-
抗生素在使用过程中由于无法被完全代谢排入水中体,这会对生态环境造成严重破坏[1-3]。目前,抗生素废水的处理方法主要有混凝法、生物法、化学氧化法、膜分离法等[4-5]。吸附法具有操作简单,适用范围广,能处理废水中低浓度的抗生素而受到广泛关注[6]。吸附剂是吸附技术的核心。HU等[7]制备了椰壳生物炭,其对左氧氟沙星和金霉素的吸附量分别为 397.7 mg·g−1和 320.9 mg·g−1。ZENG等[8]制备了活性百慕大草衍生的生物炭,对磺胺甲恶唑的吸附量为 425 mg·g−1。CHANG等[9]制备了累托石,其对金霉素的饱和吸附量为 140 mg·g−1。ZHANG等[10]制备了磁性铁矿纳米粒子,其对金霉素的饱和吸附量为 476.2 mg·g−1。AMALY等[11]制备的聚苯胺分层结构的纳米纤维吸附剂,对四环素的饱和吸附量为600 mg·g−1。LI等[12]制备的GO/TiO2 纳米复合材料,对金霉素的饱和吸附量为261.1 mg·g−1。在以上研究中,生物质炭价格低廉,但吸附性能较低,一些高分子复合材料吸附量能达到500 mg·g−1以上,但其价格较昂贵且制作工艺较为复杂。因此,需要研究一种制作工艺简单,原料来源广泛,价格低廉的吸附剂对水环境中的抗生素进行有效去除。
煤沥青是重油加工的副产品,含碳量较高,可以通过物理和化学方法将其转化为多孔碳,从而对其实现高附加值利用[13]。有研究[14]表明,煤沥青在高温下表现出一定的流动性,有助于利用模板剂和活化剂实现对其孔隙形状和结构的定向控制。由于这些优点,煤沥青基多孔材料可用于催化、电化学、生物医学、环保和军事等领域[15-16]。其中在环保领域,煤沥青基活性炭被广泛用于吸附染料、VOCs等污染物,但在吸附抗生素方面的研究鲜有报道,为此,本文以2-甲基咪唑为氮源,制备了煤沥青基掺N多孔炭,考察了其对金霉素的吸附性能[17],并使用SEM、BET、FTIR、XPS等分析方法对其物理结构和表面化学性质进行表征,且进一步阐述了可能的吸附机理。
-
主要试剂包括煤沥青(来自马钢集团有限公司)、盐酸(12 mol·L−1)、氧化镁(AR)、氢氧化钾(AR)、氯化钠(AR)、金霉素(AR)、2-甲基咪唑(AR),实验用水均为去离子水。
主要仪器包括GSL-1600X型高温真空管式炉;PHS-25型PH计;JH752型紫外分光光度计;3H-2000PS1型自动氮吸附仪(BET);Nanosem 430型场发射扫描电镜(SEM)、ESCALAB25型X-射线光电子能谱(XPS)、Zetasizer Nano ZA90型ZETA电位仪、Nicolet Is 50型傅里叶红外光谱仪(FTIR)。
-
将煤沥青块用电动破碎机研磨至可过100目筛的粉末,煤沥青:氧化镁:氢氧化钾的质量比为1:5:7.5,再按质量比为 2:0.5、2:2、2:4 (煤沥青:2-甲基咪唑)控制掺氮量,将煤沥青粉末与氧化镁、氢氧化钾、2-甲基咪唑一同放入搅拌机搅拌均匀,将混合均匀的原料放入瓷舟中,通入管式炉,以30 mL·min−1流速的氮气作为升温气氛,以5 ℃·min−1的升温速率升温到800 ℃保温60 min,再冷却至室温,取出瓷舟中的掺氮的煤沥青基炭材料,加入盛有蒸馏水的烧杯中,并少量多次加入适量盐酸溶液,搅拌16 h后,用纯水抽滤炭材料直到过滤液pH为7左右(用pH试纸检测),收集炭材料并置于烘箱100 ℃干燥6 h,冷却后过100目筛,得到的煤沥青基掺氮炭材料记为DCCx,其中x代表氮源添加量(煤沥青为2 g时2-甲基咪唑的添加量),未掺杂氮源的炭材料作为空白对照,记为CC800。
-
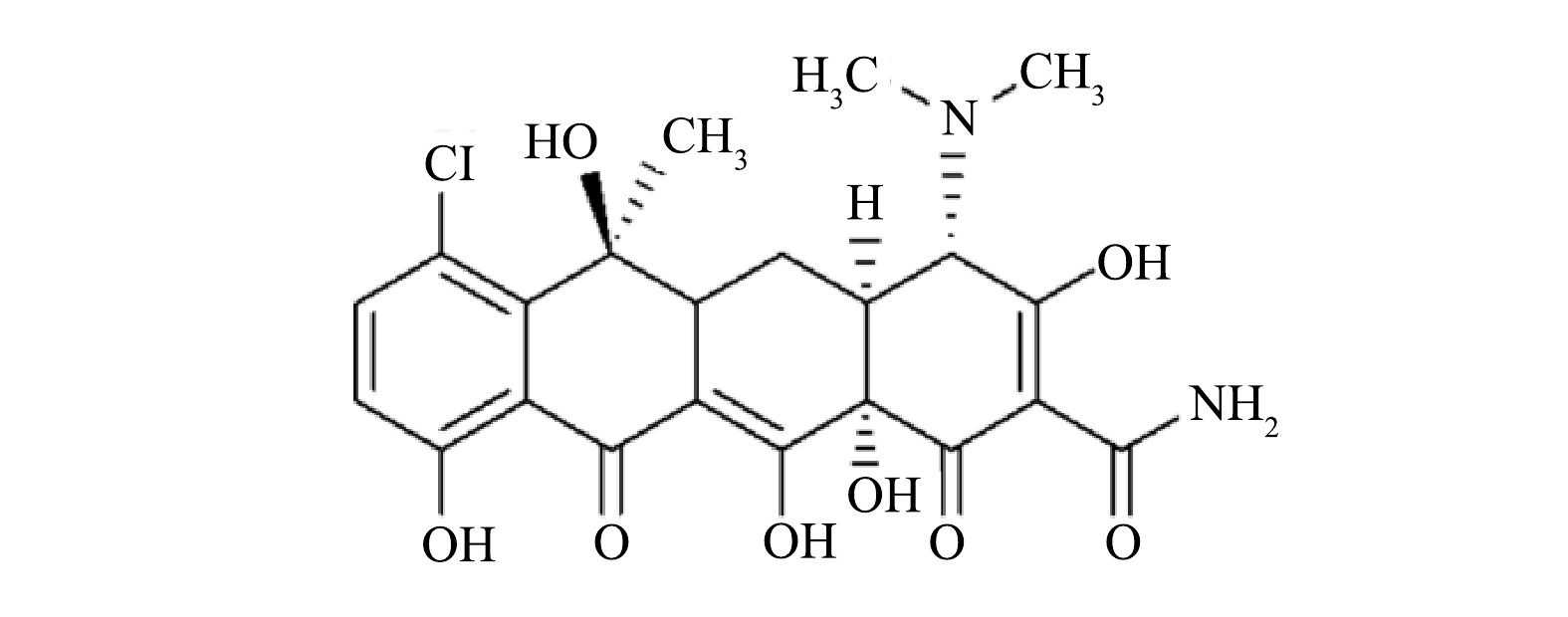
1)金霉素分子结构式及标准曲线的测定。金霉素为金黄色粉末,溶于水呈黄色,遇光颜色会变暗。其化学结构式如图1所示。配制100 mg·L−1金霉素溶液,使用紫外分光光度计测得其最大吸收波长为230 nm,在此波长下,对一系列不同浓度的金霉素溶液进行测定,得到拟合的标准方程为y=0.037 55x+0.011 71,其拟合系数R2为0.999 7,说明其在0~100 mg·L−1内,吸光度和金霉素的质量浓度有着相当好的线性关系。
2)静态吸附实验。将10 mg 吸附剂添加到 50 mL 100 mg·L−1 的 CTC 溶液中。 在空气浴恒温振荡器中以160 r·min−1 振荡24 h,使用 0.22 μm 滤膜过滤反应混合物。 在 230 nm下测定残留 CTC 溶液的吸光度。 最后,根据式(1)和式(2)计算吸附量。
式中: qe 和qt分别为反应平衡时和时间为 t 时的吸附量,mg·g−1; C0、Ce 、Ct分别为反应初始时、平衡时和 t 时的 CTC溶液质量浓度,mg·L−1; V为CTC 溶液的体积,mL;m 为吸附剂的质量,mg。
3)吸附等温线。吸附等温线是评估吸附体积和平衡浓度之间关系的有效方法。在室温条件下,将10 mg的DCCx添加进25~350 mg·L−1的金霉素溶液中,常温振荡24 h,取上清液用紫外分光光度计测试吸光度,计算浓度与吸附性能。
Langmuir 吸附等温线(式(3))[18]假设表面是完全光滑的,并且吸附不能在每个位点被占据的单层中进行。Freundlich吸附等温线(式(4)[19])是用以描述吸附过程中表面浓度关系的经验方程,适用于聚合物吸附应用和粗糙表面。
式中: qe和qm分别为代表平衡吸附量和平衡时的最大吸附量,mg·g−1; KL 为Langmuir等温线尔常数,L·mg−1。
式中: qe为平衡吸附容量,mg·g−1;KF为CTC的Freundlich等温线常数, mg1-n·g−1·L1/n;n 为吸附强度的量度。
4)吸附动力学。吸附动力学涉及对影响吸附速率的因素的研究,可用于确定涉及吸附剂和被吸附物的吸附速率常数和吸附机理[20]。
在室温条件下,将10 mg的DCCx添加进100 mg·L−1的金霉素溶液中,每隔一段时间,抽取上清液用紫外分光光度计测试吸光度,计算浓度与吸附性能。
本研究采用颗粒内扩散模型(式(5))、拟一级动力学模型(式(6))和拟二级动力学模型(式(7))来描述吸附过程[21]。
式中:qt和qe分别为反应在 t 时的吸附容量和平衡时的最大吸附容量,mg·g−1; Kd为内扩散模型分配系数,mg·g−1·min2;C为内扩散模型常数,mg·g−1;K1为拟一级动力学吸附模型常数,g·(mg·min)−1。K2为拟二级动力学吸附模型常数,g·(mg·min)−1。
-
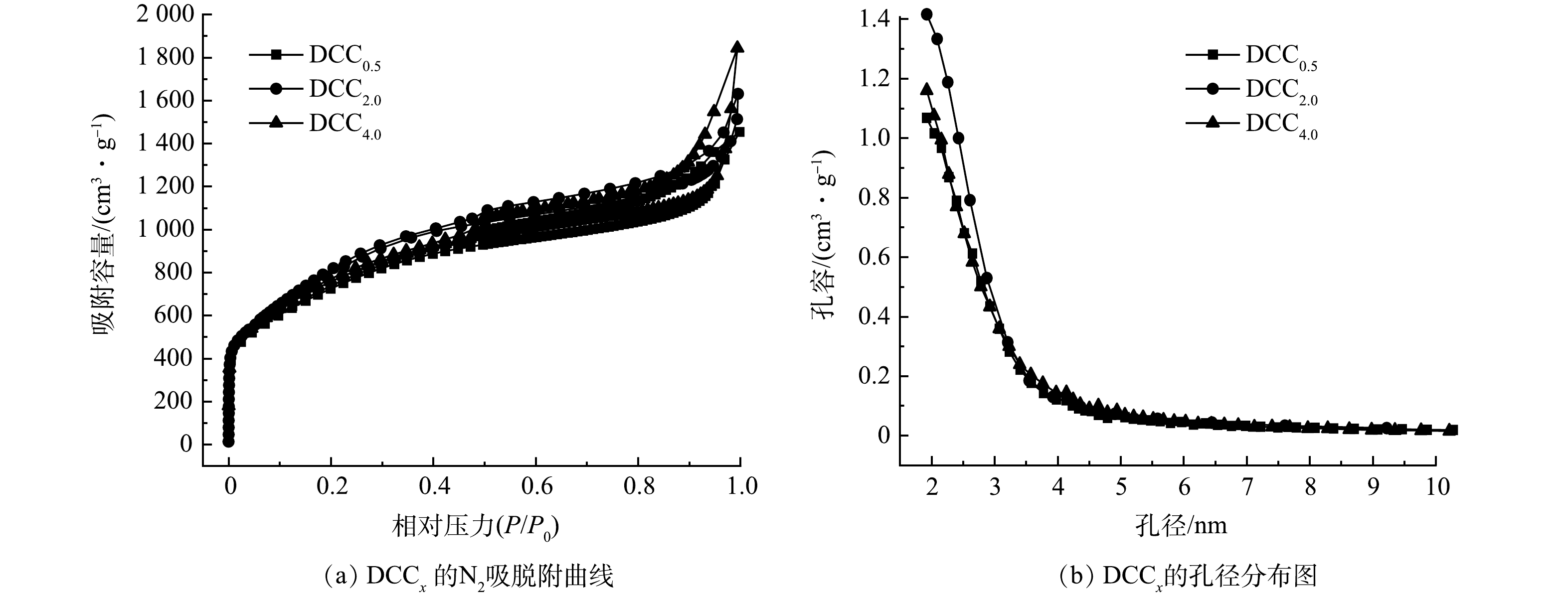
图2反映了DCCx 的N2吸脱附曲线及孔径分布结果。由图2(a)可见,3种多孔炭的氮吸脱附曲线具有明显的滞后环,属于典型的IV型等温线。这表明本文制备的多孔炭有微孔和中孔,具有明显的分级结构。在0.1~0.4 的相对压力下,多孔炭对氮气的吸附量随着压力的增加而增大,此时N2分子以单层到多层吸附在介孔的内表面。由图2(b)可见,3种多孔炭均有小于3.5 nm的小中孔存在。
DCCx的比表面积及孔结构参数见表1。在活化温度为800 ℃下,DCC2.0的比表面积、微孔比表面积和微孔孔容最高。普通的吸附剂比表面积大多小于1 000 m2·g−1,而DCCx均能达到2 500 m2·g−1以上。
-
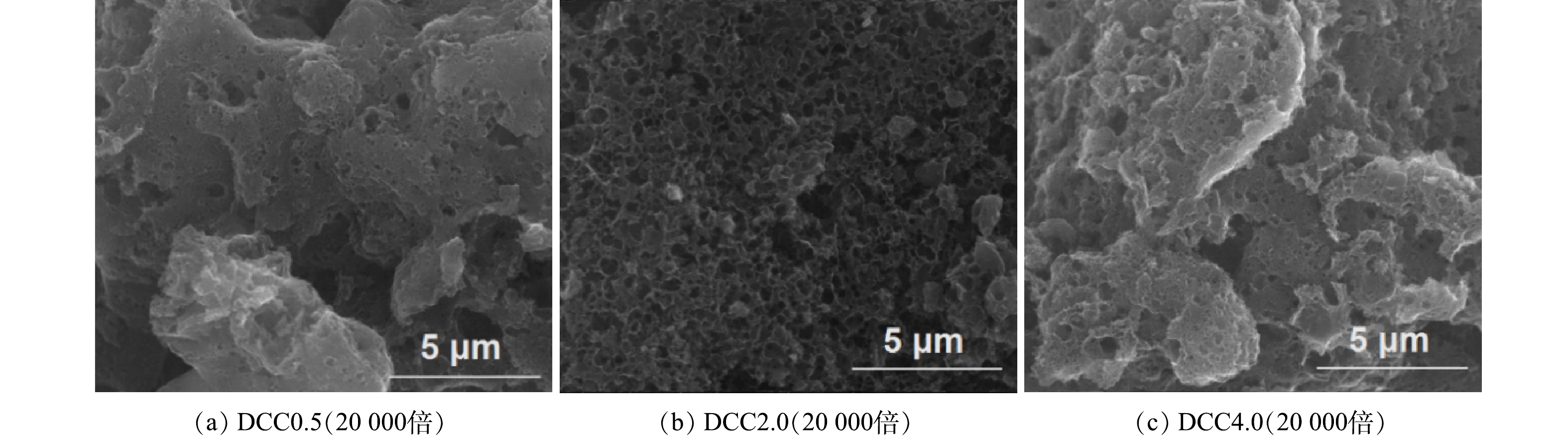
由图3可见,DCC2.0表现为密集均一的孔结构,孔壁薄且富有褶皱,而DCC0.5和DCC4.0孔结构稀疏杂乱,褶皱的片层结构可在较大程度上提升材料的比表面积[22]。
-
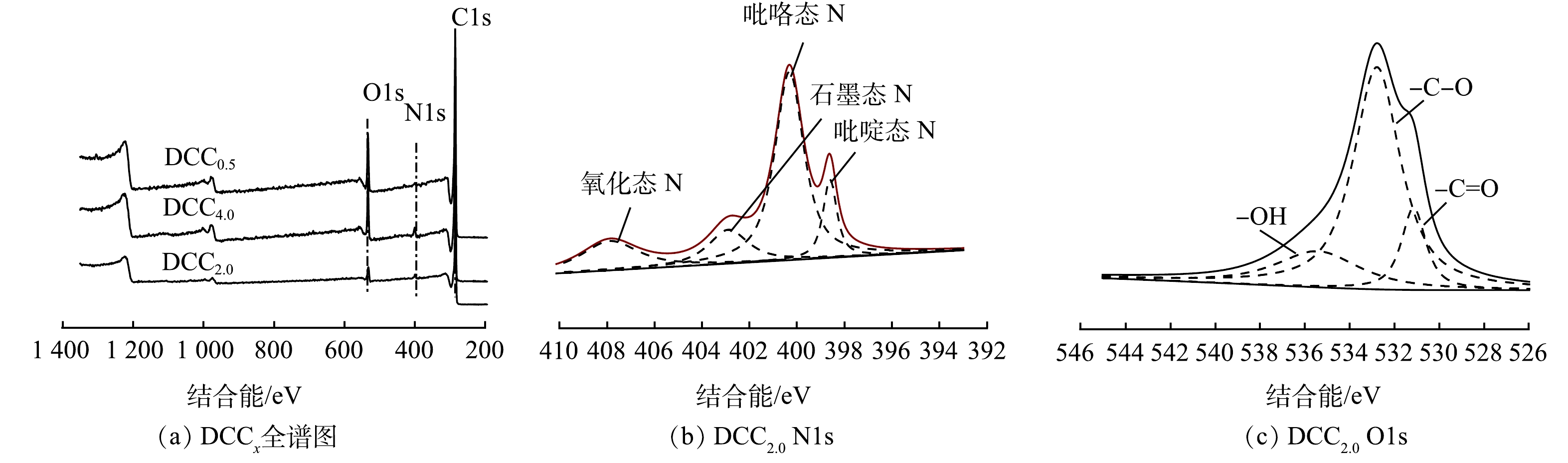
图4是DCCx的XPS全谱图及O1s、N1s的分峰拟合图。由图4(a)可知,C1s、N1s、O1s的峰均有出现,说明2-甲基咪唑的加入成功地为材料引入氮元素。DCCx的元素含量见表2。在 O1s图中,存在着—C=O、—C—O、—OH,其中DCC2.0中的—C=O、—OH含量最高。在N1s图中,存在着氧化氮、石墨氮、吡咯氮和吡啶氮,其中,DCC2.0中吡咯氮和吡啶氮的含量较高。
-
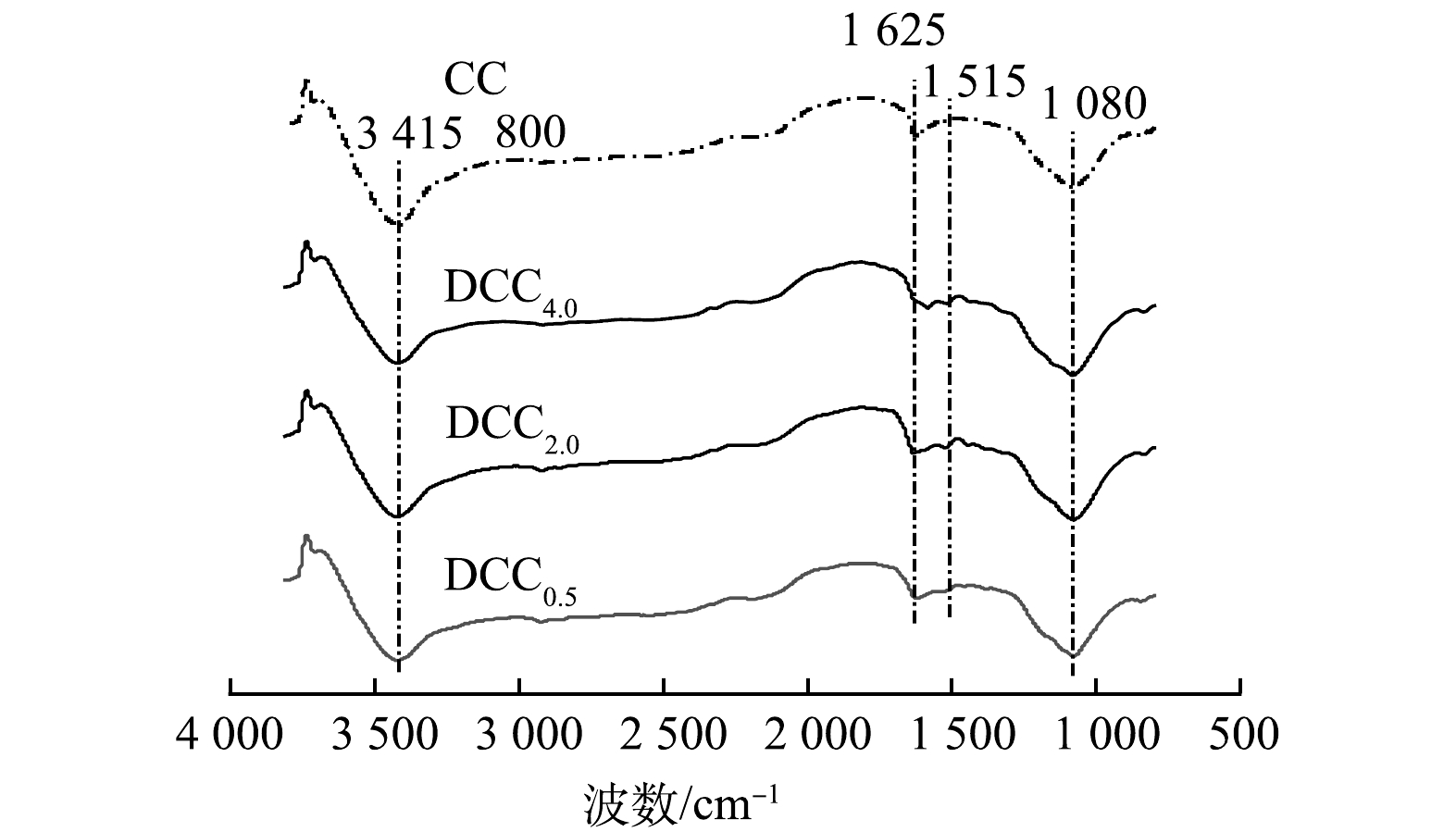
由图5可见,在3 416 cm−1处对应的吸收峰为羧酸、醇和苯酚的O-H的伸缩振动峰。1 080 cm−1附近对应的吸收峰为醇的C-O的伸缩振动峰,1 650 cm−1附近的吸收峰为苯基的C=C的伸缩振动峰。相比未添加氮源的CC800,有些区域的吸收峰产生了一定变化。其中, 1 515 cm−1产生了新的吸收峰,可能是氮掺杂引入了硝基—CO2的伸缩振动峰, 1 625处的峰加深变宽,DCC4.0还发生一定偏移,应是吡咯氮的 C=N 的伸缩振动所致。以上结果表明2-甲基咪唑的添加对炭材料表面成功进行了化学修饰[23-24]。
-
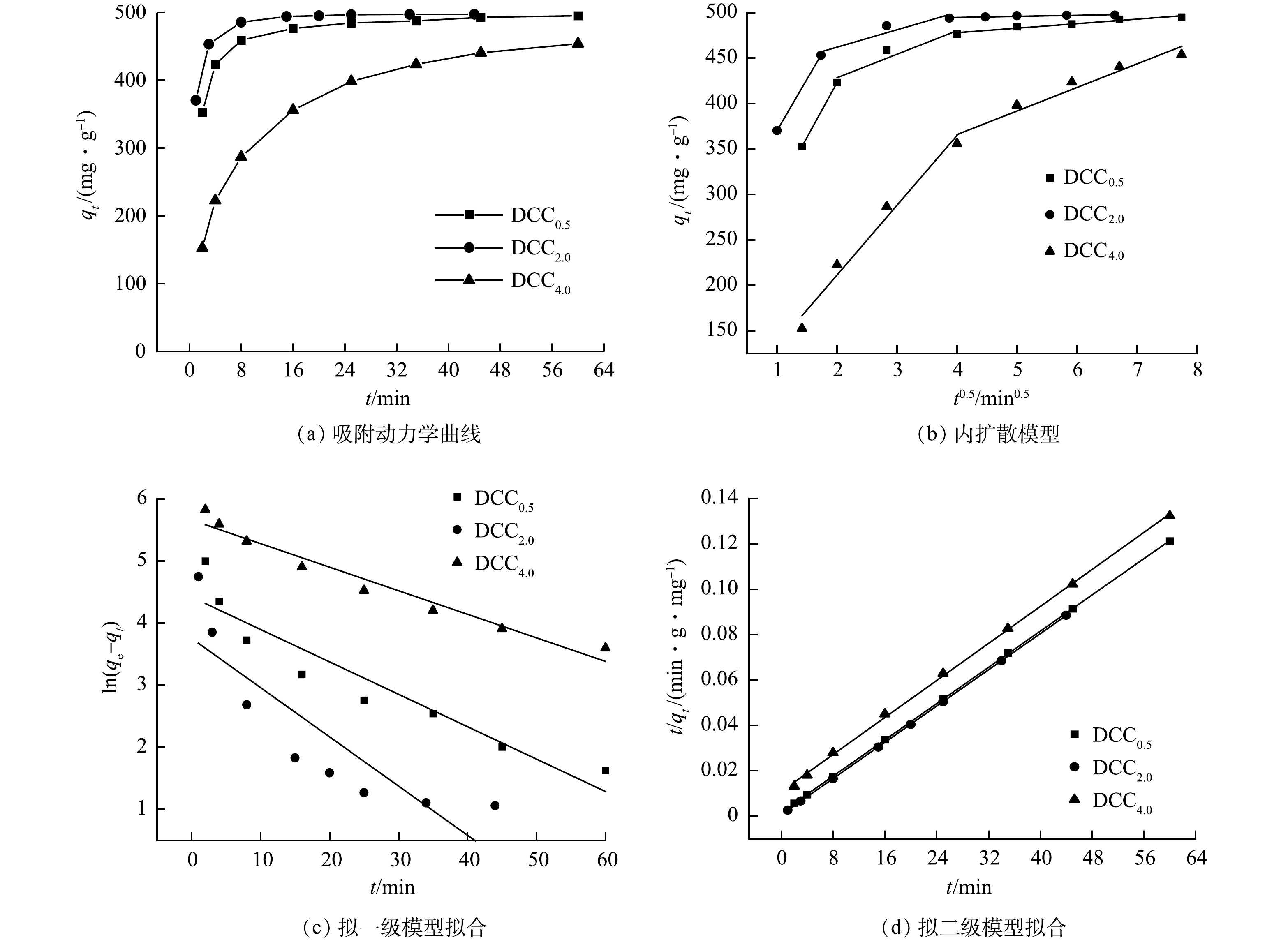
1)吸附动力学。图6反映了DCCx对金霉素的吸附动力学曲线、内扩散模型、拟一级动力学、拟二级动力学模型的拟合结果。由图6(b)可知,3种吸附剂对金霉素的吸附速率均较高。这是由于吸附初期存在大量的吸附位点,随着吸附的进行,吸附剂活性位点逐渐减少,同时,剩余溶液中的浓度驱动力也减弱,导致3种吸附剂的吸附速率逐渐降低,其中,DCC2.0的更快达到吸附平衡。DCCx吸附CTC的内扩散模型参数见表3。其中内扩散模型常数C均不为0,说明吸附过程不受内扩散控制,内扩散模型分配系数Kd的降低也解释了吸附剂吸附速率降低的原因。
DCCx吸附CTC的拟一级、二级反应动力学模型拟合参数见表4。其中,拟二级模型相关性系数更高,得到的平衡吸附量也更接近实际情况,说明化学吸附为主要控速步骤。由表中4可知,DCC2.0吸附速率最快,且3种吸附剂均能将初始质量浓度为100 mg·L−1的金霉素吸附完全。
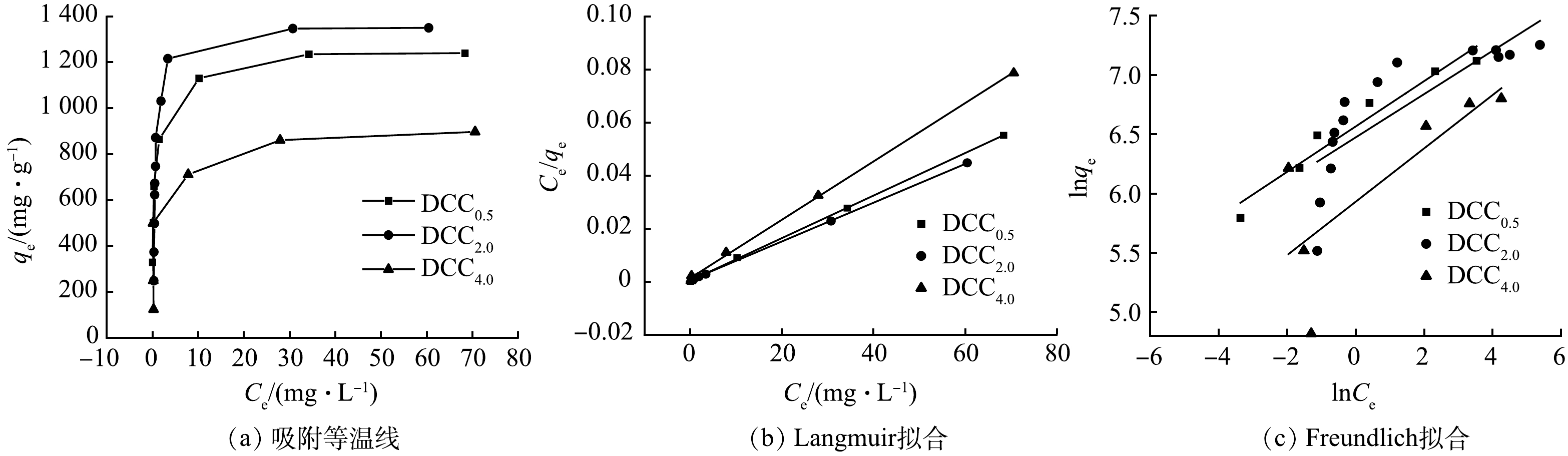
2)吸附等温线。图7反映了DCCx对金霉素的吸等温线,DCC0.5、DCC2.0、DCC4.0的饱和吸附量分别为1 247、1 368、909 mg·g−1, 明显高于目前报道的其他吸附剂的吸附性能[18-20]。用Langmuir和Freundlich模型进行拟合,2种模型的拟合结果见表5。
在Langmuir 等温方程中,KL值越大,吸附能力越强。DCC0.5的KL值最高,然而 DCC2.0有着最高的吸附容量。这说明吸附过程不只受单层吸附控制。3种材料的比表面及孔径参数所差无几,同样证明孔道结构控制的物理吸附在吸附过程中不是主导作用。故推测原子掺杂、氢键、静电吸引等原因同样了影响吸附性能。Freundlich模型的拟合系数较低,故不采用。
-
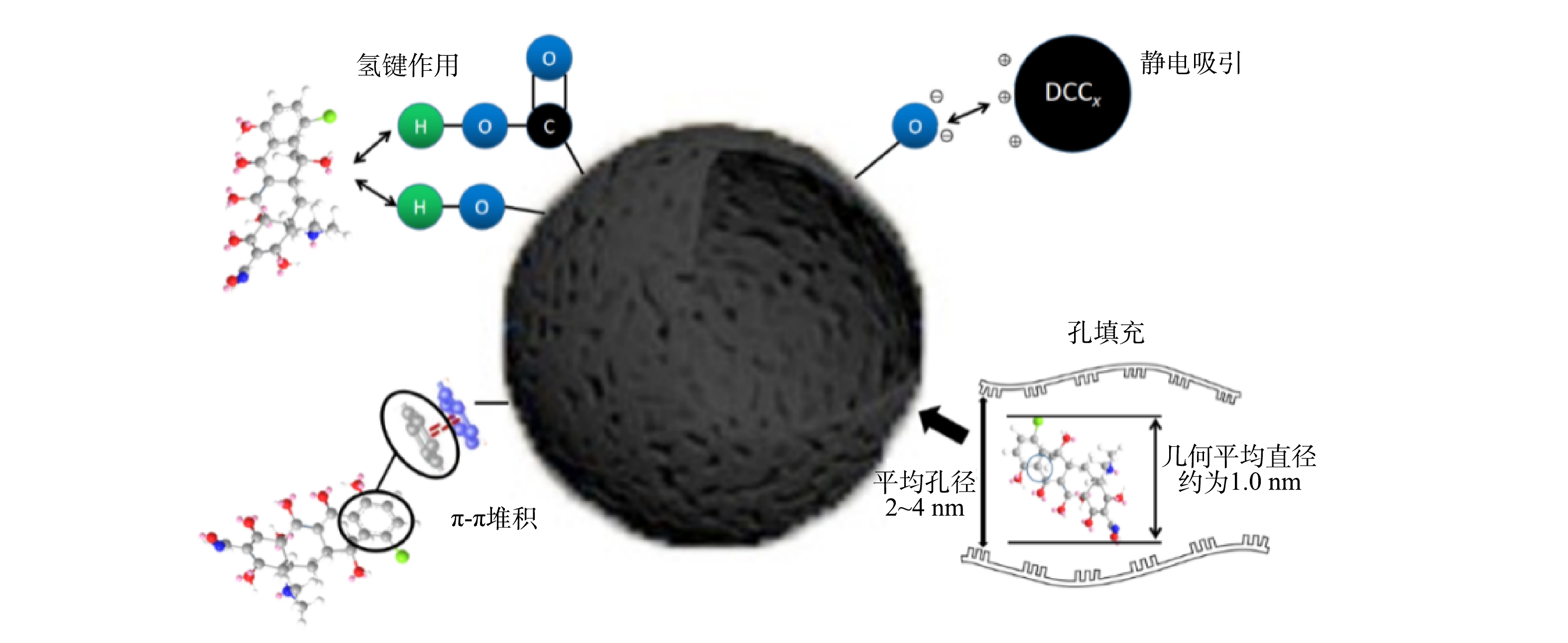
DCCx对金霉素的吸附机理如图8所示。 DCCx较高的比表面积可为金霉素提供大量的吸附位点。对金霉素分子模型进行最小能量化后得到的金霉素分子几何平均直径约为10 Å (1 nm),DCCx中大量微孔和2~4 nm的小中孔能对金霉素分子进行物理吸附。DCCx中大量的吡咯氮和吡啶氮可以提供具有静电吸引的吸附位点。较多的—C=O和—OH官能团有助于吸附剂与吸附质之间形成p-π共轭堆积和氢键。芳香性C=C、C=N在吸附金霉素的过程中发挥重要的作用。此外,金霉素的多环分子结构可与吸附剂的共轭结构实现π-π堆积的弱相互作用(范德华力/分子间力作为吸附驱动力)。
-
1)采用煤沥青为碳源,2-甲基咪唑为氮源,通过MgO模板耦合KOH活化一步制得的掺N多孔炭具有超高比表面积, DCC2.0微孔比表面积可达到2400 m2·g−1。
2) DCCx呈多孔纳米炭层堆叠结构,其中DCC2.0的孔壁为更薄的带有褶皱的纳米层,能够提供更多的金霉素吸附位点。DCCx的FTIR图谱中出现了明显的C=C和C=N的伸缩振动,且XPS数据结果表明DCC2.0中吡咯氮和吡啶氮含量较高,这些特殊的成键方式能够大幅增加化学吸附性能,包括p-π电子共轭效应、π-π堆积、氢键效应、静电吸作用等。
3) Langmuir等温线模型、内扩散模型和拟二级动力学模型能较好地描述DCCx对金霉素的吸附过程,DCC2.0对金霉素的饱和吸附容量能达到1368 mg·g−1,表现出优异的吸附性能。且10 mg DCC2.0能在20 min内将50 mL 100 mg·L−1的金霉素溶液吸附完全,吸附量为500 mg·g−1,表现出了高效的吸附速率和极高的去除率。
煤沥青基掺N多孔炭的制备及其对金霉素的吸附性能
Preparation of coal tar-based N-doped porous carbon and its adsorption performance towards chlortetracycline
-
摘要: 以煤沥青为碳源,2-甲基咪唑作为氮源,通过MgO模板耦合KOH活化一步制备得到具有高比表面积的掺N多孔炭(DCCx)。分别采用比表面积及孔径分析、扫描电子显微镜、X射线光电子能谱和傅立叶变换红外光谱仪等方法对DCCx进行了表征,并考察了其对废水中金霉素的吸附性能。结果表明,所制备的多孔炭具有层堆叠结构;制得的DCC2.0比表面积高达2 969 m2·g −1。红外光谱图中出现了明显的C=C、 C=N及硝基基团的吸收峰。DCC2.0中吡咯态氮和吡啶态氮的含量较高。DCC2.0对金霉素的饱和吸附容量高达 1 368 mg·g−1,且符合Langmuir吸附等温线模型;金霉素在多孔炭表面的吸附速度快,其符合拟二级动力学模型。Abstract: The high surface area N-doped porous carbon (DCCx) was prepared by MgO template coupled with KOH activation when coal tar and 2-methylimidazole were taken as carbon and nitrogen sources, respectively. The as-prepared DCCx was characterized by BET, SEM, XPS and FTIR, and its adsorption performance towards chlortetracycline (CTC) in wastewater was investigated. The results showed that DCCx had a type of obvious irregular layer bulk accumulation structure. The specific surface area of the obtained DCC2.0 was up to 2969 m2·g−1. The obviously tensile vibration of C=C bond C=N group appeared in FTIR spectra. DCC2.0 had a high content of pyrrole N and pyridine N. The saturated adsorption capacities of chlortetracycline on DCC2.0 was 1368 mg·g−1, the adsorption process fitted well with Langmuir isotherm model. The adsorption rate of chlortetracycline on porous carbons was fast, the kinetic adsorption process could be described by the pseudo-second-order kinetic model.
-
Key words:
- adsorption /
- antibiotic /
- porous carbon /
- nitrogen doping
-

-
表 1 DCCx 的比表面积及孔结构参数
Table 1. Specific surface area and pore structure parameters of DCCx
样本 比表面积/(m2·g−1) 微孔比表面积 /(m2·g−1) 总孔容/(mL·g−1) 微孔孔容/(mL·g−1) 平均孔径/nm DCC0.5 2 589 2 217 2.25 1.12 3.47 DCC2.0 2 969 2 400 2.52 1.21 3.40 DCC4.0 2 706 2 339 2.85 1.17 4.21 表 2 XPS元素含量表
Table 2. Element content determined by XPS analysis %
样本 C O N —C=O —CO —OH 氧化态 N 石墨化 N 吡咯态 N 吡啶态 N DCC0.5 88.22 9.88 1.90 0.21 0.46 0.33 0.37 0.09 0.47 0.07 DCC2.0 85.81 10.52 3.67 0.28 0.46 0.26 0.10 0.16 0.53 0.21 DCC4.0 90.23 6.17 3.61 0.13 0.67 0.20 0.18 0.14 0.57 0.11 表 3 DCCx吸附CTC的内扩散模型参数
Table 3. Parameters of the internal diffusion model for CTC adsorption on DCCx
样本 Kd1/
(mg·g−1·min1/2)C/
(mg·g−1)R2 Kd2 C R2 Kd3 C R2 DCC0.5 120.33 182 0.99 25.86 376 0.83 4.97 459 0.95 DCC2.0 113.20 257 0.99 19.14 424 0.81 1.19 490 0.80 DCC4.0 76.72 58 0.96 25.90 262 0.93 — — — 表 4 DCCx吸附CTC的拟一级、拟二级动力学模型参数
Table 4. Pseudo-first-order and pseudo-second-order kinetic model parameters for CTC adsorption on DCCx x
样本 拟一级动力学 拟二级动力学 qe/
(mg·g−1)K1/
(g·(mg·min)−1)R2 qe/
(mg·g−1)K2/
(g·(mg·min)−1)R2 DCC0.5 459 0.052 0.88 500 0.002 5 0.99 DCC2.0 475 0.079 0.72 500 0.007 6 0.99 DCC4.0 342 0.038 0.95 490 0.000 4 0.99 表 5 DCCx吸附CTC的Langmuir和Freundlich模型拟合参数
Table 5. Parameters of Langmuir and Freundlich model for CTC adsorption on DCCx
样本 Langmuir模型 Freundlich模型 qm/(mg·g−1) KL/( L·mg−1) R2 n KF/(mg1−n·g−1·L1/n) R2 DCC0.5 1 247 2.22 0.99 5.23 708.56 0.94 DCC2.0 1 368 1.40 0.99 5.48 646.19 0.64 DCC4.0 909 0.84 0.99 4.44 375.47 0.50 -
[1] GOU C L, WANG Y Q, ZHANG X Q, et al. Effects of chlorotetracycline on antibiotic resistance genes and the bacterial community during cattle manure composting[J]. Bioresoure Technology, 2021, 323: 124517. doi: 10.1016/j.biortech.2020.124517 [2] BILAL M, MEHMOOD S, RASHEED T, et al. Antibiotics traces in the aquatic environment: Persistence and adverse environmental impact[J]. Current Opinion in Environmental Science & Health, 2020, 13: 68-74. [3] CARDETTI M, RODRÍGUEZ S, SOLA A, et al. Use (and abuse) of antibiotics in perinatal medicine[J]. Anales de Pediatría, 2020, 93(3): 201-207. [4] DUTTA J, MALA A A. Removal of antibiotic from the water environment by the adsorption technologies: A review[J]. Water science and technology, 2020, 82(3): 401-426. [5] GOPAL G, ALEX S A, Chandrasekaran N, et al. A review on tetracycline removal from aqueous systems by advanced treatment techniques[J]. RSC Advances, 2020, 10(45): 27081-27095. doi: 10.1039/D0RA04264A [6] OUYANG J B, ZHOU L M, LIU Z R, et al. Biomass-derived activated carbons for the removal of pharmaceutical mircopollutants from wastewater: A review[J]. Separation and Purification Technology, 2020, 253: 117536. doi: 10.1016/j.seppur.2020.117536 [7] HU J T, ZHOU X, SHI Y X, et al. Enhancing biochar sorption properties through self-templating strategy and ultrasonic fore-modified pretreatment: Characteristic, kinetic and mechanism studies[J]. Science of the Total Environment, 2021, 769: 144574. doi: 10.1016/j.scitotenv.2020.144574 [8] ZENG S Q, KAN E S. Chemical activation of forage grass-derived biochar for treatment of aqueous antibiotic sulfamethoxazole[J]. ACS Omega, 2020, 5(23): 13793-13801. doi: 10.1021/acsomega.0c00983 [9] CHANG P H, JEAN J S, JIANG W T, et al. Mechanism of tetracycline sorption on rectorite[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2009, 339(1-3): 94-99. [10] ZHANG D, NIU H Y, ZHANG X L, et al. Strong adsorption of chlorotetracycline on magnetite nanoparticles[J]. Journal of Hazardous Materials, 2011, 192(3): 1088-1093. doi: 10.1016/j.jhazmat.2011.06.015 [11] AMALY N, E L-MOGHAZY A Y, SUN G, et al. Effective tetracycline removal from liquid streams of dairy manure via hierarchical poly (vinyl alcohol-co-ethylene)/polyaniline metal complex nanofibrous membranes[J]. Journal of Colloid and Interface Science, 2021, 597: 9-20. doi: 10.1016/j.jcis.2021.03.165 [12] LI Z Q, QI M Y, TU C Y, et al. Highly efficient removal of chlorotetracycline from aqueous solution using grapHene oxide/TiO2 composite: Properties and mechanism[J]. Applied Surface Science, 2017, 425: 765-775. doi: 10.1016/j.apsusc.2017.07.027 [13] FENG Y H, WANG Y H, LIU G, et al. Modification of coal-tar pitch with 10-Undecenal to reduce the content of environmental pollutants of polycyclic aromatic hydrocarbons[J]. Journal of Cleaner Production, 2018, 172: 2544-2552. doi: 10.1016/j.jclepro.2017.11.156 [14] TABASSAM R, ALVI F, ASLAM N, et al. Electrochemical investigation of LiMn2O4/asphalt and LiMn2O4/bituminous coal based cathode composites for efficient lithium-ion battery[J]. Materials Letters, 2021, 302: 130275. doi: 10.1016/j.matlet.2021.130275 [15] 余健星, 余谟鑫, 蒯乐, 等. 核桃青皮制备高含氧量多孔炭及其对Ni~(2+)的吸附性能[J]. 高等学校化学学报, 2020, 41(11): 2464-2472. [16] ZHANG C, LI Y Y, LI Y J, et al. Synthesis and Zn(II) modification of hierarchical porous carbon materials from petroleum pitch for effective adsorption of organic dyes[J]. ChemospHere, 2019, 216: 379-386. doi: 10.1016/j.chemosphere.2018.10.164 [17] 敖蒙蒙, 魏健, 陈忠林, 等. 四环素类抗生素环境行为及其生态毒性研究进展[J]. 环境工程技术学报, 2021, 11(02): 314-324. [18] QURESHI U A, HAMEED B H, AHMED M J. Adsorption of endocrine disrupting compounds and other emerging contaminants using lignocellulosic biomass-derived porous carbons: A review[J]. Journal of Water Process Engineering, 2020, 38: 101380. doi: 10.1016/j.jwpe.2020.101380 [19] HUANG X W, YANG W Q, ZHANG G S, et al. Alternative synthesis of nitrogen and carbon co-doped TiO2 for removing fluoroquinolone antibiotics in water under visible light[J]. Catalysis Today, 2021, 361: 11-16. doi: 10.1016/j.cattod.2019.10.034 [20] WANG B, XU X Y, TANG H, et al. Highly efficient adsorption of three antibiotics from aqueous solutions using glucose-based mesoporous carbon[J]. Applied Surface Science, 2020, 528: 147048. doi: 10.1016/j.apsusc.2020.147048 [21] ZHAN H Y, WANG Y T, MI X Y, et al. Effect of graphitic carbon nitride powders on adsorption removal of antibiotic resistance genes from water[J]. Chinese Chemical Letters, 2020, 31(10): 2843-2848. doi: 10.1016/j.cclet.2020.08.015 [22] HE X J, LI R C, QIU J S, et al. Synthesis of mesoporous carbons for supercapacitors from coal tar pitch by coupling microwave-assisted KOH activation with a MgO template[J]. Carbon, 2012, 50(13): 4911-4921. doi: 10.1016/j.carbon.2012.06.020 [23] JUNG K W, KIM J H, CHOI J W. Synthesis of magnetic porous carbon composite derived from metal-organic framework using recovered terephthalic acid from polyethylene terephthalate (PET) waste bottles as organic ligand and its potential as adsorbent for antibiotic tetracycline hydrochloride[J]. Composites Part B:Engineering, 2020, 187: 107867. doi: 10.1016/j.compositesb.2020.107867 [24] LIU J F, LIN H, DONG Y B, et al. The effective adsorption of tetracycline onto MoS2@Zeolite-5: Adsorption behavior and interfacial mechanism[J]. Journal of Environmental Chemical Engineering. 2021, 9(5): 105912 -



 下载:
下载: