-
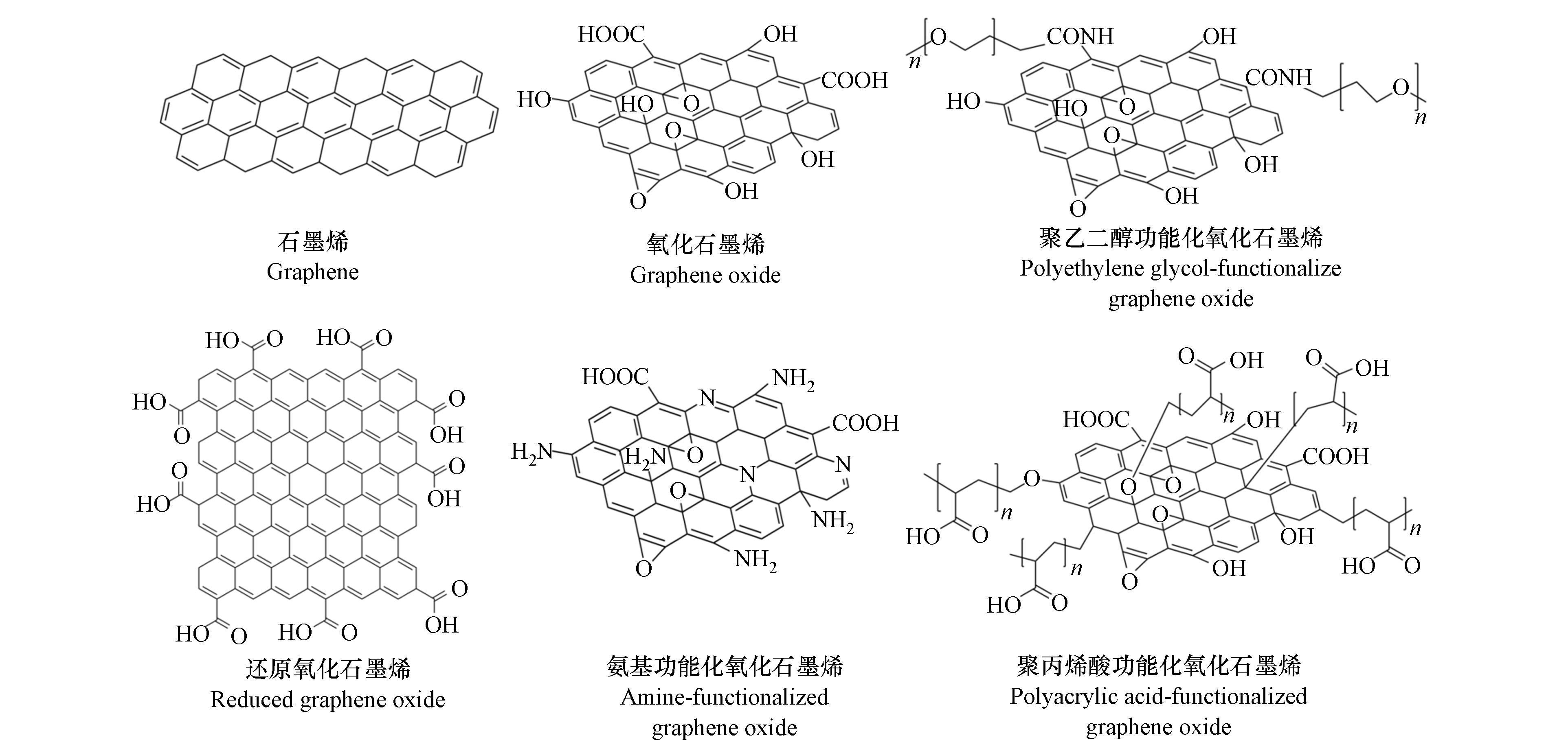
石墨烯(graphene)于2004年被英国曼彻斯特大学的两位科学家Andre Geim和Konstantin Novoselov发现[1],它是一种由单层碳原子组成六角型呈蜂巢晶格的片状结构. 石墨烯通过化学氧化反应(如Hummers法、Brodie法和Staudenmaier法等)在表面引入大量含氧官能团形成氧化石墨烯(graphene oxides,GOs). GOs的含氧官能团(如羧基、羟基、羰基等)附着在层的两侧以及平面的边缘[2 − 3],GOs分散性优异的原因在于其表面多样化的离子化基团(如羧基、羟基等)能在不同pH下电离,提供丰富的电荷,这些电荷通过静电排斥作用有效防止了GOs之间的聚集[4 − 5](图1). GOs及其衍生物主要包括原生氧化石墨烯(NGOs)、还原氧化石墨烯(RGOs)、氧化石墨烯量子点(GOQDs)和功能化氧化石墨烯(如氨基功能化GOs、聚乙二醇功能化GOs和聚丙烯酸功能化GOs等). GOs根据结构可分为单层、双层、少层(3—10层)和多层(10层以上)氧化石墨烯[6]. GOs由于其在物理、化学、生物和光电子等方面的独特优势,正越来越多地应用于废水处理[7 − 9]、光/电催化[10 − 11]、消毒[12 − 13]、电化学传感器[14 − 15]、药物传送[16 − 17]、生物成像[18 − 19]和癌症治疗[20 − 21]等领域. 然而,随着GOs产量的不断增加,其潜在的毒性问题也逐渐暴露出来,引起了研究学者们的广泛关注. 根据欧盟委员会法规(EU)No 2020/878,化学品制造商或进口商有义务根据REACH提供纳米化学物质的具体信息要求和化学安全评估(EC,2020),其中GOs的EC号为947-768-1,被归类为水生环境危害类,GOs慢性暴露后对水生生物具有长期持续影响(ECHA 2022)[22]. 本文重点关注GOs的环境转化、毒性作用以及蛋白冠介导下的生物学命运等3个方面的内容.
-
2024年5月30日,据市场研究公司Brainy Insights预测,GOs市场规模将从2023年的29亿美元增长到10年后的438亿美元. 由于GOs的大量生产和使用,在GOs和含GOs产品的制造、运输、使用和最终处置等整个生命周期中,GOs可能无意或有意地排放到环境(水、大气、土壤)中,对人类造成潜在危险[23]. 因此,了解GOs的环境转化和环境命运对于评估其工程应用和生态风险至关重要. 当GOs释放到环境中后,会经历一系列复杂的物理、化学和生物转化,随着时间的推移,这些环境转化会对它们的理化性质和行为(如GOs在环境中的迁移性、稳定性、与其他物质的相互作用以及潜在的毒性效应等)产生影响[25 − 26].
-
GOs的物理转化主要包括吸附、团聚、聚集和沉降,其中吸附是指物质分子在GOs表面通过物理作用力(如范德华力、静电相互作用等)可逆地附着或积累的过程;团聚是指在弱范德华力吸引下的可逆构象变化;聚集是指由于静电相互作用而产生的不可逆聚集现象;沉降是指在重力或其他外力作用下,GO团聚体/聚集体不可逆地沉积到底部. GOs进入到水生环境后,首先会吸附无机离子、天然有机物质(NOM)、胶体颗粒等成分,影响GOs的胶体行为,发生聚集,进而可能发生沉降[27]. 在这个过程中,主要受GOs本身的性质(大小、表面电荷、形态和官能团等)以及水环境条件(pH、温度、水环境成分等)的影响[28]. Zou等[29]研究了在水中GO(纯度:99.95%,片径:几纳米到几十纳米不等)与纳米晶Mg/Al层状双氢氧化物(LDH-CI)的相互作用. 结果显示,GO在LDH-CI上发生凝聚和沉积,这个过程受到pH和温度等因素的影响. 在pH<6时,GO在LDH-CI上的凝聚能力随着pH的增加而迅速增加,这主要是由于静电相互作用(GO的负电荷与LDH-CI的正电荷之间的吸引),而在pH>6时,GO在LDH-CI上的凝聚能力随着pH的增加而下降,这主要是范德华力和氢键仍然起作用,但静电相互作用大大减弱. 在20—30 ℃范围内,GO在LDH-Cl上的凝聚能力随着温度的升高略有增加,这主要是由于适度的温度升高能够弥补结合过程中的能量损失,促进GO与LDH-Cl表面之间的相互作用,而温度超过30 ℃时,GO在LDH-Cl上的凝聚能力开始下降,这主要是因为过高的温度加剧了GO的布朗运动,降低了GO与LDH-Cl之间结合过程的稳定性. Sadr等[30]研究了pH、温度、吸附剂(γ-环糊精功能化磁铁矿GO)含量(m)和接触时间(t)对水溶液中氟尿嘧啶去除率的影响,氟尿嘧啶通过范德华力或静电相互作用吸附在吸附剂上. 结果表明,在t=45 min、m=0.020 g、pH=7时,对氟尿嘧啶的去除率最高.
吸附胶体颗粒、生物分泌物等物质后的GOs毒性变化复杂多样. Mu等[31]研究了GO(片径:>0.5 μm,厚度:0.83 nm±0.12 nm)吸附水中斑马鱼分泌物(小分子有机物、蛋白质、核苷酸和黏多糖)前后,对斑马鱼胚胎暴露120 h的毒性影响. 结果表明,吸附分泌物后的GO(GOBS,呈纳米板状形貌,片径:19.5—282 nm,厚度:10 nm)相比于GO(0.1 mg·L−1)毒性显著增强,表现为β-半乳糖苷酶上调、线粒体膜电位显著丧失、死亡率和畸形率上升. 这一现象归因于GOBS相比于GO具有更好的分散性、更低的表面电荷状态,从而导致了更高的细胞摄取效率. 相反地,Ouyang等[32]研究1 mg·L−1GO(片径:0.5—5μm,厚度:0.8—1.2 nm)吸附地表水中普遍存在的纳米胶体(Nc)前后,对斑马鱼幼虫暴露96 h的毒性影响. 结果表明,斑马鱼出现了显著的毒性反应(如氧化应激、尾巴畸形和卵黄囊水肿),然而吸附Nc(4 mg·L−1)后的GO(GO-Nc)毒性减弱,这归因于GO-Nc相比于GO尺寸更小且亲水性增强,使得其更容易从斑马鱼体内排出,其次,Nc降低了GO的生物利用度,减少了GO与斑马鱼的直接接触,从而削弱了GO的锋利边缘会对细胞的物理损伤,进而减轻了毒性效应. 综上所述,GOs吸附不同物质后的理化性质变化决定了毒性的变化趋势,但GOs吸附物引发毒性变化的具体机制仍不完全明确.
-
化学转化是将一种化合物转化为具有不同结构、价态或组成的化合物. GOs可能与水环境中的成分发生氧化还原反应,沉淀或络合作用等[26,33]. Wang等[34]研究了GO(纯度:>99%,C/O质量比:2.9,ID/IG:0.72)与亚铁离子(Fe2+在沉积物和厌氧地下水中的浓度高达数毫摩尔[35])的相互作用. 结果表明,作为还原剂的Fe2+导致GO表面O含量大幅降低,其中GOs表面环氧/羟基和羰基减少,羧基增加. GO暴露于还原性化学物质或明亮光线下时,会转变为RGO[36]. Bortolozzo等[37]研究了GO(片径:156.8 nm±83.3 nm,厚度:1.3 nm)被次氯酸钠(NaClO)化学降解后,对秀丽隐杆线虫暴露24 h的毒性影响. 结果表明,NaClO氧化后的GO(NaClO-GO,片径:29.3 nm±5.2 nm,厚度:1.3 nm)毒性显著减轻,表现为1 mg·L−1GO导致线虫的存活率下降了60%,而相同浓度的NaClO-GO对线虫无影响,即便浓度超过50 mg·L−1时,线虫存活率仍高于80%,这一变化归因于NaClO-GO羧基官能团数量增加,尺寸减小.
光转化是自然界一种很重要的转化形式. 在自然光照射下,GOs中的π键可以吸收太阳光谱中的光. Zhang等[38]研究了新鲜的GO水分散体(片径:5 nm—1.5μm,厚度:1.5 nm,C/O质量比:1.57)暴露在空气中2年发生的变化. 结果表明,2年后GO从具有光滑线性边缘的大块薄片变成具有不均匀的分形结构的薄膜,这是由于GO的供电子活性组分(醌基团)促进O2转化为·O2−,随后转化为H2O2,最终形成·OH,GO的芳香环和发色团结构被·OH破坏,结构变得不稳定. 值得注意的是,在无光环境下,·OH的生成速率仅为光照时的一半,表明光照促进了反应的进行. 类似地,Chowdhury等[39]发现天然水环境中的硝酸盐和NOM在光照时也会产生·OH,·OH破坏GO(片径:100—800 nm,C/O质量比:1.72)结构,诱导GO的O/C含量的比值下降,GO分裂成更小的碎片. 自然环境中光转化会改变GOs的物化性质,进而引起了GOs的毒性变化. Wang等[40]研究了光驱动下GO(片径:5—8 μm,厚度:1 nm)对DNA分子(1 μmol·L−1)的损伤机制. 结果表明,在光照条件下,GO(0.01 mg·mL−1)对DNA链具有很强的切割能力,这是由于在GO经紫外光照射后,生成了单线态氧(1O2)和超氧阴离子(O2−),两者具有高度的氧化性,能够与DNA分子发生反应,最终导致DNA链的断裂或损伤.
-
生物转化包括生物降解和生物修饰. 在自然环境中,生物降解为主要的生物转化途径,生物降解是指细菌和真菌等微生物分解化合物为简单物质的过程[41]. Liu等[42]通过为期14 d的研究,探讨了萘降解菌对GO(厚度:1—1.5 nm,氧摩尔含量:34.1%)的降解能力. 结果表明,在初期阶段,GO的部分区域转化为可溶性碎片或二氧化碳,这些碎片不断脱落,缺陷区域逐渐扩展成孔洞. 由于细菌细胞尺寸的限制,形成的孔洞主要集中于亚微米级(<1 μm)范围内. 随着降解过程的持续进行,氧化作用进一步加剧,GO官能团数量显著减少并且碳骨架结构受到破坏,大块GO逐渐裂解成更小的片段. 在降解的过程中,随着GO表面官能团数量的减少,GO的毒性会减弱.
-
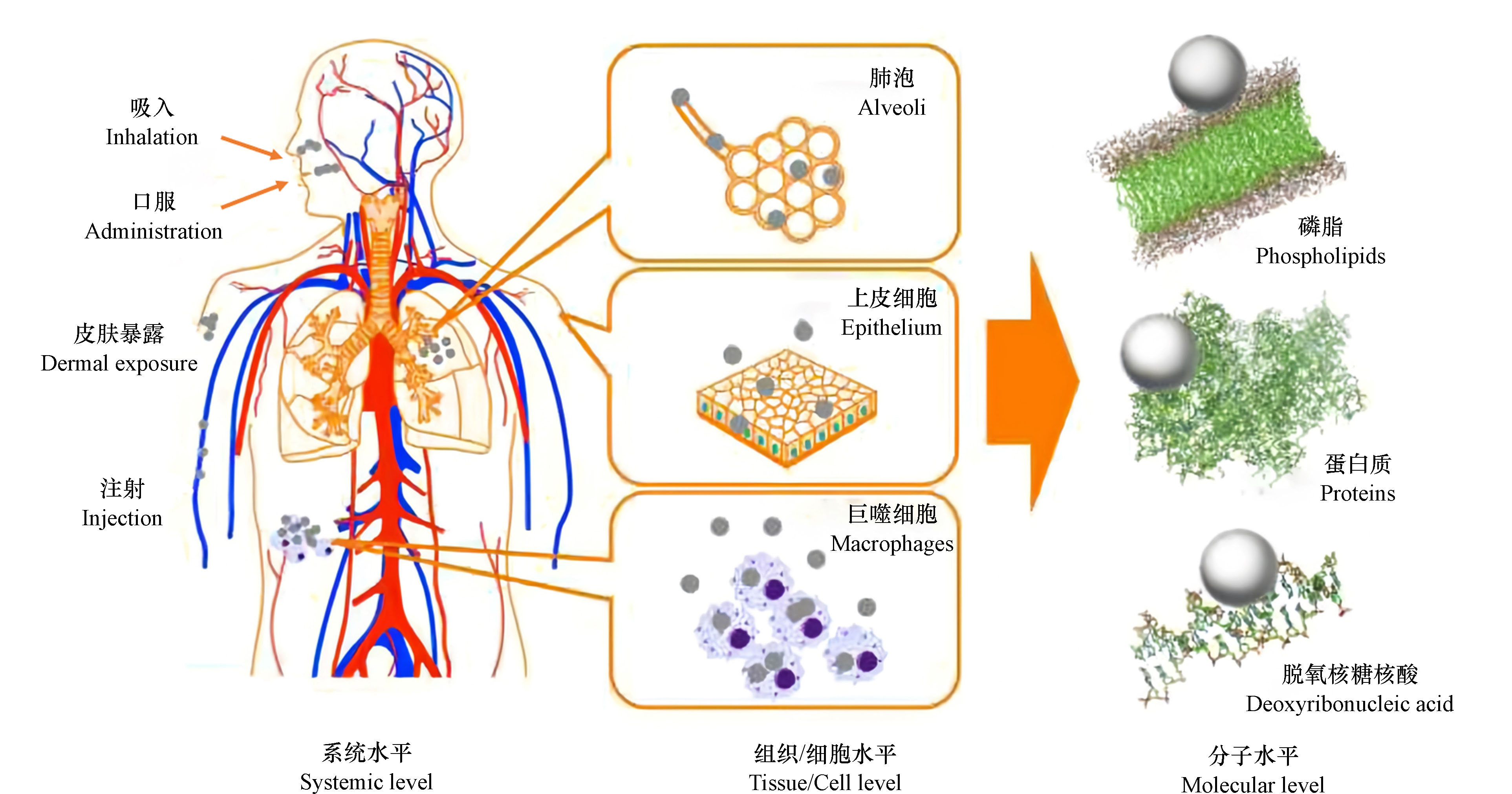
GOs能够通过呼吸系统、皮肤接触、食物摄取及药物治疗等途径进入生物体,它们不仅可以穿过细胞膜进入生物体的任何器官(如肺、肝、肾、脾、心、脑等),还可以在细胞、亚细胞、遗传和蛋白质水平上干扰正常的生命活动,从而对环境和生物体健康构成潜在风险(图2)[43 − 44]. 如果GOs在生产或使用过程中暴露途径不同,那么其在进入生物体后的迁移和分布也会有所差异,影响GOs生物分布的主要因素包括GOs的种类、纯度、尺寸、表面修饰、聚集状态以及暴露途径等[45 − 47].
-
GOs可以通过诱发炎症、肉芽肿、纤维化及过敏性免疫反应对呼吸系统、心血管系统和免疫系统等的正常生理功能造成负面效应. GOs的毒性主要表现为肺毒性[48 − 49]、肝毒性[50 − 51]、肾毒性[52 − 53]、皮肤毒性[54 − 55]、心血管毒性[56 − 57]、神经毒性[58 − 59]、生殖与发育毒性[60 − 61]和免疫毒性[62 − 63].
-
肺部作为呼吸系统的主要器官,负责气体交换,是维持生命活动不可或缺的关键部分,GOs主要通过吸入、口服等途径进入肺部. Li等[64]采用气管内滴注的方法研究GO(片径:308.4 nm±10.4 nm,厚度:1 nm)在C57BL/6小鼠体内长达3个月的生物分布和肺毒性. 结果表明,10 mg·kg−1体重GO刚注入时弥散性良好并黏附在在支气管和肺泡壁的内表面,然后GO逐渐开始聚集,最后缓慢从肺部移除. 在这个过程中,GO对肺产生过度炎症反应,肺细胞损伤,进而导致肺泡-毛细血管完整性的破坏,最终诱导慢性肺病变,特征为弥漫性肺纤维化. Loret等[65]采用单次口咽抽吸30 μg GOs观察28 d内不同尺寸的GOs对小鼠肺部影响的差异. 结果表明,SGO(片径:10—700 nm,厚度:1—2 nm,C/O质量比:2.9)会导致中性粒细胞弥漫性浸润到肺部,肺泡壁上观察到肉芽肿状结构,而LGO(片径:1—25 μm,厚度:1—2 nm,C/O质量比:2.7)主要导致支气管和血管周围有中性粒细胞的局部浸润,气道壁上观察到肉芽肿状结构,这主要是由于LGO的大尺寸会阻碍它们从上气道运输到肺泡空间. Park等[66]探究了GO(片径:1—12 μm,厚度:2 nm,ID/IG:0.67)和十二烷基胺功能化GO(DA-GO)暴露24 h对人肺癌细胞(A549)的毒性差异. 结果表明,50 μg·mL−1 GO处理的细胞表现出毒性效应,如纺锤体缩短,轻度细胞形态异常(与对照组相比细胞形状略有不规则、细胞体积略有缩小)和细胞聚集现象,但90%细胞仍然存活,而DA-GO处理的细胞高达60%死亡,变成细胞碎片,存活的少数细胞表现出异常的细胞形态,如延长的丝状足,这表明DA-GO表面修饰上的DA显著增强了其细胞毒性,导致了更高的细胞死亡率. 总之,GOs进入肺部后可能引发细胞损伤、炎症反应及纤维化等病理变化,但GOs的肺部毒性是一个复杂且多维度的过程,涉及GOs的尺寸、表面化学特性、暴露方式及时间等多个因素.
-
肝脏作为主要的代谢器官,负责解毒、蛋白质合成、储存糖分和脂肪等重要生理功能,GOs主要通过吸入、口服、注射等途径进入肝脏. Ma等[68]采用投喂含GO(ID/IG:0.91,含氧基团:C—OH、C—O—C和C=O等)的饲料的方式对罗非鱼暴露30 d. 结果表明,共摄入4 mg GO的罗非鱼的肝脏细胞中的基因表达(GST、GPX、FAS、G6、G6PD、GK和PDK-2)与对照组相比显著下调. Xiong等[69]采用向6个月大的成年斑马鱼腹腔注射的方式暴露GO(纯度:>99%,片径:90—290 nm)72 h,他们发现1 mg·L−1 GO会导致肝细胞数量减少,进而使得斑马鱼肝面积显著减小. 此外,GO还会诱导巨噬细胞和中性粒细胞的数量减少,促炎细胞因子(白细胞介素IL-1和IL-6)的表达增加. GO主要通过活性氧(ROS)和过氧化物酶体增殖物激活受体α(PPARα)介导的斑马鱼先天免疫信号通路诱导肝功能障碍. Yang等[70]通过口服或注射超小尺寸聚乙二醇化GO(nGO-PEG,片径:25 nm,厚度:1.22 nm)、聚乙二醇化还原GO(RGO-PEG,片径:50 nm,厚度:4.43 nm)和超小尺寸聚乙二醇化还原GO(nRGO-PEG,片径:27 nm,厚度:5.66 nm)的方式对雌性小鼠连续暴露7 d来探究GOs在小鼠体内的分布. 结果表明,通过注射方式连续暴露GOs(4 mg·kg−1体重)7 d后,在小鼠网状内皮系统(RES,如脾脏、肝脏等)中都发现了3种GOs的大量累积,并且小鼠脾脏和肝脏由深粉红色变为黑色,但通过口服方式并未观察到这些现象. 此外,RES对RGO-PEG的吸收量是其它两种材料的2倍,表明GOs尺寸是影响RES吸收的重要因素. 综上所述,GOs对肝脏的毒性作用是多方面的,包括基因表达的下调、细胞数量的减少、免疫功能的紊乱以及氧化应激的诱导等. 这些毒性效应不仅与GOs的物理化学性质(如尺寸、表面修饰)密切相关,还受到暴露方式、剂量和时间等因素的影响.
-
肾脏负责过滤血液,排除废物和多余水分,维持体内平衡,GOs主要通过口服、注射等途径进入肾脏. Patlolla等[71]采用喂食针给药的方式连续5 d对健康成年雄性大鼠暴露GO(片径:40 nm,在去离子水中的zeta电位:-33.2 mV). 结果表明,与对照组相比,GO(10 mg·kg−1体重)显著提高了大鼠肾脏中的抗氧化酶(如超氧化物歧化酶、过氧化氢酶和谷胱甘肽过氧化物酶)的活性并呈剂量依赖性,脂质过氧化水平升高,肾脏发生明显的形态学变化. Gurunathan等[72]研究了GO(在超纯水和DMEM培养基中的片径分别为:50 nm和90 nm,zeta电位分别为:-28.3 mV和-15 mV)对人胚胎肾细胞(HEK293)的影响. 结果表明,GO(10 μg·mL−1)显著且呈剂量依赖性地抑制细胞活力与增殖,伴随线粒体膜电位下降和ATP生成量减少. 此外,GO暴露导致DNA损伤、半胱天冬酶3活性增加,同时调控了与细胞凋亡密切相关的通路,如雌激素/IL-7/结肠直肠癌信号通路. Monroy-Torres等[73]研究了不同镧系元素修饰的GO(纯度:>99%,片径:300—800 nm,厚度:0.7—1.2 nm,氧含量45%—55%wt)对猴子肾细胞(COS-7)的毒性. 结果表明,暴露不同功能化GO(100 μg·mL−1)都会产生细胞毒性,表现为GOs对细胞膜产生物理损伤,GOs在细胞质中积累后引起细胞骨架不稳定、核膜和线粒体损伤等. 相比较而言,未修饰GO的毒性最大,镧系元素修饰后能显著降低GO的细胞毒性,细胞毒性顺序为:GO > GO + La > GO + Ho > GO + Eu > GO + Tb > GO + Gb > GO + Ce. 简言之,GOs诱导肾细胞损伤并呈剂量依赖性、肾脏形态学改变,最终导致肾功能障碍,且这种毒性作用还涉及氧化应激和炎症反应等机制的参与.
-
皮肤是生物体抵御外界侵害的第一道天然屏障. Pelin等[74]研究了不同尺寸和氧化程度的GO(GO1、GO2和GO3的片径分别为:622、845和979 nm,氧含量分别为:42.7、48.84和52.23%wt)对人类皮肤HaCaT角质形成细胞的影响. 结果表明,3种GOs(30 μg·mL−1)对细胞增殖没有影响,但在100 μg·mL−1浓度下暴露72 h后,观察到细胞增殖略有减少,尤其是大尺寸高氧化的GO3毒性最大,导致细胞增殖减少22%. 这表明只有高浓度和长时间暴露条件下,GOs才对细胞有损害. Xu等[75]研究了72 h内银修饰的RGO(Ag@rGO,片径:≤40 nm)对大鼠背部皮肤的影响. 结果显示,0.5 mL的Ag@rGO(100 μg·mL−1)未对皮肤产生明显刺激. Wu等[76]采用体内和体外实验评估GO(片径:120 nm,厚度:<1.2 nm,C/O质量比:2.4)对眼睛的损伤,他们发现GO(12.5 mg·mL−1)短期暴露(24 h)对人原代角膜上皮细胞(hCorECs)和人结膜上皮细胞(hConECs)产生了显著的细胞毒性,并产生氧化应激. 他们还对Sprague-Dawley大鼠采用滴入的方式进行了为期5 d的重复GO暴露(50 μg·mL−1),发现GO导致了大鼠可逆的轻度角膜混浊、结膜充血和角膜上皮损伤. 综上所述,尽管某些研究显示GOs在低浓度和短期暴露下对皮肤的直接毒性作用有限,但长期或高浓度暴露仍会引发细胞损伤,具体毒性效应还需进一步深入研究确认.
-
心血管系统作为血液循环的动力源泉,其健康状态直接影响全身各器官功能. Bangeppagari等[77]用不同浓度的GO分散液(片径:5.9—32.9 μm,氧含量:41%—50%wt)处理斑马鱼胚胎5 d,他们发现暴露低浓度GO(0.1—0.3 mg·mL−1)对胚胎发育无影响,但高浓度GO(0.4—1 mg·mL−1)的暴露严重影响早期心血管发育. 间充质血管(ISV)的发育是一个出芽血管生成的过程,GO会诱导ISV的异常生长(如ISV的不规则分支、延伸不完全和不对称定位等),胚胎的窦静脉(SV)与球动脉(BA)距离受到显著影响,进而导致心脏环化畸形. Xing等[78]研究了GO(厚度:1.3 nm,ID/IG:0.93)和还原氧化石墨烯(RGO,厚度:1.3 nm,ID/IG:0.95)对鲫鱼(Catlacatla)心脏细胞系(SICH)的影响,在氢氧化铵保持溶液pH12条件下,GO被悬铃木叶提取物(含多酚)还原成RGO. 结果表明,3 μg·mL−1 GO对SICH细胞系显示毒性效应,相比之下,同浓度的RGO因表面覆盖多酚而无显著影响. 此外,心血管系统细胞包括心肌细胞、内皮细胞及成纤维细胞等,GOs可诱导这些细胞损伤,干扰早期心血管和心脏发育,加速动脉硬化进程,并可能最终导致心梗.
-
神经在生物体内起着传递信息、调控生理活动的重要作用,GOs通过吸入、口服、皮肤接触等途径进入神经系统. Hu等[79]对亲代父母斑马鱼进行了GO(片径:0.55 mm,厚度:1.02 nm±0.15 nm,C/O质量比:2.18)的暴露实验,同时也观察GO对后代斑马鱼的影响. 结果表明,在亲代和后代鱼的大脑中都检测到了GO(0.01 mg·L−1)的存在,GO会导致claudin5a(神经上皮屏障系统的核心成分)显著减少. GO仅在后代中表现出显著的神经毒性,表现为多巴胺能神经元结构及功能的损害和乙酰胆碱酯酶活性的降低. 此外,后代还展现出内质网损伤、自噬促进、泛素化下调和β-半乳糖苷酶活性的升高等生物学变化. Kim等[80]采用往生长培养基中添加GO(片径:40 nm,厚度:6 nm,C/O原子数目比:2.57)的方式对秀丽隐杆线虫进行24 h暴露实验. 结果表明,GO(5 mg·L−1)在秀丽隐杆线虫头部累积,导致多巴胺能和谷氨酸能神经元的神经递质含量显著降低,并损伤化学感觉神经元(AFD),进而引发了秀丽隐杆线虫的运动行为的异常变化. Ye等[81]研究GO(片径:0.1—2 μm,厚度:1.1 nm,在DMEM培养基中zeta电位:−18.5 mV)、聚乙二醇功能化GO(GO-PEG,片径:0.1—2 μm,厚度:5.8 nm,在DMEM培养基中zeta电位:−6.4 mV)和聚乙烯亚胺功能化GO(GO-PEI,片径:0.1—2 μm,厚度:11.2 nm,在DMEM培养基中zeta电位:+34.2 mV)对小鼠小胶质细胞(BV-2,一种中枢神经系统中的免疫细胞)的毒性. 结果表明,相同浓度(50 μg·mL−1)下,BV-2细胞对GO-PEI摄取量最大. GO-PEI因其正电荷特性,诱导线粒体断裂,进一步导致线粒体功能障碍,最终诱导细胞自噬和凋亡,而GO-PEG和GO处理组未观察到此类显著影响. GO-PEI一方面促进细胞内肿瘤坏死因子-α(TNF-α)和一氧化氮(NO)的过量产生,另一方面诱导线粒体动力蛋白相关蛋白(Drp1)的募集增加,同时促进细胞中微管相关蛋白轻链1(LC3-I)向LC3-II的转化增加和细胞自噬受体蛋白(p62)的降解. 综上,GOs展现出显著的神经毒性,包括诱导神经细胞损伤、扰乱细胞周期与凋亡过程、破坏神经元结构,间接地影响神经递质含量,最终导致神经系统功能障碍.
-
生殖过程涉及生殖细胞的产生、受精和胚胎发育,发育是指从受精卵到成熟个体的整个过程. Dziewięcka等[82]采用长期往食物饲料中添加GO(片径:0.5—5 μm,厚度:1.1 nm±0.2 nm,C/O原子数目比:2.5)的方式对家蟋蟀进行暴露实验,每克饲料中含200 μg的GO. 结果表明,GO暴露导致家蟋蟀生殖功能障碍,同时破坏其肠道细胞结构,进而引发跨代效应,表现为后代细胞活性显著降低. Zhang等[83]采用往水体中添加GO(纯度:>99%,片径:0.3—2.6 μm,厚度:1.01 nm±0.05 nm)的方式对斑马鱼胚胎暴露7 d(从刚受精后开始计算). 结果表明,48 h内低浓度GO(0.001—0.1 mg·L−1)对胚胎孵化无显著影响,当浓度提升至1—100 mg·L−1时,胚胎孵化显著延迟,且心率呈双向变化:1 mg·L−1时心率显著降低,100 mg·L−1时心率反而升高. GO暴露96 h后引起明显的畸形(躯干弯曲、尾部畸形、心包水肿、卵黄囊水肿、颅面畸形和体长缩短),且畸形率随剂量增加而显著上升. 类似地,River等[84]研究了GO(片径:124 nm±26 nm,氮原子含量:0.59%±0.03%)和氨基功能化GO(GO-NH2,片径:124 nm±26 nm,氮原子含量:3.2%±0.2%)对秀丽隐杆线虫发育的影响. 结果表明,GO(100 μg·mL−1)急性处理(12 h)对线虫无显著影响,但长期(72 h)暴露显著抑制线虫的生长与繁殖,而GO-NH2无此效应. 此现象归因于线虫细胞富含胆固醇,GO通过氧化胆固醇、扰乱细胞质膜脂质构成,进而损伤线虫生殖细胞、卵母细胞、胚胎,咽部以及消化结构(咽、肠道). 总的来说,GOs具有显著的亲代与子代毒性,并引发跨代毒性累积效应. 其毒性直接靶向生殖系统,导致生殖功能障碍,严重损害生殖细胞及卵母细胞的健康,进而诱发胚胎受损与发育畸形.
-
免疫系统作为机体的防御屏障,其稳定性对于维持健康至关重要. Yang等[85]研究了单层GO(mono-GO,片径:
1554.00 nm±543.34 nm)和多层GO(multi-GO,片径:123.48 nm±47.95 nm)暴露24 h对小鼠树突状细胞系(DC2.4)的影响. 结果表明,mono-GO(100 μg·mL−1)和multi-GO(0.01 μg·mL−1)均能诱导ROS产生,并差异调控多种免疫相关基因(H2-DMb1,Ncbp3,Oas2,Men1,Fas,Cd320,Cd244和Tinagl1)表达,促进肿瘤坏死因子(TNF-α)释放并呈剂量依赖性. 相较于multi-GO,mono-GO对细胞活力影响较小,但能显著改变细胞形态. 此外,multi-GO在脂多糖(LPS)刺激下促进促炎细胞因子(IL-6)释放,而mono-GO则抑制IL-6产生. Chen等[86]利用GO(片径:>1.5 μm)处理斑马鱼14 d,发现1 mg·L−1GO显著促进脾脏中促炎细胞因子(IL-1β和IL-6)的表达,导致斑马鱼体内ROS的过量生成,从而触发炎症反应. Sun等[87]研究了聚赖氨酸功能化GO(GO-PLL,片径:数百nm到几μm之间,厚度:3.6 nm)和聚赖氨酸功能化单层GO(sGO-PLL,片径:<50 nm,厚度:10 nm)负载免疫刺激性CpG寡脱氧核苷酸后对小鼠巨噬细胞Raw264.7的影响. 结果表明,相同浓度下(12.5 μg·mL−1),与GO-PLL-CpG相比,sGO-PLL-CpG具有更好的生物相容性、更高的细胞摄取效率和更高的免疫刺激活性(刺激了肿瘤坏死因子TNF-α和促炎细胞因子IL-6的分泌). 综上,GOs诱导氧化应激,进而触发免疫反应,导致多种免疫相关基因的差异表达. 这一系列反应促进了促炎细胞因子的释放,最终加剧炎症反应. 这个过程中GOs的毒性作用受表面功能化、结构等因素的影响. -
除了对动物和人体健康的潜在威胁外,GOs对生态环境中的植物也表现出一定的毒性作用. Wu等[88]选用50 mg·L−1 GO(纯度:>95%,片径:0.42—0.85 μm,厚度:1.99—2.66 nm,氧含量:40%—50%wt)处理7 d,发现该处理显著降低水稻营养液的pH值,GO诱导水稻氧化应激,伴随细胞膜损伤及丙二醛、一氧化氮和苯丙氨酸解氨酶活性上升,最终对植株生长造成不利影响,具体表现为鲜重降低和木质素含量变化. Nogueira等[89]研究了绿藻(Raphidocelissubcapitata)在含GO(片径:120—200 nm,厚度:3.5 nm,在超纯水中的zeta电位:-46 mV)环境中暴露96 h的
变化. 结果表明,20 μg·mL−1GO可导致绿藻生长抑制率达50%,这归因于GO既降低叶绿素活性,又通过黏附在细胞表面降低了藻类细胞的光捕获效率. Malina等[90]评估了不同氧化程度的GOs(HO-GO、HU-GO和TO-GO的片径均为:3 mm,厚度均为:1 nm,C/O原子数目比分别为:2.6、1.95和1.72)在0.39 μg·mL−1浓度下暴露48 h对绿藻(Raphidocelissubcapitata)和蓝藻(Synechococcus elongatus)的毒性影响. 结果表明,相比于绿藻,GOs对蓝藻表现出更高的细胞毒性和更快的生长抑制,并且氧化程度较低的HO-GO更易引发藻类显著的机械损伤. Zhao等[91]研究了GO(纯度:>99%,片径:500—
5000 nm)和氧化石墨烯量子点(GOQD,片径:<15 nm)对铜绿微囊藻(Microcystis aeruginosa)的联合毒性效应. 结果表明,在1—10 mg·L−1浓度区间,GOQD引发毒物兴奋效应,显著提升活性氧(ROS)和超氧化物歧化酶(SOD)水平,同时维持细胞低通透性和线粒体健康,促进藻类生长. 相反,GO诱导氧化应激,抑制藻类生长,两者联合表现为拮抗作用;当浓度超过10 mg·L−1时,GOQDs和GO均剂量依赖性地诱导了氧化应激,导致细胞损伤,两者联合表现为协同作用. 总之,水稻与藻类作为探索GOs植物毒性的首选模型,其广泛应用揭示了GOs对植物生长抑制、机械损伤、氧化应激及代谢异常等多方面的影响,且不同植物种类对GOs的毒性反应程度各异.当前,GOs对人类健康构成的主要风险,主要聚焦于工业及小规模生产或废弃物处理过程中的职业暴露,这些暴露途径主要包括吸入、摄入、皮肤及眼睛直接接触. Vaquero等[92]于一座日产量达千克级生产GOs的工厂内发现在氧化和洗涤的过程中,环境中单质碳平均浓度为0.002 mg·m−3. Liu等[93]评估了一家生产石墨烯及其衍生物(如GOs)纳米材料、年产量2000吨的中国企业中,54名长期接触纳米材料的工人的体检报告,发现职业健康体检异常15例,异常率高达27.8%,其中右心功能异常7例,主要表现为窦性心率不齐;肺功能异常8例,主要表现为肺纹理增多、肺野条索影. 此外,随着年龄和工龄的增长,心功能及肺功能异常率有升高趋势. Andrews等[94]研究了吸入不同尺寸GOs后对人体的影响,GOs分别为:小尺寸GO(S-GO,片径:108—
1646 nm,厚度:1—2 nm)、超小尺寸GO(US-GO,片径:33—479 nm,厚度:1—2 nm)、不含金属等杂质的高纯GOs. 结果显示,14名健康年轻志愿者持续吸入200 μg·m−3 S-GO或US-GO 2 h后,其心率、血压、肺功能和炎症指标均无明显异常,但其血浆蛋白中的可溶性耐药相关钙结合蛋白(sorcin)的蛋白表达量显著上升,这表明暴露于GOs增加了人类患神经退行性疾病和心血管疾病的风险. 综上所述,尽管实验室研究在特定条件下揭示了GOs的轻微毒性表现,但工业环境中GOs的复杂暴露情境,尤其是伴随的重金属等杂质污染,以及工人长期持续接触的特点,共同构成了更为严峻的健康风险. 因此,对GOs生产及处理过程中的暴露风险进行持续、系统的监测显得尤为重要. -
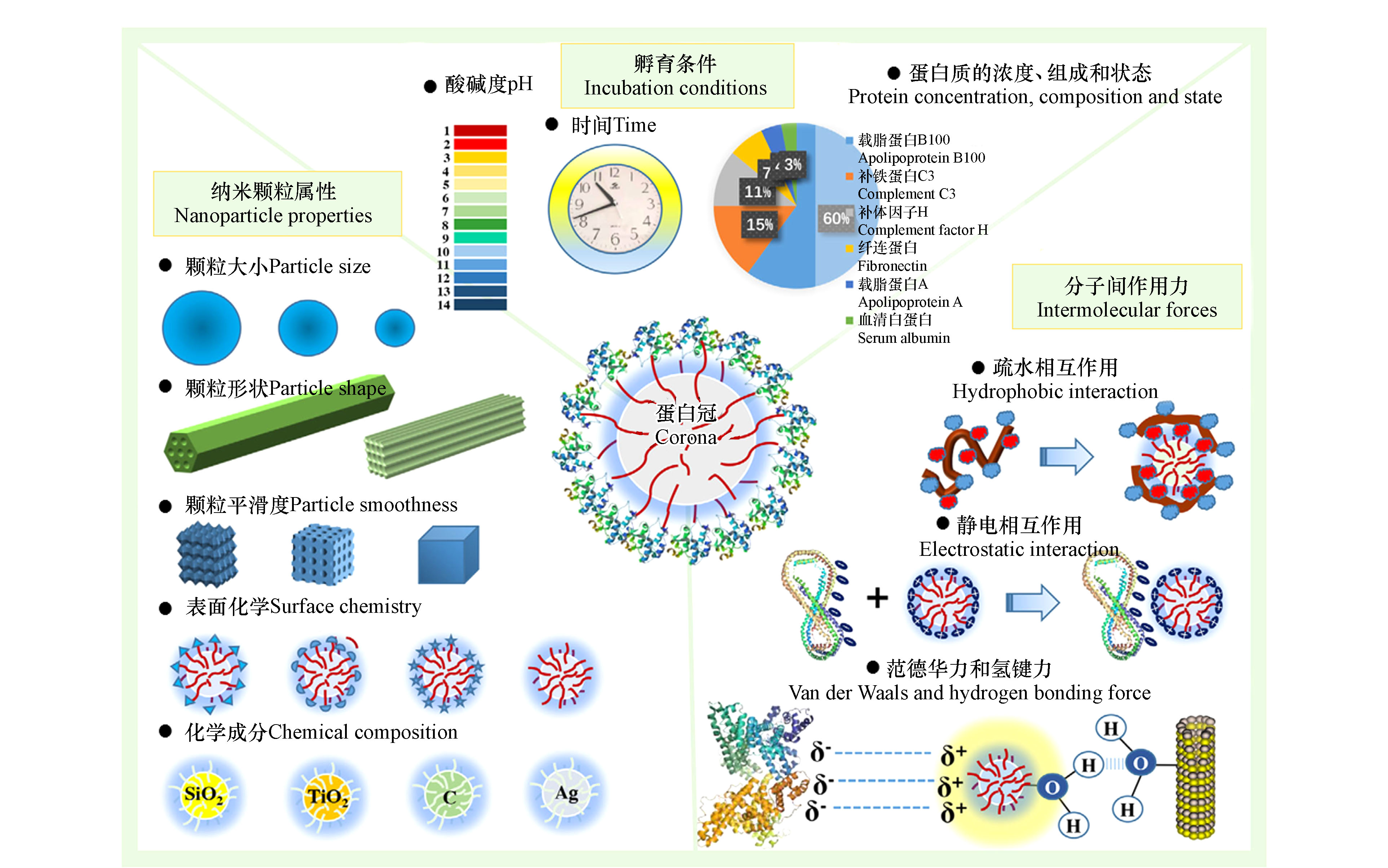
当GOs进入生物体后,蛋白质会吸附在GOs表面形成蛋白冠,蛋白冠是动态变化的[95]. 蛋白冠能够改变GOs的各种理化性质,如尺寸[96]、表面电荷[97]和表面组成成分[98]等,并赋予它们一种新的生物特性(图3),进而调节GOs的生物学行为,包括细胞摄取、分布、毒性.
-
GOs的细胞摄入既可通过能量驱动的内吞作用主动进行,也能通过直接穿透细胞膜的方式被动完成[100],蛋白冠在GOs与细胞的相互作用和细胞摄入过程中起着主导作用,进而决定GOs在生物体内的行为[101 − 102]. Duan等[103]研究了胎牛血清(FBS)蛋白包裹的GO与裸露GO(纯度:>99%,片径:0.5—3 μm,厚度:0.55—1.2 nm)对人肺癌细胞(A549)的影响差异. 结果表明,相较于裸露GO(50 μg·mL−1),被FBS(含量1%)包裹的GO显著降低了细胞毒性,这是由于蛋白冠的形成有效减少了GO的细胞摄取量. Dabrowski等[104]更进一步地研究了与血清孵育前后的GO(片径:200—800 nm,在超纯水中的zeta电位为-38.45 mV)对正常成纤维细胞(LL-24)和人肺癌细胞(A549)的影响. 结果表明,在血清中孵育后的GO发生了结构变化(sp2碳晶格的降解),相比于裸露GO(50 μg·mL−1),孵育后的GO提高了细胞活性,减少了在细胞内的积累量. 此外,A549细胞相比于LL-24细胞,对GO毒性更敏感. Alnasser等[105]利用流式细胞术研究了血清孵育后的石墨烯(片径:100—300 nm)与人胚胎肾细胞(HEK-293T)的相互作用. 结果表明,石墨烯(50 μg·mL−1)表面形成的蛋白冠中富含载脂蛋白A-I,石墨烯上的A-I蛋白冠与一种重要的膜蛋白受体——清道夫受体(SR-B1)之间存在显著的相互作用. 特别地,在SR-B1受体高表达的细胞中,这种相互作用更为强烈,揭示了石墨烯表面关键蛋白质识别基元能选择性地与特定细胞受体结合的特性. 类似地,Xu等[106]研究了GO的表面功能化对蛋白质的吸附量及网状内皮系统(RES)吞噬细胞摄取量的影响,GOs分别为:GO(片径:201 nm±9 nm,厚度:1.1 nm±0.1 nm)、氨基化GO(GO-NH2,片径:251 nm±1 nm,厚度:1.2 nm±0.2 nm)、聚丙烯酰胺功能化GO(GO-PAM,片径:363 nm±4 nm,厚度:2.9 nm±0.2 nm)、聚丙烯酸功能化GO(GO-PAA,片径:269 nm±7 nm,厚度:2.6 nm±0.2 nm)和聚乙二醇功能化GO(GO-PEG,片径:272 nm±11 nm,厚度:4.1 nm±0.3 nm). 结果表明,在含FBS的DMEM培养基中,GOs(4 μg·mL−1)的吸附量因其表面电荷而异,顺序为:GO > GO-PAM > GO-NH2> GO-PAA > GO-PEG. GOs上特定吸附蛋白(如免疫球蛋白IgG等)与吞噬细胞膜受体相互作用,促进GOs的内吞,摄取量顺序为:GO > GO-PAM > GO-PEG > GO-NH2 ≈GO-PAA. GO-NH2、GO-PAM和GO显著损伤细胞膜(如细胞明显塌陷、细胞体伸长等),而GO-PEG和GO-PAA则对细胞膜影响甚微. 总之,GOs形成蛋白冠后,其物理化学特性显著变化,直接影响细胞对GOs的摄取效率. 特定蛋白在GOs表面上选择性吸附后,通过与细胞膜受体的相互作用,促进了细胞对蛋白冠/GOs复合物的特异性识别与摄取. 此外,GO的表面功能化可有效调控蛋白冠的组成与性质,进而精细调节细胞摄取行为与毒性效应.
-
纳米毒理学评价不仅要考虑纳米材料的内在特性[107],还要考虑外在因素[108 − 109]. Côa等[110]研究牛血清白蛋白(BSA)包裹对GO(GO孵育前后厚度分别为:1.0 nm±0.1 nm和4.0 nm±0.33 nm,C/O原子数目比分别为:2.05和3.12)和多壁碳纳米管(MWCNT,直径:10—40 nm、长度:1—25 μm,孵育前后MWCNT的C/O原子数目比分别为:9.31和5.49)在秀丽隐杆线虫体内生物分布及毒性影响. 结果表明,裸露GO(0.1 mg·L−1)和裸露MWCNT(0.01 mg·L−1)均对线虫存活率呈剂量依赖性降低,并且GO的毒性在相同浓度下更大,BSA蛋白冠的存在减轻了GO和MWCNT对线虫存活率的影响. 进一步分析显示,GO和BSA-GO均被线虫内化,并广泛分布于其头部、肠道、性腺、卵等多个关键组织,相比之下,MWCNT虽也广泛分布于线虫体内,但在卵中未被检测到. 此外,BSA-MWCNT的内化主要局限于肠道、性腺,这凸显出BSA蛋白冠对MWCNT体内分布具有调控作用. BSA-MWCNT在秀丽隐杆线虫恢复进食2 h后被排出体外,而BSA-GO则持续积累在线虫肠道中,表明两者在生物体内的滞留特性存在差异. 综上,GOs在形成蛋白冠前后,其生物分布特性发生了深刻变化,这源于GOs表面物理化学性质的变化. 此外,蛋白冠的形成影响了GOs在组织中的积累模式,特别是在性腺、肠道等器官中的富集,也延长了其在生物体内的循环时间.
-
蛋白冠的形成不仅影响GOs的细胞摄取和生物分布,还影响它们的毒性[111]. Hajipour等[112]研究了GO(厚度:0.8 nm,O/C含量比:0.48)吸附不同疾病类型患者的血浆蛋白后,对人乳腺癌细胞MCF-7产生的细胞毒性变化. 结果表明,尽管蛋白冠的形成一定程度上GO减少对细胞膜的物理损伤,降低了GO(100 μg·mL−1)的细胞毒性,但疾病特异性的蛋白冠/GO复合物表现出显著的毒性差异,表现为:来自地中海贫血、妊娠、糖尿病和风湿病患者的GO刺激细胞产生较多的活性氧(ROS);来自风湿病和低纤维蛋白原血症的GO诱导细胞产生较高的脂质过氧化水平;来自高胆固醇血症和风湿病患者的GO主要诱导细胞凋亡,而来自血癌、低纤维蛋白原血症、糖尿病和蚕豆病的GO主要引发细胞坏死. Yang等[113]研究了GO(片径:100—500 nm,厚度:1.2 nm)在不同浓度FBS的培养基中孵育后暴露24 h对A549细胞的毒性的影响. 结果表明,在FBS(含量0.5%)的培养基孵育后的GO(50 μg·mL−1)毒性减弱,并且细胞毒性的减弱程度与FBS含量呈正相关. 蛋白冠通过干扰GO的表面物化性质(如尺寸、电荷等),有效降低了GO的内吞效率,进而减轻了由GO诱导的线粒体膜电位(MMP)功能障碍、细胞凋亡和细胞周期异常. Cui等[114]研究了裸露GO(片径:200 nm,在超纯水中zeta电位:−40 mV)及其经血浆孵育后对人乳腺癌细胞(SK-BR-3)的毒性效应,SK-BR-3细胞内与细胞生长和增殖有关的人表皮生长因子受体2(HER-2)过度表达. 研究表明,GO(125μg·mL−1)暴露后HER-2表达量显著减少,这与蛋白激酶B(AKT)和细胞外信号调节激酶(ERK)的表达和激活受到明显抑制有关,而血浆孵育后的GO显著减轻这一影响,蛋白冠的形成有效减少了细胞对GO的摄取,使HER-2表达和下游信号通路恢复到正常水平. Coreas等[115]研究了在含FBS的DMEM培养基中,RGO(片径:85—90 nm,厚度:1 nm)和浓缩RGO(c-RGO,片径:85—90 nm,厚度:1 nm)孵育前后对人乳腺癌细胞(MDA-MB-231)的毒性效应. 结果表明,相同浓度下(20 μg·mL−1),毒性排序为:c-RGO/蛋白冠复合物(pro-c-RGO)>c-RGO>RGO≈RGO/蛋白冠复合物(pro-RGO),这种差异归因于c-RGO相较于RGO具有更低的表面活性剂残留,导致其表面疏水区域暴露增加,增强了与细胞膜及血清蛋白的相互作用,进而提升了细胞对c-RGO及其蛋白冠复合物的摄取率,最终加剧了毒性效应. Rajasekar等[116]研究了GO(ID/IG:0.84)及其与牛血清白蛋白(BSA)复合物对大肠杆菌(Escherichia coli)的抑菌作用机制,GO通过其尖锐边缘直接破坏细菌膜,诱导氧化应激,最终导致细胞死亡. 结果表明,GO(25 μg·mL−1)及其与不同浓度BSA(1、2和4 mg·mL−1)形成的复合物产生的抑制圈直径分别为:9、13、16和17 mm,随着BSA浓度上升,BSA/GO复合物的抗菌效果显著增强,这归因于BSA蛋白冠增强了GO穿透细菌细胞膜的能力,进而提升其抗菌效能. 简言之,蛋白冠对GOs细胞毒性的影响具有双重性:一是蛋白冠通过减少GOs与细胞的直接物理接触来降低毒性;二是在特定条件下,蛋白冠能介导GOs靶向特定细胞或组织增强毒性. GOs毒性效应受蛋白冠成分(种类、数量)及GOs自身特性(如尺寸、表面修饰等)共同调控.
-
GOs是近年来研究最多的石墨烯衍生物,其在环境和医学等领域显示了良好的应用潜力,随着GOs大规模生产,其不可避免地释放到环境中并与生物体接触. 目前针对GOs环境转化、毒理学、蛋白冠等的研究方面仍然存在一些不足和挑战.
(1)当前对GOs在环境转化中的化学、物理及生物过程认知尚浅,实验室研究常受限于高浓度GOs及单一环境变量,难以准确反映复杂多变的自然环境,进而限制了低浓度GOs环境下预测建模的有效性和准确性. 此外,GOs作为污染物吸附剂可能引发的联合毒性亦需关注[117]. 基于此,未来研究应聚焦于深化环境转化机制,优化实验设计,采用更贴近实际环境的测试方法与技术.
(2)现有文献广泛指出,GOs对肺、肝、肾、皮肤、心血管神经、生殖与发育系统及免疫系统均展现出毒性. 其中,活性氧(ROS)生成、脂质过氧化及炎症反应等被认为是GOs诱导细胞毒性的主要机制,但其具体毒性路径尚未清晰界定. GOs的毒性深受其物理化学特性影响,然而,由于合成技术的多样性和复杂性,GOs产品在纯度和含氧官能团含量上差异显著,这极大地限制了不同研究中GOs毒性效应的可比性. 基于此,未来研究应聚焦于深入探索GOs的毒性机制,特别是其在不同生物系统中的具体作用路径. 同时,需要标准化GOs的合成与表征方法,以减少因材料差异带来的研究偏差,从而提高不同研究间毒性效应的可比性.
(3)当GOs与生物流体接触时,蛋白质会吸附在GOs表面形成蛋白冠,改变GOs的理化性质,进而影响到细胞摄取、生物分布、毒性,使GOs的安全性复杂化. 此外,不同类型的病人由于生理状态的差异,其体内蛋白质的表达会呈现出显著的个体化差异,这种差异导致了在GOs表面形成的蛋白冠具有高度的个性化特征[118]. 这种个性化的蛋白冠不仅增加了对GOs在生物体内行为理解的复杂性,还直接影响了其在药物传输、癌症治疗等医学应用中的有效性和安全性. 基于此,未来的研究应继续探索GOs理化性质、蛋白冠和生物学效应之间的关系以及个体间蛋白冠差异如何调控GOs的生物行为.
氧化石墨烯的环境转化与生物命运
The environmental transformation and biological fate of graphene oxide
-
摘要: 氧化石墨烯(graphene oxides,GOs)以独特的理化性能、高的比表面积和良好的生物相容性等特点被广泛应用于环境保护和生物医药等领域,随着GOs的不断生产和使用,其对环境和人类健康的潜在威胁也引起了科学家们的关注. 本文主要综述了GOs在环境中的转化机制、其暴露生物体的途径及诱导产生的毒性效应,并对复杂生物体液中蛋白冠介导的GOs生物命运(包括细胞摄取、生物分布及毒性)进行了概述,以期为探讨和评估GOs的环境行为、毒理学机制及安全性提供科学依据.Abstract: Graphene oxides (GOs) are widely used in environmental protection, biomedicine, and other fields due to their unique physicochemical properties, high specific surface area, and good biocompatibility. However, with the continuous production and use of GOs, their potential threats to the environment and human health have attracted considerable attentions. This article mainly reviews the environmental transformation mechanisms of GOs, exposure routes to living organisms, and the associated toxic effects. Additionally, it provides an overview of biological fate (including cellular uptake, biodistribution and toxicity) of GOs regulated by protein corona in complex biological fluids. The aim is to provide a scientific basis for exploring and evaluating the environmental behaviors, toxicological mechanisms, and safety of GOs.
-
Key words:
- graphene oxides /
- environmental transformation /
- toxicity /
- protein corona.
-

-
图 3 纳米颗粒表面蛋白冠的形成及影响因素[99]
Figure 3. The formation of protein corona on the surface of nanoparticles and influencing factors
-
[1] Novoselov K S, Geim A K, Morozov S V, et al. Electric field effect in atomically thin carbon films[J]. Science, 2004, 306(5696): 666-669. doi: 10.1126/science.1102896 [2] SHIN D S, KIM H G, AHN H S, et al. Distribution of oxygen functional groups of graphene oxide obtained from low-temperature atomic layer deposition of titanium oxide[J]. RSC Advances, 2017, 7(23): 13979-13984. doi: 10.1039/C7RA00114B [3] DREYER D R, PARK S, BIELAWSKI C W, et al. The chemistry of graphene oxide[J]. Chemical Society Reviews, 2010, 39(1): 228-240. doi: 10.1039/B917103G [4] KONKENA B, VASUDEVAN S. Understanding aqueous dispersibility of graphene oxide and reduced graphene oxide through pKa measurements[J]. The Journal of Physical Chemistry Letters, 2012, 3(7): 867-872. doi: 10.1021/jz300236w [5] KONKENA B, VASUDEVAN S. Covalently linked, water-dispersible, cyclodextrin: Reduced-graphene oxide sheets[J]. Langmuir, 2012, 28(34): 12432-12437. doi: 10.1021/la3020783 [6] KUMAR V, KUMAR A, LEE D J, et al. Estimation of number of graphene layers using different methods: A focused review[J]. Materials, 2021, 14(16): 4590. doi: 10.3390/ma14164590 [7] YANG K J, PAN T T, ZHAO Q, et al. Dual-function ultrafiltration membrane constructed from pure activated carbon particles via facile nanostructure reconstruction for high-efficient water purification[J]. Carbon, 2020, 168: 254-263. doi: 10.1016/j.carbon.2020.06.083 [8] CHEN C, ZHU X Y, CHEN B L. Durable superhydrophobic/superoleophilic graphene-based foam for high-efficiency oil spill cleanups and recovery[J]. Environmental Science & Technology, 2019, 53(3): 1509-1517. [9] AVORNYO A, CHRYSIKOPOULOS C V. Applications of graphene oxide (GO) in oily wastewater treatment: Recent developments, challenges, and opportunities[J]. Journal of Environmental Management, 2024, 353: 120178. doi: 10.1016/j.jenvman.2024.120178 [10] LI X H, LIU F, ZHANG W F, et al. Electrocatalytical oxidation of arsenite by reduced graphene oxide via in situ electrocatalytic generation of H2O2[J]. Environmental Pollution, 2019, 254: 112958. doi: 10.1016/j.envpol.2019.112958 [11] LIU R L, XU Y M, CHEN B L. Self-assembled nano-FeO(OH)/reduced graphene oxide aerogel as a reusable catalyst for photo-Fenton degradation of phenolic organics[J]. Environmental Science & Technology, 2018, 52(12): 7043-7053. [12] AKHAVAN O, GHADERI E. Toxicity of graphene and graphene oxide nanowalls against bacteria[J]. ACS Nano, 2010, 4(10): 5731-5736. doi: 10.1021/nn101390x [13] EGOROVA M N, TARASOVA L A, VASILIEVA F D, et al. Antimicrobial activity of graphene oxide sheets[C]//AIP Conference Proceedings, Kuala Lumpur, Malaysia, 2018: 020028-1. [14] FILIK H, AVAN A A. Review on applications of carbon nanomaterials for simultaneous electrochemical sensing of environmental contaminant dihydroxybenzene isomers[J]. Arabian Journal of Chemistry, 2020, 13(7): 6092-6105. [15] KUCINSKIS G, BAJARS G, KLEPERIS J. Graphene in lithium ion battery cathode materials: A review[J]. Journal of Power Sources, 2013, 240: 66-79. [16] NIZAMI M Z I, TAKASHIBA S, NISHINA Y. Graphene oxide: A new direction in dentistry[J]. Applied Materials Today, 2020, 19: 100576. [17] CAO W J, HE L, CAO W D, et al. Recent progress of graphene oxide as a potential vaccine carrier and adjuvant[J]. Acta Biomaterialia, 2020, 112: 14-28. doi: 10.1016/j.actbio.2020.06.009 [18] LIN J, CHEN X Y, HUANG P. Graphene-based nanomaterials for bioimaging[J]. Advanced Drug Delivery Reviews, 2016, 105: 242-254. doi: 10.1016/j.addr.2016.05.013 [19] KANG S, KIM K M, JUNG K, et al. Publisher correction: Graphene oxide quantum dots derived from coal for bioimaging: Facile and green approach[J]. Scientific Reports, 2020, 10(1): 7451. doi: 10.1038/s41598-020-60499-0 [20] WU J, LI Z F, LI Y, et al. Photothermal effects of reduced graphene oxide on pancreatic cancer[J]. Technology in Cancer Research & Treatment, 2018, 17: 1533034618768637. [21] LIU L J, MA Q M, CAO J, et al. Recent progress of graphene oxide-based multifunctional nanomaterials for cancer treatment[J]. Cancer Nanotechnology, 2021, 12(1): 18. doi: 10.1186/s12645-021-00087-7 [22] CONNOLLY M, MOLES G, CARNIEL F C, et al. Applicability of OECD TG 201, 202, 203 for the aquatic toxicity testing and assessment of 2D Graphene material nanoforms to meet regulatory needs[J]. NanoImpact, 2023, 29: 100447. doi: 10.1016/j.impact.2022.100447 [23] REN X M, LI J, CHEN C L, et al. Graphene analogues in aquatic environments and porous media: Dispersion, aggregation, deposition and transformation[J]. Environmental Science: Nano, 2018, 5(6): 1298-1340. doi: 10.1039/C7EN01258F [24] BHANUSHALI H, AMRUTKAR S, MESTRY S, et al. Shape memory polymer nanocomposite: A review on structure–property relationship[J]. Polymer Bulletin, 2022, 79(6): 3437-3493. doi: 10.1007/s00289-021-03686-x [25] CHOWDHURY I, DUCH M C, MANSUKHANI N D, et al. Colloidal properties and stability of graphene oxide nanomaterials in the aquatic environment[J]. Environmental Science & Technology, 2013, 47(12): 6288-6296. [26] HARRISON D M, BRIFFA S M, MAZZONELLO A, et al. A review of the aquatic environmental transformations of engineered nanomaterials[J]. Nanomaterials, 2023, 13(14): 2098. doi: 10.3390/nano13142098 [27] ZHAO Y C, LIU Y, ZHANG X B, et al. Environmental transformation of graphene oxide in the aquatic environment[J]. Chemosphere, 2021, 262: 127885. doi: 10.1016/j.chemosphere.2020.127885 [28] ALI J, LI Y, SHANG E X, et al. Aggregation of graphene oxide and its environmental implications in the aquatic environment[J]. Chinese Chemical Letters, 2023, 34(2): 107327. doi: 10.1016/j.cclet.2022.03.050 [29] ZOU Y D, WANG X X, AI Y J, et al. Coagulation behavior of graphene oxide on nanocrystallined Mg/Al layered double hydroxides: Batch experimental and theoretical calculation study[J]. Environmental Science & Technology, 2016, 50(7): 3658-3667. [30] SADR M K, CHERAGHI M, LORESTANI B, et al. Removal of fluorouracil from aqueous environment using magnetite graphene oxide modified with γ-cyclodextrin[J]. Environmental Monitoring and Assessment, 2024, 196(2): 116. doi: 10.1007/s10661-023-12271-w [31] MU L, GAO Y, HU X G. Characterization of biological secretions binding to graphene oxide in water and the specific toxicological mechanisms[J]. Environmental Science & Technology, 2016, 50(16): 8530-8537. [32] OUYANG S H, LI K W, ZHOU Q X, et al. Widely distributed nanocolloids in water regulate the fate and risk of graphene oxide[J]. Water Research, 2019, 165: 114987. doi: 10.1016/j.watres.2019.114987 [33] LI Y, YANG N, DU T T, et al. Transformation of graphene oxide by chlorination and chloramination: Implications for environmental transport and fate[J]. Water Research, 2016, 103: 416-423. doi: 10.1016/j.watres.2016.07.051 [34] WANG F F, DUAN L, WANG F, et al. Environmental reduction of carbon nanomaterials affects their capabilities to accumulate aromatic compounds[J]. NanoImpact, 2016, 1: 21-28. doi: 10.1016/j.impact.2016.02.001 [35] PECHER K, HADERLEIN S B, SCHWARZENBACH R P. Reduction of polyhalogenated methanes by surface-bound Fe(II) in aqueous suspensions of iron oxides[J]. Environmental Science & Technology, 2002, 36(8): 1734-1741. [36] LOH K P, BAO Q L, EDA G, et al. Graphene oxide as a chemically tunable platform for optical applications[J]. Nature Chemistry, 2010, 2(12): 1015-1024. doi: 10.1038/nchem.907 [37] BORTOLOZZO L S, CÔA F, KHAN L U, et al. Mitigation of graphene oxide toxicity in C. elegans after chemical degradation with sodium hypochlorite[J]. Chemosphere, 2021, 278: 130421. doi: 10.1016/j.chemosphere.2021.130421 [38] ZHANG Y Y, YU W T, WANG J, et al. Long-term exposure of graphene oxide suspension to air leading to spontaneous radical-driven degradation[J]. Environmental Science & Technology, 2023, 57(38): 14407-14416. [39] CHOWDHURY I, HOU W C, GOODWIN D, et al. Sunlight affects aggregation and deposition of graphene oxide in the aquatic environment[J]. Water Research, 2015, 78: 37-46. doi: 10.1016/j.watres.2015.04.001 [40] WANG X J, ZENG Z H, YANG T H, et al. DNA damage caused by light-driven graphene oxide: A new mechanism[J]. Environmental Science: Nano, 2023, 10(2): 519-527. doi: 10.1039/D2EN00948J [41] KURAPATI R, RUSSIER J, SQUILLACI M A, et al. Dispersibility-dependent biodegradation of graphene oxide by myeloperoxidase[J]. Small, 2015, 11(32): 3985-3994. doi: 10.1002/smll.201500038 [42] LIU L, ZHU C L, FAN M M, et al. Oxidation and degradation of graphitic materials by naphthalene-degrading bacteria[J]. Nanoscale, 2015, 7(32): 13619-13628. doi: 10.1039/C5NR02502H [43] XUE F M, YANG S T, CHEN L Y, et al. Quantification of sp2 carbon nanomaterials in biological systems: Pharmacokinetics, biodistribution and ecological uptake[J]. Reviews in Inorganic Chemistry, 2015, 35(4): 225-247. doi: 10.1515/revic-2015-0013 [44] LIU J H, YANG S T, WANG H F, et al. Effect of size and dose on the biodistribution of graphene oxide in mice[J]. Nanomedicine, 2012, 7(12): 1801-1812. doi: 10.2217/nnm.12.60 [45] ZHANG Y B, PETIBONE D, XU Y, et al. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine[J]. Drug Metabolism Reviews, 2014, 46(2): 232-246. doi: 10.3109/03602532.2014.883406 [46] FASERL K, CHETWYND A J, LYNCH I, et al. Corona isolation method matters: Capillary electrophoresis mass spectrometry based comparison of protein corona compositions following on-particle versus in-solution or in-gel digestion[J]. Nanomaterials, 2019, 9(6): 898. doi: 10.3390/nano9060898 [47] YARAGALLA S, MISHRA R K, THOMAS S, et al. Carbon-based nanofillers and their rubber nanocomposites: fundamentals and applications[M]. Elsevier, 2019. [48] MITTAL S, KUMAR V, DHIMAN N, et al. Physico-chemical properties based differential toxicity of graphene oxide/reduced graphene oxide in human lung cells mediated through oxidative stress[J]. Scientific Reports, 2016, 6: 39548. doi: 10.1038/srep39548 [49] ROȘU M C, PÁLL E, SOCACI C, et al. Cytotoxicity of methylcellulose-based films containing graphenes and curcumin on human lung fibroblasts[J]. Process Biochemistry, 2017, 52: 243-249. doi: 10.1016/j.procbio.2016.10.002 [50] LAMMEL T, BOISSEAUX P, NAVAS J M. Potentiating effect of graphene nanomaterials on aromatic environmental pollutant-induced cytochrome P450 1A expression in the topminnow fish hepatoma cell line PLHC-1[J]. Environmental Toxicology, 2015, 30(10): 1192-1204. doi: 10.1002/tox.21991 [51] LU K, DONG S P, PETERSEN E J, et al. Biological uptake, distribution, and depuration of radio-labeled graphene in adult zebrafish: Effects of graphene size and natural organic matter[J]. ACS Nano, 2017, 11(3): 2872-2885. doi: 10.1021/acsnano.6b07982 [52] WANG K, RUAN J, SONG H, et al. Biocompatibility of graphene oxide[J]. Nanoscale Research Letters, 2011, 6(1): 8. [53] MENDONÇA M C P, SOARES E S, de JESUS M B, et al. Reduced graphene oxide: Nanotoxicological profile in rats[J]. Journal of Nanobiotechnology, 2016, 14(1): 53. doi: 10.1186/s12951-016-0206-9 [54] WU W, YAN L, CHEN S Y, et al. Investigating oxidation state-induced toxicity of PEGylated graphene oxide in ocular tissue using gene expression profiles[J]. Nanotoxicology, 2018, 12(8): 819-835. doi: 10.1080/17435390.2018.1480813 [55] WANG X J, LIU Z. Carbon nanotubes in biology and medicine: An overview[J]. Chinese Science Bulletin, 2012, 57(2): 167-180. [56] BENGTSON S, KNUDSEN K B, KYJOVSKA Z O, et al. Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide[J]. PLoS One, 2017, 12(6): e0178355. doi: 10.1371/journal.pone.0178355 [57] BAI C C, TANG M. Progress on the toxicity of quantum dots to model organism-zebrafish[J]. Journal of Applied Toxicology, 2023, 43(1): 89-106. doi: 10.1002/jat.4333 [58] GHAZIMORADI M M, AZAD F V, JALALI F, et al. The neurotoxic mechanisms of graphene family nanomaterials at the cellular level: A solution-based approach review[J]. Current Pharmaceutical Design, 2022, 28(44): 3572-3581. doi: 10.2174/1381612829666221202093813 [59] GUO Z L, ZHANG P, CHETWYND A J, et al. Elucidating the mechanism of the surface functionalization dependent neurotoxicity of graphene family nanomaterials[J]. Nanoscale, 2020, 12(36): 18600-18605. doi: 10.1039/D0NR04179C [60] RAMAL-SANCHEZ M, FONTANA A, VALBONETTI L, et al. Graphene and reproduction: A love-hate relationship[J]. Nanomaterials, 2021, 11(2): 547. doi: 10.3390/nano11020547 [61] JIN L, DOU T T, CHEN J Y, et al. Sublethal toxicity of graphene oxide in Caenorhabditis elegans under multi-generational exposure[J]. Ecotoxicology and Environmental Safety, 2022, 229: 113064. doi: 10.1016/j.ecoenv.2021.113064 [62] AYREEN Z, KHATOON U, KIRTI A, et al. Perilous paradigm of graphene oxide and its derivatives in biomedical applications: Insight to immunocompatibility[J]. Biomedicine & Pharmacotherapy, 2024, 176: 116842. [63] YAN J Y, CHEN L L, HUANG C C, et al. Consecutive evaluation of graphene oxide and reduced graphene oxide nanoplatelets immunotoxicity on monocytes[J]. Colloids and Surfaces B: Biointerfaces, 2017, 153: 300-309. doi: 10.1016/j.colsurfb.2017.02.036 [64] LI B, YANG J Z, HUANG Q, et al. Biodistribution and pulmonary toxicity of intratracheally instilled graphene oxide in mice[J]. NPG Asia Materials, 2013, 5(4): 237-239. [65] LORET T, de LUNA L A V, LUCHERELLI M A, et al. Lung persistence, biodegradation, and elimination of graphene-based materials are predominantly size-dependent and mediated by alveolar phagocytes[J]. Small, 2023, 19(39): e2301201. doi: 10.1002/smll.202301201 [66] PARK C S, CHOI K S, SHIN J W, et al. Inhibition of viability of the respiratory epithelial cells using functionalized graphene oxide[J]. Journal of Nanoscience and Nanotechnology, 2015, 15(3): 2060-2066. doi: 10.1166/jnn.2015.9539 [67] MU Q X, JIANG G B, CHEN L X, et al. Chemical basis of interactions between engineered nanoparticles and biological systems[J]. Chemical Reviews, 2014, 114(15): 7740-7781. doi: 10.1021/cr400295a [68] MA K Y, ZHANG S P, YE B Q, et al. A new view of graphene oxide biosafety in a water environment using an eatable fish as a model[J]. RSC Advances, 2016, 6(35): 29619-29623. doi: 10.1039/C5RA26026D [69] XIONG G H, DENG Y Y, LIAO X J, et al. Graphene oxide nanoparticles induce hepatic dysfunction through the regulation of innate immune signaling in zebrafish (Danio rerio)[J]. Nanotoxicology, 2020, 14(5): 667-682. doi: 10.1080/17435390.2020.1735552 [70] YANG K, GONG H, SHI X Z, et al. In vivo biodistribution and toxicology of functionalized nano-graphene oxide in mice after oral and intraperitoneal administration[J]. Biomaterials, 2013, 34(11): 2787-2795. doi: 10.1016/j.biomaterials.2013.01.001 [71] PATLOLLA A K, RANDOLPH J, KUMARI S A, et al. Toxicity evaluation of graphene oxide in kidneys of sprague-dawley rats[J]. International Journal of Environmental Research and Public Health, 2016, 13(4): 380. doi: 10.3390/ijerph13040380 [72] GURUNATHAN S, ARSALAN IQBAL M, QASIM M, et al. Evaluation of graphene oxide induced cellular toxicity and transcriptome analysis in human embryonic kidney cells[J]. Nanomaterials, 2019, 9(7): 969. doi: 10.3390/nano9070969 [73] MONROY-TORRES B, RODRÍGUEZ-GALVÁN A, RAMÍREZ-APAN M T, et al. Lanthanide-modified graphene oxide and nanodiamond materials and their cytotoxicity[J]. Fullerenes, Nanotubes and Carbon Nanostructures, 2024, 32(6): 522-535. doi: 10.1080/1536383X.2023.2300667 [74] PELIN M, FUSCO L, LEÓN V, et al. Differential cytotoxic effects of graphene and graphene oxide on skin keratinocytes[J]. Scientific Reports, 2017, 7: 40572. doi: 10.1038/srep40572 [75] LIU L, LIU J C, WANG Y J, et al. Facile synthesis of monodispersed silver nanoparticles on graphene oxide sheets with enhanced antibacterial activity[J]. New Journal of Chemistry, 2011, 35(7): 1418-1423. doi: 10.1039/c1nj20076c [76] WU W, YAN L, WU Q, et al. Evaluation of the toxicity of graphene oxide exposure to the eye[J]. Nanotoxicology, 2016, 10(9): 1329-1340. doi: 10.1080/17435390.2016.1210692 [77] BANGEPPAGARI M, PARK S H, KUNDAPUR R R, et al. Graphene oxide induces cardiovascular defects in developing zebrafish (Danio rerio) embryo model: in-vivo toxicity assessment[J]. Science of the Total Environment, 2019, 673: 810-820. doi: 10.1016/j.scitotenv.2019.04.082 [78] XING F Y, GUAN L L, LI Y L, et al. Biosynthesis of reduced graphene oxide nanosheets and their in vitro cytotoxicity against cardiac cell lines of Catla catla[J]. Environmental Toxicology and Pharmacology, 2016, 48: 110-115. doi: 10.1016/j.etap.2016.09.022 [79] HU X G, WEI Z, MU L. Graphene oxide nanosheets at trace concentrations elicit neurotoxicity in the offspring of zebrafish[J]. Carbon, 2017, 117: 182-191. doi: 10.1016/j.carbon.2017.02.092 [80] KIM M, EOM H J, CHOI I, et al. Graphene oxide-induced neurotoxicity on neurotransmitters, AFD neurons and locomotive behavior in Caenorhabditis elegans[J]. NeuroToxicology, 2020, 77: 30-39. doi: 10.1016/j.neuro.2019.12.011 [81] YE S F, YANG P Y, CHENG K M, et al. Drp1-dependent mitochondrial fission mediates toxicity of positively charged graphene in microglia[J]. ACS Biomaterials Science & Engineering, 2016, 2(5): 722-733. [82] DZIEWIĘCKA M, WITAS P, KARPETA-KACZMAREK J, et al. Reduced fecundity and cellular changes in Acheta domesticus after multigenerational exposure to graphene oxide nanoparticles in food[J]. Science of the Total Environment, 2018, 635: 947-955. doi: 10.1016/j.scitotenv.2018.04.207 [83] ZHANG X L, ZHOU Q X, ZOU W, et al. Molecular mechanisms of developmental toxicity induced by graphene oxide at predicted environmental concentrations[J]. Environmental Science & Technology, 2017, 51(14): 7861-7871. [84] RIVE C, REINA G, WAGLE P, et al. Improved biocompatibility of amino-functionalized graphene oxide in Caenorhabditis elegans[J]. Small, 2019, 15(45): 1902699. doi: 10.1002/smll.201902699 [85] YANG Z W, PAN Y N, CHEN T T, et al. Cytotoxicity and immune dysfunction of dendritic cells caused by graphene oxide[J]. Frontiers in Pharmacology, 2020, 11: 1206. doi: 10.3389/fphar.2020.01206 [86] CHEN M J, YIN J F, LIANG Y, et al. Oxidative stress and immunotoxicity induced by graphene oxide in zebrafish[J]. Aquatic Toxicology, 2016, 174: 54-60. doi: 10.1016/j.aquatox.2016.02.015 [87] SUN J L, CHAO J, HUANG J, et al. Uniform small graphene oxide as an efficient cellular nanocarrier for immunostimulatory CpG oligonucleotides[J]. ACS Applied Materials & Interfaces, 2014, 6(10): 7926-7932. [88] WU R, FANG J, XIANG X B, et al. Graphene oxide influences transfer of plasmid-mediated antibiotic resistance genes into plants[J]. Science of the Total Environment, 2024, 911: 168652. doi: 10.1016/j.scitotenv.2023.168652 [89] NOGUEIRA P F M, NAKABAYASHI D, ZUCOLOTTO V. The effects of graphene oxide on green algae Raphidocelis subcapitata[J]. Aquatic Toxicology, 2015, 166: 29-35. doi: 10.1016/j.aquatox.2015.07.001 [90] MALINA T, MARŠÁLKOVÁ E, HOLÁ K, et al. Toxicity of graphene oxide against algae and cyanobacteria: Nanoblade-morphology-induced mechanical injury and self-protection mechanism[J]. Carbon, 2019, 155: 386-396. doi: 10.1016/j.carbon.2019.08.086 [91] ZHAO J Y, LIANG Y J, JIN L, et al. Joint toxic action and metabolic mechanisms of graphene nanomaterial mixtures in Microcystis aeruginosa[J]. Polish Journal of Environmental Studies, 2023, 32(2): 1447-1458. doi: 10.15244/pjoes/156426 [92] VAQUERO C, WENDELBO R, EGIZABAL A, et al. Exposure to graphene in a pilot production plant[J]. Journal of Physics: Conference Series, 2019, 1323(1): 012005. doi: 10.1088/1742-6596/1323/1/012005 [93] LIU H S, LI J Z, ZHANG Y T, et al. Analysis of physical examination data of cardiopulmonary function of graphene workers and health management measures[J]. Chinese Journal of Industrial Hygiene and Occupational Diseases, 2020, 38(6): 465-466. [94] ANDREWS J P M, JOSHI S S, TZOLOS E, et al. First-in-human controlled inhalation of thin graphene oxide nanosheets to study acute cardiorespiratory responses[J]. Nature Nanotechnology, 2024, 19: 705-714. doi: 10.1038/s41565-023-01572-3 [95] CEDERVALL T, LYNCH I, LINDMAN S, et al. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2007, 104(7): 2050-2055. [96] BEGUM P, IKHTIARI R, FUGETSU B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce[J]. Carbon, 2011, 49(12): 3907-3919. doi: 10.1016/j.carbon.2011.05.029 [97] TREUEL L, DOCTER D, MASKOS M, et al. Protein corona - from molecular adsorption to physiological complexity[J]. Beilstein Journal of Nanotechnology, 2015, 6: 857-873. doi: 10.3762/bjnano.6.88 [98] FRANQUI L S, de FARIAS M A, PORTUGAL R V, et al. Interaction of graphene oxide with cell culture medium: Evaluating the fetal bovine serum protein corona formation towards in vitro nanotoxicity assessment and nanobiointeractions[J]. Materials Science and Engineering: C, 2019, 100: 363-377. doi: 10.1016/j.msec.2019.02.066 [99] CUI G X, SU W T, TAN M Q. Formation and biological effects of protein corona for food-related nanoparticles[J]. Comprehensive Reviews in Food Science and Food Safety, 2022, 21(2): 2002-2031. doi: 10.1111/1541-4337.12838 [100] MU Q X, SU G X, LI L W, et al. Size-dependent cell uptake of protein-coated graphene oxide nanosheets[J]. ACS Applied Materials & Interfaces, 2012, 4(4): 2259-2266. [101] ZHANG B, WEI P, ZHOU Z X, et al. Interactions of graphene with mammalian cells: Molecular mechanisms and biomedical insights[J]. Advanced Drug Delivery Reviews, 2016, 105: 145-162. doi: 10.1016/j.addr.2016.08.009 [102] AKHTER M H, KHALILULLAH H, GUPTA M, et al. Impact of protein corona on the biological identity of nanomedicine: Understanding the fate of nanomaterials in the biological milieu[J]. Biomedicines, 2021, 9(10): 1496. doi: 10.3390/biomedicines9101496 [103] DUAN G X, KANG S G, TIAN X, et al. Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane[J]. Nanoscale, 2015, 7(37): 15214-15224. doi: 10.1039/C5NR01839K [104] DABROWSKI B, ZUCHOWSKA A, KASPRZAK A, et al. Cellular uptake of biotransformed graphene oxide into lung cells[J]. Chemico-Biological Interactions, 2023, 376: 110444. doi: 10.1016/j.cbi.2023.110444 [105] ALNASSER F, CASTAGNOLA V, BOSELLI L, et al. Graphene nanoflake uptake mediated by scavenger receptors[J]. Nano Letters, 2019, 19(2): 1260-1268. doi: 10.1021/acs.nanolett.8b04820 [106] XU M, ZHU J Q, WANG F F, et al. Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: Poly(acrylic acid)-functionalization is superior to PEGylation[J]. ACS Nano, 2016, 10(3): 3267-3281. doi: 10.1021/acsnano.6b00539 [107] MAGNE T M, de OLIVEIRA VIEIRA T, ALENCAR L M R, et al. Graphene and its derivatives: Understanding the main chemical and medicinal chemistry roles for biomedical applications[J]. Journal of Nanostructure in Chemistry, 2022, 12(5): 693-727. doi: 10.1007/s40097-021-00444-3 [108] HOLMANNOVA D, BORSKY P, SVADLAKOVA T, et al. Reproductive and developmental nanotoxicity of carbon nanoparticles[J]. Nanomaterials, 2022, 12(10): 1716. doi: 10.3390/nano12101716 [109] MA Y F, SHEN H, TU X L, et al. Assessing in vivo toxicity of graphene materials: Current methods and future outlook[J]. Nanomedicine, 2014, 9(10): 1565-1580. doi: 10.2217/nnm.14.68 [110] CÔA F, DELITE F S, STRAUSS M, et al. Toxicity mitigation and biodistribution of albumin corona coated graphene oxide and carbon nanotubes in Caenorhabditis elegans[J]. NanoImpact, 2022, 27: 100413. doi: 10.1016/j.impact.2022.100413 [111] de SOUSA M, MARTINS C H Z, FRANQUI L S, et al. Covalent functionalization of graphene oxide with d-mannose: Evaluating the hemolytic effect and protein corona formation[J]. Journal of Materials Chemistry B, 2018, 6(18): 2803-2812. doi: 10.1039/C7TB02997G [112] HAJIPOUR M J, RAHEB J, AKHAVAN O, et al. Personalized disease-specific protein corona influences the therapeutic impact of graphene oxide[J]. Nanoscale, 2015, 7(19): 8978-8994. doi: 10.1039/C5NR00520E [113] YANG Y, HAN P L, XIE X J, et al. Protein corona reduced graphene oxide cytotoxicity by inhibiting endocytosis[J]. Colloid and Interface Science Communications, 2021, 45: 100514. doi: 10.1016/j.colcom.2021.100514 [114] CUI L S, QUAGLIARINI E, XIAO S Y, et al. The protein corona reduces the anticancer effect of graphene oxide in HER-2-positive cancer cells[J]. Nanoscale Advances, 2022, 4(18): 4009-4015. doi: 10.1039/D2NA00308B [115] COREAS R, CASTILLO C, LI Z B, et al. Biological impacts of reduced graphene oxide affected by protein corona formation[J]. Chemical Research in Toxicology, 2022, 35(7): 1244-1256. doi: 10.1021/acs.chemrestox.2c00042 [116] RAJASEKAR P, RAO G, KUMAR A S, et al. Interaction of BSA with graphene oxide: Influence on the bioactivity of graphene oxide[J]. Diamond and Related Materials, 2023, 132: 109629. doi: 10.1016/j.diamond.2022.109629 [117] TANG Y L, TIAN J L, LI S Y, et al. Combined effects of graphene oxide and Cd on the photosynthetic capacity and survival of Microcystis aeruginosa[J]. Science of the Total Environment, 2015, 532: 154-161. doi: 10.1016/j.scitotenv.2015.05.081 [118] REN J Y, CAI R, WANG J, et al. Precision nanomedicine development based on specific opsonization of human cancer patient-personalized protein coronas[J]. Nano Letters, 2019, 19(7): 4692-4701. doi: 10.1021/acs.nanolett.9b01774 -



 下载:
下载: