-
目前,废水的脱氮除磷是解决环境水体富营养化问题的关键[1]。污水处理厂中氮元素的脱除主要是基于好氧硝化和缺氧反硝化的生物脱氮工艺。20世纪90年代,MULDER等[2]发现一种新型的厌氧氨氧化细菌,该菌在厌氧条件下以亚硝酸盐(
NO−2 )为电子受体,将铵(NH+4 )氧化成气态氮(N2),并产生少量硝酸盐(NO−3 )[3],这一过程称为厌氧氨氧化过程(anaerobic ammonium oxidation,Anammox)。Anammox反应与传统脱氮工艺(硝化/反硝化)系统相比,具有氮去除率更高,生物反应器体积更小,温室气体排放更低,污泥产量更低(90%)等优点[4-7]。因此,该工艺被广泛应用于高氨氨废水处理[8]。然而,厌氧氨氧化菌用于处理低基质市政废水仍然比较困难[9]。此外,JIN等[6]发现,各种底物和化学物质会影响厌氧氨氧化过程,其中包括游离氨(free ammonia,FA)、游离亚硝酸(free nitrous acid,FNA)、有机物质、盐、重金属、磷酸盐和硫化物。反应器运行方式分为连续式与间歇式2种。已有研究[10]表明,运行方式影响倒置A2/O工艺启动性能,间歇换水培养方式缩短启动时间,使污泥更快成熟,在脱氮除磷方面先达到最佳去除速率。JI等[11]和LI等[12]在氮负荷分别为630、104.8 mg·(L·d)−1时采用连续式运行,厌氧氨氧化功能菌丰度分别达到34.6%、40.2%;而DU等[13]和LI等[14]采用间歇式运行,氮负荷分别在100、280 mg·(L·d)−1时,厌氧氨氧化功能菌丰度分别为2.37%、5.01%。通过对比分析发现,连续式运行的厌氧氨氧化菌的相对丰度往往高于间歇式运行;但对于改变运行方式对于厌氧氨氧化菌影响的研究目前鲜有报道,对于厌氧氨氧化过程脱氮性能和菌群结构的影响的研究也不多。
本研究以厌氧移动床生物膜反应器为研究对象,在(25±1) ℃恒温、低基质(TN≤60 mg·L−1)条件下,由连续式运行改为间歇式运行,探究其对Anammox系统脱氮性能的影响,同时应用高通量测序方法分析系统微生物群落结构的变化,为实际污水处理厂的转型提供参考。
-
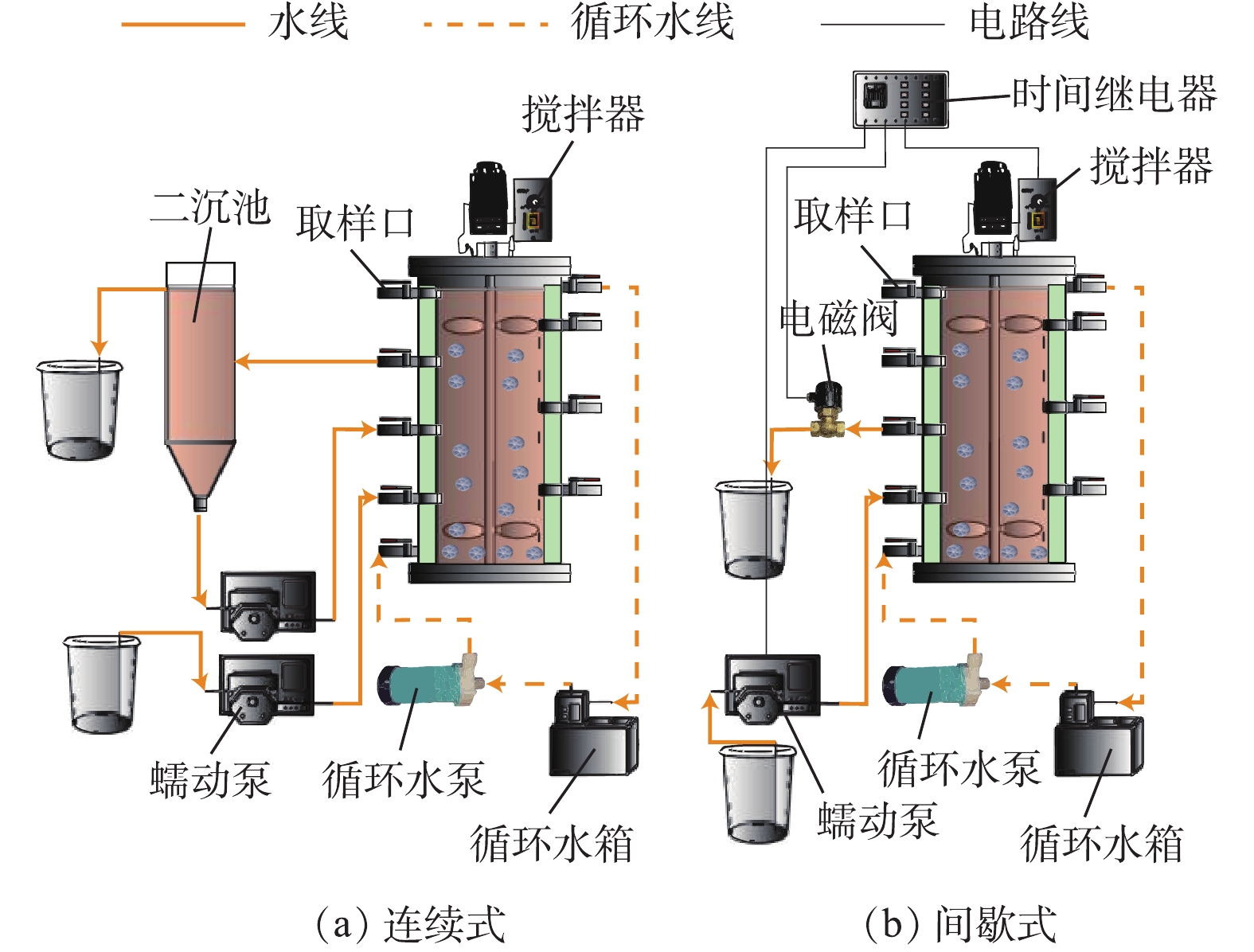
实验采用厌氧移动床生物膜反应器(anaerobic moving bed biofilm reactor,AMBBR)运行Anammox工艺。AMBBR所有部件均由有机玻璃加工而成,内径为15 cm,外径为26 cm,高为80 cm,有效体积为7.2 L。反应器用黑色遮光布覆盖,以避免光照对Anammox菌代谢的影响。反应器内部装有填料(直径为11 mm,体积分数为20%),作为微生物附着生长的载体。进水pH调节至7.2~7.8。反应器的外部为恒温水浴层,控制反应器温度为(25±1) ℃(图1)。
实验分为2个阶段:阶段1(0~39 d)采用连续式运行,流量为1.25 L·h−1,HRT为5.76 h;阶段2(40~110 d)采用间歇式运行,1个周期为3 h,进水45 min,厌氧混合105 min,沉淀15 min,出水13 min,闲置2 min。2个阶段的容积交换率均为55%。
-
反应器接种的污泥来自于长期运行的Anammox厌氧移动床生物膜反应器(AMBBR),该反应器在25 ℃下运行超过300 d。
实验使用的模拟废水为人工配制,其组成为:KHCO3 120 mg·L−1,K2CO3 27.5 mg·L−1,KH2PO4 126 mg·L−1,NH4Cl 91.7 mg·L−1,NaNO2153.8 mg·L−1,葡萄糖和淀粉各50 mg·L−1(少量有机物可以去除厌氧氨氧化产生的硝酸氮,提高总氮脱除率[15]);微量元素I和II各1 mL·L−1。微量元素浓缩液I组分为FeSO4·7H2O 9 000 mg·L−1,EDTA-Na 5 000 mg·L−1;微量元素浓缩液II组分为CoCl2·6H2O 400 mg·L−1,NiCl2·6H2O 810 mg·L−1,Na2MoO4·2H2O 250 mg·L−1,ZnCl2 210 mg·L−1,MnCl2·4H2O 360 mg·L−1。
-
水样经0.45 μm滤膜过滤后测定。水样COD经快速消解仪(5B-1B型)消解后,采用重铬酸钾法测定,pH采用雷磁PHS-3E型pH计测定,
NH+4 、NO−2 和NO−3 根据国家标准方法测定。总氮(TN)为NO−2 -N、NO−3 -N和NH+4 -N的加和。实验中所采用的污泥来自于2个阶段前后悬浮和附着2部分的污泥(第2天和第107天),一共4个样品,采样离心后,统一保存于−20 ℃冰箱中。从接种物和操作阶段结束时的污泥样品中提取DNA,用于PCR扩增,使用引物341F:CCTACGGGNGGCWGCAG和805R:GACTACHVGGGTATCTAATCC[16],委托上海生物工程有限公司完成测序。
-
厌氧氨氧化和反硝化的占比可以使用方程式[17]计算(式(1)和式(2)),平均进水氮负荷、总氮变化量、总氮的平均去除率和平均氮去除速率使用方程式[14]计算(式(3)~式(6)),功能基因的相对变化量使用方程式计算(见式(7))。
式中:u1为厌氧氨氧化比例;u2为反硝化比例;C1为进水氨氮浓度;C2为出水氨氮浓度;C3为进水总氮浓度;C4为出水总氮浓度;L为平均进水氮负荷;Vin为单位时间进水量;Vw为工作体积;T为单位时间;ΔC为总氮变化量;E为总氮的平均去除率;R1为平均氮去除速率;R为功能基因相对变化率;ΔA为功能基因相对丰度变化量;A为连续运行时功能基因的相对丰度。
-
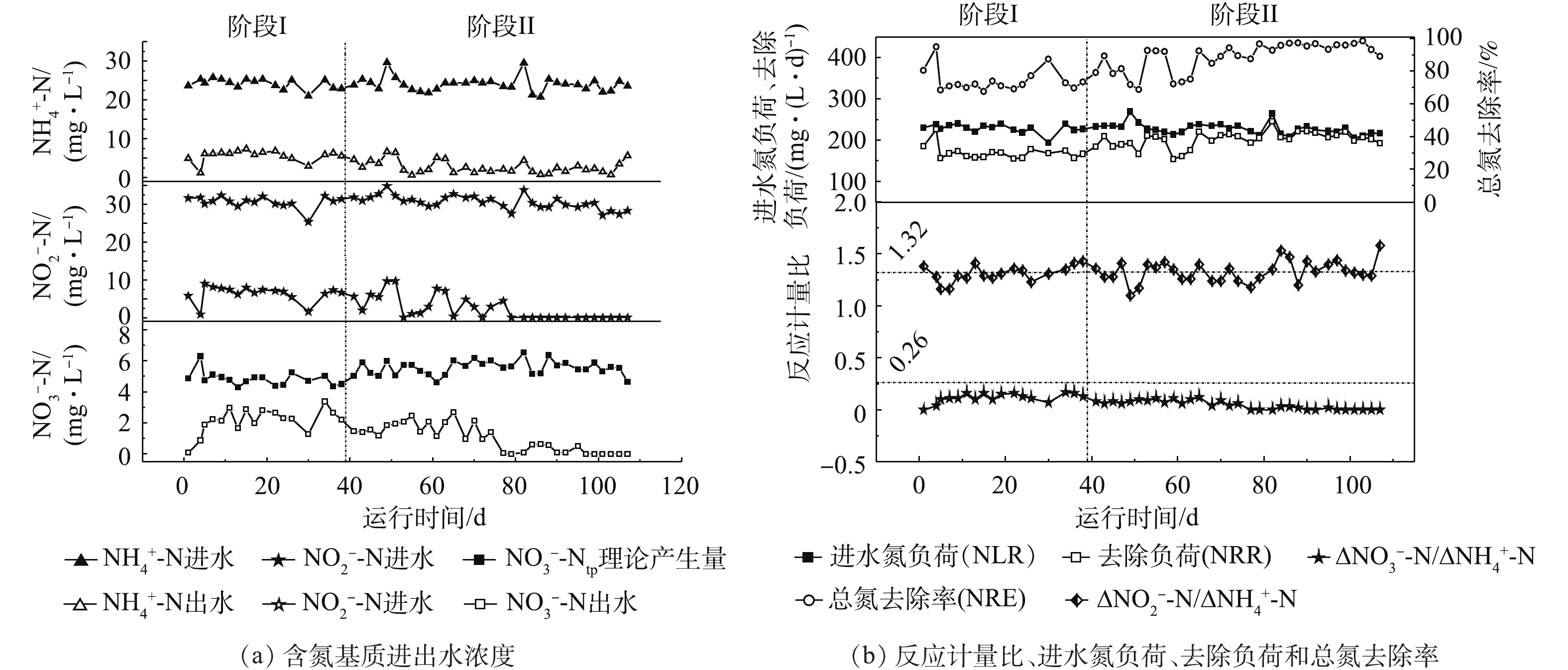
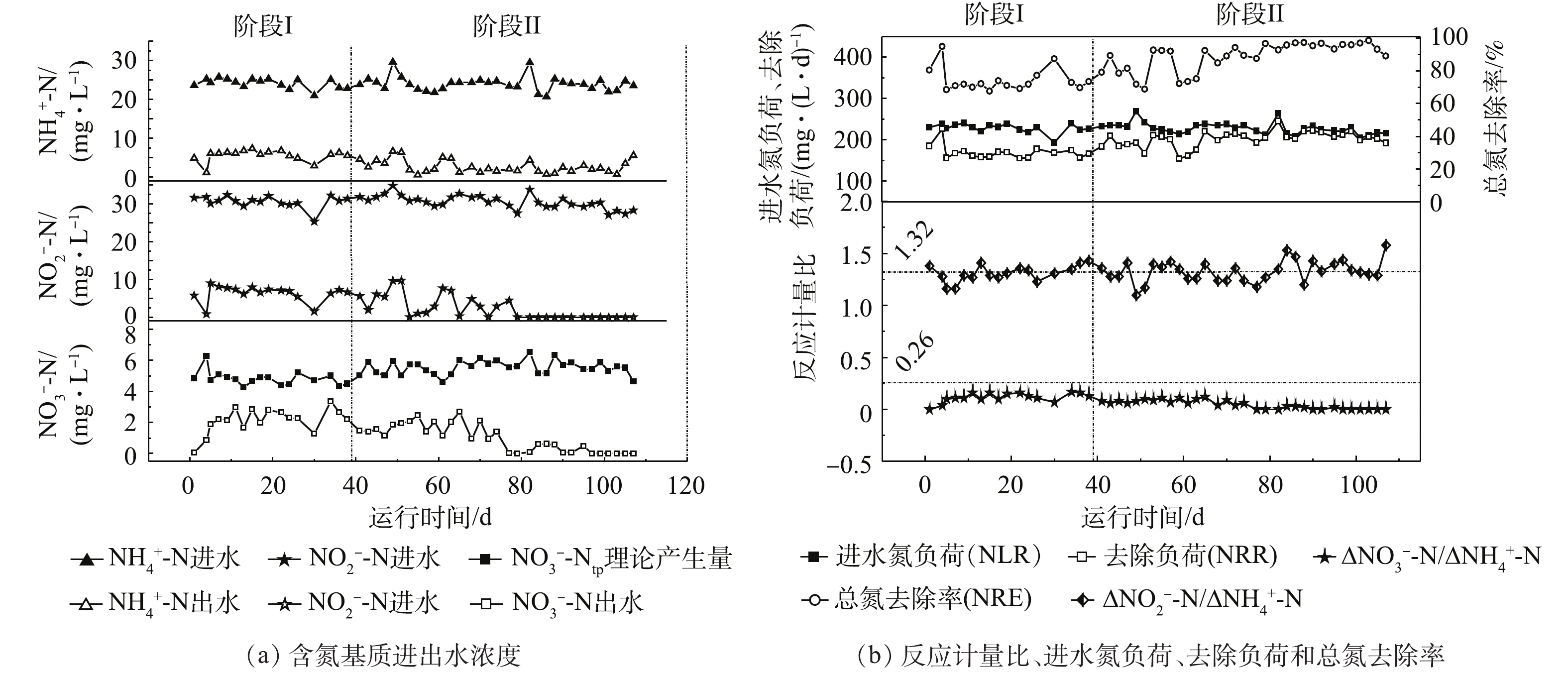
通过蠕动泵将模拟废水加入反应器中,并控制改变运行方式前后的进水水质参数、日处理水量和反应器有效工作体积不变的条件下,保证平均进水氮负荷、水力停留时间分别为(227±13) mg·(L·d)−1和5.76 h。不同阶段反应器除氮性能如图2所示。
在阶段I(1~39 d),反应器在室温(25±1) ℃下连续运行,出水氨、亚硝酸盐氮和硝酸盐氮平均浓度分别为5.6、6.4和2.12 mg·L−1;铵、亚硝酸盐和总氮的平均去除率分别为76.7%、79.3%和74.3%,平均氮去除速率为(166±20) mg·(L·d)−1。在此阶段,测定的
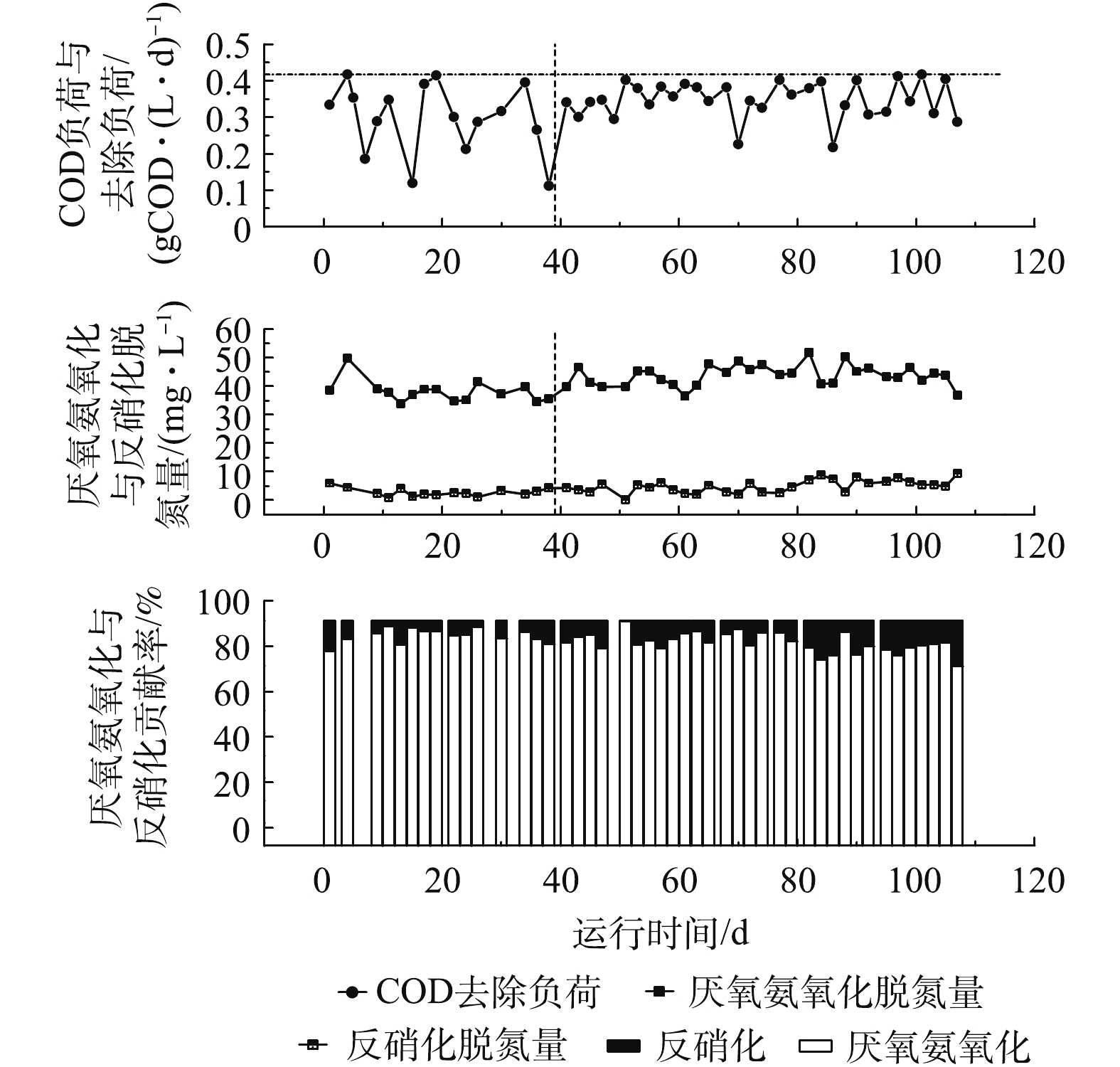
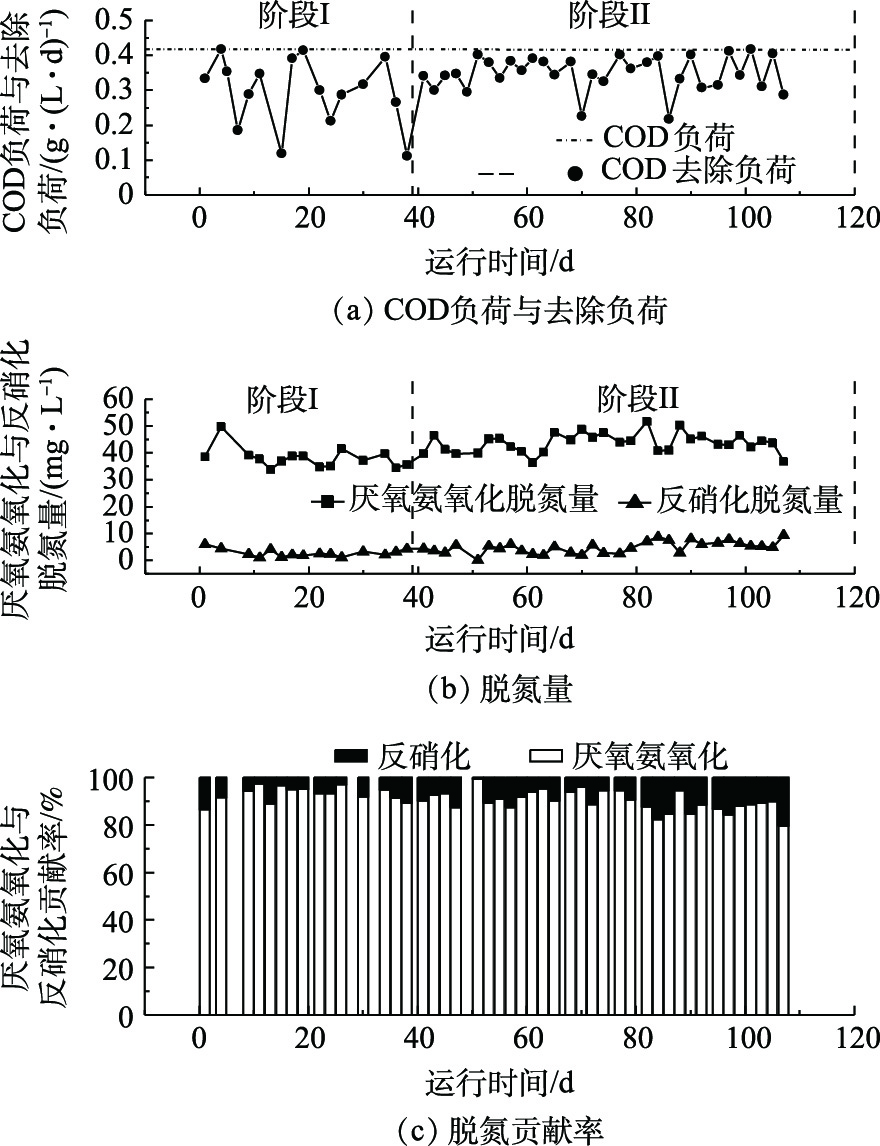
ΔNO−2 -N/ΔNH+4 -N为1.31(图2(b)),接近厌氧氨氧化过程理论值1.32[3],说明反应器内氮脱除过程主要是由厌氧氨氧化菌反应而引起的,与图3的计算结果一致。同时,所测ΔNO−3 -N/ΔNH+4 -N为0.12。根据STROUS等[3]的报道,厌氧氨氧化过程中ΔNO3-N/ ΔNH+4 -N的理论值为0.26,可得出硝酸盐平均理论产生量(NO−3 -Ntp)为4.83 mg·L−1。然而,出水中硝酸盐平均浓度仅为2.12 mg·L−1,远低于STROUS等[3]报道的理论值。分析其原因,是由于反应器进水中含有低浓度的有机物,有56%的硝酸氮(2.71 mg·L−1)被异养反硝化作用还原生成氮气。理论上,还原2.71 mg·L−1硝酸氮,异养反硝化须消耗COD(1 mg硝酸氮还原消耗2.86 mg COD)7.75 mg·L−1,反应器中去除的COD为70.98 mg·L−1,COD的去除负荷为300 mg·(L·d)−1,表明反硝化有机碳源充足。在阶段II(39~110 d),将反应器从连续式运行改成间歇式运行,容积交换率为0.55,其他操作参数负荷与阶段I保持一致。结果表明,Anammox-SBR系统的脱氮性能逐步上升。在39~63 d,平均氮去除速率和平均总氮去除率约为189.1 mg·(L·d)−1和81.8%(图2(b)),至65 d达到最高,为220.3 mg·(L·d)−1和92.5%(图2(b)),其氨氮、亚硝氮及硝酸盐出水浓度分别为1.25、0.33和2.68 mg·L−1。在64~107 d,平均氮去除速率和平均总氮去除率约为210.4 mg·(L·d)−1和93.6%(图2(b)),此时硝酸盐氮理论产生量(
NO−3 -Ntp)为5.67 mg·L−1[3],实际平均出水硝酸盐氮浓度为0.53 mg·L−1(图2(a)),有将近90.6%的硝酸氮(5.14 mg·L−1)被还原,比阶段I硝酸氮还原率提高了近1.89倍。而COD的去除负荷为350 mg·(L·d)−1(图3),与阶段I相比,只提高了50 mg·(L·d)−1。可推测出,系统硝酸盐还原能力大幅度增强。高通量测序发现,具有部分反硝化作用的Pseudomonas菌[18]丰度出现明显升高,强化了部分反硝化过程,进而降低出水硝酸氮浓度。分析导致这种现象原因有2个。第一,间歇式运行对悬浮污泥具有良好的截留作用,反应器中的搅拌增强了系统整体传质效果,提高了反应速率,进而提高了出水水质。底物与微生物之间的实际接触决定了厌氧反应器的性能,有效的搅拌促进二者的良好接触,从而提高基质的降解和转化效率[19-21]。第二,间歇式运行时,基质浓度随时间的延长由高到低变化,在连续式运行时,反应基质始终维持在较低的水平。根据一级反应动力学可知,间歇式运行时,基质降解速率较快,氮负荷相同时,间歇式出水水质效果更好。阶段II测定的化学计量系数(∆NO2-N/∆
NH+4 -N)为1.31,∆NO3-N/∆NH+4 -N(RP)由阶段I的0.12下降为0.08(39~63 d),后下降为0.02(64~107 d)(图2(b))。在阶段I,厌氧氨氧化和反硝化脱除氮浓度分别为38.15 mg·L−1和2.71 mg·L−1;在阶段II前期(39~63 d),厌氧氨氧化和反硝化脱氮浓度分别为42.12 mg·L−1和3.80 mg·L−1(图3),与阶段I相比,厌氧氨氧化和反硝化脱氮浓度分别提高了3.97 mg·L−1和1.09 mg·L−1;在阶段II后期(64~107 d),厌氧氨氧化和反硝化脱氮浓度分别为44.78 mg·L−1和5.60 mg·L−1(图3),与阶段II前期(39~63 d)相比,厌氧氨氧化和反硝化脱氮浓度分别提高了2.66 mg·L−1和1.80 mg·L−1。这说明改变运行方式后,厌氧氨氧化与反硝化协同作用得到了明显强化,同时反硝化贡献率逐渐提高(图3)。 -
连续式和间歇式运行共产生了275 797个有效序列,其附属于2 770个OTU(3%差异),398个属和17个门。OTU数量和ACE指数表明,在不同的运行方式下,生物膜具有更高的群落丰富度。Simpson指数反映了活性污泥微生物群落多样性(表1)。Simpson指数越高,意味着微生物菌种多样性越低,在不同的运行方式下,悬浮污泥Simpson指数均高于生物膜样品。生物膜样品微生物菌种多样性较高,这与WANG等[22]研究结果一致,其原因是生物膜的形成有利于增加对环境压力的抵抗力。但是,改变运行方式后,悬浮污泥Simpson指数升高,而生物膜Simpson指数却出现下降。这可能是由于改变运行方式后,装置内部污泥量基本不变,但COD去除负荷增加,导致异养菌生长速率提高,污泥产量升高[23],进而将一些生长速率较慢的细菌洗脱,导致悬浮污泥多样性降低;而填料对于活性污泥有一定的截留作用,故生物膜污泥的多样性变化不大,甚至多样性升高。
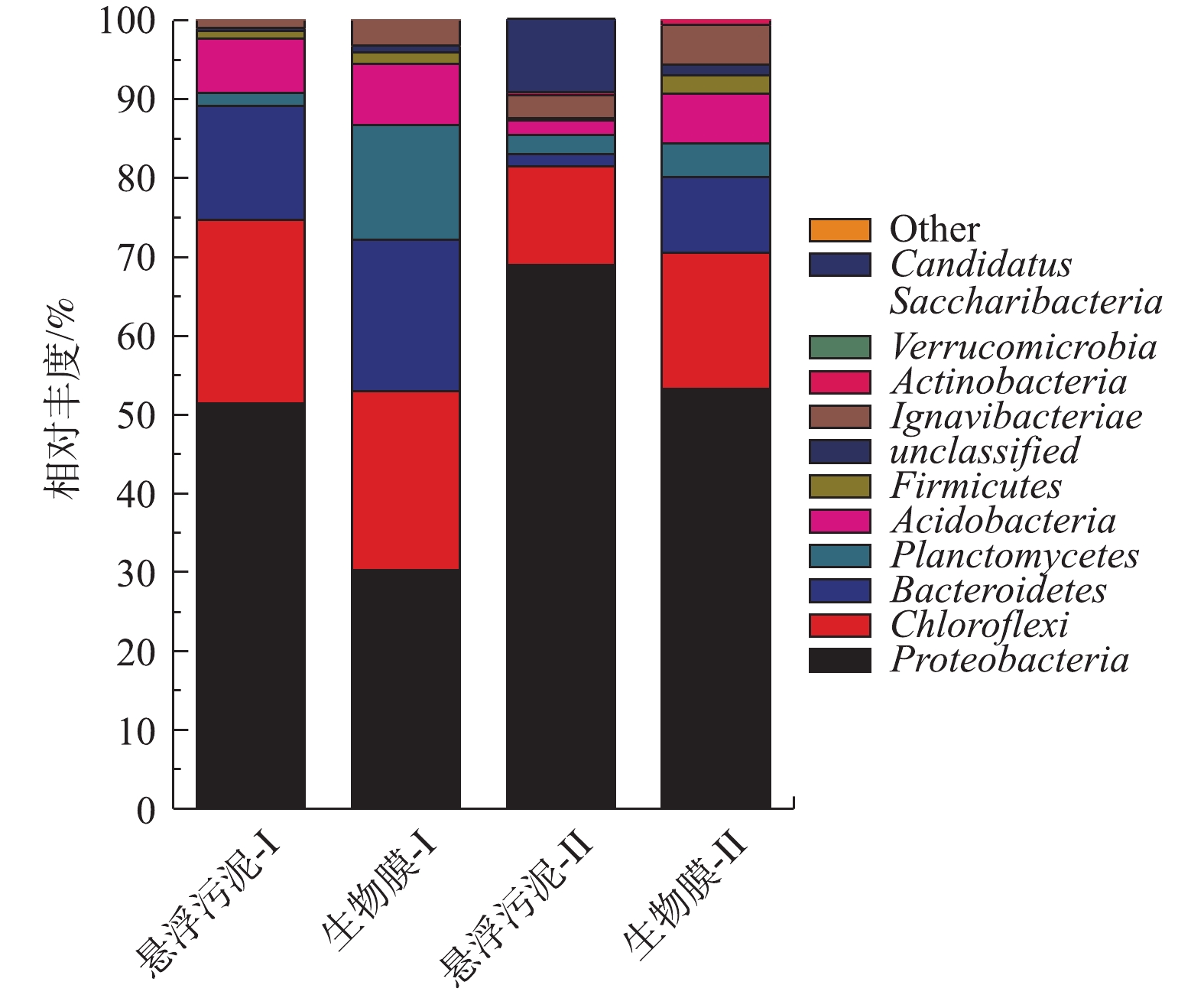
在门水平上(图4),最丰富的门是Proteobacteria,其次是Chloroflexi、Bacteroidetes、Planctomycetes和Acidobacteria,这与大多数研究结果[24-27]一致。与连续运行相比,间歇运行时,Chloroflexi、Bacteroidetes和Acidobacteria的比例同时下降,具有厌氧氨氧化作用的Planctomycetes在附着污泥上相对丰度也出现明显的降低;而Proteobacteria、Ignavibacteriae和Candidatus Saccharibacteria出现了不同程度的上升。这些结果表明,改变运行方式会影响细菌群落的组成。
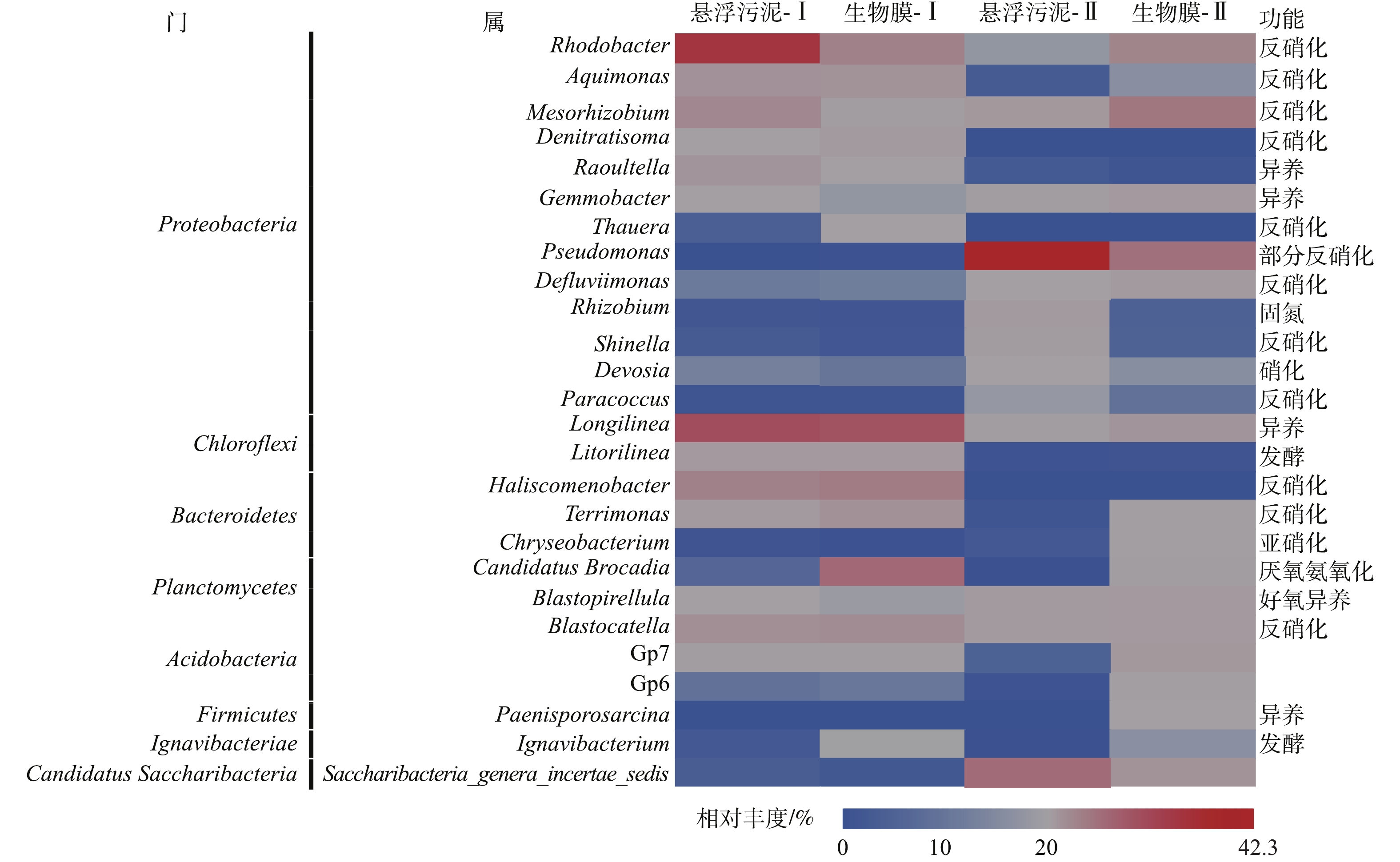
在厌氧氨氧化过程中,迄今已鉴定出5种厌氧氨氧化菌属,包括Candidatus Brocadia、Candidatus Kuenenia、Candidatus Scalindua、Candidatus Anammoxoglobus和Candidatus Jettenia[28]。 在本研究中,Candidatus Brocadia是厌氧氨氧化反应器中唯一的厌氧氨氧化菌属(图5),与大多数研究[11, 18, 29]相似。
改变运行方式后,作为厌氧氨氧化反应的功能菌Candidatus Brocadia丰度出现明显下降(图5)。推测其原因,可能是:在运行方式改变后,装置内部污泥量基本不变,但在COD去除负荷增加时,异养菌生长速率提高,导致污泥产量增加[23],从而使悬浮污泥中的Candidatus Brocadia丰度降低;同时生物膜Candidatus Brocadia丰度下降,可能是由于细胞微聚集体的Longilinea属[30]丰度下降而导致的。此外还发现,在2种不同的运行方式下,生物膜上的Candidatus Brocadia丰度高于悬浮污泥,这与LAURENI等[31]和LIU等[9]的研究结果一致。LIU等[9]认为,生长速率缓慢的微生物在废水处理系统中倾向于附着生长或形成簇,从而能承受更高的冲击负荷,并防止它们被流出物洗脱。
改变运行方式后,检测到Rhodobacter、Aquimonas、Denitratisoma、Haliscomenobacter、Terrimonas、Blastocatella[9, 18, 32-33],这些具有全程反硝化作用的菌属丰度出现不同程度降低。同时,Defluviimonas、Shinella、Paracoccus这几种具有全程反硝化作用的菌属丰度有所上升,其中具有部分反硝化作用的Pseudomonas丰度出现明显提高(生物膜11.5%,悬浮污泥42.3%)[18],因此,在间歇运行时,出水硝酸氮浓度明显降低。高通量检测结果还显示,反应器中存在少量能将氨氮氧化成亚硝酸氮的细菌(Chryseobacterium,Devosia,Chryseobacterium[34])以及能将亚硝酸氮氧化成为硝酸氮的细菌(Devosia[35])。这些好氧菌的存在可能是由于反应器上部有少量的空气,在搅拌时,反应体系有一个复氧的过程,这为好氧菌提供了一定的生存空间。此外,反应器中存在少量不具备脱氮作用的细菌。Saccharibacteria_genera_incertae_sedis属于Candidatus Saccharibacteria门,它们广泛分布于环境中[36]。Gemmobacter属包含兼性厌氧菌和异养细菌,它们可以促进大分子碳源的降解并转化为小分子碳化合物[37-38],具有降解有机化合物的能力,如糖、丙酮酸、甲醇、乙醇和酸[39]。这些好氧与异养菌的存在,消耗了反应器中存在的少量溶解氧和有机物,为厌氧氨氧化菌创造了有利的生存环境。
-
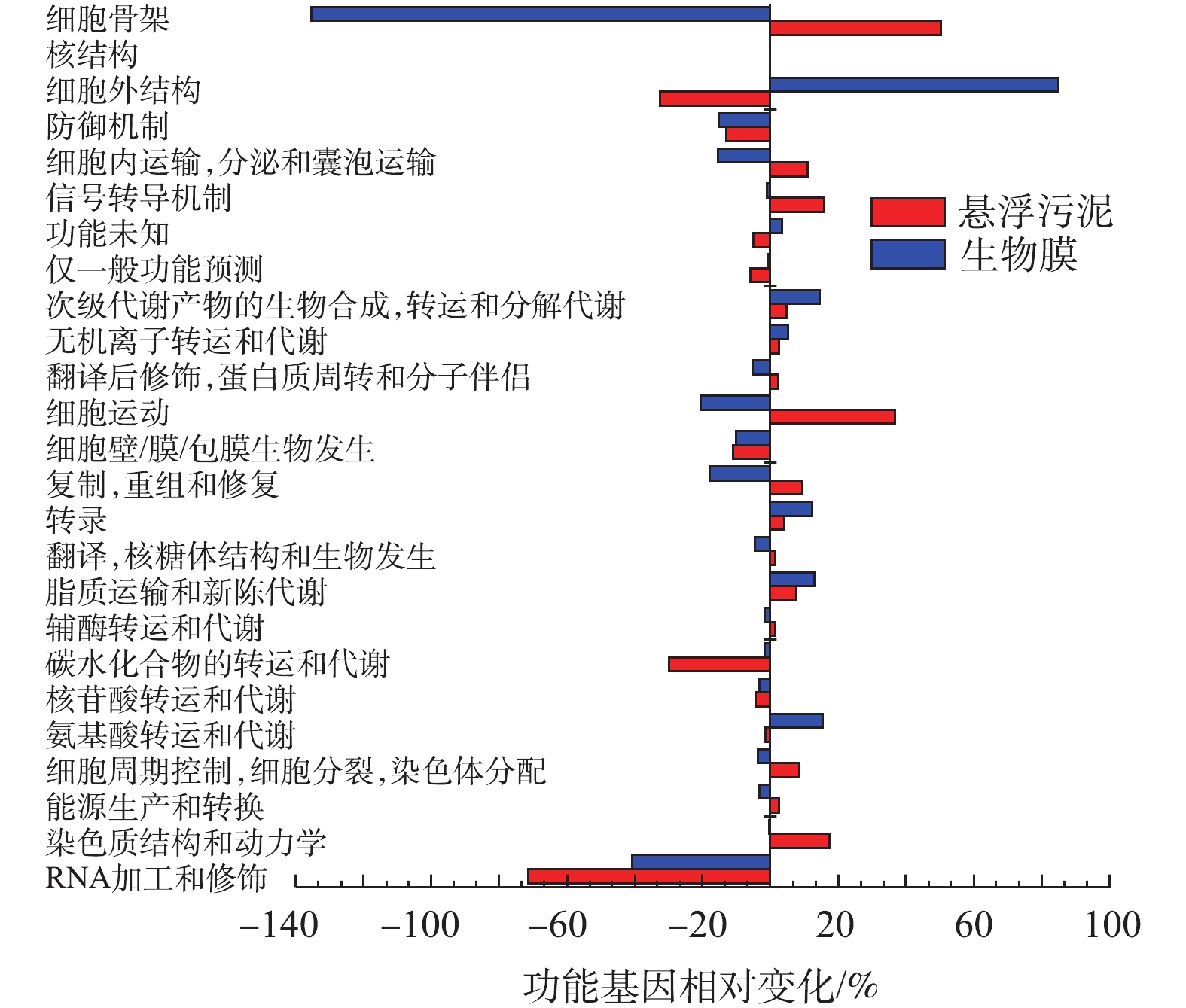
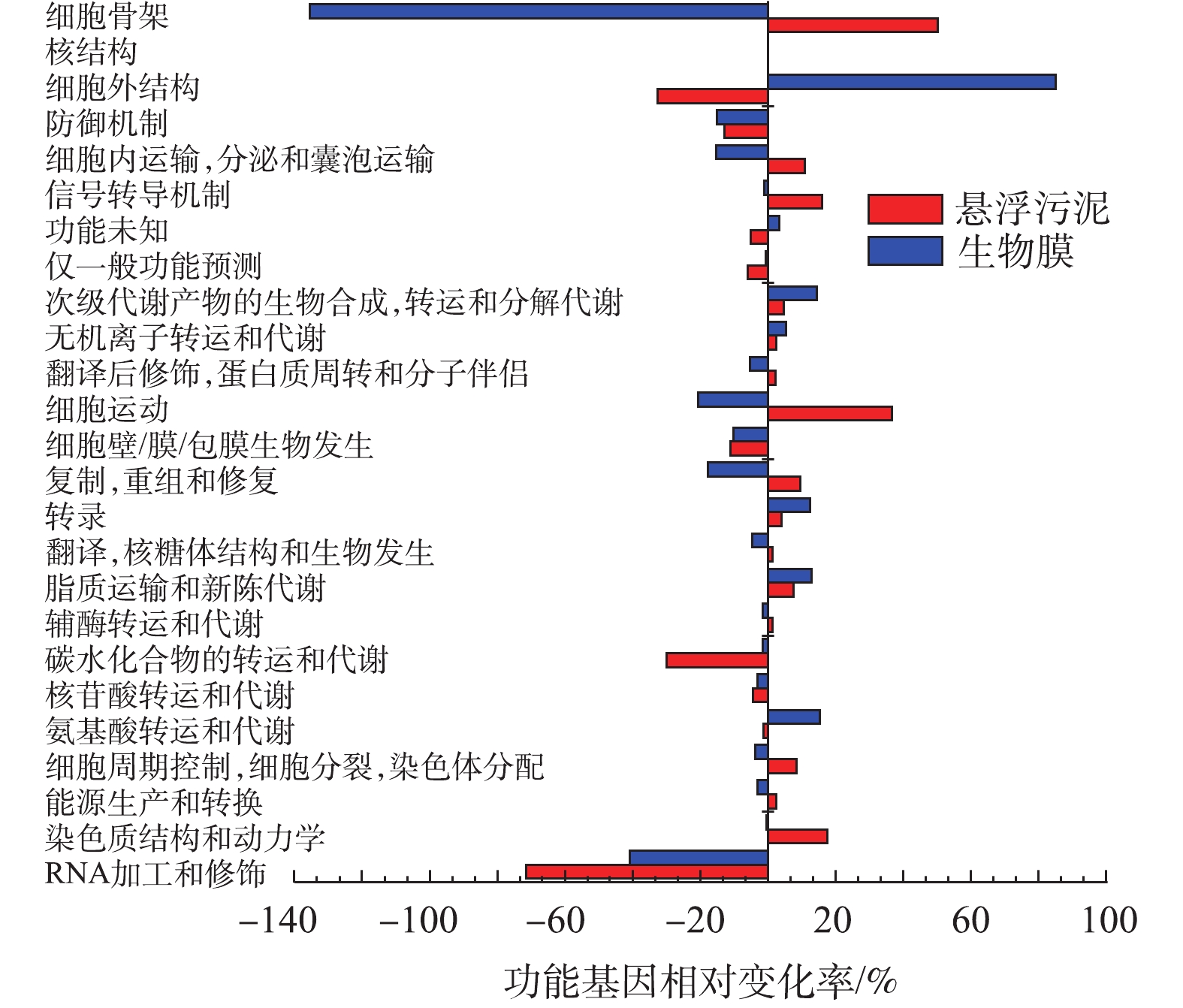
为深入了解改变运行方式后细菌微生物群的分子功能,对16S rRNA扩增子测序结果进行功能预测。基于COGs数据库,预测功能基因相对变化率,结果如图6所示。可以看出,改变运行方式后,RNA加工和修饰、碳水化合物的转运和代谢的功能基因下降,细胞外结构功能基因在悬浮污泥中出现下降而在生物膜当中出现明显上升;同时发现,细胞骨架的功能基因在悬浮污泥中出现上升而在生物膜当中出现明显下降,这与能形成细胞骨架的Longilinea属丰度降低的结论一致。而碳水化合物的转运和代谢的功能基因下降似乎与第1阶段中COD的去除负荷较低相悖,分析其原因,可能是因为连续运行时,系统COD长期处于一个较低的浓度,在这种“饥饿”的情况下,某些细菌须表达尽可能多的碳水化合物的转运和代谢的功能基因,摄取环境中的COD,来维持自身生长代谢需要。
-
1)在适量有机物的条件下,间歇式运行使具有短程反硝化功能的Pseudomonas菌的丰度明显升高,从而导致厌氧氨氧化和反硝化过程耦合作用增强,因此,系统获得更高的脱氮性能。
2)在适量有机物的条件下,间歇式运行使具有厌氧氨氧化作用的Candidatus Brocadia菌丰度降低。
3)在连续式和间歇式运行方式下,生物膜上的Candidatus Brocadia菌丰度高于悬浮污泥。
运行方式对低基质厌氧氨氧化系统脱氮及菌群结构的影响
Effect of operation modes on the nitrogen removal efficiency and microbial community structure of anammox process treating low substrate wastewater
-
摘要: 为了考察运行方式对厌氧氨氧化系统脱氮性能及菌群结构的影响,建立一套厌氧移动床生物膜反应器,在(25±1) ℃恒温、低基质(TN≤60 mg·L−1)条件下,分别以连续式和间歇式方式运行,采用高通量测序,基于直系同源蛋白簇基因(COGs),对16S rRNA扩增子测序结果进行功能预测,来表征微生物菌群结构和微生物功能的变化。结果表明:系统总氮负荷为(227±13) mg·(L·d)−1时,间歇式运行脱氮效率(90.6%)优于连续式运行效率(74.6%),生物膜厌氧氨氧化细菌的相对丰度高于悬浮污泥;反应器由连续式变为间歇式运行后,主要功能菌属Ca. Brocadia丰度降低,同时,具有部分反硝化作用的Pseudomonas菌丰度出现明显升高。进一步分析可知,在适量的有机物条件下,间歇式运行能够获得更好的厌氧氨氧化与反硝化协同处理效果。本研究结果可为污水处理厂的实际运行提供参考。Abstract: In order to investigate the effect of operation modes on the nitrogen removal performance and microbial community structure of anaerobic ammonia oxidation system, an anaerobic moving bed biofilm reactor was established, which was operated in a continuous flow mode and a batch mode at constant temperature of (25±1) ℃ and low substrate of TN≤60 mg·L−1, respectively, used high-throughput sequencing, and based on cluster of orthologous groups of proteins (COGs) gene, 16S rRNA amplicon sequencing results were functionally predicted to characterize microbial flora structure and microbial function changes. The results showed that the nitrogen removal efficiency for the batch mode was 90.6%, which was higher than 74.6% for the continuous operation mode at the total nitrogen load of 227±13 mg·(L·d)−1. And the relative abundance of anammox bacteria in biofilm was higher than that in the suspended sludge. When the continuous mode of the reactor changed to batch mode, the abundance of main functional genus Ca. Brocadia decreased, while the abundance of bacteria with partial denitrification function increased significantly. Through further analysis, the batch mode can obtain better synergistic treatment effect of anammox and denitrification than continuous mode with proper amount of organic matter. The study provides a reference for the actual operation of wastewater treatment plant.
-
Key words:
- continuous operation /
- intermittent operation /
- low substrate /
- microbial community
-
目前,废水的脱氮除磷是解决环境水体富营养化问题的关键[1]。污水处理厂中氮元素的脱除主要是基于好氧硝化和缺氧反硝化的生物脱氮工艺。20世纪90年代,MULDER[2]发现一种新型的厌氧氨氧化细菌,该菌在厌氧条件下以亚硝酸盐(
NO−2 )为电子受体,将铵(NH+4 )氧化成气态氮(N2),并产生少量硝酸盐(NO−3 )[3],这一过程称为厌氧氨氧化过程(anaerobic ammonium oxidation,Anammox)。Anammox反应与传统脱氮工艺(硝化/反硝化)系统相比,具有氮去除率更高,生物反应器体积更小,温室气体排放更低,污泥产量更低(90%)等优点[4-7]。因此,该工艺被广泛应用于高氨氨废水处理[8]。然而,厌氧氨氧化菌用于处理低基质市政废水仍然比较困难[9]。此外,JIN等[6]发现,各种底物和化学物质会影响厌氧氨氧化过程,其中包括游离氨(free ammonia,FA)、游离亚硝酸(free nitrous acid,FNA)、有机物质、盐、重金属、磷酸盐和硫化物。反应器运行方式分为连续式与间歇式2种。已有研究结果[10]表明,运行方式影响倒置A2/O工艺启动性能,间歇换水培养方式缩短启动时间,使污泥更快成熟,在脱氮除磷方面先达到最佳去除速率;JI等[11]和LI等[12]在氮负荷分别为630、104.8 mg·(L·d)−1时采用连续式运行,厌氧氨氧化功能菌丰度分别达到34.6%、40.2%;而DU等[13]和LI等[14]采用间歇式运行,氮负荷分别在100、280 mg·(L·d)−1时,厌氧氨氧化功能菌丰度分别为2.37%、5.01%。通过对比分析发现,连续式运行的厌氧氨氧化菌的相对丰度往往高于间歇式运行;但对于改变运行方式对于厌氧氨氧化菌影响的研究目前鲜有报道,对于厌氧氨氧化过程脱氮性能和菌群结构的影响的研究也不多。
本研究以厌氧移动床生物膜反应器为研究对象,在(25±1) ℃恒温、低基质(TN≤60 mg·L−1)条件下,由连续式运行改为间歇式运行,探究其对Anammox系统脱氮性能的影响,同时应用高通量测序方法分析系统微生物群落结构的变化,为实际污水处理厂的转型提供参考。
1. 材料与方法
1.1 实验装置
实验采用厌氧移动床生物膜反应器(anaerobic moving bed biofilm reactor,AMBBR)运行Anammox工艺。AMBBR所有部件均由有机玻璃加工而成,内径为15 cm,外径为26 cm,高为80 cm,有效体积为7.2 L。反应器用黑色遮光布覆盖,以避免光照对Anammox菌代谢的影响。反应器内部装有K1(直径11 mm,体积比20%)填料,作为微生物附着生长的载体。进水pH调节至7.2~7.8。反应器的外部为恒温水浴层,控制反应器温度为(25±1) ℃(图1)。
实验分为2个阶段:阶段1(0~39 d)采用连续式运行,流量为1.25 L·h−1,HRT为5.76 h;阶段2(40~110 d)采用间歇式运行,1个周期为3 h,进水45 min,厌氧混合105 min,沉淀15 min,出水13 min,闲置2 min。2个阶段的容积交换率均为55%。
1.2 接种污泥和模拟废水
反应器接种的污泥来自于长期运行的Anammox厌氧移动床生物膜反应器(AMBBR),该反应器在25 ℃下运行超过300 d。
实验使用的模拟废水为人工配制,其组成为:KHCO3 120 mg·L−1,K2CO3 27.5 mg·L−1,KH2PO4 126 mg·L−1,NH4Cl 91.7 mg·L−1,NaNO2153.8 mg·L−1,葡萄糖和淀粉各50 mg·L−1(少量有机物可以去除厌氧氨氧化产生的硝酸氮,提高总氮脱除率[15]);微量元素I和II各1 ml·L−1。微量元素浓缩液I组分为FeSO4·7H2O 9 000 mg·L−1,EDTA-Na 5 000 mg·L−1;微量元素浓缩液II组分为CoCl2·6H2O 400 mg·L−1,NiCl2·6H2O 810 mg·L−1,Na2MoO4·2H2O 250 mg·L−1,ZnCl2 210 mg·L−1,MnCl2·4H2O 360 mg·L−1。
1.3 实验方法
水样经0.45 μm滤膜过滤后测定。水样COD经快速消解仪(5B-1B型)消解后,采用重铬酸钾法测定,pH采用雷磁PHS-3E型pH计测定,
NH+4 、NO−2 和NO−3 根据国家标准方法测定。总氮(TN)为NO−2 -N、NO−3 -N和NH+4 -N的加和。实验中所采用的污泥来自于2个阶段前后悬浮和附着2部分的污泥(第2天和第107天),一共4个样品,采样离心后,统一保存于−20 ℃冰箱中。从接种物和操作阶段结束时的污泥样品中提取DNA,用于PCR扩增,使用引物341F:(CCTACGGGNGGCWGCAG)和805R:(GACTACHVGGGTATCTAATCC)[16],委托上海生物工程有限公司完成测序。
1.4 数据分析
厌氧氨氧化和反硝化的占比可以使用方程式[17]计算(见式(1)和式(2)),平均进水氮负荷、总氮变化量、总氮的平均去除率和平均氮去除速率使用方程式[14]计算(见式(3)~式(6)),功能基因的相对变化量使用方程式计算(见式(7))。
u1=(1+1.32−0.26)(C1−C2)C3−C4×100% (1) u2=100−u1 (2) L=C3⋅VinVw⋅T (3) ΔC=C3−C4 (4) E=ΔCC3 (5) R1=L⋅E (6) R=ΔAA (7) 式中:u1为厌氧氨氧化比例;u2为反硝化比例;C1为进水氨氮浓度;C2为出水氨氮浓度;C3为进水总氮浓度;C4为出水总氮浓度;L为平均进水氮负荷;Vin为单位时间进水量;Vw为工作体积;T为单位时间;ΔC为总氮变化量;E为总氮的平均去除率;R1为平均氮去除速率;R为功能基因相对变化量;ΔA为功能基因相对丰度变化量;A为连续运行时功能基因的相对丰度。
2. 结果与讨论
2.1 连续运行和间歇运行下反应器的脱氮性能
通过蠕动泵将模拟废水加入反应器中,并控制改变运行方式前后的进水水质参数、日处理水量和反应器有效工作体积不变的条件下,保证平均进水氮负荷、水力停留时间分别为(227±13) mg·(L·d)−1和5.76 h。不同阶段反应器除氮性能如图2所示。
在阶段I(1~39 d),反应器在室温(25±1) ℃下连续运行,出水氨、亚硝酸盐氮和硝酸盐氮平均浓度分别为5.6、6.4和2.12 mg·L−1;铵、亚硝酸盐和总氮的平均去除率分别为76.7%、79.3%和74.3%,平均氮去除速率为(166±20)mg·(L·d)−1。在此阶段,测定的
ΔNO−2−N/ΔNH+4 -N为1.31(图2(b)),接近厌氧氨氧化过程理论值1.32[3],说明反应器内氮脱除过程主要是由厌氧氨氧化菌反应而引起的,与图3的计算结果一致。同时,所测ΔNO−3−N/ΔNH+4 -N为0.12,根据STROUS等[3]的报道,厌氧氨氧化过程中ΔNO3−N/ ΔNH+4 -N的理论值为0.26,可得出硝酸盐平均理论产生量(NO−3 -Ntp)为4.83 mg·L−1。然而,出水中硝酸盐平均浓度仅为2.12 mg·L−1,远低于STROUS等[3]报道的理论值。分析其原因,是由于反应器进水中含有低浓度的有机物,有56%的硝酸氮(2.71 mg·L−1)被异养反硝化作用还原生成氮气,理论上,2.71 mg·L−1硝酸氮,异养反硝化须消耗COD(1 mg硝酸氮还原消耗2.86 mg COD)7.75 mg·L−1,反应器中去除的COD为70.98 mg·L−1,COD的去除负荷为300 mg·(L·d)−1,表明反硝化有机碳源充足。在阶段II(39~110 d),将反应器从连续式运行改成间歇式运行,容积交换律率为0.55,其他操作参数负荷与阶段I保持一致。结果表明,Anammox-SBR系统的脱氮性能逐步上升。在39~63 d,平均氮去除速率和平均总氮去除率约为189.1 mg·(L·d)−1和81.8%(图2(b)),至65 d达到最高,为220.3 mg·(L·d)−1和92.5%(图2(b)),其氨氮、亚硝氮及硝酸盐出水浓度分别为1.25、0.33和2.68 mg·L−1。在64~107 d,平均氮去除速率和平均总氮去除率约为210.4 mg·(L·d)−1和93.6%(图2(b)),此时硝酸盐氮理论产生量(
NO−3 -Ntp)为5.67 mg·L−1[3],实际平均出水硝酸盐氮浓度为0.53 mg·L−1(图2(a)),有将近90.6%的硝酸氮(5.14 mg·L−1)被还原,比阶段I硝酸氮还原率提高了近1.89倍。而COD的去除负荷为350 mg·(L·d)−1(图3),与阶段I相比,只提高了50 mg·(L·d)−1,可推测出,系统硝酸盐还原能力大幅度增强。高通量测序发现,具有部分反硝化作用的Pseudomonas菌[18]丰度出现明显升高,强化了部分反硝化过程,进而降低出水硝酸氮浓度。分析导致这种现象原因有2个。第一,间歇式运行对悬浮污泥具有良好的截留作用,反应器中的搅拌增强了系统整体传质效果,提高了反应速率,进而提高了出水水质。底物与微生物之间的实际接触决定了厌氧反应器的性能,有效地搅拌促进二者的良好接触,从而提高基质的降解和转化效率[19-21]。第二,间歇式运行时基质浓度随时间的延长由高到低变化,在连续式运行时,反应基质始终维持在较低的水平。根据一级反应动力学可知,间歇式运行时,基质降解速率较快,氮负荷相同时,间歇式出水水质效果更好。阶段II测定的化学计量系数(∆NO2-N/∆
NH+4 -N)为1.31,∆NO3-N/∆NH+4 -N(RP)由阶段I的0.12,下降为0.08(39~63 d),后下降为0.02(64~107 d)(图2(b))。在阶段I,厌氧氨氧化和反硝化脱除氮浓度分别为38.15 mg·L−1和2.71 mg·L−1;在阶段II前期(39~63 d),厌氧氨氧化和反硝化脱氮浓度分别为42.12 mg·L−1和3.80 mg·L−1(图3),与阶段I相比,厌氧氨氧化和反硝化脱氮浓度分别提高了3.97 mg·L−1和1.09 mg·L−1;在阶段II后期(64~107 d),厌氧氨氧化和反硝化脱氮浓度分别为44.78 mg·L−1和5.60 mg·L−1(图3),与阶段II前期(39~63 d)相比,厌氧氨氧化和反硝化脱氮浓度分别提高了2.66 mg·L−1和1.80 mg·L−1。这说明改变运行方式过后,厌氧氨氧化与反硝化协同作用得到了明显强化,同时反硝化贡献率逐渐提高(图3)2.2 微生物群落分析
连续式和间歇式运行共产生了275 797个有效序列,其附属于2 770个OTU(3%差异),398个属和17个门。OTU数量和ACE指数表明,在不同的运行方式下,生物膜具有更高的群落丰富度。Simpson指数反映了活性污泥微生物群落多样性(表1)。Simpson指数值越高,意味着微生物菌种多样性越低,在不同的运行方式下,悬浮污泥Simpson指数均高于生物膜样品。生物膜样品微生物菌种多样性较高,这与WANG等[22]研究结果一致,其原因是生物膜的形成有利于增加对环境压力的抵抗力。但是,改变运行方式后,悬浮污泥Simpson指数升高,而生物膜Simpson指数却出现下降。这可能是由于改变运行方式后,装置内部污泥量基本不变,但COD去除负荷增加,导致异养菌生长速率提高,污泥产量升高[23],进而将一些生长速率较慢的细菌洗脱,导致悬浮污泥多样性降低;而填料对于活性污泥有一定的截留作用,故生物膜污泥的多样性变化不大,甚至多样性升高。
表 1 改变运行方式后DNA样品的微生物丰富度和多样性评估Table 1. Evaluation of microbial richness and diversity of DNA samples after the change of operation mode样品 OTU ACE指数 Shannon指数 Simpson指数 悬浮污泥-I 768 1185.51 2.94 0.13 悬浮污泥t-I 586 649.83 2.74 0.19 生物膜-II 834 1216.77 3.54 0.07 生物膜-II 582 770.32 3.65 0.05 在门水平上(图4),最丰富的门是Proteobacteria,其次是Chloroflexi、Bacteroidetes、Planctomycetes和Acidobacteria,与大多数研究结果[24-28]一致。与连续运行相比,间歇运行时,Chloroflexi、Bacteroidetes和Acidobacteria的比例同时下降,具有厌氧氨氧化作用的Planctomycetes在附着污泥上相对分度也出现明显的降低;而Proteobacteria、Ignavibacteriae和Candidatus Saccharibacteria出现了不同程度的上升。这些结果表明,改变运行方式会影响细菌群落的组成。
在厌氧氨氧化过程中,迄今已鉴定出5种厌氧氨氧化菌属Candidatus Brocadia、Candidatus Kuenenia、Candidatus Scalindua、Candidatus Anammoxoglobus和Candidatus Jettenia[29]。在本次研究中,Candidatus Brocadia是厌氧氨氧化反应器中唯一的厌氧氨氧化菌属(图5),与大多数研究[11, 18, 30]相似。
改变运行方式后,作为厌氧氨氧化反应的功能菌Candidatus Brocadia丰度出现明显下降(图5)。推测其原因,可能是:在运行方式改变后,装置内部污泥量基本不变,但在COD去除负荷增加下,异养菌生长速率提高,导致污泥产量增加[23],从而使悬浮污泥当中的Candidatus Brocadia丰度降低;同时生物膜Candidatus Brocadia丰度下降,可能是由于作为细胞微聚集体的Longilinea属[31]丰度下降而导致的。此外还发现,在2种不同的运行的方式下,生物膜上的Candidatus Brocadia丰度高于悬浮污泥与LAURENI等[32]和LIU等[27]的研究结果一致。LIU等[27]认为,生长速率缓慢的微生物在废水处理系统中倾向于附着生长或形成簇,从而能承受更高的冲击负荷,并防止它们被流出物洗脱。
改变运行方式后,检测到Rhodobacter、Aquimonas、Denitratisoma、Haliscomenobacter、Terrimonas、Blastocatella[27, 33-35],这些具有全程反硝化作用的菌属丰度出现不同程度降低。同时,Defluviimonas、Shinella、Paracoccus这几种具有全程反硝化作用的菌属丰度有所上升,其中具有部分反硝化作用的Pseudomonas丰度出现明显提高(生物膜11.5%,悬浮污泥42.3%)[34],因此,在间歇运行时,出水硝酸氮浓度明显降低。高通量检测结果还显示,反应器中存在少量能将氨氮氧化成亚硝酸氮的细菌(Chryseobacterium,Devosia,Chryseobacterium[36]),以及能将亚硝酸氮氧化成为硝酸氮的细菌(Devosia[37])。这些好氧菌的存在可能是由于反应器上部有少量的空气,在搅拌时,反应体系有一个复氧的过程,这为好氧菌提供了一定的生存空间。此外,反应器中存在少量不具备脱氮作用的细菌。Saccharibacteria_genera_incertae_sedis属于Candidatus Saccharibacteria门,它们广泛分布于环境中[38]。Gemmobacter属包含兼性厌氧菌和异养细菌,它们可以促进大分子碳源的降解并转化为小分子碳化合物[39-40],具有降解有机化合物的能力,如糖、丙酮酸、甲醇、乙醇和酸[41]。这些好氧与异养菌的存在,消耗了反应器中存在的少量溶解氧和有机物,为厌氧氨氧化菌创造了有利的生存环境。
2.3 反应器的微生物功能预测
为深入了解改变运行方式后细菌微生物群的分子功能,采用PICRUSt,通过在直系同源蛋白簇(COGs)中输入可用的注释基因,来预测微生物功能的变化。基于COGs数据库,如图6所示,发现改变运行方式后,RNA加工和修饰、碳水化合物的转运和代谢的功能基因下降,细胞外结构功能基因在悬浮污泥中出现下降而在生物膜当中出现明显上升;同时发现,细胞骨架的功能基因在悬浮污泥中出现上升而在生物膜当中出现明显下降,这与能形成细胞骨架的Longilinea属丰度降低的结论一致。而碳水化合物的转运和代谢的功能基因下降似乎与第1阶段中COD的去除负荷较低相悖,分析其原因,可能是因为连续运行时,系统COD长期处于一个较低的浓度,在这种“饥饿”的情况下,某些细菌须表达尽可能多的碳水化合物的转运和代谢的功能基因,摄取环境中的COD,来维持自身生长代谢需要。
3. 结论
1)在适量有机物的条件下,间歇式运行使具有短程反硝化功能的Pseudomonas菌的丰度明显升高,从而导致厌氧氨氧化和反硝化过程耦合作用增强,因此,系统获得更高的脱氮性能。
2)在适量有机物的条件下,间歇式运行使具有厌氧氨氧化作用的Candidatus Brocadia菌丰度降低。
3)在连续式和间歇式运行方式下,生物膜上的Candidatus Brocadia菌丰度高于悬浮污泥。
-
表 1 改变运行方式后DNA样品的微生物丰富度和多样性评估
Table 1. Evaluation of microbial richness and diversity of DNA samples after the change of operation mode
样品 OTU/个 ACE指数 Shannon指数 Simpson指数 悬浮污泥-I 768 1 185.51 2.94 0.13 悬浮污泥-I 586 649.83 2.74 0.19 生物膜-II 834 1 216.77 3.54 0.07 生物膜-II 582 770.32 3.65 0.05 -
[1] XU X C, QIU L Y, WANG C, et al. Achieving mainstream nitrogen and phosphorus removal through simultaneous partial nitrification, anammox, denitrification, and denitrifying phosphorus removal (SNADPR) process in a single-tank integrative reactor[J]. Bioresource Technology, 2019, 284: 80-89. doi: 10.1016/j.biortech.2019.03.109 [2] MULDER A, VANDEGRAAF A A, ROBERTSON L A, et al. Anaerobic ammonium oxidation discovered in a denitrifying fluidized-bed reactor[J]. FEMS Microbiology Ecology, 1995, 16(3): 177-183. doi: 10.1111/j.1574-6941.1995.tb00281.x [3] STROUS M, HEIJNEN J J, KUENEN J G, et al. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms[J]. Applied Microbiology and Biotechnology, 1998, 50(5): 589-596. doi: 10.1007/s002530051340 [4] VAN DONGEN U, JETTEN M S, VAN L, et al. the SHARON-Anammox process for treatment of ammonium rich wastewater[J]. Water Science & Technology, 2001, 44(1): 153-160. [5] SIEGRIST H, SALZGEBER D, EUGSTER J, et al. Anammox brings WWTP closer to energy autarky due to increased biogas production and reduced aeration energy for N-removal[J]. Water Science & Technology, 2008, 57(33): 383-388. [6] JIN R C, YANG G F, YU J J, et al. The inhibition of the anammox process: A review[J]. Chemical Engineering Science, 2012, 197: 67-79. doi: 10.1016/j.cej.2012.05.014 [7] LACKNER S, GILBERT E M, VLAEMINCK S E, et al. Full-scale partial nitritation/anammox experiences: An application survey[J]. Water Research, 2014, 55: 292-303. doi: 10.1016/j.watres.2014.02.032 [8] TANG C J, ZHENG P, WANG C H, et al. Performance of high-loaded ANAMMOX UASB reactors containing granular sludge[J]. Water Research, 2011, 45(1): 135-144. doi: 10.1016/j.watres.2010.08.018 [9] LIU W R, YANG D H, CHEN W J, et al. High-throughput sequencing-based microbial characterization of size fractionated biomass in an anoxic anammox reactor for low-strength wastewater at low temperatures[J]. Bioresource Technology, 2017, 231: 45-52. doi: 10.1016/j.biortech.2017.01.050 [10] 凌琪, 曾麒峰, 伍昌年, 等. 不同换水方式在污泥培养驯化中的比较及在倒置A2/O启动研究[J]. 应用化工, 2015, 44: 991-994. [11] JI J T, PENG Y Z, MAI W K, et al. Achieving advanced nitrogen removal from low C/N wastewater by combining endogenous partial denitrification with anammox in mainstream treatment[J]. Bioresource Technology, 2018, 270: 570-579. doi: 10.1016/j.biortech.2018.08.124 [12] LI X J, SUN S, YUAN H Y, et al. Mainstream upflow nitritation-anammox system with hybrid anaerobic pretreatment: Long-term performance and microbial community dynamics[J]. Water Research, 2017, 125: 298-308. doi: 10.1016/j.watres.2017.08.048 [13] DU R, CAO S B, LI B K, et al. Performance and microbial community analysis of a novel DEAMOX based on partial-denitrification and anammox treating ammonia and nitrate wastewaters[J]. Water Research, 2017, 108: 46-56. doi: 10.1016/j.watres.2016.10.051 [14] LI Q, WANG S P, ZHANG P D, et al. Influence of temperature on an Anammox sequencing batch reactor (SBR) system under lower nitrogen load[J]. Bioresource Technology, 2018, 269: 50-56. doi: 10.1016/j.biortech.2018.08.057 [15] LI X, LU M Y, QIU Q C, et al. The effect of different denitrification and partial nitrification-Anammox coupling forms on nitrogen removal from mature landfill leachate at the pilot-scale[J]. Bioresource Technology, 2019, 297: 1-9. [16] ZHENG Z M, HUANG S, BIAN W, et al. Enhanced nitrogen removal of the simultaneous partial nitrification, anammox and denitrification (SNAD) biofilm reactor for treating mainstream wastewater under low dissolved oxygen (DO) concentration[J]. Bioresource Technology, 2019, 283: 213-220. doi: 10.1016/j.biortech.2019.01.148 [17] DU R, CAO S B, PENG Y Z, et al. Combined partial denitrification (PD)-anammox: A method for high nitrate wastewater treatment[J]. Environment International, 2019, 126: 707-716. doi: 10.1016/j.envint.2019.03.007 [18] WANG D P, LI T, HUANG K L, et al. Roles and correlations of functional bacteria and genes in the start-up of simultaneous anammox and denitrification system for enhanced nitrogen removal[J]. Science of the Total Environment, 2019, 655: 1355-1363. doi: 10.1016/j.scitotenv.2018.11.321 [19] KARIM K, HOFFMANN R, KLASSON T, et al. Anaerobic digestion of animal waste: Waste strength versus impact of mixing[J]. Bioresource Technology, 2005, 96: 1771-1781. doi: 10.1016/j.biortech.2005.01.020 [20] KARIM K, KLASSON T, HOFFMANN R, et al. Anaerobic digestion of animal waste: Effect of mixing[J]. Bioresource Technology, 2005, 96: 1607-1612. doi: 10.1016/j.biortech.2004.12.021 [21] 刘刈, 王智勇, 孔垂雪, 等. 沼气发酵过程混合搅拌研究进展[J]. 中国沼气, 2009, 27(3): 26-30. doi: 10.3969/j.issn.1000-1166.2009.03.006 [22] WANG C, LIU S T, XU X C, et al. Achieving mainstream nitrogen removal through simultaneous partial nitrification, anammox and denitrification process in an integrated fixed film activated sludge reactor[J]. Chemosphere, 2018, 203: 457-466. doi: 10.1016/j.chemosphere.2018.04.016 [23] 袁青彬, 郭美婷, 杨健. 污泥负荷对生物处理系统耐药细菌的影响研究: 以活性污泥法中磺胺嘧啶抗性异养菌为例[J]. 中国环境科学, 2014, 34(8): 1979-1984. [24] WANG G P, ZHANG D, XU Y, et al. Comparing two start up strategies and the effect of temperature fluctuations on the performance of mainstream anammox reactors[J]. Chemosphere, 2018, 209: 632-639. doi: 10.1016/j.chemosphere.2018.06.134 [25] GONZALEZ-GIL G, SOUGRAT R, BEHZAD A R, et al. Microbial community composition and ultrastructure of granules from a full scale anammox reactor[J]. Microbial Ecology, 2015, 70(1): 118-131. doi: 10.1007/s00248-014-0546-7 [26] LEIX C, DREWES J E, KOCH K, et al. The role of residual quantities of suspended sludge on nitrogen removal efficiency in a deammonifying moving bed biofilm reactor[J]. Bioresource Technology, 2016, 219: 212-218. doi: 10.1016/j.biortech.2016.07.134 [27] WANG S, LIU Y, NIU Q, et al. Nitrogen removal performance and loading capacity of a novel single-stage nitritation-anammox system with syntrophic micro-granules[J]. Bioresource Technology, 2017, 236: 119-128. doi: 10.1016/j.biortech.2017.03.164 [28] NARITA Y K, ZHANG L, ZENICHIRO K, et al. Enrichment and physiological characterization of an anaerobic ammonium-oxidizing bacterium ‘Candidatus Brocadia sapporoensis’[J]. Systematic and Applied Microbiology, 2017, 40(7): 448-457. doi: 10.1016/j.syapm.2017.07.004 [29] YANG H, LI D, ZENG H P, et al. Long-term operation and autotrophic nitrogen conversion process analysis in a biofilter that simultaneously removes Fe, Mn and ammonia from low-temperature groundwater[J]. Chemosphere, 2019, 222: 407-414. doi: 10.1016/j.chemosphere.2019.01.143 [30] KRAGELUND C, THOMSEN T R, MIELCZAREK A T, et al. Eikelboom’s morphotype 0803 in activated sludge belongs to the genus Caldilinea in the phylum Chloroflexi[J]. FEMS Microbiology Ecology, 2011, 76(3): 451-462. doi: 10.1111/j.1574-6941.2011.01065.x [31] LAURENI M, FALÅS P, ROBIN O, et al. Mainstream partial nitritation and anammox: Long-term process stability and effluent quality at low temperatures[J]. Water Research, 2016, 101: 628-639. doi: 10.1016/j.watres.2016.05.005 [32] LI S S, PENG C R, CHENG T S, et al. Nitrogen-cycling microbial community functional potential and enzyme activities in cultured biofilms with response to inorganic nitrogen availability[J]. Journal of Environmental Sciences, 2019, 76: 89-99. doi: 10.1016/j.jes.2018.03.029 [33] WANG S, ZHENG D, WANG S, et al. Remedying acidification and deterioration of aerobic post-treatment of digested effluent by using zero-valent iron[J]. Bioresource Technology, 2018, 247: 477-485. doi: 10.1016/j.biortech.2017.09.078 [34] KUNDU P, PRAMANIK A, DASGUPTA A, et al. Simultaneous heterotrophic nitrification and aerobic denitrification by Chryseobacterium sp. R31 isolated from abattoir wastewater[J/OL]. [2019-09-10]. BioMed Research International, 2014: 1-12. http://dx.doi.org/10.1155/2014/436056. [35] DING S, BAO P, WANG B, et al. Long-term stable simultaneous partial nitrification, anammox and denitrification (SNAD) process treating real domestic sewage using suspended activated sludge[J]. Chemical Engineering Journal, 2018, 339: 180-188. doi: 10.1016/j.cej.2018.01.128 [36] GU Y Q, LI T T, LI H Q, et al. Biofilm formation monitored by confocal laser scanning microscopy during startup of MBBR operated under different intermittent aeration modes[J]. Process Biochemistry, 2018, 74: 132-140. doi: 10.1016/j.procbio.2018.08.032 [37] WU H, GUO C Y, YIN Z H, et al. Performance and bacterial diversity of biotrickling filters filled with conductive packing material for the treatment of toluene[J]. Bioresource Technology, 2018, 257: 201-209. doi: 10.1016/j.biortech.2018.02.108 [38] PARK Y, CHO H, YU J, et al. Response of microbial community structure to pre-acclimation strategies in microbial fuel cells for domestic wastewater treatment[J]. Bioresource Technology, 2017, 233: 176-183. doi: 10.1016/j.biortech.2017.02.101 [39] HAO L P, BIZE A, CONTEAU D, et al. New insights into the key microbial phylotypes of anaerobic sludge digesters under different operational conditions[J]. Water Research, 2016, 102: 158-169. doi: 10.1016/j.watres.2016.06.014 -







 下载:
下载: