-
醋糟是食醋酿造过程的剩余固体副产物[1],其主要成分为稻壳、麸皮等[2]。我国是食醋生产大国,每年醋糟的产生量约为3.2×106 t以上[3-4]。由于醋糟具有含水率高、酸度大、难降解的特性[5],将其随意处置不仅会造成资源浪费,更易造成水土酸化等环境污染[6]。木质素是一种具有高价值的天然有机高分子化合物[7],是醋糟的重要组成部分,在其干重中占比约15%[8]。因此,将醋糟作为制备木质素的原料是一种潜在的醋糟资源化途径。然而,由于醋糟中木质纤维素结构的复杂性及稳定性导致木质素分离提纯困难,无法实现其高值化应用[9]。
目前,应用于生产的木质素分离纯化方法大多采用化学法。例如,LUCA等[10]用酸预处理和碱提取相结合的方法提纯小麦秸秆中的木质素,木质素的回收率为42%,纯度为99%。虽然化学法分离纯化木质素的效率高,然而其普遍存在成本高、二次污染隐患大且易造成木质素材料性能弱化等弊端。相比之下,生物法分离纯化木质素具备成本低、反应条件温和且无二次污染等潜在优势[11]。而且醋糟的结构松散、孔隙率大,便于微生物的附着生长与繁殖,更适合生物法纯化。据报道,好氧真菌中的白腐菌和褐腐菌分泌的木质纤维素酶系较全且酶活性较高,具备高效破坏木质纤维素结构的能力[12]。例如,LI等[13]用混合微生物对玉米秸秆发酵后发现,玉米秸秆中木质纤维素结构被大面积破坏,从而提高了酶水解的效率,纤维素、半纤维素降解率分别为34.9%和44.4%。此外,有研究发现,经定向驯化后的厌氧消化微生物体系具备高效降解纤维素和半纤维素等多糖的能力。例如,ZHOU等[14]构建了醋糟高效厌氧消化体系,反应器在5.83 g·(L·d)−1的负荷下(以VS计)运行60 d后,体系中超过40%的纤维素和90%的半纤维素被降解。
真菌发酵虽然能够有效破坏木质纤维素结构,然而其对纤维素、半纤维素等多糖的降解效率并不高;厌氧消化微生物体系虽然能高效降解多糖,然而由于厌氧微生物降解木质素能力的缺失[15],其对木质纤维素结构的破坏作用也较小。因此,现有具备木质素分离纯化潜力的生物方法普遍存在功能单一等问题,在木质纤维素结构破坏或多糖降解方面存在缺陷,无法达到高效纯化效果。本研究将醋糟作为纯化木质素的原料,提出真菌发酵联合厌氧消化的组合微生物处理的木质素生物纯化新策略,通过分析醋糟的降解情况和木质纤维素相关官能团的变化,考察该策略分离纯化木质素的效果;结合水解酶活性和碳水化合物活性酶基因的分析,深入探究木质素生物分离纯化的机制,以期为醋糟的资源化应用提供新的参考。
-
1)醋糟与厌氧体系。醋糟取自江苏省镇江市恒顺醋厂,存于-20 ℃冰箱备用。醋糟的含固率为31.61%,在干重下其纤维素、半纤维素、木质素及粗蛋白的质量分数分别为32.54%、22.48%、14.97%和10.80%。为提高醋糟的水解效率,实验采用的厌氧反应器在前期引入了瘤胃污泥,从而构建了全新的厌氧消化体系[16],该体系的含固率为13.42%。
2)供试真菌。实验采用的4种真菌分别为黄孢原毛平革菌(Phanerochaete chrysosporium)、彩绒革盖菌(Coriolus versicolor)、绿色木霉(Trichoderma viride)和青霉菌(Penicillium sp.)。其中,黄孢原毛平革菌(菌株CICC 40299)、绿色木霉(菌株CICC 13038)和青霉菌(菌株CICC 2515)购于中国工业微生物菌种保藏管理中心(CICC)。彩绒革盖菌(菌株ACCC 51171)购于中国农业微生物菌种保藏管理中心(ACCC)。
-
为准确评估真菌发酵联合厌氧消化策略纯化醋糟中木质素的效果,共设计3组实验。第1组对醋糟进行混合真菌发酵处理(FF组);第2组对醋糟进行厌氧消化处理(AD组);第3组对醋糟进行混合真菌发酵联合厌氧消化处理(FF+AD组)。将未经任何方法处理的醋糟作为对照组。将各组实验处理后的醋糟在60 ℃下烘干至恒重,用于各项指标的测定。
混合真菌发酵。采用固态发酵的方式,将黄孢原毛平革菌(Phanerochaete chrysosporium)、彩绒革盖菌(Coriolus versicolor)、绿色木霉(Trichoderma viride)和青霉菌(Penicillium sp.)4种真菌等比例混合后,以7%的接种量接入醋糟,并在25 ℃的条件下发酵18 d。设置2组平行实验。
厌氧消化处理。AD组和FF+AD组的具体运行参数见表1。2组实验的物料负荷相同。
-
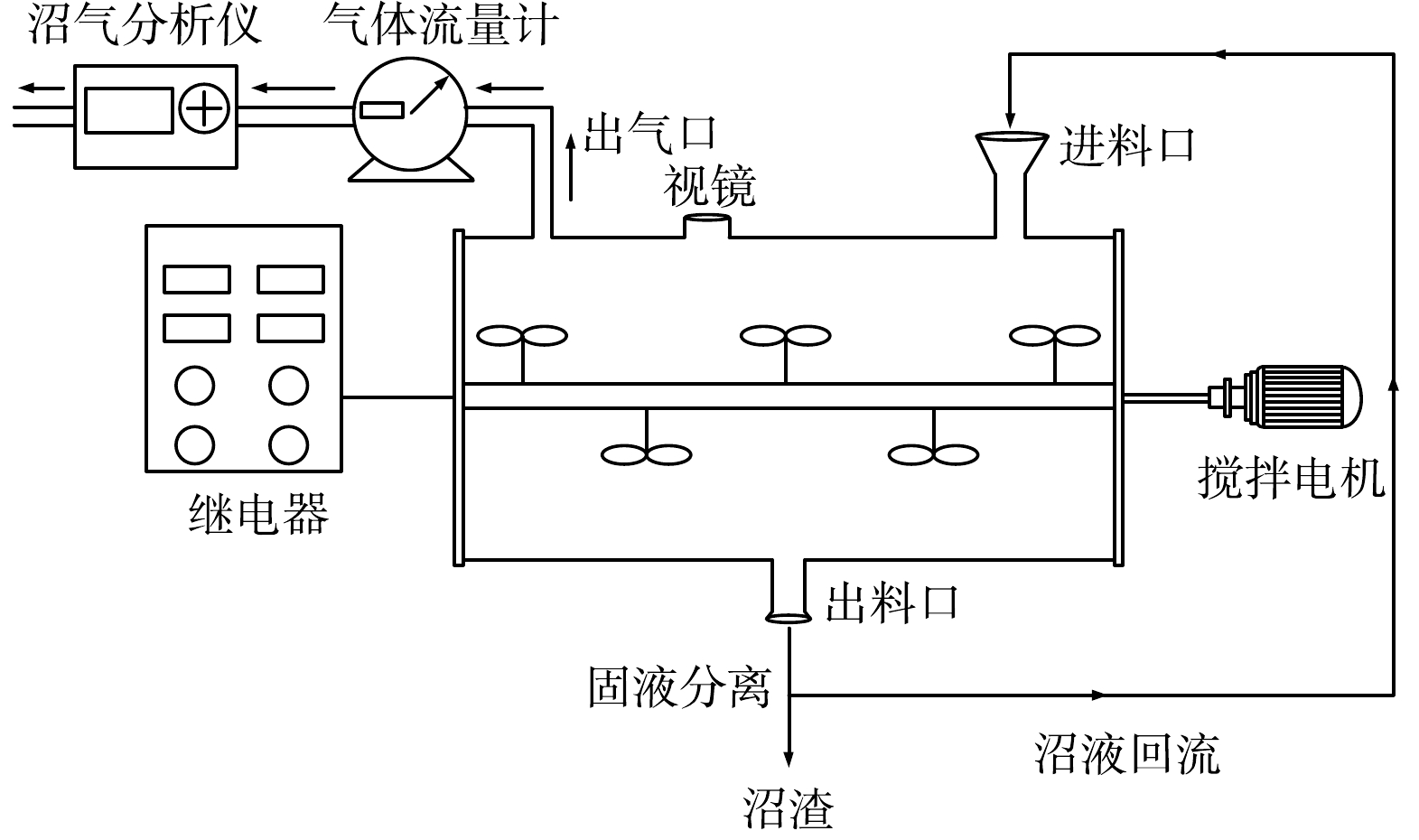
为保证进出料和沼液均匀,增加物料的停留时间,厌氧消化过程选用有效工作体积为25 L的卧式反应器,反应器装置如图1所示。反应器上口进料、下口出料,出料排出的消化液固液分离后,沼液回流补水至反应器。反应器内部配有搅拌装置,由搅拌电机带动罐体搅拌轴缓慢转动,使得料液形成垂直混合和水平推流的复合流态。反应器上方配有视镜,用以观察液面位置。出气口与气体流量计(LMF-1,长春汽车滤清器有限公司)和沼气分析仪(Gasboard-3200L,武汉立方光电有限公司)相连。整个罐体用伴热带和玻璃棉毡进行加热和保温,温度控制在(37±1) ℃。pH保持为中性。搅拌方式为间歇式搅拌,每搅拌5 min后间隔5 min,搅拌速率为4 r·min−1。每天定时进料,每2 d出料1次。
-
1)常规指标测定。TS(总固体)和VS(挥发性固体)质量分数采用干重法测定[17];粗蛋白由凯氏定氮仪(KDN-520,杭州绿博仪器有限公司)测定的凯氏氮乘以6.25后得出[18]。
2) FT-IR(傅里叶红外光谱)测定。傅里叶红外光谱采用光谱仪(vertex 70,德国Bruker公司)测定。
3)木质纤维素组分分析。纤维素、半纤维素及木质素的质量分数借助纤维分析仪(ANKOM 2000i,美国ANKOM公司),采用范式洗涤法测定[19]。木质素的纯度为样品中酸溶木质素与酸不溶木质素2者质量分数之和,酸不溶木质素质量分数测定根据GB/T 2677.8-94,酸溶木质素质量分数测定根据GB/T 10337-2008[20]。总固体和木质纤维素降解率公式见式(1)。
式中:D为总固体、纤维素、半纤维素或木质素的降解率;mbeginning为处理前添加至体系中总固体、纤维素、半纤维素或木质素的总质量,g;mout为过程中排出体系的总固体、纤维素、半纤维素或木质素的总质量,g;mend为处理后体系中总固体、纤维素、半纤维素或木质素剩余的总质量,g。木质素的回收率公式见式(2)。
式中:A为木质素的回收率;m1为处理后体系中木质素剩余的总质量,g;m2为处理前添加至体系中木质素的总质量,g。
4)水解酶活性分析。羧甲基纤维素酶(CMC)和木聚糖酶均采用DNS显色法测定,并用不含发酵液的反应体系作为空白对照[21]。1个CMC酶酶活力单位[U·(mL·min)−1]定义为每毫升粗酶液每分钟催化水解羧甲基纤维素钠(CMC-Na)生成1 μg葡萄糖的酶量,1个木聚糖酶酶活力单位[U·(mL·min)−1]定义为每毫升粗酶液每分钟分解木聚糖生成1 μg木糖的酶量。
5)碳水化合物活性酶基因的测定。分别取AD组和FF+AD组运行至稳定期的物料,存入−80 ℃保存,用于宏基因组测序。使用NEB Next® UltraTM DNA Library Prep Kit for Illumina®试剂盒来提取样品中的DNA,DNA质检合格后进行文库制备。使用IlluminaTM二代测序平台(生工生物工程股份有限公司)进行宏基因组测序,并通过FastQC和Trimmomatic软件进行数据评估与质控分析后,构建基因集。最后将基因集与CAZy数据库(http: //www. cazy. org/)进行比对,获得碳水化合物活性酶基因的功能注释信息及其相对丰度。
-
木质纤维素包含纤维素、半纤维素及木质素,其在醋糟中的质量分数约70%~80%[22]。醋糟经混合真菌发酵后,总固体的降解率为38.81%。其中,30.65%为纤维素和半纤维素,占总固体降解量的78.97%。结合表2可以看出,混合真菌发酵(FF组)处理后的醋糟中,未被降解组分(占干物质总质量的61.19%)中仍包含有质量分数28.5%的纤维素和11.33%的半纤维素等主要成分,分别占原底物中相应成分总质量的69.75%和52.63%。这表明,仍有大量物质未被降解,木质素质量分数仅为19.41%,仍处于较低水平。
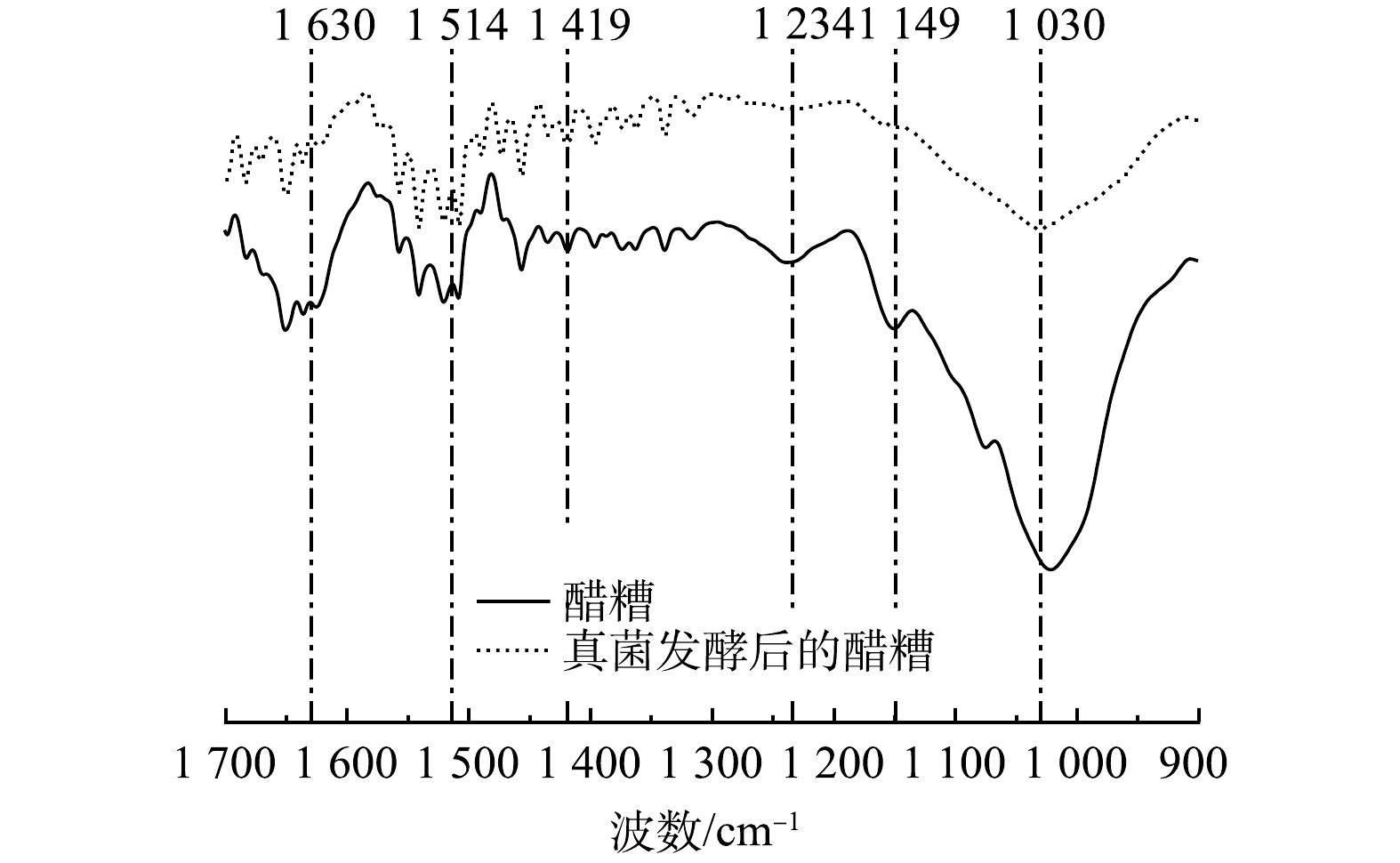
傅里叶变换红外光谱(FT-IR)能够有效分析底物固相的分子结构和官能团,可为深入探究真菌发酵过程醋糟内部木质纤维素结构的变化提供依据[23]。如图2所示,与木质素结构相关的谱峰位于1 234、1 419、1 514、1 630 cm−1处,相比于醋糟,混合真菌发酵后的醋糟在1 419、1 514 cm−1(木质素中苯环骨架C—H变形振动、木质素中苯环骨架C=C—OH伸缩振动)处的透过率增加了约3.5%,在1 234、1 630 cm−1 (木质素酚醚键 C—O—C伸缩振动、木质素中与芳香环相连的C=O伸缩振动)处的透过率增加了约5.2%。这表明,发酵过程中木质素结构的破坏主要集中在与苯环相连的侧链上,即木质素与碳水化合物之间连接键的断裂,对木质素苯环骨架的破坏作用较小。纤维素和半纤维素结构相关的谱峰位于1 030、1 149 cm−1(纤维素和半纤维素中C—O伸缩振动、纤维素和半纤维素中C—O—C伸缩振动),其透过率增加了约8.0%,峰值强度变化比木质素相关谱峰的强度变化更加明显。这表明,混合真菌发酵处理具备高效破坏纤维素和半纤维素结构的能力。综上所述,混合真菌发酵处置通过对木质素侧链结构及对纤维素、半纤维素结构的破坏,能够有效破坏木质纤维素结构,此过程有利于后续厌氧消化阶段对纤维素和半纤维素的充分水解。
-
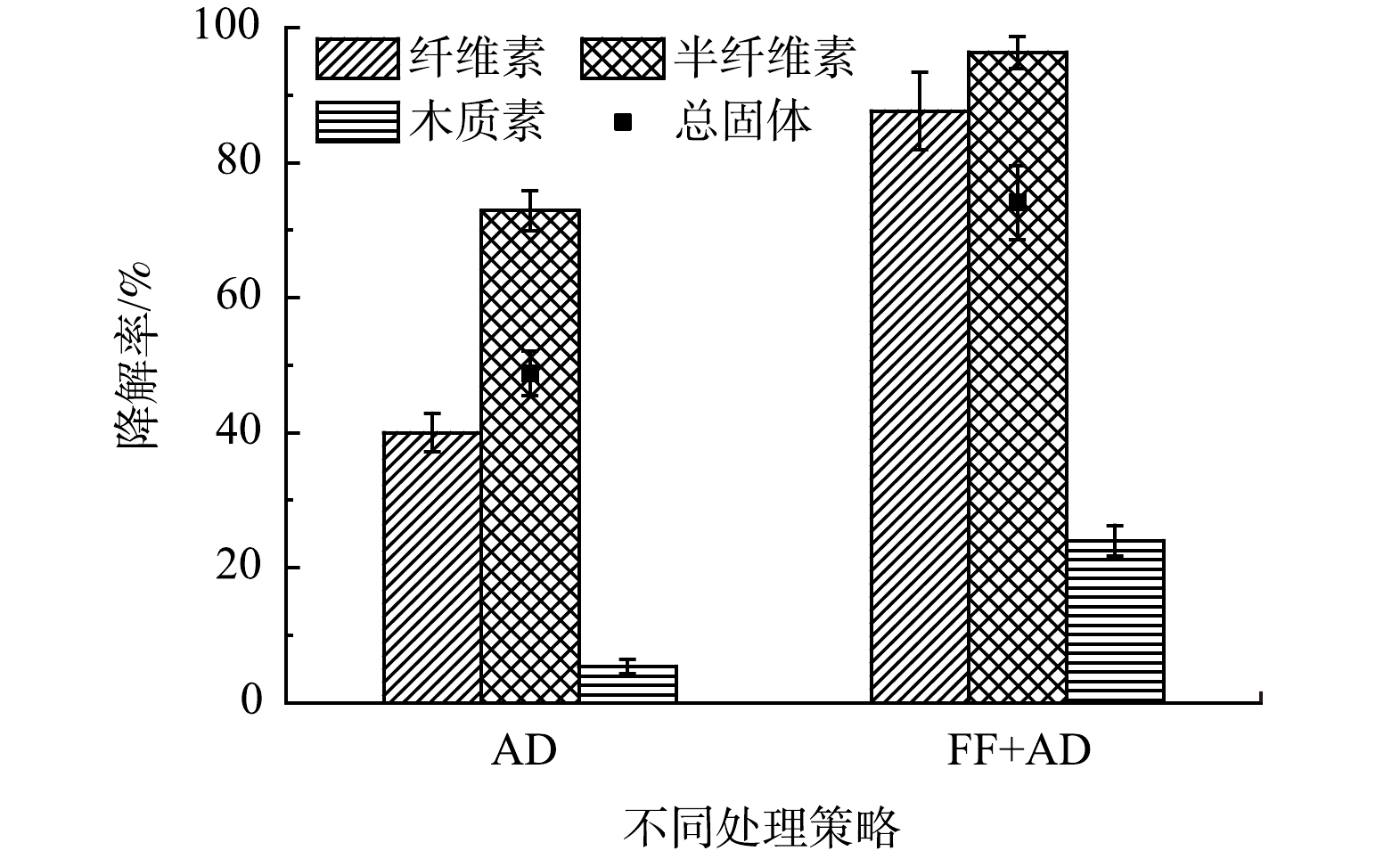
对比单一的厌氧消化处理过程(AD组)和真菌发酵联合厌氧消化处理过程(FF+AD组)的底物降解情况,以便于更精确地考察FF+AD策略分离纯化醋糟中木质素的效果。总固体降解率和木质纤维素降解率是表征厌氧消化过程底物降解的重要指标,其结果见图3。可以看出,AD组共降解了54.12%的干物质,纤维素、半纤维素和木质素相对于本物质的降解率分别为39.82%、73.63%和5.41%。FF+AD组共降解了81.51%的干物质,纤维素、半纤维素和木质素相对于本物质的降解率分别为87.65%、96.34%和23.99%。由上述数据可知,无论是底物中总固体的降解率还是单一物质的降解率,FF+AD组均高于AD组。虽然FF+AD策略处理损失了23.99%的木质素。然而,相比于化学法纯化木质素过程中木质素的损失(一般超过50%)[10],该策略仍具有优势。结合表2亦可以看出,AD组的沼渣中木质素质量分数仅为23.84%,沼渣中仍含有质量分数35.53%的纤维素、12.84%的半纤维素、7.62%的粗蛋白及其他组分未被降解。其可能的原因是,单一的厌氧过程对木质纤维素结构的破坏效果极为有限,仅能降解不被木质纤维素结构束缚的多糖。此外,FF+AD组的沼渣中纤维素、半纤维素和粗蛋白的质量分数分别为21.74%、4.45%和4.81%,相较于AD组均大幅下降,木质素纯度为62.32%,回收率为76.01%。其原因可能是,真菌发酵过程对木质纤维素结构的破坏有效地释放了纤维素和半纤维素等组分,并使其在厌氧过程得以更充分的降解。因此,FF+AD策略在高效破坏木质纤维素结构的同时可大量降解醋糟中的纤维素和半纤维素等组分,显著提升木质素纯化效率。
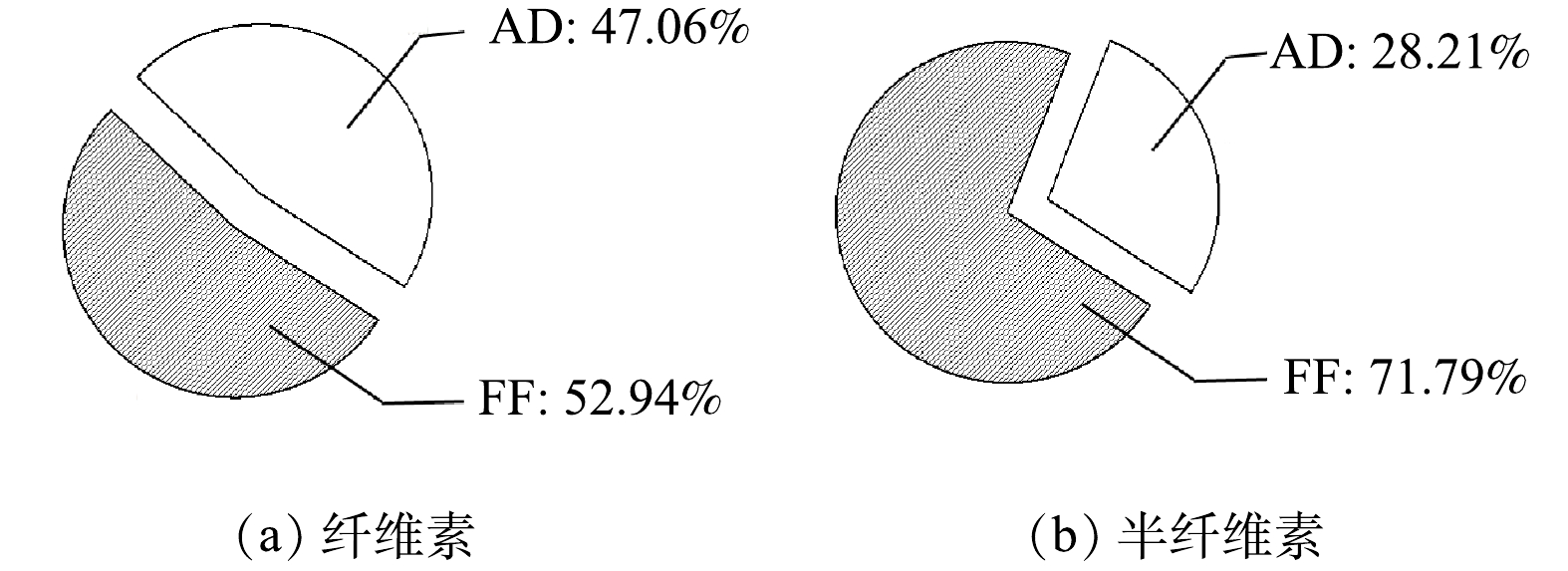
FF+AD策略中,FF阶段和AD阶段纤维素、半纤维素降解占总纤维素、半纤维素降解的比例分别如图4(a)和图4(b)所示,FF阶段和AD阶段纤维素降解占比分别为52.94%和47.06%,半纤维素降解占比分别为71.79%和28.21%。这表明,该策略的FF阶段在破坏木质纤维素结构的同时,对底物中纤维素和半纤维素有一定的降解作用,其主要体现在对半纤维素的大量降解。其原因可能是,好氧微生物的增殖需要能量供给,会优先利用纤维素和半纤维素等易降解的碳源,然而,由于纤维素具有高结晶度,且纤维素酶易被木质素所吸附。相比之下,半纤维素的分子量小且结构简单,更易被微生物所水解[24]。尽管如此,混合真菌发酵后的醋糟中仍有大量杂质残留,特别是纤维素残留量较高,占干物质总质量的28.50%。单一的FF阶段虽然能有效破坏木质纤维素结构,然而,对释放出纤维素的降解能力有限,无法满足木质素纯化要求。AD阶段能有效弥补FF阶段的功能缺陷,提高底物降解效率,进而强化木质素的纯化效果。
FF+AD策略将真菌发酵与厌氧消化2过程的微生物功能互补,显著提高了生物法分离纯化木质素的效率,最终所得木质素纯度为62.32%,回收率为76.01%。该纯度的木质素在电化学、环保、农林业、催化剂及有机高分子材料[25]等领域可被用作改性制备成木质素磺酸盐、木质素硫酸盐的粗木质素原料。
-
FT-IR图谱是一种定性分析木质纤维素结构和官能团的有效方法之一,能够从底物内部探究不同策略对木质纤维素结构的降解规律。本研究利用FT-IR技术分析了不同底物在900 ~ 1 700 cm−1波数范围内木质纤维素特征峰的变化规律,其红外光谱图如图5所示。
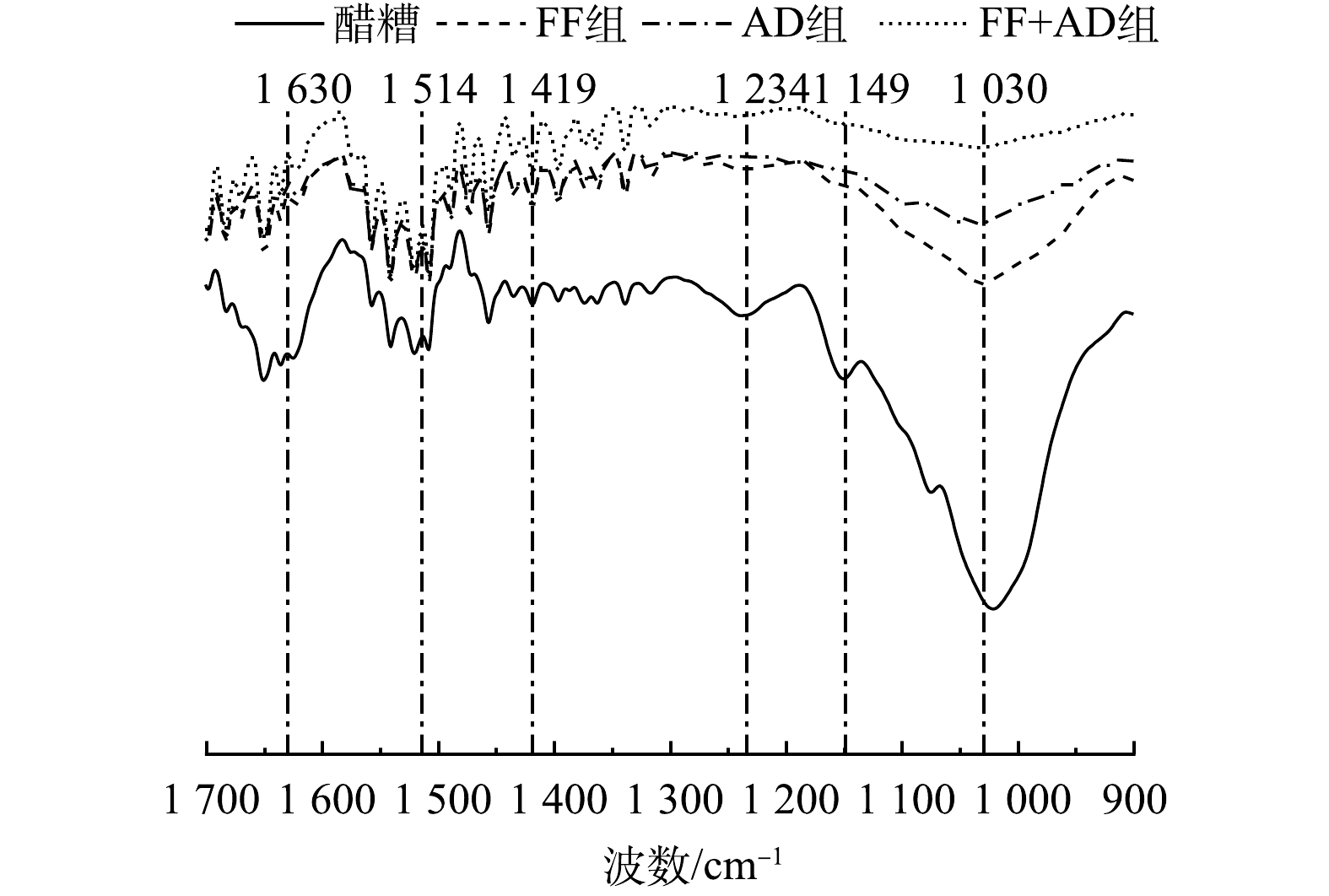
由图5可知,不同策略发酵后的沼渣与醋糟的红外光谱相比,不同吸收峰的强度变化出现明显的差异性。在1 030(纤维素和半纤维素中C—O伸缩振动)和1 149 cm−1(纤维素和半纤维素中C—O—C伸缩振动)处的峰值强度相较于醋糟变化明显,透过率均增加10%以上。这证明,底物中的纤维素和半纤维素组分被大量降解。相比之下,在1 234(木质素酚醚键C—O—C伸缩振动)、1 419(木质素中苯环骨架C—H变形振动)、1 514(木质素中苯环骨架C=C—OH伸缩振动)和1 630 cm−1(木质素中与芳香环相连的C=O伸缩振动)处的峰值强度相较于醋糟变化幅度小,透过率仅增加不到3%。这表明,木质素降解率较低,木质素损失少,其与2.2节木质纤维素的降解率结果一致。此外,各底物在1 419、1 514 cm−1处的峰值强度变化均小于在1 234、1 630 cm−1处的峰值强度变化。这表明,木质素的苯环骨架很难被降解。
不同策略发酵后沼渣之间的红外光谱图也具有明显差异。其中,FF+AD策略发酵后的沼渣与醋糟的差异性最大,全波数透过率均最高。这表明,该策略对底物中木质纤维素结构的破坏程度最高。相较于FF组和AD组,FF+AD组的峰值强度变化主要集中在1 030和1 149 cm−1这2个与纤维素、半纤维素相关的谱峰处,在1 030 cm−1处的透过率为98.75%,分别比FF组和AD组提高了4.20%和2.36%;在1 149 cm−1处的透过率为99.47%,分别比FF组和AD组提高了1.91%和1.45%。这可能是因为,FF+AD策略对木质纤维素结构的有效破坏提高了厌氧菌群对底物的水解效率,最终导致纤维素和半纤维素结构中大量C—O键和C—O—C键断裂。单一的AD处理对木质纤维素结构的破坏作用较小,该结构的存在阻碍了厌氧菌群对纤维素和半纤维素的进一步利用;单一的FF处理虽然能够破坏木质纤维素结构,然而其对纤维素的降解能力有限。因此,AD组和FF组对纤维素、半纤维素分子结构的破坏均小于FF+AD组,峰值透过率低。
综上分析,FF+AD策略在高效破坏醋糟木质纤维素结构的同时能够充分断裂纤维素、半纤维素分子结构中的C—O键和C—O—C键,降解纤维素和半纤维素,提高木质素纯度。此外,该策略对木质素苯环骨架的破坏程度较小,有利于木质素的保留回收。
-
厌氧消化体系中纤维素和半纤维素的有效脱除是醋糟中木质素高效分离纯化的关键,而纤维素和半纤维素的水解主要依靠其水解酶作用。因此,体系中水解酶的活性一定程度上能够体现纤维素、半纤维素的水解程度。羧甲基纤维素酶(CMC)是一种内切纤维素酶,是纤维素酶系中最重要的成分之一。木聚糖是半纤维素的主要组成部分,木聚糖酶是半纤维素降解过程的关键酶。体系中CMC酶和木聚糖酶的活性变化如图6所示。由图6可以看出,随着反应器的连续运行,AD组和FF+AD组的CMC酶和木聚糖酶活性虽然有波动,然而整体呈上升趋势,此趋势呈现了反应体系相关功能微生物逐渐适应物料负荷冲击的过程。对比2组厌氧体系中酶的活性可以发现,FF+AD组的CMC酶和木聚糖酶的活性均明显高于AD组。其中,CMC酶活性最高值达到62.73 U·(mL·min)−1,相较于AD组酶活性最高值提升了24.77%;木聚糖酶活性最高值达到68.89 U·(mL·min)−1,相较于AD组酶活性最高值提升了18.65%。结合FT-IR分析结果可知(2.1节),这可能是因为,真菌处理有效破坏了底物中的木质纤维素结构,使包裹在木质纤维素结构中的纤维素和半纤维素与体系中的厌氧微生物充分接触,进而提高了2种水解酶的活性,也提高了纤维素和半纤维素的降解率。综上分析,相比于单一的厌氧消化处理(AD组),真菌发酵联合厌氧消化策略(FF+AD组)的厌氧体系中CMC酶和木聚糖酶的活性显著提升,从酶活角度验证了该策略对底物中纤维素和半纤维素等组分的高效去除能力。
-
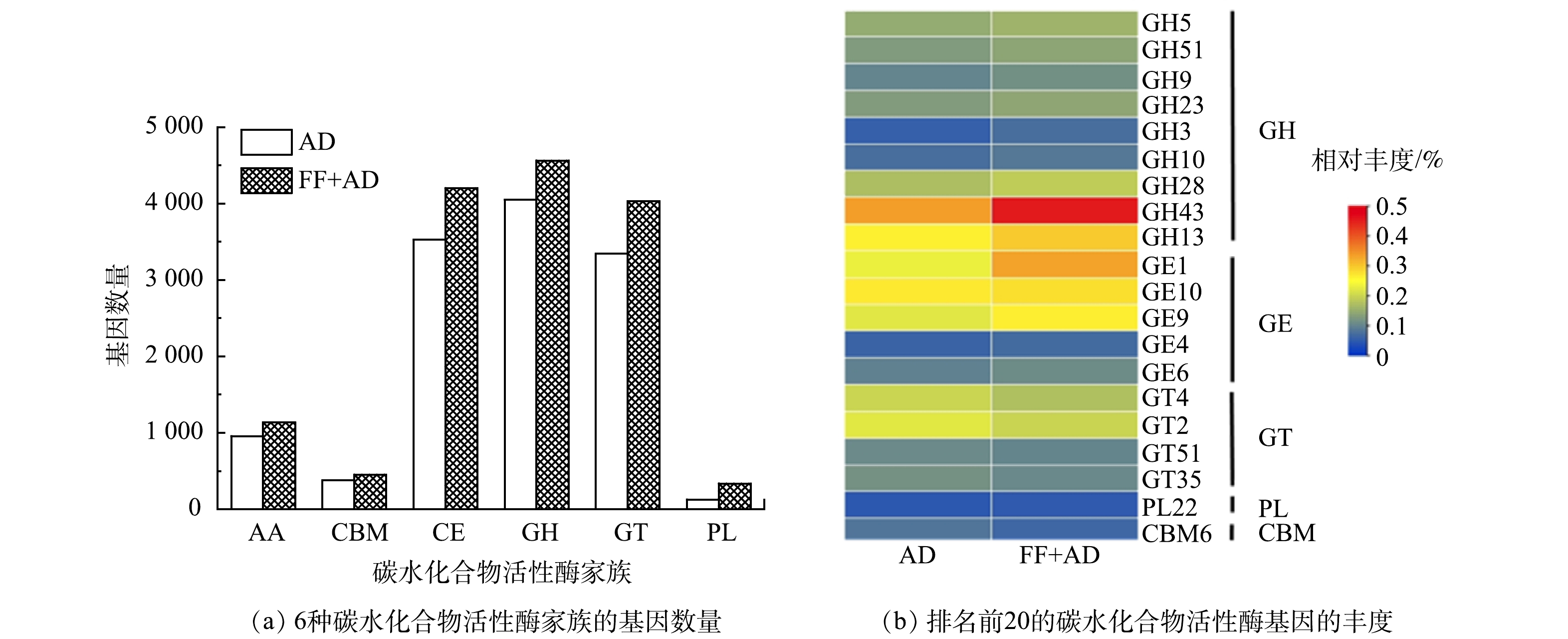
碳水化合物活性酶(CAZy)数据库是一个主要研究降解复杂碳水化合物和糖结合物的酶的数据库[26]。在水解酶活性研究的基础上,定量分析体系中碳水化合物活性酶基因的变化,对揭示微生物协同降解碳水化合物的机理有着重要意义。因此,本研究分别测定了AD组和FF+AD组厌氧体系中的碳水化合物活性酶基因,利用其来表征厌氧体系中关键水解菌群的变化情况,结果见图7。如图7(a)所示,碳水化合物活性酶根据其功能可以分为6种家族,其分别为糖苷水解酶(GH)、碳水化合物酯酶(CE)、糖基转移酶(GT)、辅助活性酶(AA)、碳水化合物结合模块(CBM)和多糖裂解酶(PL)[27]。其中,GH、CE和GT家族约占整个厌氧体系中碳水化合物活性酶基因数量的80%,其次为AA、CBM和PL家族。AD组和FF+AD组厌氧体系中碳水化合物活性酶基因总数分别为12376和14717,FF+AD组中6种水解酶家族的基因数量均高于AD组。其原因可能是,真菌发酵处置后的底物其疏松的木质纤维素结构能够释放出更多可被利用的纤维素和半纤维素等物质,进而促进了体系中水解微生物的快速增殖,碳水化合物活性酶基因数量增多。由图7(b)对比AD组和FF+AD组厌氧体系中排名前20的碳水化合物活性酶基因丰度发现,GH43(β-木糖苷酶)和CE1(乙酰木聚糖酯酶)为体系中的优势基因,主要功能均为降解半纤维素,其丰度在AD组和FF+AD组中均最高(> 0.25%)。相较于AD组,FF+AD组中的GH43和CE1的丰度均提高0.1%以上。其原因可能是,真菌发酵过程破坏底物中木质纤维素结构后,大量可被利用的半纤维素从结构中裸露出来,导致水解菌群快速生长繁殖,GH43和CE1的丰度增加。同时,裸露出来的纤维素也促进了FF+AD组中GH5、GH9和GH51等与纤维素降解有关的碳水化合物活性酶基因丰度的增加,然而由于纤维素比半纤维素更难降解,GH5、GH9和GH51的丰度之和仅上升约0.03%。GH13(α-淀粉酶)主要作用于淀粉结构中的α-1,4糖苷键,能够将淀粉降解为葡萄糖、麦芽糖等[28]。FF+AD组中GH13丰度相比于AD组提高了0.03%。这表明,FF+AD策略能够有效提高淀粉的降解率。其原因可能是,该策略对底物中木质纤维素结构的破坏减弱了该结构对淀粉酶的吸附作用。GT4、GT2、GT35和GT51分别与蔗糖、纤维素、淀粉和细胞壁的合成有关[29]。FF+AD组厌氧体系中以上4种GT家族的丰度略有下降。其原因可能是,体系中水解微生物的大量增殖,导致参与低聚糖和多糖合成的微生物数量减少。
综上所述,FF+AD策略的真菌发酵过程对底物中木质纤维素结构的破坏大幅提升了厌氧体系降解纤维素和半纤维素等物质的潜力。包含GH43、CE1、GH13等碳水化合物活性酶基因的水解微生物的大量快速富集是木质纤维素结构被破坏,纤维素和半纤维素等物质被充分释放的直接印证;同时,也表明该策略的厌氧消化过程可高效降解释放出的纤维素和半纤维素等组分,最终实现醋糟中木质素的高效分离纯化。
-
1)提出了一种生物纯化醋糟中木质素的策略(即FF+AD策略)。醋糟经真菌发酵和厌氧消化2过程纯化后,出料后的沼渣中木质素纯度为62.32%,木质素回收率为76.01%。
2)该策略能有效破坏醋糟中的木质纤维素结构。厌氧体系中CMC酶和木聚糖酶等水解酶活性的显著提升也证明了该策略具备高效去除纤维素和半纤维素等主要组分的能力。
3)厌氧体系中以GH43、CE1、GH13为代表的碳水化合物活性酶基因数量及丰度的增加,是该策略能够高效降解纤维素和半纤维素等组分,高效分离纯化醋糟中木质素的根本原因。
利用醋糟生物纯化木质素新策略的效能评估
Effectiveness assessment of a new strategy for the biopurification of lignin from vinegar residues
-
摘要: 木质素是醋糟的重要组成部分,是自然界中可直接提供芳香环结构物质的可再生资源,具有广泛的用途。然而,醋糟中木质纤维素结构稳定且难以破坏,限制了木质素的生物分离纯化效率,进而影响了醋糟的资源化利用。针对此问题,设计了一种新的木质素生物分离纯化策略,将混合真菌发酵与厌氧消化处理相结合,借助不同功能微生物的协同作用,在有效破坏木质纤维素结构的同时充分降解多糖。结果表明,经过处理后,醋糟中纤维素和半纤维素的降解率达到了87.65%和96.34%;木质素的纯度达到了62.32%,回收率为76.01%。醋糟中木质纤维素结构被破坏,从而导致体系中水解酶活性的提高。包含GH43、CE1、GH13等碳水化合物活性酶基因的微生物的大量富集是该方法实现木质素高效纯化的根本原因。本研究结果可为醋糟的资源化利用提供参考。
-
关键词:
- 醋糟资源化 /
- 木质素 /
- 生物纯化 /
- 水解酶 /
- 碳水化合物活性酶基因
Abstract: As an important component of vinegar residues, lignin is a renewable resource for providing aromatic substances in nature, and it can be widely used. However, the lignocellulosic structure of vinegar residues is very stable and the difficulty in destroying this structure leads to the low efficiency for lignin separation and purification with biological methods, and further affects the utilization of vinegar residues. For solving this problem, a novel strategy for lignin separation and purification was proposed. Based on the combination of mixed fungal fermentation and anaerobic digestion treatment process, the efficient purification of lignin can be attained through the synergistic effect of effective destruction of lignocellulosic structure and degradation of polysaccharides by microorganisms with different functions. After treatment, the degradation rate of cellulose and hemicellulose in vinegar residues reached 87.65% and 96.34%, the purity of lignin was 62.32% and the recovery rate was 76.01%. The increase of hydrolase activity and the enrichment of microorganisms containing carbohydrate-active enzyme (GH43, CE1 and GH13) genes in the treatment system after destruction of lignocellulosic structure of vinegar residues were the fundamental reason for the efficient purification of lignin by this strategy. The results showed the great potential of this strategy for the efficient separation and purification of lignin from vinegar residues, which can provide a reference for the resource utilization of vinegar residues. -

-
表 1 厌氧消化处理过程的运行参数
Table 1. Operating parameters of anaerobic digestion treatment process
实验组 物料 进料量/(g·d−1) 物料负荷(以VS计)/(g·(L·d)−1) 物料停留时间/d 运行时间/d AD 醋糟 600 7.137 6 42 50 FF+AD 真菌发酵后的醋糟 1 113 7.137 6 23 30 表 2 不同策略处理后底物成分对照
Table 2. Comparison of substrate components treated by different strategies
% 实验组 粗蛋白 木质素 纤维素 半纤维素 其他 对照组 10.80±0.53 14.97±0.12 32.54±0.84 22.48±0.34 19.21±0.11 FF组 9.45±0.10 19.41±0.11 28.50±0.46 11.33±0.14 31.31±0.24 AD组 7.62±0.08 23.84±0.23 35.53±0.33 12.84±0.11 20.17±0.14 FF+AD组 4.81±0.05 61.56±0.41 21.74±0.19 4.45±0.08 7.44±0.03 注:数值为基于干重下质量分数的平均值±标准方差。 -
[1] FENG J Y, ZHANG J Y, ZHANG J F, et al. Enhanced methane production of vinegar residue by response surface methodology (RSM)[J]. AMB Express, 2017, 7(1): 89. doi: 10.1186/s13568-017-0392-3 [2] 崔耀明, 董晓芳, 佟建明, 等. 山西老陈醋醋糟营养成分分析[J]. 饲料工业, 2015, 36(1): 24-29. doi: 10.13302/j.cnki.fi.2015.01.005 [3] WANG Z B, SHAO S P, ZHANG C S, et al. Pretreatment of vinegar residue and anaerobic sludge for enhanced hydrogen and methane production in the two-stage anaerobic system[J]. International Journal of Hydrogen Energy, 2015, 40(13): 4494-4501. doi: 10.1016/j.ijhydene.2015.02.029 [4] 王芳, 上官明军, 张变英, 等. 山西省醋糟资源现状及其在动物生产中的应用[J]. 畜禽业, 2014, 10(1): 44-46. doi: 10.3969/j.issn.1008-0414.2014.10.029 [5] 陈晓寅, 王振斌, 马海乐, 等. 醋糟的利用现状及前景[J]. 中国酿造, 2010, 29(10): 1-4. doi: 10.3969/j.issn.0254-5071.2010.10.001 [6] WEN H L, WACHEMO A C, ZHANG L, et al. A novel strategy for efficient anaerobic co-digestion based on the pretreatment of corn stover with fresh vinegar residue[J]. Bioresource Technology, 2019, 21(1): 121412. [7] 汤潜潜. 木质素的应用研究进展[J]. 内江科技, 2020, 41(5): 118-119. [8] LI L, FENG L, ZHANG R H, et al. Anaerobic digestion performance of vinegar residue in continuously stirred tank reactor[J]. Bioresource Technology, 2015, 186(3): 338-342. [9] RENDERS T, VAN DEN BOSCH S, KOELEWIJN S F, et al. Lignin-first biomass fractionation: the advent of active stabilisation strategies[J]. Energy & Environmental Science, 2017, 10(7): 1551-1557. [10] LUCA Z, ANIKA S, EVA-LISA T, et al. Valorization of side-streams from a SSF biorefinery plant: wheat straw lignin purification study[J]. Bioresources, 2017, 12(1): 1680-1696. [11] 宋春雪. 醋糟的研究与利用现状[J]. 中国调味品, 2011, 36(12): 1-4. doi: 10.3969/j.issn.1000-9973.2011.12.001 [12] 陈立祥, 章怀云. 木质素生物降解及其应用研究进展[J]. 中南林学院学报, 2003, 23(1): 79-85. [13] LI P P, HE C, LI G, et al. Biological pretreatment of corn straw for enhancing degradation efficiency and biogas production[J]. Bioengineered, 2020, 11(1): 251-260. doi: 10.1080/21655979.2020.1733733 [14] ZHOU Y L, XU Z Y, ZHAO M X, et al. Construction and evaluation of efficient solid-state anaerobic digestion system via vinegar residue[J]. International Biodeterioration & Biodegradation, 2018, 133: 142-150. [15] CHALIEUR O, MADIGOU C, CIVADE R, et al. Increasing concentrations of phenol progressively affect anaerobic digestion of cellulose and associated microbial communities[J]. Biodegradation, 2016, 27(1): 15-27. doi: 10.1007/s10532-015-9751-4 [16] 李倩, 许之扬, 周云龙, 等. 瘤胃微生物强化醋糟厌氧消化及其机制[J]. 环境科学研究, 2020, 33(10): 2370-2377. doi: 10.13198/j.issn.1001-6929.2020.02.02 [17] 国家环境保护总局. 水和废水监测分析方法[J]. 4版. 北京:中国环境科学出版社, 2002: 105-108. [18] HALL N G, SCHOENFELDT H C. Total nitrogen vs. amino-acid profile as indicator of protein content of beef[J]. Food Chemistry, 2013, 140(3): 608-612. doi: 10.1016/j.foodchem.2012.08.046 [19] VAX SOEST P J. Use of detergents in the analysis of fibrous feeds. II. a rapid method for the determination of fiber and lignin[J]. Journal of the Association of Official Agricultural Chemists, 1963, 46(5): 829-835. [20] 张通, 白富栋, 李政, 等. 酶解秸秆残渣中木质素提取方法的研究[J]. 化学研究与应用, 2018, 30(6): 901-906. doi: 10.3969/j.issn.1004-1656.2018.06.006 [21] LOWE S E, THEODOROU M K, TRINCI A P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan[J]. Applied and Environmental Microbiology, 1987, 53(6): 1216-1223. doi: 10.1128/aem.53.6.1216-1223.1987 [22] LIU J M, JIN S, BAO C H, et al. Rapid determination of lignocellulose in corn stover based on near-infrared reflectance spectroscopy and chemometrics methods[J]. Bioresource Technology, 2021, 321: 124449. doi: 10.1016/j.biortech.2020.124449 [23] YANG S G, LI J H, ZHENG Z, et al. Lignocellulosic structural changes of Spartina alterniflora after anaerobic mono- and co-digestion[J]. International Biodeterioration & Biodegradation, 2009, 63(5): 569-575. [24] CHANDRA R, TAKEUCHI H, HASEGAWA T. Methane production from lignocellulosic agricultural crop wastes: a review in context to second generation of biofuel production[J]. Renewable & Sustainable Energy Reviews, 2012, 16(3): 1462-1476. [25] 邱苏鹏, 赵会芳, 沙力争. 硬脂酰氯改性碱木质素及微纳米球的制备[J]. 中国造纸, 2021, 40(9): 1-8. [26] CANTAREL B L, COUTINHO P M, RANCUREL C, et al. The carbohydrate-active enzymes database (CAZy): an expert resource for glycogenomics[J]. Nucleic Acids Research, 2009, 37(Suppl 1): 233-238. [27] ANTUNES L P, MARTINS L F, PEREIRA R V, et al. Microbial community structure and dynamics in thermophilic composting viewed through metagenomics and metatranscriptomics[J]. Scientific Reports, 2016, 6(6): 38915. [28] LIANG J S, FANG W, WANG Q Y, et al. Metagenomic analysis of community, enzymes and metabolic pathways during corn straw fermentation with rumen microorganisms for volatile fatty acid production[J]. Bioresource Technology, 2021, 342: 126004. doi: 10.1016/j.biortech.2021.126004 [29] MA C, LO P K, XU J, et al. Molecular mechanisms underlying lignocellulose degradation and antibiotic resistance genes removal revealed via metagenomics analysis during different agricultural wastes composting[J]. Bioresource Technology, 2020, 314: 123731. doi: 10.1016/j.biortech.2020.123731 -



 下载:
下载: