-
有机污染物中丰富的共价键使其结构极为稳定,偶氮、芳香类共轭单元更是难以处理[1-2],其降解需要消耗大量能量,排入水中的有机物已造成了严重的水体污染问题[3-4],为此,可降低反应活化能的各类催化剂被用于有机物的降解过程,并成为一大热点[5]. 其中,光催化工艺更是以可再生的太阳能为能量来源,具有成本低、安全环保、清洁高效的优势[6-8],表现出巨大的研究与发展潜力.
为解决有机污染物降解难题,各类光催化剂的性能优化研究热度与日俱增. 光降解有机污染物的进行有赖于光生载流子与反应物间的电荷转移[9],但单一光催化剂通常面临着载流子难以激发、寿命过短的困境[10]. 而异质结的构建可对光催化剂的电子结构及形态特性进行调整,有效增强其光吸收能力及载流子迁移效率,并对其氧化还原能力进行调整,是改良光降解有机污染物效率的有效策略之一[11-12].
在众多异质结型光催化剂中,由TiO2与g-C3N4晶体所组成的TCN异质结在光降解领域表现出巨大潜力. TCN异质结性能的高效性来源于以下几个方面:(1)g-C3N4有着2.7 eV的适中带隙[13],光吸收谱带较宽,使TCN异质结具有优异的可见光响应能力;(2)g-C3N4晶体中sp2杂化的碳氮原子间形成共轭结构[14],同时,其良好的柔韧性有助于特殊形貌中紧密界面接触的形成[15-16],可有效促进电荷的分离与转移[17];(3)TiO2价带电势为3.1 eV[18],空穴氧化能力较强,而g-C3N4导带电势为-1.27 eV[19],具有优异的电子还原能力,为高氧化还原性系统的构建提供了基础;(4)TiO2表面丰富的羟基及g-C3N4晶体上丰富的官能团与缺陷位点有助于TCN异质结通过物理或化学作用实现各种有机污染物的捕获[20-21];(5)TiO2与g-C3N4两种晶体均具有良好的结构稳定性,生产成本较低[22-23],使TCN异质结具备工业化应用潜力.
TCN异质结中两晶体间相容的能级结构使载流子可沿Ⅱ型或Z型路径进行定向迁移. 但在Ⅱ型路径中,电子与空穴迁移向弱氧化还原能力的能带结构处,而在Z型路径中,弱氧化还原能力的载流子将在界面处复合[22]. 故而,两种TCN异质结分别存在着氧化还原能力与载流子利用效率方面的限制,且实际废水中成分极为复杂,现有的TCN异质结并不能充分解决各类污染物的降解问题. 鉴于上述问题,国内外学者开展了大量围绕TCN异质结改性策略的研究,而尺寸调控[24-26]、形貌构建[27-29]与缺陷工程[30-32]已被证明是改善TCN异质结性能的有效策略.
为此,本文比较了两种常见TCN异质结的形成机理与特性,从电子结构和微观形态的角度出发,总结了针对异质结光吸收能力、氧化还原能力、载流子迁移效率、表面活性位点及吸附能力的调控策略,讨论了TCN异质结在实际应用中可采取的优化策略,并且就目前TCN异质结在应用进程中存在的挑战进行了分析.
-
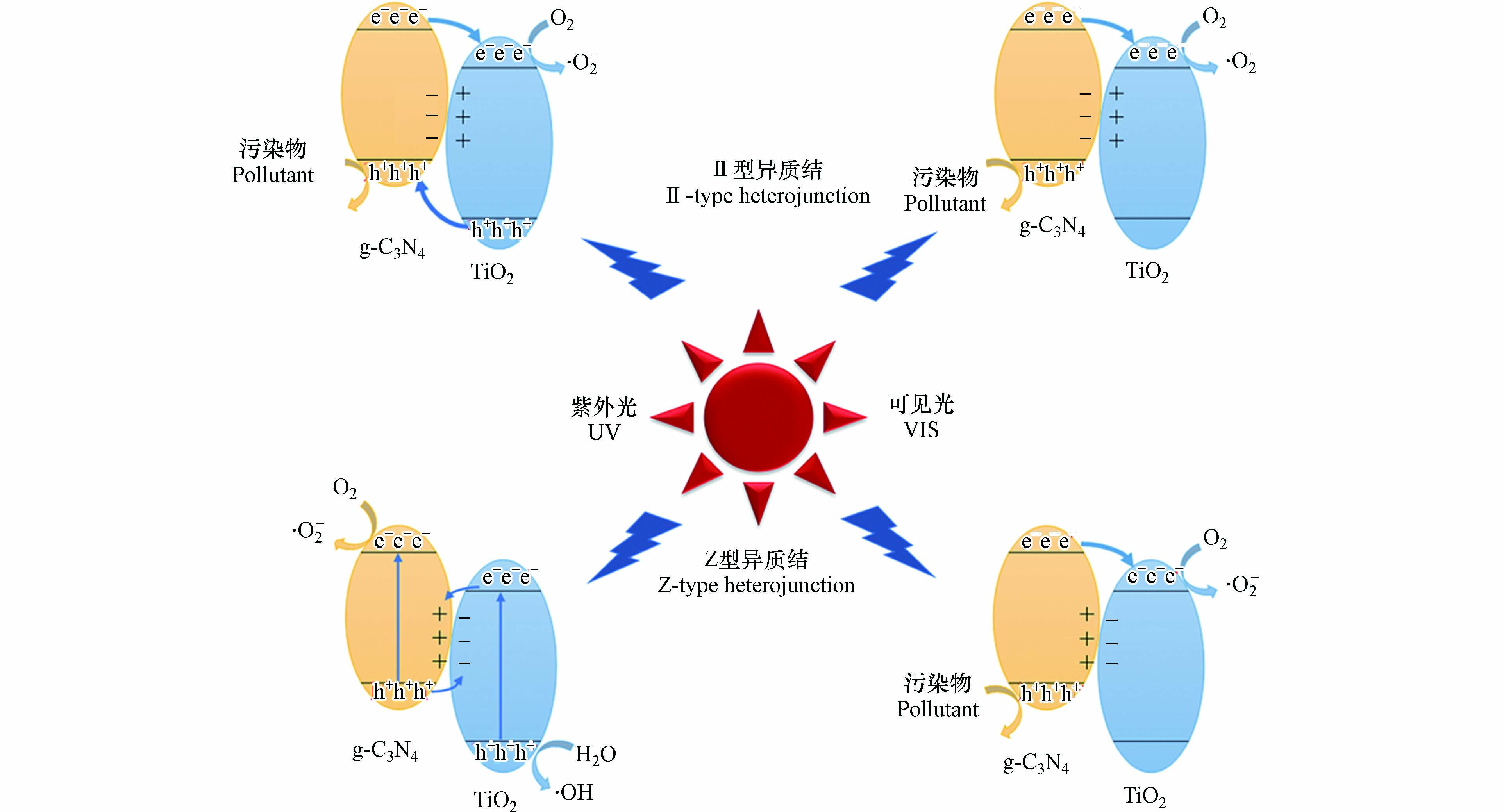
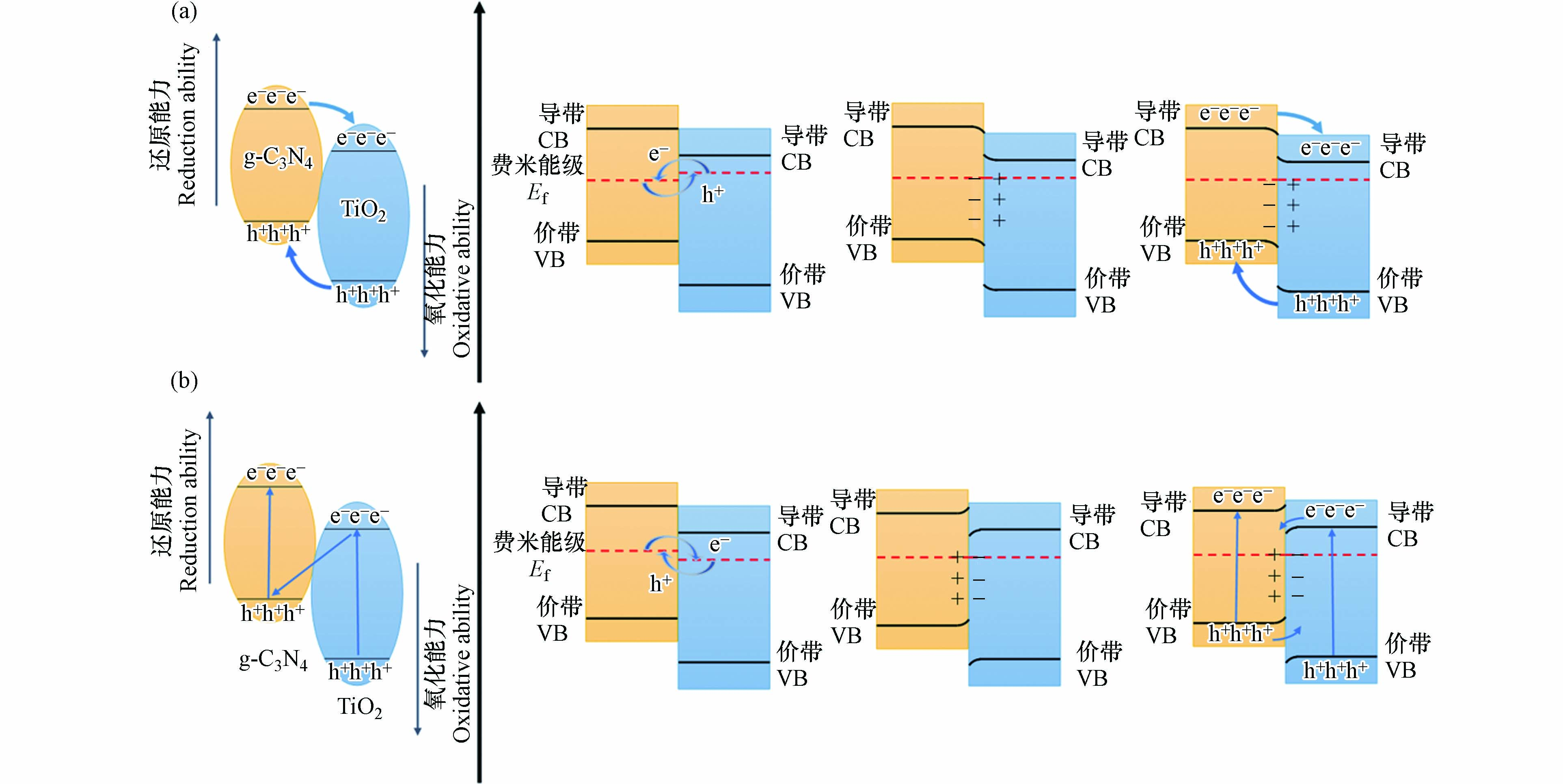
TCN异质结的类型同两晶体界面处内建电场方向有关. 如图1a所示,当g-C3N4一侧处于富电子状态时,所形成的内建电场会使光生空穴由TiO2迁移向g-C3N4的价带,而光生电子则由g-C3N4迁移向TiO2的导带,此时两晶体间形成II型异质结[33]. 而当TiO2一侧处于富电子状态时,如图1b所示,所形成的内建电场会使TiO2产生的光生电子与g-C3N4产生的光生空穴相复合,令TiO2生成的强氧化性空穴与g-C3N4生成的强还原性电子得以保留,此时TiO2与g-C3N4间形成Z型异质结[34].
TCN异质结界面处内建电场方向取决于界面两侧晶面的能级结构. 在TCN异质结的形成过程中,由于TiO2与g-C3N4晶体间存在费米能级的差异,自由电子会自发向费米能级较低处迁移,空穴沿相反方向进行迁移以使两晶体费米能级相等,并导致界面两侧电荷分布的差异[35],同时,费米能级的移动会带来能级结构的整体移动,使界面处能带发生弯曲. 而在TCN异质结形成后,由于晶体中各晶面有着不同的导带能级,光生电子倾向于在导带电位较正的晶面区域聚集,使异质结界面两侧电荷分布进一步改变[36].
TCN异质结中载流子的定向迁移趋势使载流子复合倾向受到抑制,寿命得到延长,因而载流子迁移效率较高[37]. 然而,在II型异质结中,光生电子与空穴分别集中在高电势的导带和低电势的价带上,使氧化还原能力受限[38]. 而在Z型TCN异质结晶体中,尽管TiO2生成的强氧化性空穴与g-C3N4生成的强还原性电子得到保留,但由于界面间光生电子与空穴的快速结合[39],其载流子利用效率将会降低.
g-C3N4晶体的窄带隙结构使TCN异质结表现出较宽的光响应谱带. 当光源波长处于TiO2晶体光响应范围外时,由于g-C3N4导带电位较高,其所产生的电子会转移到TiO2的导带中,而空穴则保留在价带处[40,41],本文中将该载流子迁移路径命名为VIS型路径. 在VIS路径下,TCN异质结的氧化还原能力将受到限制,但仍表现出较高的载流子迁移效率[40]. 但Z型异质结界面处的内建电场及弯曲能带会对载流子的迁移起阻碍作用,不利于载流子迁移效率的提高. 因而TCN中载流子的迁移存在3种路径,即II型、Z型及VIS型路径,其中VIS型路径存在VIS-II及VIS-Z两种情况,如图2所示.
-
TCN异质结对有机污染物的光降解作用有赖于强氧化活性物种的生成. 如图2所示,光源波长处于TiO2晶体的光响应范围内时,Z型TCN异质结中的光生载流子可将OH-及O2活化,使其电子结构不再稳定,分别生成羟基自由基(·OH)与超氧根自由基(·O-2)两种活性自由基[42]. 受空间效应影响,具有高氧化电位的空穴(h+)虽然可直接参与反应的进行,但并不是主要活性物质. 而在II型或VIS型TCN异质结中,空穴会集中在氧化电位较低的g-C3N4上,不足以将H2O氧化为·OH[41,43],此时空穴可作为主要活性物质参与光降解反应. 各活性物种的生成机理[8,44-45]如图3所示.
在·OH、·O-2等氧化活性物种的协同作用下,有机污染物将经由开环、羟基化、脱烷基化和取代反应等途径不断降解为分子量更小的中间体[43,46]. 在此过程中,降解产物所含氧原子数不断增加,其最终产物可能是羧酸、聚羧酸和羟基化羧酸类小分子有机物,也可经进一步氧化生成CO2、H2O、胺等小分子无机物[5,47-48].
-
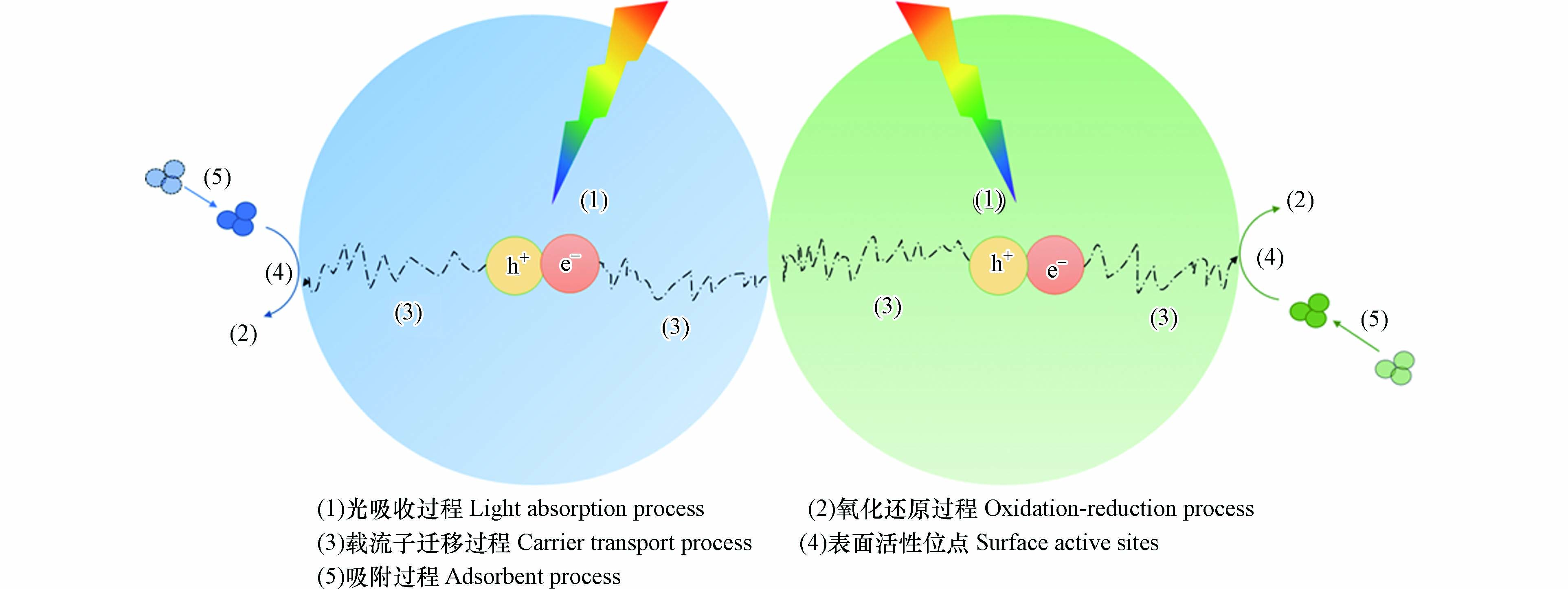
TCN异质结的性能与其结构密切相关. 图4所示为TCN异质结光降解有机污染物的理论模型图,由图可知,在有机污染物的光降解过程中,影响降解效果的性能有:(1)光吸收能力、(2)氧化还原能力、(3)载流子迁移效率、(4)表面活性位点密度、(5)吸附能力. 为此,大量的研究集中在通过尺寸调节、形貌构建及缺陷工程等手段来改善TCN异质结的上述性能,提高其对有机污染物的降解能力.
-
光吸收是光催化过程中的能量获取途径,故而光吸收能力是衡量光催化剂性能的一项重要指标. 尽管TCN异质结的光响应谱带已拓宽至可见光区甚至近红外区,但其光吸收谱带峰值通常位于近紫外区,而短波光对废水的透射能力较弱[5],故光吸收能力的增强对TCN异质结的光降解能力而言仍具有重要意义.
-
TCN异质结的能带结构主要由各原子最外层轨道或经其杂化组成[49-50]. 当晶格中原子发生变化时,会造成原子轨道的变化,使TCN异质结中生成新的杂质能级[51-52]. 这些杂质能级可能位于禁带中,也可与导带或价带重叠推动带隙的缩短[53]. 杂质能级的引入通常可利用元素在晶格中的掺杂实现,Yuan等[54]通过简单水热处理合成出N/Ti3+共掺杂TCN异质结,由于N掺杂与Ti3+自掺杂可分别在TiO2的价带上方及导带底部引入杂质能级,使价带电子可经由两中间能级分步跃迁至导带,故而异质结光响应谱带发生明显红移,光吸收强度显著提高.
空位的引入同样可诱使杂质能级的生成,还可使缺陷部位生成较弱的键合作用,阻碍成键轨道与反键轨道的分裂,使TCN异质结带隙弯曲窄化,电子跃迁所需能量减少,光响应谱带随之拓宽[55]. Kang等[56]以氢气为还原剂制备出氧空位型TiO2纳米纤维,随后通过g-C3N4的包覆合成出氧空位型TCN异质结,发现其表现出1.16 eV的价带尾,使能级带隙大为缩短,异质结光吸收能力得到显著提高. Gao等[57]以NaBH4为还原剂对TCN异质结进行混合煅烧,在TiO2与g-C3N4晶体中同时引入缺陷,所生成双缺陷型TCN异质结表现出显著增强的可见光响应能力.
-
Yan等[58]根据Mie散射定律计算发现当纳米颗粒尺寸小于16 nm时,所有与能级结构相匹配的光都将被吸收. 在此基础上,Yan等制备了TiO2尺寸分别为9 nm、12 nm与15 nm的TCN异质结,发现随着TiO2尺寸的减小,在量子约束效应的作用下,其吸光强度不断下降. 这是由于当晶体尺寸减小到纳米尺度下的一定值后,电子-空穴对间的分离将受到抑制,使带隙得到拓宽,光响应谱带随之缩小[59-60].
通过调整形态结构,利用光线的反射与折射性能增强光吸收能力[61]是另一有效措施. 据Wu等[62]报道,介孔g-C3N4材料中纳米孔的存在可加强光线的折射,使光线集中在孔隙中,而TiO2颗粒的引入则会使光能富集到TiO2周围,使光吸收强度进一步增强. 2D晶体间的合理分布是增强反射及折射效应的常用手段,Zheng等[63]通过将g-C3N4纳米片与TiO2纳米片进行物理混合制备出2D-2D Z型TCN异质结后发现,TiO2纳米片在取向上的随机分布使得光线在面间可多次反射,因而该TCN异质结具有较好的光吸收能力. 此外,将1D、2D结构组装为3D网络结构或构建中空结构也是改善光吸收能力的有效策略[12,16,64].
-
有机污染物的光降解本质上是氧化还原反应,主要依靠氧化活性物种完成. 氧化还原能力的提升可使光降解反应进程更加高效,因而氧化还原能力的大小成为影响TCN异质结性能的关键因素之一.
-
为获得足以破坏目标化合键的氧化还原能力,光催化剂应具有足够负的导带电位及足够正的价带电位[65]. 对于尺寸较小的晶体,在量子约束效应的影响下,载流子间电场相互作用将会增强,电子跃迁所需能量增大,从而推动导带或价带的移动[59,66-67]. 以g-C3N4组分为例,随厚度减小,晶体导带位置会向负方向移动,使其表现出更强的还原能力[68]. 然而这种调控手段需要以光响应能力为代价,Zhang等[26]通过控制煅烧温度制备出不同g-C3N4厚度的TCN异质结,发现g-C3N4层厚度的减小会使带隙不断加宽,这可以归因于量子约束效应与g-C3N4相对含量减小带来的协同效应.
相比而言,杂质能级的引入在调节氧化还原能力的同时还有利于光吸收能力的增强. Zhang等[69]报道称,同传统煅烧法相比,等离子体法制备出的TCN异质结有着更小的粒径尺寸,同时又会产生更多的氧空位,因而在表现出更强的氧化能力的同时有着更小的带隙. Yang等[70]通过VB XPS光谱及平带电位的分析实验发现,氮、氧空位的掺入会使g-C3N4与TiO2的能带结构整体下移,使TCN异质结的氧化能力得到增强. Wong等[71]通过微波水热处理和热缩聚同时实现了g-C3N4的氧化与TiO2晶体表面的还原,O原子在g-C3N4中的掺入推动了价带值的上移,实现能带结构的整体上移,使还原能力得到提升.
-
表面自由基是O2、H2O经载流子活化而成的产物,故而TCN异质结表面对O2及H2O的亲和力对其生成具有重要作用[72]. 据Huang等[72]报道,P、O共掺杂可以增强g-C3N4表面的电负性,使TCN异质结的亲水性得到增强,有效促进·OH的生成. 为探究空位对表面自由基的调节作用,Ning等[73]利用一次氢化处理制备出氮氧双空位Z型TCN异质结. 电子顺磁共振光谱实验结果表明,氧空位的掺入有助于促进·OH的生成,这是由于TiO2晶体表面的氧空位可诱导产生更多的羟基,促进·OH的生成,同时,氮空位的掺入可有效促进·O-2的产生.
-
载流子的迁移过程是维持光催化剂氧化还原能力的一大基础,尽管TCN异质结中两晶体交错的能带结构促进了电荷的分离以及载流子的迁移,但载流子在晶体间迁移时仍会不可避免地产生损失[74],故此提高载流子的迁移效率对TCN异质结性能的改良而言具有重要意义.
-
元素的掺杂易引起偶极矩的变化,导致局部电场的生成并起到捕获载流子的作用,从而促进电荷的分离传输[53]. Shi等[75]通过金属有机框架及尿素的原位热解将Co2+掺杂进TCN异质结,位于晶体中的Co2+可起到电子俘获位点的作用,当其处于界面处时还可起到电子媒介的作用,加速晶体间电荷转移. 当元素掺杂所诱生的杂质能级位于禁带中时,还可为载流子的迁移提供新的弛豫路径[55],有效提高载流子的分离效率. Lin等[34]通过混合泛函计算得出,C及B元素的掺入可使禁带中心产生杂质能级,加速电荷的传输. 但位于带隙深处的杂质能级将形成强载流子俘获位点,对载流子的迁移起到阻碍作用[50,76].
氮、碳、氧空位的引入同样可引入中间能级[77,78],形成浅俘获位点. Shi等[79]报道了一种通过MOF前体与三聚氰胺的原位热解法合成出氧空位型TCN异质结的方式,氧空位作为浅俘获位点,有效介导了载流子的复合弛豫,促进了光生电荷的分离与转移,使电子转移可在亚皮秒的时间尺度内进行. 此外,空位的引入还可有效促进缺陷附近电荷的再分配[80]. 据Zhang等[78]报道,阳离子与阴离子空位分别会诱导空位与电子浓度的提高,使缺陷附近电导率发生变化,形成内部电场,有效促进载流子的迁移.
位于界面处的空位还可促进异质结界面间的紧密连接[33]. 分层异质结结构界面处相互作用通常较弱,不利于载流子的界面间迁移,为此,Li等[81]通过在g-C3N4纳米片中引入碳空位并进行表面羟基化,促进了TiO2在g-C3N4上的原位组装,极大地改善了异质结界面间的相互作用. 同时,界面空位的引入还可引发界面两侧电荷的再分配,有效调节异质结界面间的内建电场强度及方向,为异质结类型的改变提供了可能[33]. 晶面间耦合作用的加强也可通过增大晶面间的接触面积来实现. Xiao等[82]通过质子化预处理的方式减小了g-C3N4的晶体尺寸,使其同TiO2纳米管阵列之间的接触面积得到增大,施主密度随之提升,促进了TCN异质结界面处的电荷转移.
-
载流子迁移路径主要与晶体形态结构有关,是决定载流子迁移效率的又一重要因素. 晶体尺寸的减小可使电荷传输路径缩短,体复合随之受到抑制,使载流子迁移能力得到增强[83]. 传统的凝胶法中通常通过控制胶体悬浮液浓度及煅烧温度来实现厚度的控制[25],但这种方式难以实现厚度的精准控制,实验重复性较差. 故Lv等[84]利用原子层沉积技术,通过控制TiO2在g-C3N4上的循环沉积次数来控制TiO2纳米颗粒的厚度,结果表明过量负载的TiO2会延长电荷传输路径,阻碍电子转移. 但随后的ESI测试结果表明,在循环沉积次数为5-65对应的TCN异质结中,循环次数为35的样品表现出最小的电子转移电阻,这是由于过小的尺寸会使电子空穴对间相互作用将会得到增强.
在各形态中,0D晶体载流子迁移路径极短[85],因而载流子迁移能力较强. Guo等[86]通过g-C3N4与TiO2两种量子点间的简单机械搅拌混合,制备了0D-0D II型TCN异质结,发现该TCN异质结的光电流强度最终达到TiO2的25倍. 相比而言,二维及一维纳米晶体有着受限的载流子迁移路径方向,表现出优异的定向迁移能力[87]. 其中,二维晶体材料有着快速面内传输移运的能力[88],Ni等[47]通过将氧空位型TiO2纳米颗粒负载于g-C3N4纳米片两侧制备出三明治状的TCN异质结,这种三明治结构的形成有效缩短了载流子的传输路径,且g-C3N4纳米片的存在使TCN异质结表现出较强的电荷转移传输能力. 而一维半导体更是有着优异的载流子单向传输能力[89],可有效阻止光生电子空穴对间的复合,促进载流子分离及传输效率的提高,Cui等[90]通过水热法将TiO2纳米棒加载到g-C3N4纳米片上制备出TCN异质结,该样品有着电荷快速输运的特性,且可使载流子间复合受到限制.
-
光氧化还原反应通常发生在活性位点上,活性位点数目的增多可避免载流子的过度累积,提高载流子利用率,加快光降解过程的进行. 故而活性位点密度的调节同样可以促进光降解有机污染物反应的进行.
比表面积的增大可带来表面活性位点密度的提高,是调节表面活性位点的最常用手段[64]. 传统的一维结构相邻晶体单元间空间较大,比表面积较小,而Nasir等[91]采用静电纺丝、碱水热法制备出了支化TiO2纤维,而后利用气相沉积技术得到1D-0D II型TCN异质结. 其中,支化TiO2纤维有着高达205.5 m2·g−1的比表面积,但由于量子点负载位点难以控制,不可避免地造成了量子点在平面间隙间的分布,使TCN异质结的比表面积降低到92.39 m2·g−1.
对于纳米片等2D结构而言,孔道结构的引入不仅可以促进光吸收能力的提升及晶面间接触的增强[62],其增大的比表面积还可赋予TCN异质结更多的活性位点. 通过模板法引入孔道结构是一种常用手段,但模板的去除条件却相对苛刻,不利于实际工业应用[92]. 为此,Zheng等[63]通过简单的NH3自蚀刻使g-C3N4纳米片晶体结构中的N-C3键部分断裂,从而生成更多的孔结构,使其比表面积高达224 m2·g−1,使活性位点密度大为提升,随后,具有大比表面积的超薄尺寸TiO2纳米片的负载使活性位点密度得到进一步提升. 尺寸的减小则是增大比表面积的通用手段[83],但过小的尺寸会造成光吸收能力的减弱.
表面空位生成后,晶体表面晶格将会无序化,使表面能得到增加,可起到表面活性位点的作用,使活性位点密度得到提升[55]. Li等[64]通过g-C3N4纳米棒间的相互缠绕合成出3D型g-C3N4,随后通过氧空位型TiO2的负载制备成TCN异质结. 由于TiO2晶体中的氧空位可起到表面活性位点的作用,且g-C3N4的三维网络结构提供了大的比表面积,该TCN异质结的表面活性位点密度要显著高于普通TCN异质结. 此外,晶体比表面积的增大会使表面金属空位形成能降低,促使阳离子空位的形成从而进一步增大活性位点密度[93].
-
反应物与载流子间的催化反应时间在ns-μs之间,而载流子的复合通常发生在ps-ns的时间尺度上[94],故而传质过程是制约载流子利用率的重要一环. 吸附过程可使目标污染物富集到TCN异质结附近,缩短光降解过程中的传质距离[95],促进光催化过程的进行,并实现吸附与光降解协同去除有机污染物,因而吸附能力的调控也是提高TCN异质结性能的常用手段.
-
在各种吸附形式中,静电吸引不仅可带来较强的作用力,还可实现有机污染物的选择性吸附. 在TCN异质结中,g-C3N4晶体表面通常呈负电荷,对阳离子型污染物的选择吸附性较高[21,96]. Liu等[97]利用再结晶退火策略制备出表面碱化TCN,使晶体表面负电荷得到进一步增强,使其对亚甲基蓝吸附能力得到大幅提升,降解效率随之大幅增长,而未经碱化的TCN对亚甲基蓝吸附效果不明显,且降解效率极低. 与此相反,Wang等[21]通过酸性水热处理的方法使g-C3N4表面电荷发生转变,使其所制备出的TCN异质结对阴离子染料表现出较强的选择吸附性.
表面空位对H2O和O2等分子具有极强的吸附能力,可促进氧化活性物种的形成,有效增强异质结的光降解能力[98]. 但过强的结合会导致这些物种难以释放进行下一步反应,甚至可通过捕获氧原子消除缺陷,造成空位失活[99]. 据Liu等[98]报道,表面氧空位易受到氧物种的影响,结构稳定性较差,相比而言,亚表面氧空位对含氧物种有着适中的吸附能力,可降低表面催化反应能垒,且结构较为稳定,可有效促进氧化活性物种的生成.
-
相比于静电吸附相对有限的适用性,通过增大比表面积使吸附位点增多,进一步促使吸附性的提升更具普适性[100]. Hu等[100]通过g-C3N4纳米片在TiO2微球上的原位生长及TiO2的自组装制备出三维花状g-C3N4/TiO2复合微球,g-C3N4纳米片的加入使光生电子富集到TiO2晶体表面,改变了TiO2微球的表面电荷,使其对阳离子型有机物表现出高选择吸附性. 同时,该TCN异质结有着239.75 m2·g−1的高比表面积,使其吸附能力得到进一步增强.
微孔结构可使TCN异质结的比表面积大为提升,但其较小的孔道却不利于传质过程的进行,故通常采用介孔或大孔结构来提高其传质能力[62]. 为此,Zou等[101]以聚乙烯吡咯烷酮(PVP)及液态石蜡为模板剂,采用一步静电纺丝和煅烧法相结合的方式制备出分级介孔/大孔TCN异质结,这种中孔大孔互联通孔结构不仅提供了高的比表面积,还为分子的扩散提供了极为有效的传输路径,使吸附活性位点数量大为提升.
-
为增强TCN异质结的光降解能力,多种TCN异质结已通过各类调控策略被构建而出,并用于各种有机污染物的去除研究. 表1展示了近3年来TCN异质结在光降解有机污染物的代表性研究成果.
目前所制备出的TCN异质结多呈粉末状,可直接投置于废水中进行污染物的降解. 在此过程中,除异质结自身结构外,TCN异质结的性能还会受到外部环境的影响. 以溶液pH值为例,Hu等[43]报道称,溶液pH值对TCN异质结的吸附性能有着较大影响. 当溶液pH值小于等电点时,TCN异质结表面呈正电荷状态,对阳离子状态下的有机物呈排斥作用,而当其大于等电点时,则表现出逆转的吸附.
适宜的预处理可使TCN异质结的性能达到最佳值,而精确的建模则有助于更快地探索出最适宜参数. 为探索各工艺参数对光降解过程的影响程度,Ali等[112]对有机污染物初始浓度、反应温度、光照强度及催化剂用量同降解速率参数间的关系进行了建模,并提出在各参数中,有机污染物初始浓度会影响到光吸收过程,对光降解性能影响最大.
TCN异质结光降解有机污染物的一大目的在于消除其毒性. Guo等[48]以大鼠为标本对四环素及其光降解中间体进行了毒性分析,发现大部分四环素中间体毒性降低,相似的结论在Dou等[7]对阿莫西林和头孢噻肟光降解中间产物进行评估时被得出. 然而,Dou等[7]发现某些中间产物存在诱变为高毒性中间体的可能. 因此建立起光降解过程后续毒性评估工艺具有一定的必要性.
目前而言,单一的光催化技术适用场合有限,而光催化技术同其它技术的联用则可使其应用范围得到拓展. 如,粉末状的TCN异质结面临着难以回收的问题,不能很好地适用于流动系统. 而膜技术与光催化的结合则可以很好地解决这个问题. Zhang等[113]利用恒电位阳极氧化法将g-C3N4量子点组装到TiO2纳米管阵列(TNA)膜上,制备出具有直通道和高度有序排列的TCN异质结膜材料. 经检测,样品膜具有极高的孔隙率,其纯水通量约为250 L·m−2·h−1,且具有较高的抗压性. 在可见光下,可去除60%以上的罗丹明B.
-
综上所述,TCN异质结具有较宽的光吸收谱带与较低的载流子复合率,且可通过尺寸调控、形貌构建及缺陷引入的策略使其吸附能力、氧化还原能力、载流子迁移能力等性能进一步提升,因而在光降解污染物等领域具有巨大前景. 目前,TCN异质结的构建研究已取得许多重大进展,但仍然存在一些问题需要解决:(1)为探索TCN异质结的构-效关系,许多高成本或高风险的合成方式被应用,但并不适用于实际工业生产,未来应注重低成本大批量制备方式的研发;(2)TCN异质结在微塑料降解中的研究相对不足,制约其光降解活性的一大因素在于异质结对微塑料的弱吸附性,以异质结光氧化还原能力促进强氧化剂(如H2O2)循环再生可以达成异质结对微塑料的间接氧化作用,将有助于解决微塑料难传质的问题.
光降解有机污染物用TiO2/g-C3N4异质结的构建策略
Study on the strategy of TiO2/ g-C3N4 heterostructure construction for photodegradable organic pollutants
-
摘要: 由TiO2与g-C3N4晶体所组成的TiO2/g-C3N4异质结(TCN异质结)有着成本低廉、带隙较窄、光响应谱带宽、载流子迁移效率高等优点,可有效解决有机污染物降解过程耗能高的问题. 然而,常见的两种TCN异质结存在着载流子利用率或氧化还原能力上的限制. 为此,需要对TCN异质结的构建策略进行改良以满足实际应用需求. 本文主要围绕影响TCN异质结各项性能的因素,对近年来TCN异质结的构建策略及其在光降解有机污染物领域中的研究进展进行了总结. 最后,对TCN异质结研究中的未来发展方向提出了展望.Abstract: The TiO2/g-C3N4 heterojunction (TCN heterojunction) composed of TiO2 and g-C3N4 crystals has the advantages of low cost, narrow band gap, wide spectral response bandwidth and high carrier migration efficiency, which can effectively solve the problem of high energy consumption in the degradation process of organic pollutants. However, the two common TCN heterojunctions have limitations in carrier utilization or redox ability. Therefore, the construction strategy of TCN heterojunction needs to be improved to meet the practical application requirements. This review mainly focuses on the factors affecting the performance of TCN heterojunction, and summarizes the construction strategies of TCN heterojunction and its research progress in the field of photodegradation of organic pollutants in recent years. Finally, the future development direction of TCN heterojunction research is prospected.
-
Key words:
- TiO2 /
- g-C3N4 /
- heterojunction /
- photodegradation /
- organic pollutants /
- reaction mechanism
-

-
表 1 TCN异质结的构建策略及应用
Table 1. Construction strategy and application of TCN heterojunction
目标
Target策略
Strategy合成方法
Synthetic Method载流子迁移途径
Carrier migration path催化剂用量
Catalyst dosage应用
Application参考
文献
Ref载流子迁移 控制g-C3N4壳层厚度 溶胶凝胶法 II型 50 mg,100 mL(10 mg·L−1) 罗丹明B(93 %,90 min) [102] 表面活性位点载流子迁移 合成超细TiO2纳米颗粒 电化学腐蚀法 Z型 10 mg,40 mL(20 mg·L−1) 盐酸四环素(99.4 %,120 min) [103] 载流子迁移 控制沉积尺寸与位置 原子层沉积技术 II型 20 mg,20 mL(5 mg·L−1) 对硝基苯酚(100 %,180 min) [84] 表面活性位点 在g-C3N4中引入介孔结构 氨气自腐蚀 Z型 10mg,60 mL(10 mg·L−1) 罗丹明B(99.2 %,8 min) [63] 载流子迁移 引入多孔结构 热分解MOF VIS型 20 mg,40 mL(10 mg·L−1) 橙黄II(95 %,3 h) [104] 表面活性位点 构建一维核壳结构 静电纺丝工艺;气相沉积法 VIS型 30mg,30 mL(13.3 mg·L−1) 罗丹明B(96 %,45 min) [40] 光吸收载流子迁移 构建空心核壳结构 模板法 II型 30 mg,100 mL(50 mg·L−1) 四环素(94.5 %,60 min) [16] 表面活性位点载流子迁移 构建三明治结构;引入氧空位 行星研磨和原位还原法 Z型 50 mg,50 mL(10 mg·L−1) 盐酸四环素(87.7 %,60 min) [47] 载流子迁移 构建1D-2D结构 超声混合法 Z型 10 mg,50 mL(15 µmol·L−1) 环丙沙星(93.4 %,60 min) [43] 载流子迁移 增强界面结合力 热缩聚法 II型 30 mg,100 mL(20 mg·L−1) 恩诺沙星(99.6 %,90 min) [38] 载流子迁移 在TiO2中掺杂Y3+ 溶胶凝胶法 Z型 30 mg,30 mL(20 mg·L−1) 罗丹明B(99.5 %,90 min) [105] 光吸收载流子迁移 在TiO2中掺杂Zr4+和N 溶胶凝胶法 Z型 50 mg,50 mL(20 mg·L−1) 罗丹明B(98 %,75 min) [106] 表面活性位点 在TiO2中掺杂C 水热联合煅烧法 Z型 100 mg,100 mL(20 mg·L−1)
100 mg,100 mL(20 mg·L−1)罗丹明B(97 %,90 min)苯酚(92 %,60 min) [107] 光吸收 在TiO2中掺杂C、N、S 水热法 Z型 20 mg,50 mL(20 mg·L−1) 甲基橙(99.8 %,80 min)苯酚(97.6 %,60 min) [108] 光吸收 在TiO2中掺杂N;在VN-g-C3N4中掺杂O 一步法煅烧;溶剂热法 Z型 40 mg,100 mL(30 mg·L−1) 盐酸四环素(79.9 %,120 min) [46] 载流子迁移 在g-C3N4中掺杂C 热缩聚法 II型 100 mg,100 mL(10 mg·L−1)100 mg,100 mL(10 mg·L−1) 双氯芬酸(98.92 %,30 min)卡马西平(99.77 %,6 h) [109] 载流子迁移 在g-C3N4中掺杂B 热缩聚法 type-Z 50 mg,50 mL(10 mg·L−1)
50 mg,50 mL(10 mg·L−1)罗丹明B(99.8 %,40 min)左氧氟沙星(98.2 %,50 min) [110] 载流子迁移 在g-C3N4中掺杂S 溶胶凝胶法 II型 10 mg,100 mL(10 mg·L−1) 盐酸四环素(98.1 %,60 min) [111] 载流子迁移 在g-C3N4中掺杂P和O 溶胶凝胶法 Z型 50 mg,50 mL(10 mg·L−1) 恩诺沙星(98.5 %,60 min) [72] 吸附传质 碱化g-C3N4表面 溶胶凝胶法 - 100 mg,50 mL(20 mg·L−1) 亚甲基蓝(100 %,120 min) [97] 吸附传质 调节表面基团 酸性水热法 VIS型 40 mg,80 mL(20 mg·L−1) 四环素(82 %,60 min) [21] -
[1] LI J, ZHU K M, LI R M, et al. The removal of azo dye from aqueous solution by oxidation with peroxydisulfate in the presence of granular activated carbon: Performance, mechanism and reusability [J]. Chemosphere, 2020, 259: 127400. doi: 10.1016/j.chemosphere.2020.127400 [2] ZHANG Y Y, WANG F, HUDSON-EDWARDS K A, et al. Characterization of mining-related aromatic contaminants in active and abandoned metal(loid) tailings ponds [J]. Environmental Science & Technology, 2020, 54(23): 15097-15107. [3] NARZARY S, ALAMELU K, RAJA V, et al. Visible light active, magnetically retrievable Fe3O4@SiO2@g-C3N4/TiO2 nanocomposite as efficient photocatalyst for removal of dye pollutants [J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104373. doi: 10.1016/j.jece.2020.104373 [4] LIU Y C, YU Z X, LI X H, et al. Super hydrophilic composite membrane with photocatalytic degradation and self-cleaning ability based on LDH and g-C3N4 [J]. Journal of Membrane Science, 2021, 617: 118504. doi: 10.1016/j.memsci.2020.118504 [5] PARVULESCU V I, EPRON F, GARCIA H, et al. Recent progress and prospects in catalytic water treatment [J]. Chemical Reviews, 2022, 122(3): 2981-3121. doi: 10.1021/acs.chemrev.1c00527 [6] 陈侣存, 崔雯, 陈鹏, 等. 宽带隙金属氧化物材料光催化降解苯系物: 反应机理和改性策略 [J]. 材料导报, 2021, 35(21): 21001-21011. CHEN L C, CUI W, CHEN P, et al. Photocatalytic degradation for benzene series in wide-band gap metal oxide: Reaction mechanism and modification strategies [J]. Materials Reports, 2021, 35(21): 21001-21011(in Chinese).
[7] DOU M M, WANG J, GAO B R, et al. Photocatalytic difference of amoxicillin and cefotaxime under visible light by mesoporous g-C3N4: Mechanism, degradation pathway and DFT calculation [J]. Chemical Engineering Journal, 2020, 383: 123134. doi: 10.1016/j.cej.2019.123134 [8] ZHAO W, YANG X R, LIU C X, et al. Facile construction of all-solid-state Z-scheme g-C3N4/TiO2 thin film for the efficient visible-light degradation of organic pollutant [J]. Nanomaterials (Basel, Switzerland), 2020, 10(4): 600. doi: 10.3390/nano10040600 [9] LIU L C, CORMA A. Structural transformations of solid electrocatalysts and photocatalysts [J]. Nature Reviews Chemistry, 2021, 5(4): 256-276. doi: 10.1038/s41570-021-00255-8 [10] WANG X Y, LIU M Y. Photocatalytic enhancement mechanism of direct Z-scheme heterojunction O-g-C3N4@Fe-TiO2 under visible-light irradiation [J]. Applied Surface Science, 2019, 485: 353-360. doi: 10.1016/j.apsusc.2019.04.207 [11] SHEN S, FU J J, WANG H B. Unravelling the favorable photocatalytic effect of hydrogenation process on the novel g-C3N4-TiO2 catalysts for water purification [J]. Diamond and Related Materials, 2021, 114: 108292. doi: 10.1016/j.diamond.2021.108292 [12] KUMAR D P, RANGAPPA A P, SHIM H S, et al. Nanocavity-assisted single-crystalline Ti3+ self-doped blue TiO2(B) as efficient cocatalyst for high selective CO2 photoreduction of g-C3N4 [J]. Materials Today Chemistry, 2022, 24: 100827. doi: 10.1016/j.mtchem.2022.100827 [13] THANH TRUC N T, GIANG BACH L, THI HANH N, et al. The superior photocatalytic activity of Nb doped TiO2/g-C3N4 direct Z-scheme system for efficient conversion of CO2 into valuable fuels [J]. Journal of Colloid and Interface Science, 2019, 540: 1-8. doi: 10.1016/j.jcis.2019.01.005 [14] YANG X J, ZHAO L, WANG S M, et al. Recent progress of g-C3N4 applied in solar cells [J]. Journal of Materiomics, 2021, 7(3): 728-741. [15] XIA Y, XU L, PENG J H, et al. TiO2@g-C3N4 core/shell spheres with uniform mesoporous structures for high performance visible-light photocatalytic application [J]. Ceramics International, 2019, 45(15): 18844-18851. doi: 10.1016/j.ceramint.2019.06.118 [16] CHEN P R. Rational design of TiO2 hollow nanospheres decorated with ultrathin g-C3N4 nanosheets for enhanced photocatalytic degradation of tetracycline [J]. Journal of Materials Science:Materials in Electronics, 2021, 32(20): 24845-24855. doi: 10.1007/s10854-021-06944-w [17] LI W C, ZHOU L X, XIE L K, et al. N-Fe-Gd co-doped TiO2/g-C3N4 nanosheet hybrid composites with superior photocatalytic dye degradation [J]. Advanced Composites and Hybrid Materials, 2022, 5(1): 481-490. doi: 10.1007/s42114-021-00326-w [18] FANG Z M, XING L, LIU Y B, et al. Ternary heterojunction stabilized photocatalyst of Co-TiO2/g-C3N4 in boosting sulfite oxidation during wet desulfurization [J]. Applied Surface Science, 2021, 551: 149478. doi: 10.1016/j.apsusc.2021.149478 [19] IBRAHIM Y O, GONDAL M A. Visible-light-driven photocatalytic performance of a Z-scheme based TiO2/WO3/g-C3N4 ternary heterojunctions [J]. Molecular Catalysis, 2021, 505: 111494. doi: 10.1016/j.mcat.2021.111494 [20] MAO C C, WANG X, ZHANG W, et al. Super-hydrophilic TiO2-based coating of anion exchange membranes with improved antifouling performance [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 614: 126136. doi: 10.1016/j.colsurfa.2021.126136 [21] WANG Q, ZHANG L X, GUO Y K, et al. Multifunctional 2D porous g-C3N4 nanosheets hybridized with 3D hierarchical TiO2 microflowers for selective dye adsorption, antibiotic degradation and CO2 reduction [J]. Chemical Engineering Journal, 2020, 396: 125347. doi: 10.1016/j.cej.2020.125347 [22] SARI F N I, YEN D T K, TING J M. Enhanced photocatalytic performance of TiO2 through a novel direct dual Z-scheme design [J]. Applied Surface Science, 2020, 533: 147506. doi: 10.1016/j.apsusc.2020.147506 [23] 卫思颖, 马建中, 范倩倩. 量子点/TiO2复合光催化材料的研究进展 [J]. 复合材料学报, 2021, 38(3): 712-721. WEI S Y, MA J Z, FAN Q Q. Research advances on quantum dots/TiO2 composite photocatalytic materials [J]. Acta Materiae Compositae Sinica, 2021, 38(3): 712-721(in Chinese).
[24] WANG Y Y, YANG W J, CHEN X J, et al. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer [J]. Applied Catalysis B:Environmental, 2018, 220: 337-347. doi: 10.1016/j.apcatb.2017.08.004 [25] LI J, ZHANG M, LI X, et al. Effect of the calcination temperature on the visible light photocatalytic activity of direct contact Z-scheme g-C3N4-TiO2 heterojunction [J]. Applied Catalysis B:Environmental, 2017, 212: 106-114. doi: 10.1016/j.apcatb.2017.04.061 [26] ZHANG G H, ZHANG T Y, LI B, et al. An ingenious strategy of preparing TiO2/g-C3N4 heterojunction photocatalyst: In situ growth of TiO2 nanocrystals on g-C3N4 nanosheets via impregnation-calcination method [J]. Applied Surface Science, 2018, 433: 963-974. doi: 10.1016/j.apsusc.2017.10.135 [27] LI F X, XIAO X D, ZHAO C, et al. TiO2-on-C3N4 double-shell microtubes: In-situ fabricated heterostructures toward enhanced photocatalytic hydrogen evolution [J]. Journal of Colloid and Interface Science, 2020, 572: 22-30. doi: 10.1016/j.jcis.2020.03.071 [28] PAN J Q, DONG Z J, WANG B B, et al. The enhancement of photocatalytic hydrogen production via Ti3+ self-doping black TiO2/g-C3N4 hollow core-shell nano-heterojunction [J]. Applied Catalysis B:Environmental, 2019, 242: 92-99. doi: 10.1016/j.apcatb.2018.09.079 [29] LI Y L, WANG J S, YANG Y L, et al. Seed-induced growing various TiO2 nanostructures on g-C3N4 nanosheets with much enhanced photocatalytic activity under visible light [J]. Journal of Hazardous Materials, 2015, 292: 79-89. doi: 10.1016/j.jhazmat.2015.03.006 [30] LI Z L, YANG Y, WANG S X, et al. High-density ruthenium single atoms anchored on oxygen-vacancy-rich g-C3N4-C-TiO2 heterostructural nanosphere for efficient electrocatalytic hydrogen evolution reaction [J]. ACS Applied Materials & Interfaces, 2021, 13(39): 46608-46619. [31] MOHAMED M A, JAAFAR J, M ZAIN M F, et al. In-depth understanding of core-shell nanoarchitecture evolution of g-C3N4@C, N co-doped anatase/rutile: Efficient charge separation and enhanced visible-light photocatalytic performance [J]. Applied Surface Science, 2018, 436: 302-318. doi: 10.1016/j.apsusc.2017.11.229 [32] XIAO L M, LIU T F, ZHANG M, et al. Interfacial construction of 0D/1D g-C3N4 nanoparticles/TiO2 nanotube arrays with Z-scheme heterostructure for improved photoelectrochemical water splitting [J]. ACS Sustainable Chemistry & Engineering, 2018, 7(2): 2483-2491. [33] YU X H, XIE J, LIU Q Q, et al. The origin of enhanced photocatalytic activity in g-C3N4/TiO2 heterostructure revealed by DFT calculations [J]. Journal of Colloid and Interface Science, 2021, 593: 133-141. doi: 10.1016/j.jcis.2021.02.103 [34] LIN Y M, WANG Q, MA M T, et al. Enhanced optical absorption and photocatalytic water splitting of g-C3N4/TiO2 heterostructure through C&B codoping: A hybrid DFT study [J]. International Journal of Hydrogen Energy, 2021, 46(14): 9417-9432. doi: 10.1016/j.ijhydene.2020.12.114 [35] YAN M Y, JIANG Z Y, ZHENG J M, et al. Theoretical study on transport-scheme conversion of g-C3N4/TiO2 heterojunctions by oxygen vacancies [J]. Applied Surface Science, 2020, 531: 147318. doi: 10.1016/j.apsusc.2020.147318 [36] WU Y X, LIU L M, AN X Q, et al. New insights into interfacial photocharge transfer in TiO2/C3N4 heterostructures: Effects of facets and defects [J]. New Journal of Chemistry, 2019, 43(11): 4511-4517. doi: 10.1039/C9NJ00027E [37] LIANG D, HUANG Y L, WU F, et al. In situ synthesis of g-C3N4/TiO2 with{001}and{101}facets coexposed for water remediation [J]. Applied Surface Science, 2019, 487: 322-334. doi: 10.1016/j.apsusc.2019.05.088 [38] ZHANG R, YU Y Q, WANG H B, et al. Mesoporous TiO2/g- C3N4 composites with O-Ti-N bridge for improved visible-light photodegradation of enrofloxacin [J]. The Science of the Total Environment, 2020, 724: 138280. doi: 10.1016/j.scitotenv.2020.138280 [39] QI F, AN W J, WANG H, et al. Combing oxygen vacancies on TiO2 nanorod arrays with g-C3N4 nanosheets for enhancing photoelectrochemical degradation of phenol [J]. Materials Science in Semiconductor Processing, 2020, 109: 104954. doi: 10.1016/j.mssp.2020.104954 [40] CUI L, LIU S L, WANG F K, et al. Growth of uniform g-C3N4 shells on 1D TiO2 nanofibers via vapor deposition approach with enhanced visible light photocatalytic activity [J]. Journal of Alloys and Compounds, 2020, 826: 154001. doi: 10.1016/j.jallcom.2020.154001 [41] JANG E, KIM W J, KIM D W, et al. Atomic layer deposition with rotary reactor for uniform hetero-junction photocatalyst, g-C3N4@TiO2 core-shell structures [J]. RSC Advances, 2019, 9(57): 33180-33186. doi: 10.1039/C9RA05958J [42] WANG C L, HU L M, CHAI B, et al. Enhanced photocatalytic activity of electrospun nanofibrous TiO2/g-C3N4 heterojunction photocatalyst under simulated solar light [J]. Applied Surface Science, 2018, 430: 243-252. doi: 10.1016/j.apsusc.2017.08.036 [43] HU K, LI R Q, YE C L, et al. Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin [J]. Journal of Cleaner Production, 2020, 253: 120055. doi: 10.1016/j.jclepro.2020.120055 [44] MEI P, WANG H H, GUO H, et al. The enhanced photodegradation of bisphenol A by TiO 2/C3N4 composites [J]. Environmental Research, 2020, 182: 109090. doi: 10.1016/j.envres.2019.109090 [45] RAJA V, JAFFAR ALI B M. Synergy of photon up-conversion and Z-scheme mechanism in graphitic carbon nitride nanoparticles decorated g-C3N4-TiO2 [J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2021, 611: 125862. doi: 10.1016/j.colsurfa.2020.125862 [46] WANG Y X, RAO L, WANG P F, et al. Photocatalytic activity of N-TiO2/O-doped N vacancy g-C3N4 and the intermediates toxicity evaluation under tetracycline hydrochloride and Cr(VI) coexistence environment [J]. Applied Catalysis B:Environmental, 2020, 262: 118308. doi: 10.1016/j.apcatb.2019.118308 [47] NI J X, WANG W, LIU D M, et al. Oxygen vacancy-mediated sandwich-structural TiO2−x/ultrathin g-C3N4/TiO2−x direct Z-scheme heterojunction visible-light-driven photocatalyst for efficient removal of high toxic tetracycline antibiotics [J]. Journal of Hazardous Materials, 2021, 408: 124432. doi: 10.1016/j.jhazmat.2020.124432 [48] KıLıÇ D, SEVIM M, EROĞLU Z, et al. Strontium oxide modified mesoporous graphitic carbon nitride/titanium dioxide nanocomposites (SrO-mpg-CN/TiO2) as efficient heterojunction photocatalysts for the degradation of tetracycline in water [J]. Advanced Powder Technology, 2021, 32(8): 2743-2757. doi: 10.1016/j.apt.2021.05.043 [49] PATNAIK S, SAHOO D P, PARIDA K. Recent advances in anion doped g-C3N4 photocatalysts: A review [J]. Carbon, 2021, 172: 682-711. doi: 10.1016/j.carbon.2020.10.073 [50] ZHANG W, HE H L, LI H Z, et al. Visible‐light responsive TiO2‐based materials for efficient solar energy utilization [J]. Advanced Energy Materials, 2021, 11(15): 2003303. doi: 10.1002/aenm.202003303 [51] OUYANG L K, ZHANG Y, WANG Y, et al. Insights into the adsorption and photocatalytic oxidation behaviors of boron-doped TiO2/g-C3N4 nanocomposites toward As(III) in aqueous solution [J]. Industrial & Engineering Chemistry Research, 2021, 60(19): 7003-7013. [52] ZHOU S H, LIU S K, SU K, et al. Graphite carbon nitride coupled S-doped hydrogenated TiO2 nanotube arrays with improved photoelectrochemical performance [J]. Journal of Electroanalytical Chemistry, 2020, 862: 114008. doi: 10.1016/j.jelechem.2020.114008 [53] ZHAO Z Y, ZHAO X, YI J, et al. Effects of nonmetal doping on electronic structures and optical property of anatase TiO2 from first-principles calculations [J]. Rare Metal Materials and Engineering, 2015, 44(7): 1568-1574. doi: 10.1016/S1875-5372(15)30094-1 [54] YUAN X J, SUN M X, YAO Y, et al. N/Ti3+-codoped triphasic TiO2/g-C3N4 heterojunctions as visible-light photocatalysts for the degradation of organic contaminants [J]. New Journal of Chemistry, 2019, 43(6): 2665-2675. doi: 10.1039/C8NJ04595J [55] KUMAR A, RAIZADA P, HOSSEINI-BANDEGHARAEI A, et al. C-, N-Vacancy defect engineered polymeric carbon nitride towards photocatalysis: viewpoints and challenges [J]. Journal of Materials Chemistry A, 2021, 9(1): 111-153. doi: 10.1039/D0TA08384D [56] KANG S, IM T, KOH M, et al. Facile fabrication of electrospun black titania nanofibers decorated with graphitic carbon nitride for the application of photocatalytic CO2 reduction [J]. Journal of CO2 Utilization, 2020, 41: 101230. doi: 10.1016/j.jcou.2020.101230 [57] GAO H H, CAO R Y, XU X T, et al. Construction of dual defect mediated Z-scheme photocatalysts for enhanced photocatalytic hydrogen evolution [J]. Applied Catalysis B:Environmental, 2019, 245: 399-409. doi: 10.1016/j.apcatb.2019.01.004 [58] YAN J Q, LI P, BIAN H, et al. Synthesis of a nano-sized hybrid C3N4/TiO2 sample for enhanced and steady solar energy absorption and utilization [J]. Sustainable Energy & Fuels, 2017, 1(1): 95-102. [59] EFROS A L, BRUS L E. Nanocrystal quantum dots: From discovery to modern development [J]. ACS Nano, 2021, 15(4): 6192-6210. doi: 10.1021/acsnano.1c01399 [60] OLADEMEHIN O P, ELLINGTON T L, SHUFORD K L. Toward quantum confinement in graphitic carbon nitride-based polymeric monolayers [J]. The Journal of Physical Chemistry A, 2021, 125(35): 7597-7606. doi: 10.1021/acs.jpca.1c04597 [61] LIU X L, MA R, ZHUANG L, et al. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants [J]. Critical Reviews in Environmental Science and Technology, 2021, 51(8): 751-790. doi: 10.1080/10643389.2020.1734433 [62] WU W B, LI X, RUAN Z H, et al. Fabrication of a TiO2 trapped meso/macroporous g-C3N4 heterojunction photocatalyst and understanding its enhanced photocatalytic activity based on optical simulation analysis [J]. Inorganic Chemistry Frontiers, 2018, 5(2): 481-489. doi: 10.1039/C7QI00751E [63] ZHENG L C, SUN X J, ZHANG R, et al. Enhanced photocatalytic performance of ammonia self-etched holely g-C3N4 decorated with anatase nanoflakes by a facile synthesis process [J]. Applied Surface Science, 2021, 542: 148580. doi: 10.1016/j.apsusc.2020.148580 [64] LI Z X, GE K, YANG K, et al. Z-scheme 3 D g-C3N4/TiO2−x heterojunctions with high photocatalytic efficiency [J]. ChemistrySelect, 2020, 5(36): 11159-11169. doi: 10.1002/slct.202003150 [65] YANG L, PENG Y T, LUO X D, et al. Beyond C3N4 π-conjugated metal-free polymeric semiconductors for photocatalytic chemical transformations [J]. Chemical Society Reviews, 2021, 50(3): 2147-2172. doi: 10.1039/D0CS00445F [66] SHARMA K, RAIZADA P, HASIJA V, et al. ZnS-based quantum dots as photocatalysts for water purification [J]. Journal of Water Process Engineering, 2021, 43: 102217. doi: 10.1016/j.jwpe.2021.102217 [67] GROMMET A B, FELLER M, KLAJN R. Chemical reactivity under nanoconfinement [J]. Nature Nanotechnology, 2020, 15(4): 256-271. doi: 10.1038/s41565-020-0652-2 [68] SONG X H, WANG M, LIU W T, et al. Thickness regulation of graphitic carbon nitride and its influence on the photocatalytic performance towards CO2 reduction [J]. Applied Surface Science, 2022, 577: 151810. doi: 10.1016/j.apsusc.2021.151810 [69] ZHANG B, PENG X F, WANG Z. Noble metal-free TiO2-coated carbon nitride layers for enhanced visible light-driven photocatalysis [J]. Nanomaterials, 2020, 10(4): 805. doi: 10.3390/nano10040805 [70] YANG X J, LIU T F, ZHANG M, et al. Interfacial dual vacancies modulating electronic structure to promote the separation of photogenerated carriers for efficient CO2 photoreduction [J]. Applied Surface Science, 2021, 551: 149305. doi: 10.1016/j.apsusc.2021.149305 [71] WONG K T, KIM S C, YUN K, et al. Understanding the potential band position and e-/ h+ separation lifetime for Z-scheme and type-II heterojunction mechanisms for effective micropollutant mineralization: Comparative experimental and DFT studies [J]. Applied Catalysis B:Environmental, 2020, 273: 119034. doi: 10.1016/j.apcatb.2020.119034 [72] HUANG J X, LI D G, LI R B, et al. One-step synthesis of phosphorus/oxygen co-doped g-C3N4/anatase TiO2 Z-scheme photocatalyst for significantly enhanced visible-light photocatalysis degradation of enrofloxacin [J]. Journal of Hazardous Materials, 2020, 386: 121634. doi: 10.1016/j.jhazmat.2019.121634 [73] NING P, CHEN H Y, PAN J H, et al. Surface defect-rich g-C3N4/TiO2 Z-scheme heterojunction for efficient photocatalytic antibiotic removal: Rational regulation of free radicals and photocatalytic mechanism [J]. Catalysis Science & Technology, 2020, 10(24): 8295-8304. [74] ZHANG B, MA X H, MA J, et al. Fabrication of rGO and g-C3N4 co-modified TiO2 nanotube arrays photoelectrodes with enhanced photocatalytic performance [J]. Journal of Colloid and Interface Science, 2020, 577: 75-85. doi: 10.1016/j.jcis.2020.05.031 [75] SHI H N, DU J, HOU J G, et al. Solar-driven CO2 conversion over Co2+ doped 0D/2D TiO2/g-C3N4 heterostructure: Insights into the role of Co2+ and cocatalyst [J]. Journal of CO2 Utilization, 2020, 38: 16-23. doi: 10.1016/j.jcou.2020.01.005 [76] LI J, LI B W, LI Q Y, et al. The effect of N-doped form on visible light photoactivity of Z-scheme g-C3N4/TiO2 photocatalyst [J]. Applied Surface Science, 2019, 466: 268-273. doi: 10.1016/j.apsusc.2018.10.035 [77] LIU X Q, KANG W, ZENG W, et al. Structural, electronic and photocatalytic properties of g-C3N4 with intrinsic defects: A first-principles hybrid functional investigation [J]. Applied Surface Science, 2020, 499: 143994. doi: 10.1016/j.apsusc.2019.143994 [78] ZHANG Y C, AFZAL N, PAN L, et al. Structure-activity relationship of defective metal-based photocatalysts for water splitting: Experimental and theoretical perspectives [J]. Advanced Science, 2019, 6(10): 1900053. doi: 10.1002/advs.201900053 [79] SHI H N, LONG S R, HU S, et al. Interfacial charge transfer in 0D/2D defect-rich heterostructures for efficient solar-driven CO2 reduction [J]. Applied Catalysis B:Environmental, 2019, 245: 760-769. doi: 10.1016/j.apcatb.2019.01.036 [80] SARKAR A, KHAN G G. The formation and detection techniques of oxygen vacancies in titanium oxide-based nanostructures [J]. Nanoscale, 2019, 11(8): 3414-3444. doi: 10.1039/C8NR09666J [81] LI Y N, CHEN Z Y, WANG M Q, et al. Interface engineered construction of porous g-C3N4/TiO2 heterostructure for enhanced photocatalysis of organic pollutants [J]. Applied Surface Science, 2018, 440: 229-236. doi: 10.1016/j.apsusc.2018.01.106 [82] XIAO L M, ZHU H H, ZHANG M, et al. Enhanced photoelectrochemical performance of g-C3N4/TiO2 heterostructure by the cooperation of oxygen vacancy and protonation treatment [J]. Journal of the Electrochemical Society, 2020, 167(6): 066513. doi: 10.1149/1945-7111/ab84f7 [83] MAI H X, CHEN D H, TACHIBANA Y, et al. Developing sustainable, high-performance perovskites in photocatalysis: design strategies and applications [J]. Chemical Society Reviews, 2021, 50(24): 13692-13729. doi: 10.1039/D1CS00684C [84] LV P, ZHAO C Y, LEE W J, et al. Less is more: Enhancement of photocatalytic activity of g-C3N4 nanosheets by site-selective atomic layer deposition of TiO2 [J]. Applied Surface Science, 2019, 494: 508-518. doi: 10.1016/j.apsusc.2019.07.131 [85] ZHOU B X, DING S S, WANG Y, et al. Type-II/type-II band alignment to boost spatial charge separation: A case study of g-C3 N4 quantum dots/a-TiO2/r-TiO2 for highly efficient photocatalytic hydrogen and oxygen evolution [J]. Nanoscale, 2020, 12(10): 6037-6046. doi: 10.1039/D0NR00176G [86] GUO R B, ZENG D D, XIE Y, et al. Carbon nitride quantum dots (CNQDs)/TiO2 nanoparticle heterojunction photocatalysts for enhanced ultraviolet-visible-light-driven bisphenol a degradation and H2 production [J]. International Journal of Hydrogen Energy, 2020, 45(43): 22534-22544. doi: 10.1016/j.ijhydene.2020.06.096 [87] HAO Q, JIA G H, WEI W, et al. Graphitic carbon nitride with different dimensionalities for energy and environmental applications [J]. Nano Research, 2020, 13(1): 18-37. doi: 10.1007/s12274-019-2589-z [88] XU C Q, LI D Z, LIU X L, et al. Direct Z-scheme construction of g-C3N4 quantum dots/TiO2 nanoflakes for efficient photocatalysis [J]. Chemical Engineering Journal, 2022, 430: 132861. doi: 10.1016/j.cej.2021.132861 [89] PANDEY B, RANI S, ROY S C. A scalable approach for functionalization of TiO2 nanotube arrays with g-C3N4 for enhanced photo-electrochemical performance [J]. Journal of Alloys and Compounds, 2020, 846: 155881. doi: 10.1016/j.jallcom.2020.155881 [90] LUAN S L, QU D, AN L, et al. Enhancing photocatalytic performance by constructing ultrafine TiO2 nanorods/g-C3N4 nanosheets heterojunction for water treatment [J]. Science Bulletin, 2018, 63(11): 683-690. doi: 10.1016/j.scib.2018.04.002 [91] NASIR M S, YANG G R, AYUB I, et al. Hybridization of g-C3N4 quantum dots with 1D branched TiO2 fiber for efficient visible light-driven photocatalytic hydrogen generation [J]. International Journal of Hydrogen Energy, 2020, 45(27): 13994-14005. doi: 10.1016/j.ijhydene.2020.03.129 [92] VIDYASAGAR D, BALAPURE A, GHUGAL S G, et al. Template‐free macro‐mesoporous TiO2/carbon nitride interface for visible‐light‐driven photocatalysis [J]. Physica Status Solidi (a), 2019, 216(20): 1900212. doi: 10.1002/pssa.201900212 [93] WANG B, LIU J W, YAO S, et al. Vacancy engineering in nanostructured semiconductors for enhancing photocatalysis [J]. Journal of Materials Chemistry A, 2021, 9(32): 17143-17172. doi: 10.1039/D1TA03895H [94] 陈鹏, 周莹, 董帆. 二维光催化材料电子结构和性能调控策略研究进展 [J]. 物理化学学报, 2021, 37(8): 43-57. CHEN P, ZHOU Y, DONG F. Advances in regulation strategies for electronic structure and performance of two-dimensional photocatalytic materials [J]. Acta Physico-Chimica Sinica, 2021, 37(8): 43-57(in Chinese).
[95] SHENG Y Q, WEI Z, MIAO H, et al. Enhanced organic pollutant photodegradation via adsorption/photocatalysis synergy using a 3D g-C3N4/TiO2 free-separation photocatalyst [J]. Chemical Engineering Journal, 2019, 370: 287-294. doi: 10.1016/j.cej.2019.03.197 [96] LI J, XIONG Y, WAN H Q, et al. In-situ investigation of dye pollutant adsorption performance on graphitic carbon nitride surface: ATR spectroscopy experiment and MD simulation insight [J]. Journal of Hazardous Materials, 2021, 418: 126297. doi: 10.1016/j.jhazmat.2021.126297 [97] LIU H, YU D Q, SUN T B, et al. Fabrication of surface alkalinized g-C3N4 and TiO2 composite for the synergistic adsorption-photocatalytic degradation of methylene blue [J]. Applied Surface Science, 2019, 473: 855-863. doi: 10.1016/j.apsusc.2018.12.162 [98] LIU Y, ZHU Q, LI X Y, et al. Combining high photocatalytic activity and stability via subsurface defects in TiO2 [J]. The Journal of Physical Chemistry, 2018, 122(30): 17221-17227. [99] ZHANG Y J, XU Z F, LI G Y, et al. Direct observation of oxygen vacancy self-healing on TiO2 photocatalysts for solar water splitting [J]. Angewandte Chemie, 2019, 58(40): 14229-14233. doi: 10.1002/anie.201907954 [100] HU K K, LEI E, HU C Y, et al. G-C3N4/TiO2 composite microspheres: in situ growth and high visible light catalytic activity [J]. CrystEngComm, 2020, 22(42): 7104-7112. doi: 10.1039/D0CE01154A [101] ZOU X X, YANG Y L, CHEN H J, et al. Hierarchical meso/macro-porous TiO2/graphitic carbon nitride nanofibers with enhanced hydrogen evolution [J]. Materials & Design, 2021, 202: 109542. [102] AR S R, WILSON H M, MOMIN B M, et al. TiO2 nanosheet/ultra-thin layer g-C3N4 core-shell structure: Bifunctional visible-light photocatalyst for H2 evolution and removal of organic pollutants from water [J]. Applied Surface Science, 2020, 528: 146930. doi: 10.1016/j.apsusc.2020.146930 [103] ZHANG B, HE X, MA X H, et al. In situ synthesis of ultrafine TiO2 nanoparticles modified g-C3N4 heterojunction photocatalyst with enhanced photocatalytic activity [J]. Separation and Purification Technology, 2020, 247: 116932. doi: 10.1016/j.seppur.2020.116932 [104] TATYKAYEV B, CHOUCHENE B, BALAN L, et al. Heterostructured g-CN/TiO2 photocatalysts prepared by thermolysis of g-CN/MIL-125(Ti) composites for efficient pollutant degradation and hydrogen production [J]. Nanomaterials, 2020, 10(7): 1387. doi: 10.3390/nano10071387 [105] PAK S, RI K, XU C M, et al. Fabrication of g-C3N4/Y-TiO2 Z-scheme heterojunction photocatalysts for enhanced photocatalytic activity [J]. New Journal of Chemistry, 2021, 45(42): 19903-19916. doi: 10.1039/D1NJ03691B [106] PAHI S, SAHU S, SINGH S K, et al. Visible light active Zr- and N-doped TiO2 coupled g-C3N4 heterojunction nanosheets as a photocatalyst for the degradation of bromoxynil and Rh B along with the H2 evolution process [J]. Nanoscale Advances, 2021, 3(22): 6468-6481. doi: 10.1039/D1NA00460C [107] LI X B, XIONG J, XU Y, et al. Defect-assisted surface modification enhances the visible light photocatalytic performance of g-C3N4@C-TiO2 direct Z-scheme heterojunctions [J]. Chinese Journal of Catalysis, 2019, 40(3): 424-433. doi: 10.1016/S1872-2067(18)63183-3 [108] HUANG Z, JIA S, WEI J, et al. A visible light active, carbon-nitrogen -sulfur co-doped TiO2/g-C3N4 Z-scheme heterojunction as an effective photocatalyst to remove dye pollutants [J]. RSC Advances, 2021, 11(27): 16747-16754. doi: 10.1039/D1RA01890F [109] HU Z Z, CAI X W, WANG Z R, et al. Construction of carbon-doped supramolecule-based g-C3N4/TiO2 composites for removal of diclofenac and carbamazepine: A comparative study of operating parameters, mechanisms, degradation pathways [J]. Journal of Hazardous Materials, 2019, 380: 120812. doi: 10.1016/j.jhazmat.2019.120812 [110] BALAKUMAR V, SELVARAJAN S, BAISHNISHA A, et al. In-situ growth of TiO2@B-doped g-C3N4 core-shell nanospheres for boosts the photocatalytic detoxification of emerging pollutants with mechanistic insight [J]. Applied Surface Science, 2022, 577: 151924. doi: 10.1016/j.apsusc.2021.151924 [111] DIVAKARAN K, BAISHNISHA A, BALAKUMAR V, et al. Photocatalytic degradation of tetracycline under visible light using TiO2@sulfur doped carbon nitride nanocomposite synthesized via in situ method [J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105560. doi: 10.1016/j.jece.2021.105560 [112] ALI I, PARK S, KIM J O. Modeling the photocatalytic reactions of g-C3N4-TiO2 nanocomposites in a recirculating semi-batch reactor [J]. Journal of Alloys and Compounds, 2020, 821: 153498. doi: 10.1016/j.jallcom.2019.153498 [113] ZHANG Q, QUAN X, WANG H, et al. Constructing a visible-light-driven photocatalytic membrane by g-C3N4 quantum dots and TiO2 nanotube array for enhanced water treatment [J]. Scientific Reports, 2017, 7: 3128. doi: 10.1038/s41598-017-03347-y -



 下载:
下载: