-
抗生素是一类具有抑菌或者杀菌活性的化合物,它可以是微生物或高等动植物的次级代谢物,也可以是由人工或半人工合成的有机化合物. 按照化学结构和性质的不同,抗生素可以分为β-内酰胺类(β-lactam)、四环素类(tetracycline)、氨基糖苷类(aminoglycoside)、大环内酯类(macrolide)、喹诺酮类(quinolone)、磺胺类(sulfonamide)、糖肽类(glycopeptide)和多粘菌素类(polymyxin)等. 近年来,随着抗生素用量的不断增多,其在不同环境介质及食品(土壤、底泥、地表水、肉制品等)中频繁检出,对生态环境和人体健康均带来较大风险[1-3]. 抗生素会经过环境暴露和食物链传递进入人体,在人体内蓄积,进而造成一系列人体健康危害. 另外,抗生素会进一步导致抗性基因和耐药性问题产生,其危害远超抗生素本身[4]. 鉴于此,目前越来越多的研究聚焦于抗生素的人体内暴露水平、特征分析及健康风险研究. 加之分析仪器设备的发展和进步,抗生素的检测技术、内暴露特征和人体健康风险已成为近几年的研究热点,本文分别对其进行了系统综述.
-
抗生素因其具有独特的杀菌或抑菌特性,被广泛应用于临床治疗、畜牧业、农业和水产养殖业等领域中,发挥了前所未有的重要作用[5-6]. 在临床中,抗生素被用于人体疾病的预防或治疗,主要是由细菌、真菌等微小病原菌引起的感染性疾病. 在养殖业中,抗生素作为良好的促生长剂而被广泛应用,1950年,美国食品药品监督管理局首次批准允许在饲料中添加抗生素,截止目前,全球约有三分之二的抗生素被应用到畜牧业中[7]. 其中,四环素类、磺胺类、喹诺酮类抗生素因价格低廉、抗菌谱宽,以及可加速幼崽生长、缩短饲养周期、增加经济效益等优势,被广泛应用在畜牧养殖中[8].
为满足生产与治疗的需求,近年来全球各类抗生素的使用量呈逐年增长的趋势. 2000年至2015年,全球抗生素消耗量增加了65%,其中中低收入国家作为全球抗生素消耗的主要推动者,其抗生素消耗量增加了114%[9]. 以医用抗生素为例,西方国家抗生素药物处方占总处方的30%,而在我国这一比例则高达70%[10]. 2017年,全球约有9.3万t抗生素用于鸡、牛和猪(占所有食用动物的93.75%),预计2030年将增加11.5%[11]. 中国作为抗生素生产和消费的第一大国,2020年抗生素的年产量高达22.3万t[12],2013年抗生素的年使用量为16.2万t,其中人用抗生素用量7.8 t,兽用抗生素用量8.4万t,约占全球抗生素总使用量的50%[13]. 预计2030年,我国仍然是世界抗生素的最大消费国(占比43%)[11-12].
-
相对于食品和环境介质,关于人体抗生素检测技术的研究相对较少. 早在1978年,T.B. VREE等就采用高效液相色谱(HPLC)对人体体液(血浆、唾液和尿液)中阿莫西林和氨苄西林进行测定[14]. 在此后的30多年内,人体中抗生素的检测技术无显著进步,存在目标物种类少、方法灵敏度低等问题. 近十年来,随着抗生素的健康风险逐渐受到关注,以及前处理方法和质谱等痕量分析技术的发展和创新,国内外针对抗生素检测技术的研究也逐渐增多.
-
由于抗生素种类较多,不同类型抗生素间的官能团、酸碱性、极性和pKa差异较大,若采用单一前处理方法同时分析多种不同类型抗生素,难度较大,因此已有研究多针对一种[15-16]、一类[17-20]、亦或是临床/非临床领域中使用频繁但种类有限的抗生素[21-22]. 常用的前处理技术有蛋白沉淀法、固相分散萃取、固相萃取、固相微萃取等.
-
蛋白沉淀法多适用于富含蛋白质的血浆或母乳样本,其主要优势为操作简便、成本低且易于实施. Xie等[23]利用甲醇沉淀蛋白质,对血浆中7种常用抗生素(美罗培南、头孢噻肟、头孢哌酮、哌拉西林、利奈唑胺、莫西沙星和替加环素)进行提取,HPLC分析,定量限为0.05—0.8 μg·mL−1. Baietto等[24]采用乙腈沉淀蛋白质,离心后稀释进样,开发了同时测定血浆中达托霉素、阿米卡星、庆大霉素和利福平的方法,可用于临床药代动力学研究. 另有研究[25]采用蛋白沉淀法结合高效液相色谱串联三重四极杆质谱测定血浆和母乳中达托霉素,以评估母乳喂养的安全性. 但仅采用蛋白沉淀法无法完全去除基质中其他杂质,会导致基质效应偏高,所以不适用于浓度低的目标物和质谱响应偏低的情况,且长期连续进样可能影响色谱柱寿命,造成仪器污染等.
-
DSPE主要包括QuEChERS方法和基质固相分散萃取(MSPD). QuEChERS方法最初用于水果及蔬菜中农药的前处理,现已广泛应用于不同基质样本中多种目标化合物的分析[26-29]. QuEChERS溶剂消耗少、检测速度快、所需样品量少,其准确度和精密度均较高[30]. 有研究者[31]利用QuEChERS提取法结合HPLC,建立了尿液中6类18种抗生素的分析方法,被应用于青藏高原地区学龄前儿童体内抗生素负荷水平的检测中. 但是,QuEChERS的灵敏度差、检出限较高[31],且对于极性目标物的提取效率差[32]. MSPD一般适用于固体、半固体基质的处理,故常应用于食品检测中,其操作步骤简便,但是易受到样品类型、分散剂种类与比例、净化填料类型、洗涤剂种类和体积等因素的影响[33]. Sun等[34]采用MSPD对血清样本中6种喹诺酮类抗生素进行预处理,采用HPLC对目标物进行分析,该方法定量限为8—13 ng·mL−1,由于血清样本量小且粘度较大,会附着于容器,导致目标物损失.
-
SPE是利用被萃取物质在液-固两相间的分配不同进行样品前处理的一种分离技术,是目前人体生物样本(尿液、血浆)抗生素前处理中最常用的方法. 鉴于不同种类抗生素化学性质差异较大,而HLB萃取小柱对酸性、碱性和中性化合物均有较好的选择性,因此是抗生素分析中常用的SPE小柱类型. 2008年,Supattanapong等[16]采用HLB的SPE小柱对人体血浆中阿奇霉素进行萃取. 2010年,Fernandez-Torres等[35]比较了溶剂提取和SPE两种前处理方法对尿液中4类11种抗生素及其代谢物的前处理效果,结果显示SPE方法检出限明显低于溶剂提取,灵敏度更高. 除此之外,在部分血清样品的前处理中,有研究采用CBA、Strata-X-C或MCX固相萃取小柱对抗生素进行提取. Kaale等采用CBA固相萃取小柱结合毛细管电泳法对血清中庆大霉素4种组分进行分析,回收率范围为78%—93%[36]. Zhang等采用Strata-X-C固相萃取小柱对血清中万古霉素萃取,结合LC–MS分析,该方法定量限为0.001 μg·mL−1,回收率为89.2%—98.1%[37]. Oertel等采用MCX固相萃取小柱结合ESI-MS/MS对新霉素进行分析,该方法为新霉素的临床和药理研究奠定基础[38]. 然而,相对于HLB型小柱,以上三种萃取小柱适用范围小,仅满足特定类型抗生素的分析. 近年来,为了提高前处理效率,缩短前处理时间,实现大批量生物样本的高通量分析目的,96孔固相萃取板和在线SPE被应用到抗生素的前处理中. 2011年,Dotsikas等[39]采用全自动前处理技术结合96孔固相萃取板对血浆中粘菌素A和粘菌素B进行提取,后进行质谱分析,实现了高通量和高效率分析的目的. 国内以Wang等研究[40]为代表,采用HLB的96孔萃取板进行前处理,建立了人体尿液中6类共14种抗生素的靶向分析及74种抗生素的非靶向筛查方法,提高了分析效率. 在该方法基础之上,Liu等[40-41]采用HLB的96孔萃取板,扩增目标抗生素类型,建立了8类41种抗生素及2种代谢物的分析方法,是目前人体尿液抗生素靶向分析中涵盖抗生素类别最多的分析方法. Xie等[42]利用在线SPE结合HPLC建立了人体血浆和尿液中法罗培南的分析方法,样本进行简单预处理后,经在线SPE装置浓缩净化,最后进行检测分析,血浆和尿液中定量限分别为0.02 μg·mL−1和0.05 μg·mL−1. 在线SPE既减少了人工操作的误差,又极大提高了样品处理效率,因此被应用于大批量样本的检测,但是,该方法中柱填料重复使用,使得净化效率下降,且分析高浓度样品后存在交叉污染的风险.
-
SPME是集取样、萃取、浓缩和进样于一体的微萃取新技术. 作为固相微萃取的优质填料,分析印迹聚合物(MIPs)可以对目标物进行特异性吸附,从而将样品各组分选择性分离与富集. Buszewski等[15]采用SPME对血浆中阿莫西林进行提取,后进行高效液相色谱-紫外检测器(HPLC-UV)分析;另有研究[15,43]采用SPME方法对血浆中阿莫西林、头孢噻肟、甲硝唑及其代谢物进行前处理. SPME样品用量少、操作简便、无需有机溶剂,但需要根据待测物质的种类来选择萃取纤维涂层[44],当目标物种类多、化学性质差异大时,较难满足分析要求.
-
此外,分散液液微萃取(DLLME)、酸碱提取法和振荡提取法也被应用于人体生物样本中抗生素的分析. Pastor-Belda等[45]采用DLLME方法提取尿液中甲砜霉素、氟苯尼考和氯霉素,并结合液相色谱-四极杆飞行时间质谱测定,该方法回收率为83%—104%. DLLME法多用于提取化学性质相似的几种或同类抗生素,对多种不同类型抗生素同时提取的适用性差. 有研究者在人体体液(血浆、唾液和尿液)中加入高氯酸后,进行涡旋提取,离心后上清液进行仪器分析,可用于阿莫西林和氨苄西林检测分析[14]. Mizuno等[46]建立了可同时测定人发中的氧氟沙星、诺氟沙星和环丙沙星的方法,该方法先利用NaOH对头发中抗生素进行提取,然后结合高效液相色谱-荧光检测器(HPLC-FLD)检测,结果显示回收率为77.6%—95.4%. Cazorla-Reyes等[21]建立了人体尿液、脑脊液、血清和痰液中6类21种抗生素的前处理方法,其中尿液和脑脊液采用流动相稀释,振荡提取的方法;血清和痰液分别采用乙腈和二硫苏糖醇振荡提取,离心,取上清液加入流动相稀释的方法,结果表明所有基质中目标物的检出限和回收率均较为理想. 酸碱提取法和振荡提取法操作简便,耗时短,但相较于选择性更高的DSPE、SPE、SPME等方法净化效率相对较低,残留的杂质会影响目标物响应,易造成仪器污染.
-
测定抗生素的方法包括微生物分析、免疫分析、高效液相色谱法(HPLC)和高效液相色谱串联质谱法(HPLC-MS)等. 微生物检测操作简便、耗时短,可实现对多种抗生素的分析,但对部分抗生素灵敏度较低,难以准确定量. 免疫分析法耗时短、成本低,但操作繁琐. 因此,HPLC和HPLC-MS是目前测定抗生素的常用方法.
-
鉴于HPLC分析成本低,可以与紫外线检测器(UV)、电化学检测器(ECD)、荧光检测器(FLD)等串联使用,其应用较为广泛. 1978年, VREE等[14]建立了人体体液(血浆、唾液和尿液)中阿莫西林和氨苄西林的HPLC分析方法,该方法与微生物学等早期报告方法的结果相当. 1994年,Mizuno等[46]利用NaOH对头发中抗生素进行提取,然后结合HPLC-FLD测定人发中的氧氟沙星、诺氟沙星和环丙沙星. 2008年,Supattanapong等[16]采用SPE结合HPLC-ECD对血浆中阿奇霉素进行分析,该方法检出限为10 ng·mL−1. 2010年,Fernandez-Torres等[35]开发了基于HPLC-FLD对尿液中4类11种抗生素及其代谢物进行检测的方法,该方法已应用于实际样本测定中,但样品分析时间长(39 min/样品),检测效率低. 2011年,Buszewski等[15]采用HPLC-UV对血浆中阿莫西林进行分析,但该方法检出限较高(1.21 μg·mL−1).
-
普通人体内抗生素含量多为痕量水平,与HPLC相比,HPLC-MS灵敏度高、分析速度快、分析效率高. 因此,目前在抗生素分析中,应用最为广泛的检测方法是HPLC-MS,常用的有液相色谱-三重四极杆串联质谱(HPLC-QqQ-MS/MS)、液相色谱-四极杆串联飞行时间质谱(HPLC-Q/TOF MS)、液相色谱-静电场轨道阱质谱(HPLC-Orbitrap MS)等.
HPLC-QqQ-MS/MS属于低分辨质谱,常用的扫描模式为选择反应监测(SRM)和多反应监测(MRM),最多可同时分析150—200种化合物,且灵敏度高,可满足环境和人体中抗生素的定量分析需求. 在质谱检测时,应根据抗生素质谱行为的不同选择合适的离子扫描模式,如氯霉素类在负离子模式下信号较强,而大环内酯类、喹诺酮类、四环素类等在正离子模式下信号较强;另外,为增强电离、改善峰形,通常在流动相中加入一定比例的甲酸或甲酸铵、乙酸铵等缓冲盐[21,40];鉴于大多数抗生素具有极性,而HSS T3色谱柱内纯二氧化硅填料能更好地保留极性抗生素,因此其分离效果更优[40-41]. 2014年,Cazorla-Reyes等[21]采用HPLC-MS/MS对尿液、血清、和支气管痰液中6类21种抗生素进行分析,检出限为0.01—1.00 μg·mL−1(尿液、血清和脑脊液)和0.02—0.67 μg·g−1(痰液). Baietto等[24]采用HPLC-MS建立了血浆中达托霉素、阿米卡星、庆大霉素和利福平的分析方法,该方法定量限为0.63—2.34 μg·mL−1. Liu等[41]采用固相萃取结合HPLC-QqQ-MS/MS的方法建立了人体尿液中41种抗生素及2种代谢物的分析方法,该方法正、负离子模式运行时间分别是18.5 min和5.0 min,检出限为0.016—1.481 ng·mL−1,灵敏度较之前提高了10—1000倍. Li等[47]采用HLB固相萃取技术对尿液进行前处理,结合 HPLC-QqQ-MS/MS对抗生素进行分析,该方法检出限为0.01—1.0 ng·mL−1. 总之,QqQ-MS/MS灵敏度高、定量能力强,在靶向分析中应用广泛,但是其分辨率低,无法满足对未知化合物的定性分析需求.
Q/TOF MS和Orbitrap MS属于高分辨质谱(HRMS),可通过精确质量数对目标物进行定性识别,是未知物筛查和识别的可靠工具[48]. 目前,抗生素的疑似靶标分析或非靶标分析在人体生物样本的相关研究较少,主要集中于土壤[49]、地表水[50-51]、废水[52]、动物粪便[53]、蜂蜜[54]等环境或食品样本中抗生素及其代谢物的筛查. 唯一一篇关于人体中抗生素非靶向分析的研究来自于2014年,Wang等[40]首次利用Q/TOF MS,建立了人体尿液中74种抗生素(环境和食品中检出率较高)的非靶标筛查方法,成功应用于人群抗生素内暴露监测,在实际样本中筛查出磺胺二甲嘧啶和头孢克洛. 普通人群抗生素的暴露途径为食品和饮用水,加之抗生素在人体中半衰期较短,其检出率和检出浓度相对于环境和食品样本均较低,这也制约了人体中抗生素筛查研究. 人体生物样本相对环境和食品样本基质更为宝贵而复杂,因此优化前处理步骤和仪器分析方法以降低检出限尤为关键.
-
近年来,更多研究者从抗生素的环境和食品外暴露研究逐渐聚焦到人体抗生素的内暴露研究. 人体生物样本中抗生素测定结果能够真实反映出不同暴露途径的抗生素总量,尤其是纳入了之前调查中未考虑的经食品摄入的兽用抗生素的暴露量[55]. 另外,抗生素进入人体后,除了以原型或结合物的形式排出体外,一部分还会在人体中进行代谢,这也是之前外暴露研究未考量的问题[56]. 了解人群抗生素内暴露特征,对抗生素的科学管理、防范耐药性风险、开展健康风险研究具有重要意义.
-
抗生素经过环境暴露和食物链传递引起在人体中的积累[57-58]. 经口摄入是抗生素最主要的暴露途径,普通人群中抗生素主要通过饮食和饮水摄入[59],其中,动物源性食品的摄入是主要的暴露途径[60]. 目前,在人体和动物中常用的抗生素超过100种[61-62],已有80种抗生素及其代谢物在食品和饮用水中检出,其很有可能已经经过食物链进入人体[62]. 此外,人体也会经皮肤接触(痤疮等皮肤病治疗药物)[63]以及呼吸接触(药物、灰尘等)等方式暴露于抗生素. 对于病人而言,摄入途径一般通过服用抗生素类药物[62,64].
-
据报道,30%—90%的抗生素不能被人体和动物体吸收,其会随着尿液和粪便排出体外[12,65]. 有研究发现,人体尿液中抗生素浓度是血液中的数十至数百倍. 因此,在人体内暴露研究中,除部分研究采用血浆[66]、血清[19]、脑脊液[21]、器官组织[67,68]等生物样本外,大多数研究均采用尿液作为基质. 在临床中,监测尿液中抗生素的浓度有助于研判病人的预后发展[31]. 此外,尿液作为非损伤性生物样本采集方式,具有采样成本低、采样对象依从性高等特点,尤其适用于一些以婴儿或未成年人为目标人群的研究,有助于增加样本量和提高应答率.
-
抗生素内暴露水平的研究,对于了解抗生素人体赋存水平,评估其暴露风险,以及制定相应政策、法规均具有重要意义. 目前,全球范围内关于人体中抗生素的内暴露研究已经有一系列报道,但是相对于环境和食品中抗生素的研究仍然较少. 另外,已有研究仅局限于某地区或特定人群(儿童、孕妇、老年人、成年人),涵盖不同地区、不同年龄层的相关调查研究亟待开展. 近20年来我国相继出台了许多政策以应对抗生素滥用问题,抗生素人体内暴露水平理应有所下降,但是其时间变化趋势研究却鲜有报道.
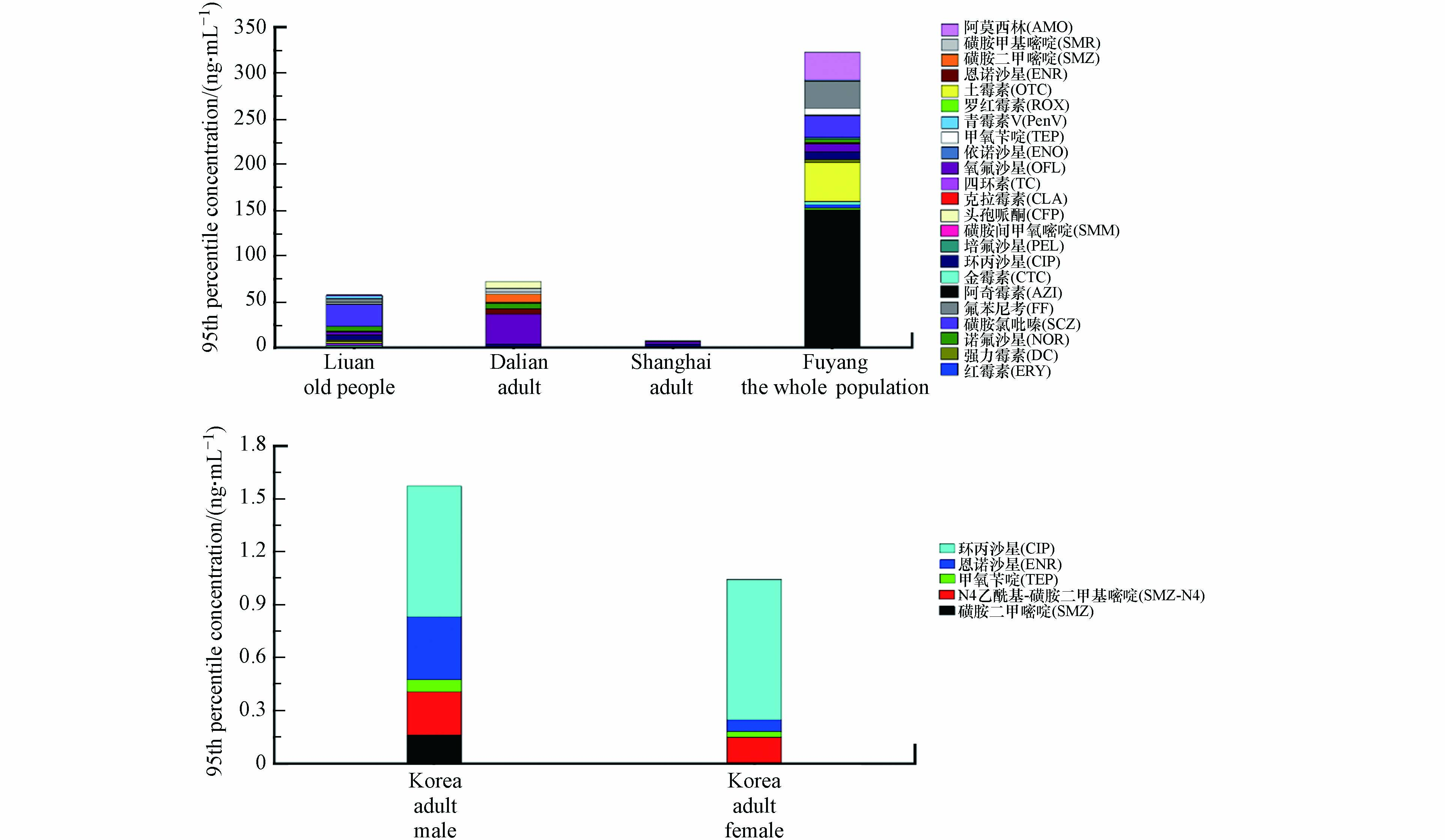
现有研究结果表明,年龄、性别、临床用药、饮食习惯、经济水平、民族等因素均有可能影响抗生素内暴露水平. 不同地区、人群中抗生素的检出率和95th浓度如图1和图2所示. 通过分析儿童(含新生儿)[47,55-56,64,69-72]、孕妇[72-75]、成人[76-78]、老年人[62,79]及全人群[80-82]生物基质(尿液、血清、粪便)中抗生素检出率发现,抗生素在我国和韩国人群中检出率均较高,尤其是阿奇霉素、环丙沙星、氧氟沙星、甲氧苄啶、氟苯尼考[71]. 另外,我国安徽地区抗生素检出率[82]高于韩国,其中老年人和孕妇抗生素的总体检出率较高,其次为儿童和成人,这里需要指出的是,检出率的高低与检测方法密切相关,所以检出浓度可能更具有实际意义. 就检出浓度而言,在全人群中阿奇霉素检出浓度(95th浓度150.42 ng·mL−1)最高,氧氟沙星、环丙沙星、青霉素的检出浓度相对较高. 我国大陆地区人群中抗生素95th浓度普遍高于我国香港地区和韩国[77],此外,安徽和西藏内陆地区抗生素检出浓度普遍高于香港、上海、江苏、浙江等地区,说明抗生素的使用情况与经济发达程度密切相关,经济发达地区可能具有更高水平的抗生素管理、食品安全和环境保护意识[56]. 值得注意的是,抗生素在老年人、成人和儿童中的浓度显著高于孕妇,这可能是由于孕妇在妊娠期间,饮食和用药均比较谨慎导致. 对于老年人而言,临床用药[83]和代谢能力下降[84]是其抗生素检出率和检出浓度均较高的原因. 成人每日摄食肉类和奶制品量通常较高[76],从而增加抗生素的暴露风险. 有研究发现,普通成年人在进行为期5d的素食饮食后,研究对象尿液中抗生素含量明显下降(如环丙沙星)[77]. 另有研究发现,男性中磺胺类抗生素检出率比女性高[56,78-79],可能与男性摄入更多的动物源食物,从而增加食物源VAs或PVAs的更高暴露风险. 有研究发现,在过去三个月内使用过抗生素药物的儿童体内HAs、人/兽共用抗生素(H/VAs)和总抗生素的检出率均显著高于未使用抗生素的儿童[64],因此,个别人体尿液中抗生素检出浓度较高的原因可能是持续摄入药物、严重污染的食物或饮用水[79]. 藏族儿童体内的恩诺沙星和土霉素,以及回族儿童体内的诺氟沙星含量都明显高于汉族儿童,可见民族也是影响抗生素检出差异的影响因素,这可能与生活和饮食习惯相关[69].
-
抗生素的过量使用甚至滥用导致耐药菌甚至多重耐药菌的滋生,使抗生素现有效力下降,增加感染或炎症的风险,甚至可能危及人类生命. 现如今,抗生素耐药性问题已经成为世界各国政府和社会关注的重大公共卫生问题. 关于抗生素毒理学和流行病学研究,此前多是基于动物、细胞模型,或是基于环境和食品外暴露、问卷调查和处方药记录中抗生素数据,以上研究表明抗生素可能会导致耐药性、肥胖/超重、血糖异常、肾毒性、神经系统疾病、牙齿变色、过敏性疾病等多种不良健康结局. 近十年来,抗生素人体内暴露研究陆续展开,基于内暴露监测数据研究也表明抗生素内暴露会造成多种健康风险. 本节对抗生素可能造成的人体健康风险进行了综述.
-
绩效奖励[85]、缺乏安全意识、管理制度不完善等,均会造成医院或门诊抗生素处方不合理问题出现[86-88],进一步导致抗生素的过量使用甚至滥用问题[56]. 韩国一项来自6所医院的研究发现,与2004年相比,2012年广谱抗生素和抗多重耐药菌抗生素消耗量分别增加了10.2%和70.7%,且与总体耐药性增加呈显著正相关[89]. 一项来自中国山东地区关于ICU患者(n=454,≥18岁)的研究同样发现,接受抗生素治疗后,有30.44%患者出现耐药细菌感染,因此治疗中应谨慎使用抗生素[90]. 另外,在当前COVID-19流行的背景下,抗生素在新冠感染患者中频繁使用[91-93],在普通人群中的使用也有升高趋势[94],这也是导致近几年我国人群中耐药性细菌感染的发生率增加的重要原因之一[95]. 此外,食物和环境中的抗生素可以通过食物链进入人体,导致抗生素在人体内累积[79]. 研究发现,短期服用抗生素会使耐药细菌在人体内稳定存在并持续数年[96]. 即使是低剂量的抗生素,也可能导致耐药性产生[97],进一步导致抗生素抗性基因增殖[47,98]. 不仅如此,环境中抗生素也会导致抗性基因产生,抗性基因通过在不同微生物间的水平传递和食物链/食物网的动态转移,使人类和动物对抗生素产生普遍耐药性,其产生的危害远超抗生素本身[99].
各种抗生素耐药细菌甚至多重耐药菌的产生,使得全球耐药情况不容乐观,在全球204个国家,下呼吸道感染造成的与耐药性相关的死亡人数超150万人[100],美国每年由于感染多重耐药性细菌而死亡的人数为2.3万人,欧盟为2.5万人[101]. 若仍不采取措施,预计到2050年,抗生素耐药性导致的死亡人数可能达到每年1000万人[102]. 为积极响应世卫组织(WHO,2015年)开展的《控制微生物耐药全球行动计划》,中国、美国、日本、英国、挪威等国发布了遏制抗生素耐药性国家行动计划,旨从国家层面实施综合治理的策略和措施,提高抗菌药物的科学管理水平. 我国农业农村部密集出台“禁抗”政策,2015年,禁止洛美沙星、培氟沙星、氧氟沙星、诺氟沙星4种人兽共用抗生素物用于动物类食品中[103]. 2016年,禁止硫酸黏菌素预混剂用于动物促生长[104]. 2019年,禁止饲料生产企业生产含有促生长类药物饲料添加剂(如土霉素钙预混剂、阿维拉霉素预混剂、恩拉霉素预混剂)的商品饲料[105].
-
抗生素已被证实在一定剂量水平下具有生长促进作用,且其与体重或BMI指数改变的相关性已成为目前研究的热点. 有研究发现,早期低剂量抗生素治疗可调节肠道微生物群,并诱导小鼠肝脏脂肪酸和脂质代谢的改变[106];在一个关键的发育窗口期,暴露于低剂量青霉素可能会导致小鼠在晚年肥胖[107]. 另有多项基于内暴露水平的研究发现,肥胖/超重的成人中金霉素、四环素、环丙沙星和磺胺甲噁唑的检出率高于正常体重者[76];婴幼儿生长早期暴露于抗生素与体重增加有关[108];儿童的肥胖/超重与VAs和PVAs浓度显著相关[109]. 然而,有研究发现超重/肥胖与HAs和优先作为人用的抗生素(PHAs)内暴露浓度无显著相关性[109]. 其原因可能是,与VAs或PVAs的暴露来源不同,人体中HAs或PHAs内暴露水平与药物的使用剂量、使用频率和持续时间等息息相关,所以抗生素浓度在一定时间内波动较大,加之抗生素在人体内半衰期短且为不连续暴露,因此HAs或PHAs对人体健康的影响不显著. 综上所述,研究对象的个体间差异,以及混杂因素的纳入,均会影响统计分析结果,因此,抗生素与肥胖/超重的因果关系仍需进一步研究.
-
现有研究发现喹诺酮类抗生素的使用可能造成血糖稳态的改变,导致低血糖症或高血糖症的发生. 细胞和动物模型研究结果发现,喹诺酮类药物会通过促进胰岛素产生而导致血糖下降,其可能机制是通过阻断KATP通道(ATP敏感性钾通道)或者增强β细胞营养素的刺激作用而实现[110-111]. 另有研究表明,当大鼠暴露于加替沙星后,胰腺β细胞中的分泌颗粒减少,胰岛素合成/运输受损,使得血清胰岛素水平降低,造成高血糖症的发生[112]. Althaqafi等[113]系统综述了截至到2019年9月喹诺酮类药物对糖尿病患者(30—78岁)作用的相关研究,总共检索到735篇文章,其中16篇符合检索标准,发现在8项研究(4663名患者)中,1588名患者服用喹诺酮类药物后出现高血糖;但是,在11项研究(6208名患者)中,2179名患者服用喹诺酮类药物后出现低血糖. 另外该研究表明血糖异常通常与糖尿病本身无关,而与喹诺酮类药物摄入相关,在加替沙星、莫西沙星、环丙沙星、左氧氟沙星4种抗生素中,莫西沙星与血糖异常的相关性最高,环丙沙星相关性最低. 但该文献存在数据缺失等问题可能会影响统计分析结果;喹诺酮类药物不良反应的严重程度也可能影响糖尿病患者血液中胰岛素含量.
-
抗生素治疗人类疾病的同时,其(氨基糖苷类、万古霉素、粘杆菌素等)导致的肾毒性也不容忽视. 氨基糖苷类抗生素(如庆大霉素和妥布霉素),在儿科临床治疗中仍然普遍使用. 但是,有研究表明20%至33%儿童暴露于氨基糖苷类药物后,可能发生急性肾损伤,因此研究和发现新的肾功能生物标志物对于预测急性肾损伤有重要意义[114]. 万古霉素被广泛应用于治疗葡萄球菌导致的感染,有研究发现,分别有10%—20%和30%—40%的患者接受常规和大剂量万古霉素治疗后出现肾毒性,另有研究表明,万古霉素与哌拉西林和他唑巴坦联合使用时,肾毒性或急性肾损伤的发生率增加[115-116],然而其导致肾毒性的机制仍不明确,可能与氧化应激和过敏反应有关[117-118]. 人群和动物模型实验表明粘杆菌素也可导致肾毒性,在泰国381名患者(51—62岁)的回顾性研究中发现,粘菌素给药后患者的肾毒性发生率为74% [119],其机制可能与肾小管上皮细胞膜通透性增加、细胞肿胀和细胞溶解有关,在此过程中也有氧化应激和炎性反应参与[120]. Chang等对中国14家医院接受多粘菌素 B 治疗的患者进行多中心、回顾性队列研究,发现患者中急性肾损伤的发病率为33.5%,且多粘菌素B的负荷剂量是急性肾损伤中的危险因素[121]. 另有研究发现,在对印度110名连续入院的1个月至12岁的儿童进行氨基糖苷类药物治疗(≥4 d),发现有71名(64.5%)儿童发展为复合肾毒性,其中42名儿童(38.2%)为急性肾损伤,并且17人(15.5%)的急性肾损伤明确归因于氨基糖苷类药物的使用,因此,在低血压和危重儿童中使用氨基糖苷类药物时必须谨慎[122].
-
抗生素所致的肠道菌群失调可进一步引起神经系统疾病,如抑郁、焦虑、失眠等. 有研究表明,大鼠反复服用药物剂量的环丙沙星会导致神经系统毒性,其中,脑神经递质水平的改变和氧化应激是环丙沙星诱导神经毒性的可能潜在机制[123]. 抗生素暴露与神经疾病的相关性在流行病学研究中也被证实. Zhu等[79]基于人体抗生素内暴露水平,探究了抗生素暴露与老年人抑郁之间的关系. 该研究对990名中国安徽地区老人(≥60岁)尿液中9类抗生素进行检测,发现阿奇霉素、磺胺氯吡嗪、四环素等与老年人抑郁症风险升高存在显著关联,且存在性别和年龄差异. 但是,由于该研究为横断面设计,故抗生素暴露与抑郁症之间的因果关系不确定;其次,抗生素的使用和暴露水平因地理区域的不同而存在很大差异,而本研究人群仅来自单一地区,所以缺乏全国代表性. Lurie等[124]基于1995—2013年英国医疗数据库信息进行了3项嵌套病例对照研究,该研究纳入了202974名抑郁症患者、14570名焦虑症患者以及2690名精神病患者,每一病例按发病率密度再抽样匹配4名对照组成员,使用Logistic回归分析精神疾病与抗生素暴露的相关性,发现使用单一抗生素治疗可增加抑郁/焦虑风险,且抑郁/焦虑风险随疗程增加而增加. 一项前瞻性研究表明,124名母亲在怀孕期间和怀孕后14d内的抗生素暴露与产后1—2个月的产后抑郁存在显著关联. 但是,该研究中抗生素暴露信息来自问卷调查,可能对分析结果有一定影响[125]. 此外,多项研究表明米诺环素可作为改善抑郁症状的辅助药物[126-127]. 一项在巴基斯坦为期3个月的随机对照试验中,将40名参与者随机分配接受米诺环素或照常治疗,结合抑郁量表和血液生物标志物对治疗效果进行分析,结果显示米诺环素可能具有减轻抑郁症状的作用,但是本研究研究对象数量较少,仍需要大样本量的流行病学研究加以验证[128].
-
抗生素的使用也会产生其它健康危害. 在过去几十年中,全球范围内过敏性疾病的流行率呈上升趋势[129],妊娠期是胎儿发育的关键时期,Geng等[130]对安徽马鞍山2543对母子尿液样本中41种抗生素及2种代谢物进行分析,发现母亲孕早期磺胺二甲嘧啶和孕中期环丙沙星的暴露会增加儿童(4岁)患湿疹的风险,孕晚期土霉素的暴露与儿童哮喘风险增加有关. 一项对1401名美国儿童的研究,评估了2003年至2007年间在出生后6个月内使用抗生素与6岁时哮喘之间的关系,结果表明,早期使用抗生素与6岁时的哮喘有关(OR=1.52, 95%CI: 1.07, 2.16)[131]. 据报道许多人对β-内酰胺类药物青霉素过敏,老年患者和住院患者报告的过敏率较高[132],另有研究发现儿童对大环内脂类药物也会出现过敏反应[133]. 四环素于1948年问世,其作为广谱抗生素可用于治疗儿童和成人的许多常见感染疾病. 但是,四环素能够螯合钙离子,并结合到牙齿、软骨和骨骼中,导致乳牙和恒牙变色,这种永久性变色从黄色或灰色到棕色不等. 然而,目前的大部分研究均是病例报告,纵向研究较少,纵向研究可以提供关于四环素染色在人群中的患病率、严重程度、病因和临床表现的确切信息,为如何有效管理四环素导致的染色提供科学依据[134-135]. 美国一项包括37516名无心血管疾病和癌症的前瞻队列研究表明,成年后期(年龄≥60岁)长期使用(≥2月)抗生素可能是全因死亡率和心血管死亡率升高的危险因素,因此需要考虑抗生素暴露对慢性病死亡风险的不利影响[136]. 但是,该研究纳入人群的抗生素使用情况通过问卷形式获得,未收集抗生素类别信息,因此无法评估特定类型抗生素与健康结局的关联性;抗生素使用情况是自我报告的方式,其可能被高估或低估. 另有研究发现,口服抗生素不仅会引起人体肠道菌群的改变,其还与慢性肠道炎症的发生相关,可进一步导致结肠癌发生风险升高,但是其因果关系和生物学机制需要进一步探讨[137-138].
-
抗生素自问世以来,在临床治疗、畜牧养殖和农业种植等领域发挥了重要作用,但随着抗生素用量的增多甚至滥用,其带来了一系列环境与健康问题. 目前研究多针对环境外暴露分析,其来源广泛、赋存高,受人为活动影响大,针对人体内暴露水平的研究相对较少,且多集中于抗生素母体化合物的暴露特征和来源分析,或是基于问卷调查、处方药记录和抗生素的产量和使用量调查等开展的流行病学研究,而抗生素的实际负荷水平研究及其人体健康风险研究仍然有限.
近年来,抗生素在不同介质中检测技术的发展为抗生素暴露水平的研究提供了有力保障. 目前人体内抗生素的检测方法中,靶向分析的目标抗生素最多为43种(含2种代谢物)[40-41,79],仅有一项关于74种抗生素非靶向分析的报道[40]. 据调查,当前人体和动物中常用的抗生素已超过100种[61-62],所以亟需优化和创新前处理和仪器分析方法,开发涵盖更多抗生素种类的前处理技术及靶向、疑似靶向和非靶向分析方法. 另外,由于抗生素不稳定,在人体中容易代谢和转化,所以进入人体中的抗生素,除了以原型或结合物的形式排除体外,一部分还会在人体中进行代谢,因此针对人体中抗生素代谢物的研究同样具有科学意义.
抗生素的人体健康风险、内暴露特征及检测技术研究进展
Research progress on human health risk, internal exposure characteristics and analysis technologies of antibiotics
-
摘要: 抗生素自问世以来,被广泛应用于临床治疗、畜牧业、农业和水产养殖业等领域中,在治疗感染性疾病和促进生长方面功不可没. 但是,抗生素的大量使用甚至滥用,使得环境外暴露和人体内暴露水平升高,进而对生态环境和人体健康造成严重威胁. 本文对抗生素的使用现状进行概述,并系统综述了尿液、血清、母乳、唾液等介质中抗生素的检测方法、内暴露特征及人体健康风险的研究进展. 目前液相色谱串联质谱法是抗生素最常用的分析方法,固相萃取法是人体生物样本抗生素分析中最常用的前处理方法. 人体抗生素内暴露靶向分析中,已有报道涵盖抗生素类别最多的分析方法包括41种抗生素及2种代谢物,而疑似靶向和非靶向分析方法鲜有报道,此后需进一步优化抗生素的提取和分析方法,开发涵盖更多抗生素及其代谢物类型的前处理技术及靶向、疑似靶向和非靶向分析方法. 已有流行病学研究多是基于问卷调查、处方药记录和抗生素的产量和使用量调查等开展,而基于抗生素的实际负荷水平的人体健康风险研究和流行病学研究仍然有限,因此亟待开展涵盖不同地区、不同人群的抗生素内暴露特征和队列研究.Abstract: Since the appearance of antibiotics, they have been widely used in clinical therapeutics, animal husbandry, agriculture, aquaculture, and other fields, especially in treating of infectious diseases and promoting growth. However, the extensive use or even abuse of antibiotics increases the levels of external exposure and internal exposure, which poses a serious threat to the ecological environment and human health. In this review, the current usage situation of antibiotics had been summarized, and the detection methods in human matrix (urine, serum, breast milk, saliva), internal exposure characteristics and human health risks of antibiotics, had been systematically reviewed. At present, liquid chromatography-tandem mass spectrometry (LC-MS/MS) is the most commonly used determination method for antibiotics, and solid phase extraction (SPE) is the most common pretreatment method of antibiotics in human biological samples. In targeted analysis of antibiotics in human biological matrix, the reported method covering the most types of antibiotics included 41 antibiotics and 2 metabolites. However, there are few reports on methods of suspected targeted and non-targeted analysis of antibiotics. Therefore, it is necessary to further optimize the extraction and analysis methods of antibiotics, and develop targeted, suspected targeted and non-targeted analysis methods that covering more types of antibiotics and their corresponding metabolites in the future. Most of the existing epidemiological studies are based on questionnaires, prescription drug records, antibiotic production and usage surveys, but human health risk studies and epidemiological studies based on the actual levels of antibiotics internal exposure are still limited. Therefore, it is urgent to carry out studies on internal exposure characteristics and of antibiotics in different regions and populations.
-

-
-
[1] ZHOU L J, YING G G, ZHAO J L, et al. Trends in the occurrence of human and veterinary antibiotics in the sediments of the Yellow River, Hai River and Liao River in Northern China [J]. Environmental Pollution, 2011, 159(7): 1877-1885. doi: 10.1016/j.envpol.2011.03.034 [2] PAN M, CHU L M. Fate of antibiotics in soil and their uptake by edible crops [J]. Science of the Total Environment, 2017, 599/600: 500-512. doi: 10.1016/j.scitotenv.2017.04.214 [3] BEN Y J, HU M, ZHONG F X, et al. Human daily dietary intakes of antibiotic residues: Dominant sources and health risks [J]. Environmental Research, 2022, 212: 113387. doi: 10.1016/j.envres.2022.113387 [4] CHEN X L, YANG Y Y, KE Y C, et al. A comprehensive review on biodegradation of tetracyclines: Current research progress and prospect [J]. Science of the Total Environment, 2022, 814: 152852. doi: 10.1016/j.scitotenv.2021.152852 [5] MOSER C, LERCHE C J, THOMSEN K, et al. Antibiotic therapy as personalized medicine - general considerations and complicating factors [J]. APMIS, 2019, 127(5): 361-371. doi: 10.1111/apm.12951 [6] MCMANUS P S, STOCKWELL V O, SUNDIN G W, et al. Antibiotic use in plant agriculture [J]. Annual Review of Phytopathology, 2002, 40: 443-465. doi: 10.1146/annurev.phyto.40.120301.093927 [7] ROBLES-JIMENEZ L E, ARANDA-AGUIRRE E, CASTELAN-ORTEGA O A, et al. Worldwide traceability of antibiotic residues from livestock in wastewater and soil: A systematic review [J]. Animals:an Open Access Journal from MDPI, 2021, 12(1): 60. [8] MARTINS M T, MELO J, BARRETO F, et al. A simple, fast and cheap non-SPE screening method for antibacterial residue analysis in milk and liver using liquid chromatography-tandem mass spectrometry [J]. Talanta, 2014, 129: 374-383. doi: 10.1016/j.talanta.2014.04.049 [9] KLEIN E Y, van BOECKEL T P, MARTINEZ E M, et al. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015 [J]. Proceedings of the National Academy of Sciences of the United States of America, 2018, 115(15): E3463-E3470. [10] 邵彬彬. 高效三元复合光催化剂的制备及其去除水体中抗生素污染物的机理研究[D]. 长沙: 湖南大学, 2020. SHAO B B. Synthesis of efficient ternary composite photocatalysts and their mechanism study of removing antibiotic pollutants from water[D]. Changsha: Hunan University, 2020(in Chinese).
[11] TISEO K, HUBER L, GILBERT M, et al. Global trends in antimicrobial use in food animals from 2017 to 2030 [J]. Antibiotics (Basel, Switzerland), 2020, 9(12): 918. [12] 蔡东明, 欧阳洁, 丁锦建, 等. 抗生素消毒副产物的分析检测及毒性效应研究进展 [J]. 分析化学, 2022, 50(3): 327-340. doi: 10.19756/j.issn.0253-3820.210811 CAI D M, OUYANG J, DING J J, et al. Research progress on identification and toxic effects of antibiotics disinfection by-products [J]. Chinese Journal of Analytical Chemistry, 2022, 50(3): 327-340(in Chinese). doi: 10.19756/j.issn.0253-3820.210811
[13] 邹秀萍, 李振玮, 张丛林, 等. 构建中国特色新污染物风险防控体系[J]. 环境生态学, 2022, 4(S1): 111-115. ZOU X P, LI Z W, ZHANG C L, et al. Building on ecological risk prevention and control system of emerging contaminants with Chinese characteristics[J]. Environmental Ecology, 2022, 4(Sup 1): 111-115(in Chinese).
[14] VREE T B, HEKSTER Y A, BAARS A M, et al. Rapid determination of amoxycillin (clamoxyl) and ampicillin (penbritin) in body fluids of many by means of high-performance liquid chromatography [J]. Journal of Chromatography, 1978, 145(3): 496-501. doi: 10.1016/S0378-4347(00)81384-5 [15] BUSZEWSKI B, SZULTKA M, OLSZOWY P, et al. A novel approach to the rapid determination of amoxicillin in human plasma by solid phase microextraction and liquid chromatography [J]. Analyst, 2011, 136(12): 2635-2642. doi: 10.1039/c1an00005e [16] SUPATTANAPONG S, KONSIL J. Solid phase extraction and high performance liquid chromatography for the determination of azithromycin in human plasma [J]. The Southeast Asian Journal of Tropical Medicine and Public Health, 2008, 39(6): 978-987. [17] YıLDıRıM S, KARAKOÇ H N, YAŞAR A, et al. Determination of levofloxacin, ciprofloxacin, moxifloxacin and gemifloxacin in urine and plasma by HPLC-FLD-DAD using pentafluorophenyl core-shell column: Application to drug monitoring [J]. Biomedical Chromatography:BMC, 2020, 34(10): e4925. [18] FATICA E, FABER J, GAFFRON C, et al. Quantification of serum sulfamethoxazole and trimethoprim by ultra-fast solid-phase extraction-tandem mass spectrometry [J]. Therapeutic Drug Monitoring, 2020, 42(5): 724-732. doi: 10.1097/FTD.0000000000000785 [19] WANG X L, GUO T, WEI Y B, et al. Determination of quinolone antibiotic residues in human serum and urine using high-performance liquid chromatography/tandem mass spectrometry [J]. Journal of Analytical Toxicology, 2019, 43(7): 579-586. doi: 10.1093/jat/bkz034 [20] SIME F B, ROBERTS M S, ROBERTS J A, et al. Simultaneous determination of seven β-lactam antibiotics in human plasma for therapeutic drug monitoring and pharmacokinetic studies [J]. Journal of Chromatography B, 2014, 960: 134-144. doi: 10.1016/j.jchromb.2014.04.029 [21] CAZORLA-REYES R, ROMERO-GONZÁLEZ R, FRENICH A G, et al. Simultaneous analysis of antibiotics in biological samples by ultra high performance liquid chromatography-tandem mass spectrometry [J]. Journal of Pharmaceutical and Biomedical Analysis, 2014, 89: 203-212. doi: 10.1016/j.jpba.2013.11.004 [22] 陈聪, 严慧, 沈保华, 等. 超高效液相色谱-串联质谱法同时测定尿液中16种抗生素 [J]. 法医学杂志, 2011, 27(1): 25-29. CHEN C, YAN H, SHEN B H, et al. Simultaneous determination of sixteen antibiotics in human urine with ultra performance liquid chromatography-tandem mass spectrometry [J]. Journal of Forensic Medicine, 2011, 27(1): 25-29(in Chinese).
[23] XIE F F, LIU L Y, WANG Y, et al. An UPLC-PDA assay for simultaneous determination of seven antibiotics in human plasma [J]. Journal of Pharmaceutical and Biomedical Analysis, 2022, 210: 114558. doi: 10.1016/j.jpba.2021.114558 [24] BAIETTO L, D’AVOLIO A, de ROSA F G, et al. Development and validation of a simultaneous extraction procedure for HPLC-MS quantification of daptomycin, amikacin, gentamicin, and rifampicin in human plasma [J]. Analytical and Bioanalytical Chemistry, 2010, 396(2): 791-798. doi: 10.1007/s00216-009-3263-1 [25] DEI CAS M, CASAGNI E, GAMBARO V, et al. Determination of daptomycin in human plasma and breast milk by UPLC/MS-MS [J]. Journal of Chromatography. B, 2019, 1116: 38-43. doi: 10.1016/j.jchromb.2019.03.036 [26] PERESTRELO R, SILVA P, PORTO-FIGUEIRA P, et al. QuEChERS - Fundamentals, relevant improvements, applications and future trends [J]. Analytica Chimica Acta, 2019, 1070: 1-28. doi: 10.1016/j.aca.2019.02.036 [27] SUNYER-CALDÚ A, DIAZ-CRUZ M S. Development of a QuEChERS-based method for the analysis of pharmaceuticals and personal care products in lettuces grown in field-scale agricultural plots irrigated with reclaimed water [J]. Talanta, 2021, 230: 122302. doi: 10.1016/j.talanta.2021.122302 [28] ACOSTA-DACAL A, RIAL-BERRIEL C, DÍAZ-DÍAZ R, et al. Validation of a method scope extension for the analysis of POPs in soil and verification in organic and conventional farms of the canary Islands [J]. Toxics, 2021, 9(5): 101. doi: 10.3390/toxics9050101 [29] PETRARCA M H, BRAGA P A D C, REYES F G R, et al. Exploring miniaturized sample preparation approaches combined with LC-QToF-MS for the analysis of sulfonamide antibiotic residues in meat- and/or egg-based baby foods [J]. Food Chemistry, 2022, 366: 130587. doi: 10.1016/j.foodchem.2021.130587 [30] PÉREZ-BURGOS R, GRZELAK E M, GOKCE G, et al. Quechers methodologies as an alternative to solid phase extraction (SPE) for the determination and characterization of residues of cephalosporins in beef muscle using LC-MS/MS [J]. Journal of Chromatography B, 2012, 899: 57-65. doi: 10.1016/j.jchromb.2012.05.002 [31] HUANG W J, QIU Q J, CHEN M Y, et al. Determination of 18 antibiotics in urine using LC-QqQ-MS/MS [J]. Journal of Chromatography B, 2019, 1105: 176-183. doi: 10.1016/j.jchromb.2018.12.019 [32] di ROCCO M, MOLONEY M, O’BEIRNE T, et al. Development and validation of a quantitative confirmatory method for 30 β-lactam antibiotics in bovine muscle using liquid chromatography coupled to tandem mass spectrometry [J]. Journal of Chromatography A, 2017, 1500: 121-135. doi: 10.1016/j.chroma.2017.04.022 [33] TU X J, CHEN W B. A review on the recent progress in matrix solid phase dispersion [J]. Molecules (Basel, Switzerland), 2018, 23(11): 2767. doi: 10.3390/molecules23112767 [34] SUN H W, QIAO F X, LIU G Y, et al. Simultaneous isolation of six fluoroquinolones in serum samples by selective molecularly imprinted matrix solid-phase dispersion [J]. Analytica Chimica Acta, 2008, 625(2): 154-159. doi: 10.1016/j.aca.2008.07.025 [35] FERNANDEZ-TORRES R, CONSENTINO M O, LOPEZ M A B, et al. Simultaneous determination of 11 antibiotics and their main metabolites from four different groups by reversed-phase high-performance liquid chromatography-diode array-fluorescence (HPLC-DAD-FLD) in human urine samples [J]. Talanta, 2010, 81(3): 871-880. doi: 10.1016/j.talanta.2010.01.031 [36] KAALE E, LONG Y H, FONGE H A, et al. Gentamicin assay in human serum by solid-phase extraction and capillary electrophoresis [J]. Electrophoresis, 2005, 26(3): 640-647. doi: 10.1002/elps.200410012 [37] ZHANG T, WATSON D G, AZIKE C, et al. Determination of vancomycin in serum by liquid chromatography-high resolution full scan mass spectrometry [J]. Journal of Chromatography B, 2007, 857(2): 352-356. doi: 10.1016/j.jchromb.2007.07.041 [38] OERTEL R, RENNER U, KIRCH W. Determination of neomycin by LC-tandem mass spectrometry using hydrophilic interaction chromatography [J]. Journal of Pharmaceutical and Biomedical Analysis, 2004, 35(3): 633-638. doi: 10.1016/j.jpba.2004.01.018 [39] DOTSIKAS Y, MARKOPOULOU C K, KOUNDOURELLIS J E, et al. Validation of a novel LC-MS/MS method for the quantitation of colistin A and B in human plasma [J]. Journal of Separation Science, 2011, 34(1): 37-45. doi: 10.1002/jssc.201000680 [40] WANG H X, WANG B, ZHOU Y, et al. Rapid and sensitive screening and selective quantification of antibiotics in human urine by two-dimensional ultraperformance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2014, 406(30): 8049-8058. doi: 10.1007/s00216-014-8197-6 [41] LIU K Y, ZHANG J J, GENG M L, et al. A stable isotope dilution assay for multi-class antibiotics in pregnant urines by LC-MS/MS [J]. Chromatographia, 2020, 83(4): 507-521. doi: 10.1007/s10337-020-03866-3 [42] XIE R, WEN J, WEI H, et al. High-throughput determination of faropenem in human plasma and urine by on-line solid-phase extraction coupled to high-performance liquid chromatography with UV detection and its application to the pharmacokinetic study [J]. Journal of Pharmaceutical and Biomedical Analysis, 2010, 52(1): 114-121. doi: 10.1016/j.jpba.2009.12.010 [43] SZULTKA-MLYNSKA M, POMASTOWSKI P, BUSZEWSKI B. Application of solid phase microextraction followed by liquid chromatography-mass spectrometry in the determination of antibiotic drugs and their metabolites in human whole blood and tissue samples [J]. Journal of Chromatography B, 2018, 1086: 153-165. doi: 10.1016/j.jchromb.2018.04.013 [44] 陈波. 新型样品前处理技术在环境有机污染物分析检测中的应用研究[D]. 重庆: 西南大学, 2012. CHEN B. Application of new sample pre-treatment technology in the analysis and detection of environmental organic pollutants[D]. Chongqing: Southwest University, 2012(in Chinese).
[45] PASTOR-BELDA M, CAMPILLO N, ARROYO-MANZANARES N, et al. Determination of amphenicol antibiotics and their glucuronide metabolites in urine samples using liquid chromatography with quadrupole time-of-flight mass spectrometry [J]. Journal of Chromatography B, 2020, 1146: 122122. doi: 10.1016/j.jchromb.2020.122122 [46] MIZUNO A, UEMATSU T, NAKASHIMA M. Simultaneous determination of ofloxacin, norfloxacin and ciprofloxacin in human hair by high-performance liquid chromatography and fluorescence detection [J]. Journal of Chromatography B:Biomedical Sciences and Applications, 1994, 653(2): 187-193. doi: 10.1016/0378-4347(93)E0440-2 [47] LI N, HO K W K, YING G G, et al. Veterinary antibiotics in food, drinking water, and the urine of preschool children in Hong Kong [J]. Environment International, 2017, 108: 246-252. doi: 10.1016/j.envint.2017.08.014 [48] 李明, 马家辰, 李红梅, 等. 静电场轨道阱质谱的进展 [J]. 质谱学报, 2013, 34(3): 185-192. LI M, MA J C, LI H M, et al. Progress on electrostatic orbitrap mass spectrometer [J]. Journal of Chinese Mass Spectrometry Society, 2013, 34(3): 185-192(in Chinese).
[49] QIU M, HU A L, HUANG Y M M, et al. Elucidating degradation mechanisms of florfenicol in soil by stable-isotope assisted nontarget screening [J]. Journal of Hazardous Materials, 2021, 403: 123974. doi: 10.1016/j.jhazmat.2020.123974 [50] FENG X X, LI D, LIANG W Q, et al. Recognition and prioritization of chemical mixtures and transformation products in Chinese estuarine waters by suspect screening analysis [J]. Environmental Science & Technology, 2021, 55(14): 9508-9517. [51] VERGEYNST L, van LANGENHOVE H, JOOS P, et al. Suspect screening and target quantification of multi-class pharmaceuticals in surface water based on large-volume injection liquid chromatography and time-of-flight mass spectrometry [J]. Analytical and Bioanalytical Chemistry, 2014, 406(11): 2533-2547. doi: 10.1007/s00216-014-7672-4 [52] GAGO-FERRERO P, SCHYMANSKI E L, BLETSOU A A, et al. Extended suspect and non-target strategies to characterize emerging polar organic contaminants in raw wastewater with LC-HRMS/MS [J]. Environmental Science & Technology, 2015, 49(20): 12333-12341. [53] SOLLIEC M, ROY-LACHAPELLE A, SAUVÉ S. Development of a suspect and non-target screening approach to detect veterinary antibiotic residues in a complex biological matrix using liquid chromatography/high-resolution mass spectrometry [J]. Rapid Communications in Mass Spectrometry, 2015, 29(24): 2361-2373. doi: 10.1002/rcm.7405 [54] GÓMEZ-PÉREZ M L, PLAZA-BOLAÑOS P, ROMERO-GONZÁLEZ R, et al. Comprehensive qualitative and quantitative determination of pesticides and veterinary drugs in honey using liquid chromatography-Orbitrap high resolution mass spectrometry [J]. Journal of Chromatography A, 2012, 1248: 130-138. doi: 10.1016/j.chroma.2012.05.088 [55] WANG H X, TANG C X, WANG Y P, et al. Urinary antibiotic level of school children in Shanghai, East China, 2017-2020 [J]. Environmental Pollution, 2021, 291: 118167. doi: 10.1016/j.envpol.2021.118167 [56] WANG H X, WANG B, ZHAO Q, et al. Antibiotic body burden of Chinese school children: A multisite biomonitoring-based study [J]. Environmental Science & Technology, 2015, 49(8): 5070-5079. [57] YUE F L, LI F L, KONG Q Q, et al. Recent advances in aptamer-based sensors for aminoglycoside antibiotics detection and their applications [J]. Science of the Total Environment, 2021, 762: 143129. doi: 10.1016/j.scitotenv.2020.143129 [58] LAN L Y, YAO Y, PING J F, et al. Recent advances in nanomaterial-based biosensors for antibiotics detection [J]. Biosensors and Bioelectronics, 2017, 91: 504-514. doi: 10.1016/j.bios.2017.01.007 [59] LIU Y J, WANG S Q, PAN J L, et al. Antibiotics in urine of the general population: Exposure, health risk assessment, and food factors [J]. Journal of Environmental Science and Health, Part B, 2022, 57(1): 1-12. doi: 10.1080/03601234.2021.2017211 [60] SIMIN J, FORNES R, LIU Q, et al. Antibiotic use and risk of colorectal cancer: A systematic review and dose–response meta-analysis [J]. British Journal of Cancer, 2020, 123(12): 1825-1832. doi: 10.1038/s41416-020-01082-2 [61] WANG H X, WANG N, WANG B, et al. Antibiotics in drinking water in Shanghai and their contribution to antibiotic exposure of school children [J]. Environmental Science & Technology, 2016, 50(5): 2692-2699. [62] LIU X J, ZHANG J J, SANG Y R, et al. Antibiotic exposure and potential risk of depression in the Chinese elderly: A biomonitoring-based population study [J]. Environmental Science and Pollution Research, 2021, 28(21): 26794-26806. doi: 10.1007/s11356-021-12560-2 [63] XU H X, LI H Y. Acne, the skin microbiome, and antibiotic treatment [J]. American Journal of Clinical Dermatology, 2019, 20(3): 335-344. doi: 10.1007/s40257-018-00417-3 [64] WANG H X, TANG C X, YANG J Q, et al. Predictors of urinary antibiotics in children of Shanghai and health risk assessment [J]. Environment International, 2018, 121: 507-514. doi: 10.1016/j.envint.2018.09.032 [65] ZHANG Q Q, YING G G, PAN C G, et al. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance [J]. Environmental Science & Technology, 2015, 49(11): 6772-6782. [66] DECOSTERD L A, MERCIER T, TERNON B, et al. Validation and clinical application of a multiplex high performance liquid chromatography - tandem mass spectrometry assay for the monitoring of plasma concentrations of 12 antibiotics in patients with severe bacterial infections [J]. Journal of Chromatography. B, 2020, 1157: 122160. doi: 10.1016/j.jchromb.2020.122160 [67] FREITAS A, BARBOSA J, RAMOS F. Multidetection of antibiotics in liver tissue by ultra-high-pressure-liquid-chromatography-tandem mass spectrometry [J]. Journal of Chromatography B, 2015, 976/977: 49-54. doi: 10.1016/j.jchromb.2014.11.008 [68] SZERKUS O, JACYNA J, GIBAS A, et al. Robust HPLC-MS/MS method for levofloxacin and ciprofloxacin determination in human prostate tissue [J]. Journal of Pharmaceutical and Biomedical Analysis, 2017, 132: 173-183. doi: 10.1016/j.jpba.2016.10.008 [69] HUANG Y S, ZHANG Z H, HOU T C, et al. Antibiotic burden of school children from Tibetan, Hui, and Han groups in the Qinghai-Tibetan Plateau [J]. PLoS One, 2020, 15(2): e0229205. doi: 10.1371/journal.pone.0229205 [70] ZHANG J J, LIU K Y, SUN L, et al. Exposure to antibiotics and mental disorders in children: A community-based cross-sectional study [J]. Environmental Geochemistry and Health, 2021, 43(8): 3237-3253. doi: 10.1007/s10653-021-00840-2 [71] ZHAO Y Y, ZHOU Y H, ZHU Q Y, et al. Determination of antibiotic concentration in meconium and its association with fetal growth and development [J]. Environment International, 2019, 123: 70-78. doi: 10.1016/j.envint.2018.11.053 [72] ZHOU Y J, ZHU F, ZHENG D Y, et al. Detection of antibiotics in the urine of children and pregnant women in Jiangsu, China [J]. Environmental Research, 2021, 196: 110945. doi: 10.1016/j.envres.2021.110945 [73] GENG M L, LIU K Y, HUANG K, et al. Urinary antibiotic exposure across pregnancy from Chinese pregnant women and health risk assessment: Repeated measures analysis [J]. Environment International, 2020, 145: 106164. doi: 10.1016/j.envint.2020.106164 [74] ZENG X X, ZHANG L Y, CHEN Q, et al. Maternal antibiotic concentrations in pregnant women in Shanghai and their determinants: A biomonitoring-based prospective study [J]. Environment International, 2020, 138: 105638. doi: 10.1016/j.envint.2020.105638 [75] WANG H X, WANG N, QIAN J H, et al. Urinary antibiotics of pregnant women in Eastern China and cumulative health risk assessment [J]. Environmental Science & Technology, 2017, 51(6): 3518-3525. [76] WANG H X, YANG J Q, YU X, et al. Exposure of adults to antibiotics in a Shanghai suburban area and health risk assessment: A biomonitoring-based study [J]. Environmental Science & Technology, 2018, 52(23): 13942-13950. [77] JI K, KHO Y L, PARK Y, et al. Influence of a five-day vegetarian diet on urinary levels of antibiotics and phthalate metabolites: A pilot study with “Temple Stay” participants [J]. Environmental Research, 2010, 110(4): 375-382. doi: 10.1016/j.envres.2010.02.008 [78] LIU S S, ZHAO G D, ZHAO H X, et al. Antibiotics in a general population: Relations with gender, body mass index (BMI) and age and their human health risks [J]. Science of the Total Environment, 2017, 599/600: 298-304. doi: 10.1016/j.scitotenv.2017.04.216 [79] ZHU Y T, LIU K Y, ZHANG J J, et al. Antibiotic body burden of elderly Chinese population and health risk assessment: A human biomonitoring-based study [J]. Environmental Pollution, 2020, 256: 113311. doi: 10.1016/j.envpol.2019.113311 [80] JI K, KHO Y, PARK C, et al. Influence of water and food consumption on inadvertent antibiotics intake among general population [J]. Environmental Research, 2010, 110(7): 641-649. doi: 10.1016/j.envres.2010.06.008 [81] WANG Q, DUAN Y J, WANG S P, et al. Occurrence and distribution of clinical and veterinary antibiotics in the faeces of a Chinese population [J]. Journal of Hazardous Materials, 2020, 383: 121129. doi: 10.1016/j.jhazmat.2019.121129 [82] ZHANG J J, LIU X J, ZHU Y T, et al. Antibiotic exposure across three generations from Chinese families and cumulative health risk [J]. Ecotoxicology and Environmental Safety, 2020, 191: 110237. doi: 10.1016/j.ecoenv.2020.110237 [83] HAYWARD G N, MOORE A, MCKELVIE S, et al. Antibiotic prescribing for the older adult: Beliefs and practices in primary care [J]. Journal of Antimicrobial Chemotherapy, 2018, 74(3): 791-797. [84] AN R, WILMS E, MASCLEE A A M, et al. Age-dependent changes in GI physiology and microbiota: Time to reconsider? [J]. Gut, 2018, 67(12): 2213-2222. doi: 10.1136/gutjnl-2017-315542 [85] CURRIE J, LIN W C, ZHANG W. Patient knowledge and antibiotic abuse: Evidence from an audit study in China [J]. Journal of Health Economics, 2011, 30(5): 933-949. doi: 10.1016/j.jhealeco.2011.05.009 [86] FLEMING-DUTRA K E, HERSH A L, SHAPIRO D J, et al. Prevalence of inappropriate antibiotic Prescriptions among US ambulatory care visits, 2010-2011 [J]. JAMA, 2016, 315(17): 1864-1873. doi: 10.1001/jama.2016.4151 [87] DAVIES S C. Reducing inappropriate prescribing of antibiotics in English primary care: Evidence and outlook [J]. Journal of Antimicrobial Chemotherapy, 2018, 73(4): 833-834. doi: 10.1093/jac/dkx535 [88] WANG J, WANG P, WANG X H, et al. Use and prescription of antibiotics in primary health care settings in China [J]. JAMA Internal Medicine, 2014, 174(12): 1914-1920. doi: 10.1001/jamainternmed.2014.5214 [89] KIM B, KIM Y, HWANG H, et al. Trends and correlation between antibiotic usage and resistance pattern among hospitalized patients at university hospitals in Korea, 2004 to 2012: A nationwide multicenter study [J]. Medicine, 2018, 97(51): e13719. doi: 10.1097/MD.0000000000013719 [90] LI Y X, XIA X Y, LI X H, et al. Correlation between the use of antibiotics and development of a resistant bacterial infection in patients in the ICU [J]. Bioscience Trends, 2018, 12(5): 517-519. doi: 10.5582/bst.2018.01130 [91] XU X W, WU X X, JIANG X G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: Retrospective case series [J]. BMJ (Clinical Research Ed. ), 2020, 368: m606. [92] ZHOU F, YU T, DU R H, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study [J]. The Lancet, 2020, 395(10229): 1054-1062. doi: 10.1016/S0140-6736(20)30566-3 [93] HUANG C L, WANG Y M, LI X W, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [J]. The Lancet, 2020, 395(10223): 497-506. doi: 10.1016/S0140-6736(20)30183-5 [94] HU Y, WEI X P, ZHU Q Q, et al. COVID-19 pandemic impacts on humans taking antibiotics in China [J]. Environmental Science & Technology, 2022, 56(12): 8338-8349. [95] MIRZAEI R, GOODARZI P, ASADI M, et al. Bacterial co-infections with SARS-CoV-2 [J]. IUBMB Life, 2020, 72(10): 2097-2111. doi: 10.1002/iub.2356 [96] JAKOBSSON H E, JERNBERG C, ANDERSSON A F, et al. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome [J]. PLoS One, 2010, 5(3): e9836. doi: 10.1371/journal.pone.0009836 [97] KOHANSKI M A, DEPRISTO M A, COLLINS J J. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis [J]. Molecular Cell, 2010, 37(3): 311-320. doi: 10.1016/j.molcel.2010.01.003 [98] WRIGHT G D. The antibiotic resistome: The nexus of chemical and genetic diversity [J]. Nature Reviews Microbiology, 2007, 5(3): 175-186. doi: 10.1038/nrmicro1614 [99] PRUDEN A, PEI R T, STORTEBOOM H, et al. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado [J]. Environmental Science & Technology, 2006, 40(23): 7445-7450. [100] MURRAY C J L, IKUTA K S, SHARARA F, et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis [J]. The Lancet, 2022, 399(10325): 629-655. doi: 10.1016/S0140-6736(21)02724-0 [101] BLAIR J M A, WEBBER M A, BAYLAY A J, et al. Molecular mechanisms of antibiotic resistance [J]. Nature Reviews Microbiology, 2015, 13(1): 42-51. doi: 10.1038/nrmicro3380 [102] JASOVSKÝ D, LITTMANN J, ZORZET A, et al. Antimicrobial resistance-a threat to the world's sustainable development [J]. Upsala Journal of Medical Sciences, 2016, 121(3): 159-164. doi: 10.1080/03009734.2016.1195900 [103] 中华人民共和国农业部公告第2292号[EB/OL].[2015-9-1]. http://www.moa.gov.cn/nybgb/2015/jiuqi/201712/t20171219_6103873.htm [104] 中华人民共和国农业部公告第246号[EB/OL].[2016-7-26]. http://www.moa.gov.cn/nybgb/2016/dibaqi/201712/t20171219_6102822.htm [105] 中华人民共和国农业部公告第2428号[EB/OL].[2019-12-12]. http://www.moa.gov.cn/nybgb/2020/202003/202004/t20200419_6341878.htm [106] CHO I, YAMANISHI S, COX L, et al. Antibiotics in early life alter the murine colonic microbiome and adiposity [J]. Nature, 2012, 488(7413): 621-626. doi: 10.1038/nature11400 [107] COX L M, YAMANISHI S, SOHN J, et al. Altering the intestinal Microbiota during a critical developmental window has lasting metabolic consequences [J]. Cell, 2014, 158(4): 705-721. doi: 10.1016/j.cell.2014.05.052 [108] SAARI A, VIRTA L J, SANKILAMPI U, et al. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life [J]. Pediatrics, 2015, 135(4): 617-626. doi: 10.1542/peds.2014-3407 [109] WANG H X, WANG N, WANG B, et al. Antibiotics detected in urines and adipogenesis in school children [J]. Environment International, 2016, 89/90: 204-211. doi: 10.1016/j.envint.2016.02.005 [110] MAEDA N, TAMAGAWA T, NIKI I, et al. Increase in insulin release from rat pancreatic islets by quinolone antibiotics [J]. British Journal of Pharmacology, 1996, 117(2): 372-376. doi: 10.1111/j.1476-5381.1996.tb15201.x [111] GHALY H, KRIETE C, SAHIN S, et al. The insulinotropic effect of fluoroquinolones [J]. Biochemical Pharmacology, 2009, 77(6): 1040-1052. doi: 10.1016/j.bcp.2008.11.019 [112] YABE K, YAMAMOTO Y, SUZUKI T, et al. Functional and morphological characteristics of pancreatic islet lesions induced by quinolone antimicrobial agent gatifloxacin in rats [J]. Toxicologic Pathology, 2019, 47(1): 35-43. doi: 10.1177/0192623318809062 [113] ALTHAQAFI A, ALI M, ALZAHRANI Y, et al. How safe are fluoroquinolones for diabetic patients?A systematic review of dysglycemic and neuropathic effects of fluoroquinolones [J]. Therapeutics and Clinical Risk Management, 2021, 17: 1083-1090. doi: 10.2147/TCRM.S284171 [114] MCWILLIAM S J, ANTOINE D J, SMYTH R L, et al. Aminoglycoside-induced nephrotoxicity in children [J]. Pediatric Nephrology, 2017, 32(11): 2015-2025. doi: 10.1007/s00467-016-3533-z [115] BURGESS L D, DREW R H. Comparison of the incidence of vancomycin-induced nephrotoxicity in hospitalized patients with and without concomitant piperacillin-tazobactam [J]. Pharmacotherapy, 2014, 34(7): 670-676. doi: 10.1002/phar.1442 [116] MOUSAVI M, ZAPOLSKAYA T, SCIPIONE M R, et al. Comparison of rates of nephrotoxicity associated with vancomycin in combination with piperacillin-tazobactam administered as an extended versus standard infusion [J]. Pharmacotherapy, 2017, 37(3): 379-385. doi: 10.1002/phar.1901 [117] ELYASI S, KHALILI H, DASHTI-KHAVIDAKI S, et al. Vancomycin-induced nephrotoxicity: Mechanism, incidence, risk factors and special populations [J]. European Journal of Clinical Pharmacology, 2012, 68(9): 1243-1255. doi: 10.1007/s00228-012-1259-9 [118] KAN W C, CHEN Y C, WU V C, et al. Vancomycin-associated acute kidney injury: A narrative review from pathophysiology to clinical application [J]. International Journal of Molecular Sciences, 2022, 23(4): 2052. doi: 10.3390/ijms23042052 [119] SANGTHAWAN P, GEATER A F, NAORUNGROJ S, et al. Characteristics, influencing factors, predictive scoring system, and outcomes of the patients with nephrotoxicity associated with administration of intravenous colistin [J]. Antibiotics (Basel, Switzerland), 2021, 11(1): 2. [120] JAVAN A O, SHOKOUHI S, SAHRAEI Z. A review on colistin nephrotoxicity [J]. European Journal of Clinical Pharmacology, 2015, 71(7): 801-810. doi: 10.1007/s00228-015-1865-4 [121] CHANG K, WANG H B, ZHAO J P, et al. Risk factors for polymyxin B-associated acute kidney injury [J]. International Journal of Infectious Diseases, 2022, 117: 37-44. doi: 10.1016/j.ijid.2022.01.055 [122] SRAVANI M, KRISHNAMURTHY S, PARAMESWARAN N, et al. Assessment of causality in hospitalized children with aminoglycoside-related nephrotoxicity [J]. Indian Pediatrics, 2022, 59(3): 226-229. doi: 10.1007/s13312-022-2475-8 [123] ILGIN S, CAN O D, ATLI O, et al. Ciprofloxacin-induced neurotoxicity: Evaluation of possible underlying mechanisms [J]. Toxicology Mechanisms and Methods, 2015, 25(5): 374-381. doi: 10.3109/15376516.2015.1026008 [124] LURIE I, YANG Y X, HAYNES K, et al. Antibiotic exposure and the risk for depression, anxiety, or psychosis: A nested case-control study [J]. The Journal of Clinical Psychiatry, 2015, 76(11): 1522-1528. doi: 10.4088/JCP.15m09961 [125] MURPHY J R, PAUL S, DUNLOP A L, et al. Maternal peripartum antibiotic exposure and the risk of postpartum depression [J]. Research in Nursing & Health, 2018, 41(4): 369-377. [126] DEAN O M, KANCHANATAWAN B, ASHTON M, et al. Adjunctive minocycline treatment for major depressive disorder: A proof of concept trial [J]. The Australian and New Zealand Journal of Psychiatry, 2017, 51(8): 829-840. doi: 10.1177/0004867417709357 [127] ZAZULA R, HUSAIN M I, MOHEBBI M, et al. Minocycline as adjunctive treatment for major depressive disorder: Pooled data from two randomized controlled trials [J]. Australian & New Zealand Journal of Psychiatry, 2021, 55(8): 784-798. [128] HUSAIN M I, CHAUDHRY I B, RAHMAN R R, et al. Minocycline as an adjunct for treatment-resistant depressive symptoms: Study protocol for a pilot randomised controlled trial [J]. Trials, 2015, 16: 410. doi: 10.1186/s13063-015-0933-5 [129] ASHER M I, GARCÍA-MARCOS L, PEARCE N E, et al. Trends in worldwide asthma prevalence [J]. The European Respiratory Journal, 2020, 56(6): 2002094. doi: 10.1183/13993003.02094-2020 [130] GENG M L, TANG Y, LIU K Y, et al. Prenatal low-dose antibiotic exposure and children allergic diseases at 4 years of age: A prospective birth cohort study [J]. Ecotoxicology and Environmental Safety, 2021, 225: 112736. doi: 10.1016/j.ecoenv.2021.112736 [131] RISNES K R, BELANGER K, MURK W, et al. Antibiotic exposure by 6 months and asthma and allergy at 6 years: Findings in a cohort of 1, 401 US children [J]. American Journal of Epidemiology, 2010, 173(3): 310-318. [132] PONGDEE T, LI J T. Evaluation and management of penicillin allergy [J]. Journal of the American Medical Association, 2019, 321(2): 188-199. doi: 10.1001/jama.2018.19283 [133] MORI F, PECORARI L, PANTANO S, et al. Azithromycin anaphylaxis in children [J]. International Journal of Immunopathology and Pharmacology, 2014, 27(1): 121-126. doi: 10.1177/039463201402700116 [134] SÁNCHEZ A R, ROGERS R S, SHERIDAN P J. Tetracycline and other tetracycline-derivative staining of the teeth and oral cavity [J]. International Journal of Dermatology, 2004, 43(10): 709-715. doi: 10.1111/j.1365-4632.2004.02108.x [135] ZHU Z Y, YU Q, QI G G, et al. Tigecycline-induced tooth discoloration in children younger than eight years [J]. Antimicrobial Agents and Chemotherapy, 2021, 65(9): e0085421. doi: 10.1128/AAC.00854-21 [136] HEIANZA Y, MA W J, LI X, et al. Duration and life-stage of antibiotic use and risks of all-cause and cause-specific mortality: Prospective cohort study [J]. Circulation Research, 2020, 126(3): 364-373. doi: 10.1161/CIRCRESAHA.119.315279 [137] ZHANG J J, HAINES C, WATSON A, et al. 2845. oral antibiotic use and risk of colorectal cancer in the UK, 1989-2012: A matched case-control study [J]. Gut, 2019, 68(11): 1971-1978. doi: 10.1136/gutjnl-2019-318593 [138] NITZAN O, ELIAS M, PERETZ A, et al. Role of antibiotics for treatment of inflammatory bowel disease [J]. World Journal of Gastroenterology, 2016, 22(3): 1078-1087. doi: 10.3748/wjg.v22.i3.1078 -




 下载:
下载: