-
偶氮染料是印染工业中最常用且应用范围最广的一类染料,是印染废水的主要组成部分[1]. 染料废水具有色度高、毒性大、可生化性差等特点,如果处理不当排放到环境中,将会对周围生态环境和人体造成严重危害[2]. 目前常用的处理方法包括生物法[3]、膜分离法[4]、吸附法[5]、高级氧化法[6]等. 膜分离法虽分离效果好、出水水质高但存在膜材料的成本高,容易被污染等问题;吸附法所用的吸附剂容易达到饱和且再生费用较高,限制了该方法在染料废水中的应用;此外,采用生物法处理前期投资大,处理周期长. 高级氧化(AOPs)工艺作为一种功能强大且具有良好的发展前景的技术,因其应用广泛、反应速度快、氧化能力强等优点备受关注[7]. 由于硫酸盐自由基具有强的氧化性,以及更广泛的pH适用性[8 − 9],近年来基于过硫酸盐(PS)的AOPs技术受到关注.
由于PS的稳定性,在不添加活化剂的情况下,PS很难与有机物发生反应. 目前对PS催化活化的手段包括紫外光活化[10]、过渡金属活化[11]以及碳材料活化[12]等. 紫外活化在实际应用中对污染物的去除率较低,过渡金属活化会引入金属离子,容易产生二次污染,因此碳材料催化活化技术开始受到广泛关注. 目前已经证实了包括氧化还原石墨烯、碳纳米管、纳米金刚石和生物炭等碳材料,可以用作过二硫酸盐(PDS)和过一硫酸盐(PMS)活化的催化剂,以增强污染物的降解[13 − 14]. 相比于碳纳米管、纳米金刚石等催化剂造价昂贵,以生物废弃物为原料制备的生物炭具有成本低,制作简单和良好的活化性能等优点,其催化应用潜力近年来备受关注[14].
研究已证实,以剩余活性污泥[15]、竹子[16]等废弃生物质制成的生物炭,具有良好的活化PDS的效果. 生物炭的制备主要采用热处理法,包括热解法和水热法,其中热解法需要对原料进行干化预处理,且热解温度高,材料制备能耗大[17],而原料无需干化的水热处理具有反应温度低、能耗低的特点,成为低成本制备生物炭的更优选择,此外,为提高生物炭的催化效能,常对生物炭进行改性处理,如杂原子掺杂[18]、表面含氧官能化[19]、单原子掺杂[20]等. 生物炭表面的含氧官能团决定了生物炭催化剂的性质,如羧基决定了生物炭的表面电荷[21],酚基决定了生物炭表面的氧化还原性质[22]. 其中简单的酸碱改性可实现生物炭表面含氧官能化,使表面含氧官能团发生改变,从而实现生物炭催化活化效能的提升[23]. 除了使用化学性碱如NaOH进行改性,壳聚糖作为目前自然界中唯一发现的带正电荷的天然碱性多糖[24],具有良好的降解性、生物相容性和生物药理学活性[25],因此可作为生物炭改性的碱性物质.
本研究以剩余活性污泥经水热法制备生物炭,经氢氧化钠(NaOH)碱改性、壳聚糖改性及联合改性后活化PDS,基于对RB5染料废水的降解效能确定最优改性方法. 考察水热处理条件、改性剂投加量、生物炭活化PDS反应条件、系统中的阴离子等对污染物降解效能的影响,并通过自由基淬灭和电子顺磁共振波谱仪(EPR)自由基捕获,探究参与污染物降解的主要活性物质,以确定反应机理.
-
(1)未改性生物炭的制备
将从上海市某污水处理厂二沉池取回的污泥进行重力沉降,浓缩后在105 ℃下干燥4 h,用粉碎机破碎后过100目聚乙烯筛,将干燥污泥按照1.8 g挥发性悬浮固体(VSS)的量加入60 mL去离子水中,混合均匀后转移至密闭的水热反应釜内,在温度为220 ℃的条件下加热4 h. 将水热后的产物进行固液分离,固相物质即为制备所得的水热炭,命名为BC.
(2)经NaOH碱改性生物炭的制备
对于采用碱改性的生物炭,将从上海市某污水处理厂二沉池取回的污泥进行重力沉降,浓缩后在105 ℃下干燥4 h,用粉碎机破碎后过100目聚乙烯筛,将干燥污泥按照1.8 g VSS的量加入60 mL去离子水中,使用NaOH和H2SO4调节体系的pH至11,混合均匀后转移至密闭的水热反应釜内,在温度为220 ℃的条件下加热4 h,将水热后的产物进行固液分离,并将固体物质干燥后命名为BC-pH=11. 水热反应前添加0.18 g壳聚糖,并用NaOH和H2SO4调节体系pH至11,相同水热条件下制备的生物炭命名为10CBC-pH=11.
(3)壳聚糖改性生物炭的制备
将从上海市某污水处理厂二沉池取回的污泥进行重力沉降,浓缩后在105 ℃下干燥4 h,用粉碎机破碎后过100目聚乙烯筛,将干燥污泥按照1.8 g VSS的量加入60 mL去离子水中,将0.018 、0.072、0.144、0.18 g的壳聚糖添加至1.8 g VSS的干燥污泥中(壳聚糖质量占污泥VSS质量的1%、4%、8% 和10%),混合均匀后置于密闭的水热反应釜中,并在温度为220 ℃的条件下加热4 h. 将水热后的产物进行固液分离,固相物质即为制备所得的水热炭,分别命名为1CBC、4 CBC、8CBC、10CBC.
(4)实验试剂
L-组氨酸(C6H9N3O2)和PDS(Na2S2O8)购于阿拉丁试剂有限公司;重水(D2O)、壳聚糖((C6H11NO4)n,脱乙酰度=80.0%—95.0%)、氯化钾(KCl)、磷酸二氢钾(KH2PO4)、硝酸钾(KNO3)、氯仿(CHCl3)购于国药化学试剂有限公司;5-二甲基-1-吡咯啉-N-氧化物(DMPO)、2,2,6,6-四甲基-4-哌啶酮(TEMP)、甲醇(CH3OH)、叔丁醇(C4H10O)、碳酸氢钠(NaHCO3)购于上海易恩化学技术有限公司;RB5购于Sigma-Aldrich公司.
-
(1)对RB5染料废水的吸附去除实验
取100 mL浓度为40 mg·L−1的RB5溶液,向溶液中加入5 g·L−1的生物炭(BC,BC-pH=11,10CBC,10CBC-pH=11),置于恒温摇床(LYGZ-2102C, 金坛良友仪器(常州))内以160 r·min−1转速在((25±1)℃)下振荡. 在设定时间取样,样品经0.45 μm滤膜过滤后采用紫外可见分光光度计(TU-1810, 普析通用有限责任公司(北京))在597 nm的波长下测定RB5浓度. 每次实验均进行3组平行实验,将所得结果的平均值进行数据分析.
(2)改性和未改性生物炭活化PDS对RB5染料废水的去除实验
取100 mL浓度为40 mg·L−1的RB5溶液. 向溶液中加入5 g·L−1的生物炭(BC、BC-pH=11、10CBC、10CBC-pH=11)和1 mmol·L−1的PDS,置于恒温摇床内以160 r·min−1转速在((25±1)℃)下振荡. 在设定时间取样,样品经0.45 μm滤膜过滤后采用紫外可见分光光度计在597 nm的波长下测定RB5浓度. 每次实验均进行3组平行实验,将所得结果的平均值进行数据分析.
(3)壳聚糖改性生物炭活化PDS降解RB5染料废水的影响因素研究
在探究不同壳聚糖添加量所制备的生物炭对活化PDS降解RB5废水的实验中,取100 mL浓度为40 mg·L−1的RB5溶液. 向溶液中加入5 g·L−1的生物炭(BC、1CBC、4CBC、8CBC、10CBC)和1 mmol·L−1的PDS,置于恒温摇床内以160 r·min−1转速在((25±1)℃)下振荡. 在设定时间取样,样品经0.45 μm滤膜过滤后采用紫外可见分光光度计在597 nm的波长下测定RB5浓度. 每次实验均进行3组平行实验,将所得结果的平均值进行数据分析.
在最佳壳聚糖添加量制备的生物炭(1CBC)基础上,探究1CBC的制备条件(水热时间和水热温度)及活化PDS的反应条件(PDS的投量,1CBC的投量,初始RB5的浓度,初始pH,不同阴离子)的影响,采用控制变量法,除研究变量外,其它变量均保持不变,以选取最佳条件. 每次实验均进行3组平行实验,将所得结果的平均值进行数据分析.
本实验反应速率采用伪一级动力学公式进行拟合. 通过得到不同反应条件下的ln(C0/Ct)与t之间的关系,即可得到其反应速率.
式中,k为反应速率常数,min−1;Ct为反应t时刻对应的污染物浓度,mg·L−1;C0为反应初始时刻污染物的浓度,mg·L−1.
-
将5 g·L−11CBC和2 mmol·L−1PDS置于100 mL锥形瓶中,分别加入40 mg·L−1 RB5和50 mmol·L−1 的淬灭剂(甲醇、叔丁醇、氯仿和L-组氨酸),然后置于恒温摇床振荡,在设定时间取样,经0.22 μm滤膜过滤后采用紫外可见分光光度计在597 nm的波长下测定剩余RB5的浓度. 每次实验均进行3组平行实验,将所得结果的平均值进行数据分析.
-
使用EPR(EMXnano Bench Top,德国布鲁克)检测反应体系的活性物质. DMPO用于捕获·OH和
${\rm{SO}}_4^{-} $ ·;TEMP用于捕获1O2,${\rm{O}}_2^- $ ·使用DMPO在纯甲醇体系中捕获. 在反应速率最快的时刻进行取样,经0.22 μm的膜过滤后,取2滴待测溶液,加入20 μL对应捕获剂(DMPO或TEMP),然后将溶液置于毛细管中进行EPR自由基检测分析.在D2O中捕获1O2时,使用TEMP在纯D2O体系中进行. 在反应速率最快的时刻取样,经0.22 μm的膜过滤后,取2滴待测溶液并加入20 μL的TEMP,然后将溶液置于毛细管中进行EPR自由基检测分析.
-
浸出液中金属浓度的测定:将5 g·L−1的生物炭和2 mmol·L−1的PDS加入100 mL的去离子水中,置于恒温摇床内以160 r·min−1转速在((25±1)℃)下振荡. 分别在30、60、120、180 min取样,样品经0.45 μm滤膜过滤后采用原子发射光谱仪(Prodigy-ICP,美国)进行Fe、Cu、Al、Mn、Cr、As、Cd、Zn、Hg、Pb金属浓度的检测.
浸出液活化PDS降解RB5实验:将5 g·L−1的生物炭加入100 mL的去离子水中,置于恒温摇床内以160 r·min−1转速在((25±1)℃)下震荡3 h后得到浸出液. 然后将浸出液过滤后加入40 mg·L−1的RB5和2 mmol·L−1的PDS进行反应,在设定时间取样,样品经0.45 μm滤膜过滤后采用紫外可见分光光度计在597 nm的波长下测定RB5浓度. 每次实验均进行3组平行实验,将所得结果的平均值进行数据分析.
-
研究污泥基生物炭经不同改性方式后,单独生物炭以及生物炭活化PDS系统对RB5的去除效果. 生物炭本身对RB5的吸附效果如图1(a)所示,发现未进行改性的生物炭(BC)和只进行碱改性的生物炭(BC-pH=11)对RB5的吸附效果甚微,但经过壳聚糖改性后的生物炭(10CBC, 10 CBC-pH=11),表现出一定的吸附效果,RB5的去除效率在4 %—18 %,去除效果有限. 可知,污泥基生物炭及改性生物炭均难以通过吸附方式对RB5进行有效去除. 此外,在仅投加PDS时,RB5亦不能被氧化去除.
当生物炭投加PDS后,从图1(b)中可知,RB5的降解显著提升,说明生物炭可有效活化PDS降解RB5. 虽然经不同方式改性后的生物炭均在180 min达到了对RB5的完全降解,但降解速率有显著差异,依次为10CBC/PDS> 10CBC- pH=11/PDS> BC-pH=11/PDS> BC/PDS,这一现象说明改性生物炭可更有效的活化PDS,且壳聚糖改性比碱改性有更好的活化效果,此外,碱和壳聚糖同时改性对于生物炭活化PDS无协同作用,甚至比单独壳聚糖改性的生物炭效果略差. 壳聚糖的良好改性效果可能得益于其表面丰富的官能团,有研究利用壳聚糖掺杂的生物炭微球催化剂实现对阿莫西林的有效降解[26]. 因此,本研究确定以壳聚糖改性的方式制备污泥基生物炭CBC,并进行条件优化.
-
壳聚糖改性生物炭制备时,壳聚糖的添加量决定着催化剂所能提供的活性位点数量. 图2(a)表明了壳聚糖在污泥中不同掺杂比例条件下制备的生物炭,对RB5降解的影响,图2(b)进一步表明了对RB5降解反应速率的影响. 在壳聚糖的掺杂比例从1%(1CBC)增加至10%(10CBC)的过程中,RB5的降解速率先加快后减慢,在投加量为4%时降解速率最快(降解速率为0.0677 min−1),在此基础上增加壳聚糖的投量,反应速率反而降低,在投加量为10%(10CBC)时,降解速率降低至0.0491 min−1. 造成该现象的原因可能是:过量的壳聚糖负载在生物炭上会堵塞相关的活性位点,导致降解速率有一定程度下降. 除此之外,壳聚糖的加入使反应时间从180 min缩短至60 min. 根据上述分析并考虑到经济适用性,本文选取1%(1CBC)作为最佳掺杂量进行后续的研究.
-
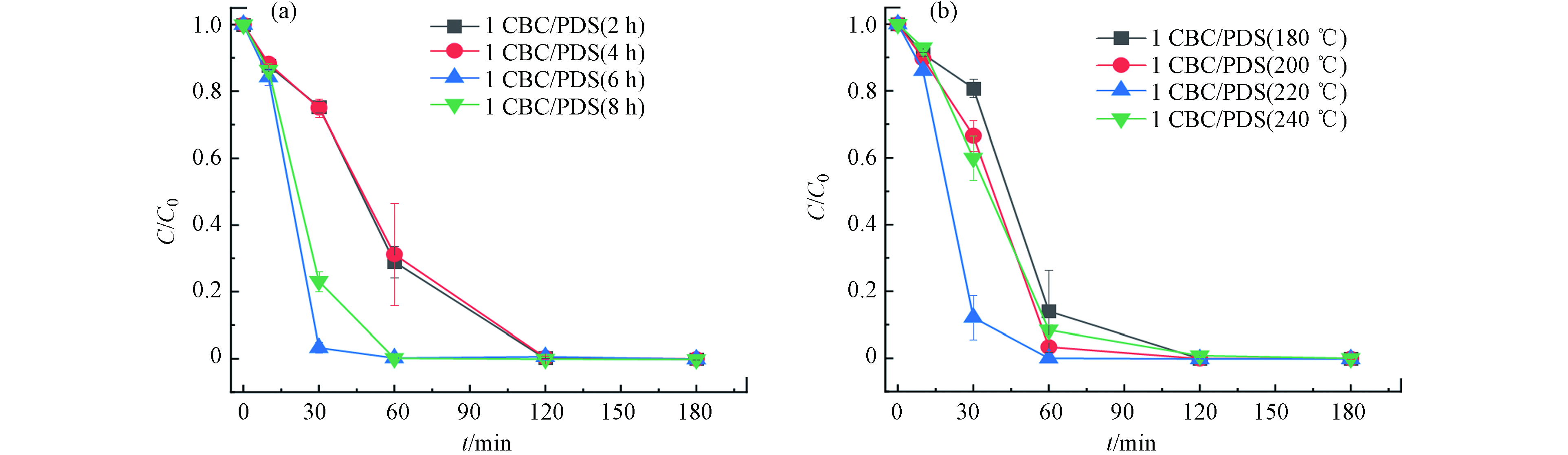
研究表明,水热条件对于生物炭的制备以及催化性能有显著的影响[27]. 因此,本研究探讨不同的水热温度和水热时间制备出的生物炭,对活化PDS降解RB5的影响,结果见图3.
图3(a)展示了水热时间对RB5降解的影响. 在水热时间为2 h和4 h ,反应温度为220 ℃时,体系对RB5的降解速率差别不大,分别为0.0479 min−1和0.0454 min−1,可能是由于在此水热条件下污泥尚未完全碳化,导致没有显著的降解差异. 但随着水热时间的延长,反应体系对RB5的降解速率有了明显的改善,并在水热时间为8 h时达到最优,降解速率为0.084 min−1,且反应时间从120 min缩短至60 min. 此外,在水热时间为6 h时,亦能在60 min实现RB5的完全去除,因此,确定最优反应时间为6 h.
图3(b)显示了水热温度对RB5的降解影响. 设置水热反应时间为6 h,水热温度从180 ℃增至240 ℃时,体系对RB5的降解速率呈先增加后减小的趋势. 在反应时间为60 min时,180 ℃、200 ℃、220 ℃、240 ℃条件下制备的1CBC活化PDS,对RB5的去除率分别为86%、97%、100%和92%. 综合RB5降解效果与经济因素,本研究选取最佳水热温度为200 ℃,水热时间为6 h,采用此条件制备的1CBC进行后续研究.
-
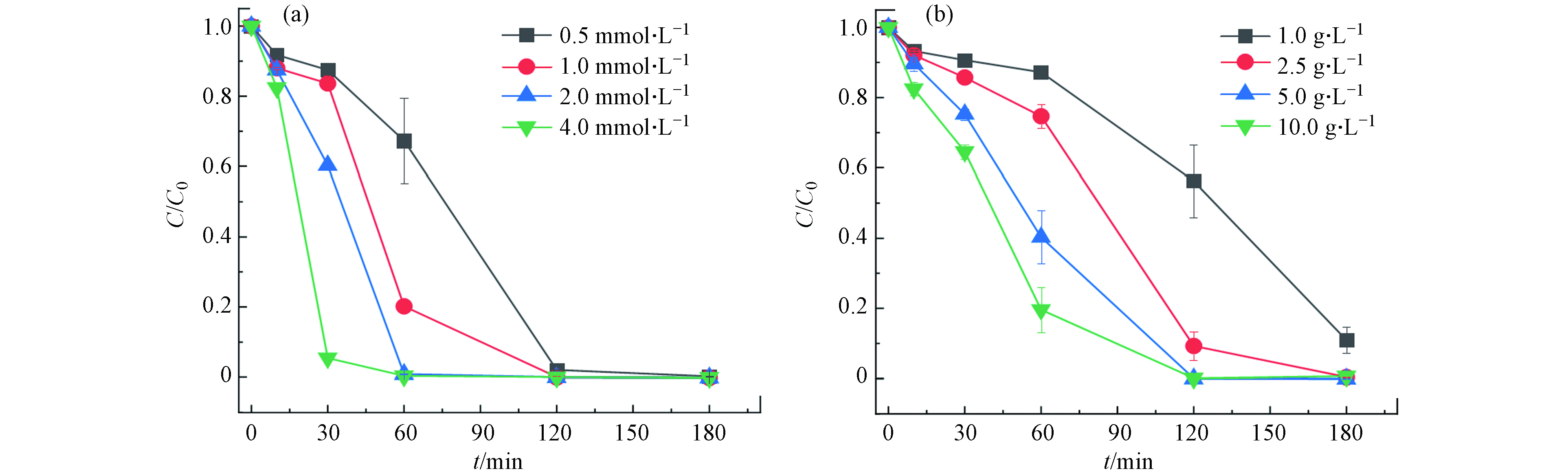
除生物炭的制备条件会对RB5的去除产生影响外,反应条件亦直接影响污染物的去除表现. PDS投量对RB5降解的影响如图4(a)所示,RB5的降解速率随PDS投量的增加(由0.5 mmol·L−1增至4.0 mmol·L−1)逐渐提高,降解速率由0.0313 min−1增至0.0706 min−1,可能由于PDS投量的增加使体系产生了更多的活性物质,从而加快反应速率,由于PDS投量为2 mmol·L−1和4 mmol·L−1时,均表现出RB5的快速高效去除,因此确定PDS的最优投量为2 mmol·L−1.
同样,催化剂1CBC的投量影响RB5的降解效果(图4(b)),随着1CBC投量从1.0 g·L−1增加至10.0 g·L−1,体系中催化活化PDS的活性位点增多,引起RB5的降解速率由0.0093 min−1逐步增加至0.0487 min−1. 由于1CBC投量为5.0 g·L−1和10.0 g·L−1时,反应均在120 min完成,因此选择生物炭的最佳投量为5.0 g·L−1.
-
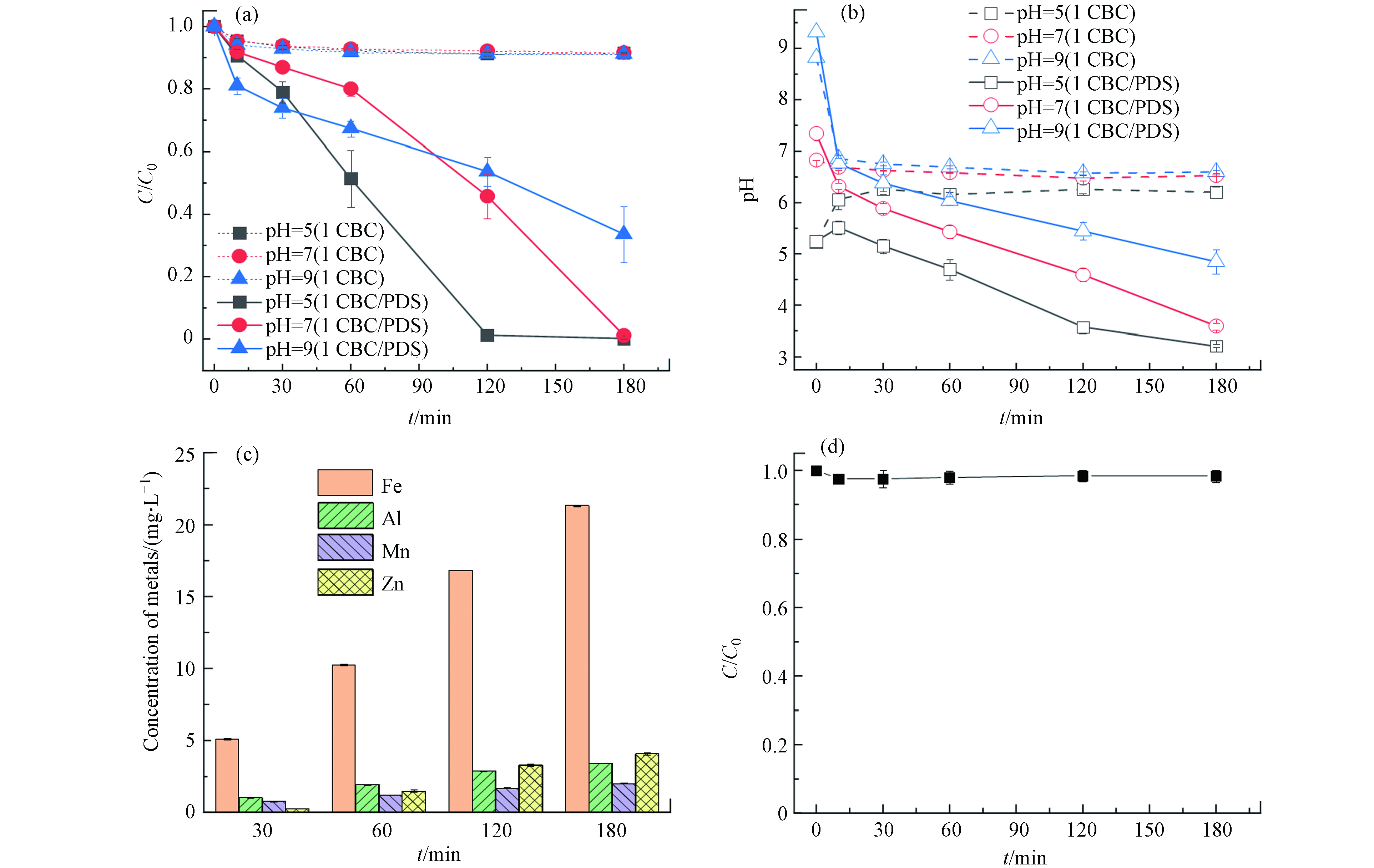
溶液pH变化会对体系中PDS的存在状态及材料的表面电荷产生影响[28],进而影响污染物去除表现. 图5展示了初始pH及反应中pH的变化对RB5降解的影响. 图5(a)为不同初始pH对RB5降解的影响,可知,在不同初始pH下,1CBC在180 min时对RB5的吸附效果相同,均为10%. 在1CBC/PDS体系,RB5的降解效果显著提升,其中在初始pH=5时去除效率最高,为0.0113 min−1;随着初始pH的升高,反应速率逐渐降低. 当pH=9时,在120 min时RB5的去除率仅为49%,1CBC活化PDS的能力下降. 酸性条件利于RB5的降解,可能是由于壳聚糖具有去质子化的胺基,在酸性条件下与带负电荷的RB5染料之间强烈的静电相互作用导致;而在碱性条件下,静电相互作用受抑制,导致降解速率下降[29].
为了进一步探究不同初始pH对BR5降解效果差异的原因,测定了反应过程中pH的变化(图5(b)). 在1CBC吸附实验中,不同初始pH条件下,反应180 min后,pH均接近6—7,趋于中性. 这可能是由于生物炭成分复杂,具有一定的缓冲能力导致. 然而,在1CBC/PDS系统中,无论初始pH呈酸性还是碱性,体系在180 min时的pH均呈酸性. 可能原因如式(3)、式(4)和式(5)所示[30 − 31],在初始pH=5时,
${\rm{S}}_2 {\rm{O}}_8^{2-} $ 在酸性条件下与H+结合,最后又产生H+,导致pH下降. 然而在初始pH=9时,${\rm{S}}_2 {\rm{O}}_8^{2-} $ 与H2O结合导致H+的产生,使反应过程中的pH不断下降.此外,由于污泥中可能含有多种金属,目前许多金属已经证实具有活化PS的效果[32]. 因此本研究为进一步探究污泥生物炭在反应中释放出的金属离子是否具有催化作用,在初始pH=5时,测定了1CBC/PDS体系中多种金属(Fe、Cu、Al、Mn、Cr、As、Cd、Zn、Hg、Pb)的浸出浓度,由于Cu、Cr、As、Cd、Hg、Pb的浓度低于检出限,图5(c)中只显示了浓度高于检出限的Fe、Al、Mn、Zn的浸出浓度,图5(d)为浸出液活化PDS降解RB5的去除效果. 在图5(c)中金属浓度随着反应时间的延长不断增加,其中在180 min时Al、Mn、Zn浓度均在5 mg·L−1以下,而Fe的浓度已达到了20 mg·L−1左右. 为进一步验证反应过程中浸出的金属是否会对RB5的降解产生影响,将1CBC在去离子水中浸出180 min后,在浸出液中加入2 mmol·L−1的PDS对RB5进行降解,结果如图5(d)所示. 尽管浸出液中有较高的金属含量,但是浸出液中的金属离子未表现出PDS的活化及对RB5的降解.
在1CBC/PDS反应系统中,不同初始RB5浓度下,污染物的浓度随反应时间的变化如图6所示. 在反应时间为30 min时,RB5初始浓度为40 mg·L−1、80 mg·L−1、160 mg·L−1和240 mg·L−1的情况下,降解效率分别为100%、100%、84%和70%;图6(b)表明了不同初始浓度下的RB5对反应速率的影响,发现随着RB5浓度的增加,体系的降解速率有所降低,但是在60 min的反应时间内,不同浓度的RB5染料废水均能完全降解,说明1CBC/PDS体系受到RB5浓度的影响较小,进一步体现该体系在处理高浓度RB5废水中的优势.
-
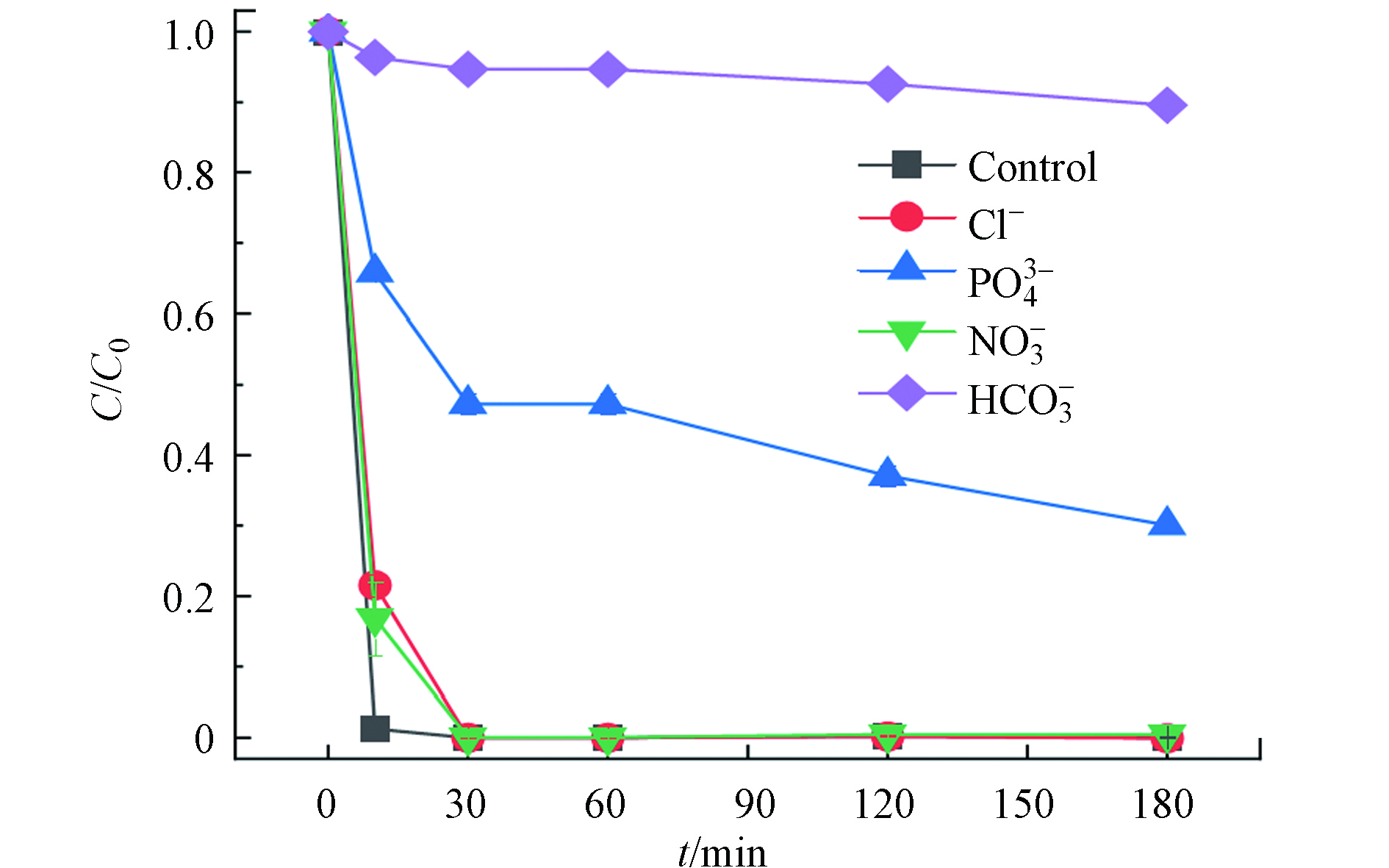
印染废水是一个复杂的水体,通常含有各种阴离子,这些离子的存在可能会对PDS体系造成一定的影响[33]. 本研究探究了Cl-、
${\rm{PO}}_4^{3-} $ 、${\rm{NO}}_3^{-} $ 和${\rm{HCO}}_3^{-} $ 对1CBC/PDS体系降解RB5的影响. 结果如图7所示,20 mmol·L−1 的Cl− 和${\rm{NO}}_3^{-} $ 对RB5的降解速率几乎没有影响. 甚至还有研究表明在以过一硫酸盐(PMS)为氧化剂的AOPs系统中,适量的Cl−可以与PMS反应生成HClO和Cl2,进一步增强体系的氧化强度[34]. 然而,${\rm{PO}}_4^{3-} $ 和${\rm{HCO}}_3^{-} $ 对体系产生明显的抑制作用,结合2.4.2中pH对反应的影响,可能是因为${\rm{PO}}_4^{3-} $ 属于碱性阴离子,导致溶液的pH升高,因此产生污染物降解抑制的现象[35]. 此外,有研究表明${\rm{HCO}}_3^{-} $ 可以促进PMS的分解,并且可以与·OH和SO4−·反应生成氧化能力较弱的HCO3·和${\rm{CO}}_3^{-} $ ·自由基[36],在本体系中可能是由于${\rm{HCO}}_3^{-} $ 消耗了体系中的PDS导致抑制效果达到90%. -
基于硫酸根自由基的AOPs技术,污染物的降解途径主要包括自由基途径和非自由基途径. 在自由基反应途径中起主要作用的是·OH、SO4−·、O2−·等自由基,而非自由基途径起主导作用的是1O2、电子转移和表面活性氧物种[37].
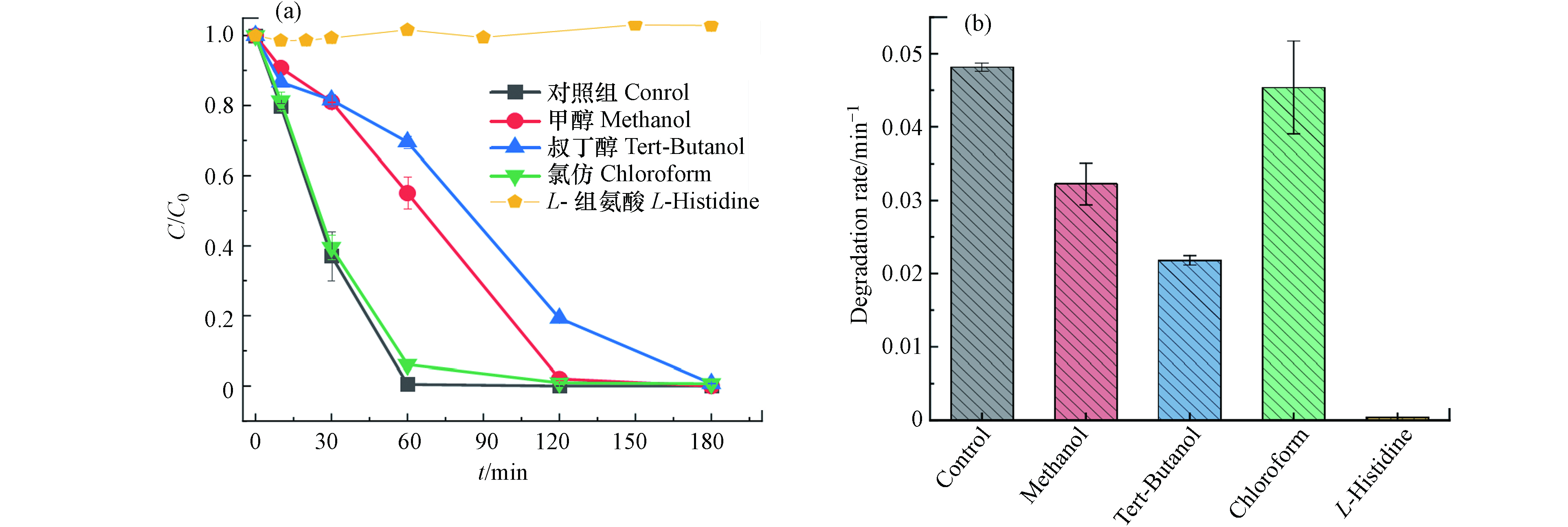
为确定参与RB5降解的活性物种,本研究对1CBC/PDS体系进行自由基淬灭和EPR自由基捕获,如图8所示. 使用4种常见的自由基淬灭剂,其中,甲醇通常用于·OH和
${\rm{SO}}_4^{-} $ ·的淬灭,叔丁醇通常用于淬灭·OH;氯仿和L-组氨酸是${\rm{O}}_2^- $ ·和1O2的有效淬灭剂. 结果发现,甲醇和叔丁醇对反应速率有抑制作用,且降解速率与对照组相比分别降低了33.0%和54.7%,但最终仍可实现RB5的全部去除. 氯仿对反应体系几乎没有抑制作用,而L-组氨酸的加入对体系产生强烈的抑制作用,使降解速率由0.048 min−1降至0.0003 min−1. 从自由基淬灭的实验结果推测,反应中的活性物种可能是·OH 、${\rm{SO}}_4^{-} $ ·和1O2.为进一步验证参与反应的活性物种,以 DMPO 和 TEMP 作为自旋捕获剂,采用EPR进行活性物质捕获,通过测定反应体系中未成对电子的顺磁性来鉴定自由基的存在[38].
图9(a)为1CBC/PDS体系及其对照组的·OH 和
${\rm{SO}}_4^{-} $ ·的自由基捕获图谱,在1CBC系统中无明显的·OH 和${\rm{SO}}_4^{-} $ ·的自由基信号;单独PDS体系中检测到了显著的${\rm{SO}}_4^{-} $ ·信号,而·OH 信号不明显. 在1CBC/PDS体系,信号较杂乱,无明显的·OH 和${\rm{SO}}_4^{-} $ ·自由基信号. 实验结果表明,单独PDS可以通过自身分解产生${\rm{SO}}_4^{-} $ ·自由基,但在1CBC/PDS系统中,尽管有高效的RB5降解效能,但降解效果不是由·OH 和${\rm{SO}}_4^{-} $ ·引起.据文献报道,D2O可以大大延长1O2的寿命[39],即体系中如果产生1O2,在D2O中将检测到更强的信号. 图9(b)为1CBC/PDS体系及其对照组的1O2捕获图谱. 在单独的1CBC系统和单独PDS系统中无1O2信号, 1CBC/PDS中检测到微弱的1O2信号. 为近一步确认1O2的存在,用D2O替换H2O再次检测,如图9(b)所示,在D2O中检测到了高强度的1O2信号,结合自由基淬灭实验,进一步确认1O2为1CBC/PDS体系的活性物种,即1CBC/PDS体系是以为1O2为主的非自由基氧化体系,RB5的降解主要通过1O2氧化实现.
-
以剩余污泥为原料,通过水热反应制备生物炭,并采用不同的改性方式优化水热炭活化PDS降解RB5染料废水的降解效能,得到以下主要结论.
(1)相比于采用NaOH改性,使用壳聚糖改性后的污泥水热炭对活化PDS降解RB5染料废水具有更快的降解速率.
(2)确定了1CBC/PDS体系中水热炭制备的最佳水热温度为200 ℃,最佳水热时间为6 h;最佳PDS投量为2 mmol·L−1;最佳的1CBC投量为5 g·L−1;最优反应初始pH为5.
(3)反应体系中,Cl−和
${\rm{NO}}_3^{-} $ 对RB5的去除效率影响很小;而${\rm{HCO}}_3^{-} $ 和${\rm{PO}}_4^{3-} $ 对RB5的去除产生显著的抑制作用.(4)自由基淬灭实验和EPR自由基捕获实验结果证明了1O2是1CBC/PDS体系降解RB5起主要贡献的活性物种.
壳聚糖改性污泥水热炭活化过硫酸盐降解活性黑5染料废水
Chitosan modified sludge hydrochar activated peroxydisulfate to degradate activated black 5 dyeing wastewater
-
摘要: 印染废水可生化性低,生物处理难度大. 通过在剩余污泥中掺杂壳聚糖,经水热处理制备出壳聚糖改性生物炭(CBC),并用于活化过二硫酸盐(PDS),实现对活性黑5(RB5)染料废水的高效降解. 在水热温度为200 ℃,水热时间为6 h时制备的CBC对RB5的去除效果最优,反应60 min去除率可达到100 %. 此外,PDS投量为2 mmol·L−1,CBC投量为5 g·L−1,初始pH=5时反应效果最佳. 反应系统中的Cl−和
${\rm{NO}}_3^{-} $ 对RB5去除几乎无影响,而${\rm{PO}}_4^{3-} $ 、${\rm{HCO}}_3^{-} $ 有明显抑制作用. 机理研究证实了单线态氧(1O2)对RB5的降解起主要贡献,而不是PDS氧化体系常见的硫酸根自由基.Abstract: Printing and dyeing wastewater is difficult to treat biologically due to property of low biodegradability. By doping chitosan in the wasted sludge, modified biochar (CBC) was prepared by hydrothermal treatment and was used to activate peroxydisulfate (PDS) to achieve efficient degradation of activated black 5 (RB5) dyeing wastewater. The CBC prepared under the condition of hydrothermal temperature of 200 °C and hydrothermal time 6 h showed the best RB5 removal effect, achieving a removal rate of 100 % at 60 min. In addition, the best performance was obtained with a PDS dosage of 2 mmol·L−1, a CBC dosage of 5 g·L−1 and an initial pH of 5. The Cl− and${\rm{NO}}_3^{-} $ in the reaction system had almost no effect on RB5 removal, while the${\rm{PO}}_4^{3-} $ and${\rm{HCO}}_3^{-} $ exhibited a significant inhibitory impact. The mechanism study confirmed that the singlet oxygen (1O2) played a major contribution to the degradation of RB5 rather than sulfate radical, which is common in PDS oxidation systems.-
Key words:
- advanced oxidation /
- hydrochar /
- dye wastewater /
- singlet oxygen
-

-
-
[1] PARMAR N D, SHUKLA S R. Decolourization of dye wastewater by microbial methods- A review [J]. Indian Journal of Chemical Technology, 2018, 25(4): 315-323. [2] ZENG Q Q, LIU Y, SHEN L G, et al. Facile preparation of recyclable magnetic Ni@filter paper composite materials for efficient photocatalytic degradation of methyl orange [J]. Journal of Colloid and Interface Science, 2021, 582(Pt A): 291-300. [3] BERKESSA Y W, YAN B H, LI T F, et al. Treatment of anthraquinone dye textile wastewater using anaerobic dynamic membrane bioreactor: Performance and microbial dynamics [J]. Chemosphere, 2020, 238: 124539. doi: 10.1016/j.chemosphere.2019.124539 [4] AĞTAŞ M, YıLMAZ Ö, DILAVER M, et al. Hot water recovery and reuse in textile sector with pilot scale ceramic ultrafiltration/ nanofiltration membrane system [J]. Journal of Cleaner Production, 2020, 256: 120359. doi: 10.1016/j.jclepro.2020.120359 [5] 袁思杰, 张芮铭. 染料废水处理技术研究进展[J]. 染料与染色, 2022, 59(4): 55-62. YUAN S J, ZHANG R M. Research progress of dye wastewater treatment technology[J]. Dyestuffs and Coloration, 2022, 59(4): 55-62(in Chinese).
[6] dos SANTOS A J, BRILLAS E, CABOT P L, et al. Simultaneous persulfate activation by electrogenerated H2O2 and anodic oxidation at a boron-doped diamond anode for the treatment of dye solutions [J]. Science of the Total Environment, 2020, 747: 141541. doi: 10.1016/j.scitotenv.2020.141541 [7] LUTZE H V, BIRCHER S, RAPP I, et al. Degradation of chlorotriazine pesticides by sulfate radicals and the influence of organic matter [J]. Environmental Science & Technology, 2015, 49(3): 1673-1680. [8] GIANNAKIS S, LIN K Y A, GHANBARI F. A review of the recent advances on the treatment of industrial wastewaters by Sulfate Radical-based Advanced Oxidation Processes (SR-AOPs) [J]. Chemical Engineering Journal, 2021, 406: 127083. doi: 10.1016/j.cej.2020.127083 [9] 谷得明, 郭昌胜, 冯启言, 等. 基于硫酸根自由基的高级氧化技术及其在环境治理中的应用[J]. 环境化学, 2018, 37(11): 2489-2508. doi: 10.7524/j.issn.0254-6108.2018012102 GU D M, GUO C S, FENG Q Y, et al. Sulfate radical-based advanced oxidation processes and its application in environmental remediation [J]. Environmental Chemistry, 2018, 37(11): 2489-2508 (in Chinese). doi: 10.7524/j.issn.0254-6108.2018012102
[10] IKE I A, LINDEN K G, ORBELL6 J D, et al. Critical review of the science and sustainability of persulphate advanced oxidation processes [J]. Chemical Engineering Journal, 2018, 338: 651-669. doi: 10.1016/j.cej.2018.01.034 [11] MIAO J, ZHU Y, LANG J Y, et al. Spin-state-dependent peroxymonosulfate activation of single-atom M-N moieties via a radical-free pathway [J]. ACS Catalysis, 2021, 11(15): 9569-9577. doi: 10.1021/acscatal.1c02031 [12] TANG L, LIU Y N, WANG J J, et al. Enhanced activation process of persulfate by mesoporous carbon for degradation of aqueous organic pollutants: Electron transfer mechanism [J]. Applied Catalysis B: Environmental, 2018, 231: 1-10. doi: 10.1016/j.apcatb.2018.02.059 [13] CHEN X, OH W D, LIM T T. Graphene- and CNTs-based carbocatalysts in persulfates activation: Material design and catalytic mechanisms [J]. Chemical Engineering Journal, 2018, 354: 941-976. doi: 10.1016/j.cej.2018.08.049 [14] WANG J, WANG S. Preparation, modification and environmental application of biochar: A review [J]. Journal of Cleaner Production, 2019, 227: 1002-1022. doi: 10.1016/j.jclepro.2019.04.282 [15] YIN R, GUO W, WANG H, et al. Singlet oxygen-dominated peroxydisulfate activation by sludge-derived biochar for sulfamethoxazole degradation through a nonradical oxidation pathway: Performance and mechanism [J]. Chemical Engineering Journal, 2019, 357: 589-599. doi: 10.1016/j.cej.2018.09.184 [16] MA D, YANG Y, LIU B, et al. Zero-valent iron and biochar composite with high specific surface area via K2FeO4 fabrication enhances sulfadiazine removal by persulfate activation [J]. Chemical Engineering Journal, 2021, 408: 127992. doi: 10.1016/j.cej.2020.127992 [17] ZHU S, HUANG X, MA F, et al. Catalytic removal of aqueous contaminants on N-doped graphitic biochars: Inherent roles of adsorption and nonradical mechanisms [J]. Environmental Science & Technology, 2018, 52(15): 8649-8658. [18] HUANG Q, SONG S, CHEN Z, et al. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review [J]. Biochar, 2019, 1(1): 45-73. doi: 10.1007/s42773-019-00006-5 [19] ODINGA E S, WAIGI M G, GUDDA F O, et al. Occurrence, formation, environmental fate and risks of environmentally persistent free radicals in biochars [J]. Environment International, 2020, 134: 105172. doi: 10.1016/j.envint.2019.105172 [20] SHANG Y N, XU X, GAO B Y, et al. Single-atom catalysis in advanced oxidation processes for environmental remediation [J]. Chemical Society Reviews, 2021, 50(8): 5281-5322. doi: 10.1039/D0CS01032D [21] WANG R Z, HUANG D L, LIU Y G, et al. Recent advances in biochar-based catalysts: Properties, applications and mechanisms for pollution remediation [J]. Chemical Engineering Journal, 2019, 371: 380-403. doi: 10.1016/j.cej.2019.04.071 [22] YUAN Y, BOLAN N, PRÉVOTEAU A, et al. Applications of biochar in redox-mediated reactions [J]. Bioresource Technology, 2017, 246: 271-281. doi: 10.1016/j.biortech.2017.06.154 [23] LI F, XIE Y, WANG Y, et al. Improvement of dyes degradation using hydrofluoric acid modified biochar as persulfate activator [J]. Environmental Pollutants and Bioavailability, 2019, 31(1): 32-37. doi: 10.1080/26395940.2019.1578185 [24] DING S, WANG Y, LI J, et al. Progress and prospects in chitosan derivatives: Modification strategies and medical applications [J]. Journal of Materials Science & Technology, 2021, 89: 209-224. [25] KAMEL E M, AHMED O M, ABD EL-SALAM H M. Fabrication of facile polymeric nanocomposites based on chitosan-gr-P2-aminothiophenol for biomedical applications [J]. International Journal of Biological Macromolecules, 2020, 165: 2649-2659. doi: 10.1016/j.ijbiomac.2020.09.140 [26] QI H, PAN G, SHI X, et al. Cu-Fe-FeC3@nitrogen-doped biochar microsphere catalyst derived from CuFe2O4@chitosan for the efficient removal of amoxicillin through the heterogeneous electro-Fenton process [J]. Chemical Engineering Journal, 2022, 434: 134675. doi: 10.1016/j.cej.2022.134675 [27] 段安冉, 王海龙, 王丹. 反应温度和时间对水热法制备Li2FeSiO4的影响[J]. 广州化工, 2021, 49(1): 22-23. doi: 10.3969/j.issn.1001-9677.2021.01.009 DUAN A R, WANG H L, WANG D. Effect of temperature and time on preparation of Li2FeSiO4 by hydrothermal method[J]. Guangzhou Chemical Industry, 2021, 49(1): 22-23(in Chinese). doi: 10.3969/j.issn.1001-9677.2021.01.009
[28] ZHAO R, LI X, SUN B, et al. Preparation of phosphorylated polyacrylonitrile-based nanofiber mat and its application for heavy metal ion removal [J]. Chemical Engineering Journal, 2015, 268: 290-299. doi: 10.1016/j.cej.2015.01.061 [29] ARAMESH N, BAGHERI A R, BILAL M. Chitosan-based hybrid materials for adsorptive removal of dyes and underlying interaction mechanisms [J]. International Journal of Biological Macromolecules, 2021, 183: 399-422. doi: 10.1016/j.ijbiomac.2021.04.158 [30] WANG J L, WANG S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants [J]. Chemical Engineering Journal, 2018, 334: 1502-1517. doi: 10.1016/j.cej.2017.11.059 [31] JOHNSON R L, TRATNYEK P G, JOHNSON R O. Persulfate persistence under thermal activation conditions [J]. Environmental Science & Technology, 2008, 42(24): 9350-9356. [32] 刘路明, 高志敏, 邓兆雄, 等. 过硫酸盐的活化及其在氧化降解水中抗生素的机理和应用[J]. 环境化学, 2022, 41(5): 1702-1717. doi: 10.7524/j.issn.0254-6108.2021010601 LIU L M, GAO Z M, DENG Z X, et al. Activation of persulfate and its mechanism and application in oxidative degradation of antibiotics in water[J]. Environmental Chemistry, 2022, 41(5): 1702-1717 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021010601
[33] HUO J X, PANG X H, WEI X Y, et al. Efficient degradation of printing and dyeing wastewater by Lotus leaf-based nitrogen self-doped mesoporous biochar activated persulfate: Synergistic mechanism of adsorption and catalysis [J]. 2022, 12(9): 1004. [34] LUO R, LI M Q, WANG C H, et al. Singlet oxygen-dominated non-radical oxidation process for efficient degradation of bisphenol A under high salinity condition [J]. Water Research, 2019, 148: 416-424. doi: 10.1016/j.watres.2018.10.087 [35] DUAN X, AO Z, ZHOU L, et al. Occurrence of radical and nonradical pathways from carbocatalysts for aqueous and nonaqueous catalytic oxidation [J]. Applied Catalysis B: Environmental, 2016, 188: 98-105. doi: 10.1016/j.apcatb.2016.01.059 [36] LIU J G, JIANG S J, CHEN D D, et al. Activation of persulfate with biochar for degradation of bisphenol A in soil [J]. Chemical Engineering Journal, 2020, 381: 122637. doi: 10.1016/j.cej.2019.122637 [37] CHEN Y D, WANG R, DUAN X, et al. Production, properties, and catalytic applications of sludge derived biochar for environmental remediation [J]. Water Research, 2020, 187: 116390. doi: 10.1016/j.watres.2020.116390 [38] 蒋国斌, 徐莉, 曹福亮, 等. 电子顺磁共振法(EPR)对银杏叶提取物及其卷烟捕获自由基能力研究(英文)[J]. 光谱学与光谱分析, 2017, 37(4): 1322-1328. JIANG G B, XU L, CAO F L, et al. Electron paramagnetic resonance (EPR) studies on free radical scavenging capacity of EGB and EGB cigarette[J]. Spectroscopy and Spectral Analysis, 2017, 37(4): 1322-1328(in Chinese).
[39] WANG H, GUO W, LIU B, et al. Edge-nitrogenated biochar for efficient peroxydisulfate activation: An electron transfer mechanism [J]. Water Research, 2019, 160: 405-414. doi: 10.1016/j.watres.2019.05.059 -










 下载:
下载: