-
随着铁路深埋隧道需求量的增加,高温高氟地下水涌出问题越来越突出,如果施工过程中产生的高温高氟废水处理不当,不仅会对当地环境造成严重影响[1],还会对当地居民的饮用水安全造成隐患[2]。氟是人体内必需的微量元素之一,人体日常饮用水的含氟量为0.4~0.6 mg·L−1[3],当超过人体正常需求量时,会引起全身性中毒疾病(即地氟病)。世界卫生组织(WHO)规定,饮用水中氟离子质量浓度上限为1.5 mg·L−1。长期接触和摄入高剂量(>1.5 mg·L−1)的氟化物会导致氟斑牙、免疫缺陷等疾病[4],甚至可能损害神经系统、内分泌系统、生殖系统、肝脏等的功能,从而对人体健康产生危害,还会影响食物链和生态系统的平衡[5]。因此,控制高氟水中氟的含量对保护自然环境和人体健康具有十分重要的意义。
目前处理含氟废水方法主要有沉淀法[6]、膜分离法[7]、离子交换法[8]和吸附法[9]。吸附法因材料成本低、操作条件可控、不产生2次污染、具有再利用潜力和再生可能性等优点[10],被认为是目前应用最广泛的除氟方法。国内外常用的吸附剂有活性氧化铝[11]、活性炭[12]、活性锯末[13]、活性椰壳炭[14]、骨炭[15]、细菌[16]、土壤吸附剂[17]、稀土氧化物[18]、活性粘土[19]、赤泥、废催化剂和飞灰等固体工业废物[20-21]、生物炭[22-23]、钢渣[24]等,其中钢渣具有来源丰富、成本低廉、疏松多孔、耐腐蚀抗冲刷等优点,可作为一种优异的环境功能除氟材料。但钢渣自身的物理性质和化学活性低的性能限制了其吸附性能,需制备一种新型且高效的改性钢渣吸附剂用以处理隧道施工排放的高氟废水。
张龙强[25]利用钢渣做吸附剂,研究了其对水中的铅离子和铬离子的吸附特征,结果表明,初始pH和温度对钢渣吸附重金属离子的影响不大,且钢渣能够适应废水的变化。张峻搏[26]以钢渣为原料对其进行改性,制备出一种多层状金属氢氧化物,研究其对含磷废水的吸附效果,结果表明,钢渣对生物池出水和湿地系统中总磷的去除率较高。纪鹏华[27]以钢渣为基材,通过添加改性剂、粘接剂和扩孔剂对钢渣进行改性,制备出具有易于分离且除磷效果好等优点的吸附剂。刘平[28]利用盐酸和硫酸对钢渣进行改性,发现利用改性钢渣除氟时,可以有效的提高钢渣的除氟效率,但相较于其他材料,除氟效果稍差(吸附容量为0.86 mg·g−1)。基于此,本研究采用与硬碱的氟离子具有强配位能力的硬酸La3+对钢渣进行改性,制备一种新型高效除氟吸附剂,并探究改性钢渣吸附水中氟离子的热力学和动力学性能,以期最大限度地发挥钢渣资源的回收利用价值。
-
主要试剂为:硝酸镧(La(NO3)3·6H2O)、氢氧化钠(NaOH)、碳酸氢钠(NaHCO3)(优级纯)、无水硫酸钠(Na2SO4)、柠檬酸三钠(C6H5Na3O7·2H2O)、硝酸钠盐酸均为购于成都市科隆化学品有限公司。氟化钠购于成都金山化学试剂有限公司。无水氯化镁购于天津百伦斯生物技术有限公司。以上试剂除碳酸氢钠外均为分析纯。去离子水实验室自制。
主要仪器为:DHG系列电热鼓风干燥箱(上海一恒科学仪器有限公司),SHA-B型水浴恒温振荡器(上海力辰邦西仪器科技有限公司)、LC-85DL型隔膜真空泵(上海力辰邦西仪器科技有限公司),DZS-706型雷磁多参数分析仪(上海仪电科学仪器有限公司)。
-
改性钢渣的制备。称取一定量的清洗并烘干后的钢渣,以固液比为1:10加入硝酸镧溶液中,以200 r·min−1振荡。将改性后的钢渣清洗、过滤、烘干后密封保存备用。改性过程中其余反应条件:硝酸镧的质量浓度分别为0、12.996、25.992、64.98、129.96、259.92 g·L−1;振荡时间分别为3、6、12、24、36 h。
缓冲溶液的配制:将57 mL HAc、58.0 g NaCl和58.8 g C6H5Na3O7·2H2O加入500 mL去离子水中,搅拌溶解。将烧杯置于冷水浴中,搅拌过程中缓慢加入240 g·L−1NaOH,调节该过程pH在5.0~5.5,定容至1 L。F−储备液的配制:氟化钠于110 ℃下干燥2 h,备用。准确称取上述0.221 g NaF溶解于500 mL去离子水中,定容至1 L,保存于干净的聚乙烯试剂瓶中备用。取上述溶液100 mL定容至1 L,得10 mg·L−1氟化钠标准溶液。
-
使用雷磁多参数水质分析仪测量溶液中F− 浓度,根据标准曲线、稀释倍数及测量电位,计算溶液中F− 浓度。含氟溶液浓度的测定:吸取适量样品于塑料烧杯中,用稀盐酸或乙酸钠溶液调节pH至中性,加入20 mL总离子强度缓冲溶液,定容至100 mL,摇匀后移入塑料烧杯中测量。采用扫描电子显微镜SEM(INSPECT-F50,美国)、比表面积测试仪BET(F-Sorb 2 400,北京)、射线电子能谱EDS、X射线衍射分析(XRD)及傅里叶红外光谱(FTIR)对改性前后钢渣进行表征。
-
1)初始pH对除氟效果的影响。改性钢渣吸附剂对氟离子的吸附与溶液pH有关,将0.2 g改性钢渣加入10 mL质量浓度为10 mg·L−1的氟化钠溶液中调节pH分别为3、5、7、9、11,在25 ℃恒温水浴环境下,以200 r·min−1振荡40 min,静置1 h,取上清液测定氟离子浓度,探究pH对吸附性能的影响。
2)吸附热力学实验。将0.8 g改性钢渣加入10 mL含不同质量浓度的氟离子溶液中,调节pH至3,分别在30、45和60 ℃恒温水浴环境下,以200 r·min−1振荡40 min,静置1 h,取上清液测定氟离子浓度。采用式(1)计算F−吸附率,采用式(2)计算吸附容量。采用Langmuir模型(式(3))、Freundlich模型(式(4))和Temkin模型(式(5))拟合改性钢渣吸附剂对F−的等温吸附过程。
式中:
$ \eta \mathrm{为}\mathrm{吸}\mathrm{附}\mathrm{效}\mathrm{率},\mathrm{\%}; $ qe为吸附容量,mg·g−1;C0为吸附前浓度,mg·L−1;Ct为吸附平衡后的浓度,mg·L−1;V为溶液体积,L;m为吸附剂质量,g。式中:
$ {{q}}_{\text{max}} $ 为最大吸附容量,mg·g−1;b为吸附常数,L·g−1;Ce为吸附平衡时的氟离子浓度,mg·L−1;KF为反映吸附量吸附常数,L·g−1;n为吸附强度指数;R为热力学常数 8.314×10−3 kJ·(mol·K)−1;T为热力学温度,K;bT为与吸附热有关的常数,kJ·mol−1;aT为平衡键能常数,L·g−1。3)吸附动力学实验。将0.8 g改性钢渣加入氟离子质量浓度为10、50、100 mg·L−1的溶液中。调节pH至3,在30 ℃恒温水浴环境下,以200 r·min−1分别振荡5、10、30、60、120、240、360、1 440、2 160 min取样,静置1 h,取上清液测定氟离子质量浓度。为了描述改性钢渣对氟的吸附特征,探讨其吸附机理,分别以伪一级动力学模型(式(6))、伪二级动力学模型(式(7))对实验数据进行拟合。
式中:qe为平衡时刻的吸附量,mg·g−1;qt为t时刻的吸附量,mg·g−1;t为吸附时间,min;k1为伪一级吸附反应速率常数,min−1;k2为伪二级吸附反应速率常数,g·(mg·min)−1。
-
改性钢渣对氟离子的吸附效率,除了与吸附剂本身的性质和pH有关外,还与溶液中共存阴离子有关。基于此,本研究根据当地水质情况,在氟离子溶液中加入不同质量浓度的氯离子、硫酸根离子和碳酸氢根离子,研究共存离子对除氟效率的影响,具体操作方法是将0.8 g改性钢渣加入到不同浓度含共存阴离子的50 mg·L−1的氟化钠溶液中。调节pH至3,在45 ℃恒温水浴环境下,以200 r·min−1振荡40 min,静置1 h,取上清液测定氟离子质量浓度。
-
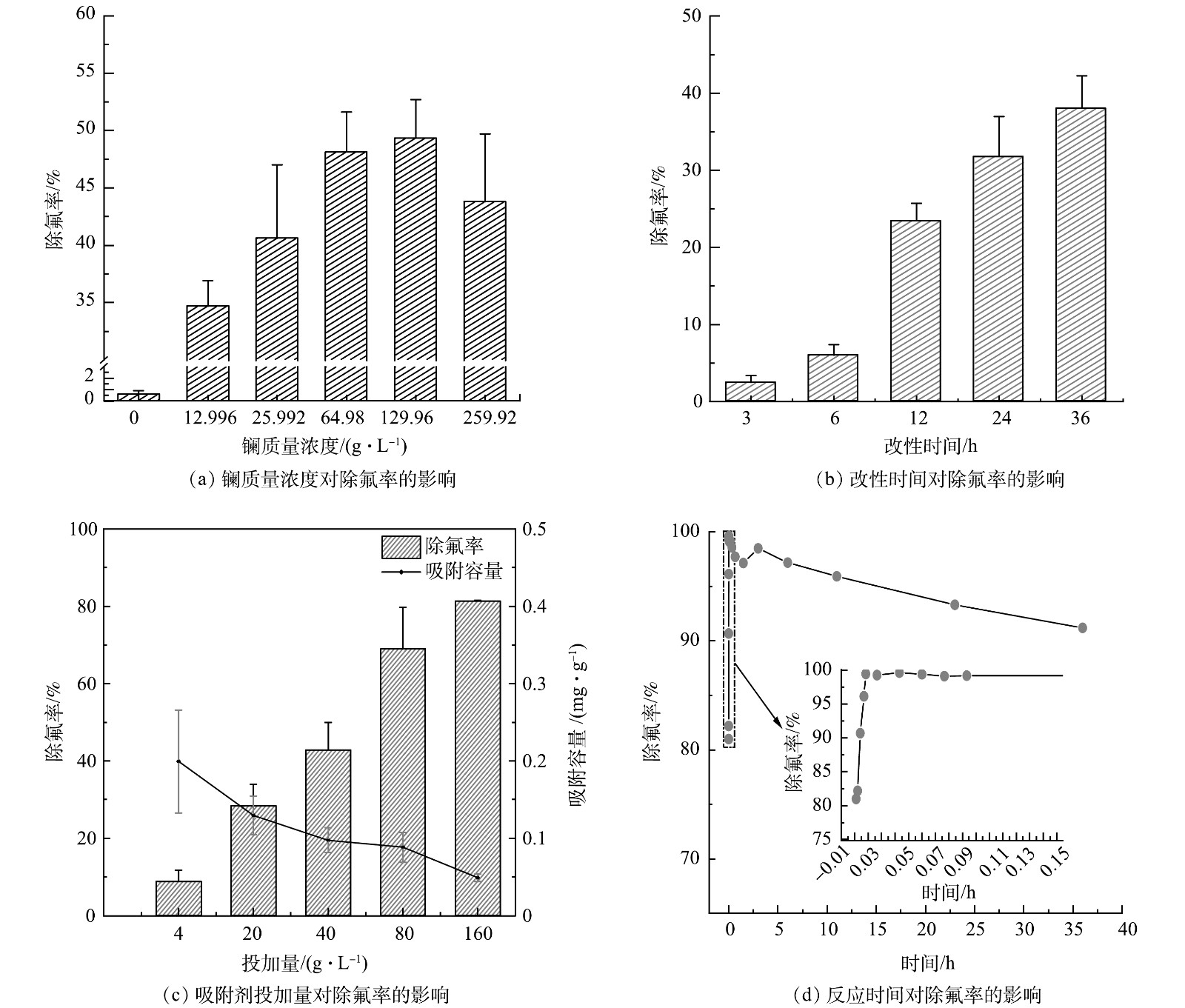
改性钢渣的除氟效率与改性时间、硝酸镧的用量、吸附剂的投加量及吸附时间有关。将0.2 g钢渣加入10 mL离子质量浓度为10 mg·L−1的氟离子溶液中。如图1(a)所示,当改性时间达到12 h时,除氟率较改性6 h明显提高,达到25.71%。随着时间的增加,除氟率继续提高,当改性时间为36 h时,除氟率达到41.70%。随着时间的延长,更多La负载到钢渣表面,可增加La的吸附位点。将0.2 g钢渣加入10 mL氟离子质量浓度为10 mg·L−1的溶液中。如图1(b)所示,随着硝酸镧质量浓度的增加,改性钢渣的除氟率在整体上先增大后减小,在硝酸镧质量浓度为129.96 g·L−1时达到峰值,为49.33%。因为硝酸镧质量浓度的增加,更多的La元素留在了钢渣表面,提供了更多的活性点位,但硝酸镧质量浓度过高时,使钢渣在浸渍过程中不稳定,一方面可能引起了钢渣内部的坍塌导致孔隙和通道的堵塞,阻碍其与F−的接触;另一方面可能形成了过多的硝酸镧晶体充斥在材料内部孔隙及通道中,阻碍了F−在内部孔隙和通道中的扩散,导致除氟效率下降。因此,综合考虑吸附效率和经济成本,以固液比为1∶10时,选择硝酸镧质量浓度为64.98 g·L−1对钢渣进行改性,能够达到较好的除氟效果。图1(c)为吸附剂投加量对吸附效果的影响,改性钢渣的除氟效果呈现出随投加量的增加而升高的趋势,并在投加量为160 g·L−1时达到峰值,此时的除氟率为81.33%。ERIC等[29]指出,随着吸附剂量的增加,溶液中吸附F− 的活性点位增多。相应地,随着投加量的增加,吸附材料的吸附容量由0.199 mg·g−1,下降至0.046 mg·g−1。因为吸附剂投加量增加,吸附材料对F− 吸附不饱和,单位质量的吸附剂吸附F− 的质量下降,吸附剂效率降低。由图1(d)可见,选择经制备条件优化后的钢渣,将0.8 g吸附剂加入10 mL氟离子质量浓度为10 mg·L−1的溶液中,随着吸附时间的增加,其除氟率先增大再减小。吸附过程十分迅速,在4 s内就能达到80.98%的除氟率,之后除氟率继续上升,在2 min时达到最大值,为99.58%,在10 min左右达到吸附平衡。
-
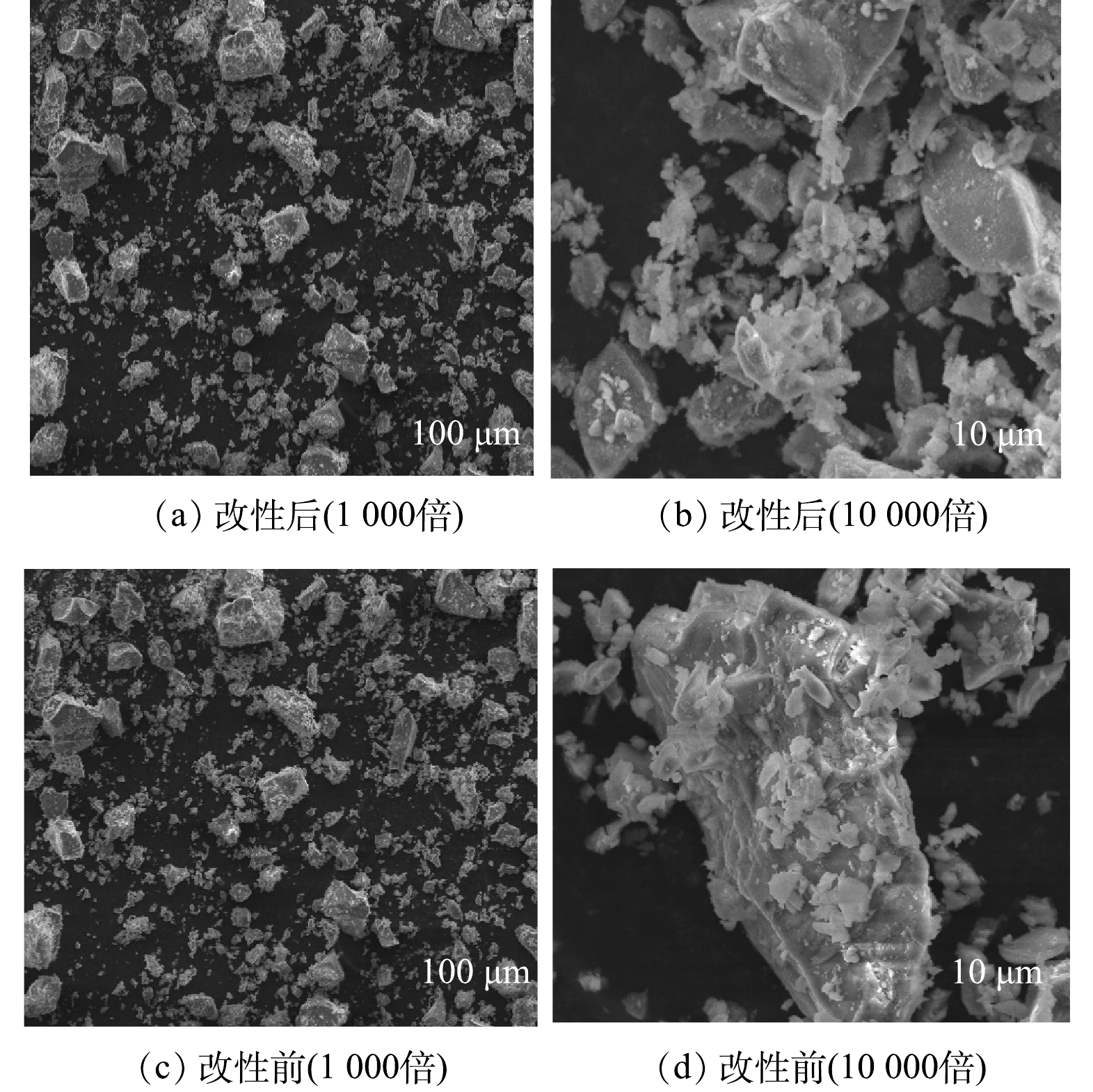
图2(a)、图2(b)分别为镧改性后钢渣的SEM图;图2(c)、图2(d)分别为未改性钢渣的SEM图。可见,改性前,钢渣的形状不规则,粒径大小各异。大块的钢渣表面有不平整的凸起;在改性后,小粒径钢渣的比例升高,钢渣表面的附着物与凸起增多,增大了表面粗糙度及比表面积。
-
图3(a)和图3(b)分别为钢渣改性前后的能谱图,表1是钢渣改性前后的元素组成。从表中可以看出,未改性钢渣主要由氧、钙、碳、硅等元素组成,而改性后的钢渣,大部分元素的含量发生变化,如硅、铝、镁元素含量增加,以及钙、铁和锰减少,可能由于改性时发生的离子反应引起的[30]。La元素的能谱出现,含量大约为2.63%。说明通过改性可成功的将镧负载于钢渣表面。
-
钢渣改性前后的BET结果表明,比表面积由0.549 9 m2·g−1增大到23.367 5 m2·g−1,平均孔径估算值由0.934 nm减小到0.135 1 nm。根据SEM扫描结果,改性后的钢渣表面突起增多,表面粗糙度增大,这与BET表征结果一致。根据查阅对比国际纯化学与应用化学联盟的规定,微孔大小<2 nm,介孔为2~50 nm,大孔>50 nm[31]。本研究中的改性钢渣是以微孔为主的吸附材料。
-
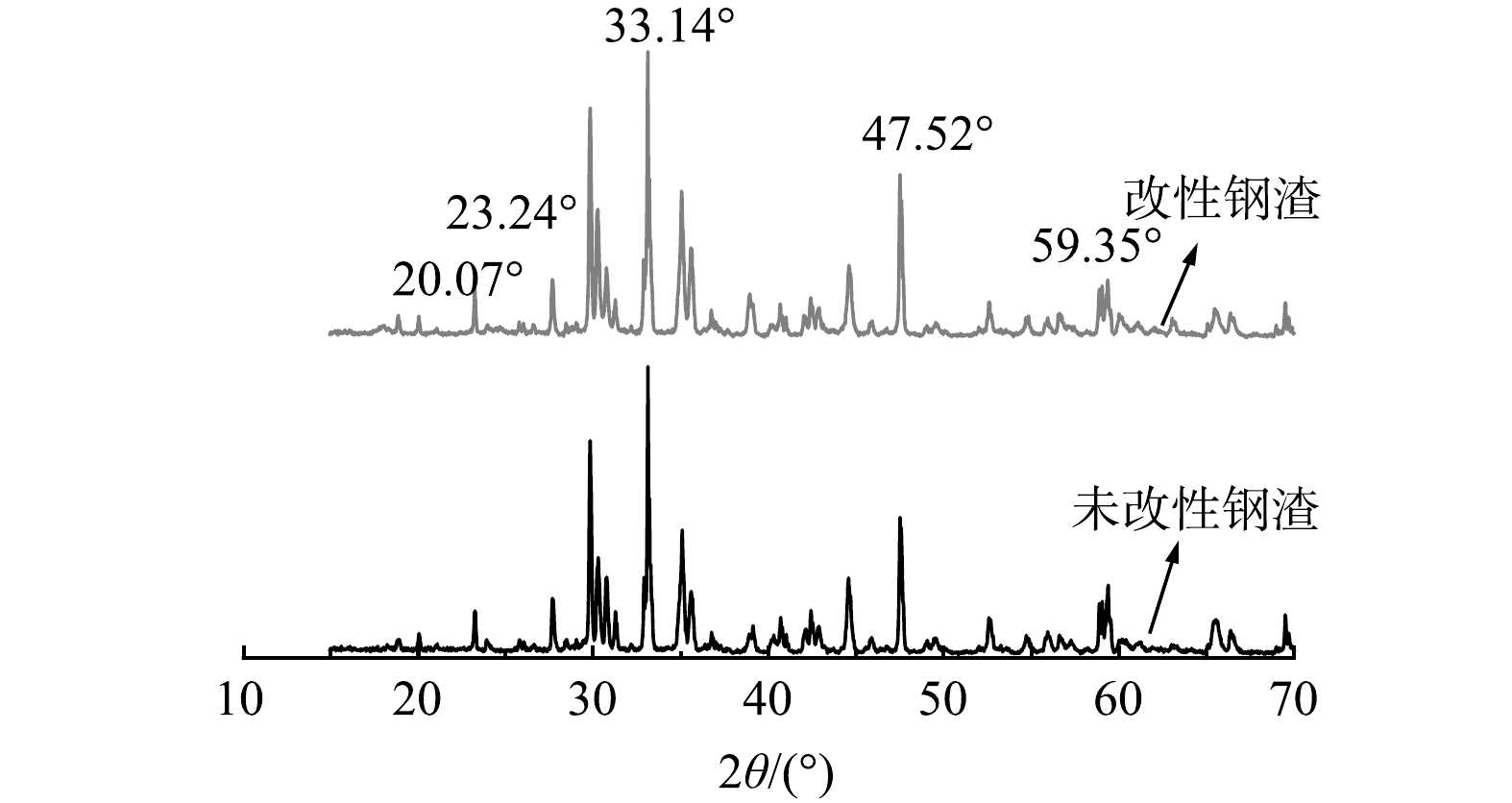
钢渣改性前后的XRD谱图如图4所示。用JADE软件分析该钢渣的物相,主要有Ca(TiO3)、CaO、MgO、Ca2Mg(Si2O7)、Al2O3等,钢渣的成分十分复杂,但所含的元素类型与EDS测定的结果一致。在30°~35°的位置有较高且尖锐的峰,钢渣的物相组成主要取决于其化学成分,特别是其碱度(CaO/SiO2)[32]。改性后的钢渣与PDF标准卡对比发现在2θ为20.07o、23.24o、33.14o、47.52o、59.35o处出现(La0.4Ca0.6)(MnO3)的特征峰。以上结果证明镧元素被成功地负载于钢渣表面,为吸附除氟提供了更多的吸附位点。此外,改性前后XRD谱图变化不大,说明钢渣改性后的晶体结构并没有发生变化。
-
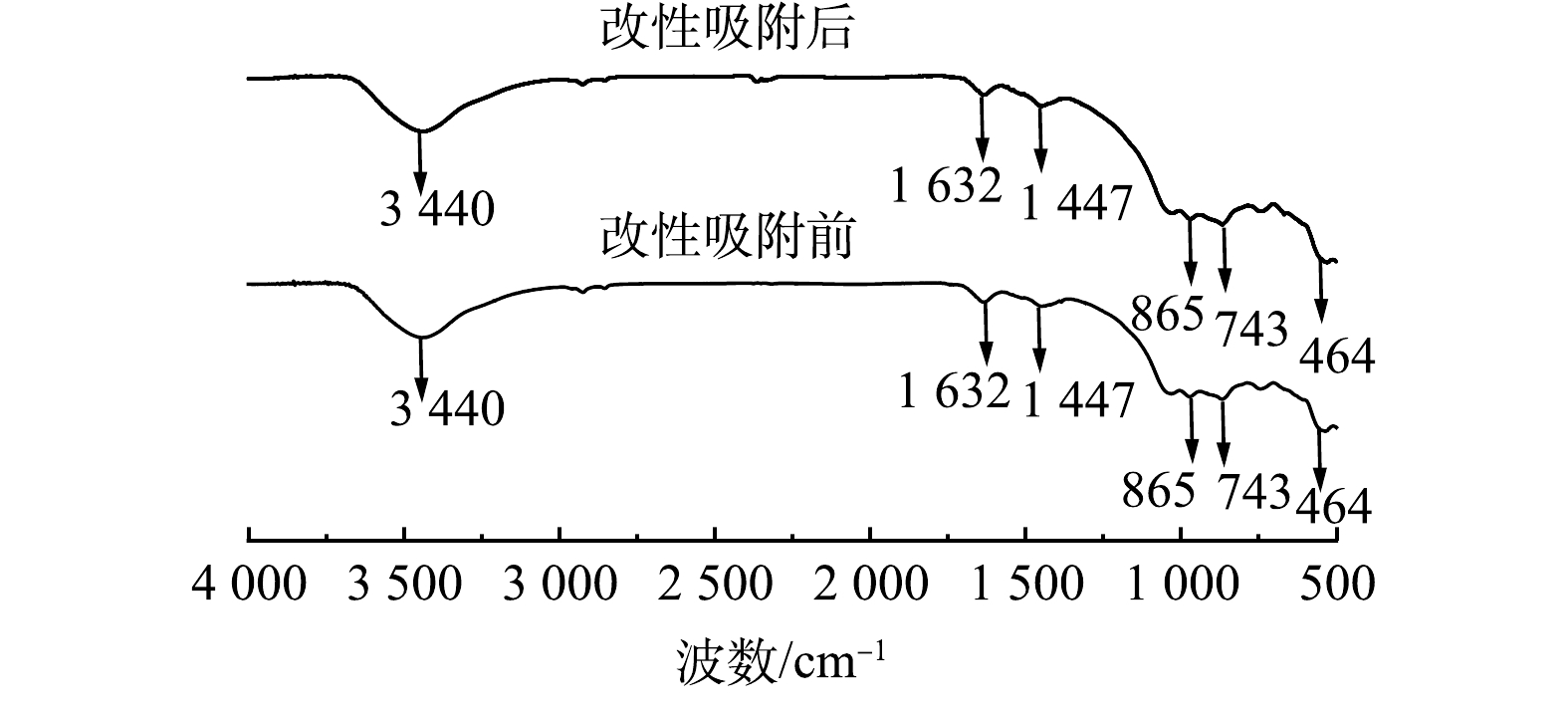
由图5可见,在3 440 cm−1处出现的吸收峰是由O—H振动引起的,1 632 cm−1附近为—OH键的弯曲振动吸收峰,1 490~1 350 cm−1为C—H的弯曲振动峰[33]。865 cm−1左右处为AlO45−的非对称伸缩振动峰[34]。在1 200~800 cm−1对应Si—O—Si伸缩振动峰, 800~600 cm−1区域的吸收带对应是(TO4)四面体的T—O—T(T=Si,Al)对称振动峰[35],464 cm−1左右处是Si—O—Si(SiO4四面体)的弯曲振动峰[36]。对比改性钢渣吸附氟离子前后的谱图发现,改性钢渣吸附氟离子前后的图谱几乎没有变化。这说明改性钢渣对水体中的氟离子吸附量非常小,进一步说明改性钢渣除氟可能是基于钢渣表面负载的镧水化后的化合物部分进入液相,与氟离子结合生成沉淀而去除。
-
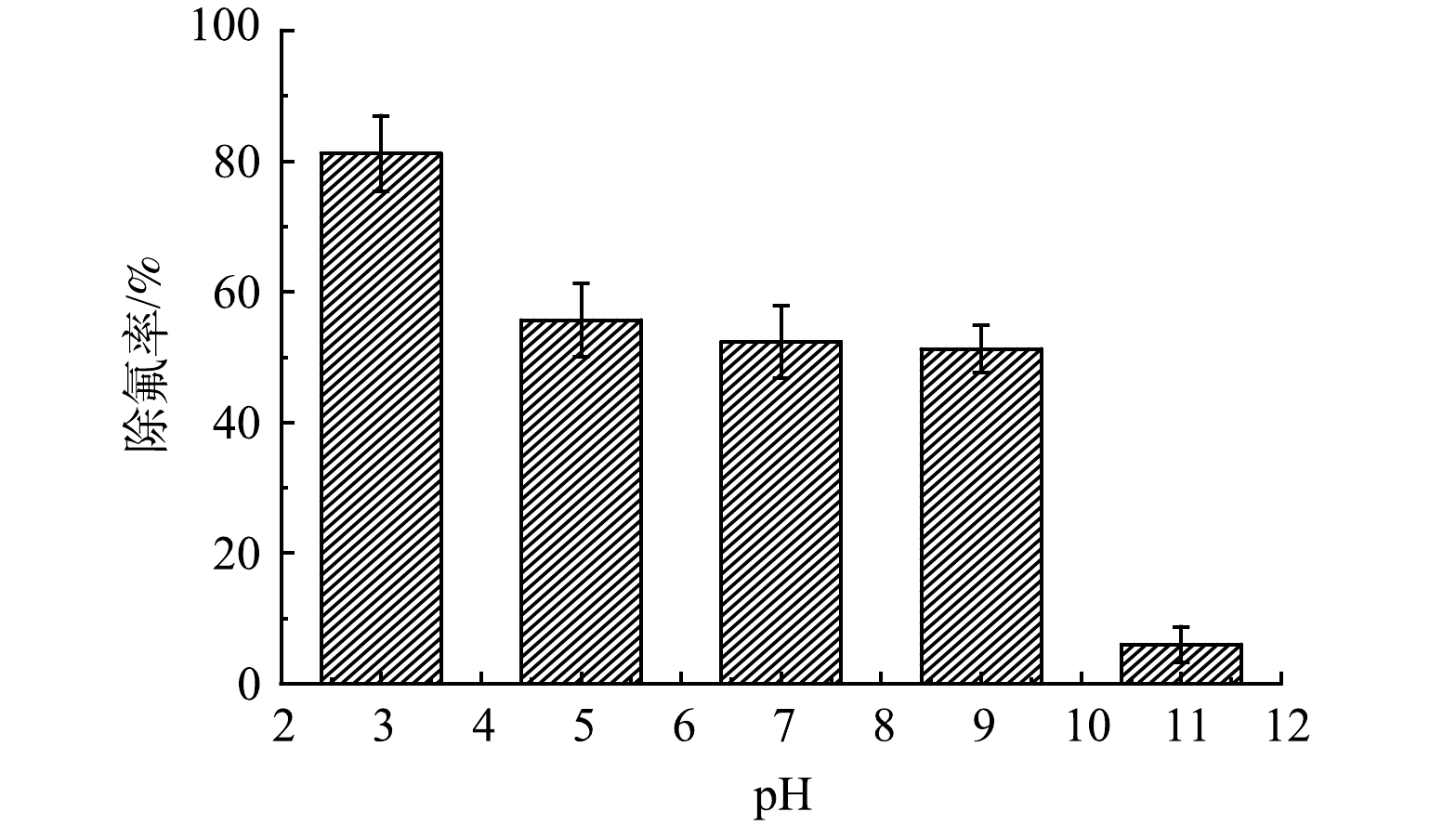
如图6所示,溶液中F−的去除效率受溶液pH的影响较为明显,因pH影响F−在水溶液中的存在形式,且溶液pH的改变能直接影响改性钢渣的表面电荷及表面官能团的质子化或去质子化过程。由图6可见,改性钢渣的除氟效果随着初始pH的增加而降低。溶液初始pH为强酸性时,改性钢渣的除氟率较高,达到80%左右;当pH=5.0~9.0,除氟率变化不大;当溶液为强碱性时,改性钢渣的除氟率明显下降,这一结论与刘平的研究相似[28]。这是因为在酸性条件下,带负电荷的钢渣与H+和F−形成一个电离层复合层,可促进钢渣对F−的吸附;另一方面,改性钢渣表面的活性点位发生质子化作用带正电,有利于对F−的吸附。在碱性环境中时,溶液中含有较多的OH−与F−存在竞争吸附,影响其吸附效率[37],而当pH上升至超过钢渣等电点时,电荷吸附作用减弱或消失,不利于对氟离子的去除。另外钢渣能够使吸附除氟后的溶液pH升高,吸附饱和后的钢渣可以用于肥料和水泥的生产,因此,处理污水的成本也未必升高。
-
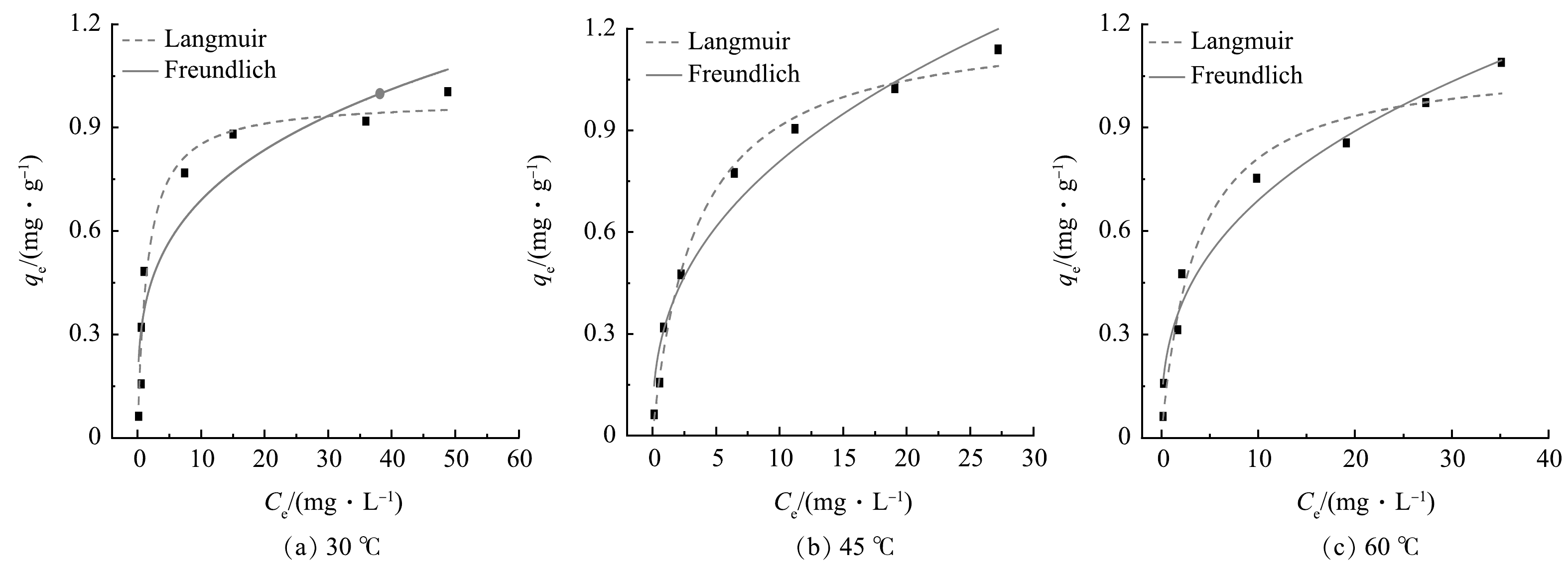
Langmuir吸附等温线和Freundlich吸附等温方程的拟合结果如图7及表2所示。Langmuir吸附数据表明,R2最高为0.991,这表明在吸附过程中,存在大多数单分子层吸附。吸附剂的理论最大单层分子吸附量为1.230 mg·g−1。Freundlich吸附数据表明,1/n的值最高为0.394,所以吸附反应过程能够进行,R2最高为0.976,表明吸附过程也不是标准的单层吸附,可能存在多层吸附[38]。Temkin吸附等温模型拟合结果如图8及表2所示。由R2值可知,Temkin吸附等温线拟合吸附过程准确度较高。根据拟合结果得到的bT的值分别为15.048、12.301、15.113 kJ·mol−1,表明吸附过程主要以化学吸附过程。
吉布斯自由能(
$ \Delta {G}^{0} $ )、焓变($ {\Delta }{\text{H}}^{\text{0}} $ )和熵变($ {\Delta }{\text{S}}^{\text{0}} $ )之间的关系如式(8)、式(9)和式(10)所示。式中:
${\Delta }{{G}}^{\text{0}}$ 为吉布斯自由能变,KJ·mol−1;R为理想气体常数,kJ·(mol·K)−1;T为温度,K;Ka为吸附平衡常数[39]; qe为平衡吸附量,mg·g−1;Ce为氟离子的平衡浓度,mg·L−1$;{\Delta }{{H}}^{\text{0}}$ 为焓变,kJ·mol−1;${\Delta }{{S}}^{\text{0}}$ 为熵变,J·mol−1。Ka随温度的上升而降低,表明该过程为放热过程,即温度升高会阻碍吸附的进行。做
${\Delta }{{G}}^{\text{0}}$ 对T的拟合线,通过计算截距和斜率可分别求得${\Delta }{{H}}^{\text{0}}$ 和${\Delta }{{S}}^{\text{0}}$ (表3)。结果表明,随着温度的增加,体系的${\Delta }{{G}}^{\text{0}}$ 升高,证明在吸附过程中,温度的增加会使吸附反应受阻。拟合线的截距得到${\Delta }{{H}}^{\text{0}}$ <0(-23.804 kJ·mol−1),说明该反应过程放热。根据Le Chatelier原理,升高温度会降低反应速率。拟合线的斜率得到${\Delta }{{S}}^{\text{0}}$ <0(-0.083 kJ·(mol·K)−1),表明在吸附过程中固体溶解水平的自由度下降[40]。${\Delta }{{S}}^{\text{0}}$ <0可能由于在较高温度下反应效率降低导致的[41];同理与未改性钢渣对比发现,由拟合截距得到${\Delta }{{H}}^{\text{0}}$ >0,说明未改性的钢渣除氟反应过程为吸热过程,而拟合斜率得${\Delta }{{S}}^{\text{0}}$ <0,表明未改性钢渣在吸附过程固体溶解水平的自由度也下降。 -
由表4可见,伪二级动力学模型的拟合结果优于伪一级动力学模型。因此,使用伪二级动力学模型可以更好地描述改性钢渣吸附F−的过程,表明吸附过程主要为化学吸附,而表面吸附是动力学控制的主要步骤[39]。随着初始氟浓度的上升,F−更容易与吸附剂上的活性点位结合,吸附速率上升,速率常数k2增大。
-
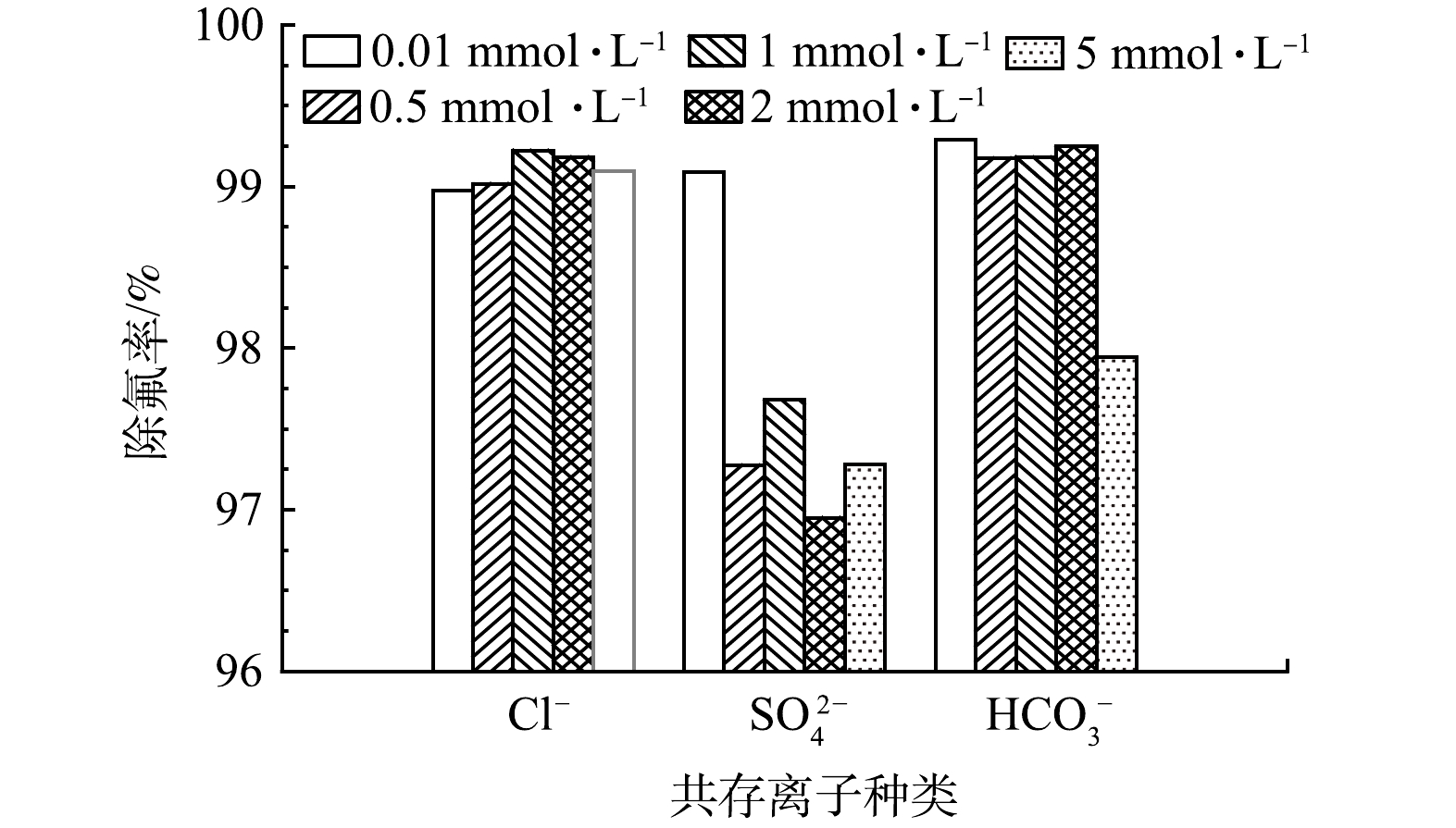
如图9所示,Cl−浓度的增加对改性钢渣除氟效率的影响并不明显。这是由于Cl−本身的选择性比F−低,难以和F−进行有效竞争。低浓度的SO42−对钢渣除氟效果的影响不大,但当SO42−的浓度大于或等于0.048 g·L−1时,钢渣的除氟效率明显受到抑制。这可能是因为随SO42−浓度的增加,其会与钢渣表面的铝、钙等物质形成少量难溶物,一方面会堵塞材料内部通道,另一方面会减少表面的吸附位点,从而影响除氟效果。HCO3−浓度的增加对改性钢渣除氟效率的影响一开始并不明显,但在其浓度达到0.3 g·L−1时抑制除氟过程,可能是因大量HCO3−的存在可改变溶液pH。同时,在酸性条件下HCO3−反应生成碳酸根离子(CO32−),高价阴离子会与F− 产生竞争吸附,使F−在改性钢渣吸附剂表面的吸附位点大幅度减少,从而影响改性钢渣的除氟效果。
-
1)使用30 g·L−1硝酸镧对钢渣改性36 h后得到改性钢渣,控制投加量为160 g·L−1,pH=3,吸附含氟浓度为10 mg·L−1的溶液。氟离子去除率达到81.33%。在吸附过程中,温度过高会抑制吸附。
2)改性钢渣对氟离子的去除率在4 s内达到80.98%,2 min时达到最高,为99.58%,约10 min达到吸附平衡。随着共存离子浓度的增大,除Cl−对氟吸附的影响不明显外,SO42−、HCO3−均会不同程度地抑制氟的吸附。镧改性钢渣的比表面积为23.367 5 m2·g−1,表面空隙以微孔为主。
3)准二级动力学模型能更好地描述吸附过程,说明化学吸附是主要的吸附过程。氟的初始浓度越高,F−越容易与钢渣表面的活性点位结合,从而提高吸附速率。Temkin吸附等温线模型拟合结果表明,吸附机理主要以化学吸附为主。由Langmuir吸附等温线模型的结果得到最大理论氟吸附容量为1.230 mg·g−1。
镧改性钢渣对水中氟离子的吸附性能
Adsorption performance of lanthanum-modified steel slag towards fluoride ion in water
-
摘要: 为有效去除铁路隧道开挖过程中涌出的地下水中的氟离子,避免对当地环境和居民身体健康造成危害,利用镧改性钢渣得到一种除氟材料,通过扫描电镜、比表面积测定、能量散射光谱、X射线衍射及傅里叶红外光谱等方法对材料进行表征。此外,结合吸附热力学和吸附动力学模型拟合,探究了改性钢渣对水中氟离子的吸附机理。结果表明:改性钢渣的比表面积由未改性的0.549 9 m2·g−1增大到23.367 5 m2·g−1,小粒径的钢渣比例增大且表面粗糙程度增强。能谱分析表明通过改性,可成功的将镧负载于钢渣表面。吸附拟合模型表明,钢渣对氟离子的吸附遵循Langmuir模型,说明钢渣对氟离子的吸附更接近于单层吸附,且主要为化学吸附。热力学参数表明,吸附吉布斯自由能(∆G0)>0,焓变(∆H0)和熵变(∆S0)<0,表明该反应是放热过程,改性钢渣的除氟过程符合伪二级动力学过程。改性钢渣有望成为一种具有应用前景的除氟材料。Abstract: To effectively remove fluoride ions in the groundwater gushed from Sichuan-tibet railway tunnel excavation process, and avoid the harm to the local environment and physical health of residents, a new type of fluoride removal material was prepared by using lanthanum modified steel slag. Scanning electron microscope and the measurement of specific surface area analysis, X-ray energy spectrum were used to characterize the material. The adsorption mechanism of fluoride ions in water by modified steel slag was investigated by adsorption thermodynamics and kinetics model fitting. The results showed that the specific surface area of modified steel slag increased from 0.5499m2·g−1 of unmodified steel slag to 23.3675 m2·g−1, the proportion of steel slag with small particle size and the surface roughness increased. Energy spectrum analysis showed that lanthanum could be successfully loaded on the surface of steel slag by modification. The adsorption fitting model showed that the adsorption of fluoride ions by steel slag followed the Langmuir model, indicating that the adsorption process was closer to monolayer one, and was dominated by chemical adsorption. Thermodynamic parameters showed that the adsorption Gibbs free energy (∆G0) was greater than 0, enthalpy change (∆H0) and entropy change (∆S0) were lower than 0, indicating that the reaction was an exothermic and entropy reduction process, and the fluorine removal process by modified steel slag fitted well with the pseudo second-order kinetics process. Modified steel slag is expected to be a kind of fluoride removal material with a good application prospect.
-
Key words:
- slag /
- lanthanum /
- fluoride removal /
- character
-

-
表 1 改性前后钢渣的元素组成及百分含量
Table 1. Elemental composition of steel slags before and after modification %
样品 C O Mg Al Si K Ca Ti Mn Fe La 改性前 14.15 35.75 3.87 4.39 9.09 0.55 20.77 8.29 1.02 2.11 — 改性后 45.85 — 13.93 27.73 3.57 0.86 2.66 2.78 — — 2.78 表 2 吸附等温线模型拟合参数
Table 2. Fitting parameters of adsorption isotherm model
温度/ ℃ Langmuir Freundlich Temkin qmax/(mg·g−1) B/(L·mg−1) R2 1/n KF/(mg·g−1) R2 aT/(L·g−1) bT/(kJ·mol−1) R2 30 0.980 0.661 0.970 0.277 0.365 0.893 9.662 15.048 0.961 2 45 1.230 0.287 0.991 0.394 0.326 0.970 5.719 12.301 0.971 3 60 1.101 0.279 0.971 0.369 0.294 0.976 6.764 15.113 0.968 4 表 3 热力学参数计算结果
Table 3. Calculation results of thermodynamic parameters
吸附剂 温度/K Ka/(mL·g−1) $ \Delta {G}^{0}/ $ $ \Delta {H}^{0}/( $ $ \Delta {S}^{0}/( $ 改性钢渣 303.15 0.661 1.043 −23.804 30.68 318.15 0.287 3.302 333.15 0.279 3.536 未改性钢渣 303.15 1.202 −0.009 −0.083 −1.038 2 318.15 4.404 −3.921 333.15 1.459 −3.124 表 4 吸附动力学模型参数
Table 4. Parameters of adsorption kinetics model
初始氟浓度/(mg·L−1) 伪一级动力学 伪二级动力学 qe1/(mg·g−1) k1/min−1 R2 qe2/(mg·g−1) k2/(g·(mg·min)−1) R2 10 0.002 −0.000 479 0.017 0.062 −4.293 0.999 7 50 0.015 −0.000 762 0.113 0.262 −20.872 1 100 0.069 −0.000 108 0.067 0.474 76.858 0.999 9 -
[1] 王贵玲, 张发旺, 刘志明. 国内外地热能开发利用现状及前景分析[J]. 地质学报, 2000, 21(2): 134-139. doi: 10.3321/j.issn:0001-5717.2000.02.005 [2] 刘伟. 西藏典型高温水热系统水文地球化学及地热利用引发的水污染问题[D]. 武汉: 中国地质大学, 2008. [3] 李克斌, 唐兴礼, 国丽, 等. 载镧壳聚糖去除水中氟离子的吸附特性研究[J]. 湖南科技大学学报. (自然科学版), 9, 24(3): 113-117.
[4] 雅茹, 陈姝璇, 奥妮琪, 等. 水中氟离子去除方法的研究进展[J]. 三峡生态环境监测, 2022, 7(3): 1-13. [5] HE J, YANG Y, WU Z, et al. Review of fluoride removal from water environment by adsorption[J]. Journal of Environmental Chemical Engineering, 2020, 8(6): 104516. doi: 10.1016/j.jece.2020.104516 [6] 李永富, 孟范平, 姚瑞华. 饮用水除氟技术开发应用现状[J]. 水处理技术, 2010, 36(7): 10-13. doi: 10.16796/j.cnki.1000-3770.2010.07.004 [7] 王月. 一种新型纳米氧化铝材料净水效果的初步研究[D]. 长春: 吉林大学, 2006. [8] 邹雄, 杨大锦, 刘俊场, 等. 硫酸锌溶液离子交换除氟氯工业化试验[J]. 有色金属(冶炼部分), 2021(11): 11-14. [9] 付娆, 张文龙, 冯江涛, 等. 锐钛矿型二氧化钛的低温合成及其吸附除氟性能的研究[J]. 环境工程, 2020, 38(2): 70-76. doi: 10.13205/j.hjgc.202002009 [10] DHANASEKARAN P, SAI P M S, GNANSEKAR K I. Fixed bed adsorption of fluoride by Artocarpus hirsutus based a dsorbent[J]. Journal of Fluorine Chemistry, 2017(195): 37-46. [11] MILLAR G J, COUPERTHWAITE S J, DAWES L A, et al. Activated alumina for the removal of fluoride ions from high alkalinity groundwater: New insights from equilibrium and column studies with multicomponent solutions[J]. Separation and Purification Technology, 2017, 187: 14-24. doi: 10.1016/j.seppur.2017.06.042 [12] RAVULAPLLI S, KUNTA R. Defluoridation studies using active carbon derived from the barks of Ficus racemosa plant[J]. Journal of Flourine Chemistry, 2018, 193: 58-66. [13] YE C, YAN B, JI X, et al. Adsorption of fluoride from aqueous solution by fly ash cenospheres modified with paper mill lime mud: experimental and modeling[J]. Ecotoxicology Environment Safety, 2019, 180: 366-373. doi: 10.1016/j.ecoenv.2019.04.086 [14] CHOONG C E, WONG K T, JANG S B, et al. Fluoride removal by palm shell waste based powdered activated carbon vs. functionalized carbon with magnesium silicate: implications for their application in water treatment[J]. Chemosphere, 2019, 239: 124765. [15] ALKURDI S S A, Al-JUBOORI R A, BUNDSCHUH J, et al. Bone char as a green sorbent for removing health threatening fluoride from drinking water[J]. Environment International, 2019, 127: 704-719. doi: 10.1016/j.envint.2019.03.065 [16] TCHOMGUI-KAMGA E, ALONZO V, NANSEU-NJIKI C P, et al. Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water[J]. Carbon, 2009, 48(2): 333-343. [17] WAMBU E W, AMBUSSO W O, ONINDO C, et al. Review of fluoride removal from water by adsorption using soil adsorbents – an evaluation of the status[J]. Journal of Water Reuse and Desalination, 2016, 6(1): 1-29. doi: 10.2166/wrd.2015.073 [18] RAICHUR A, BASU M. Adsorption of fluoride onto mixed rare earth oxides[J]. Separation and Purification Technology, 2001, 24(1/2): 121-127. [19] AGARWAL M, RAI K, SHRIVASTAV R, et al. Defluoridation of water using amended clay[J]. Journal of Cleaner Production, 2003, 11(4): 439-444. doi: 10.1016/S0959-6526(02)00065-3 [20] CENGELOGLU Y, KIR E, ERSOZ M. Removal of fluoride from aqueous solution by using red mud[J]. Separation and Purification Technology, 2002, 28(1): 81-86. doi: 10.1016/S1383-5866(02)00016-3 [21] PIEKOS R, PASLAWSKA S. Fluoride uptake characteristic of fly ash[J]. Flouride, 1999, 32(1): 14-19. [22] 李春鹭. 聚吡咯负载花生壳质生物质炭去除水中氟的研究[D]. 北京: 中国地质大学, 2017. [23] 王建国. 镧改性柚子皮生物碳除氟性能及机理研究[D]. 北京: 中国地质大学, 2018. [24] SCOTT I S P C, PENN C J. Estimating the variability of steel slag properties and their influence in phosphorus removal ability[J]. Chemosphere, 2021, 276: 130205. doi: 10.1016/j.chemosphere.2021.130205 [25] 张龙强. 钢渣处理含铅、镉废水的性能研究[D]. 南京: 南京理工大学, 2015. [26] 张峻搏. 钢渣对含磷废水及染料废水的吸附性能及应用研究[D]. 石家庄: 河北科技大学, 2020. [27] 纪鹏华. 钙/铝改性钢渣陶粒的制备及其除磷性能的研究[D]. 保定: 河北大学, 2017. [28] 刘平. 含氟废水的吸附处理研究[D]. 南宁: 广西大学, 2005. [29] TCHOMGUI-K E, ALONZO V, NANSEU-NJIKI C P, et al. Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water[J]. Carbon, 2010, 48(2): 333-343. doi: 10.1016/j.carbon.2009.09.034 [30] 陈琼. 改性钢渣去除铀(VI)的实验研究[D]. 南华: 南华大学, 2020. [31] 胡家朋, 刘瑞来, 林皓, 等. 改性羟基磷灰石制备及除氟特性研究[J]. 人工晶体学报, 2019, 48(1): 81-89. doi: 10.16553/j.cnki.issn1000-985x.2019.01.014 [32] 冯珊珊, 汪凯举, 章蓝月, 等. 钢渣对Ni~(2+)与Cu~(2+)的竞争吸附研究[J]. 硅酸盐通报, 2022, 41(5): 1750-1757. [33] 牛晨雨. 铁氧化菌改性钢渣水体除砷性能研究[D]. 武汉: 武汉理工大学, 2018. [34] 卢明阳, 庞来学, 杨达, 等. 钢渣-煤基固废可控低强度材料制备及热力学分析[J]. 硅酸盐通报, 2022, 41(7): 2344-2351. doi: 10.16552/j.cnki.issn1001-1625.2022.07.024 [35] 罗智宏, 何峰, 张文涛, 等. 熔融法转炉钢渣微晶玻璃的结构与性能研究[J]. 人工晶体学报, 2018, 47(3): 514-521. doi: 10.3969/j.issn.1000-985X.2018.03.011 [36] 何环宇, 虎涛涛, 刘虹灵, 等. 钢渣-废FCC催化剂耦合制备地质聚合物及其性能表征[J]. 武汉科技大学学报, 2022, 45(2): 87-92. [37] 魏惠. 钢渣对磷的吸附与解吸特性及影响因素研究[D]. 郑州: 郑州大学, 2010. [38] 卢琪愿, 张卫民, 王玉罡. 钢渣负载纳米零价铁-羟基磷灰石的制备及其对锰的吸附性能[J]. 有色金属(冶炼部分), 2022(5): 86-94. [39] HAI N Y T, YOU S J, HOSSEINI-BANDEGHARAEI A, et al. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review[J]. Water Research, 2017, 120(9): 88-116. [40] VAN L-N, SJOBERG V, NGOC P D, et al. Removal mechanism of arsenic (V) by stainless steel slags obtained from scrap metal recycling[J]. Journal of Environmental Chemical Engineering, 2020, 8(4): 103833. doi: 10.1016/j.jece.2020.103833 [41] AHMADI S, MOHAMMADI L, RAHADR A, et al. Acid dye removal from aqueous solution by using Neodymium(III) oxide nanoadsorbents[J]. Nanomaterials, 2020, 10(3): 556. doi: 10.3390/nano10030556 -



 下载:
下载: