-
氮氧化物(NOx)不仅具有很强的毒性,而且还是酸雨和光化学烟雾的重要源头之一,给人类健康和生态环境带来极大威胁[1-2] 。烟气脱硝技术一直是环境治理领域的重要研究方向之一。目前,工业中较成熟的NOx的脱除方法主要包括选择性催化还原法(selective catalytic reduction, SCR)[3]和选择性非催化还原法(selective non-catalytic reduction, SNCR)[4]。但这2种烟气脱硝技术方法存在一些弊端,如设备占地面积大、反应成本高以及二次污染等[5-6]。相比之下,光催化氧化技术作为一种环境友好、投资费用少、反应条件温和的污染物去除方法,在NOx的净化中具有巨大潜能[7]。
在众多的光催化剂中,TiO2因其廉价易得、环境友好和催化活性高等特点颇受关注。近期研究表明,TiO2能够对NOx实现有效的光催化氧化降解[8],但是TiO2催化剂依然存在缺陷,其中难以利用可见光以及光生电子-空穴复合率高等问题严重制约了其在光催化脱硝过程中的应用和发展[9]。因此,对TiO2进行改性,使其能够在可见光驱动下实现有效的光生电子对分离具有重要的科学和现实意义。在过去的10年中,人们已成功实现了非金属元素以及过渡金属元素对TiO2的掺杂,研究发现,这些元素的掺杂不仅能够有效改善TiO2对可见光的吸收,还能在一定程度上缓解光生电子对复合率高的问题。NGUGEN等[9]成功制备了Cu掺杂TiO2纳米片,用于降解有机染料,发现Cu离子可作为界面电荷迁移的介质,从而降低电子-空穴复合率;TREVISAN等[10]研究发现,N元素的掺杂可有效提升TiO2的价带位置,使得带隙宽度有效降低,从而实现了对可见光的响应。然而对TiO2进行改性,仍然存在催化剂比表面积低的缺点,这将影响光催化性能的进一步提高。
鉴于此,将TiO2与碳材料复合,可在降解有机污染物方面表现出优异的性能[11]。碳纳米管(carbon nanotubes, CNTs)的高导电性可以提供电子通道,从而增加了电子湮灭长度,以防止电子-空穴的结合;可以在TiO2晶格中引入杂质能级,从而加速电子-空穴的分离[12];可以在TiO2晶格中引入杂质能级,从而加速电子-空穴的分离[13-14],同时碳纳米管可以有效增大TiO2的比表面积。因此,将CNTs与TiO2进行复合是一个很好的思路。
本研究将具有良好导电性的CNTs与具有良好储氧能力的Ce耦合,采用溶胶-凝胶法,制备出CNTs-Ce/TiO2系列复合光催化剂,并对其光催化脱硝性能进行测试;通过扫描电子显微镜 (SEM)、全自动微孔物理吸附-脱附 (BET)、X射线衍射 (XRD)、拉曼光谱(Raman)、X射线光电子能谱(XPS)和紫外可见漫反射谱(DRS)等技术,对复合材料进行结构表征和性能分析,以探讨光催化在烟气脱硝方面的应用潜力,为湿法脱硫后的脱硝的研究提供参考。
全文HTML
-
催化剂制备所需材料:钛酸丁酯(Ti(OCH3)4,98.56%);无水乙醇(C2H5OH,99%);硝酸(HNO3,98.02%);硫酸(H2SO4,浓度98.08%);碳纳米管(CNTs,纯度95.12%);硝酸铈六水化合物(Ce(NO3)3·6H2O,99.55%);硝酸铜三水化合物(Cu(NO3)2·3H2O,99.04%);尿素(H2NCONH2,99.02%)。
-
在制备TiO2光催化剂时,利用钛酸丁酯(Ti(OCH3)4)作为钛源,将20 mL钛酸丁酯加入7 mL乙酸和3 mL HNO3的混合液中,搅拌均匀后得到A液;将20 mL无水乙醇与4 mL去离子水混合后,用HNO3调pH≈2.0,得B液;将B液逐滴加入到A中,搅拌得到凝胶状产物,在80 ℃下干燥,并将产物在500 ℃焙烧2 h,冷却后,制备出TiO2光催化剂。
在制备金属与非金属掺杂的钛基光催化剂时,将20 mL钛酸丁酯加入70 mL乙酸和3 mL HNO3的混合液中,搅拌均匀后得到A液;称取一定量的含有元素M (M占Ti的比例为6%)的前驱物,溶于20 mL无水乙醇与4 mL去离子水中,并用HNO3调pH≈2.0,得到B液;将B液逐滴加入到A中,搅拌得到凝胶状产物,干燥后,在500 ℃下焙烧2 h,得到金属与非金属掺杂的Ti基光催化剂,分别记为N/TiO2、Cu/TiO2、Ce/TiO2。
在制备CNTs掺杂的钛基光催化剂时,称取0.68 g CNTs,加入到40 mL无水乙醇溶液中,超声分散,再加入20 mL钛酸丁酯、30 mL无水乙醇和3 mL HNO3到CNTs悬浊液中,得到A液;称取一定量的含有元素M(M占Ti的比例为6%)的前驱物溶于20 mL无水乙醇与4 mL超纯水中,并用HNO3调pH≈2.0,得到B液;将B液逐滴加入到A中,搅拌干燥、焙烧,冷却得到CNTs掺杂的Ti基光催化剂,分别记为CNTs-TiO2、CNTs-N/TiO2、CNTs-Cu/TiO2、CNTs-Ce/TiO2。
-
采用扫描电子显微镜(SEM)观察催化剂的形貌特征;使用全自动微孔物理吸附-脱附分析测试仪 (BET)分析内外比表面积和孔分布情况;采用X射线粉末衍射仪 (XRD)测定催化剂的晶体结构;拉曼光谱仪(Raman)确定催化剂的组成结构和理化特性;X射线光电子能谱仪(XPS)分析材料的元素组成及价态;紫外可见漫反射谱分光光度计(DRS)测试催化剂的光响应特性。
-
实验系统主要由配气系统、光催化氧化脱硝系统以及气体分析测试系统构成,如图1所示。
在模拟烟气中,SO2、NO、N2、O2和CO2按不同流量喷入气体缓冲罐,混匀,体积空速为10 000 h−1,总气体流量为100 mL·min−1,H2O(g)体积分数为4%~6%,CO2体积分数为6%,O2体积分数为4%~6%,N2体积分数为80%~90%,NO和SO2进口浓度为0.006%,反应器中催化剂为0.6 mL,将光催化反应器中的温度调节到50 ℃,开启光源。反应后的模拟烟气进入干燥瓶进行干燥处理,利用烟气分析仪(英国凯恩,940MKⅡ)连续采样测定NO浓度,NO氧化效率计算方法见式(1)。
式中:η为NO氧化效率;Cin为进口浓度;Cout为出口浓度。
1.1. 实验原料
1.2. 催化剂制备方法
1.3. 催化剂表征
1.4. 实验与分析方法
-
针对制备的8种光催化剂:TiO2、N/TiO2、CNTs-TiO2、CNTs-N/TiO2、Cu/TiO2、Ce/TiO2、CNTs-Cu/TiO2、CNTs-Ce/TiO2,进行光催化氧化NO活性测试。通过4种方式对催化剂进行灯光照射实验:紫外线短波UVB(290~320 nm)单独照射,紫外线短波UVB与紫外线长波UVA(320~400 nm)同时照射,紫外线短波UVB、紫外线长波UVA和可见光vis(380~780 nm)同时照射和无光源照射。光催化氧化烟气脱硝效率对比结果如图2所示。
由图2可知,当紫外线短波UVB(290~320 nm)、紫外线长波UVA(320~400 nm)和可见光vis(380~780 nm) 3种灯同时打开时,NO氧化效率最高;当UVB(290~320 nm)和UVA(320~400 nm)同时打开与UVA(320~400 nm)单独打开时,氧化效率有所降低;当3种灯全部关闭时,NO氧化效率明显迅速下降,经过10 min左右,对NO氧化效率接近于0。可见,打开灯数越多,所产生的能量越强,更易于产生空穴。而空穴具有很强的氧化性,可以和水蒸气生成具有很高活性的自由基。这些自由基和污染物发生氧化还原反应,所以NO氧化效率大大提升。关灯后,因为没有能量受体,并且反应温度只有50 ℃,根本达不到钛基催化剂的活化温度,因此,关灯后NO氧化效率较低。
当3种灯同时打开时,可以明显观察到,CNTs-Ce/TiO2的NO氧化效率相对于TiO2参与的光催化氧化效率显著提升。这可能是由于形成了CeO2杂质能级,从而有效地捕获光生电子和空穴[15],抑制了光生载流子的复合,提高了光催化活性;并且引入CNTs后,不仅有效改善了其自身的导电性,从而有利于电子转移,以促进电子-空穴的分离,同时还提供了更大的比表面积和孔径。
-
用扫描电镜(SEM)观察了CNTs-Ce/TiO2的形貌。由图3可看出,纯TiO2样品表面结构紧致光滑,Ce/TiO2表面出现了活性组分团聚现象,溶胶的高黏度可能是引起此现象的原因之一[16];而CNTs-Ce/TiO2团聚现象得以改善,颗粒均匀,分散程度较高,其原因是CNTs多孔的特性促进了催化剂表面空隙的形成,有利于气体扩散,从而更好地与气体污染物发生反应。
-
研究表明,比表面积越大,越有利于促进反应的进行[17]。由表1可知,对于TiO2,Cu/TiO2比表面积均有所下降,这是由于TiO2的孔隙被团聚的CuO颗粒所覆盖造成的[18];但是Ce/TiO2的比表面积却显著增大。XIAO等[19]也发现,氧化铈的存在有利于有效地增强催化剂比表面积。加入适量CNTs后,各催化剂比表面积和平均孔径均有不同程度的增大,其中CNTs-Ce/TiO2的比表面积和平均孔径最大。相对于纯TiO2,CNTs-Ce/TiO2比表面积提高了81.5%,CNTs-Ce/TiO2的大孔径有利于反应气体及产物的扩散及转移[20],产生的少量中间产物的沉积也不会造成孔堵塞。
-
图4显示了锐钛矿TiO2以及CNTs-Ce/TiO2催化剂的XRD图谱。由图4可见,N/TiO2、CNTs-TiO2、CNTs-N/TiO2、Cu/TiO2、Ce/TiO2和CNTs-Cu/TiO2各样品在2θ为25.36°、37.85°、48.08°、53.99°、55.20°、62.74°、68.92°、70.35°、75.26°、82.70°的位置上都出现了衍射峰。与标准卡片(PDF#65-5714)对比,各样品皆成TiO2锐钛矿。在复合材料的光谱范围内,没有出现CNTs的特征衍射峰,这是由于TiO2锐钛矿型主峰位置处(2θ=25.36°)和CNTs的特征衍射峰(2θ=26.23°)发生了重叠,且TiO2的质量分数远高于CNTs所导致[21-22]。CNTs-Ce/TiO2和Ce/TiO2相对于TiO2,在锐钛矿主峰位置处(2θ=25.36°),峰形明显宽化且衍射峰强度减弱,这是由于Ce的掺杂使TiO2晶格发生了膨胀(畸变)。Ce/TiO2和CNTs-Ce/TiO2的XRD图谱中没有检测到与Ce相关的衍射峰,表明Ce4+离子可能以小团簇形式的CeO2分散均匀地覆盖在催化剂表面,这种覆盖可以有效地分离电荷载体,从而阻碍了电子空穴对的复合,以提高光催化活性[23]。
-
由图5可知,0~800 cm−1的拉曼峰是TiO2的特征峰,在144、197、394.2、515.2和636.4 cm−1处的振动峰分别从属于锐钛矿型TiO2的Eg(144 cm−1)、Eg(198 cm−1)、B1g(398 cm−1)、A1g(515 cm−1)和Eg(640 cm−1)的特征信号峰[24],证明TiO2在复合材料中是以锐钛矿型TiO2存在的,这和XRD的测试结果相吻合。如图5(a)所示,144 cm−1为Ti—O键伸缩振动的特征峰,与纯相TiO2相比,Ce/TiO2的Ti—O振动的特征峰显示出明显的蓝移、拓宽和削弱,这表明Ce的掺杂为TiO2引入了缺陷[25]。如图5(b)所示,在1 353 cm−1和1 586 cm−1处分别对应于CNTs的无序碳(D峰)和石墨碳(G峰),复合材料均出现了这2个特征峰,由此证明了复合材料中CNTs的存在[26]。拉曼位移和键长之间的关系[27]见式(2)。
式中:ν为拉曼位移,cm−1;RM-O为金属、非金属原子和氧原子之间的M—O键长,nm。
由式(2)得到掺杂CNTs后各样品的Ti—O键长度变化,结果如表2所示。通过计算可知,CNTs-Ce/TiO2的Ti—O键长最短,表明其构建出了紧密的界面接触关系,从而有利于光生电子沿Ti—O键向CNTs转移,以促进电子-空穴的分离。
-
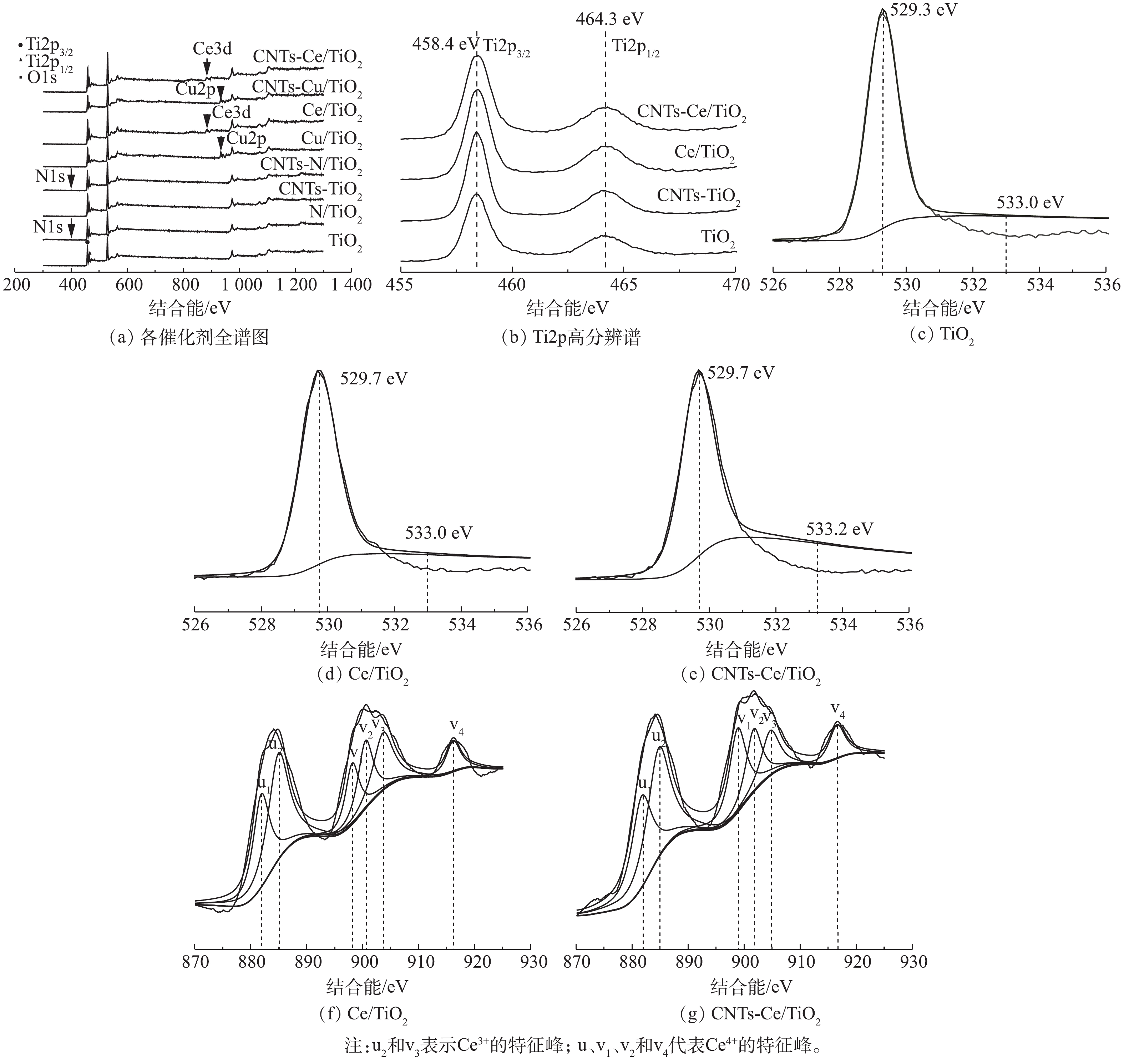
图6为各催化剂的X射线光电子能谱,用于进一步确定合成物质的元素组成及其价态。图6(a)为各催化剂的全谱图,通过CNTs-Ce/TiO2全谱可以确认,CNTs-Ce/TiO2中包含元素Ti、O和Ce,同时也对XRD中没有检测到Ce的存在作了补充说明。图6(b)为 Ti2p的高分辨谱,458.4 eV和464.3 eV处的2个特征峰,分别归属于Ti2p3/2和Ti2p1/2,对应于TiO2中的Ti4+。图6(c)~图6(e)分别为TiO2、Ce/TiO2和CNTs-Ce/TiO2的O1s高分辨谱,利用Gaussian-Lorentzian函数结果,对O1s峰进行拟合去卷积处理,3种催化剂均可以拟合2个O特征峰:结合能在529.3~530.3 eV的拟合峰对应于催化剂的晶格氧(Oβ),包括Ti—O和Ce—O键等;结合能在532.4~533.2 eV的拟合峰对应于化学吸附氧(Oα)。有研究[28]表明,Oα非常活跃,具有更强的氧传递和供给能力,从而有利于提高催化氧化效率。对比图6(c)~(e)可以明显看出,CNTs-Ce/TiO2吸附氧的特征峰最强,这主要得益于Ce元素优良的氧化性能和储氧能力以及CNTs强大的电子转移性能。图6(f)~图6(g)为Ce/TiO2和CNTs-Ce/TiO2催化剂的Ce3d的XPS图谱,Ce3d光谱可拟合成6个特征峰。结果表明,Ce3+和Ce4+共同存在于催化剂表面,其中Ce4+为主要存在状态。根据峰面积计算,Ce/TiO2中Ce3+/Ce4+的比例为 62.1%,而CNTs-Ce/TiO2中Ce3+/Ce4+的比例为 67.4%,由此说明,CNTs的引入能够促进催化剂形成更多的三价铈氧化物。还有研究[29]表明,Ce3+导致催化剂的电子分布不均,形成氧空位和不饱和化学键,此特性有利于催化剂表面的氧吸附,进而促进整个催化氧化反应,这一结论与CNTs-Ce/TiO2的O1s XPS图谱信息相吻合。
-
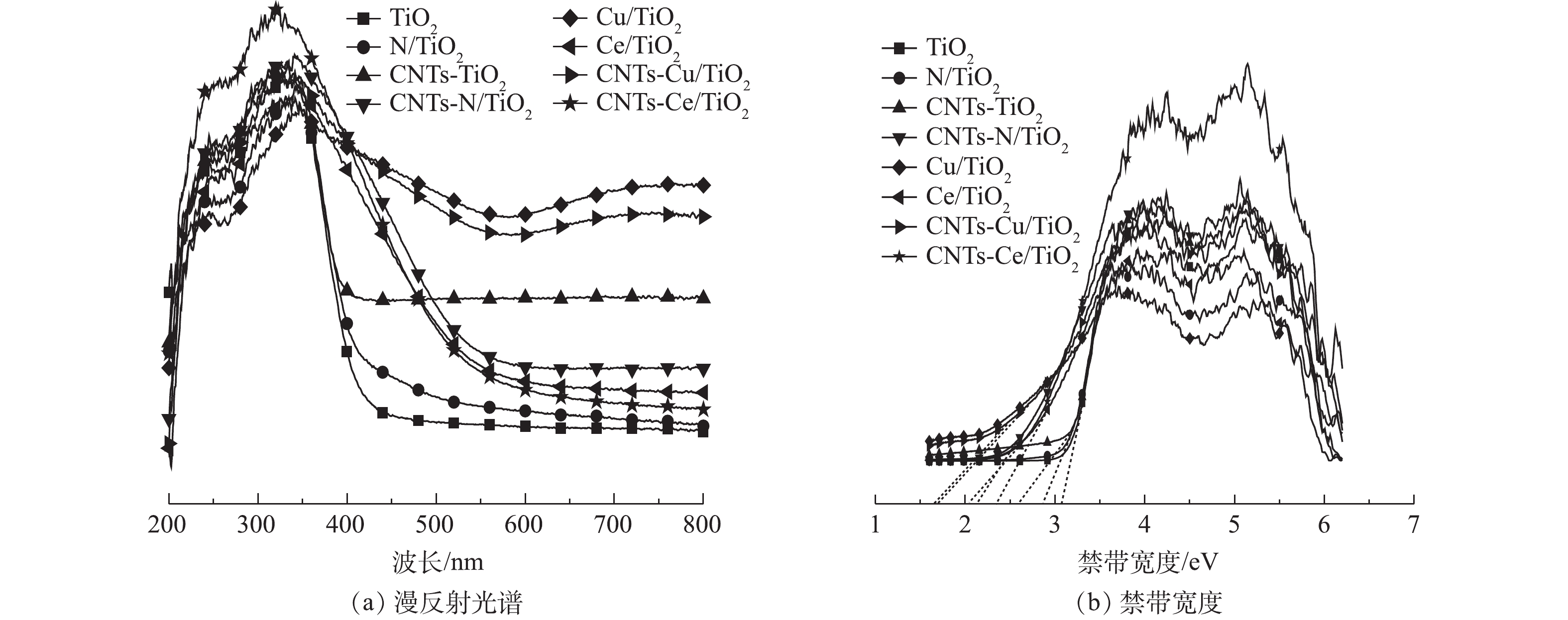
图7(a)显示了CNTs-Ce/TiO2的紫外-可见漫反射光谱。在200~400 nm的紫外光区域内,TiO2加入Ce 和CNTs后,Ce/TiO2和CNTs-TiO2的吸光性能没有较明显的区别,但CNTs-Ce/TiO2吸光性能却有了大幅度的提升,这是由于Ce在TiO2基体中的嵌入以及CNTs与TiO2在复合催化剂中良好的连接,从而导致了强相互作用[23];在400~800 nm的可见光区域内,CNTs-Ce/TiO2对可见光的吸收范围较大,这种红移是由于Ce完全结合在TiO2基体中,从而改变了其电子结构和晶体结构[23]。CNTs-Ce/TiO2的带隙宽度可根据光吸收能带方程[30]计算得到,计算方法见式(3)。
式中:α为吸收系数;h为普朗克常数;ν为吸收光频率;Eg为禁带宽度值;A为常数。
由式(3)计算出CNTs-Ce/TiO2的带隙宽度为2.06 eV,对比纯相TiO2(3.09 eV)和Ce/TiO2(2.15 eV),明显变窄,对可见光谱的响应较强,主要原因是 CeO2杂质能级的形成降低了光生电子迁跃所需能量[31]。
2.1. 光催化性能测试
2.2. 扫描电子显微镜分析
2.3. N2吸附/脱附分析
2.4. X射线衍射分析
2.5. 拉曼光谱分析
2.6. X射线光电子能谱分析
2.7. 紫外-可见漫反射光谱分析
-
1)采用溶胶-凝胶法制备了新型的CNTs-Ce/TiO2光催化剂,成功地在金属掺杂改性TiO2的基础上复合CNTs。
2)在可见及紫外光同时照射下,CNTs-Ce/TiO2催化剂光催化氧化NO的效率最高。这是由于Ce和CNTs的协同作用使NO在可见及紫外光照射下的氧化效率比制备所得其他催化剂均有所提高。
3) Ce高度分散在催化剂表面,有利于催化性能提高;CNTs的掺杂提高了催化剂比表面积,促进了催化剂表面空隙的形成,有利于气体扩散;拉曼光谱(Raman)分析表明,CNTs-Ce/TiO2的Ti—O键长最短,有利于光生电子沿Ti—O键向CNTs转移,以促进电子-空穴的分离;XPS说明CNTs-Ce/TiO2具有更高的氧化效率,并且CNTs的引入能够促进催化剂形成更多的三价铈氧化物,形成的氧空位有利于催化剂表面的氧吸附,从而促进整个催化氧化反应。




 下载:
下载: