-
滴滴涕(DDT)是一类具有很强迁移性和生物富集性的持久性有机污染物(POPs)[1],广泛分布于各个国家和地区,甚至通过食物链和食物网富集到人体内,因此早已被列入各国优先控制污染物的名单。
机械化学球磨法通过剪切、磨擦、冲击、挤压等机械力作用,降低反应活化能,从而轻易地诱发化学反应[2-3],相比于传统的焚烧和非焚烧处置技术,其具有操作简单、适用范围广、二次污染少等诸多优点[4]。如今,机械化学球磨法能够实现POPs的高效脱氯,且大部分球磨产物可直接填埋,但高效球磨药剂中存在的少量金属单质仍未得到充分利用。为此,不少学者展开研究。例如,有学者利用机械化学球磨PVC后的产物制氢[5-6];也有学者利用Fe-Ni-SiO2混合药剂球磨降解五氯硝基苯(PCNB)的球磨产物对水相中的4-氯酚实现催化脱氯[7];此外,Cagnetta等[8]利用La2O3药剂球磨处理全氟化合物(PFCs),并利用球磨产物制备发光材料;同时提出利用Bi2O3和La2O3混合药剂球磨溴化持久性有机污染物,以此制备具有催化和电性能的化合物(如铋或镧溴氧化物)[9-10],从而探索出机械化学球磨法处理溴化持久性有机污染物“从废物到材料”的新方法[11-12]。
Cagnetta等[13]发现,行星球磨仪降解污染物的工艺参数中物料比(m球磨药剂:m污染物)非常高,本团队前期研究[14—15]同样发现,Fe-Zn药剂降解DDTs所用的物料比也很高,大约为10∶1到50∶1(m药剂∶m滴滴涕)。过量的药剂易造成浪费和二次污染,为此本研究拟对铁锌双金属球磨产物开展资源化利用,机械化学球磨降解高浓度DDTs污染土壤后的球磨产物可对水相中四氯化碳(CCl4)的有效去除。
1994年,Gillham等[16]发现零价铁粉可还原降解水相中氯代烃;近年来,零价铁降解水相中的氯代烃的研究已发展至纳米双金属领域[17]。此外,Tseng等[18]发现使用活性炭和零价铁的复合材料能够更加高效地去除水相中三氯乙烯。因此,本研究以CCl4作为重质非水相液体(DNAPL)代表成分,选择Fe-Zn双金属球磨处理高浓度DDTs污染土壤后的球磨产物作为修复材料,发现球磨产物中剩余的铁粉以及球磨过程产生的石墨和不定型碳可以成功去除水相中的CCl4。
目前,国内的机械化学球磨技术仍处于实验室阶段,而国外已经实现商用。例如,德国Tribochem公司于1996年在挪威利用机械化学法处理了多氯联苯(PCBs)污染土壤;于1998年在德国处理了PCBs变压器油;新西兰EDL公司更是在近年来研发了机械化学脱卤(MCD)工艺技术[19],成功在多国修复了多个POPs高浓度污染场地,处理效率达到15 t·h−1,修复效果达到新西兰相关用地标准[20]。因此,当我国的机械化学球磨技术完全投入到规模化应用后,球磨产物的安全处置和资源化利用必定是值得考虑的问题。
本文主要研究了球磨产物对水相中CCl4的降解与吸附,以此探索了机械化学球磨产物再利用的途径,为处理机械化学技术商业化应用后产生的大量残渣提供了理论基础和研究方向。
-
DDTs污染土壤样品:采自华北地区某化工厂,其中,6种单体o, p’-DDE、p, p’-DDE、o, p’-DDD、p, p’-DDD、o, p’-DDT、p, p’-DDT的质量浓度如表1。
实验所用药剂与仪器:10—100 μm零价铁粉和锌粉;分析纯正己烷、丙酮和四氯化碳;色谱纯正己烷、二氯甲烷和甲醇;PM400行星球磨仪;超声仪;HGC-12A 氮吹仪;四通道色谱净化仪。
-
本团队之前的研究[14]发现,国产行星式球磨仪降解高浓度DDTs污染土壤的最佳工艺参数为球料比27∶1,转速500 r·min−1,铁锌比7∶3,物料比2∶1。本研究使用的球磨仪是德国Retsch公司生产的PM 400型行星式球磨机,公转∶自转=1∶−3,与前期研究所用国产球磨仪不同(公转∶自转=1∶−2)。因此,在球料、物料和铁锌比例等工艺条件不变的情况下,针对PM 400行星球磨仪,设置不同转速,测定土壤中DDTs的去除率随球磨时间的变化。
称取10 g DDTs污染土壤置于250 mL球磨罐,称取3.5 g铁粉和1.5 g锌粉加入球磨罐,使用13颗磨球,磨球总重约400 g,球料比27∶1。分别设置球磨仪转速为300、250、200、150 r·min−1,运行间隔15 min(每运行15 min后停止15 min),在0—6 h内设置采样点,每组实验均设置4个重复。
-
首先筛选球磨产物作为水相中CCl4脱氯剂的最佳用量。以甲醇为溶剂,制备浓度为4000 mg·L−1的CCl4-CH3OH母液,分别取0.1、0.2、0.3、0.4、0.5、0.8、1.0、1.5 g的球磨产物加入到40 mL棕色试剂瓶中并缓慢注入去离子水至满瓶。使用微量注射器移取CCl4-CH3OH母液至各瓶中,保证每瓶溶液中CCl4浓度为10 mg·L−1,密封后放置往复式变速振荡器振荡24 h。
本实验的液相反应体系全程在40 mL棕色vial瓶中进行。首先在棕色试剂瓶中添加定量的球磨产物,然后缓慢注入去离子水至满瓶,用气相进样针移取一定量CCl4-CH3OH母液至棕色试剂瓶,迅速盖上并拧紧含有聚四氟乙烯薄膜的盖子,在瓶体周身包裹数层保鲜膜。取样分析前,首先在待上机的vial瓶中装入定量的去离子水,反应完全后打开反应瓶,通过微量取样针迅速取样至待上机的vial瓶,拧紧聚四氟乙烯薄膜盖后移至配有吹扫捕集器的GC-MS,进行检测分析。整个反应体系及上机检测过程近乎不存在挥发损失,而前期注射污染溶液和后期取样的过程也通过气相进样针进行,最大程度地减少CCl4及其产物的挥发。
为探究球磨产物去除CCl4的机理,使用脱氯剂用量为1 g,分别为在0、1、2、3、5、6、8、10、12、24、48、72 h等时间点取样,利用GC-MS测定其中间产物。
为考察球磨产物对水相中的CCl4吸附情况,使用无药剂添加的污染土壤和加入SiO2球磨助剂的污染土壤进行球磨,并取其球磨产物开展水相中CCl4吸附实验。
-
DDT的6种单体:取0.01 g球磨产物置于20 mL的正己烷-丙酮(等体积)混合液,超声提取30 min,经低速离心机(2000 r·min−1)离心10 min,取上清液经漏斗过滤于茄形瓶中,重复3次。后将滤液通过旋转蒸发仪浓缩至1—2 mL,移入四通道色谱净化仪去除滤液中的杂质[14—15]。
装填四通道色谱净化仪的净化柱后,设置两侧压力分别为167 kPa和70 kPa。用2 mL正己烷-二氯甲烷的混合液(等体积)润洗4次。将上述浓缩液移入净化柱净化9次,收集于尾形瓶。最后将尾形瓶内溶液通过旋转蒸发仪浓缩,浓缩样品经氮吹仪吹干,用色谱纯正己烷定容至1 mL,稀释后上机[14—15]。
CCl4:取200 μL污染水样,用600 μL正己烷(色谱纯)涡旋萃取3 min后,准确移取200 μL上清液,与800 μL正己烷(色谱纯)混合均匀后上机。
-
DDTs定量分析:采用Agilent 7890B 型气相色谱仪。色谱柱型号:DB-35MS。升温程序:初始80 ℃,以50 ℃·min−1升至210 ℃,再以1 ℃·min−1升至220 ℃,保留1 min,最后以2 ℃·min−1升至230 ℃,保留10 min。载气(高纯氮)流量0.8 mL·min−1;进样体积1 µL,分流(10:1)进样,进样口温度200 ℃,检测器温度300 ℃[14—15]。
CCl4及其降解产物的定性定量分析:采用GC-MS。色谱柱型号DB-VRX;载气气流1.2 mL·min−1;离子源温度230 ℃,四极杆温度150 ℃。升温程序:40 ℃保持2 min,以20 ℃·min−1升至250 ℃,保持1 min;进样口温度230 ℃。
拉曼光谱分析:采用DXR532型拉曼光谱仪。配置532 nm和780 nm双氦氖激光器系统,拉曼位移范围: 100—3500 cm−1。
比表面积及孔隙度分析:采用NOVAtouch LX1型全自动比表面积及孔隙度分析仪。
-
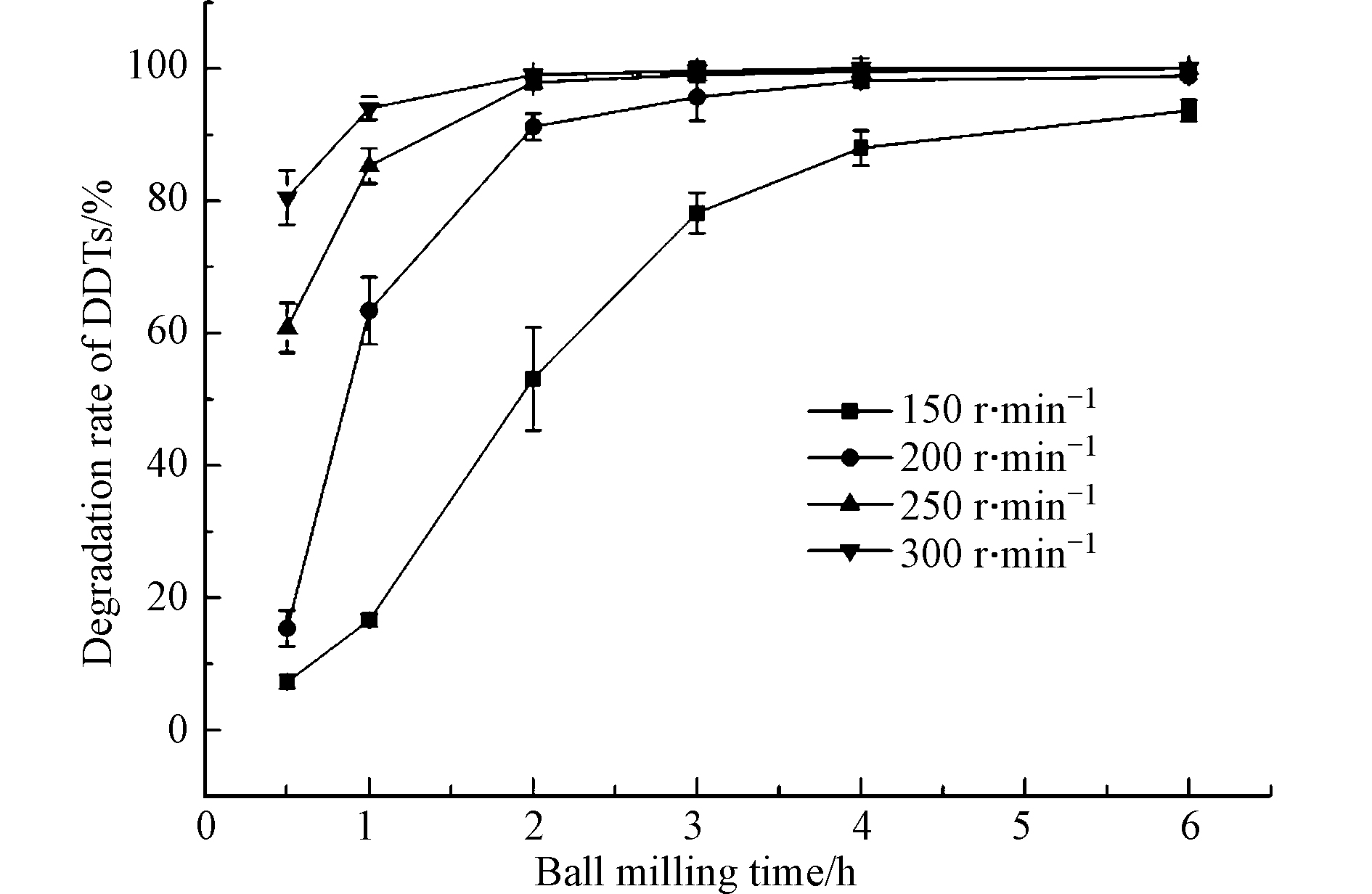
不同转速下,土壤中DDTs去除率随时间的变化如图1所示,结果表明,DDTs去除率随着转速的提高而增加。本研究团队之前的研究[14—15]也表明,转速的提升能够增加磨球碰撞频率,并提高磨球碰撞的瞬时速度,从而增加体系的碰撞能量。对比200、250、300 r·min−1发现,250 r·min−1和300 r·min−1的最高去除率相似且都比200 r·min−1更高,都在2 h内达到95%以上。当球磨转速高于250 r·min−1后,转速增加对DDTs去除率的提升已不显著。此外,更高的转速会带来更多的能量消耗,因此本研究设置250 r·min−1作为机械化学球磨降解土壤DDTs实验的最佳转速。张冬格等[21]在DEM软件模拟中发现,在250 r·min−1和300 r·min−1的同等转速情况下,球料比(35:1、30:1和25:1)的改变对法向碰撞速率影响较小,当球磨转速大于250 r·min−1后,罐内碰撞能量的增加逐渐缓慢,该实验结论与本研究结果相似。因此,在兼顾DDTs去除率和经济效益的角度综合分析,本研究中球磨法处理土壤DDTs时的转速均设置为250 r·min−1。
-
本团队之前的研究发现[21],机械化学球磨降解土壤中DDTs所添加的零价铁相对于土壤是过量的,即球磨反应降解污染土壤后的球磨产物中仍有少量铁粉的剩余,因此可以利用球磨产物中剩余的零价铁降解水相中的CCl4。本实验的球磨药剂为铁锌双金属,因此球磨产物中的Zn元素的作用及存在形式如何,也是值得考虑的问题。分别通过XRD和XPS检测发现球磨4 h后,锌单质全部参与机械化学反应,产物中锌元素均以Zn2+的形式存在[22—23]。因此,在相同球磨工艺参数下,本研究的水相中CCl4脱氯主要依靠产物中剩余零价铁的还原性,无锌单质或锌离子参与还原降解反应。另外,本研究使用锌单质1.5 g(0.023 mol),在机械化学球磨作用下全部转为Zn2+溶于40 mL棕色试剂瓶中,即CCl4降解反应体系初期的Zn2+浓度为0.575 mol·L−1。根据溶度积常数表可知,常温下
${K\rm{s}\rm{p}}_{{\rm{Zn}\left(\rm{OH}\right)}_{2}}= $ $ 1.2\times {10}^{-17} $ 。由${K{\rm{sp}}_{{\rm{Zn}\left(\rm{OH}\right)}_{2}}={C}_{{\rm{Z}\rm}}^{2+}}\times {C}^{2}_{{\rm{O}\rm{H}}^{-}}$ 和$ {C}_{{\rm{Z}\rm{n}}^{2+}}=0.575 $ mol·L−1,可计算Zn(OH)2开始沉淀时的pH为5.66;由$ {K\rm{s}\rm{p}}_{{\rm{Zn}\left(\rm{OH}\right)}_{2}}={C}_{{\rm{Z}\rm{n}}^{2+}}\times {C}^{2}_{\rm{OH}^{-}} $ 和$ {C}_{{\rm{Z}\rm{n}}^{2+}}={10}^{-6} $ mol·L−1,可计算Zn(OH)2沉淀完全时的pH为7.72;由${Ksp}_{\rm{Zn\left(OH\right)}_{2}}= $ $ {C}_{{\rm{Z}\rm{n}\left(\rm{O}\rm{H}\right)}_{4}^{2-}}/{C}^{2}_{\rm{OH}^{-}} $ 和$ {C}_{{{\rm{Zn}}\left({\rm{OH}}\right)}_{4}^{2-}}={10}^{-6} $ mol·L−1,可计算Zn(OH)2沉淀开始溶解时的pH=9.46。因此,Zn(OH)2沉淀完全的pH值范围为7.72—9.46。本实验反应完全后,多次测量pH取平均值的结果为8.78,可知溶液中的Zn2+均以Zn(OH)2沉淀的形式存在。因此,本研究接下来主要讨论零价铁对水相中CCl4的降解与吸附,暂不考虑锌元素在水相中的溶出。球磨产物投加量对水相中CCl4去除率的影响如图2。当球磨产物与CCl4反应24 h后,在一定范围内,球磨产物投加量的增加能够显著提高水相中CCl4的去除率;当球磨产物的投加量超过7.5 g·L−1后,水相中CCl4的去除率存在明显上升;当球磨产物投加量超过20 g·L−1后,经过24 h充分反应,水相中CCl4能够被全部去除。因此,为保证反应的充分进行,本研究选择25 g·L−1球磨产物的投加量开展试验,接下来研究反应时间对水相中CCl4去除率的影响。
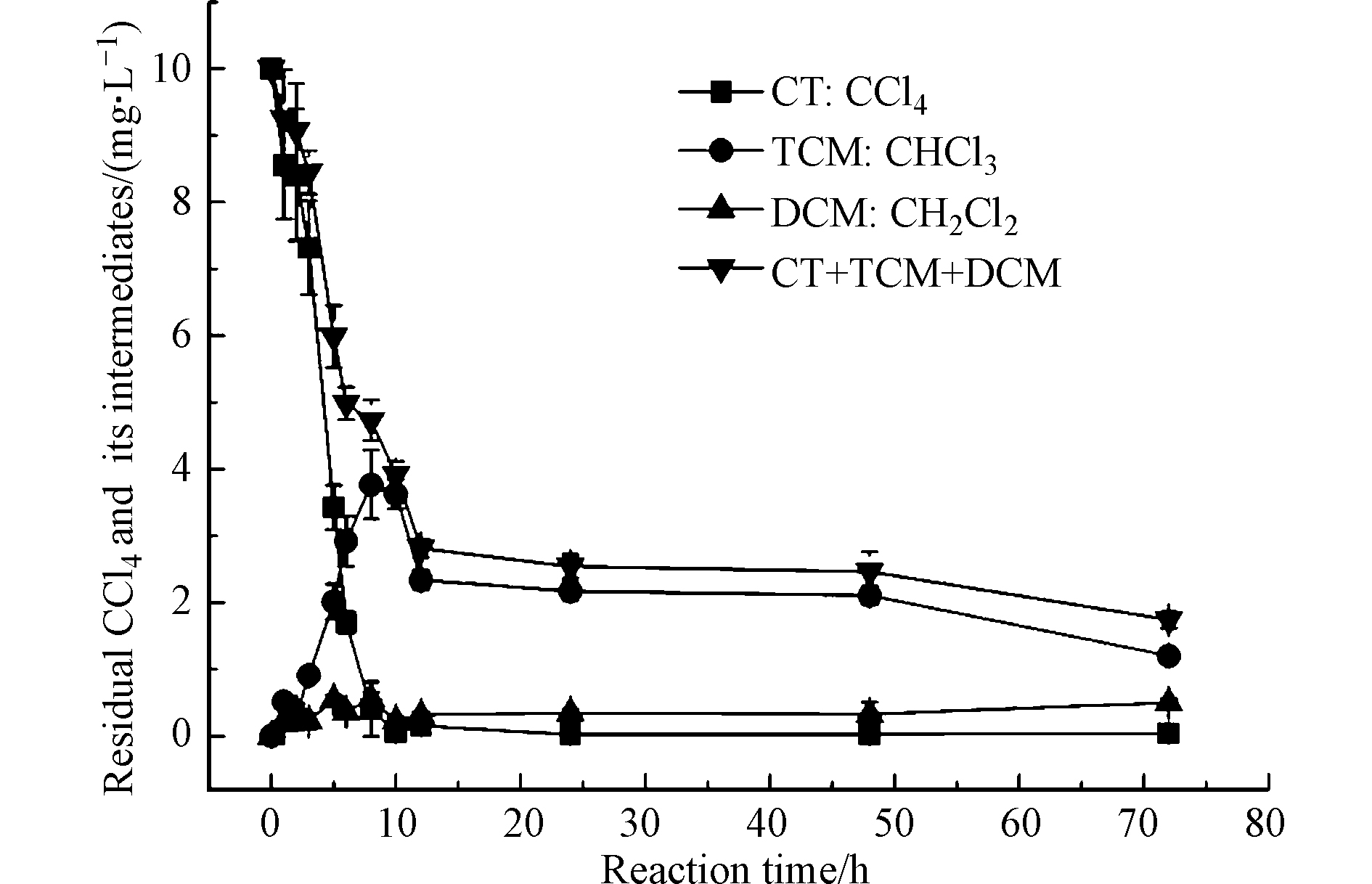
如图3所示,CCl4在前10 h内快速降解,12 h后达到平衡且无法检出。随着CCl4的降解,三氯甲烷逐渐生成,且二氯甲烷浓度缓慢上升,但氯甲烷一直未检出。由此可见,CCl4在球磨产物中剩余零价铁的还原作用下发生了脱氯反应,这与Matheson等[24]利用零价铁粉降解CCl4发现降解产物为氯仿和二氯化碳的结果相似,均为逐级脱氯,且还原到二氯甲烷后再难以继续脱氯。其中,氯仿的产生表明CCl4存在大量降解,而二氯甲烷的少量存在与氯甲烷的未检出表明氯仿并未有大量的降解。
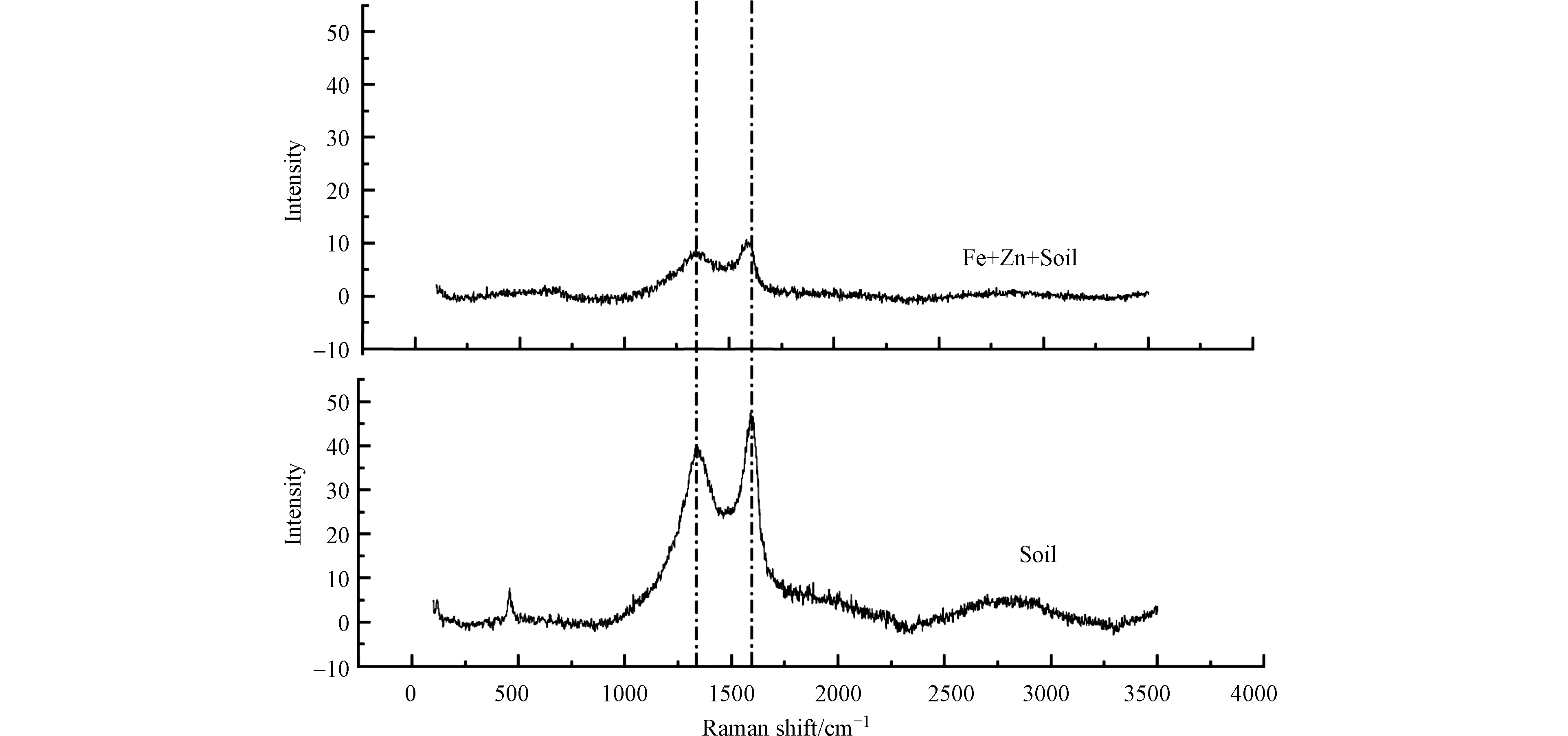
张望[25]分别使用同比例的Fe+SiO2+C(石墨)、Fe+SiO2+PCNB、Fe+SiO2进行球磨,发现上述三者球磨产物的脱氯性能为Fe+SiO2+C(石墨)> Fe+SiO2+PCNB> Fe+SiO2,并利用XRD发现机械化学球磨能够生成部分Fe-C合金,在水相中形成微电极促进脱氯反应的进行。本研究通过拉曼光谱对实验中Fe+Zn+Soil的球磨产物定性发现土壤也同样发生了碳化,如图4所示。
从图4可以看出,添加球磨药剂的土壤与纯土壤经过机械化学球磨后都发生碳化,其中1320 cm−1处为无定形碳峰,1580 cm−1处为石墨峰。这与之前的研究[25—26]结果一致。同时,添加铁锌双金属球磨药剂的污染土壤经过球磨后的拉曼峰相比于球磨纯土壤,变得更加平缓单一,这与球磨过程中的反应激烈程度有关。金属药剂活泼性较高且质地较硬,反应较为激烈,对土壤中有机物组分的降解更为彻底,因此也会产生更多的官能团。根据以往零价铁脱氯的研究[24]可知,零价铁脱氯主要存在3种途径:
1)氢解:铁表面电子转移给有机物发生脱氯反应
2)还原消除:零价铁腐蚀产生的Fe2+的还原脱氯
3)加氢还原:厌氧状态下,H2O作为电子受体与零价铁反应生成H2,H2还原氯代有机物。
研究表明[24, 27—28],在没有催化剂的作用下,生成的H2难以发生还原脱氯反应。因此,本实验中CCl4的脱氯机制主要是零价铁的氢解、Fe2+还原消除。此外,本实验中污染土壤球磨后发生碳化且球磨产物中存在零价铁剩余,在机械力的高强度作用下,也可能如张望[25]之前的研究所述,生成少量的Fe-C合金,从而在水溶液中能够形成微电极加速零价铁对水中氯代烃的降解。
此外,从文献[29—30]可知,零价铁降解CCl4是一个逐级脱氯至二氯甲烷且氯代烃总量守恒的过程。但是本实验中的图3表明,CCl4及其降解产物的总量一直在降低,并未达到守恒。因此,可以推断本实验中球磨产物对CCl4存在吸附作用。
-
分别对纯土壤、添加Fe-Zn双金属的土壤、添加SiO2的土壤的3种球磨产物进行比表面积和孔隙度分析,结果见表2和图5。
由表2可知,3种球磨产物的比表面积和孔隙度相比于同类吸附剂要小很多,其中纯土壤球磨之后的产物的比表面积和孔隙度是最发达的,铁锌双金属作为添加剂的土壤球磨后的产物孔隙度是最不发达,三者介孔的孔径类似,都是8 nm左右。从图5也可以看出,三者的吸脱附曲线属于Ⅱ类,即其物理吸附的模型为非孔型吸附,表面吸附作用力较弱。而从图3的结果看来,铁锌双金属作为添加剂的土壤球磨产物对水中氯代烃的吸附还是较大的。因此,本实验不能单纯考虑发达孔隙的吸附作用,也应考虑3种球磨产物对水相中氯代烃是否存在化学键的吸附。从文献中可知,乔本志等[31]利用1HNMR发现氯仿与丙酮、环己酮及甲基丙烯酸甲酯之间都能形成氢键,并测得其氢键缔合常数,而氯仿又是本实验中CCl4降解生成的关键性中间产物,极有可能与球磨产物中的某些含氧官能团形成氢键。
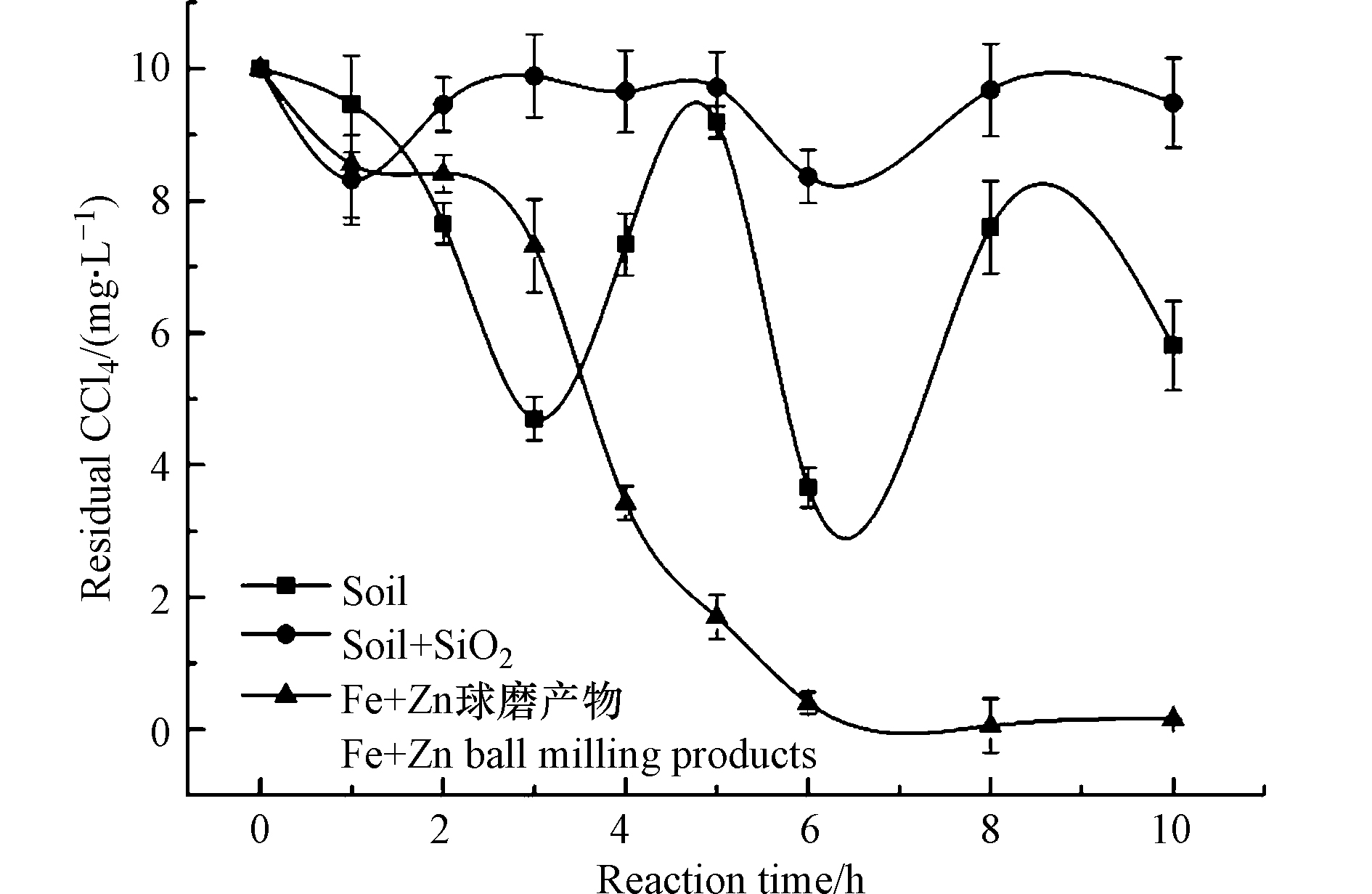
为考察球磨产物中对水中氯代烃是否存在成键吸附,以纯土壤和添加SiO2的土壤球磨产物作为对照,研究其对水中四氯化碳的吸附,实验结果如下图6所示。
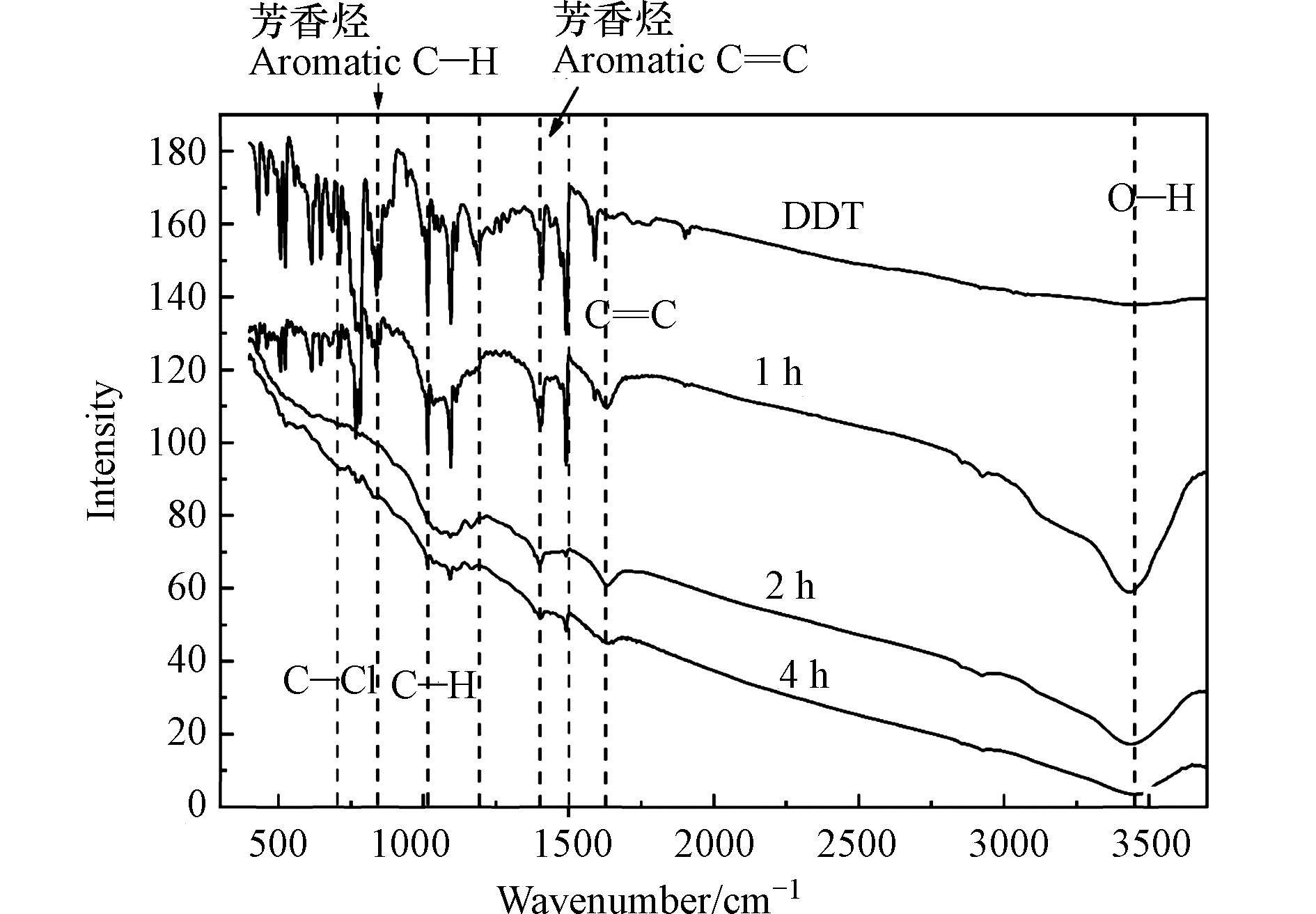
由图6可知,纯污染土壤的球磨产物对CCl4的吸附极不稳定,在旋转混匀过程中很容易脱附,而SiO2作为添加剂的污染土壤球磨产物对四氯化碳几乎没有吸附,这也与表2中球磨产物比表面积和孔隙度不发达的结论相一致。由此可见,SiO2也仅是因为质地较硬,在球磨过程中摩擦剧烈使得土壤被球磨得更加粉碎,从而减小了产物的比表面积和孔隙度。因此,上述两种球磨产物(无金属药剂)对于水中的CCl4的吸附属于依靠土壤孔隙的物理吸附,极易脱附,而铁锌双金属球磨产物对四氯化碳及其产物的吸附主要依靠其中的金属反应而非土壤吸附。张望[25]和本团队之前的研究[22]通过红外光谱(如图7)分析发现凡是有零价铁参与的球磨反应,因为零价金属的活泼性和铁粉质地较硬使得有机物在球磨过程中降解较为彻底,相比于其他金属氧化物而言,能够将有机物中的苯环裂解生成小分子化合物,在此过程中就产生了更多的官能团和自由基。
本团队之前的研究[14]发现,将干净土壤作为球磨药剂对DDT纯化学品进行球磨,4 h能达到57%的去除率,而将高浓度DDT污染土壤单独球磨8 h后,也能达到85%的去除率,然后两者球磨产物中DDE、DDD的含量很高。就像添加CaO球磨药剂一样,球磨纯土壤仅仅是在球磨过程对DDT简单地脱氯化氢形成DDE,而未能彻底破坏其苯环结构。而Fe-Zn双金属由于性质活泼且质地较硬,对污染物的降解较为彻底。本研究通过取不同时间点DDT化学品的球磨产物,测其红外光谱发现,随球磨时间增长,脂肪烃和芳香烃上的碳氢、碳氯等键逐渐断裂,仅有3300 cm−1左右的羟基官能团逐渐增多。由此可见,DDTs污染物在苯环裂解的同时产生了更多的含氧官能团,而这些含氧官能团(主要为羟基)能够与CCl4的主要降解产物(氯仿)形成微弱的氢键,从而增强球磨产物对水中氯仿的吸附,这与图3中氯仿含量先升后降相吻合,也与乔本志等[31]的研究成果相似。因此,Fe-Zn双金属的球磨产物并非直接对水中CCl4进行吸附,而是对剩余零价铁降解CCl4生成的氯仿进行吸附。
-
(1)铁锌双金属球磨产物处理水相中四氯化碳的反应,存在逐级脱氯和吸附两个过程。
(2)铁锌双金属球磨产物中剩余的零价铁能够对水中四氯化碳进行逐级脱氯,且主要脱氯至二氯甲烷。
(3)铁锌双金属球磨处理DDTs污染土壤的过程产生一些含氧官能团(主要为羟基),能够与水中极性较大的氯仿形成微弱的氢键,发生吸附。
Fe-Zn双金属球磨法处置污染土壤后的球磨产物去除水中四氯化碳
Removal of carbon tetrachloride in water by ball milling products after disposal of contaminated soil with Fe-Zn bimetal ball milling
-
摘要: 机械化学球磨是一种高效处理POPs污染土壤的非焚烧处置方式,具有操作简易性和广泛适用性等独特优势。当机械化学球磨法投入到实际污染场地的土壤修复工程,产生的大量球磨产物如何处置是值得研究的问题。根据现有文献和本团队之前的研究发现,添加的球磨药剂(如铁、锌和氧化钙等)总是过量的。由于零价铁去除水相中氯代烃的研究较为成熟,因此本研究拟利用球磨产物中剩余的零价铁去除水中CCl4,一方面发现球磨产物中的剩余零价铁能对CCl4逐级脱氯至二氯甲烷;另一方面对零价铁降解CCl4的中间产物分析,发现CCl4降解产物的总量并不守恒,存在吸附现象。因此,本研究以球磨高浓度DDTs污染土壤和添加SiO2的高浓度污染土壤的球磨产物作为对照,考察铁锌双金属球磨产物对水中CCl4及其降解产物的吸附情况,研究发现铁锌双金属在机械化学球磨过程中反应较为激烈,从而产生了更多的含氧官能团能(主要为羟基)与水相中强极性的氯仿形成微弱氢键,发生吸附。Abstract: Mechanochemical ball milling is an efficient non-incineration disposal method with unique advantages such as easy operation and wide applicability. When the mechanochemical ball milling is put into the actual contaminated site, how to dispose of the ball milling products will be a problem worthy of study. According to the existing literature and previous research, the added ball milling agents (such as iron, zinc, calcium oxide, etc.) are always in excess. Due to the large amount of research on the removal of chlorinated hydrocarbons in aqueous phase by zero valent iron (ZVI), this experiment mainly used the remaining ZVI in the ball milling product to remove CCl4 in aqueous phase. On one hand, it was found that the residual ZVI in ball milling products can dechlorinate CCl4 to dichloromethane step by step; on the other hand, intermediate product of CCl4 was analyzed, and it was found that the total amount of degradation products of CCl4 was not conserved and there was adsorption. Therefore, in this study, the contaminated soil without reagent and contaminated soil with added SiO2 were used as controls to investigate the adsorption of Fe-Zn bimetal ball milling products on CCl4 and its by-products in water. At the same time, it was found that the Fe-Zn bimetal reaction was more intense during the mechanochemical ball milling, and the degradation of DDTs in contaminated soil was more thorough. As a result, more oxygen functionality (hydroxyl) was generated and weak hydrogen bonds were formed with strong polar chloroform in the aqueous phase, and adsorption occured.
-

-
表 1 DDT污染土壤中各衍生物的浓度
Table 1. The concentration of derivative in DDT contaminated soil
单体
Monomerp, p’-DDE o, p’-DDE o, p’-DDD p, p’-DDD o, p’-DDT p, p’-DDT 合计
Total含量/(mg·kg−1) 1451.04 1224.42 205.71 0 3913.47 782.62 7577.27 表 2 3种球磨产物的比表面积及孔隙度
Table 2. Specific surface area and porosity of the three ball milling products
指标Index 土壤 Soil Fe+Zn+Soil SiO2+Soil as, BET/(m2·g−1) 23.70 10.74 35.28 Vp /(cm3·g−1) 0.04 0.02 0.04 Mean pore diameter /nm 8.12 8.93 8.67 -
[1] 栾晓琳, 乔田峰, 吕敏, 等. 近百年来大辽河口潮间带中滴滴涕(DDTs)的沉积记录及其对人类活动的响应 [J]. 环境化学, 2020, 39(1): 119-127. doi: 10.7524/j.issn.0254-6108.2019043001 LUAN X L, QIAO T F, LV M, et al. Sediment records of DDTs in intertidal sediment core of Daliao River Estuary and their respones to anthropogenic activities in the past century [J]. Environmental Chemistry, 2020, 39(1): 119-127(in Chinese). doi: 10.7524/j.issn.0254-6108.2019043001
[2] 李冷, 曾宪滨. 粉碎机械力化学的进展及其在材料开发中的应用 [J]. 武汉工业大学学报, 1993, 15(1): 23-26. LI L, ZENG X B. Progress of mechanochemistry in comminution and its application in material development [J]. Journal of Wuhan University of Technology, 1993, 15(1): 23-26(in Chinese).
[3] 杨南如. 机械力化学过程及效应(Ⅰ)——机械力化学效应 [J]. 建筑材料学报, 2000, 3(1): 26-33. YANG N R. Mechanochemical process and effect (Ⅰ) - mechanochemical effect [J]. Journal of Buliding and Materials, 2000, 3(1): 26-33(in Chinese).
[4] 陈志良, 陆胜勇, 毛琼晶, 等. 水平式球磨机用于POPs机械化学处置的能量传递 [J]. 环境化学, 2016, 35(10): 2134-2145. doi: 10.7524/j.issn.0254-6108.2016.10.2016031603 CHEN Z L, LU S Y, MAO Q J, et al. Energy transfer in mechanochemical treatment of POPs in a horizontal ball mill [J]. Environmental Chemistry, 2016, 35(10): 2134-2145(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.10.2016031603
[5] TONGAMP W, ZHANG Q, SAITO F. Mechanochemical decomposition of PVC by using La2O3 as additive [J]. Journal of Hazardous Materials, 2006, 137(2): 1226-1230. doi: 10.1016/j.jhazmat.2006.04.013 [6] TONGAMP W, ZHANG Q, SHOKO M, et al. Generation of hydrogen from polyvinyl chloride by milling and heating with CaO and Ni(OH)2 [J]. Journal of Hazardous Materials, 2009, 167(1): 1002-1006. [7] ZHANG T, HUANG J, ZHANG W, et al. Coupling the dechlorination of aqueous 4-CP with the mechanochemical destruction of solid PCNB using Fe–Ni–SiO2 [J]. Journal of Hazardous Materials, 2013, 250-251: 175-180. doi: 10.1016/j.jhazmat.2013.01.072 [8] CAGNETTA G, ZHANG Q, HUANG J, et al. Mechanochemical destruction of perfluorinated pollutants and mechanosynthesis of lanthanum oxyfluoride: A Waste-to-Materials process [J]. Chemical Engineering Journal, 2017, 316: 1078-1090. doi: 10.1016/j.cej.2017.02.050 [9] HUANG S T, JIANG Y R, CHOU S Y, et al. Synthesis, characterization, photocatalytic activity of visible-light-responsive photocatalysts BiOxCly/BiOmBrn by controlled hydrothermal method [J]. Journal of Molecular Catalysis a-Chemical, 2014, 391: 105-120. doi: 10.1016/j.molcata.2014.04.020 [10] YAN D, LEI B, CHEN B, et al. Synthesis of high-quality lanthanide oxybromides nanocrystals with single-source precursor for promising applications in cancer cells imaging [J]. Applied Materials Today, 2015, 1(1): 20-26. doi: 10.1016/j.apmt.2015.06.001 [11] CAGNETTA G, LIU H, ZHANG K, et al. Mechanochemical conversion of brominated POPs into useful oxybromides: a greener approach [J]. Scientific Reports, 2016, 6: 28394. doi: 10.1038/srep28394 [12] ZHANG K, HUANG J, WANG H, et al. Mechanochemical destruction of decabromodiphenyl ether into visible light photocatalyst BiOBr [J]. Rsc Advances, 2014, 4(28): 14719-14724. doi: 10.1039/C3RA47738J [13] CAGNETTA G, ROBERTSON J, HUANG J, et al. Mechanochemical destruction of halogenated organic pollutants: A critical review [J]. Journal of Hazardous Materials, 2016, 313: 85-102. doi: 10.1016/j.jhazmat.2016.03.076 [14] 隋红, 李海波, 宋静, 等. 高浓度DDTs污染土壤机械化学球磨试剂筛选 [J]. 环境科学研究, 2015, 28(8): 1227-1233. SUI H, LI H B, SONG J, et al. Selection of milling reagents for mechanochemical degradation of high concentrations of DDTs in contaminated soil [J]. Research of Environmental Sciences, 2015, 28(8): 1227-1233(in Chinese).
[15] 张冬格, 隋红, 宋静, 等. CaO机械化学法去除土壤中DDTs的工艺参数优化 [J]. 环境科学研究, 2016, 29(9): 1336-1343. ZHANG D G, SUI H, SONG J, et al. Optimization of the operational parameters for mechanochemical degradation of DDTs in containated soil with calcium oxide [J]. Research of Environmental Sciences, 2016, 29(9): 1336-1343(in Chinese).
[16] GILLHAM R W, MAJOR L, WADLEY S L, et al. Advances in the application of zero-valent iron for the treatment of groundwater containing VOCs [J]. IAHS Publication(International Association of Hydrological Sciences), 1998, 250: 475-481. [17] O’CARROLL D, SLEEP B, KROL M, et al. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation [J]. Advances in Water Resources, 2013, 51: 104-122. doi: 10.1016/j.advwatres.2012.02.005 [18] TSENG H H, SU J G, LIANG C. Synthesis of granular activated carbon/zero valent iron composites for simultaneous adsorption/dechlorination of trichloroethylene [J]. Journal of hazardous materials, 2011, 192(2): 500-506. doi: 10.1016/j.jhazmat.2011.05.047 [19] 毛琼晶, 陆胜勇, 卫樱蕾, 等. 水平球磨机械化学法处置多氯联苯污染土壤的试验 [J]. 环境化学, 2016, 35(4): 607-614. doi: 10.7524/j.issn.0254-6108.2016.04.2015090101 MAO Q J, LU S Y, WEI Y L, et al. Mechanochemical decomposition of polychlorinated biphenyls contaminated soil using a horizontal ball mill [J]. Environmental Chemistry, 2016, 35(4): 607-614(in Chinese). doi: 10.7524/j.issn.0254-6108.2016.04.2015090101
[20] 肖松文, 肖骁. 持久性有机污染物机械化学无害化处理的研究进展 [J]. 矿冶工程, 2006, 26(2): 53-56. doi: 10.3969/j.issn.0253-6099.2006.02.014 XIAO S W, XIAO X. Advances in study of mechanochemical process for persistent organic pollutants treatments [J]. Mining and Metallurgical Engineering, 2006, 26(2): 53-56(in Chinese). doi: 10.3969/j.issn.0253-6099.2006.02.014
[21] 戎宇舟. 铁-锌双金属机械化学法处理土壤中滴滴涕的过程机理及再利用研究[D]. 天津: 天津大学, 2018: 80. RONG Y Z. Process, mechanism and reuse study on the mechanochemical treatment of DDT in soil with iron-zinc bimetal[D]. Tianjin: Tianjin University, 2018: 80 (in Chinese).
[22] SUI H, RONG Y, SONG J, et al. Mechanochemical destruction of DDTs with Fe-Zn bimetal in a high-energy planetary ball mill [J]. Journal of hazardous materials, 2018, 342: 201-209. doi: 10.1016/j.jhazmat.2017.08.025 [23] SONG J, GAO X, RONG Y, et al. Mechanism for degradation of dichlorodiphenyltrichloroethane by mechano-chemical ball milling with Fe-Zn bimetal [J]. Journal of environmental management, 2019, 247: 681-687. [24] MATHESON L J, TRATNYEK P G. Reductive dehalogenation of chlorinated methanes by iron metal [J]. Environmental Science & Technology, 1994, 28(12): 2045-2053. [25] 张望. 基于Fe-SiO2的POPs废物机械化学处置工艺及机理研究[D]. 北京: 清华大学, 2012: 115. ZHANG W. Process and mechanism study of mechanochemical destruction of POPs wastes using Fe-SiO2[D]. Beijing: Tsinghua University, 2012: 115 ( in Chinese) .
[26] TANAKA Y, ZHANG Q, SAITO† F. Mechanochemical dechlorination of trichlorobenzene on oxide surfaces [J]. Journal of Physical Chemistry B, 2003, 107(40): 11091-11097. doi: 10.1021/jp0276808 [27] LIU C-C, TSENG D-H. WANG C-Y. Effects of ferrous ions on the reductive dechlorination of trichloroethylene by zero-valent iron [J]. Journal of Hazardous Materials, 2006, 136(3): 706-713. doi: 10.1016/j.jhazmat.2005.12.045 [28] LOOKMAN R, BASTIAENS L, BORREMANS B, et al. Batch-test study on the dechlorination of 1, 1, 1-trichloroethane in contaminated aquifer material by zero-valent iron [J]. Journal of Contaminant Hydrology, 2004, 74(1-4): 133-144. doi: 10.1016/j.jconhyd.2004.02.007 [29] 陈静, 陈海, 金歆, 等. 纳米零价铁降解水中四氯化碳的试验研究 [J]. 环境科学学报, 2017, 37(2): 610-616. CHEN J, CHEN H, JIN X, et al. Degradation of aqueous carbon tetrachloride by nanoscale zero-valent iron [J]. Acta Scientiae Circumstantiae, 2017, 37(2): 610-616(in Chinese).
[30] 孟亚锋. 零价铁还原降解四氯化碳废水研究[D]. 杭州: 浙江大学, 2010: 116. MENG Y F. Reduction degradation of carbon tetrachloride wastewater by zero-valent iron[D]. Hangzhou: Zhejiang University, 2010: 116 (in Chinese) .
[31] 乔本志, 张季君. 1HNMR法研究氯仿与某些化合物的氢键作用 [J]. 太原工业大学学报, 1992, 23(2): 33-36. QIAO B Z, ZHANG J J. The 1HNMR study of hydrogen-bond associations between chloroform and some compounds [J]. Journal of Taiyuan University of Technology, 1992, 23(2): 33-36(in Chinese).
-



 下载:
下载: