-
黑碳是由化石燃料或生物质不完全燃烧形成的含碳固体物质,其水溶性部分称为溶解性黑碳(dissolved black carbon,DBC)[1]. DBC在地表水体普遍存在,约占淡水体系中溶解性有机碳的10%,被认为是天然溶解性有机质(dissolved organic matter,DOM)的重要来源[2]. DBC具有较高的芳香性和丰富的官能团(尤其是羧基和酚基),可导致强烈的光化学活性. 在光照条件下DBC能高效产生单线态氧(1O2)、羟基自由基(·OH)和超氧自由基(O2·−)等活性氧物种(reactive oxygen species,ROS),从而可以促进抗生素(如克林霉素、四环素)[2 − 3]、内分泌干扰物(如17-β雌二醇)[4]、塑化剂(如邻苯二甲酸二乙酯)[5]和杀虫剂(如吡虫啉)[6]等多种有机污染物的降解,对水环境中微污染物的环境转化起到至关重要的作用[4, 7]. 此外,水环境组分也会影响DBC的胶体稳定性和环境行为[8],因此,研究环境因子对DBC环境行为的影响对水体中微污染物的降解转化具有重要意义.
微塑料具有易吸附、难降解、参与环境中其他污染物迁移转化等特点,成为重要的环境问题之一[9 − 11]. DOM广泛存在于水环境中,水中微塑料与DOM不可避免地会相互接触并作用[12]. 二者的相互作用不仅能改变微塑料对共存污染物的吸附特性,也会影响微塑料和DOM的环境行为和生态效应[13]. 有相关报道,聚乙烯(PE)微塑料会诱导DOM组分中的蛋白质物质转化为腐殖质,提高DOM的腐殖化程度;聚苯乙烯(PS)微塑料能提高河岸带沉积物DOM的腐殖化,促进了大分子组分的形成,从而使DOM的生物可利用度降低[14 − 16]. DBC作为DOM的重要组分也会与微塑料相互作用. Song等人研究发现DBC中具有酸/碱性官能团的小质量分子和蛋白样荧光团更易与聚氯乙烯(PVC)相互作用[17]. 目前关于DOM和微塑料相互作用的研究主要集中在量化DOM在微塑料上的吸附效率,然而,关于微塑料与DBC共存条件下,微塑料对DBC可能产生的光化学活性变化研究尚未见报道.
因此,本文以小麦秸秆生物质在400 ℃温度下无氧热解制备的DBC和3种典型塑料:聚苯乙烯微塑料(PS)、聚乳酸微塑料(PLA)、回收轮胎碎屑颗粒(RTC)为研究对象,在DBC和微塑料共存条件下,探究DBC与微塑料作用后产生的光化学活性变化,同时对微塑料老化情况进行分析. 为DBC受微塑料影响产生的环境行为变化提供理论研究基础.
-
甲醇(色谱纯)、乙腈(色谱纯)、磷酸二氢钠(分析纯)、磷酸氢二钠(分析纯)、氢氧化钠(分析纯)购自上海阿拉丁生化科技股份有限公司. 2,4,6-三甲基苯酚(TMP,纯度98%)、糠醇(FFA,纯度98%)购自J&K Scientific公司. 聚乳酸(PLA,白色塑料)购自美国Natureworks公司,聚苯乙烯(PS,白色塑料)购自镇江奇美化工有限公司,轮胎回收碎屑颗粒(RTC,黑色塑料)购自华益橡胶有限公司,所有塑料均过筛200目后用于实验. 超纯水(18 MΩ·cm,天津市兰力科化学电子高科技有限公司生产的超纯水器制备).
-
小麦秸秆经高速粉碎机(DFT-200A,中国林大仪器有限公司)粉碎过筛后放入管式炉,在氮气氛围下400 ℃热解3 h后自然冷却获得黑碳. 将3.00 g黑碳溶于300 mL纯净水混合均匀,并用超声波机(SB-500D,中国宁波新智超声波仪器)超声30 min,超声结束后采用0.45 μm水系滤膜过滤,得到实验所需的溶解性黑碳(DBC). TOC仪器(CD-800S,中国杭州启鲲科学仪器)测定DBC的总有机碳(total organic carbon,TOC)浓度. 此外,DBC储备液严格避光低温保存.
-
将PLA、PS、RTC 3种微塑料分别用电子天平(AL204,梅特勒脱利多公司)准确称量0.02 g放入石英管,加入10 mg·L−1C的DBC溶液并定容至20 mL. 用Qsun光老化仪(Qsun Xe-1,美国Q-Panel公司)进行光老化试验,分别老化22、44、91 h. 溶液过滤后获得老化微塑料颗粒和DBC溶液,避光低温保存,以备实验使用. 微塑料对照组准确称量0.02 g微塑料放入石英管中并用纯净水定容至20 mL,Qsun老化91 h. 将10 mg·L−1C的DBC溶液加入石英管并定容到20 mL,Qsun老化22、44、91 h,作为DBC对照组.
老化后的微塑料分别命名为PLA(DBC)22~91、PS(DBC)22~91、RTC(DBC)22~91,对照组的微塑料命名为PLA91、PS91、RTC91. 与微塑料共光照的DBC溶液命名为DBC(PLA) 22~91、DBC(PS)22~91、DBC(RTC)22~91;DBC对照组命名为DBC22、DBC44、DBC91,其中初始DBC命名DBC0. 最后采用TOC仪器测定各个DBC溶液的含碳量.
-
采用紫外分光光度计(Evolution 201,安捷伦科技有限公司)测定DBC溶液的紫外-可见吸收光谱,200—700 nm扫描波段、波长间隔1 cm,根据紫外扫描结果得出SUVA254吸光值;采用三维荧光光谱仪(F-
7000 ,HITACHI),激发波长Ex和发射波长Em均为200—500 nm、扫描速度2400 nm·min−1、光学狭缝为5 nm、波长间隔为5 nm,获得DBC溶液的三维荧光光谱.分别选用TMP和FFA为3DBC*和1O2的探针试剂. 将TMP和FFA加入DBC溶液中进行光照,实验所用浓度为:10 mgC·L−1DBC,10 μmol·L−1TMP,10 μmol·L−1 FFA. 通过pH计(雷磁PHS-3E)测定溶液pH,用H2SO4和NaOH调节至溶液pH=7. 每隔固定时间取样放入高效液相色谱仪(Agilent 1260HPLC)进行检测,检测条件如下:色谱柱为Agilent Eclipse XDB-C18柱,进样量20 μL,柱温35 ℃,流速1 mL·min−1,检测TMP的流动相比例为30 %水和70 %乙腈,检测FFA的流动相比例为40 %水和60 %乙腈,其紫外检测波长均为220 nm.
-
采用扫描电子显微镜(SEM,捷克TESCAN MIRA LMS)观察老化前后PLA、PS、RTC颗粒的表面形貌变化;采用傅里叶红外光谱(FTIR,美国Thermo Scientific Nicolet
6700 )测定紫外老化前后PLA、PS、RTC颗粒的官能团变化. -
有/无微塑料共存光照的DBC紫外吸收光谱如图1所示. 由图1可知,光照后DBC溶液的紫外吸光度大幅度下降. DBC吸收紫外辐射后会发生一系列光化学反应,当吸收紫外光造成吸光度及荧光强度出现损失的情况称为光漂白[18]. 在经过长时间紫外辐照后,DBC溶液都发生了不同程度的光漂白,光漂白会破坏DBC的共轭结构,导致吸光性能降低,吸收光谱向短波移动[18]. 值得注意的是,与微塑料共光照后的DBC溶液的吸光度均大于DBC单独光照后的吸光度. 微塑料会吸收紫外辐射发生自由基反应以促进自身老化[19],当微塑料与DBC共存时,微塑料会与DBC产生光竞争效应,减弱DBC的光漂白效果,使得DBC的紫外吸光度损失降低. RTC为黑色塑料,PLA、PS为白色塑料,它们对DBC的光竞争效应存在差异性. 图1(a)显示DBC(RTC)22的吸光度要远大于DBC(PLA)22和DBC(PS)22,黑色塑料吸光性比白色塑料更强,更高效缓解DBC光漂白损失. 光照44 h和91 h后,DBC(PLA)的吸光度更大. PLA聚合物材料在光辐照下会破坏分子链的醚部分,发生解聚反应[20],PLA更先分解为更小粒径的颗粒或碎片,在溶液中分布更广泛,光竞争能力更强.
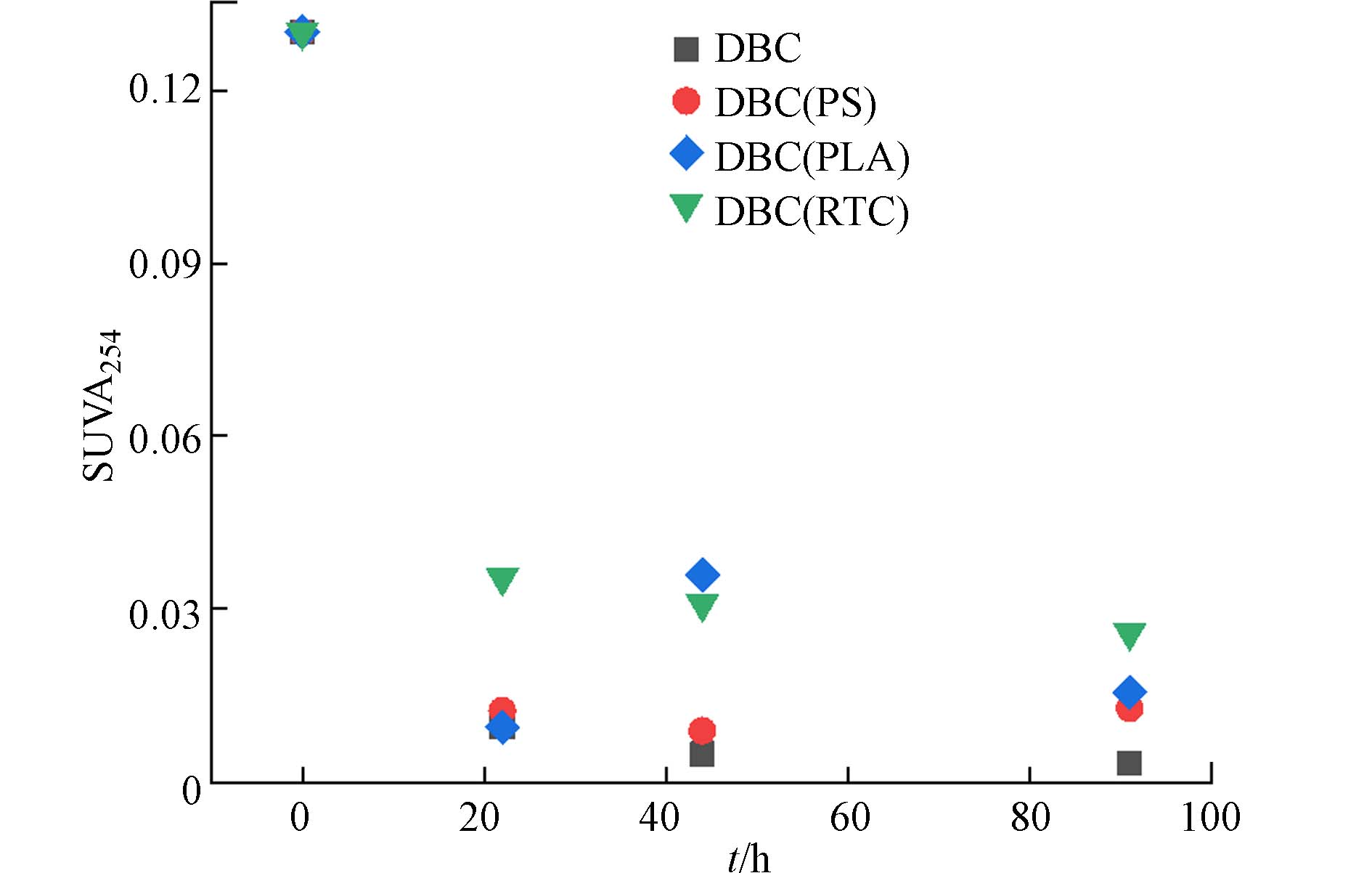
以往研究表明,紫外吸收光谱的特征参数可以描述物质的光活性,其中SUVA254通常与DBC的分子量和芳香性呈正相关[21 − 22]. 有/无微塑料共存光照的DBC的SUVA254值如图2所示,SUVA254随光照时间增长而减小,DBC分子量减小,芳香性减弱,这与光漂白造成的结果一致[23]. 但总体而言,DBC(RTC)的值要略大,这是因为RTC黑色塑料对DBC的光竞争效应要更强,与紫外吸光光谱结果相符.
光漂白不仅会使DBC吸光度降低,也会破坏DBC的荧光强度,为了探究DBC荧光组分的变化对其进行了三维荧光光谱分析(图3). DBC0在荧光Ⅱ、Ⅲ区有最大的荧光吸收峰,说明DBC的主要成分是芳香蛋白和黄腐酸类[24 − 25]. 经过紫外辐照后,荧光Ⅱ、Ⅲ区的信号明显减弱,而且芳香蛋白的荧光信号向发射波长(Em)的短波方向移动,出现荧光蓝移现象. 紫外光照相同时间后的DBC(PLA)、DBC(PS)和DBC(RTC)的荧光强度都要比DBC大. 光照22 h后,DBC(PS)22和DBC(RTC)22的荧光丰度大于DBC(PLA)22和DBC22,其中DBC(RTC)22荧光组分最为丰富. 一方面,黑色塑料吸光度更大,减弱DBC光漂白;另一方面,RTC是由橡胶、增强剂、抗氧化剂等组成的复杂物质,光老化促进RTC添加剂的释放通量和速率[26 − 27]. 光照44 h后,所有溶液中DBC(PLA)44的荧光特征峰最为显著. PLA是乳酸单体聚合物,光老化会造成PLA主链断裂解聚为乳酸,形成更小单体,释放出部分可溶物质并与天然有机质有相似的腐殖质荧光吸收特性[28 − 29]. 光照91 h后DBC溶液的荧光信号都很微弱,由于实验光照时间的限制导致了微塑料在光老化过程产生的有机物质比DBC光漂白造成的损失小,因而整体的荧光强度减弱.
-
DBC通过所含的芳香羧基、羟基和羰基等发色基团可吸收太阳光介导产生激发三重态DBC(3DBC*)、单线态氧(1O2)等活性氧物种,用TMP和FFA分别作为3DBC*和1O2探针试剂以探究与微塑料共存光照后DBC的光化学活性变化[22]. TMP降解速率如图4所示. 所有溶液的TMP降解速率都呈降低趋势. DBC0的TMP降解速率最大,3DBC*生成能力最强,随着光照时间增加,DBC(PS)、DBC(RTC)和DBC(PLA)的TMP降解速率降低,即使微塑料与DBC共存能够竞争光子以减弱DBC的共轭结构破坏程度和荧光组分的损失,但也只能减缓DBC紫外辐照后光化学活性减弱的程度. DBC溶液的FFA降解速率如图4所示,DBC0溶液的FFA降解最快,光照后溶液的FFA降解速率均降低,与TMP的降解速率变化趋势一致.
光照后DBC的光化学活性被破坏,微塑料对DBC的光竞争能较好的减弱DBC的光漂白效应,使得DBC的光化学活性部分保留. 虽然3种微塑料的化学成分及组成和颜色各有差异,但由于过长时间的光漂白严重破坏了DBC的活性官能团,导致不同微塑料对DBC光化学活性影响的差异显著性较低.
-
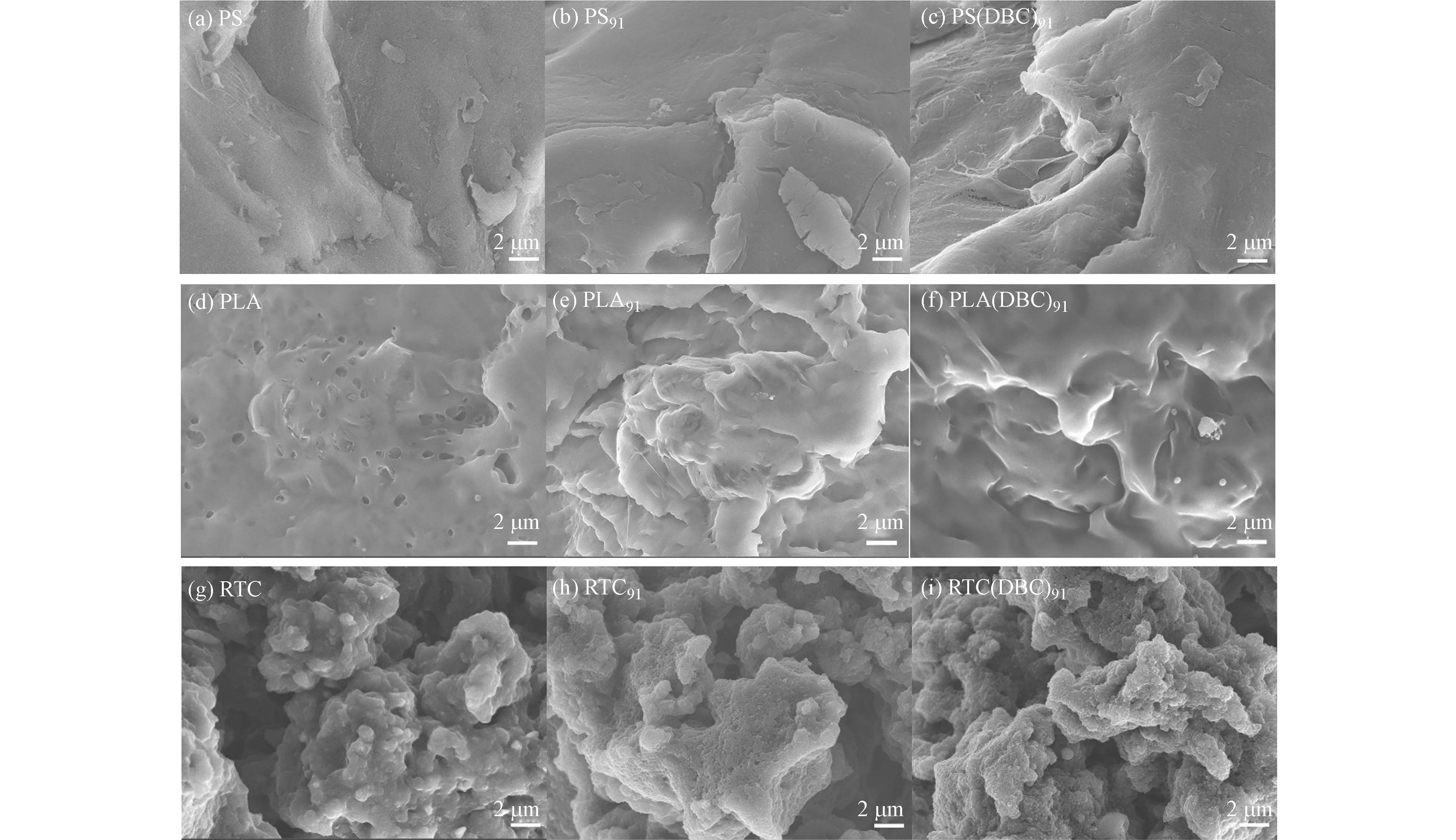
为了进一步观察微塑料老化情况,对在水/DBC溶液中老化前后的3种微塑料进行SEM扫描(图5). 初始PLA、PS、RTC颗粒的表面分布着大小不一的孔径或裂缝、凸起、剥落碎屑等,这是由于实验所采用的商业微塑料在生产过程中机械磨损导致的. 紫外光照老化91 h后,PLA颗粒表面粗糙程度增大,裂纹、断层多,形成凹凸不平的纹理. 同样,PS颗粒光照91 h后表面也出现了更多的裂缝、裂纹加深,并有更多剥落的小碎屑,这是因为PS更易脆化和碎裂[30]. 光照后的RTC颗粒表面也发生了明显变化,出现鳞屑状,产生了更多凹坑. 有研究报道紫外辐射会导致微塑料结构产生变化,光照引起微塑料产生断裂反应并伴随活性氧生成,活性氧又会继续破坏微塑料的结构[19]. 此外,在不同溶液介质中,微塑料的形貌变化也有所不同. 如图5e和f所示,PLA91和PLA(DBC)91表观形貌都产生了裂纹或凹凸不平的纹理,PLA(DBC)91比PLA91更光滑,PLA91出现许多断层,表面粗糙,PLA(DBC)91表面有许多凸起或凹陷并有微小球状或碎片颗粒附着在表面. 同样的,在DBC溶液氛围下进行光照的PS和RTC颗粒均比水溶液氛围中的颗粒产生了更深的裂痕和更多的碎屑. DBC作为水中重要的光敏剂可以产生活性氧物种,而活性氧正是微塑料老化的关键,可以促进微塑料的老化.
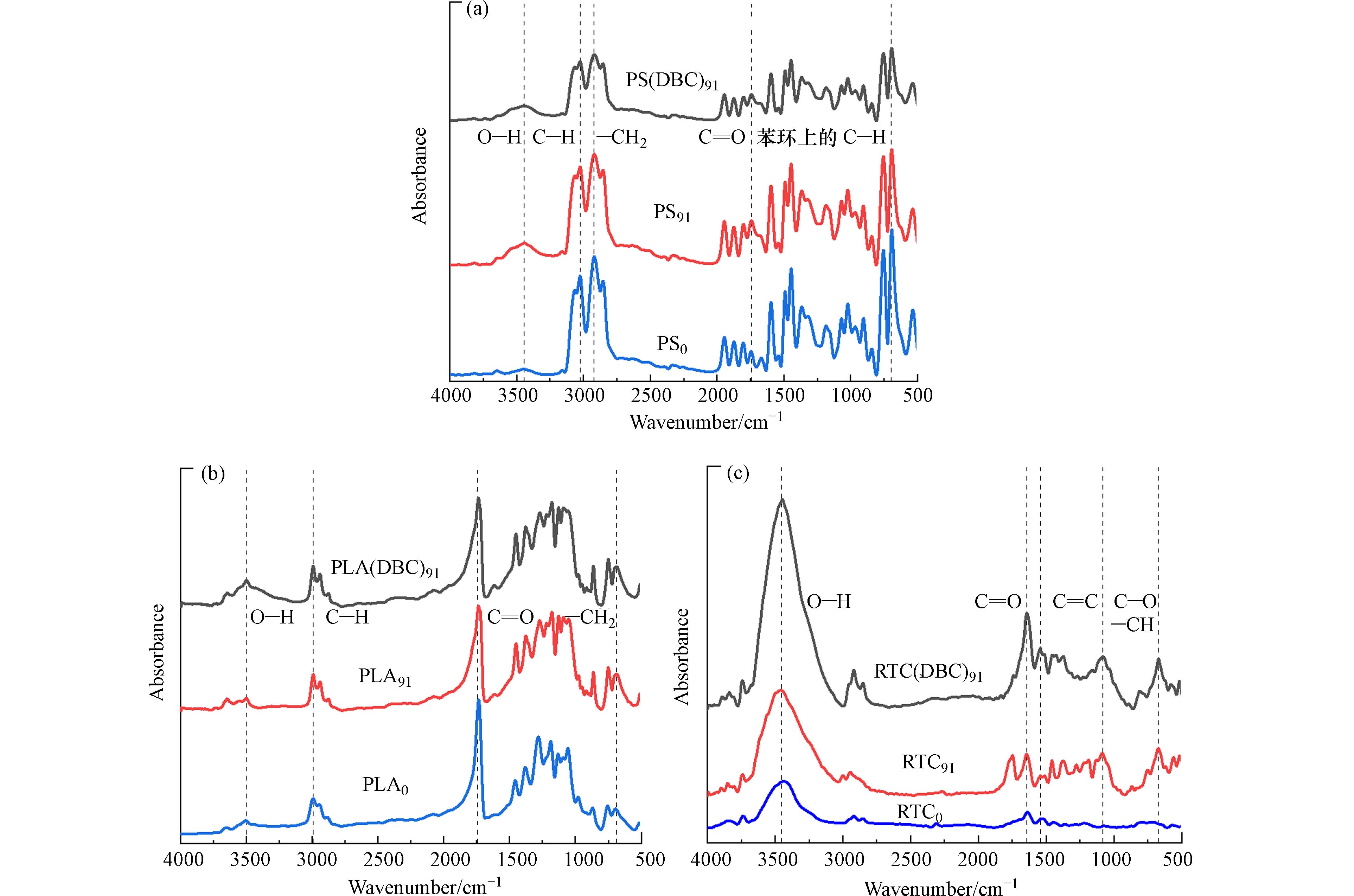
老化不仅会改变微塑料的形貌,也会改变微塑料的官能团. 通过傅里叶红外光谱(FTIR)可以得出微塑料的分子组成和官能团的变化[31 − 32]. 从图6(a)中可以看到老化后的PS91和PS(DBC)91在
3342 cm−1处—OH的拉伸振动吸收峰有明显的增大[33],紫外光的能量促使塑料中的C—H(420 kJ·mol−1)和C—C(375 kJ·mol−1)键断裂并释放自由基,经过一系列复杂的扩链、支化和链终止反应后,最终塑料会引入更多的含氧官能团,使含氧组分增加[34]. 老化后的PS在3020 cm−1和2918 cm−1处的吸收峰也产生了显著的变化,分别是由苯环的不饱和C—H拉伸振动和—CH2的不对称拉伸振动造成的,老化后单取代苯环上氢原子的面外变形振动也引起了698 cm−1吸收峰的变化. PS91在1747 cm−1处的羰基吸收峰要明显大于PS的羰基吸收峰,说明碳链内部和端位出现了更高程度的氧化,苯环的吸电子效应使氧分子有更多能量克服空间位阻氧化内部碳原子,同时由于苯环的空间效应使端位碳原子比内部碳原子的优势更少,因此生成更多的酮/醛物质[35].图6(b)为PLA老化前后的FTIR图,图谱显示在
3500 cm−1处有—OH拉伸振动引起的特征吸收峰,且PLA(DBC)91的峰面积最大. 塑料的降解反应主要是光降解氧化过程,涉及自由基链式反应,在紫外光照射下塑料样品中部分C—C、C—H发生断裂形成自由基,与氧反应产生含氧基团(C—O)从而进一步的分解为其他产物[31]. DBC光照产生的活性氧物种使PLA老化更加剧烈,引入更多—OH. 在2995 cm−1处的C—H伸缩振动和750 cm−1处的亚甲基弯曲振动都发生了不同程度的变化. 值得关注的是,1735 cm−1的酯羰基特征吸收峰随着老化时间增加而降低,表明酯键的断裂,这意味着PLA发生降解[36].RTC在
3346 、1648 、1548 、1089 、673 cm−1处有明显的吸收峰(图6(c)),其分别属于O—H伸缩振动吸收峰、C=O特征吸收峰、C=C典型特征吸收峰、酚羟基或脂肪醇的C—O拉伸吸收峰、芳香C—H弯曲[37 − 40]. 与RTC0相比,RTC(DBC)91和RTC91在3346 、1648 、1089 cm−1处含氧官能团的吸收峰明显要更高,其中RTC(DBC)91的峰面积要大于RTC91. RTC由橡胶及其他复杂组分组成,老化破坏了乙烯基,导致橡胶中羰基的生成峰面积增大,与前人的研究一致[40]. 具有共轭双键的化合物如橡胶等更易发生加成、聚合等反应,随着老化时间的增长,C=C峰面积增加明显. 光照条件下DBC会产生大量的活性氧物种,促进RTC的自由基链式反应,氧化效果更加显著,引入更多的含氧官能团使RTC的老化程度更高[19]. -
本研究将DBC与微塑料共存光照,通过测定各个DBC溶液的紫外吸收光谱和三维荧光光谱发现与微塑料共存光照的DBC溶液的紫外吸光度和荧光丰度均大于单独光照的DBC,探针实验发现前者光致3DBC*和1O2的能力也大于后者. 微塑料对DBC的光竞争有效削减了DBC光漂白效应,减弱DBC的光化学活性失活程度. 其中黑色微塑料RTC比白色微塑料PLA、PS的光竞争效应更强,对DBC光漂白影响更大. 此外,微塑料在DBC光致活性氧物种作用下结构被破坏,释放有机质进入溶液导致溶液荧光组分更加复杂.
光老化使PS、PLA、RTC 3种微塑料的微观形貌出现更多裂纹裂缝、褶皱现象,表面粗糙程度增加,并引入更多的含氧官能团. 环境因子DBC存在的光老化体系下,PS、PLA、RTC的形貌变化更加显著,含氧官能团变化更为突出,促进了微塑料老化.
微塑料对溶解性黑碳光化学活性的影响机制
Effect mechanism of microplastics on photochemical activity of dissolved black carbon
-
摘要: 溶解性黑碳(DBC)是表层水体中的重要光活性组分,受水中共存物质的影响. 微塑料是一类新污染物,在水环境中不断被检出. 研究证实了微塑料可与DBC发生吸附等作用影响DBC的分散、团聚等环境行为,但微塑料对DBC光化学活性的影响尚未见报道. 本文研究了微塑料对DBC光谱参数及光化学活性的影响,结果表明,在微塑料存在条件下光照后的DBC溶液的紫外吸光度、光化学活性均强于单独光照的DBC,且前者芳香蛋白的荧光丰度损失程度也小于后者. 此外,黑色微塑料存在时对DBC溶液紫外吸光度、光化学活性及荧光丰度变化的影响大于另外两种白色微塑料. 相同光照时间下,DBC存在的光照体系下微塑料表面产生的碎屑、裂纹多于微塑料单独光照产生的;傅里叶红外光谱(FTIR)结果也显示DBC作用条件下微塑料光照后引入了O-H等含氧官能团,说明DBC促进了微塑料的光老化.Abstract: Dissolved black carbon (DBC) was an important photoactive component in surface waters and was influenced by co-occurring substances in the water. Microplastics were a new class of pollutants that are constantly detected in the aqueous environment. Studies had confirmed that microplastics can affect the environmental behavior of DBC such as dispersion and agglomeration by adsorption and other interactions with DBC, but the effect of microplastics on the photochemical activity of DBC was not reported. In this paper, we investigated the effects of microplastics on the spectral parameters and photochemical activity of DBC. The results showed that the UV absorbance and photochemical activity of DBC solutions after illumination in the presence of microplastics were stronger than those of DBC illuminated alone, and the loss of fluorescence abundance of aromatic proteins in the former was also smaller than that in the latter. In addition, the effect of the presence of black microplastics on the changes of UV absorbance, photochemical activity and fluorescence abundance of DBC solution was greater than that of the other two white microplastics. Under the same light time, more debris and cracks were produced on the surface of microplastics in the light system in the presence of DBC than those produced by microplastics alone; Fourier transform infrared spectroscopy (FTIR) results also showed that oxygen-containing functional groups such as O—H were introduced into microplastics after light exposure under the conditions of DBC action, indicating that DBC promoted the photoaging of microplastics.
-
Key words:
- dissolved black carbon /
- photochemistry /
- microplastics /
- photoaging.
-

-
-
[1] CHEN H R, WANG J J, ZHAO X T, et al. Occurrence of dissolved black carbon in source water and disinfection byproducts formation during chlorination[J]. Journal of Hazardous Materials, 2022, 435: 129054. doi: 10.1016/j.jhazmat.2022.129054 [2] WANG L, LI J, ZHAO J, et al. Photodegradation of clindamycin by the dissolved black carbon is simultaneously regulated by ROS generation and the binding effect[J]. Water Research, 2023, 233: 119784. doi: 10.1016/j.watres.2023.119784 [3] 张宵, 刘一帆, 刘强, 等. 溶解性黑碳促进水环境中四环素的光降解[J]. 环境化学, 2023, 42(6): 2064-2075. doi: 10.7524/j.issn.0254-6108.2021122001 ZHANG X, LIU Y F, LIU Q, et al. Dissolved black carbon enhanced the photodegradation of tetracycline in aqueous solution[J]. Environmental Chemistry, 2023, 42(6): 2064-2075 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021122001
[4] ZHOU Z C, CHEN B N, QU X L, et al. Dissolved black carbon as an efficient sensitizer in the photochemical transformation of 17β-estradiol in aqueous solution[J]. Environmental Science & Technology, 2018, 52(18): 10391-10399. [5] FANG G D, LIU C, WANG Y J, et al. Photogeneration of reactive oxygen species from biochar suspension for diethyl phthalate degradation[J]. Applied Catalysis B:Environmental, 2017, 214: 34-45. doi: 10.1016/j.apcatb.2017.05.036 [6] ZHANG P, SHAO Y F, XU X J, et al. Phototransformation of biochar-derived dissolved organic matter and the effects on photodegradation of imidacloprid in aqueous solution under ultraviolet light[J]. Science of the Total Environment, 2020, 724: 137913. doi: 10.1016/j.scitotenv.2020.137913 [7] LIU H T, GE Q, XU F C, et al. Dissolved black carbon induces fast photo-reduction of silver ions under simulated sunlight[J]. Science of the Total Environment, 2021, 775: 145897. doi: 10.1016/j.scitotenv.2021.145897 [8] XU Y H, OU Q, LIU C H, et al. Aggregation and deposition behaviors of dissolved black carbon with coexisting heavy metals in aquatic solution[J]. Environmental Science:Nano, 2020, 7(9): 2773-2784. doi: 10.1039/D0EN00373E [9] HE H, LIU K Q, GUO Z W, et al. Photoaging mechanisms of microplastics mediated by dissolved organic matter in an iron-rich aquatic environment[J]. Science of the Total Environment, 2023, 860: 160488. doi: 10.1016/j.scitotenv.2022.160488 [10] 陈苏, 刘颖, 张晓莹, 等. 微塑料老化行为及其对重金属吸附影响的研究进展[J]. 生态与农村环境学报, 2023, 39(1): 12-19. CHEN S, LIU Y, ZHANG X Y, et al. Progress in the study on ageing behavior of microplastics and its effect on heavy metal adsorption[J]. Journal of Ecology and Rural Environment, 2023, 39(1): 12-19 (in Chinese).
[11] XIAO Y H, WANG Q J, LI P H, et al. Impact of light-aged microplastic on microalgal production of dissolved organic matter[J]. Science of the Total Environment, 2023, 889: 164312. doi: 10.1016/j.scitotenv.2023.164312 [12] XU B L, LIU F, BROOKES P C, et al. Microplastics play a minor role in tetracycline sorption in the presence of dissolved organic matter[J]. Environmental Pollution, 2018, 240: 87-94. doi: 10.1016/j.envpol.2018.04.113 [13] ABDURAHMAN A, CUI K Y, WU J, et al. Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis[J]. Ecotoxicology and Environmental Safety, 2020, 198: 110658. doi: 10.1016/j.ecoenv.2020.110658 [14] CHEN M L, LIU S S, BI M H, et al. Aging behavior of microplastics affected DOM in riparian sediments: From the characteristics to bioavailability[J]. Journal of Hazardous Materials, 2022, 431: 128522. doi: 10.1016/j.jhazmat.2022.128522 [15] HUNG C M, CHEN C W, HUANG C P, et al. Ecological responses of coral reef to polyethylene microplastics in community structure and extracellular polymeric substances[J]. Environmental Pollution, 2022, 307: 119522. doi: 10.1016/j.envpol.2022.119522 [16] SUN Y Z, JI J H, TAO J G, et al. Current advances in interactions between microplastics and dissolved organic matters in aquatic and terrestrial ecosystems[J]. TrAC Trends in Analytical Chemistry, 2023, 158: 116882. doi: 10.1016/j.trac.2022.116882 [17] SONG F H, LI T T, WU F C, et al. Temperature-dependent molecular evolution of biochar-derived dissolved black carbon and its interaction mechanism with polyvinyl chloride microplastics[J]. Environmental Science & Technology, 2023, 57(18): 7285-7297. [18] 高洁, 江韬, 闫金龙, 等. 天然日光辐照下两江交汇处溶解性有机质(DOM)光漂白过程: 以涪江-嘉陵江为例[J]. 环境科学, 2014, 35(9): 3397-3407. GAO J, JIANG T, YAN J L, et al. Photobleaching of dissolved organic matter(DOM) from confluence of two rivers under natural solar radiation: A case study of Fujiang River-Jialingjiang River[J]. Environmental Science, 2014, 35(9): 3397-3407 (in Chinese).
[19] 左林子, 侯婉儿, 王飞, 等. 微塑料的光老化过程及其携带内源污染物释放的研究进展[J]. 环境化学, 2022, 41(7): 2245-2255. doi: 10.7524/j.issn.0254-6108.2021082001 ZUO L Z, HOU W E, WANG F, et al. Research progress on photo-aging of microplastics and their effects on the release of endogenous pollutants[J]. Environmental Chemistry, 2022, 41(7): 2245-2255 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021082001
[20] WANG L, PENG Y W, XU Y L, et al. An in situ depolymerization and liquid chromatography-tandem mass spectrometry method for quantifying polylactic acid microplastics in environmental samples[J]. Environmental Science & Technology, 2022, 56(18): 13029-13035. [21] WEISHAAR J L, AIKEN G R, BERGAMASCHI B A, et al. Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon[J]. Environmental Science & Technology, 2003, 37(20): 4702-4708. [22] 屠依娜, 石凤丽, 李英杰, 等. 水中不同热解温度溶解性黑碳的光化学活性[J]. 环境化学, 2022, 41(6): 2094-2102. doi: 10.7524/j.issn.0254-6108.2021012804 TU Y N, SHI F L, LI Y J, et al. Photochemical activity of dissolved black carbon from different pyrolysis temperature in aqueous solution[J]. Environmental Chemistry, 2022, 41(6): 2094-2102 (in Chinese). doi: 10.7524/j.issn.0254-6108.2021012804
[23] MORAN M A, SHELDON W M Jr, ZEPP R G. Carbon loss and optical property changes during long-term photochemical and biological degradation of estuarine dissolved organic matter[J]. Limnology and Oceanography, 2000, 45(6): 1254-1264. doi: 10.4319/lo.2000.45.6.1254 [24] MOUNIER S, BRAUCHER R, BENAı̈M J Y. Differentiation of organic matter’s properties of the Rio Negro Basin by cross-flow ultra-filtration and UV-spectrofluorescence[J]. Water Research, 1999, 33(10): 2363-2373. doi: 10.1016/S0043-1354(98)00456-4 [25] DETERMANN S, REUTER R, WAGNER P, et al. Fluorescent matter in the eastern Atlantic Ocean. Part 1: Method of measurement and near-surface distribution[J]. Deep Sea Research Part I:Oceanographic Research Papers, 1994, 41(4): 659-675. doi: 10.1016/0967-0637(94)90048-5 [26] XU Q, LI G, FANG L, et al. Enhanced formation of 6PPD-Q during the aging of tire wear particles in anaerobic flooded soils: The role of iron reduction and environmentally persistent free radicals[J]. Environmental Science & Technology, 2023, 57(14): 5978-5987. [27] XIAO L H, ZHENG Z Y, IRGUM K, et al. Studies of emission processes of polymer additives into water using quartz crystal microbalance-a case study on organophosphate esters[J]. Environmental Science & Technology, 2020, 54(8): 4876-4885. [28] SHI Y Q, LIU P, WU X W, et al. Insight into chain scission and release profiles from photodegradation of polycarbonate microplastics[J]. Water Research, 2021, 195: 116980. doi: 10.1016/j.watres.2021.116980 [29] LEE Y K, MURPHY K R, HUR J. Fluorescence signatures of dissolved organic matter leached from microplastics: Polymers and additives[J]. Environmental Science & Technology, 2020, 54(19): 11905-11914. [30] DING L, MAO R F, MA S R, et al. High temperature depended on the ageing mechanism of microplastics under different environmental conditions and its effect on the distribution of organic pollutants[J]. Water Research, 2020, 174: 115634. doi: 10.1016/j.watres.2020.115634 [31] MAO R F, LANG M F, YU X Q, et al. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals[J]. Journal of Hazardous Materials, 2020, 393: 122515. doi: 10.1016/j.jhazmat.2020.122515 [32] DING L, LUO Y Y, YU X Q, et al. Insight into interactions of polystyrene microplastics with different types and compositions of dissolved organic matter[J]. Science of the Total Environment, 2022, 824: 153883. doi: 10.1016/j.scitotenv.2022.153883 [33] 范秀磊, 常卓恒, 邹晔锋, 等. 可降解微塑料对铜和锌离子的吸附解吸特性[J]. 中国环境科学, 2021, 41(5): 2141-2150. FAN X L, CHANG Z H, ZOU Y F, et al. Adsorption and desorption properties of degradable microplastic for Cu2+ and Zn2+[J]. China Environmental Science, 2021, 41(5): 2141-2150 (in Chinese).
[34] 王林, 王姝歆, 曾祥英, 等. 老化作用对微塑料吸附四环素的影响及其机制[J]. 环境科学, 2022, 43(10): 4511-4521. WANG L, WANG S X, ZENG X Y, et al. Effect of aging on adsorption of tetracycline by microplastics and the mechanisms[J]. Environmental Science, 2022, 43(10): 4511-4521 (in Chinese).
[35] 刘少通, 程文华, 彭文山, 等. 聚乙烯和聚苯乙烯塑料在青岛海洋大气环境中的自然老化行为研究[J]. 合成材料老化与应用, 2019, 48(2): 24-29,37. LIU S T, CHENG W H, PENG W S, et al. Natural weathering of typical plastics(PE, PS) under Qingdao marine atmospheric environment[J]. Synthetic Materials Aging and Application, 2019, 48(2): 24-29,37 (in Chinese).
[36] WANG N, YU J G, CHANG P R, et al. Influence of formamide and water on the properties of thermoplastic starch/poly(lactic acid) blends[J]. Carbohydrate Polymers, 2008, 71(1): 109-118. doi: 10.1016/j.carbpol.2007.05.025 [37] QIU X R, MA S R, ZHANG J X, et al. Dissolved organic matter promotes the aging process of polystyrene microplastics under dark and ultraviolet light conditions: The crucial role of reactive oxygen species[J]. Environmental Science & Technology, 2022, 56(14): 10149-10160. [38] LUO L, CHEN Z E, LV J T, et al. Molecular understanding of dissolved black carbon sorption in soil-water environment[J]. Water Research, 2019, 154: 210-216. doi: 10.1016/j.watres.2019.01.060 [39] 毕晨曦. 聚乳酸塑料在高温下水解降解的研究[D]. 大连: 大连理工大学, 2020. BI C X. Study on hydrolytic degradation of polylactic acid plastic at high temperature[D]. Dalian: Dalian University of Technology, 2020 (in Chinese).
[40] WU S W, QIU M, GUO B C, et al. Nanodot-loaded clay nanotubes as green and sustained radical scavengers for elastomer[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(2): 1775-1783. -




 下载:
下载: