-
活性污泥作为污水处理厂的主要副产物,其产量在2017年已达4.328×107 t (以含水率80%计)[1],且处理费用可占污水处理厂总运行费用的60%[2-3]。由于多环芳烃(polycyclic aromatic hydrocarbons,PAHs)具有较低的溶解性和较高的辛醇/脂水分配系数,因此,在污水处理过程中,PAHs容易吸附到活性污泥上[4]。虽然PAHs在污水处理过程中的去除率能达到90%,但由于自身的疏水特征会使得PAHs聚集在活性污泥中[5]。根据MENG等[6]对过去14年间我国污泥中有机污染物的统计,干污泥中16种PAHs(∑PAHs)含量为0.1×103~17×103 μg·kg−1,平均含量为159 μg·kg−1。因此,活性污泥中不仅含有大量的有机质[7],而且还含有大量污染物质[8-9]。本课题组前期研究结果表明,秸秆、纤维素与污泥在不同配比下进行联合厌氧消化均能促进污泥中∑PAHs降解,其降解速率可达到29.86%~51.33%和14.82%~20.75%[10-11]。可见,秸秆对PAHs的促进能力强于纤维素。而根据CHANDRA等[12]的统计,一些常见作物(小麦、玉米、水稻)的秸秆的纤维素、半纤维素及木质素含量分别为25%~44.3%、30%~50%和10%~21%。其中,纤维素和半纤维素在厌氧条件下容易水解形成葡萄糖,从而为微生物的生长提供碳源。因此,为了解秸秆和纤维素为共基质时污泥中PAHs的降解机制,可利用秸秆和纤维素的主要水解产物葡萄糖为共基质。
本研究以葡萄糖为共基质,研究污泥与葡萄糖在不同配比下联合厌氧消化对污泥中PAHs去除效能及细菌群落的影响,并优化最佳配比,为深入了解秸秆和纤维素与污泥进行联合厌氧消化过程中PAHs的降解机制提供参考和技术支撑。
-
实验污泥采集于贵阳市某污水处理厂(SBR处理工艺)浓缩池新鲜污泥,在采集过程中,利用粒径为100目(0.15 mm)的钢筛进行过滤,以去除污泥中的大颗粒物。污泥取回后,静置沉淀一段时间,倾去上清液后置于4 ℃冰箱,保存待用。在美国环保署(US EPA)公布的优先控制的16种PAHs中,本研究所用实验污泥仅检出13种PAHs,且污泥中PAHs以3~4环PAHs为主,其含量如表1所示。
-
实验分为4组,每组各2个7 L的厌氧消化反应器。反应器的有效容积为5 L,采用机械搅拌,并利用温度为(35±1) ℃的恒温流动水进行保温;搅拌轴与容器间采用水封,确保密闭。首先向反应器中投入1/3的消化污泥作为接种污泥,之后按有效容积10%(500 mL)投加至有效容积,以后每天按有效容积5%(250 mL)投加污泥,并排出等量消化污泥。在投加污泥过程中,向反应器中鼓吹N2,保持反应器处于厌氧状态。第1组为空白实验(CK);第2组按VS污泥∶VS葡萄糖=1∶0.1投加葡萄糖(P1);第3组按VS污泥∶VS葡萄糖=1∶0.3投加葡萄糖(P2);第4组按VS污泥∶VS葡萄糖=1∶0.5投加葡萄糖(P3)。每间隔7 d,取反应器内消化污泥,检测污泥中PAHs的含量变化,共取10次;采集第70 天的消化污泥,分析微生物群落结构。
-
消化污泥经冷冻干燥后,碾碎过100目筛,避光保存。称取约5.0 g过筛污泥,以二氯甲烷为提取剂,索氏抽提24 h。采用内标法,用Agilent GC6890N/5973C气相色谱-质谱联用仪对13种PAHs的残留量进行定量分析。
消化污泥样品冷冻后进行16S rRNA高通量测序,实验在上海美吉生物医药科技有限公司完成,在Illumina公司的MiseqPE300平台上完成分析。
-
通过方法空白、空白加标、基质加标、样品平行和添加回收率指示物对实验分析过程进行质量控制。样品方法空白中未检出目标化合物。空白加标16种PAHs标准样品的回收率为51.73%~127.00%,基质加标的平均回收率为51.86%~120.53%。回收率指示物NAP-d8、ACE-d10、PHE-d10、CHR-d12和PERY-d12的回收率分别为(61.85±23.88)%、(95.68±22.59)%、(95.22±32.07)%、(103.53±46.45)%和(62.16±22.78)%。线性方程R2为0.990~0.999,均满足定量分析要求。
-
实验数据采用Microsoft Office Excel 2016进行处理和分析,利用Origin 9进行作图。消化污泥中PAHs数据采用单因素方差(One-way ANOVA)(P<0.05)进行分析;微生物数据利用上海美吉云平台(www.i-sanger.com)进行检查分析,筛选有效序列并将相似性≥97%的序列归为同一分类操作单元(OTU),计算多样性指数,并绘制韦恩图(Venn)、群落结构柱状图等。PAHs降解速率计算方法见式(1)。
式中:R为PAHs降解速率;SCK为未添加葡萄糖实验组消化污泥中的多环芳烃含量;SCI为添加葡萄糖实验组消化污泥中的多环芳烃含量。
-
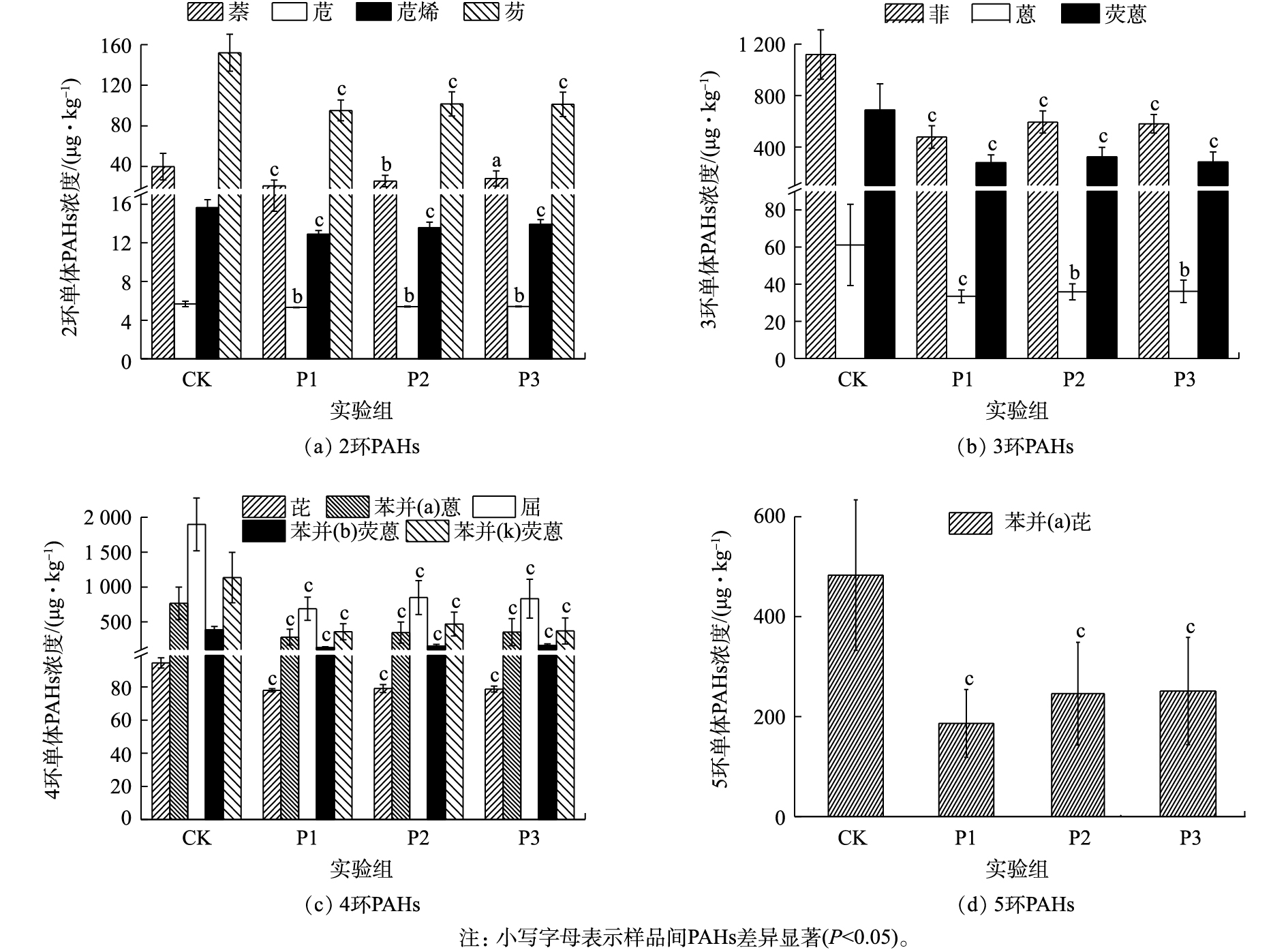
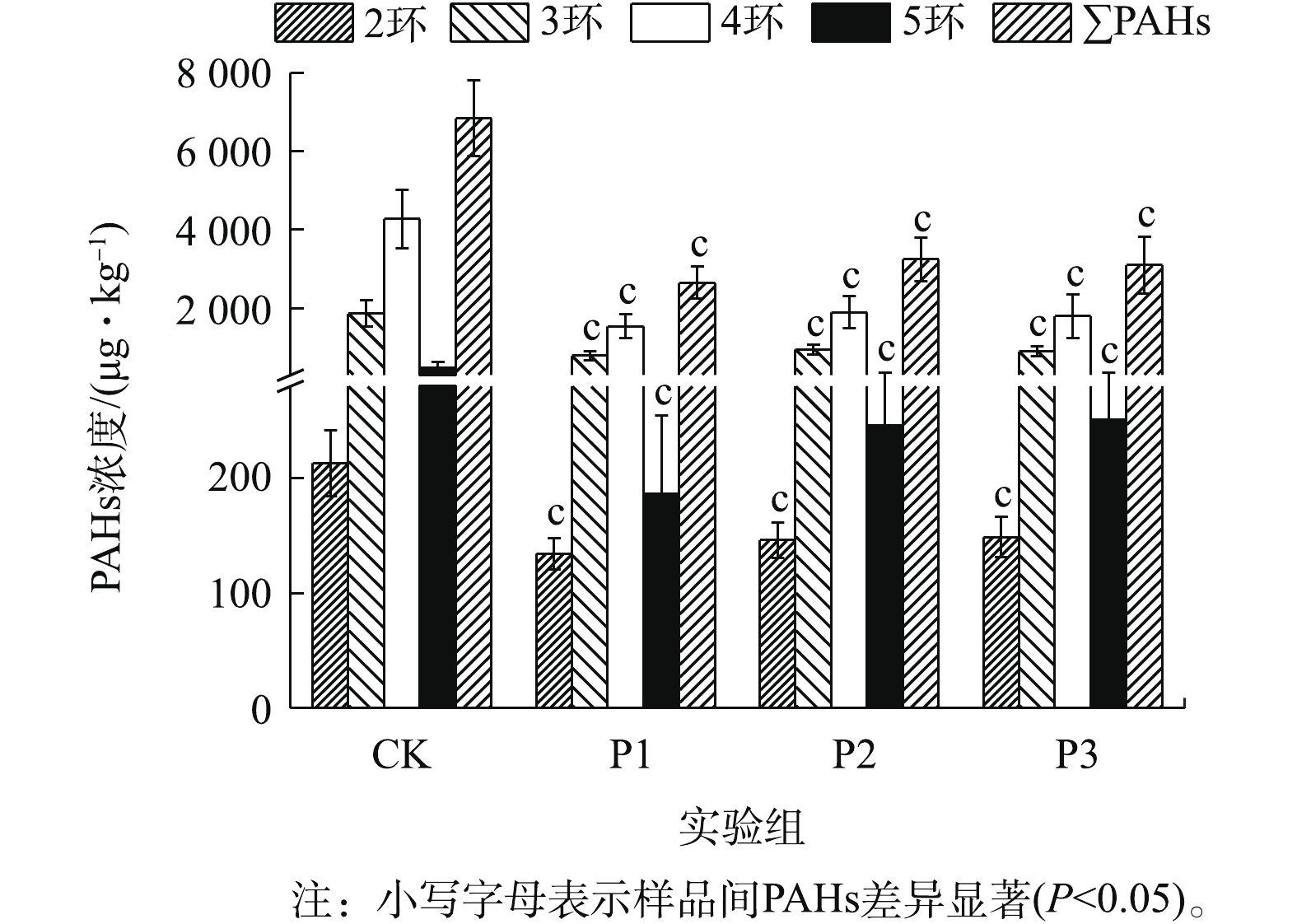
图1为厌氧降解期间消化污泥中PAHs含量的变化情况。各实验组中∑PAHs浓度为3 627.62~8 039.89 μg·kg−1(CK)、1 966.59~3 364.01 μg·kg−1(P1)、2 299.60~4 021.82 μg·kg−1(P2)和2 248.19~4 564.46 μg·kg−1(P3)。可见,葡萄糖的添加能显著促进污泥中∑PAHs的降解(P<0.05)(图1)。其中,P1实验组对2~5环及∑PAHs的降解能力较强,其降解速率可达到(36.08±9.88)%、(56.26±11.31)%、(63.36±8.19)%、(59.60±14.05)%、(60.56±8.10)%。由此可见,PAHs的去除能力并未随葡萄糖添加量的增加而增加,这主要是因为过量添加共基质会抑制微生物细胞活性,造成微生物细胞的衰竭,从而导致共代谢系统效率下降[13]。从图1中可以看出,各实验组中4环PAHs的降解速率均为最高,而2环PAHs的降解速率较低。其中,4环PAHs的平均降解速率均大于50%。
-
图2为不同实验组消化污泥中各单体PAHs的浓度。显然,葡萄糖的添加能显著降低污泥中各单体PAHs的浓度(P<0.05)。在2环PAHs中,萘的降解速率随着葡萄糖添加量的增加逐渐降低;但P1实验组对萘的降解能力最强,可达到(43.00±18.51)%。与CK相比,各实验组中苊的浓度虽发生显著变化(P<0.05),但苊的降解速率仍然较低,仅达到(6.08±4.30)%(P1)、(4.61±4.60)%(P2)、(4.56±4.49)%(P3);这可能是由于进样污泥中苊的浓度较低,从而导致葡萄糖对苊的促进作用较低。由图2(b)可知,葡萄糖对菲、蒽和荧蒽具有较好的促进能力(P<0.05)。其中,荧蒽的平均降解速率均大于50%;而菲的降解速率为(56.01±11.47)%(P1)、(45.48±12.78)%(P2)和(46.65±11.71)%(P3);蒽的降解速率为(40.81±16.11)%(P1)、(36.78±16.56)%(P2)和(36.96±15.96)%(P3)。可见,菲的去除能力明显强于蒽,这可能是由于蒽在水中的溶解度较低,不易于被微生物利用[14-15],从而导致微生物对蒽的降解能力较弱。此外,MCNALLY等[16]研究也表明,在萘和菲共存条件下,萘可以促进菲的降解。因此,菲的降解速率强于蒽。
与低分子质量PAHs(2~3环)不同,葡萄糖对高分子质量PAHs(≥4环)的促进作用更为显著(P<0.05)。在4环PAHs中,虽然葡萄糖对芘的促进作用较弱,但平均降解速率相对稳定。与芘不同,葡萄糖能显著促进苯并(a)蒽、䓛、苯并(b)荧蒽和苯并(k)荧蒽的降解,其平均降解速率均大于50%。其中,P1实验组对苯并(a)蒽、䓛、苯并(b)荧蒽和苯并(k)荧蒽的平均降解速率均大于62%。此外,葡萄糖的添加也能显著促进污泥中苯并(a)芘的降解,其降解速率可达到(59.60±14.05)%(P1)、(46.05±23.94)%(P2)和(44.21±26.06)%(P3)。由于污泥中致癌性PAHs主要为4~6环PAHs,但在实验污泥中未发现6环PAHs。因此,致癌性PAHs均为4~5环芳烃。由此可见,按VS污泥∶VS葡萄糖=1∶0.1的比例向污泥中添加葡萄糖不仅能极大地促进高分子质量PAHs的降解,而且还能降低处理成本。
-
在本次实验中,各点位微生物的序列统计结果见表2。对4个实验组消化污泥中微生物样品中获得的序列进行处理,得到有效序列总计123 056条,其中P2实验组中有效序列最少,为29 114条,CK实验组中有效序列最多,为35 232条。将这些序列以97%的相似性作为一个单元来划分,进行OTU (operational taxonomic unit)聚类分析,共得到5 475个运算的分类单位(OTU)。其中,P1实验组的OTU数明显高于P2和P3,由于每个OTU可对应不同的种群[17],因此,P1实验组中微生物种群高于P2和P3。可见,随着葡萄糖添加量的增加,消化污泥中微生物种群数呈递减趋势。对序列进行随机抽样,统计抽样的重复样本数和OTU数,分别计算香农指数(Shannon)和辛普森(Simpson)指数,以进行多样性分析。由于Shannon指数[18]和Simpson指数[19]能反映样品中微生物群落的多样性及受样品群落中物种丰富度和物种均匀度的影响,且Shannon指数越大,Simpson指数越小,则表明样品中物种越丰富。因此,通过Shannon指数和Simpson指数均可直观看出消化污泥中细菌多样性。其中,P1实验组的物种最丰富,但丰富度较低。
Venn图用于统计样本之间所共有以及独有的OTU数目,可以直观地比较样品中OTU数目组成相似性及重叠情况[20]。如图3所示,4个实验组所共有的OTU总数为790个,其中CK、P1、P2和P3特有的OTU数目分别为3、13、4和3个。P1实验组中特有的OTU数目较多,预示有较多特有的细菌种类。
-
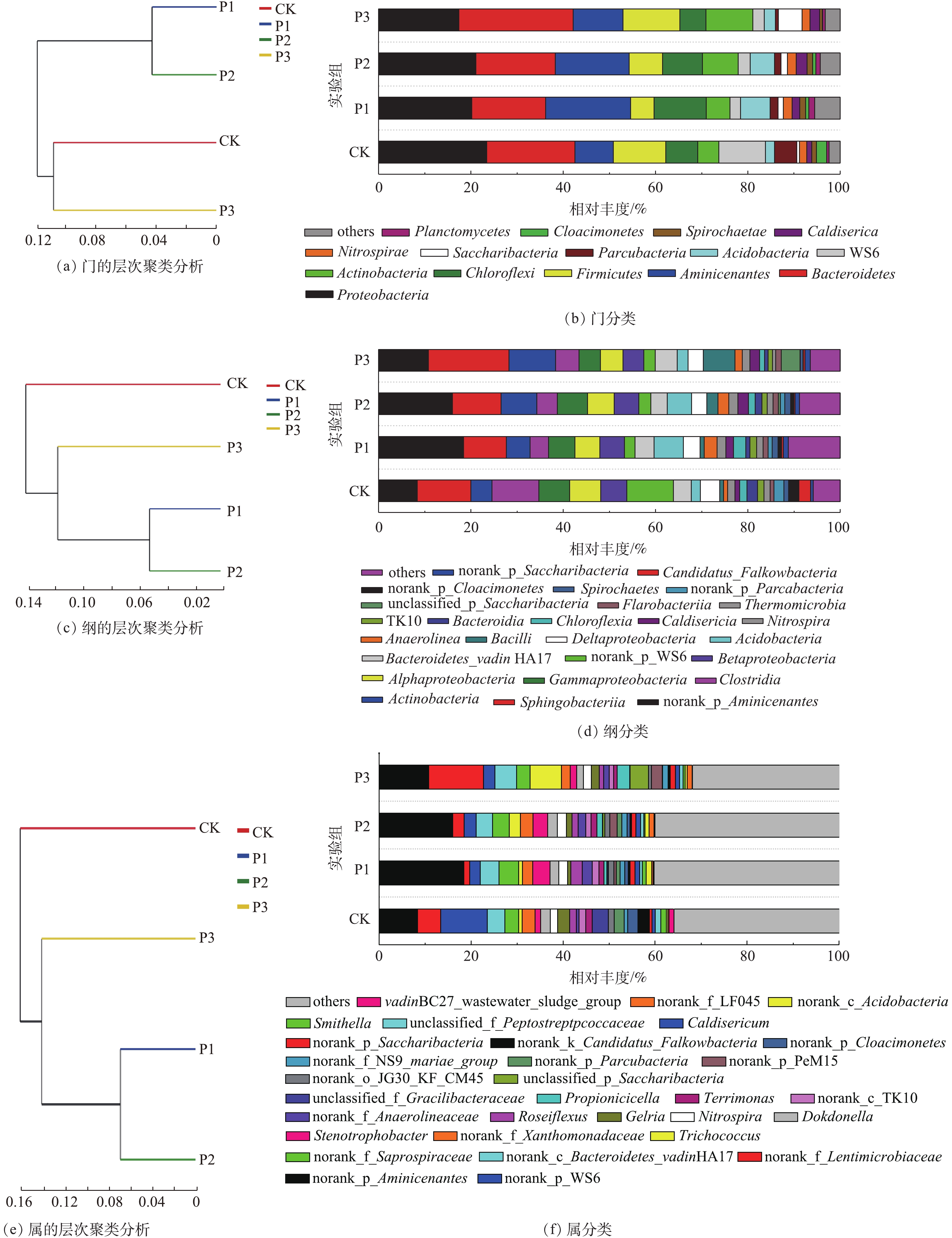
根据分类学分析结果可知样品在各分类程度上的比对情况。结果包含了2个信息:样品中含有何种菌群;某菌群在此样品中所占的比例。因此,使用这种方法能够直观地观察出不同样品物种组成及分类状况[21]。如图4所示,在门、纲和属水平上,均采用多样性相似度树与组成成分柱状图组合的方法分析消化污泥中细菌组成成分,图4(a)、图4(c)和图4(e)是样品间基于群落组成的Bray-Curtis层次聚类分析[22],图4(b)、图4(d)、图4(f)是样品的群落结构柱状图。经物种注释,绝大部分基因信息均能找到相对应的菌种。结果显示:消化污泥中细菌隶属42个门、94个纲和365个属。
在门水平上,主要优势种群(>5%)以Proteobacteria(变形菌门)、Bacteroidetes(拟杆菌门)、Aminicenantes、Chloroflexi(绿弯菌门)和Firmicutes(厚壁菌门)为主(图4(b));这一结果与以往的研究[23-24]相似。Proteobacteria和Bacteroidetes所占比例更是达到17%和16%以上。根据门聚类结果发现,Aminicenantes在P1与P2实验组中相对丰度较高,而在CK与P3中较低,分别为18.25%、15.95%、10.81%和8.35%;P1与P2实验组中Proteobacteria相对丰度高于P3,分别为20.18%和21.05%。Bacteroidetes在P1和P2实验组中相对丰度较低,分别为15.80%和17.02%,而在P3与CK中相对丰度较高,达到24.73%和18.96%。可见,当污泥与葡萄糖的配比较低时,不利于Bacteroidetes生长,而添加过量葡萄糖能促进Bacteroidetes生长。此外,Bacteroidetes不仅对复杂的碳化合物具有降解能力[25],而且大多数属于Bacteroidetes的细菌在有机物降解过程中会产生各种裂解酶[24],以促进污泥中有机物的水解。除Bacteroidetes外,Firmicutes和Actinobacteria (放线菌门)对PAHs可能也具有一定的降解能力[26-27]。但在本研究中,Firmicutes相对丰度仅在P3实验组中高于CK;而Actinobacteria相对丰度均呈增加趋势,分别由4.54%(CK)增至5.16%(P1)、7.74%(P2)和10.14%(P3)。可见,在该体系中,Actinobacteria相对丰度的增加可能对PAHs降解具有促进作用。此外,有研究[28-29]发现,部分相对丰度较低的群落,如Spirochaetes、Planctomycetes、Lentisphaerae、Deferribacteres和Verrucomicrobia,对PAHs、二氯甲烷、原油中污染物和含氯乙烯均具有一定的促进作用。而在本研究中,Planctomycetes相对丰度由0.52%(CK)分别增至1.27%(P1)、0.97%(P2)和0.56%(P3),表明Planctomycetes相对丰度的增加可能会促进污泥中PAHs降解,但Actinobacteria和Planctomycetes与PAHs去除能力之间是否具有线性关系仍需要大量数据进行论证。
在纲(图4(d))水平上,优势菌群以norank_p_Aminicenates、Sphingobacteria、Actinobacteria、Clostridia、Gammaproteobacteria、Alphaproteobacteria、Betaproteobacteria和Bacteroidetes_vadin HA17为主。norank_p_Aminicenates相对丰度随葡萄糖添加量的增加而减少;P1、P2和P3相对丰度分别为18.41%、15.99%和10.81%。此外,葡萄糖添加量的不同也会导致反应器中部分对难降解有机物具有降解能力的细菌产生影响,如Spirochaetes和Bacteroidetes_vadin HA17。Spirochaetes在P1和P2实验组中相对丰度较高,分别为1.30%和1.21%;而在CK与P3中较低,分别为1.06%和0.55%。与Spirochaetes不同,Bacteroidetes_vadin HA17在P1和P3中相对丰度较高,分别为4.01%和4.66%;而在CK与P3中,仅为3.79%和3.48%。以往的研究表明,Bacteroidetes_vadin HA17对难降解有机物具有一定的降解能力[30],因此,Spirochaetes和Bacteroidetes_vadin HA17相对丰度的增加可能会促进污泥中PAHs的降解。同时,在本研究中,P1和P2具有的Betaproteobacteria相对丰度差异较小,分别为5.31%和5.33%;而CK与P3的Betaproteobacteria相对丰度差异较大,分别为5.67%和4.51%;Sphingobacteria在CK与P3中相对丰度较高,分别为11.63%和17.42%;而在P1和P2中,相对丰度较低,分别为9.24%和10.52%。因此,根据纲水平聚类结果,P1和P2的细菌主要组成成分更为接近。
相对丰度较低的菌属(others)是细菌在属(图4(f))水平上的优势菌群,在P1和P2实验组中,相对丰度较高,分别达40.23%和40.01%,而在CK与P3实验组中,仅为35.86%和31.87%。在检出的菌属中,norank_p_Aminicenantes为主要菌属,且在P1和P2中相对丰度较高,分别达18.41%和15.99%;而在CK与P3中,相对丰度较低,分别为8.38%和10.81%。在本研究中,P1和P2实验组中的Dokdonella、Nitrospira、Stenotrophobacter、norank_f_Anaerolineaceae和Caldisericum等菌属的相对丰度差异较小,而在CK和P3实验组中的差异较大。在上述菌属中,Stenotrophobacter和norank_f_Anaerolineaceae相对丰度会显著增加,分别由1.09%和0.69%(CK)增至3.38%和2.27%(P1)、2.89%和1.77%(P2)、1.26%和1.33%(P3)。以往的研究结果表明,Anaerolineaceae不仅能在厌氧条件下降解烃类化合物[31],而且还能用于修复PAHs污染严重的区域[32]。因此,norank_f_Anaerolineaceae相对丰度的增加可能也会促进污泥中PAHs的降解。同时,属水平聚类结果也表明,P1和P2实验组中细菌主要组成成分更为接近。由此可见,向污泥中添加不同配比的葡萄糖会对体系中的细菌群落产生较大的影响,当污泥与葡萄糖的配比为1∶0.1和1∶0.3时,体系中的细菌组成成分接近,但随着配比的进一步增加,体系中的细菌组成成分会发生显著的变化。
-
1)向污泥中添加葡萄糖均能显著促进PAHs的降解。P1(VS污泥∶VS葡萄糖=1∶0.1)实验组对2~5环和∑PAHs去除能力最强(P<0.05);降解速率可达到(36.08±9.88)%、(56.26±11.31)%、(63.36±8.19)%、(59.60±14.05)%和(60.56±8.10)%。
2)向污泥添加不同比例的葡萄糖,均能显著提高污泥中高分子质量PAHs(≥4环)的降解速率(P<0.05)。P1(VS污泥∶VS葡萄糖=1∶0.1)实验组对苯并(a)蒽、䓛、苯并(b)荧蒽和苯并(k)荧蒽的平均降解速率均大于62%;而对苯并(a)芘的降解速率可达到(59.60±14.05)%。
3)在门、纲和属水平上,消化污泥中主要的优势菌群有Proteobacteria、Bacteroidetes、Aminicenates、Chloroflexi、Firmicutes、norank_p_Aminicenates、Sphingobacteria、Actinobacteria、Clostridia、Gammaproteobacteria、Alphaproteobacteria、Betaproteobacteria、Bacteroidetes_vadin HA17和norank_p_Aminicenantes。
4)葡萄糖的添加能促进Actinobacteria、Planctomycetes、Spirochaetes、Bacteroidetes_vadin HA17和norank_f_Anaerolineaceae菌群的生长,从而促进污泥中PAHs降解。
污泥与葡萄糖不同配比联合厌氧消化对污泥中多环芳烃去除效能及细菌群落的影响
Effect of different ratio of sludge to glucose combined with anaerobic co-digestion on polycyclic aromatic hydrocarbons removal efficiency and bacterial community in sludge
-
摘要: 为分析污泥与葡萄糖不同配比进行联合厌氧消化对污泥中多环芳烃(PAHs)去除效能及细菌群落的影响,在中温(35±1) ℃条件下,以未添加葡萄糖的污泥厌氧消化为对照(CK),研究了活性污泥与葡萄糖按不同有机质含量(挥发性固体(VS)质量比)分别为1∶0.1、1∶0.3和1∶0.5对PAHs去除效能及细菌群落的影响。结果表明,葡萄糖添加量的增加并未进一步提高PAHs的降解能力。P1实验组(VS污泥∶VS葡萄糖=1∶0.1)对消化污泥中∑PAHs的去除能力最强;降解速率可达到(60.56±8.10)%;且高分子质量PAHs(≥4环)的降解速率显著高于低分子质量PAHs(2~3环)(P<0.05)。苯并(a)蒽、䓛、苯并(b)荧蒽和苯并(k)荧蒽的平均降解速率均大于62%;而苯并(a)芘的降解速率达到(59.60±14.05)%。此外,使用16S rRNA技术,检测消化污泥中细菌群落发现,向污泥中添加葡萄糖,可能通过促进Actinobacteria、Bacteroidetes_vadin HA17、Spirochaetes、Planctomycetes和norank_f_Anaerolineaceae菌群的生长,从而提高污泥中PAHs的去除能力。Abstract: In order to analyze the effects of the different ratio of sludge to glucose combined with anaerobic co-digestion on the removal efficiency of polycyclic aromatic hydrocarbons (PAHs) and bacterial communities in sludge, at medium temperature (35±1) ℃, the effects of different organic matter contents (as volatile solids (VS) mass ratio) for sludge and glucose of 1∶0.1, 1∶0.3 and 1∶0.5 on the removal efficiency of PAHs and bacterial community were compared with anaerobic digestion of sludge without added glucose (CK). The results showed that the increase of glucose amount did not further improve the degradation ability of PAHs. Among them, P1 experimental group (VSsludge∶VSglucose=1∶0.1) had the strongest removal ability of ∑PAHs in digested sludge, and degrading rate could reach (60.56±8.10)%. And the removal efficiency of high molecular weight PAHs (≥4 ring) was significantly higher than that of the low molecular weight PAHs (2~3 rings) (P<0.05). All the average degradation rates of benzo(a)pyrene, anthracene, benzo(b)fluoranthene and benzo(k)fluoranthene were higher than 62%, while the degradation rate of benzo(a)pyrene reached (59.60±14.05)%. In addition, 16S rRNA technology was used to detect the bacterial community in the digested sludge. It was found that the addition of glucose to the sludge could enhance PAHs removal in the sludge by promoting the growth of Actinobacteria, Bacteroidetes_vadin HA17, Spirochaetes, Planctomycetes and norank_f_Anaerolineaceae.
-
Key words:
- sludge /
- anaerobic co-digestion /
- different ratio /
- PAHs /
- bacterial community
-

-
表 1 实验污泥中PAHs含量
Table 1. Concentration of PAHs in experimental sludge
化合物中文名称 英文简写 PAHs含量/(μg·kg−1) 萘 NaP 41.72±12.89 苊 Ace 5.72±0.14 苊烯 Acy 14.95±0.88 芴 Flu 114.14±9.46 菲 Phe 669.85±52.13 蒽 Ant 43.57±4.42 荧蒽 Fluo 447.67±77.75 芘 Pyr 89.70±2.40 苯并(a)蒽 BaA 85.21±35.81 䓛 Chry 345.26±27.93 苯并(b)荧蒽 BbF 299.71±49.09 苯并(k)荧蒽 BkF 582.88±337.19 苯并(a)芘 BaP 305.56±117.10 总PAHs ∑PAHs 3 116.06±454.23 表 2 各样本序列统计
Table 2. Sequence statistics of each sample
样品名称 序列数量/条 OTU/个 Shannon Simpson CK 35 232 1 301 5.28 0.017 P1 29 228 1 455 5.51 0.030 P2 29 114 1 408 5.49 0.023 P3 29 482 1 311 4.97 0.033 -
[1] 杭世珺, 傅涛, 戴晓虎, 等. 技术路线没有走通, 产业没有融通, 政策缺乏贯通 污泥出路困境如何破?[J]. 环境经济, 2019(2): 34-39. [2] ZHAO J, GUI L, WANG Q, et al. Aged refuse enhances anaerobic digestion of waste activated sludge[J]. Water Research, 2017, 123: 724-733. doi: 10.1016/j.watres.2017.07.026 [3] WANG Q, WEI W, GONG Y, et al. Technologies for reducing sludge production in wastewater treatment plants: State of the art[J]. Science of the Total Environment, 2017, 587-588: 510-521. doi: 10.1016/j.scitotenv.2017.02.203 [4] CLARKE B O, SMITH S R. Review of 'emerging' organic contaminants in biosolids and assessment of international research priorities for the agricultural use of biosolids[J]. Environment International, 2011, 37(1): 226-247. doi: 10.1016/j.envint.2010.06.004 [5] APARICIO I, SANTOS J L, ALONSO E. Limitation of the concentration of organic pollutants in sewage sludge for agricultural purposes: A case study in South Spain[J]. Waste Management, 2009, 29(5): 1747-1753. doi: 10.1016/j.wasman.2008.11.003 [6] MENG X Z, VENKATESAN A K, NI Y L, et al. Organic contaminants in Chinese sewage sludge: A meta-analysis of the literature of the past 30 years[J]. Environmental Science & Technology, 2016, 50(11): 5454-5466. [7] 李素慧, 许智华, 樊小军. 污水处理厂污泥处理处置现状分析及建议[J]. 能源研究与信息, 2011, 27(4): 187-192. doi: 10.3969/j.issn.1008-8857.2011.04.001 [8] LAVADO R S, RODRIGUEZ M B, TABOADA M A. Treatment with biosolids affects soil availability and plant uptake of potentially toxic elements[J]. Agriculture Ecosystems & Environment, 2005, 109(3/4): 360-364. [9] SHOBER A L, STEHOUWER R C, MACNEAL K E. Chemical fractionation of trace elements in biosolid-amended soils and correlation with trace elements in crop tissue[J]. Communications in Soil Science & Plant Analysis, 2007, 38(7): 1029-1046. [10] International Journal of Environmental Science and Technology. Effect of straw on microbial community composition and degradation efficiency of polycyclic aromatic hydrocarbons in sludge digester[J]. International Journal of Environmental Science and Technology, 2019, 16(12): 7973-7986. [11] 李新. 纤维素对污泥中多环芳烃厌氧生物降解的影响研究[D]. 贵州: 贵州大学, 2017. [12] CHANDRA R, TAKEUCHI H, HASEGAWA T. Methane production from lignocellulosic agricultural crop wastes: A review in context to second generation of biofuel production[J]. Renewable and Sustainable Energy Reviews, 2012, 16(3): 1462-1476. doi: 10.1016/j.rser.2011.11.035 [13] HENRY S M, GRBIC-GALIC D. Influence of endogenous and exogenous electron donors and trichloroethylene oxidation toxicity on trichloroethylene oxidation by methanotrophic cultures from a groundwater aquifer[J]. Applied and Environmental Microbiology, 1991, 57(1): 236-244. [14] MILTON L L, MILOS V N, KEITH D B. Analytical Chemistry of Polycyclic Aromatic Compounds[M]. New York: Academic Press, 1981. [15] LAHA S, LUTHY R G. Effects of nonionic surfactants on the solubilization and mineralization of phenanthrene in soil-water systems[J]. Biotechnology and Bioengineering, 1992, 40(11): 1367-1380. doi: 10.1002/(ISSN)1097-0290 [16] MCNALLY D L, MIHELCIC J R, LUEKING D R. Biodegradation of mixtures of polycyclic aromatic hydrocarbons under aerobic and nitrate-reducing conditions[J]. Chemosphere, 1999, 38(6): 1313-1321. doi: 10.1016/S0045-6535(98)00532-3 [17] RODRIGUES V D, TORRES T T, OTTOBONI L M M. Bacterial diversity assessment in soil of an active Brazilian copper mine using high-throughput sequencing of 16S rDNA amplicons[J]. Antonie Van Leeuwenhoek International Journal of General and Molecular Microbiology, 2014, 106(5): 879-890. doi: 10.1007/s10482-014-0257-6 [18] MOLLER A K, SOBORG D A, Al SOUND W A, et al. Bacterial community structure in high-arctic snow and freshwater as revealed by pyrosequencing of 16S rRNA genes and cultivation[J]. Polar Research, 2013, 32(1): 1-11. [19] WANG Y, SHENG H F, HE Y, et al. Comparison of the levels of bacterial diversity in freshwater, intertidal wetland, and marine sediments by using millions of illumina tags[J]. Applied and Environmental Microbiology, 2012, 78(23): 8264-8271. doi: 10.1128/AEM.01821-12 [20] SCHLOSS P D, GEVERS D, WESTCOTT S L. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies[J]. Plos One, 2011, 6(12): e27310. doi: 10.1371/journal.pone.0027310 [21] FOUTS D E, SEBASTIAN S, JANAKI P, et al. Next generation sequencing to define prokaryotic and fungal diversity in the Bovine Rumen[J]. Plos One, 2012, 7(11): e48289. doi: 10.1371/journal.pone.0048289 [22] OBERAUNER L, ZACHOW C, LACKNER S, et al. The ignored diversity: Complex bacterial communities in intensive care units revealed by 16S pyrosequencing[J]. Scientific Reports, 2013, 3: 1413-1424. doi: 10.1038/srep01413 [23] NELSON M C, MORROSON M, YU Z. A meta-analysis of the microbial diversity observed in anaerobic digesters[J]. Bioresource Technology, 2011, 102(4): 3730-3739. doi: 10.1016/j.biortech.2010.11.119 [24] RIVERE D, DESVIGNES V, PELLETIER E, et al. Towards the definition of a core of microorganisms involved in anaerobic digestion of sludge[J]. ISME Journal, 2009, 3(6): 700-714. doi: 10.1038/ismej.2009.2 [25] BURNS A S, PUGH C W, SEGID Y T, et al. Performance and microbial community dynamics of a sulfate-reducing bioreactor treating coal generated acid mine drainage[J]. Biodegradation, 2012, 23(3): 415-429. doi: 10.1007/s10532-011-9520-y [26] NIEPCERON M, MARTIN-LAURENT M, CRAMPON F, et al. GammaProteobacteria as a potential bioindicator of a multiple contamination by polycyclic aromatic hydrocarbons (PAHs) in agricultural soils[J]. Environmental Pollution, 2103, 180: 199-205. [27] ROGERS S W, ONG S K, MOORMAN T B. Mineralization of PAHs in coal-tar impacted aquifer sediments and associated microbial community structure investigated with FISH[J]. Chemosphere, 2007, 69: 1563-1573. doi: 10.1016/j.chemosphere.2007.05.058 [28] IMFELD G, ARAGONES C E, FETZER I, et al. Characterization of microbial communities in the aqueous phase of a constructed model wetland treating 1, 2-dichloroethene-contaminated groundwater[J]. FEMS Microbiology Ecology, 2010, 72(1): 74-88. doi: 10.1111/fem.2010.72.issue-1 [29] WANG L, ZHENG B, LEI K. Diversity and distribution of bacterial community in the coastal sediments of Bohai Bay, China[J]. Acta Oceanologica Sinica, 2015, 34(10): 122-131. doi: 10.1007/s13131-015-0719-3 [30] BALDWIN S A, KHOSHNOODI M, REZADEBASHI M, et al. The microbial community of a passive biochemical reactor treating arsenic, zinc and sulfate-rich seepage[J]. Frontiers in Bioengineering and Biotechnology, 2015, 3: 27. [31] TISCHER K, KLEINSTEUBER S, SCHLEINITZ K M, et al. Microbial communities along biogeochemical gradients in a hydrocarbon-contaminated aquifer[J]. Environmental Microbiology, 2013, 15(9): 2603-2615. doi: 10.1111/emi.2013.15.issue-9 [32] YAN Z S, HAO Z, WU H F, et al. Co-occurrence patterns of the microbial community in polycyclic aromatic hydrocarbon-contaminated riverine sediments[J]. Journal of Hazardous Materials, 2019, 367: 99-108. doi: 10.1016/j.jhazmat.2018.12.071 -



 下载:
下载: