-
三氯生(5-氯-2-(2,4-二氯苯氧基)苯酚)常在药品和个人护理产品(肥皂、除臭剂和牙膏)中用作防腐剂和抗菌剂,在生产及使用过程中,通过各种途径排放到水体环境[1,2]. 其在各国污水处理厂的进口和出口中均有检出[3],Zheng等[4]对中国郑州4个污水厂的监测发现TCS的进水和出水浓度分别为(322.5 ± 22.3) – (467.2 ± 39.4) ng∙L−1和(5.0 ± 4.2) – (11.3 ± 6.4) ng∙L−1. TCS不仅影响陆地和水生生物生长,且可作为内分泌干扰物导致免疫功能障碍[4,5].
基于硫酸根自由基(SO4·−)和羟基自由基(∙OH)的高级氧化技术(AOPs)是水处理中有机污染物的有效去除方法之一[6]. SO4·−比∙OH具有更强的选择性,因此基于SO4·−的AOPs在水处理中得到了广泛的关注[7]. Wang等[8]用污泥衍生生物炭活化过一硫酸盐降解废水中TCS,实验发现脱氯和羟基化是TCS降解的主要途径. 李青松等[9]利用紫外光激活过硫酸钠去除水中TCS,并通过竞争动力学计算自由基与TCS的二级反应速率常数. 目前大多数此类研究偏重于宏观,对SO4·−诱导TCS转化的微观反应动力学和机制较少涉及.
本文以三氯生为研究对象,采用266 nm激光闪光光解技术研究了三氯生与SO4·−反应中出现的中间产物和这些瞬态物种的生长与衰减规律,结合GC-MS对转化产物进行分析,探讨了SO4·−诱导TCS氧化过程中的转化途径,以便对被研究对象之间的交叉反应的微观反应过程有较完整的认识,从而更加深刻地了解其降解机理中的关键环节,并且可为AOPs技术在实际废污水处理中的应用提供理论参考.
-
三氯生(AR,天津市光复精细化工研究所),过硫酸钾(K2S2O8,AR)、叔丁醇(TBA,>99.5%)和甲醇(>99.5%)均购于天津市致远化学试剂有限公司. 高纯氮气(>99.999%)和氧气(>99.99%)均购于南京上元工业气体厂. 反应溶液均采用超纯水新鲜制备,实验温度均为室温.
-
使用英国爱丁堡公司的LP920型纳秒级激光闪光光解光谱仪[10-11]进行SO4·−和TCS的瞬态光解实验. 激发光源为Nd-YAG激光器,工作波长为266 nm,激光能量为 (8 ± 2) mJ∙pulse−1,探测光源为450 W氙灯. 在激光激发前,用高纯氮气或氧气连续鼓泡20 min,以得到N2或O2饱和溶液.
-
紫外-可见(UV-vis)吸收光谱由岛津UV-1750分光光度计记录. 光化学反应主要稳态产物使用气相色谱质谱联用仪(GC-MS)进行测定分析,HP-5MS毛细管柱(30 m × 0.25 mm,0.25 μm),载气为氦气,流速1.5 mL∙min−1,离子源温度为230 ℃. 柱温箱初始温度为40 ℃保持5 min,然后以10 ℃∙min−1的速度升至200 ℃保持5 min,最后以5 ℃∙min−1的速度升至280 ℃并保持5 min.
-
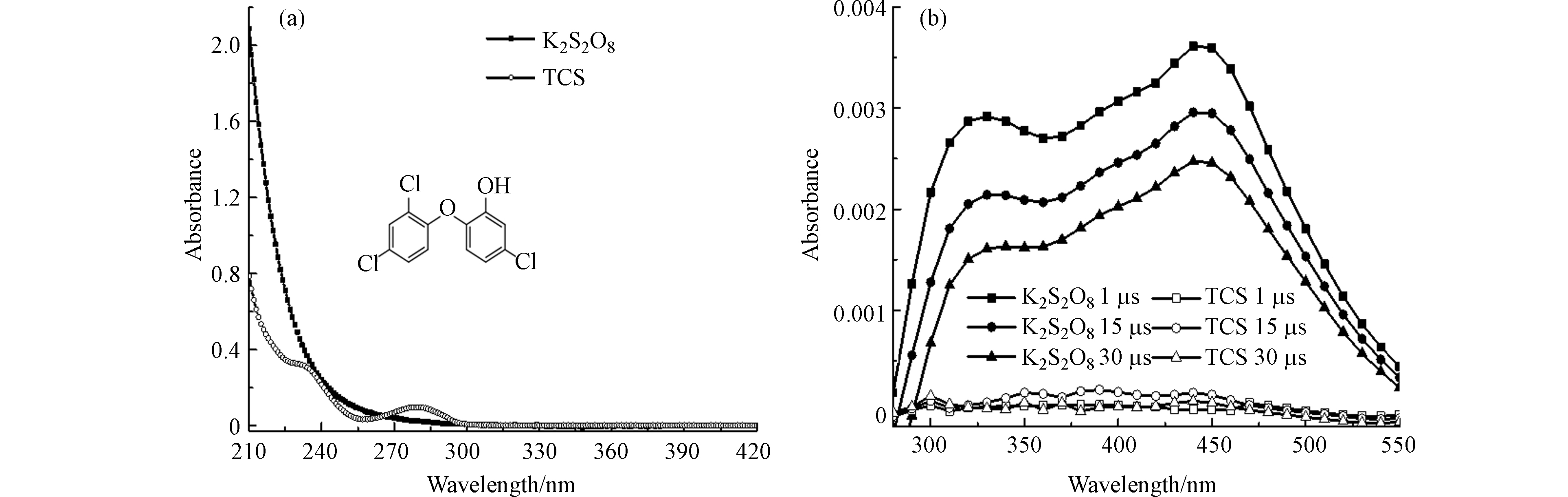
1.2 × 10−4 mol∙L−1 TCS和5 × 10−3 mol∙L−1 K2S2O8溶液的紫外-可见吸收光谱如图1a所示. K2S2O8和TCS的吸收都在< 300 nm范围内,这表明TCS和K2S2O8均能在266 nm光下被激发.
考虑到TCS在266 nm处存在微弱吸收,因此脉冲激光能量调为 (8 ± 2) mJ∙pulse−1,以减少直接光解对反应产物的影响. 图1b显示了1 × 10−2 mol∙L−1 K2S2O8和3.11 × 10−5 mol∙L−1 TCS溶液分别在266 nm激光激发下的瞬态吸收光谱. 结果表明,TCS在266 nm激光直接光解过程中未产生明显的瞬态吸收. 而K2S2O8在300 – 550 nm处有明显的吸收带,其吸收峰值分别位于330 nm和450 nm处. 450 nm处的瞬态物质可归属于SO4·−[12],而330 nm处的吸收峰归属于SO4·−与S2O82−反应生成的S2O8·−,这与Schuchmann等[13]研究过硫酸钾在中性水溶液中的脉冲辐解,并将340 nm瞬态物质归为S2O8·−而430 nm归为SO4·−的吸收相近.
-
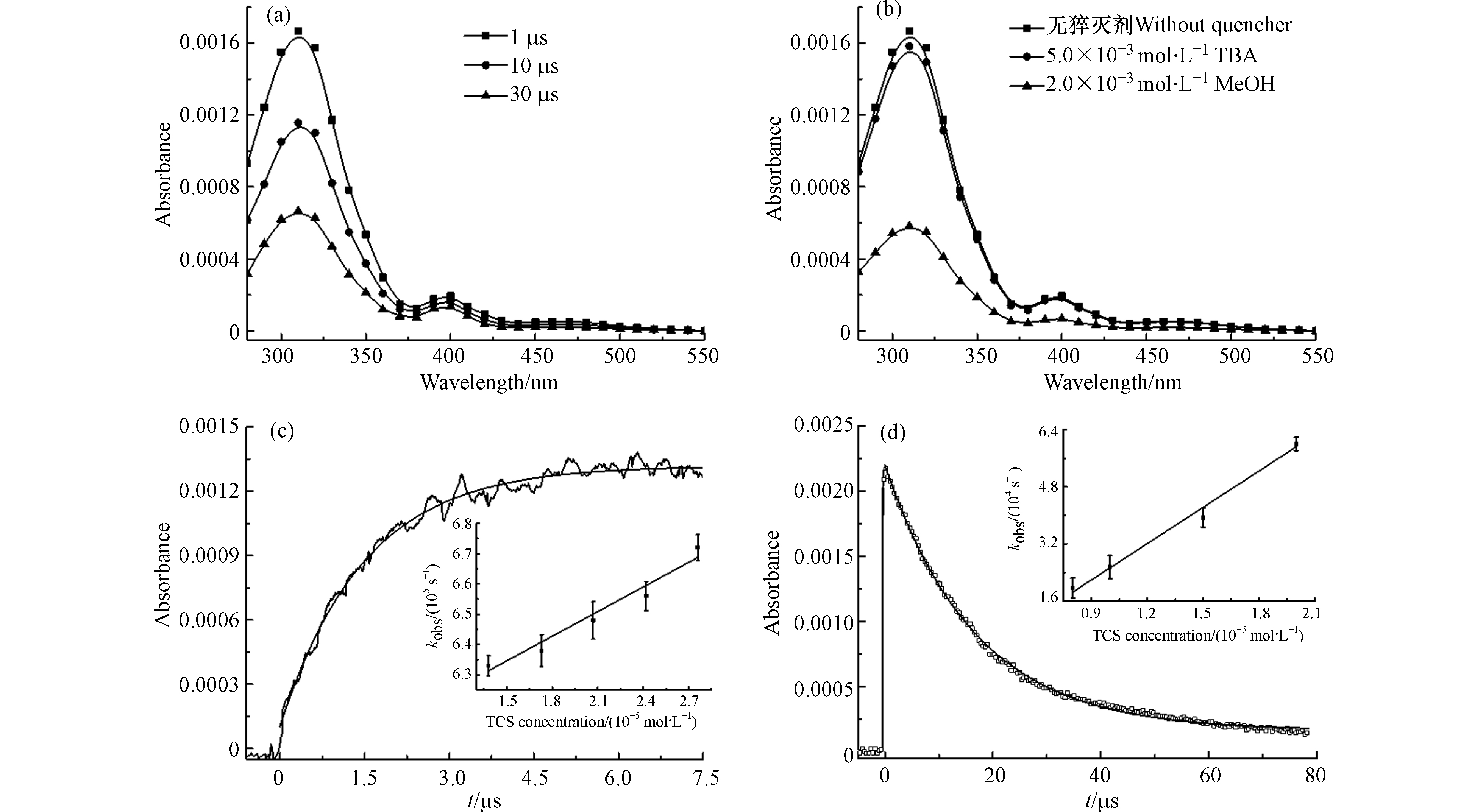
N2饱和条件下1 × 10−2 mol∙L−1 K2S2O8和3.11 × 10−5 mol∙L−1 TCS混合溶液的净瞬态吸收光谱如图2a所示,出现310 nm的吸收峰和400 nm的弱吸收带. 这些“净”吸收光谱是由混合溶液的原始吸收光谱扣除K2S2O8光离解的背景信号得到的. 因为SO4·−与水分子反应可以生成∙OH[14](式3),因此体系中可能存在两种活性物质参与反应. 叔丁醇(TBA)是有效的∙OH猝灭剂,但与SO4·−反应活性较低[15].
当加入5.0 × 10−3 mol∙L−1 TBA后,∙OH的猝灭率超过90%,而SO4·−的猝灭率仅为5%左右. 添加TBA后(图2b),混合溶液(TCS和K2S2O8)的瞬态吸收没有显著变化,由此可见,这些瞬态物质并非来源于∙OH的反应.
排除了∙OH的贡献后,使用有效SO4·−猝灭剂甲醇来确认SO4·−的存在. 在反应溶液中加入甲醇2.0 × 10−2 mol∙L−1,约70%的SO4·−被猝灭. 在加入甲醇后(图2b),310 nm和400 nm处吸收带都有相同比例的减小,表明这些瞬态中间体直接或间接来源于SO4·−的反应.
Yang等[7]的研究表明,SO4·−作为亲电子自由基,可以通过亲电加成反应直接攻击富电子基团. Yuan等[16]研究了酸性橙7在UV/PS过程中的降解,指出SO4·−诱导反应过程中将首先产生SO4·−加合物. Merga等[17]研究了SO4·−与一些卤代甲苯衍生物的反应,发现SO4·−对芳环的攻击可以生成SO4·− adducts,并且在315 nm处显示出最大吸收峰. 因此将310 nm吸收峰处的瞬态物质归因于以下反应产生的TCS−SO4·− adduct.
对310 nm处TCS−SO4·− adduct进行动力学分析(图2c),得到其生成准一级速率常数kgrowth= 6.72 × 105 s−1. 通过拟合310 nm处瞬态物质的准一级生成速率与TCS浓度的线性关系(图2c插图),得到加合物形成的二级反应速率常数为k= (2.71 ± 0.24) × 109 L∙mol−1∙s−1,这个数值代表SO4·−加成到TCS分子苯环上的反应速率常数. 而这并不能代表整体的反应速率,因此拟合SO4·−在450 nm下的衰减速率与TCS浓度的线性关系(图2d),得到SO4·−与TCS反应的总速率常数为 (3.39 ± 0.22) × 109 L∙mol−1∙s−1. 通过比较这两个速率常数,可以得出SO4·−加成途径占总反应的79.9% (2.71/3.39). 李青松等[9]通过稳态竞争动力学方法得到较大的反应速率常数(9.86 × 109 L∙mol−1∙s−1),首先加入高浓度TBA掩蔽∙OH反应,然后通过TCS与苯甲酸竞争SO4·−(已知苯甲酸与TCS的反应速率常数为1.2 × 109 L∙mol−1∙s−1)得到TCS与SO4·−的反应速率常数. 实验方法和条件的不同可能导致实验结果之间的差异. 但是本实验结果在Neta等[18]报道的SO4·−与芳香族化合物反应速率常数数值范围内。
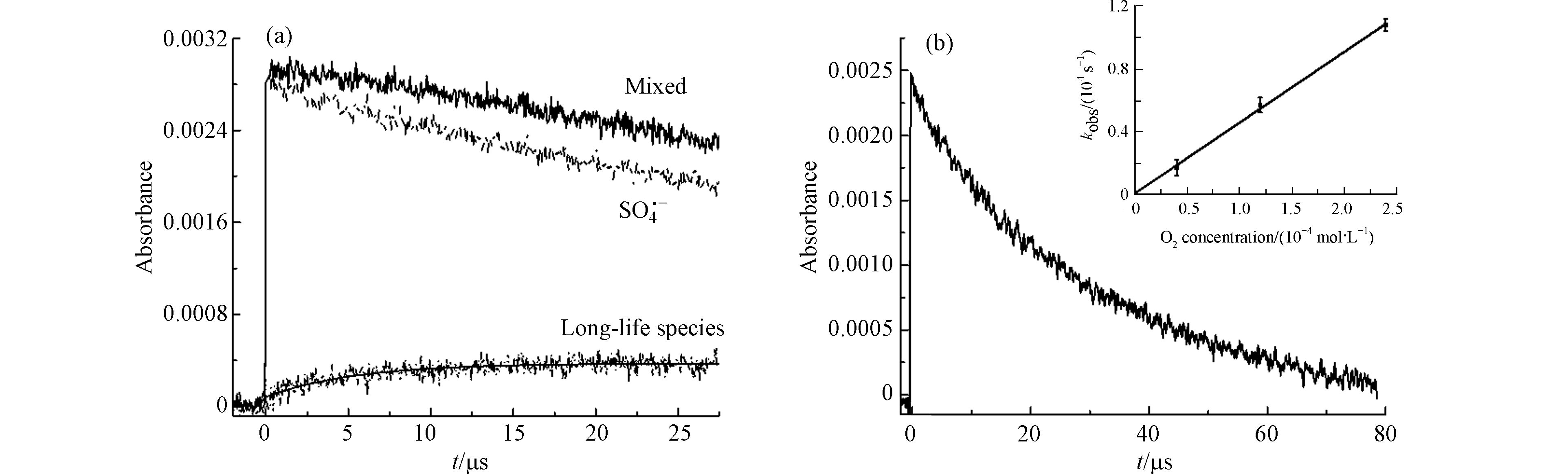
进一步分析400 nm的吸收峰,其生长符合准一级反应,并且拟合得到其速率kgrowth= 1.85 × 105 s−1(图3a),从图3看出其寿命远远比TCS−SO4·− adduct和SO4·−长。从上述的猝灭实验可知,在400 nm的瞬态物种也与SO4·−有关. 一方面,SO4·−易于通过单电子转移(single electron transfer, SET)途径攻击富电子基团,而TCS的羟基和醚官能团是强给电子基团[19-20],SO4·−通过SET与TCS反应形成阳离子自由基,阳离子自由基迅速脱质子生成酚氧基(式10和11). 另一方面,SO4·−也可以从TCS的OH基团中提取1个H原子,直接形成酚氧基(式12). 这与Chen等[6]描述的苯酚在SO4·−作用下通过电子转移转化为酚氧基的结果类似. Nakanishi等[21]提到α-生育酚的苯氧基吸收谱带在400 nm左右. Merga等[17]提出SO4·−与某些卤代苯和卤代甲苯的反应也会在400 nm处生成强度较低的峰,并将这些峰归为酚氧型自由基. 根据以上分析,在400 nm处的瞬态物质可归属为酚氧自由基. 考虑到酚氧自由基会与O2反应,因此向混合溶液中通入不同浓度的溶解氧,并拟合400 nm处酚氧自由基的衰减速率和O2浓度的线性关系(图3b),得到酚氧自由基和O2反应的二级速率常数为 (4.50 ± 0.19) × 107 L∙mol−1∙s−1,这与Anderson等[22]报道的卡拉芬净酚氧基和O2的反应速率常数 (4.0 × 107 L∙mol−1∙s−1) 接近. 并且酚氧自由基可能进行双分子反应生成二聚体,但本实验产物分析未观测到相关产物,同时Lucarini等[23]实验得出α-生育酚苯氧基生成二聚体的反应速率较小 (1.1× 102 L∙mol−1∙s−1),Plimmer等[24]认为酚氧基通常只有不超过5%会转化成二聚体产物,因此不考虑酚氧自由基的双分子反应.
-
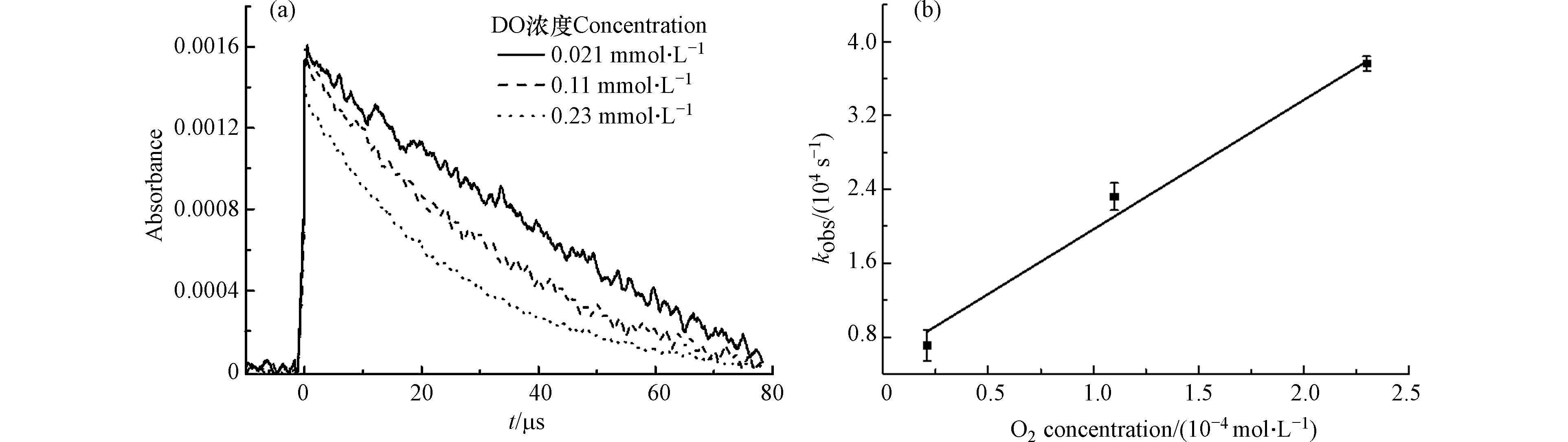
不同溶解氧浓度下TCS−SO4·− adduct的衰减曲线如图4a所示. 随着溶解氧浓度从0.21 × 10−4 mol∙L−1增加到2.3 × 10−4 mol∙L−1, 310 nm处的准一级衰减速率常数从0.71 × 104 s−1增加到3.76× 104 s−1,说明TCS−SO4·− adduct可以被O2氧化. 通过拟合TCS−SO4·− adduct的衰减速率和O2浓度的线性关系(图4b),得到TCS−SO4·− adduct和O2反应的二级速率常数为 (1.40 ± 0.14) × 108 L∙mol−1∙s−1. 这与Zhu等[25]使用激光闪光光解得到BPA−SO4·− adduct和O2反应的二级速率常数 (1.28 ± 0.14) × 108 L∙mol−1∙s−1相符.
-
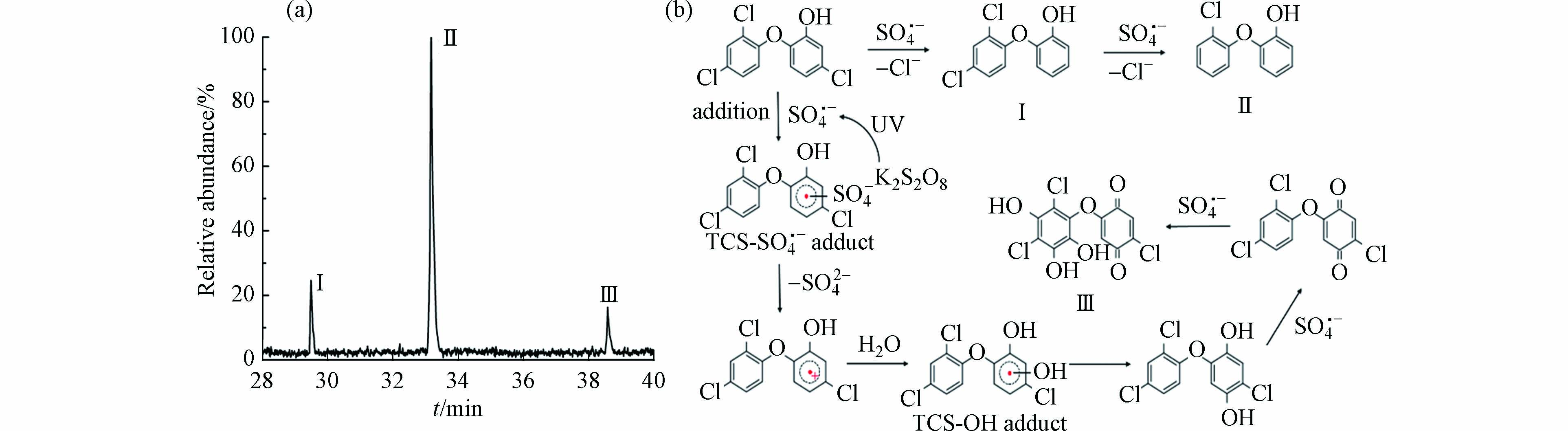
配制100 mL含1.5 × 10−5 mol∙L−1 TCS和1 × 10−3 mol∙L−1 K2S2O8混合溶液作为实验样品,向溶液中连续通入高纯氮气使其达到N2饱和状态. 在氮气保护下分次转移到4 mL石英样品池中. 将激光调至Q-Switch模式,频率为10 Hz、单脉冲能量约为8 mJ的266 nm激光. 石英池内样品经过约4—5 s的照射,在照射过程中不停地上下左右移动样品池,以保证样品均匀受到激光照射,光照后的溶液使用100 mL的二氯甲烷萃取两次. 将萃取后的有机部分转移至250 mL避光烧杯中,加入100 g无水Na2SO4脱水. 密封静置24 h以后,通过旋转蒸发将样品浓缩至1 mL进行GC-MS分析. 得到TCS与SO4·−反应后产物的总离子色谱图如图5a所示,主要产物为(Ⅰ)2-(2,4-二氯苯氧基)苯酚、(Ⅱ)2-(2-氯苯氧基)苯酚、(Ⅲ)2-氯-5(2,4-二氯-3,5,6-三羟基苯氧基)-1,4-苯醌.
第一个降解途径为SO4·−通过脱氯途径直接攻击TCS上的氯取代基,生成2-(2,4-二氯苯氧基)苯酚(m/z 255),随后继续脱氯生成2-(2-氯苯氧基)苯酚(m/z 220). 这一途径与Zhou等[26]通过零价Cu活化过硫酸盐产生SO4·−并以脱氯为主要途径降解二氯苯酚的结果相吻合. 第二个途径为SO4·−作为亲电子自由基,可以通过亲电加成反应攻击TCS的芳环形成TCS-SO4·− adduct,随后TCS-SO4·− adduct通过脱SO42−形成正离子自由基. 由于正离子自由基的不稳定性,可迅速与H2O发生反应生成TCS-OH adduct,随后稳定化为2-氯-5(2,4-二氯苯氧基)-1,4-对苯二酚,对苯二酚极易被SO4·−氧化成相应的对苯醌. 而产物m/z = 355刚好与这种对苯醌的分子量相差48,结合蒋梦迪等[27]对热活化过硫酸盐降解TCS的研究,可推测产物为2-氯-5(2,4-二氯-3,5,6-三羟基苯氧基)-1,4-苯醌(m/z 355). 综上,TCS和SO4·−的光化学反应途径如图5b所示.
-
采用266 nm激光闪光光解和GC-MS分析技术研究了SO4·−与TCS反应的动力学和机理. 实验结果表明,K2S2O8光解产生的SO4·−主要通过亲电加成攻击TCS芳环形成TCS-SO4·− adduct,反应的速率常数为 (2.71 ± 0.24) × 109 L∙mol−1∙s−1,SO4·−的加成途径占总反应的79.9% (2.71/3.39). 同时TCS-SO4·− adduct可与溶解氧发生反应,其反应速率常数为 (1.40 ± 0.14) × 108 L∙mol−1∙s−1. TCS与SO4·−反应会生成多种酚类物质,这些二次污染物可能会对环境造成危害,因此在采用高级氧化技术进行水体污染防治时,需对有机污染物之间的交叉反应和转化予以高度关注.
水体中三氯生与硫酸根自由基的反应动力学和机理
Reaction kinetics and mechanism of triclosan with sulfate radical in aqueous solution
-
摘要: 利用266 nm激光闪光光解瞬态吸收光谱技术探讨了水体中三氯生(TCS)与硫酸根自由基(SO4·−)的光化学反应机制,考察了其反应瞬态物种的生长和衰减动力学,并利用气相色谱质谱联用(GC-MS)技术分析了稳态光解产物. 结果表明,SO4·−与TCS反应的总速率常速为 (3.39 ± 0.22) × 109 L∙mol−1∙s−1,其主要途径是SO4·−亲电加成攻击TCS的芳环形成TCS-SO4·− 加合物(TCS-SO4·− adduct),二级反应速率常数为 (2.71 ± 0.24) × 109 L∙mol−1∙s−1. 并且TCS-SO4·− adduct可与溶解氧发生反应,二级速率常数为 (1.40 ± 0.14) × 108 L∙mol−1∙s−1. TCS与SO4·−的转化产物主要有2-(2, 4-二氯苯氧基)苯酚和2-氯-5(2, 4-二氯-3, 5, 6-三羟基苯氧基)-1, 4-苯醌等. 从产物分析可知,TCS与SO4·−的反应途径主要有两种,一种是SO4·−直接攻击TCS上的氯取代基并且脱氯成分子量更小的苯酚. 另一种是SO4·−通过亲电加成生成TCS-SO4·− adduct,接着生成相应的酚和醌,最后被SO4·−氧化生成羟基化产物.Abstract: The photochemical reaction mechanism of triclosan (TCS) and sulfate radical (SO4·−) in water was studied by using 266 nm laser flash photolysis transient absorption spectroscopy techniques. Its growth and decay kinetics of the reaction transient species were systematically investigated. The reaction products were analyzed by gas chromatography mass spectrometry (GC-MS). The results revealed that the overall reaction rate constant of SO4·− with TCS was (3.39 ± 0.22) × 109 L∙mol−1∙s−1, while the main pathway was SO4·− attacked the aromatic ring of TCS through electrophilic addition and formed TCS-SO4·− adduct, the second-order reaction rate constant was (2.71 ± 0.24) × 109 L∙mol−1∙s−1. In addition, TCS-SO4·− adduct could react with dissolved oxygen, and the second-order rate constant was (1.40 ± 0.14) × 108 L∙mol−1∙s−1. The transformation products of TCS and SO4·− mainly included 2- (2, 4-dichlorophenoxy) phenol and 2-chloro-5 (2, 4-dichloro-3, 5, 6-trihydroxy-phenoxy)-1, 4-benzoquinone, etc. From the analysis of products, there were two main reaction pathways between TCS and SO4·−. One was that SO4·− directly attacked the chlorines in TCS to generate phenols with smaller molecular weight via dechlorination. The other option was SO4·− created TCS-SO4·− adduct through electrophilic addition, then generated corresponding phenol and quinone, which were finally oxidized by SO4·− to generate hydroxylated products.
-
Key words:
- triclosan /
- sulfate radical (SO4·−) /
- laser flash photolysis /
- reaction kinetics
-

-
图 2 (a) K2S2O8和TCS混合溶液的瞬态吸收光谱, (b) 混合溶液在添加不同猝灭剂时的瞬态吸收光谱, (c) 310 nm处瞬态物质的生成曲线, 插图: 310 nm处瞬态物质的准一级生成速率与TCS浓度的线性关系, (d) 450 nm处SO4•−的衰减曲线, 插图: 450 nm处SO4•−的准一级衰减速率与TCS浓度的线性关系.
Figure 2. (a) Transient absorption spectra of mixed K2S2O8 and TCS solutions, (b) Transient absorption spectra of mixed solutions with different quenchers, (c) Growth curve of the transient species at 310 nm, Inset: Plot of the pseudo-first-order formation rate of transient species at 310 nm against TCS concentrations, (d) Decay curve of SO4•− at 450 nm, Inset: Plot of the pseudo-first-order decay rate of SO4•− at 450 nm against TCS concentrations.
-
[1] 高海萍, 周雪飞, 张亚雷, 等. 三氯生对水生生物的毒性效应研究进展 [J]. 环境化学, 2012, 31(8): 1145-1150. GAO H P, ZHOU X F, ZHANG Y L, et al. The toxic effect of triclosan on the aquatic organisms [J]. Environmental Chemistry, 2012, 31(8): 1145-1150(in Chinese).
[2] YUEH M F, HE F, CHEN C, et al. Triclosan leads to dysregulation of the metabolic regulator FGF21 exacerbating high fat diet-induced nonalcoholic fatty liver disease [J]. PNAS, 2020, 117(49): 31259-31266. [3] YANG Y, OK Y S, KIM K H, et al. Occurrences and removal of pharmaceuticals and personal care products (PPCPs) in drinking water and water/sewage treatment plants: A review [J]. Science of the Total Environment, 2017, 596/597: 303-320. doi: 10.1016/j.scitotenv.2017.04.102 [4] ZHENG G D, YU B, WANG Y W, et al. Removal of triclosan during wastewater treatment process and sewage sludge composting—A case study in the middle reaches of the Yellow River [J]. Environment International, 2020, 134: 105300. doi: 10.1016/j.envint.2019.105300 [5] CHEN J, HARTMANN E M, KLINE J, et al. Assessment of human exposure to triclocarban, triclosan and five parabens in US indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry [J]. Journal of Hazardous Materials, 2018, 360: 623-630. doi: 10.1016/j.jhazmat.2018.08.014 [6] CHEN C Y, WU Z H, ZHENG S S, et al. Comparative study for interactions of sulfate radical and hydroxyl radical with phenol in the presence of nitrite [J]. Environmental Science & Technology, 2020, 54(13): 8455-8463. [7] YANG Y, LU X L, JIANG J, et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate [J]. Water Research, 2017, 118: 196-207. doi: 10.1016/j.watres.2017.03.054 [8] WANG S Z, WANG J L. Activation of peroxymonosulfate by sludge-derived biochar for the degradation of triclosan in water and wastewater [J]. Chemical Engineering Journal, 2019, 356: 350-358. doi: 10.1016/j.cej.2018.09.062 [9] 李青松, 李学艳, 姚宁波, 等. UV/SPS降解水中三氯生的效能及动力学 [J]. 环境科学, 2017, 38(4): 1467-1476. doi: 10.13227/j.hjkx.201609211 LI Q S, LI X Y, YAO N B, et al. Efficiency and kinetics of triclosan degradation in aqueous solution by UV/sodium persulfate [J]. Environmental Science, 2017, 38(4): 1467-1476(in Chinese). doi: 10.13227/j.hjkx.201609211
[10] MA J Z, ZHU C Z, LU J, et al. Photodissociation of peroxynitric acid (HO2NO2) aqueous solution at 266 nm [J]. Journal of Photochemistry and Photobiology A:Chemistry, 2017, 342: 35-41. doi: 10.1016/j.jphotochem.2017.03.033 [11] 雷宇, 陆军, 马建中, 等. 355 nm光照下液相中甲苯与亚硝酸的交叉反应 [J]. 环境化学, 2017, 36(8): 1693-1699. doi: 10.7524/j.issn.0254-6108.2016113003 LEI Y, LU J, MA J Z, et al. Cross reaction of toluene with nitrous acid in aqueous phase initiated by irradiation of 355 nm UV light [J]. Environmental Chemistry, 2017, 36(8): 1693-1699(in Chinese). doi: 10.7524/j.issn.0254-6108.2016113003
[12] WANG D G, SCHUCHMANN H P, von SONNTAG C. Phenylalanine: its ·OH and SO4·−-induced oxidation and decarboxylation. A pulse radiolysis and product analysis study [J]. Zeitschrift Für Naturforschung B, 1993, 48(6): 761-770. [13] SCHUCHMANN H P, DEEBLE D J, OLBRICH G, et al. The SO4•−-induced chain reaction of 1, 3-dimethyluracil with peroxodisulphate [J]. International Journal of Radiation Biology and Related Studies in Physics, Chemistry, and Medicine, 1987, 51(3): 441-453. [14] KHATAEE A R, MIRZAJANI O. UV/peroxydisulfate oxidation of C. I. Basic Blue 3: Modeling of key factors by artificial neural network [J]. Desalination, 2010, 251(1/2/3): 64-69. [15] GAO H P, CHEN J B, ZHANG Y L, et al. Sulfate radicals induced degradation of Triclosan in thermally activated persulfate system [J]. Chemical Engineering Journal, 2016, 306: 522-530. [16] YUAN R X, WANG Z H, HU Y, et al. Probing the radical chemistry in UV/persulfate-based saline wastewater treatment: Kinetics modeling and byproducts identification [J]. Chemosphere, 2014, 109: 106-112. doi: 10.1016/j.chemosphere.2014.03.007 [17] MERGA G, RAO B S M, MOHAN H, et al. Reactions of ∙OH and SO4•− with some halobenzenes and halotoluenes: A radiation chemical study [J]. The Journal of Physical Chemistry, 1994, 98(37): 9158-9164. [18] NETA P, MADHAVAN V, ZEMEL H, et al. Rate constants and mechanism of reaction of sulfate radical anion with aromatic compounds [J]. Journal of the American Chemical Society, 1977, 99(1): 163-164. doi: 10.1021/ja00443a030 [19] XIAO R Y, YE T T, WEI Z S, et al. Quantitative structure: Activity relationship (QSAR) for the oxidation of trace organic contaminants by sulfate radical [J]. Environmental Science & Technology, 2015, 49(22): 13394-13402. [20] LUO S, WEI Z S, DIONYSIOU D D, et al. Mechanistic insight into reactivity of sulfate radical with aromatic contaminants through single-electron transfer pathway [J]. Chemical Engineering Journal, 2017, 327: 1056-1065. [21] NAKANISHI I, FUKUHARA K, SHIMADA T, et al. Effects of magnesium ion on kinetic stability and spin distribution of phenoxyl radical derived from a vitamin E analogue: Mechanistic insight into antioxidative hydrogen transfer reaction of vitamin E [J]. Journal of the Chemical Society Perkin Transactions, 2002(9): 1520-1524. [22] ANDERSON R F, BRIMBLE M A, NAIRN M R, et al. Reactions of semiquinones in aqueous solution. A comparison of the one electron reduction of kalafungin and analogues with other semiquinones using pulse radiolysis [J]. Journal of the Chemical Society, Perkin Transactions 2, 1999(3): 475-480. [23] LUCARINI M, PEDULLI G F, CIPOLLONE M. Bond dissociation enthalpy of. alpha.-tocopherol and other phenolic antioxidants [J]. The Journal of Organic Chemistry, 1994, 59(17): 5063-5070. [24] PLIMMER J R, KLINGEBIEL U I. Riboflavin photosensitized oxidation of 2, 4-dichlorophenol: Assessment of possible chlorinated dioxin formation [J]. Science, 1971, 174(4007): 407-408. [25] ZHU Y C, ZHU M Y, XIE J J, et al. Photochemical reaction kinetics and mechanism of bisphenol A with K2S2O8 in aqueous solution: A laser flash photolysis study [J]. Canadian Journal of Chemistry, 2021, 99(1): 43-50. [26] ZHOU P, ZHANG J, ZHANG Y L, et al. Degradation of 2, 4-dichlorophenol by activating persulfate and peroxomonosulfate using micron or nanoscale zero-valent copper [J]. Journal of Hazardous Materials, 2018, 344: 1209-1219. [27] 蒋梦迪, 张清越, 季跃飞, 等. 热活化过硫酸盐降解三氯生 [J]. 环境科学, 2018, 39(4): 1661-1667. doi: 10.13227/j.hjkx.201707201 JIANG M D, ZHANG Q Y, JI Y F, et al. Degradation of triclosan by heat activated persulfate oxidation [J]. Environmental Science, 2018, 39(4): 1661-1667(in Chinese). doi: 10.13227/j.hjkx.201707201
-



 下载:
下载: