-
近年来,以冶金工业产生的含砷固废和垃圾焚烧后产生的垃圾焚烧飞灰为代表的危险固体废弃物日益增多,严重危害着生产地周边的生态环境[1-2]。含砷固废的大量堆存会污染土地,或造成滑坡、泥石流等灾害;风化形成的碎屑和尾矿,或被水冲刷进入水域,或溶解后渗入地下水,或被风刮入大气,以水、气为媒介污染环境[3]。目前,对含砷废渣的处理方法主要有2种:1)固化/稳定化,即将游离的砷转化成化学性质稳定的砷酸盐形式存放或采用惰性材料进行包裹;2)资源化利用,回收利用废渣中的砷和其他有价金属,以实现含砷废渣无害化和资源化利用的双重目的,但这对技术和设备均有较高要求[4-5]。针对上述问题,徐伟航等[6]以矿渣、钢渣、脱硫灰和磷酸淤渣为原材料制备冶金渣基胶凝材料,并探究其对垃圾焚烧飞灰中重金属的固化性能,结果表明,垃圾焚烧飞灰固化体的抗压强度和重金属浸出浓度均满足进入填埋场的要求。目前,含砷废渣和垃圾焚烧飞灰的最佳处置方式仍是固化填埋[4, 7]。垃圾焚烧飞灰固化/稳定化含砷废渣已有研究,张理群等[8]针对尾渣中砷的化学结构及赋存形态进行研究,发现尾渣中的砷主要以金属砷酸盐的形式存在,如果长期堆放,存在砷释放风险。另外,LI等[9]采用FeSO4和H2SO4联合处理砷渣,揭示了Fe可以形成稳定的Fe-O-As络合物(FeAsO4∙xFe(OH)3)来减少砷的释放。LI等[10]利用垃圾焚烧飞灰和赤泥对含砷废渣进行固化/稳定化处理,固砷体的抗压强度和浸出效果良好,固砷率高达99.9%。但是,固砷体的长期稳定性和潜在危害性还未得到应有的重视,固砷体在填埋或临时堆放过程中存在重金属浸出风险,须考虑其长期稳定性。
含砷固化体长期稳定性的评价方法有浸泡实验[11]、动态淋溶实验[12]、半动态浸出实验[13]、高温加速实验[14]、稳定性判别法[15]、释放机理法[16]和浸出分析法[17]等。何品晶等[18]、HYKS等[19]通过柱式浸出法对飞灰及其稳定化合物的长期浸出特性进行了研究,结果表明,短期测试可能低估稳定化处理飞灰在填埋初期的重金属浸出风险。但有学者[11, 20]指出,短期测试并不能准确描述飞灰固化体进入实际填埋场后的动态浸出行为。TANG等[21]对燃煤飞灰中的重金属残留量及环境危害进行了研究,采用2种稀硝酸对飞灰进行浸提,发现飞灰中重金属的浸出率不超过5%。LI等[22]采用终点pH淋溶试验方法,对不同酸性环境下的重金属淋溶形态、化学形态及环境危害进行了分析,结果显示:采用硅酸盐水泥和螯合剂进行固化剂处理,能够有效地降低大部分重金属的淋溶,而飞灰固化体中的重金属则会由于生物作用而被浸出,从而增加了对环境的危害。
本研究以飞灰基胶凝固砷体为长期稳定性风险评价对象,以(浓硫酸∶浓硝酸∶去离子水=4∶1∶40)混合酸调至pH=3.2和5.0制备淋溶液,模拟不同酸度的酸雨进行动态淋溶实验。实验采用柱浸法进行淋溶,分析不同pH淋溶液对飞灰固砷体中砷溶出量的影响,并探讨固砷体中砷在模拟酸雨作用下的淋溶累积释放特征;通过改进多级连续提取法(Sequential Extraction Procedure,简称SEP)分析飞灰固砷体中砷的结合形态,并利用潜在生态风险指数进行长期稳定性风险评价,以期为飞灰固砷体在不同侵蚀作用下的安全处置提供参考。
-
实验所用飞灰取自湖南岳阳某生活垃圾焚烧厂,该厂垃圾焚烧炉型为炉排炉,样品从焚烧设施的烟气净化系统以及烟道和烟囱底部的沉积物中收集。水淬渣为云南某钢铁冶炼厂采用冷转鼓渣处理系统处理得到的水渣。含砷废渣取自云南某铜冶炼厂,是烟尘经净化处理形成污酸后采用石灰铁盐法处置得到的废弃物。硅粉购自河南某材料公司。上述材料均于电热恒温鼓风干燥箱(DHG-9123A型,上海龙跃仪器设备有限公司)60 ℃下烘干至恒重,用粉碎机振磨后过200目标准筛,存入自封袋备用。含砷废渣、水淬渣和硅粉进行XRF检测(PANalytical Axios型,帕纳科有限公司),其化学组成见表1。
按飞灰(80%)、水淬渣(8%)、硅粉(2%)、含砷废渣(10%)占固体材料总质量比值取料,加入10% 的2.00 M水玻璃(3.30M,山东优索化工科技有限公司,加入氢氧化钠调节模数)作为碱激发剂,以0.47的液固比加去离子水搅拌均匀,浇筑到20 mm×20 mm×20 mm的钢型模具,标准条件下(20 ℃±准 ℃、湿度95%±1%)养护7 d,于水泥胶砂抗折抗压实验机(TYE-300F型,无锡建仪仪器机械有限公司)测定抗压强度后烘至恒重。再用密封式系列化验制样粉碎机(LC-GJK100-2DT型,上海力辰仪器科技有限公司)粉碎后于20目、60目和100目筛网进行筛分,得到粒径为250~850 μm、150~250 μm和<150 μm的样品,置于密封袋中备用。
-
为模拟西南地区降雨对固砷体的淋溶现象,统计了近几年的降雨量取平均值,根据降雨量和自然降雨强度调整实验中的淋溶量和恒流泵的流出速率[23-24]。本实验模拟1年的降雨量,淋溶时间为12 d,即1 d代表1个月,调节流出速率为1 mL·min−1。具体淋溶量见表2。
分别称取不同粒径的样品(250~850 μm、150~250 μm 和<150 μm)20 g置于自制淋溶柱中,柱长1 000 mm,由上至下依次填充石英砂(50 mm)、飞灰固砷体(600 mm)、棉花(50 mm),下接锥形瓶收集淋出液。实验前取300 mL去离子水浸润,浸出液于pH计(PHS-3C型,上海精密科学仪器有限公司)测定pH值,并在电感耦合等离子体光谱仪(ICP-OES Optima 8000型,上海硅仪生化科技有限公司)进行重金属离子浓度测定。
-
对淋溶前后的飞灰固砷体进行改进多级连续提取(Sequential Extraction Procedure,SEP)实验,得到酸可提取态(F1)、可还原态(F2)、可氧化态(F3)和残渣态(F4)的4种赋存形态下的重金属含量。具体步骤参照文献[25]。
-
浸出液重金属累积释放量计算公式如式(1)~式(2)所示。
式中:
$ q $ 为模拟酸雨时含砷固砷体中重金属的累积释放量,mg·kg−1;$ {\rho }_{i} $ 为第$ i $ 次重金属的质量浓度,mg·L−1;V为淋溶液体积,L;m为供试样品质量,kg;K为重金属释放率;$ \omega $ 为重金属初始质量分数,mg·kg−1。 -
通过淋出液pH以及淋溶前后重金属形态分析,评估飞灰固砷体环境风险。目前,用于重金属风险评价的方法有单因子污染指数法、地累积指数法、潜在风险评估指数法[26-27]。由于潜在生态指数法不仅考虑了重金属的含量,还综合考虑了多种元素协同作用、毒性水平、污染浓度以及环境敏感性。故采用潜在风险评估指数(Potential Ecological Risk Index, PERI)评价重金属的稳定性和潜在风险。其计算公式如式(3)~式(5)所示。
式中:
$ {C}_{\mathrm{f}}^{i} $ 代表每种重金属的单项式潜在生态风险因子;$ {C}_{\mathrm{D}}^{i} $ 和$ {C}_{\mathrm{n}}^{i} $ 分别代表每种重金属的测量含量和背景参考值。此处根据《土壤环境质量建设用地土壤污染风险管控标准(试行)》(GB36600-2018)[28],取砷的Ⅰ类建设用地筛选值20 mg·kg−1和Ⅱ类建设用地筛选值60 mg·kg−1作为参考值;$ {\mathrm{E}}_{\mathrm{r}}^{i} $ 是单个重金属的潜在风险,$ {\mathrm{T}}_{\mathrm{r}}^{i} $ 是对应单个重金属的毒性反应因子,砷取值10;PERI即为多种重金属的潜在危害指数总和[29]。Hakanson定义了5类$ {\mathrm{E}}_{\mathrm{r}}^{i} $ 值和4类PERI值(表3、表4)。 -
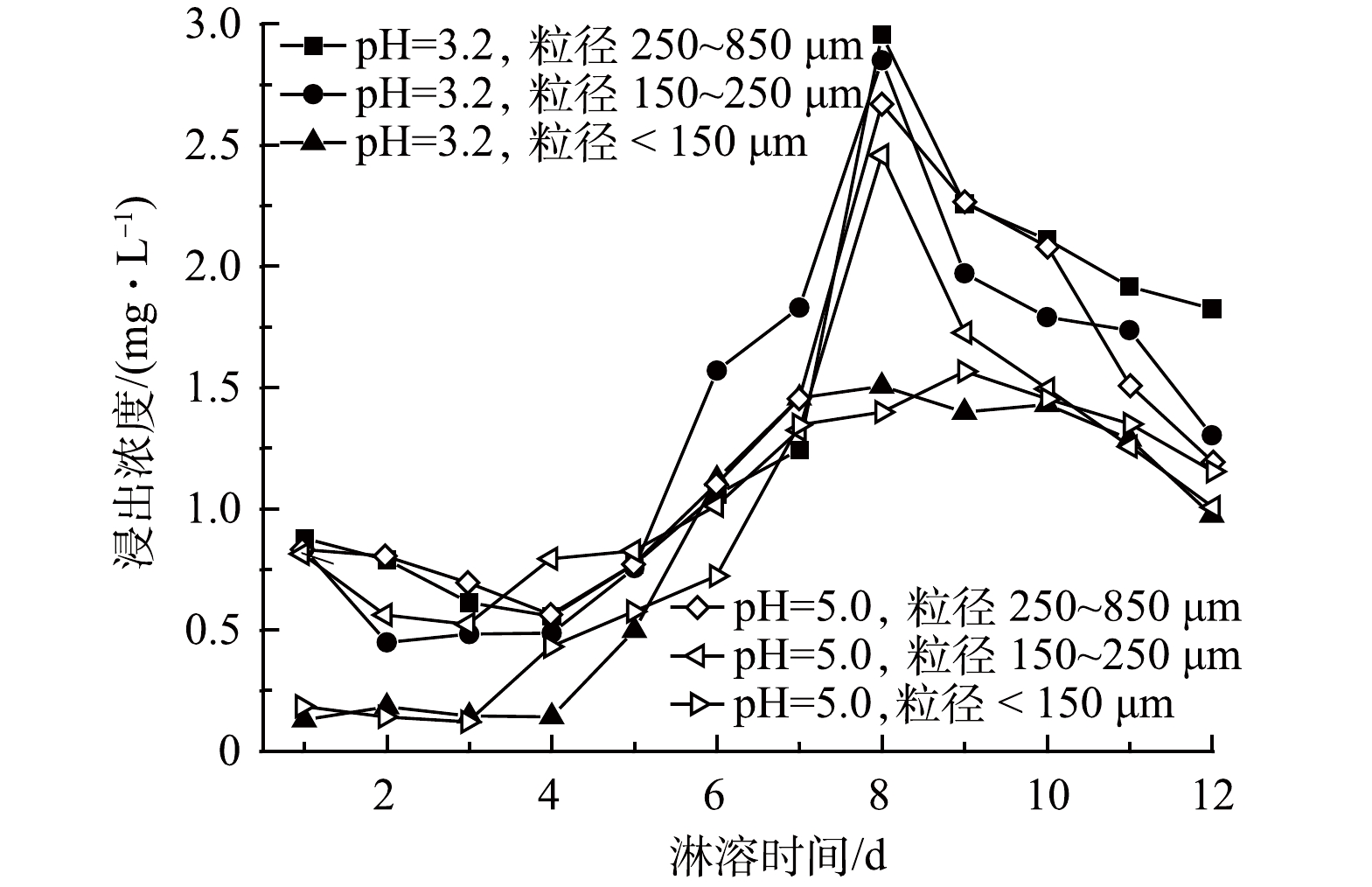
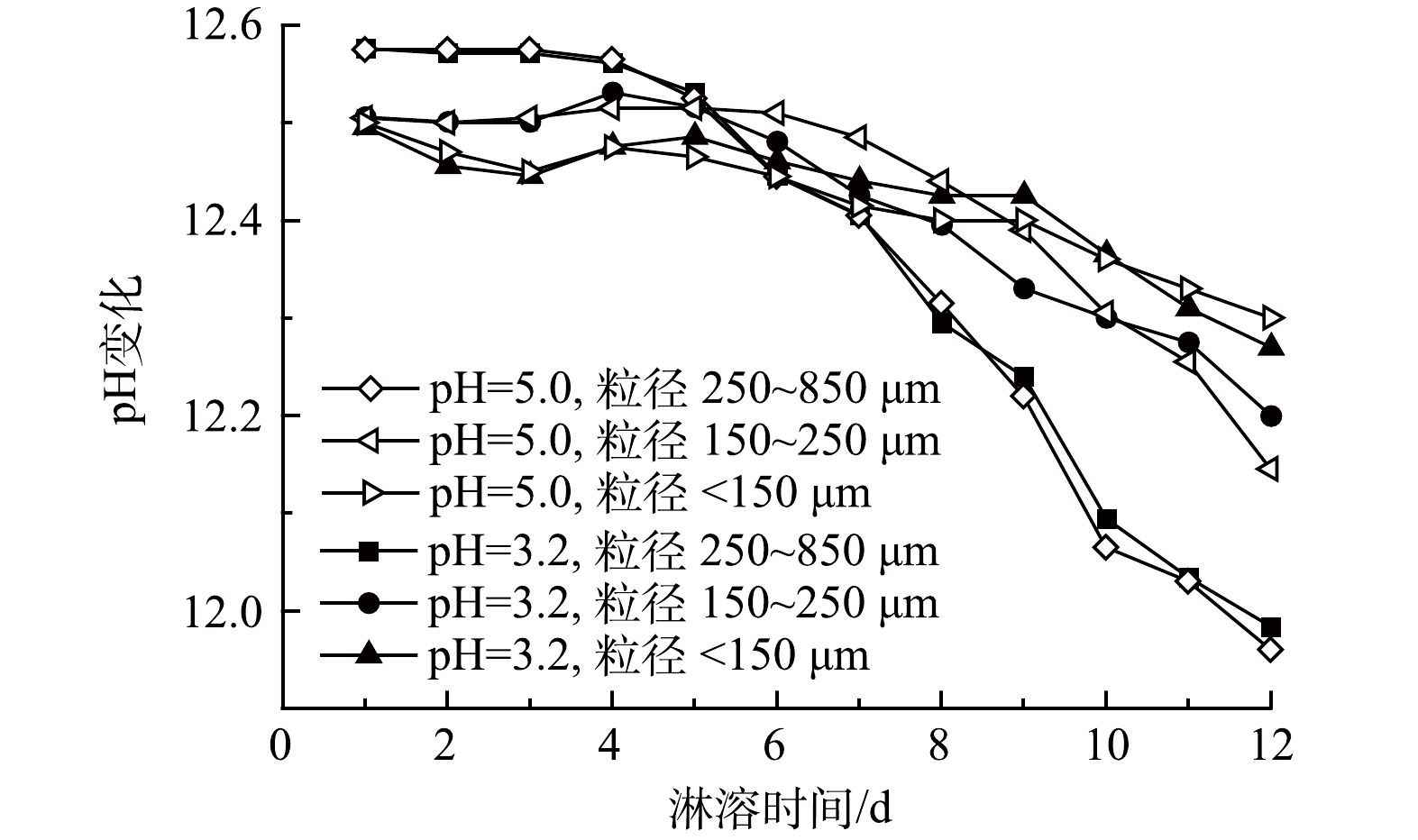
在垃圾焚烧炉的烟气处理系统中,为了去除其中的酸性气体,会注入石灰[30],另外,飞灰含有金属氧化物(CaO、MgO等),与水接触时会发生水解作用释放大量的OH−,呈现出强碱性[31],pH为12.40,故而以飞灰作为基材的固砷体也呈强碱性,浸出液pH在11.90~12.70,变化范围小。从淋溶时间来看,随着时间的增加,pH呈缓慢下降趋势;浸出pH的变化与粒径大小也有关系,但与淋溶液初始pH联系不密切(图1)。
飞灰具有强碱性,添加酸性淋溶液时,飞灰与淋溶液发生化学反应,导致浸出液的pH呈下降趋势。在前期,由于淋溶液的侵蚀加剧了飞灰基质的破坏,淋溶液中的酸还不足以中和固砷体中的碱性物质,对酸雨的缓冲能力较强,呈现出相对稳定甚至出现上升趋势;随着淋溶时间的延长,浸出液的pH减小幅度逐渐增大,因为固砷体中含有较多Al3+,长时间淋溶释放出能与溶液中的OH−反应生成Al(OH)3胶体,且尾矿中含有少量硫化矿物,会氧化生成H2SO4、Fe2(SO4)3等氧化剂,进一步加剧硫化物的氧化溶解,使得后期浸出液pH下降明显[32-34]。粒径大小与浸出液pH以6 d为转折点呈现前期粒径大、pH高,后期粒径大、pH低的趋势。其中,粒径为250~850 μm的样品浸出液pH下降趋势最为明显。这是因为,粒径较大,空隙不均匀,下渗速度快,与飞灰固砷体接触时间短,反应量少,随着时间的延长,pH下降趋势变缓[34]。浸出液pH居高不下(11.90~12.70)的主要原因在于:强酸性的淋溶液与飞灰样品接触、下渗过程中,H+会侵蚀飞灰,释放出飞灰中的碱性物质。另外,飞灰中含有大量Cl−,可促进含砷废渣中钙砷化合物Ca5(AsO4)3OH和Ca5(AsO4)3Cl的形成和生长,而这些稳定的钙砷化合物形成的最佳pH正好在12~13[25]。
-
飞灰固砷体在不同酸度(pH=3.2、pH=5.0)、不同粒径(250~850 μm、150~250 μm、<150 μm)的淋溶液下模拟动态淋溶得到的浸出液含有微量重金属。其中,砷最高质量浓度为2.42 mg·L−1,低于危险废物鉴别标准(GB 5085.3—2007)[35]中的5 mg·L−1。砷的释放与淋溶液的淋溶量之间的关系为:淋溶量越大,砷浸出浓度越高(图2)。这与刘平[36]的研究有所差异,其研究表明,砷浸出浓度会随淋溶量的增加而减小。这可能是由于淋溶量的增加,使砷浓度得以稀释。
通过模拟酸雨作用下的飞灰固砷体的动态淋溶实验,得到了不同淋溶量与浸出液砷浓度之间的关系:淋溶量越大,浸出浓度越高,5~8 d尤为明显。这与重金属快速释放阶段相吻合。加入碱激发剂使飞灰表面积增大,从而提高其对砷的吸附能力[37],故前期固化效果良好。随着淋溶量的增加,固砷体表面的碱金属离子被溶解消耗,砷溶解增加,表现为浸出浓度快速上升。在后期,固砷体表面金属氧化物能够被碳酸化形成碳酸盐,不易被溶解,这与浸出液呈强碱性相吻合[38]。不同粒径的尾矿比表面积、带电荷数等不同。粒径越小,砷的赋存能力越强;粒径越大,浸出浓度越高。其主要原因可能为,粒径小的飞灰固砷体拥有更大的比表面积,更容易吸附、络合共沉淀重金属,产生环境风险更高。
-
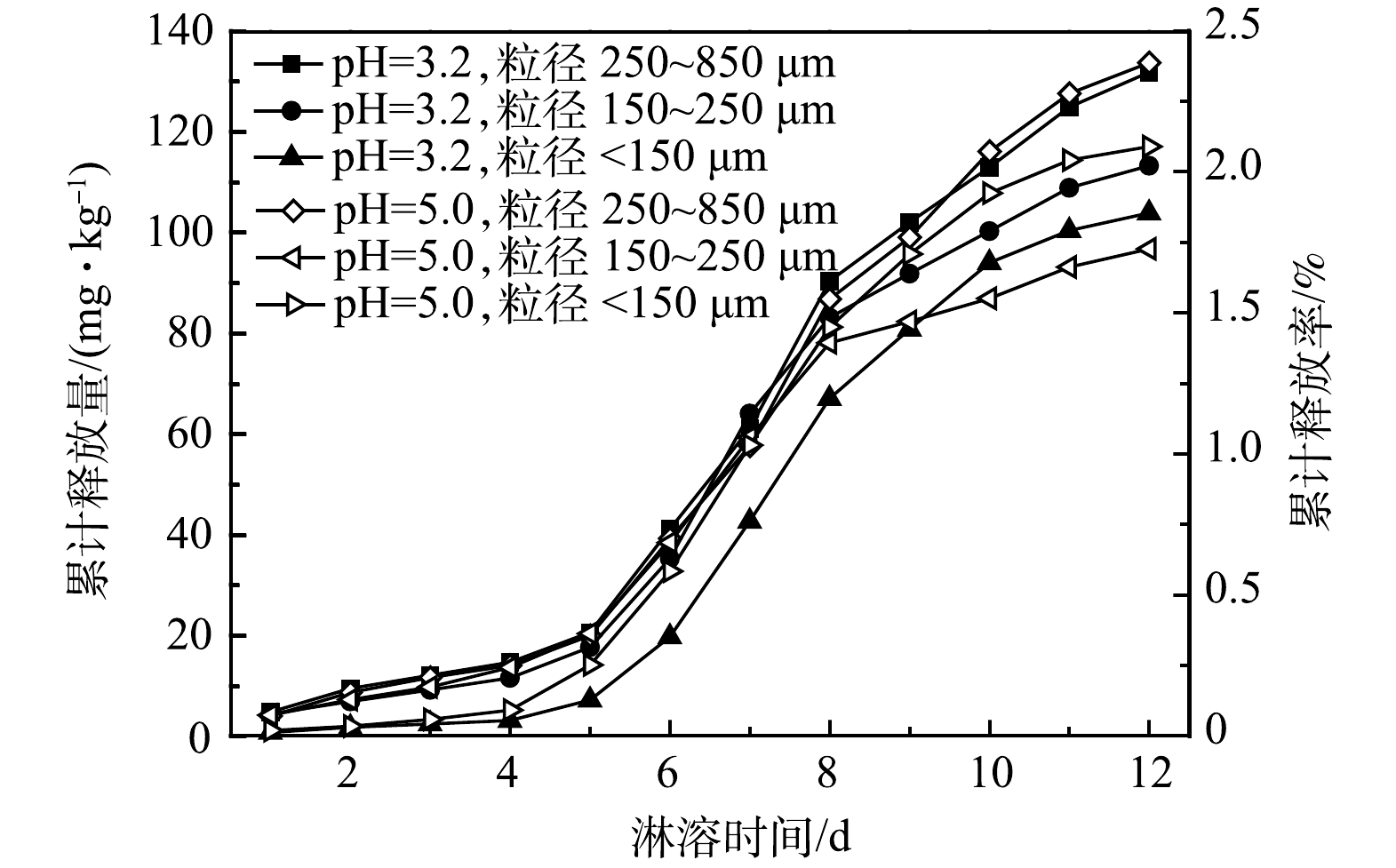
根据1.4中的式(1)、式(2)计算可知,飞灰固砷体在为期12 d的动态淋溶实验中累计释放的砷质量分数达133.78 mg·kg−1,释放率为2.32%(通过全元素分析得到砷的初始质量分数为5 771.25 mg·kg−1)。这一过程中释放量(率)呈现出如下趋势(见图3):同一酸度(pH=3.2)淋溶液下,粒径越小,释放量越低;同一粒径范围(<150 μm)内,淋溶液pH越低,砷释放量越高;总体呈现缓慢增加后,出现一个快速释放阶段(5~8 d)后趋于稳定的趋势。这与重金属浸出液浓度变化规律相符,也与已有研究结果[33]相近。
动态淋溶实验结果表明,飞灰固砷体中砷累计释放量符合国家标准,固化/稳定化效果好,固砷率≥97.68%,对其他重金属的固化效果更佳。根据图4,可将重金属的释放过程大致分为3个阶段,即初始阶段、快速释放阶段和慢速释放阶段。在初始阶段,重金属与酸性淋溶液接触,产生了石膏晶体,对重金属有一定的吸附作用[36];另外,飞灰中含有的NaCl、KCl等可溶性盐会与酸快速反应,与重金属形成竞争效应,减少了重金属的释放[39]。随着淋溶时间增长,重金属的释放进入快速释放阶段,固砷体表面吸附的离子交换态重金属会快速的溶解流出,淋溶液中大量的H+将重金属置换溶出,导致固砷体中离子交换态重金属大量溶解,重金属快速释放[40]。在离子交换态重金属释放尽后,重金属释放进入慢速释放阶段,在这一阶段,随着淋溶时间的延长尾矿与酸溶液、空气等相互作用,将更稳定的重金属形态还原态物和有机结合态物中重金属释放出来。由于稳定态重金属释放速率较慢,释放的重金属量也有限,所以到淋溶中后期重金属的溶出速率和溶出量都在减少[41]。不同pH条件下、不同金属元素的释放特征和迁移能力与重金属在尾砂中的赋存形态具有重要关系。除了淋溶液的pH,重金属的释放量还与淋溶量、淋溶速率、固砷体的组成和性质等因素有关[42]。
-
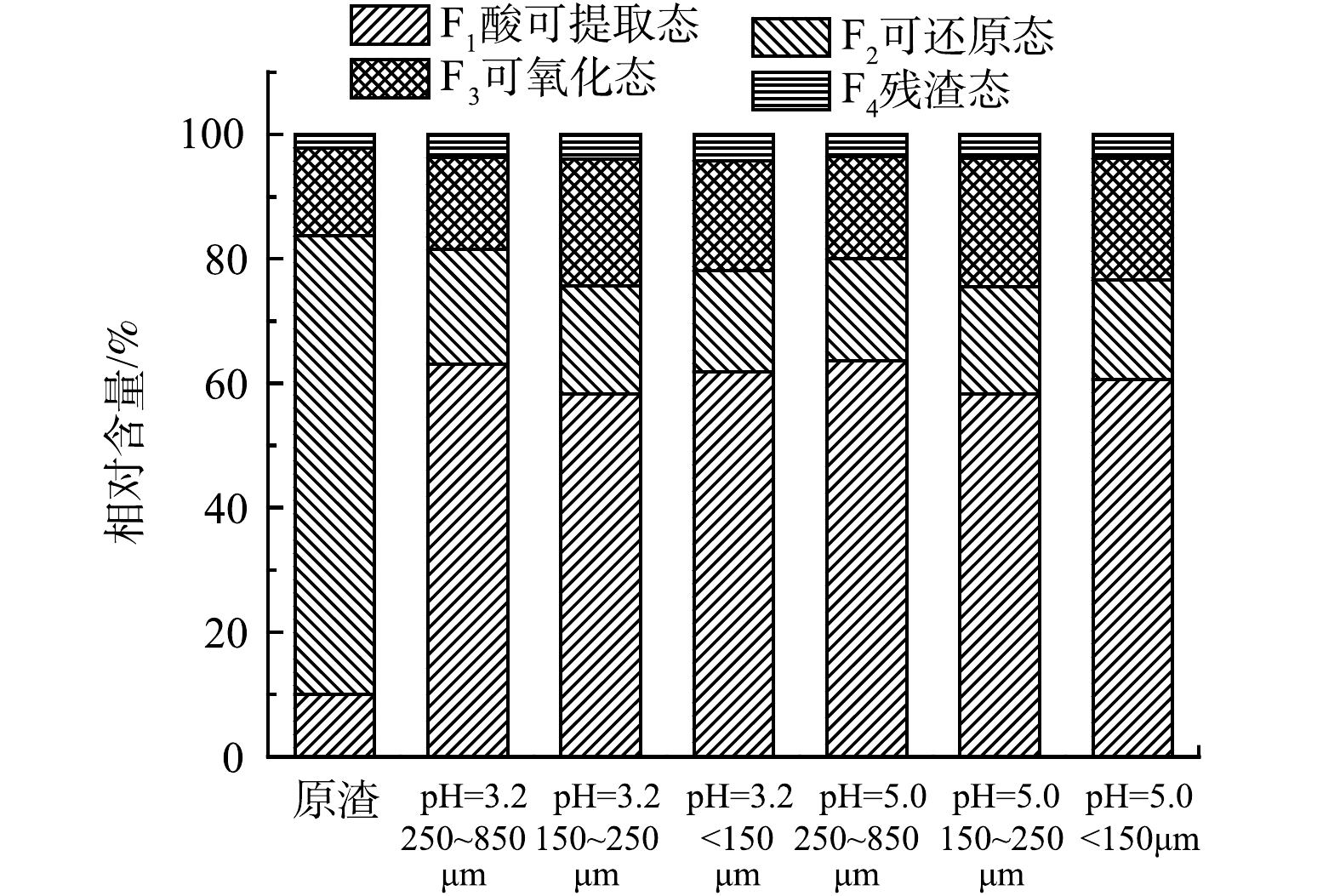
通过SEP改进多级连续提取法进行实验结果分析,可得到动态淋溶前后飞灰固砷体中重金属的化学形态分布(图4)。利用潜在风险评估指数(PERI)公式(式3~式5)进行计算,得到表5中的潜在风险指数(表5)。其中,由于低级环境风险状态固体废物中只有砷是不达标危害元素,因此砷的潜在风险指数即为固体废物的综合潜在风险指数。
从砷的化学形态来看,原渣中的砷大部分以可还原态存在,其次是可氧化态和可还原态,残渣态砷的化学形态占比仅为2.1%。这说明,固砷体中97.9%的砷易发生迁移转化,在环境中存在较大风险。通过模拟酸雨动态淋溶发现,在酸雨作用下,可还原态砷的比例大幅下降,从73.7%下降到16.5%;可还原态砷与酸雨中的酸发生作用转化为酸可提取态,使酸可提取态砷由10.0%上升到60.0%,可氧化态砷的含量则没有明显变化,而残渣态砷则略有升高。这说明,飞灰固化含砷废渣后仍需要远离酸雨淋溶,否则砷会大量溶出,造成二次溶出危害[36]。淋溶过程使原渣中的砷化学形态发生变化,不同粒径的飞灰固砷体中残渣态砷含量均在pH=3.2条件下比pH=5.0条件下高。粒径<150 μm的飞灰固砷体在pH=3.2动态淋溶下的残渣态砷相对含量最高,风险最低。不同粒径的钙砷渣在不同pH的酸雨侵蚀下,不仅砷化学形态发生了一定程度的转变,同时部分不稳定的有效态砷被浸出,具有潜在的环境风险。这与已有研究结果[33]一致。
分析飞灰固砷体在模拟酸雨动态浸出作用下的PERI值(表5)可知,当固砷体以Ⅰ类建设用地筛选值(20 mg·kg−1)作为砷背景参考值时,原渣的PERI值小于150。这表明,该侵蚀环境下对环境的潜在生态危害轻微。经模拟酸雨淋溶后的固砷体的PERI值处于150≤PERI <300,属于中等风险,对环境的潜在生态危险中等。综合分析可知,在动态侵蚀环境下,以Ⅱ类建设用地筛选值作为背景参考值时,固砷体对环境的潜在生态危害轻微;以Ⅰ类建设用地筛选值作为背景参考值时,固砷体对环境的潜在生态危害为中等风险。
-
1)模拟酸雨动态淋溶条件下,飞灰固砷体呈碱性,对酸雨具有缓冲性。相同条件、相同粒径时,酸雨pH越小,浸出液pH变化越小,固砷体稳定性越好;酸雨pH相同时,固砷体粒径越大,固砷体稳定性相对越差。
2)砷浸出过程呈现出初始、快速释放和慢速释放3个阶段的浸出特征,且浸出浓度与淋溶量成正比例关系。淋溶实验表明,飞灰具有良好的固砷效果,固化率高达97.68%,浸出液最高砷质量浓度仅为2.42 mg·L−1。
3)动态侵蚀作用下,原渣中的还原态砷大幅度降低,酸可提取态砷和残渣态砷增加,有利于降低固砷体的风险,分别以Ⅰ类和Ⅱ类建设用地筛选值作为砷背景参考值,固砷体潜在生态危害程度由中等危害转为轻微生态危害,为飞灰固砷体在不同环境下的安全处置提供参考。
飞灰基胶凝固砷体长期稳定性风险评价
Risk assessment of long-term stability of fly ash-based cementitious material containing arsenic
-
摘要: 为明确酸雨条件下飞灰基胶凝固砷体的长期稳定性,模拟2种酸度的酸雨对3种粒径状态下的固砷体进行动态淋溶实验,并运用改进多级连续提取法(Sequential Extraction Procedure, SEP)和潜在风险评估指数对固砷体进行长期稳定性风险评价。结果表明,随着浸出时间的增加,固砷体中的Al3+溶出并与溶液中的OH−反应生成Al(OH)3胶体,且废渣中的硫化矿物氧化生成H2SO4、Fe2(SO4)3等氧化剂,进一步加剧硫化物的氧化溶解,使得浸出液pH呈下降趋势;而且,由于飞灰固砷体粒径大小不同,表面积大小和吸附位点变化,使砷浸出过程呈现出初始、快速释放和慢速释放3个阶段的浸出特征,最高浸出质量浓度为2.42 mg·L−1,累计释放量达133.78 mg·kg−1,累计释放率为2.32%。SEP实验发现,原渣中的还原态砷大幅度降低,酸可提取态砷和残渣态砷增加,有利于降低固砷体的风险。潜在生态风险评价表明,在Ⅰ类和Ⅱ类建设用地筛选值为背景时,潜在生态危害程度由中等危害转为轻微危害。本研究结果可为飞灰固砷体在不同侵蚀作用下的安全处置提供参考。Abstract: This paper presents a dynamic leaching test of arsenic fixation in three particle sizes based on an innovative simulation of two acid rainfall conditions in southwest China to investigate the long-term stability of fly ash-based cementitious materials that contain arsenic under acid rain conditions. Furthermore, the long-term stability risk of arsenic fixation is evaluated using a Sequential Extraction Procedure (SEP) and the potential risk assessment index. Results showed that the Al3+ in the solid arsenic bodies dissolved and reacted with the OH− in solution to form Al(OH)3 colloids as the leaching time increased. Moreover, the oxidation of sulfide minerals in the slag produced oxidants, such as H2SO4 and Fe2(SO4)3, which further aggravated the oxidative dissolution of sulfides, thereby resulting in an overall decreasing pH value of the leachate. In addition, due to the varying particle sizes of the fly ash solid arsenic bodies, surface area size, and adsorption site changes, the arsenic leaching process showed three stages of leaching characteristics, namely, initial, rapid, and slow release, with a maximum leaching concentration of 2.42 mg·L−1, the cumulative release of 133.78 mg·kg−1, and the cumulative release rate of 2.32%. The SEP test revealed that the reduced state of arsenic in the raw slag was lowered substantially, and the acid extractable state and residual state of arsenic were increased, which was conducive to lessening the risk of arsenic fixation bodies. The evaluation of the potential ecological risk showed that the potential ecological hazard when the screening value of Class Ⅰ and Class Ⅱ construction sites was the background changed from moderate hazard to slight hazard.
-
Key words:
- fly ash /
- arsenic slag solidification /
- simulated acid rain /
- leaching /
- risk assessment
-

-
表 1 实验材料各化学成分质量分数
Table 1. Chemical composition of raw materials (mass fraction)
% 供试原料 CaO SiO2 Al2O3 As2O3 SO3 ZnO Fe2O3 飞灰 43.00 3.17 0.63 — 6.02 0.31 0.50 水淬渣 38.52 33.19 12.93 — 1.13 0.83 0.60 含砷废渣 36.31 1.81 0.95 31.61 12.31 6.69 3.07 硅粉 0.36 98.18 0.20 — 0.25 — 0.05 注:“—”表示未检出。 表 2 动态模拟酸雨实验降雨分布
Table 2. Distribution of dynamic simulated rainfall
月份 降雨量/mm 淋溶量/mL 月份 降雨量/mm 淋溶量/mL 1 75.9 104 7 229.8 315 2 80.3 110 8 236.4 324 3 69.3 95 9 143.7 197 4 71.5 98 10 135.0 185 5 118.2 162 11 72.2 99 6 163.4 224 12 52.5 72 表 3 单一重金属潜在生态风险等级
Table 3. Potential ecological risk level of single heavy metal
Eir值 单一潜在
生态风险等级Eir<40 轻微 40≤Eir<80 中等 80≤Eir<160 强 160≤Eir<320 很强 Eir≥320 极强 表 4 沉积物重金属潜在生态风险等级
Table 4. Potential ecological risk rating of sediment heavy metals
PERI值 综合潜在
生态风险PERI<150 轻微 150≤PERI<300 中等 300≤PERI<600 高 PERI≥600 严重 表 5 动态淋溶前后砷的潜在风险评估指数(PERI)
Table 5. Potential ecological risk index (PERI) of arsenic before and after dynamic leaching
材料或处理条件 潜在风险评估指数 Ⅰ类PERI Ⅱ类PERI 原渣 86.19 28.73 pH=3.2,250~850 μm 169.86 56.62 pH=3.2,150~250 μm 169.82 56.61 pH=3.2,<150 μm 163.72 54.57 pH=5.0,250~850 μm 154.36 51.45 pH=5.0,150~250 μm 164.2 54.73 pH=5.0,<150 μm 170.53 56.84 -
[1] HOJSAK I, BRAEGGER C, BRONSKY J, et a1. Arsenic in Rice: A Cause for Concern[J]. Journal of pediatric gastroenterology andnutrition, 2015, 60(1): 142-145. doi: 10.1097/MPG.0000000000000502 [2] PODGORSKI J, BERG M. Global threat of arsenic in groundwater[J]. Science, 2020, 368(6493): 845-850. doi: 10.1126/science.aba1510 [3] LIU G, SHI Y, GUO G, et al. Soil pollution characteristics and systemic environmental risk assessment of a large-scale arsenic slag contaminated site[J]. Journal of Cleaner Production, 2020, 251(1/2/3): 119721. [4] 龚傲, 陈丽杰, 吴选高, 等. 含砷废渣处理现状及研究进展[J]. 有色金属科学与工程, 2019, 10(4): 28-33. doi: 10.13264/j.cnki.ysjskx.2019.04.005 [5] 郑锦一, 张学洪, 刘杰, 等. 广西老厂铅锌尾矿重金属动态纵向释放迁移规律[J]. 桂林理工大学学报, 2020, 40(3): 580-586. doi: 10.3969/j.issn.1674-9057.2020.03.018 [6] 许伟航, 傅平丰, 方贵稳, 等. 冶金渣基胶凝材料固化生活垃圾焚烧飞灰的性能研究[J]. 矿产保护与利用, 2021, 41(3): 10-16. doi: 10.13779/j.cnki.issn1001-0076.2021.03.002 [7] 倪海凤, 旦增, 周鹏, 等. 国内城市生活垃圾焚烧飞灰研究现状及进展[J]. 再生资源与循环经济, 2021, 14(3): 24-30. doi: 10.3969/j.issn.1674-0912.2021.03.009 [8] 张理群, 周慧慧, 郑刘根, 等. 尾渣中砷的精细化学结构、赋存形态[J]. 环境化学, 2021, 40(5): 1611-1618. doi: 10.7524/j.issn.0254-6108.2019121202 [9] LI E P, YANG T, WANG Q, et al. Long-term stability of arsenic calcium residue (ACR) treated with FeSO4 and H2SO4: Function of H+ and Fe(II)[J]. Journal of Hazardous Materials, 2021, 420: 126549. doi: 10.1016/j.jhazmat.2021.126549 [10] LI Y C, MIN X B, KE Y, et al. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation[J]. Waste Management, 2019, 83: 202-208. doi: 10.1016/j.wasman.2018.11.019 [11] 曹珊珊, 吴光红, 苏睿先. 模拟中性和酸性降雨及垃圾渗滤液浸泡粉煤灰及渣重金属浸出特征[J]. 环境科学, 2011, 32(6): 1831-1836. doi: 10.13227/j.hjkx.2011.06.045 [12] 刘建, 何亮, 骆成杰, 等. 生活垃圾焚烧飞灰固化体重金属动态浸出规律[J]. 中国环境科学, 2019, 39(3): 1087-1093. doi: 10.3969/j.issn.1000-6923.2019.03.023 [13] 孙胤涛, 姚科, 郑虞琪, 等. 飞灰基碱激发胶凝材料中重金属的一维半动态浸出行为分析[J]. 环境科学与技术, 2021, 44(1): 24-31. doi: 10.19672/j.cnki.1003-6504.2021.01.004 [14] 盛农. 加速碳酸化法稳固化工业危险废物焚烧飞灰重金属的研究[J]. 安徽农学通报, 2018, 24(5): 74-79. doi: 10.3969/j.issn.1007-7731.2018.05.034 [15] 王娜娜. 水中重金属的快速判别与铜铬镍快速检测方法研究[D]. 哈尔滨: 哈尔滨工业大学, 2014. [16] BU Y W, DAI X, LU P, et al. Release characteristics of semi-volatile heavy metals during co-combustion of sewage sludge and coal under the O2/CO2 atmosphere[J]. Journal of Thermal Analysis & Calorimetry, 2018, 133(2): 1041-1047. [17] FAN C, WANG B, ZHANG T. Review on cement stabilization/solidification of municipal solid waste incineration fly ash[J]. Advances in Materials Science and Engineering, 2018, 10(2): 1-7. [18] 何品晶, 吴长淋, 章骅, 等. 生活垃圾焚烧飞灰及其稳定化产物的长期浸出行为[J]. 环境化学, 2008, 27(6): 786-790. doi: 10.3321/j.issn:0254-6108.2008.06.018 [19] HYKS J, ASTRUP T, CHRISTENSEN T H. Long-term leaching from MSWI air-pollution-control residues: Leaching characterization and modeling[J]. Journal of Hazardous Materials, 2009, 162(1): 80-91.21. doi: 10.1016/j.jhazmat.2008.05.011 [20] 李清毅, 宋凯, 何亮, 等. 生活垃圾填埋场中飞灰固化体动态/半动态浸出行为[J]. 环境工程, 2019, 37(11): 149-154. doi: 10.13205/j.hjgc.201911024 [21] TANG Y, PAN J S, LI B Q, et al. Residual and ecological risk assessment of heavy metals in fly ash from co-combustion of excess sludge and coal[J]. Scientific Reports, 2021, 11(1): 2499. doi: 10.1038/s41598-021-81812-5 [22] LI W H, SUN Y J, HUANG Y M, et al. Evaluation of chemical speciation and environmental risk levels of heavy metals during varied acid corrosion conditions for raw and solidified/stabilized MSWI fly ash[J]. Waste Management, 2019, 87: 407-416. doi: 10.1016/j.wasman.2019.02.033 [23] 潘建华. 中国西南地区时雨量分布特征与时月变化[J]. 灾害学, 2019, 34(4): 113-120. doi: 10.3969/j.issn.1000-811X.2019.04.020 [24] 潘建华. 中国西南地区小时雨量概率分布特征[J]. 干旱气象, 2020, 38(2): 226-233. [25] 费讲驰. 含砷废渣固砷体环境稳定性及其潜在风险评价[D]. 长沙: 中南大学, 2019. [26] 杜梅, 杨俊, 刘君, 等. 矿区尾矿重金属环境风险评价方法综述[J]. 环境与可持续发展, 2021, 6: 143-151. doi: 10.19758/j.cnki.issn1673-288x.202106143 [27] 胡煜欣, 宋炜, 周瑞静. 某金属冶炼企业土壤As污染特征及潜在生态风险评价[J]. 中国环境监测, 2021, 37(6): 147-155. doi: 10.19316/j.issn.1002-6002.2021.06.16 [28] 中华人民共和国生态环境部, 中华人民共和国国家市场监督管理总局. 土壤环境质量建设用地土壤污染风险管控标准(试行): GB36600-2018[S]. 北京: 中国环境科学出版社, 2018. [29] HAKANSON L. An ecological risk index for aquatic pollution control a sedimentological approach[J]. Water Research. 1980, 14(8): 975-1001. [30] WEIBEL G, EGGENBERGER U, SCHLUMBERGER S, et al. Chemical associations and mobilization of heavy metals in fly ash from municipal solid waste incineration[J]. Waste Management, 2017, 62: 147-159. doi: 10.1016/j.wasman.2016.12.004 [31] LI W H, GU K, YU Q W, et al. Leaching behavior and environmental risk assessment of toxic metals in municipal solid waste incineration fly ash exposed to mature landfill leachate environment[J]. Waste Management, 2021, 120(1): 68-75. [32] LUBNA A A I. Chemical characterization and mobility of metal species in fly ash-water system[J]. Water Science, 2015, 29(2): 109-122. doi: 10.1016/j.wsj.2015.10.001 [33] XUE-HUA P Y, DENG R Y, WANG C C, et al. Leaching characteristics of heavy metals from tailings under simulated rainfall in dexing copper mine[J]. Environmental Engineering, 2019, 37(5): 61-65. [34] 杨玉婷. 尾矿材料重金属释放机理及其运移规律研究[D]. 昆明: 昆明理工大学, 2020. [35] 中华人民共和国环境保护总局, 中华人民共和国国家质量监督检验检疫总局. 危险废物鉴别标准-浸出毒性鉴别: GB 5085.3-2007[S]. 北京: 中国环境科学出版社, 2007. [36] 刘平. 煤矸石及其燃后灰渣中砷、硒、锑的淋溶释放研究[D]. 南昌: 南昌大学, 2013. [37] HUANG X R, ZHAO H H, ZHANG G B, et al. Potential of removing Cd(II) and Pb(II)from contaminated water using a newly modified fly ash[J]. Chemosphere, 2020, 242: 125148. doi: 10.1016/j.chemosphere.2019.125148 [38] WANG Y T, ZHANG H, WU X Y, et al. Ecotoxicity assessment of sodium dimethyl dithio carbamate and its micro-sized metal chelates in Caenorhabditis elegans[J]. Science of The Total Environment, 2020, 720: 137666. doi: 10.1016/j.scitotenv.2020.137666 [39] KIM H S, SEO B H, KUPPUSAMY S, et al. A DOC coagulant, gypsum treatment cansimultaneously reduce As, Cd and Pb uptake by medicinal plants grown in contaminated soil[J]. Ecotoxicology and Environmental Safety, 2018, 148: 615-619. doi: 10.1016/j.ecoenv.2017.10.067 [40] 肖劲光, 刘喜, 肖武, 等. 高浓度砷渣土稳定化/固化效果及其影响因素研究[J]. 节能与环保, 2018(12): 74-75. doi: 10.3969/j.issn.1009-539X.2018.12.025 [41] 钱玲, 李冰, 陈希, 等. 黄金尾砂重金属淋溶释放规律[J]. 东南大学学报:自然科学版, 2020, 50(6): 1084-1089. [42] LI X H, WU Y G, LIU M F, et al. Effects of simulated acid rain on heavy metal release and biological toxicity of lead and zinc smelting waste residues[J]. Journal of Agro-Environment Science, 2020, 39(11): 2504-2514. -



 下载:
下载: