应,Pb(Ⅱ)的形态在酸性环境中以Pb2+为主,而随着溶液中OH−的增加,逐渐生成Pb(OH)2沉淀。FRANCIS等[18]在进行理论模拟后得出,当pH为10.5左右时,Pb(OH)2的比例最大,即铅的去除率最大。
-
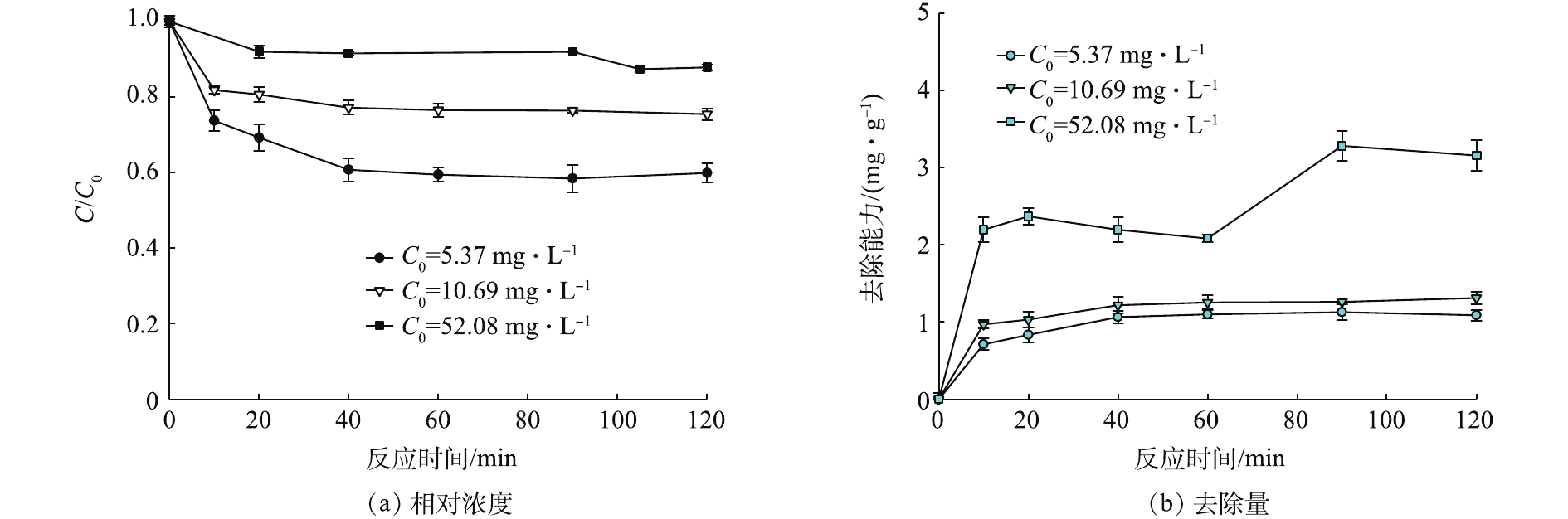
为进一步探讨赤泥对Pb(Ⅱ)的去除机理,展开了赤泥去除Pb(Ⅱ)的动力学实验。如图9所示,当pH=4.0±0.15,去除反应快速进行,随后在90~120 min趋于平衡。平衡时刻3个初始浓度水溶液中Pb(Ⅱ)的残余浓度分别3.25、8.17和45.77 mg·L−1,去除率为41.5%、24.5%和12.6%,赤泥对水溶液中Pb(Ⅱ)去除能力分别为1.09、1.26和3.16 mg·g −1。
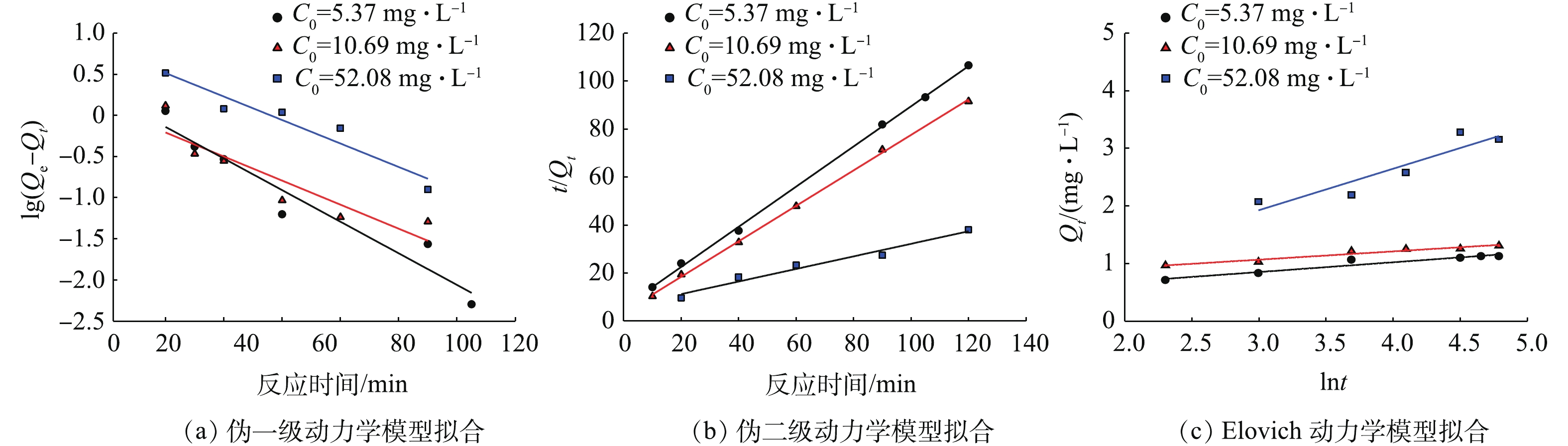
为定量描述赤泥对Pb(Ⅱ)的去除动力学,采用3种动力学模型来对初始浓度为10 mg·L−1的Pb(Ⅱ)的去除过程进行拟合[19-21],这3种模型分别是Lagergren伪一级动力学模型,见式(1);Lagergren伪二级动力学模型,见式(2);Elovich 动力学模型,见式(3)。模型拟合如图10所示。
式中:Qe和Qt分别代表平衡时刻吸附量和t时刻的吸附量,mg·g−1;k1、k2和a为各自模型的反应速率常数;C为常数。
如表2所示,对比3种动力学模型的拟合效果,伪二级动力学模型的拟合效果优于 Elovich 动力学以及伪一级动力学,具有更高的拟合系数。伪二级动力学模型假设吸附速率由吸附剂表面未被占有的吸附空位数目的平方值决定,吸附过程受化学吸附机理的控制[20]。由此说明赤泥去除Pb(Ⅱ)的机理包括化学吸附。
2.1. 赤泥特性
2.2. Pb(Ⅱ)去除效果
2.3. Pb(Ⅱ)初始浓度影响
2.4. pH的影响
2.5. 机理分析
-
1)联合法赤泥的主要化学成分有CaO、SiO2、Al2O3和Fe2O3;矿物组成主要有方解石、水钙铝榴石、钙霞石、铝酸钙和赤铁矿;平均比表面积为43.8 m2·g−1,表面积平均粒径为0.137 μm;赤泥粉末的颗粒形状不规则,表面凹凸不平,存在孔隙结构,且部分赤泥颗粒相互黏结形成较大聚集体。赤泥中含有大量碱性物质,有很强的酸中和能力,赤泥酸消耗能力约为1.875 mol·kg−1。
2)赤泥对水溶液中的Pb(Ⅱ)具有较强的去除能力。当赤泥投加量为2.0 g·L−1时,在pH=4.0,初始浓度为5~50 mg·L−1时,反应在90~120 min时达到平衡,去除率为10%~45%,去除能力为1.06~5.73 mg·g−1。初始pH=4,且不控制pH的条件下,赤泥粉末与Pb(Ⅱ)迅速反应,反应10 min时,Pb(Ⅱ)去除率超过98%,平衡时水溶液中Pb(Ⅱ)浓度小于0.2 mg·L−1 远低于污水综合排放标准中第一类污染物最高允许排放浓度1.0 mg·L−1的要求。
3)水溶液pH和初始浓度都会对Pb(Ⅱ)的去除效果产生较大影响。在中性和碱性的条件下,赤泥对Pb(Ⅱ)的去除效果较好,去除率可以达到90%以上。当初始浓度为1~100 mg·L−1时,初始浓度越高,去除率越低,赤泥的去除能力越强。
4)赤泥对Pb(Ⅱ)的吸附符合拟二级动力学模型,去除过程受化学吸附机理的控制。



 下载:
下载: