-
为减少SO2的排放量,许多国内外学者[1-4]对脱硫技术进行了探索和研究。据统计,我国每年SO2的排放量均超过了2×107 t[5-7],SO2已成为当前排放量较大的主要大气污染物。在环境保护部颁布《火电厂大气污染物排放标准》[8]后,一些脱硫技术[9-10]对减少SO2的排放起到了一定的作用。根据脱硫剂形态的不同,可将烟气脱硫技术分为干法烟气脱硫、半干法烟气脱硫以及湿法烟气脱硫技术3种[11]。其中,湿法烟气脱硫技术被认为是最有效的控制技术之一,目前在全世界范围内得到了广泛的应用[12-15],湿法烟气脱硫技术主要包括石灰石-石膏法[16]、海水烟气脱硫法、双碱法、氨法[17]、氧化镁法[18-20]、微生物法[21],但这些方法具有占地面积大、脱硫效率低等缺点。
磷矿浆脱硫是一种新型的脱硫方法,磷矿浆中的过渡金属Fe3+对脱硫反应起催化作用[22-23],它比目前企业常用的石灰石-石膏法脱硫的经济成本低,适合有燃煤锅炉、磷化工的企业。该方法以磷矿浆作为吸收剂,SO2通入磷矿浆后,在O2及H2O的作用下生成硫酸,生成的硫酸与磷矿浆里的Fe2O3和CaMg(CO3)2进一步反应生成Fe3+、Ca2+和Mg2+等。这些产物可进行回收利用,在脱硫过程中磷矿浆得到了资源再利用[24-26]。
超声波具有方向性好、功率大、穿透力强、能引起空化作用等特点[27]。超声波雾化器利用电子高频振荡,通过雾化片的高频谐振产生超声波,在超声波的空化作用下,液体分子间的作用力被破坏,雾化介质被打散,形成分散均匀的微米级的高密度微小雾滴[28-32]。超声波雾化所产生的雾滴喷射速度低,因而初速度为零,能维持较稳定的状态,易产生高浓度细小的液滴流,此时,雾滴与烟气接触面积增大,停留时间延长,有利于烟气中SO2与雾滴的反应[33-34]。超声波强化气固相催化过程的报道[35-37]表明,超声波能使多相催化反应的单程转化率提高近10倍,对多相催化反应效果非常明显。
本研究采用超声波雾化技术对磷矿浆湿法脱硫进行强化,针对吸收温度、进气流量、固液比、雾化功率、pH,探讨超声波雾化作用对磷矿浆脱硫效果的影响,为实现烟气中SO2的净化与磷矿的资源化提供参考。
全文HTML
-
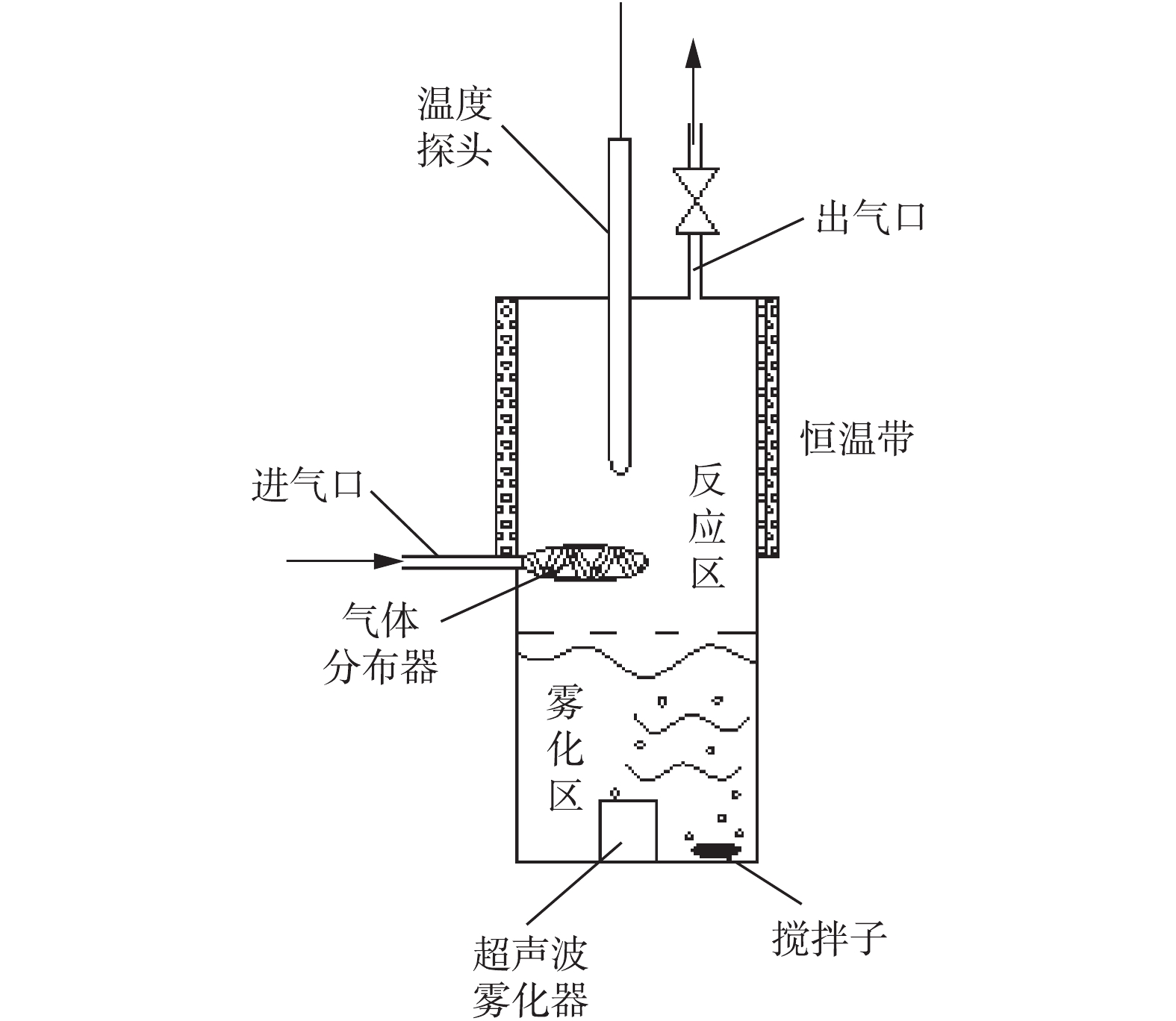
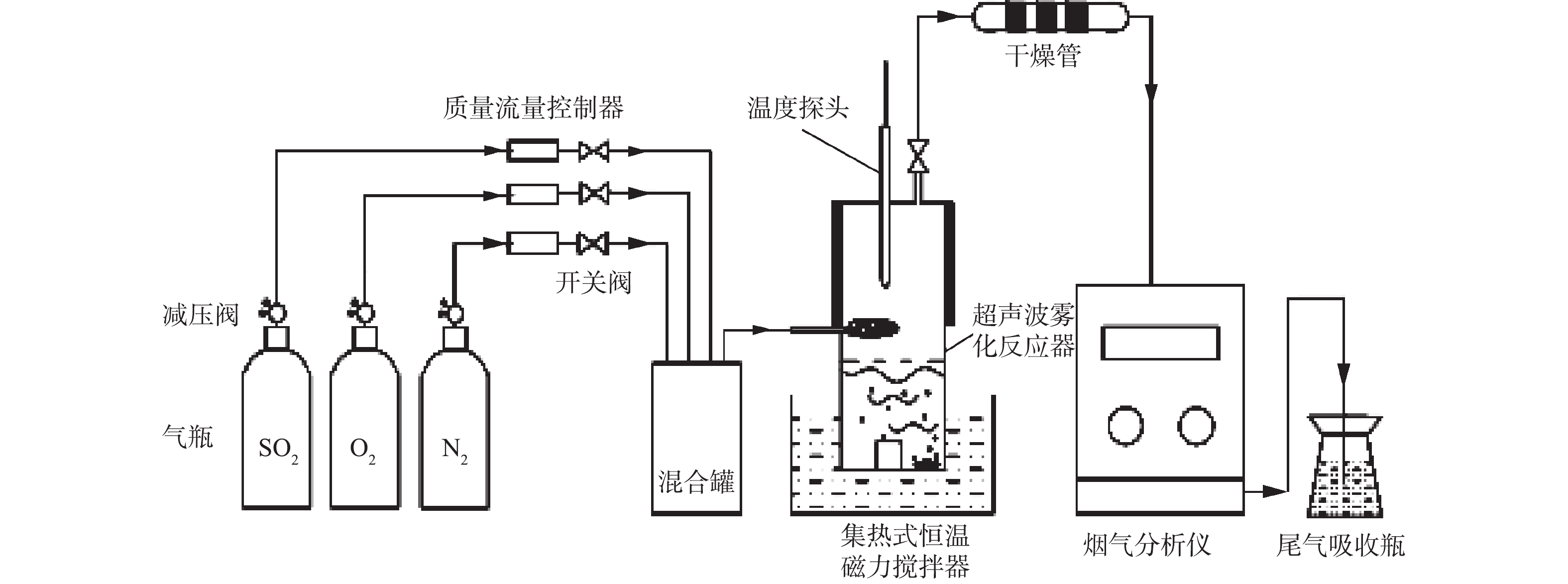
玻璃反应器内径为60 mm、外径为70 mm、高为300 mm(云南瑞祥化玻教仪研发有限公司);AR224CN型电子天平(奥豪斯上海有限公司);STARTER 2 100型pH计(奥豪斯上海有限公司);DF-101S集热式恒温加热磁力搅拌器(巩义市予华仪器有限责任公司);D07型质量流量控制器(北京七星华创电子股份有限公司);EDX-8 000能量色散型X射线荧光光谱仪(日本岛津SHIMADZU);ecom-J2KN型多功能烟气分析仪(德国RBR公司);Nova Nano SEM450型场发射扫描电镜(美国FEI公司);ICP-MS电感耦合等离子体质谱仪(美国安捷伦公司)。实验采用的磷矿来自于云南省某磷肥厂,用球磨机将磷矿磨细,用200目标准筛进行筛分,取筛下矿粉。
-
超声波雾化磷矿浆脱除SO2的实验流程和超声波雾化磷矿浆脱硫的反应器结构分别如图1和图2所示。实验采用动态配气,采用浓度为9 999.7 mg·m−3的SO2标准气、高纯N2和99.5%的O2,配制SO2浓度为1 500 mg·m−3、氧含量为15%的模拟烟气。使用质量流量计控制配制好的模拟烟气,经混合罐混合均匀后,进入超声波雾化反应吸收装置。磷矿浆在下部雾化区,经超声波雾化器雾化后,形成雾滴,上升至上部反应区,气体进入反应器后,与上部反应区的雾滴接触并发生反应。反应后的气体经出气口,通过干燥管干燥后,用烟气分析仪进行检测,尾气用高锰酸钾溶液吸收。
SO2脱除率计算方法如式(1)所示。
式中:η为脱除率;c1和c2分别为进口和出口SO2浓度,mg·m−3。
1.1. 仪器和原料
1.2. 实验方法
-
影响超声波雾化磷矿浆催化氧化SO2的工艺参数有很多。本研究选择吸收温度、固液比、进气流量作为影响因素进行考察,对吸收温度、固液比、进气流量进行正交实验,采用L16 (43)正交表设计实验,选取的因素水平如表1所示。
表2为超声波雾化磷矿浆催化氧化SO2的正交实验结果。表2中k1~k4为各因素水平下的平均值,平均值代表同一因素下不同水平对实验结果(脱硫率)影响的程度,可凭借平均值的大小来确定该因素最佳水平的取值。表2中R值表示k1~k4之间最大平均值与最小平均值的差值,称为极差,表现为相应因素对实验结果(脱硫率)影响的重要程度。R的大小体现了不同因素水平变动时对脱硫率影响的程度。实验考察指标为反应10 h时磷矿浆的脱硫效果,用脱硫率表示。
由正交实验结果可知,A4B4C1为反应的最优方案,即在SO2浓度为1 500 mg·m−3、氧含量为15%时,最佳实验条件为吸收温度35 ℃,固液比25∶100,进气流量0.3 L·min−1。
-
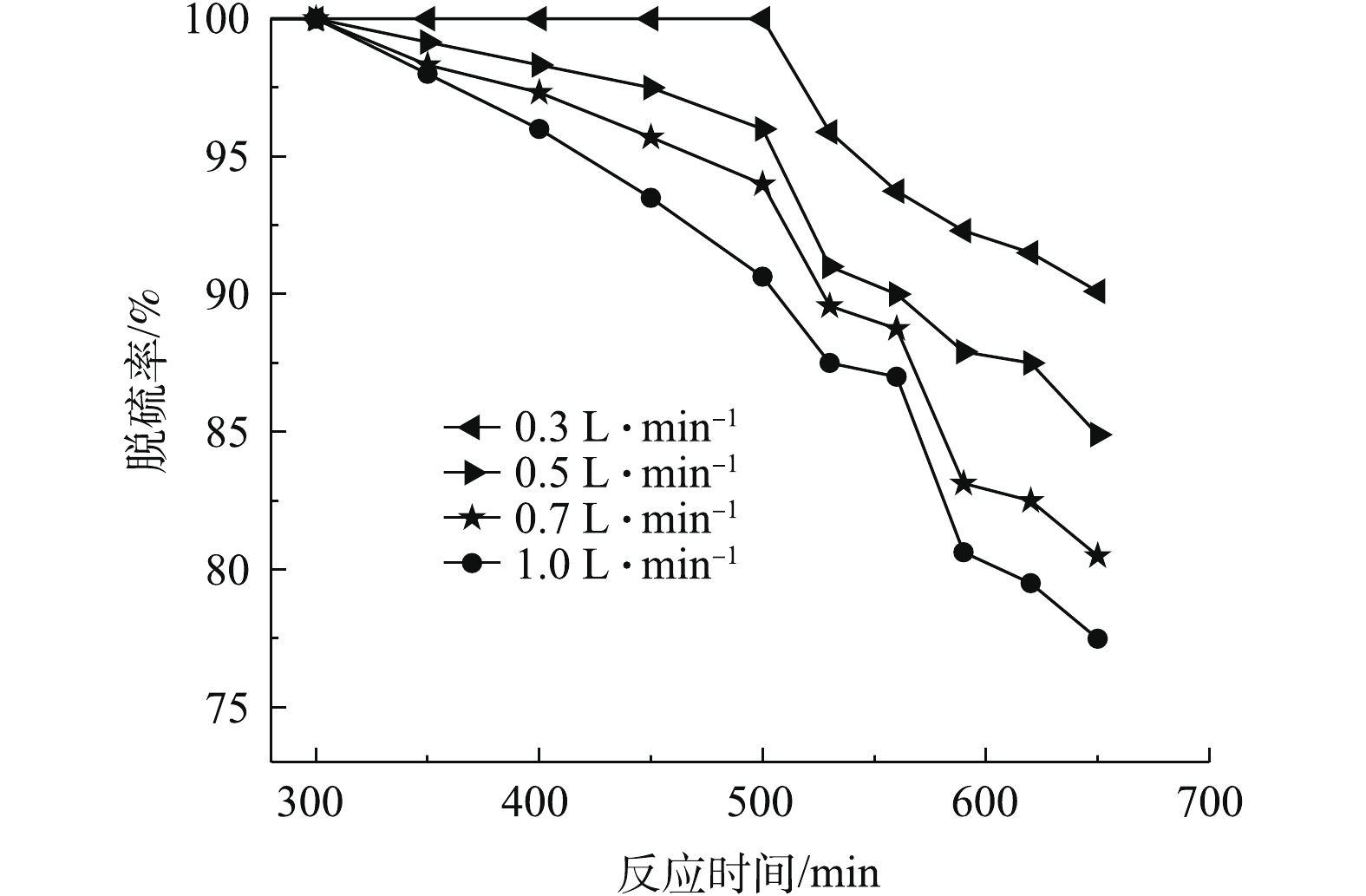
进气流量对SO2脱除率的影响如图3所示。在吸收温度为35 ℃、固液比为25∶100的实验条件下,考察进气流量分别为0.3、0.5、0.7和1.0 L·min−1时脱硫率随反应时间的变化规律。
如图3所示,随着反应时间的延长,脱硫率随进气流量的增加而降低,这是因为进气流量会影响烟气在反应器里的停留时间。流速增加,停留时间减短,烟气与雾滴接触时间缩短。此外,进气流量的增加会导致雾滴状态不稳定,不易停留在反应器内,致使雾滴对模拟烟气中的SO2和O2的吸收不充分,从而导致脱硫率下降。因此,为了得到较好的脱硫效果,实验选用0.3 L·min−1的进气流量。
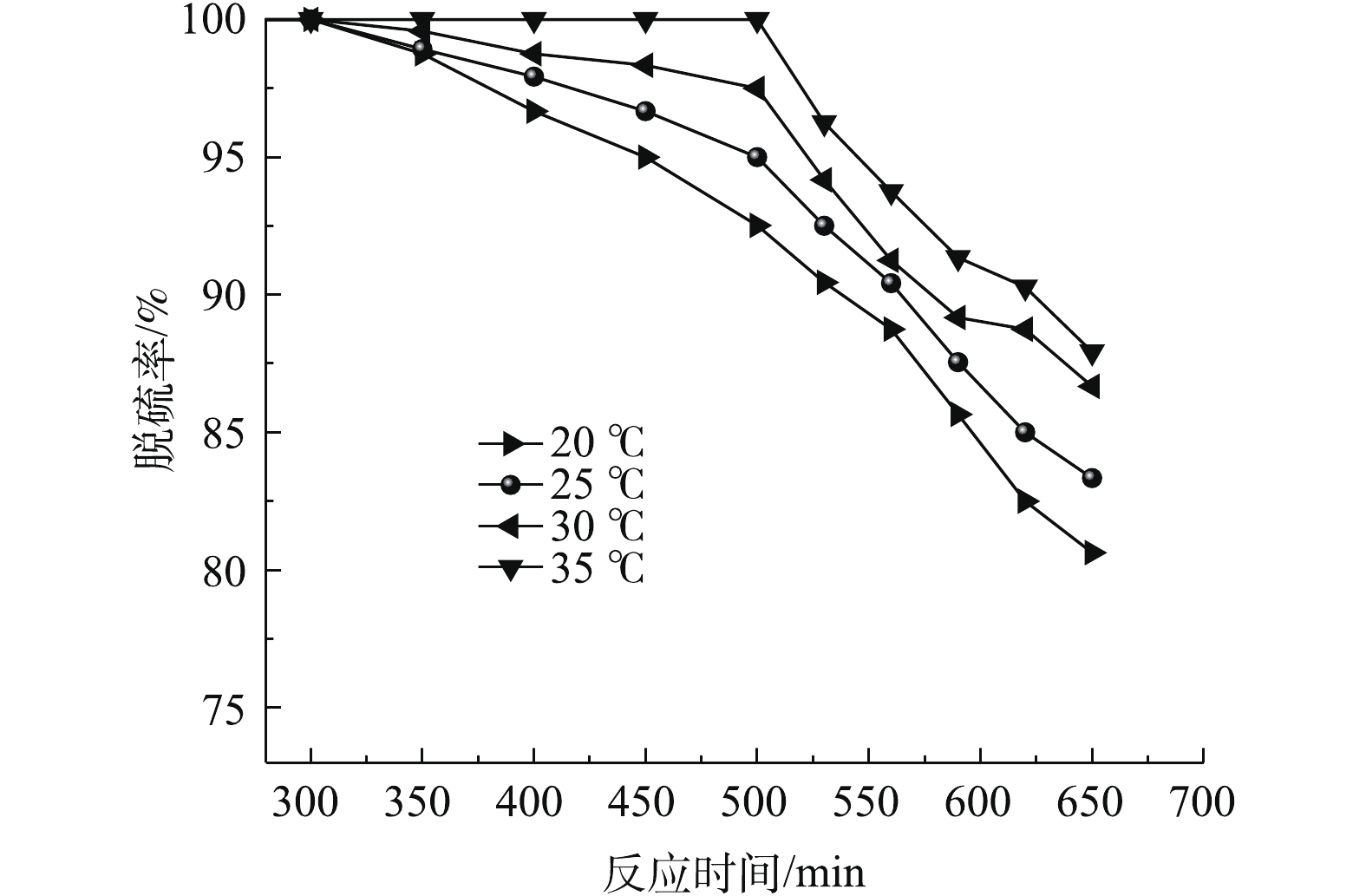
吸收温度对SO2脱除率的影响如图4所示。在进气流量为0.3 L·min−1,固液比为25∶100的实验条件下,考察吸收温度分别为20、25、30和35 ℃时脱硫率随反应时间的变化规律。
随着反应时间的延长,脱硫率随吸收温度的增大而增大。吸收温度对超声波雾化磷矿浆催化氧化SO2的影响主要在于模拟烟气中SO2、O2的液膜溶解过程和SO2液相催化氧化的反应速率。吸收温度升高,磷矿浆脱硫反应的扩散系数增大,超过平均活化能的分子数也相应增加,SO2的液相催化氧化速率加快,从而导致脱硫效率的增加。为得到较好的脱硫效果,选取吸收温度为35 ℃进行实验。
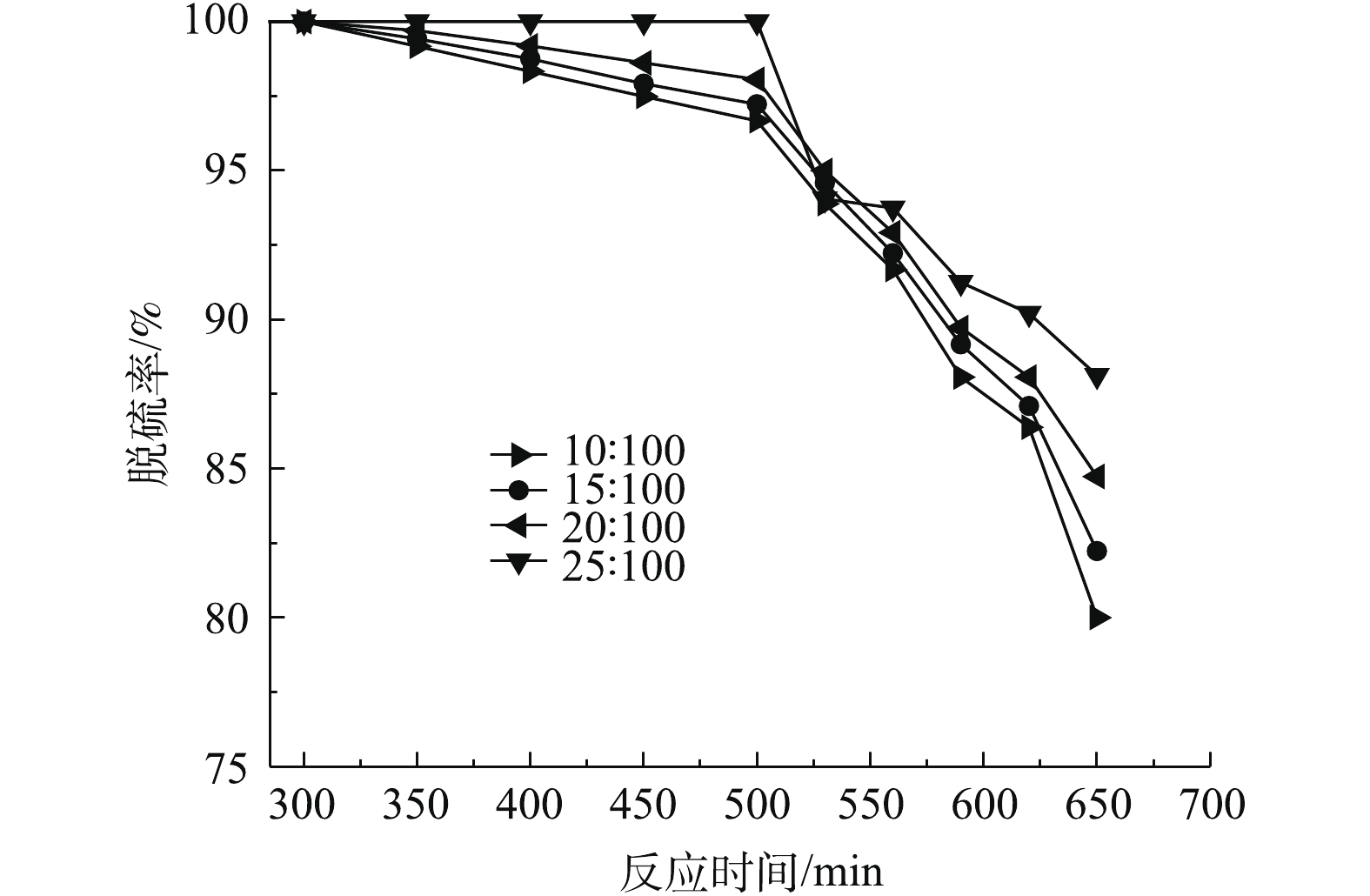
固液比对SO2脱除率的影响如图5所示。在进气流量为0.3 L·min−1,吸收温度为35 ℃的实验条件下,考察固液比分别为10∶100、15∶100、20∶100和25∶100时脱硫率随反应时间的变化规律。
随着固液比的增大,矿浆吸收液中磷矿粉的比例增大,浆液黏度增大,浆液中SO2的扩散速率减慢。吸收液中固体含量的增加有利于提高磷矿粉在溶液中的分散程度,使得雾化液混合均匀,有利于反应的进行,实验选取固液比为25∶100。
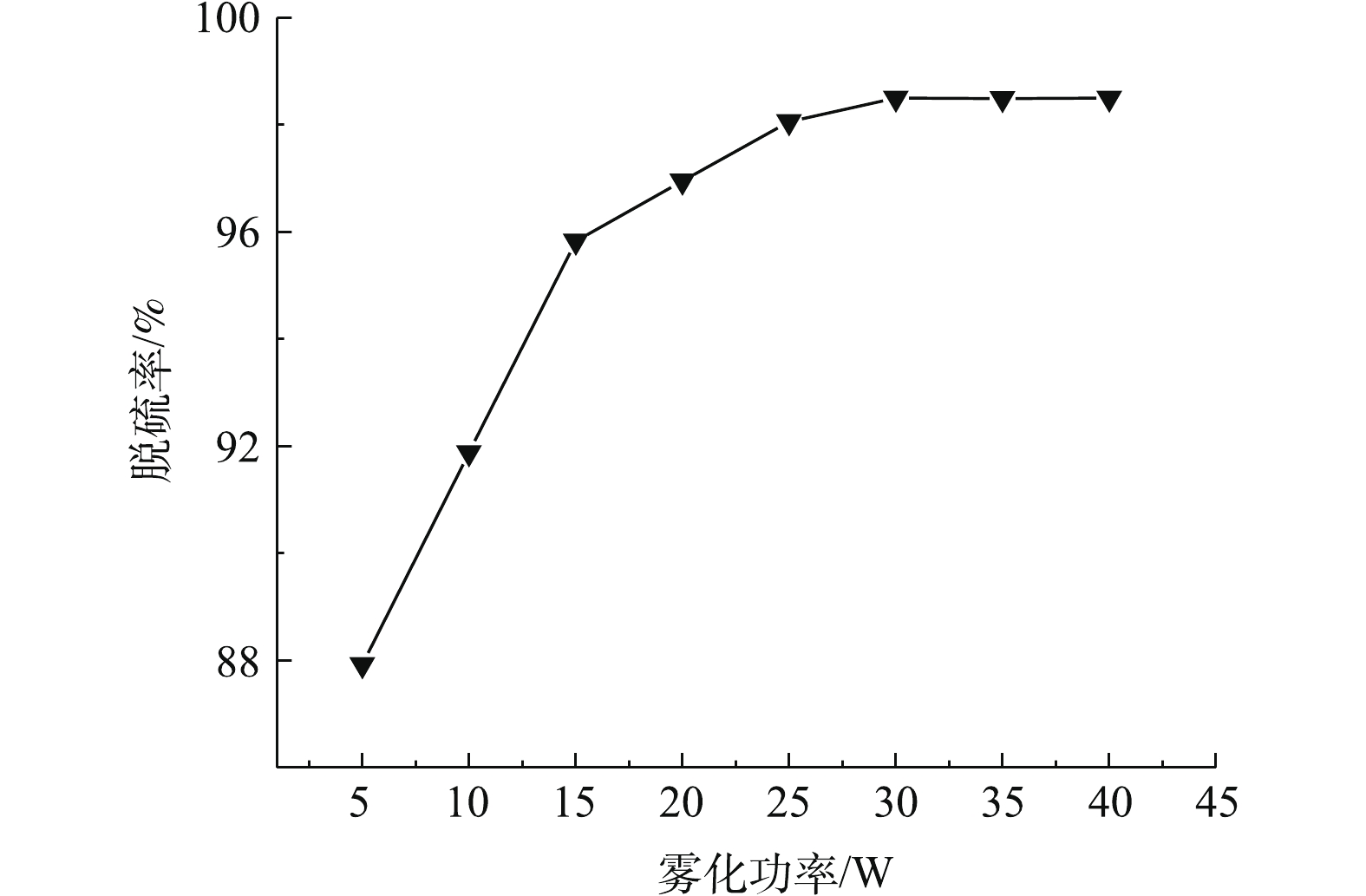
雾化功率对SO2脱除率的影响如图6所示。在吸收温度为35 ℃、固液比为25∶100、进气流量为0.3 L·min−1的实验条件下,考察反应进行240 min时脱硫率随雾化功率的变化规律。
当雾化功率从5 W增大到30 W时,脱硫率从87.9%提高至98.5%。雾化功率增大,超声波雾化器做功越大,超声波对雾滴的空化作用增强,使反应器中超声波雾化后的雾滴量增大,SO2与雾滴发生有效碰撞的概率增大,雾化速率提高,脱硫率随之提高。当雾化功率大于30 W时,脱硫率维持在98%,趋于一个稳定的数值,故实验选取30 W为适宜的雾化功率。
-
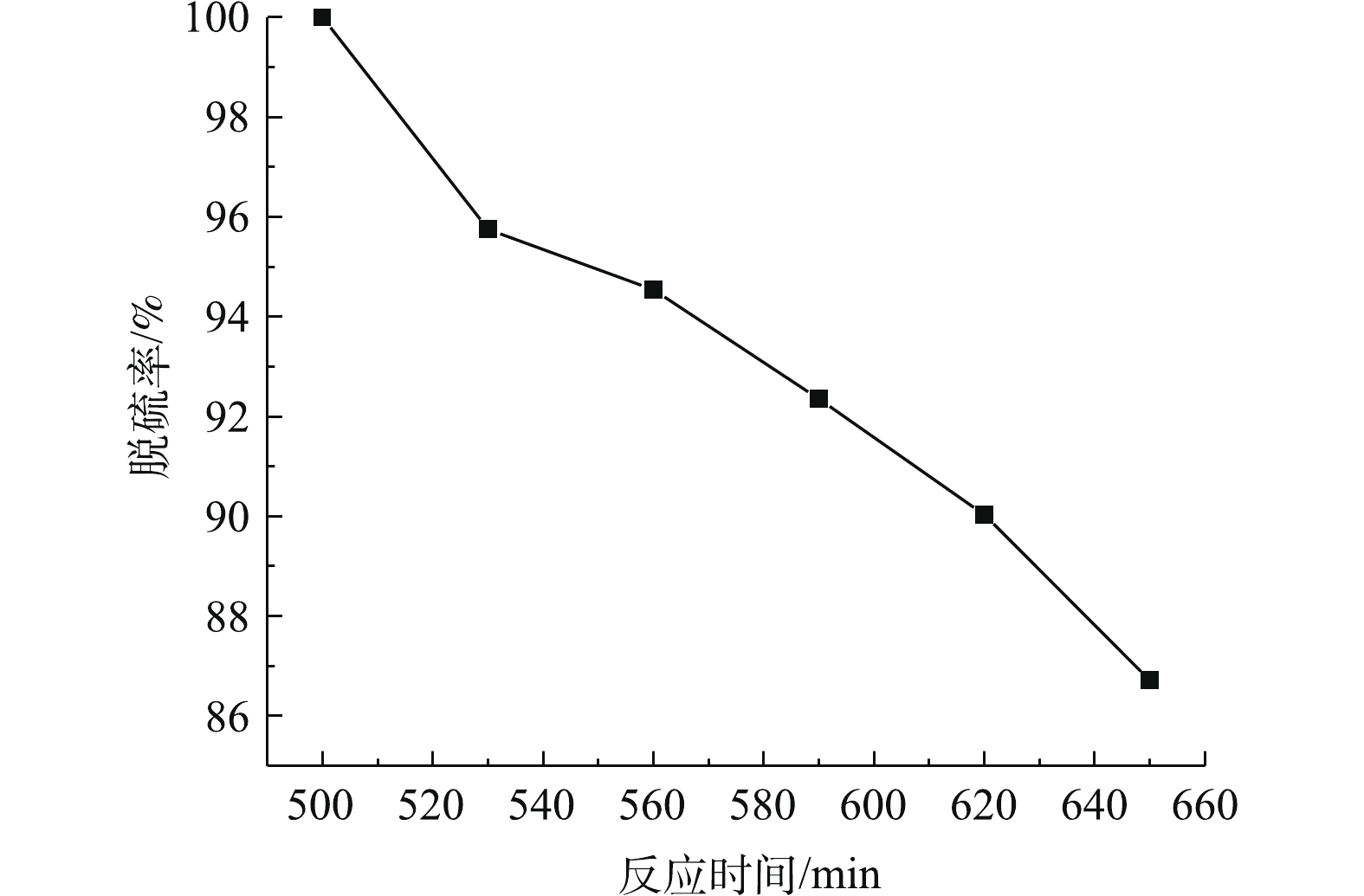
在吸收温度为35 ℃、固液比为25∶100、进气流量为0.3 L·min−1、雾化功率为30 W条件下进行实验。如图7所示,在最佳反应条件下,脱硫率为100%的反应时间持续500 min,脱硫率≥90%的反应时间可持续620 min。与已有报道[38]中没有加超声波雾化作用下磷矿浆催化氧化SO2的实验结果对比,脱硫率维持在100%的反应时间明显延长100 min。实验过程中,随着反应时间的增加,脱硫率逐渐下降。这是因为随着反应的进行,溶液pH逐渐下降,pH的下降降低了SO2在吸收液中的溶解度,从而进一步导致反应过程中脱硫率的下降。
-
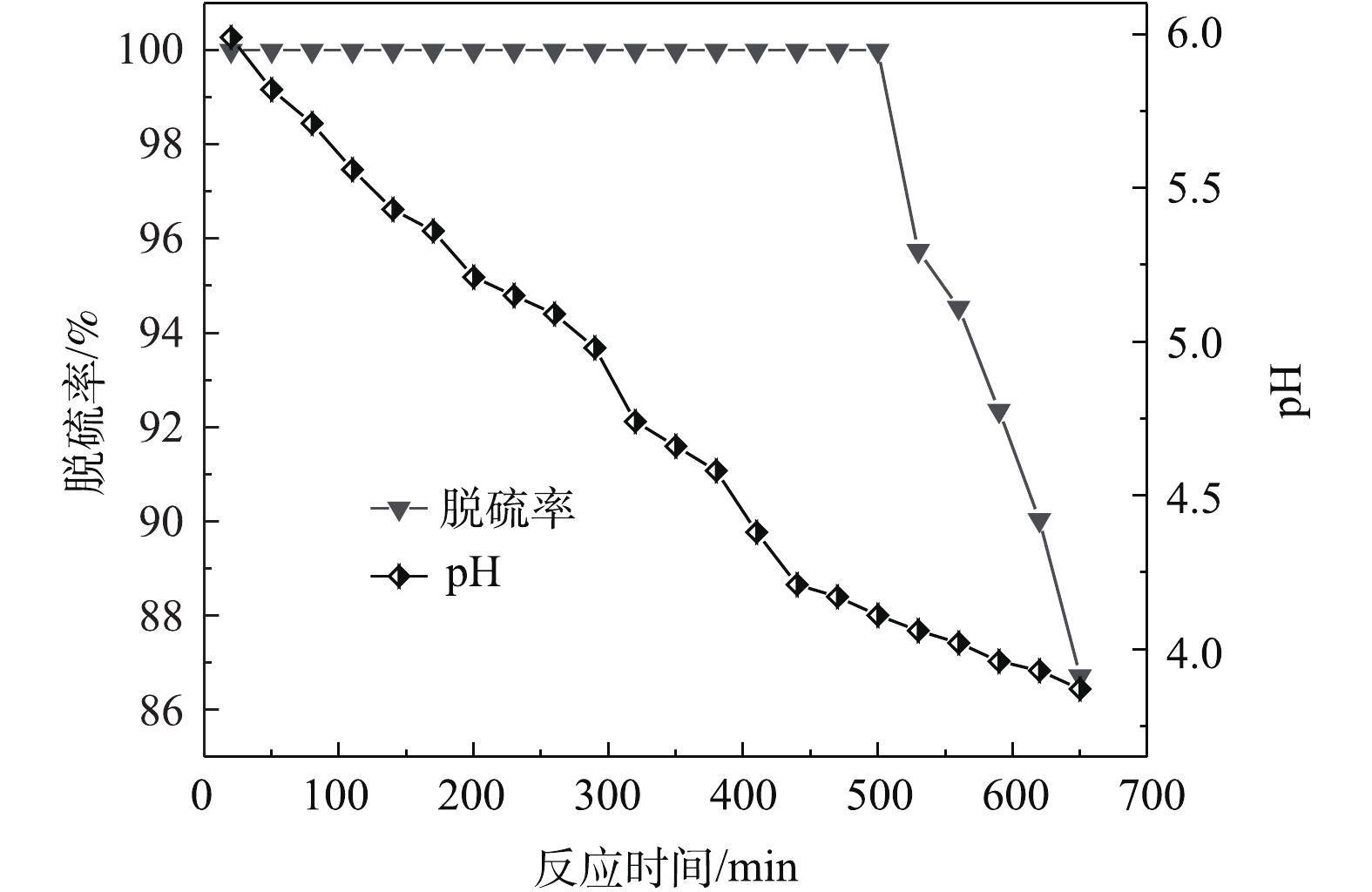
在吸收温度为35 ℃、固液比为25∶100、进气流量为0.3 L·min−1、雾化功率为30 W的最佳条件下进行实验。由图8看出,反应过程中磷矿浆吸收液pH随反应时间的增加而逐渐降低,脱硫率也逐渐下降。这是由于随着反应的进行,不断生成H2SO4,且生成的一部分H2SO4又与磷矿中的CaMg(CO3)2反应生成CO2[25],CO2易溶于水生成H2CO3。另外,磷矿粉中的P2O5能与热水反应生成磷酸,导致吸收液的pH逐渐下降,从而进一步导致了脱硫率的下降。
2.1. 超声波雾化磷矿浆催化氧化SO2单因素实验
2.2. 最佳反应条件下的超声波雾化磷矿浆脱硫效果
2.3. 最佳反应条件下反应脱硫效率及过程中吸收液pH的变化
-
将反应后的磷矿浆进行沉淀、沥水、烘干后得到矿粉,对反应前后的矿粉进行SEM、XRF表征;对反应前后的吸收液进行ICP和IC测定。
-
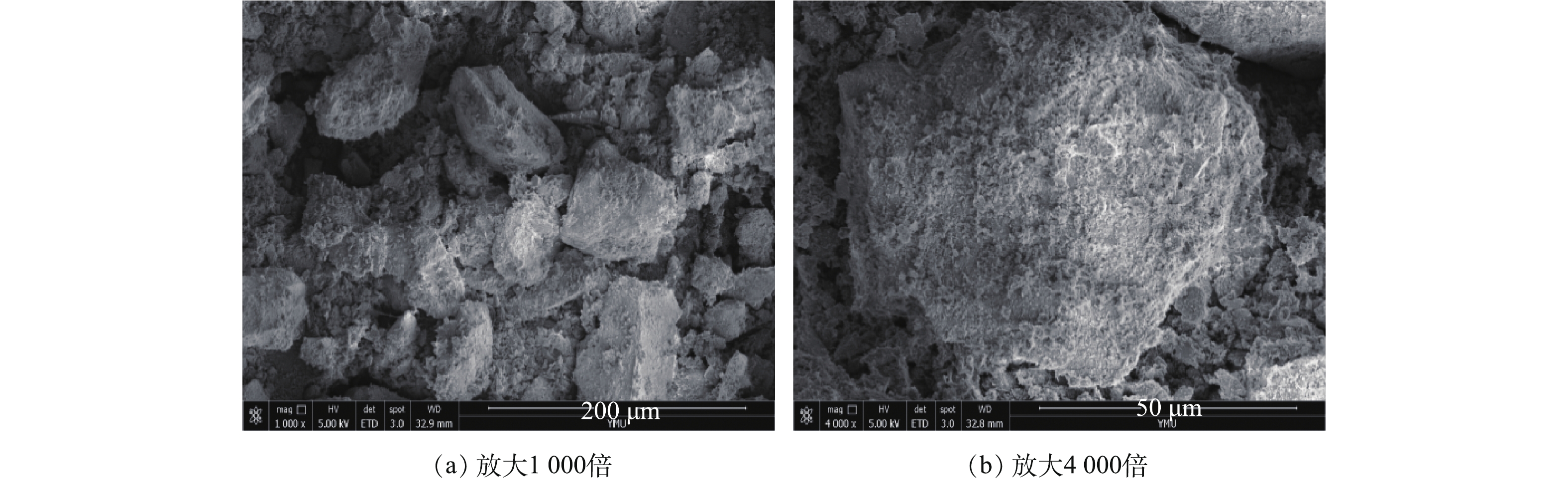
反应前和反应后矿粉的SEM如图9和图10所示。可以看出,磷矿粉由不规则的块状颗粒堆叠而成,反应前矿粉颗粒间距较小,颗粒表面附着一层粗糙的絮状物,与反应前矿粉相比,反应后的矿粉颗粒表面明显光滑。一方面是由于反应过程中矿粉颗粒的溶解;另一方面,在反应过程中,SO2通入磷矿浆中,与H2O和O2反应生成H2SO4,生成的H2SO4与磷矿中的CaMg(CO3)2反应生成了CaSO4,反应方程见式(2)和式(3)。
-
用XRF测反应前后磷矿成分,结果如表3所示。可以看出,CaMg(CO3)2、P2O5、Fe2O3和Al2O3在反应后含量都有所下降。CaMg(CO3)2的减少是由于在反应过程中生成了H2SO4,且生成的一部分H2SO4会与其发生反应;P2O5的减少是由于P2O5能溶于水,与热水反应生成磷酸;Fe2O3和Al2O3的含量减少是由于生成的H2SO4又与Fe2O3和Al2O3反应,反应方程见式(4)~式(8)。
-
分别用ICP、IC对反应前、后的吸收液进行测定,反应前、后吸收液中的离子浓度如表4所示。可以看出,吸收液中Ca2+、Mg2+、Fe3+、
${\rm{SO}}_4^{2 - }$ 的浓度逐渐增大。这是由于实验过程中,随着反应的进行,生成了H2SO4,H2SO4与磷矿粉中的Fe2O3反应,生成了Fe3+;生成的H2SO4与CaMg(CO3)2发生反应,生成了MgSO4和CaSO4;CaSO4微溶于水,导致溶液中Ca2+浓度逐渐增加,MgSO4易溶于水,溶液中Mg2+浓度也会增加。由于${\rm{HSO}}_3^ - $ 和${\rm{SO}}_3^{2 - }$ 的不稳定性,在溶液中易发生氧化,难以在吸收液中检测到${\rm{HSO}}_3^ - $ 和${\rm{SO}}_3^{2 - }$ ,这也是吸收液中${\rm{SO}}_4^{2 - }$ 浓度逐渐增大的原因之一。反应方程见式(9)~式(16)。
3.1. SEM表征
3.2. XRF表征
3.3. 反应前、后吸收液成分的测定
-
1)本研究采用超声波雾化技术将磷矿浆转化为细小雾滴,使模拟烟气与雾化液滴充分接触发生反应,实现净化SO2的效果。与磷矿浆脱硫相比,超声波雾化磷矿浆的脱硫率维持在100%的反应时间增加110 min。
2)超声波雾化磷矿浆催化氧化SO2在很大程度上受吸收温度、固液比、进气流量的影响,它们对脱硫率的影响顺序为R进气流量>R吸收温度>R固液比。进气流量越小,体系脱硫效果越好;吸收温度升高,固液比越大,体系脱硫效率增大。超声波雾化器功率为30 W时脱硫效果最好。随着反应的进行,吸收液pH逐渐降低。在SO2进气浓度为1 500 mg·m−3、氧含量为15%、进气流量为0.3 L·min−1、吸收温度为35 ℃、固液比为25∶100、雾化功率为30 W的最佳条件下,超声波雾化磷矿浆催化氧化SO2脱硫率≥90%的反应时间可维持在620 min以上。
3)从反应前、后磷矿粉的表征结果及吸收液成分的变化可知,随着反应的进行,矿粉表面逐渐变光滑,溶液中Ca2+、Mg2+、Fe3+、
${\rm{SO}}_4^{2 - }$ 浓度逐渐增大。



 下载:
下载: