-
甲醛作为主要室内空气污染物[1],其来源主要为装修过程中使用的含有粘合剂的人造板材、劣质油漆和地毯等的缓慢释放[2]。控制室内低浓度甲醛的途径有2种 [3]:一是源头控制,如使用环保材料以降低室内空气中甲醛浓度;二是末端治理,即去除空气中游离的甲醛气体。催化氧化法被认为是最为有效的吸附处理技术,包括常温催化氧化和光催化氧化技术,具有脱除效率高、无二次污染等优点,可将HCHO直接转化为CO2和H2O[4],其核心在于研制出能在低温潮湿环境下高效催化氧化甲醛的催化剂。
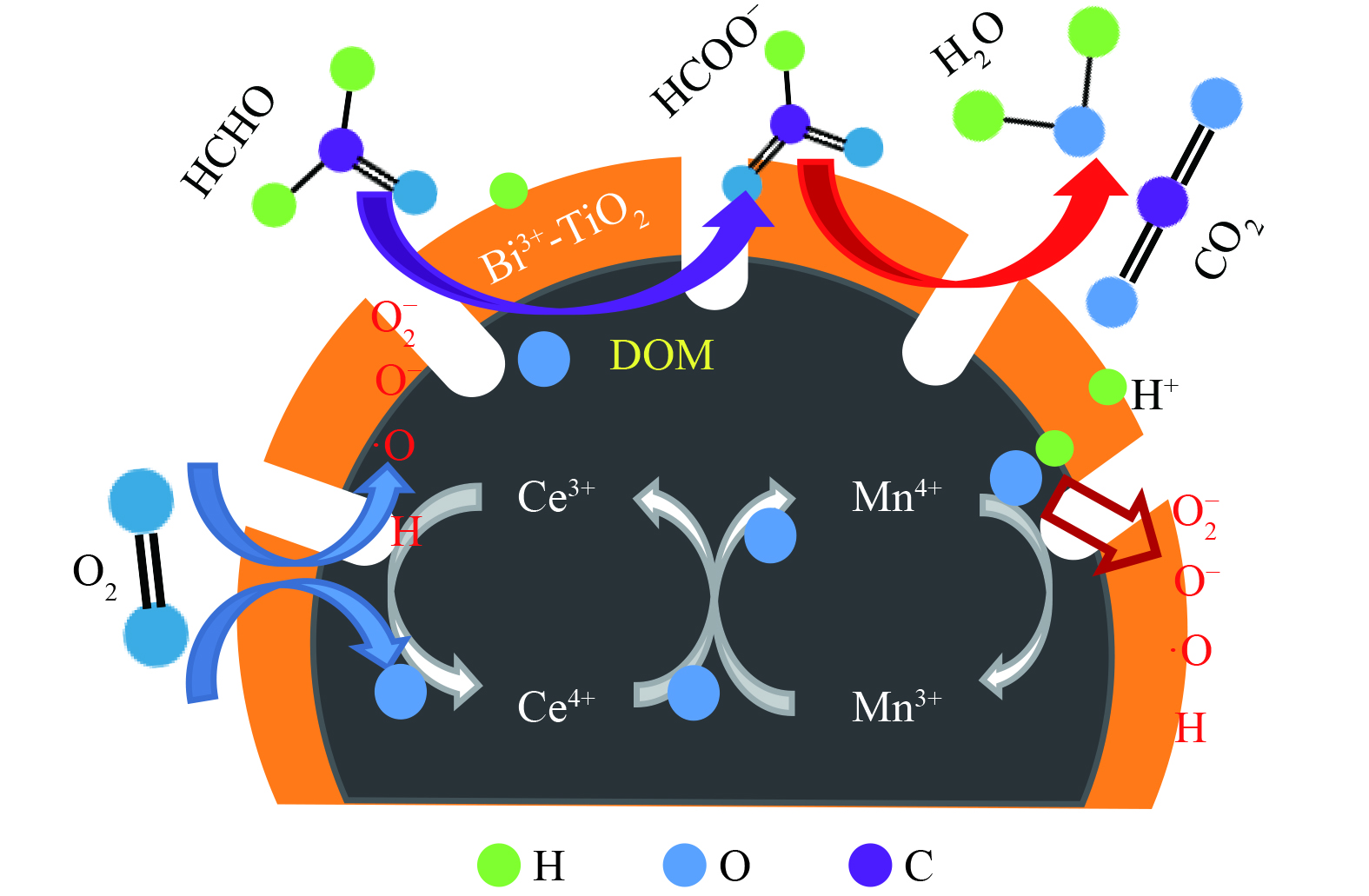
二氧化钛(TiO2)为最常用的半导体材料,具有较高的太阳光敏感性、化学稳定性和低毒性等优点[5],且TiO2的高能带态密度能实现高效的光电流转换,使得其比ZnO、SnO2、ZrO2、CdS和g-C3N4等半导体材料具有更高的氧化活性[6],可在紫外光激发下生成强氧化性的羟基自由基(·OH)和超氧阴离子自由基(·O2−)等[7-8]。然而,TiO2仅能吸收紫外光,无法吸收更多的可见光,且较低的电子转移率和较高的光致电子与空穴复合率亦会严重影响整体量子产率。通常采用2种解决策略:一是将TiO2辐射吸收范围扩展到可见光区域;二是抑制激发电子和正空穴的复合[9]。为此需要对TiO2光催化剂进行改性,如贵金属沉积[10],半导体耦合[11]等。TiO2与半导体Bi2O3耦合[12]亦可将吸收光谱扩展到可见光区域,显著增强光催化氧化活性 [13]。掺杂的Bi离子以化合物形式部分取代了一些钛原子形成的锐钛矿TiO2呈现出更大的可见光偏移,且Bi和Y共掺杂致使光生空穴和电子的复合速率降低,有利于促进催化剂氧化性能[14]。但由于Bi2O3-TiO2受光照条件限制,且Bi2O3和TiO2亦可作为光生电子和空穴的复合中心而降低其氧化性能。
锰基催化剂为一种高效、深度的常温催化氧化催化剂。隐钾锰矿型和水钠锰矿型氧化锰中存在多种价态的锰,易相互转化,使得氧化锰具有较高的催化氧化活性[15],但锰基催化剂亦会出现结晶性差、易团聚、易失活等现象,会直接影响催化剂稳定性和再生活性[16]。结构型、电子型CeO2助催化剂有较强的储氧能力[17],可在高空速条件下为氧化反应提供充足的氧,并借助Ce4+/Ce3+离子偶的Redox循环,有效改善活性位点间的电子传递。易变价的Ce又导致晶界处存在各种非化学计量缺陷,因在混合氧化物存在下易形成更多的晶格缺陷,增加活性位点。TANG等[18-19]通过改进的共沉淀方法制备了一系列MnOx-CeO2催化剂,在100 ℃反应温度下实现了100%的HCHO降解。这是通过氧转移机制有效激活分子氧,MnOx-CeO2固溶体的形成致使氧化锰具有更高的氧化状态,且表面更丰富的晶格氧物种对低温催化氧化甲醛发挥着至关重要的作用。ZHANG等[20]采用溶胶-凝胶柠檬酸法制备了一系列高效的MnOx-Co3O4-CeO2三效催化剂,当三者摩尔比为16∶19∶1,反应温度为100 ℃时,催化剂展示出对HCHO的最佳去除效率,但MnOx-CeO2存在稳定性差,一段时间后可能由于大量吸附空气中水蒸气致使HCHO去除效率大幅降低,且显示室温下氧化锰表现为HCHO的氧化物,而非催化剂[21]。
本研究以常温催化氧化性能的MnCeOx氧化物为载体,起到吸附和常温催化作用,以具有可见光催化氧化性能的Bi3+-TiO2为活性组分,采用不同制备方法研制Bi3+-TiO2与MnCeOx相耦合的可见光光热协同催化氧化催化剂,以实现光生电子和活性氧物种(O2−, O−, ·OH )的转移与氧化,同时利用MnOx-CeO2的黑色组分吸光促使Bi3+-TiO2光催化性能提升或升高温度进而提升其常温催化氧化性能,并探索制备方法、负载量、组分配比以及结构形貌等对催化剂性能的影响,研究考察Bi3+-TiO2/MnCeOx耦合情况下的催化氧化性能及可能机理,以期为低浓度HCHO治理提供参考。
-
1) MnCeOx活性粉末的制备。取55.0 mL硝酸锰溶液(质量分数为50%)和250 mL蒸馏水于烧杯中,依次投加25.5 g硝酸铈和16.0 g柠檬酸至上述溶液中,搅拌至完全溶解,再将上述溶液置于60 ℃水浴锅蒸煮直至形成溶胶状,自然陈化24 h,将所得样品置于100 ℃烘箱干燥直至形成粉末状,再经550 ℃,7 h煅烧制得MnCeOx活性粉末备用。
2) Bi3+-TiO2/MnCeOx催化剂的制备。研究采用浸渍法、水热法和物理混合等3种方法将Bi3+-TiO2(Bi2O3负载至MnCeOx上形成复合催化剂,最佳负载量为7.0%(质量分数)。浸渍法是先取7.1 mL钛酸四丁酯、8.5 mL乙醇和丙三醇混合溶液(体积比1:1)混合搅拌形成溶液A,另取8.5 mL乙醇和丙三醇混合溶液并加入适量乙酸、碳酸铵和硝酸铋搅拌制成溶液B;再将溶液B缓慢滴加至溶液A中形成混合溶液,并向其中投加2.0 g MnCeOx活性粉末形成悬浊液C,持续搅拌10 min后置于80 ℃烘箱中干燥直至形成粉末状;研磨成细粉状并经450 ℃,8.5 h焙烧制得10.0%(质量分数)Bi3+-TiO2/MnCeOx催化剂。不同Bi3+-TiO2负载量(质量分数1.0%、2.0%、5.0%、20.0%)的Bi3+-TiO2/MnCeOx催化剂亦可采用上述方法制得。在10.0%Bi3+-TiO2/MnCeOx基础上,探索了不同焙烧温度(350、550、和650 ℃)对催化剂性能的影响。同时,将乙醇和丙三醇混合溶液替换为纯乙醇,亦采用上述方法制备10.0%Bi3+-TiO2/MnCeOx(EtOH)催化剂(550 ℃)。水热法则是将上述所得悬浊液C置于具有聚四氟乙烯内胆的高压反应釜中110 ℃陈化48 h,再经多次洗涤(水和乙醇)、干燥和550 ℃焙烧所得。混合法是将分别制得的Bi3+-TiO2光催化剂粉末和MnCeOx活性粉末按照适当比例通过简单物理混合而成。与此同时,将浸渍法所制的10.0%Bi3+-TiO2/MnCeOx(550 ℃)进行NaOH改性处理。具体方法如下:取适量Bi3+-TiO2/MnCeOx粉末与0.1 mol·L−1 NaOH水溶液混合均匀,置于反应釜110 ℃陈化,用蒸馏水洗涤至中性,在HCl溶液中浸泡2 h,经蒸馏水洗涤、干燥制得Bi3+-TiO2/MnCeOx(NaOH)催化剂。
-
本实验为模拟室内环境条件下的光催化耦合常温催化HCHO性能评价,在尺寸为60 cm×60 cm×60 cm 密闭、含盖的玻璃反应器中进行,以反应器底部正上方10 cm处悬挂的36W LED灯为可见光光源,并通过反应器内HCHO的质量浓度变化来确定催化剂活性。首先将质量分数为38%的甲醛溶液滴加至培养皿中,并直接置入玻璃反应器内待其充分挥发,并将装填量为1.0 g催化剂均匀涂抹于Φ=5 cm培养皿中,并置于反应器内LED灯正下方,迅速取出装有甲醛溶液的培养皿并采用凡士林密封玻璃盖,连续测量反应器内HCHO质量浓度,始终保持其初始质量浓度为 (1.05±0.05) mg·m−3。HCHO质量浓度直接由甲醛分析仪(PPM-400st)测量得出,每次连续测定3次并取平均值。甲醛转化率按如式 (1) 计算。
式中:φ表示为催化氧化HCHO转化率;Ct表示每反应12 h后反应器内的气态甲醛质量浓度,mg·m−3;C0表示反应器内起始甲醛质量浓度,mg·m−3。
-
为研究考察催化剂微观结构在催化氧化反应过程的作用,用AXSD8衍射仪对催化剂进行了XRD检测,选用Cu靶射线管,扫描速率为4°·min−1,2θ衍射角范围为10°~80°;催化剂的比表面积、孔径大小及其分布情况采用以N2吸附的Autosorb-iQ-AG-MP表面积分析仪检测。表面形貌采用日本Hitachi公司生产的SU1510扫描电镜检测分析。材料纳米尺度的结构、晶格面以及晶格间距由日本JEOL公司生产的200 kV场发射透射电子显微镜(JEM-2100F)检测分析。样品傅里叶变换红外(FTIR)光谱分析是在美国Thermo Scientific Nicolet 6700 FTIR光谱仪上进行,催化剂粉末与KBr混合压片后置于DRIFT池中,分辨率为4 cm−1,扫描范围为4 000~400 cm−1,每个光谱均需扣除背景值。样品紫外-可见吸收光谱由日本岛津仪器公司生产的UV-3600 Plus紫外-可见-近红外分光光度计检测分析。
-
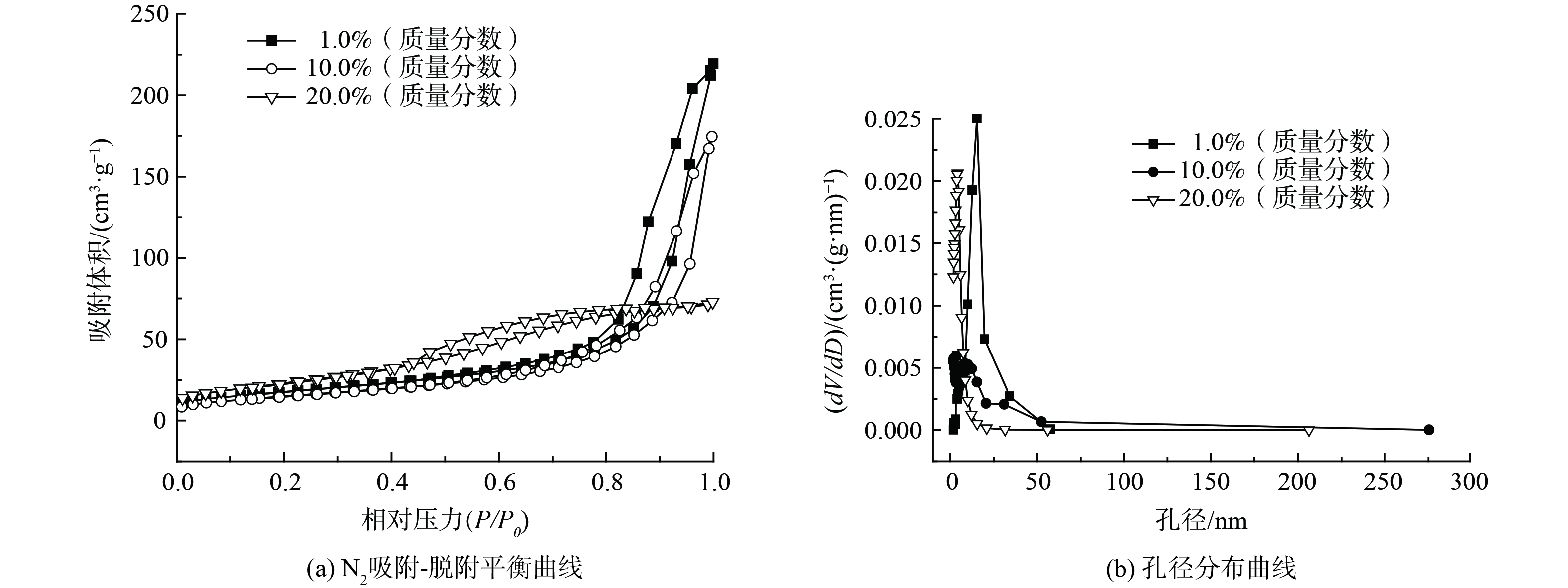
1) 结构分析。为考察不同负载量的Bi3+-TiO2对催化剂比表面积及孔容孔径产生的影响,对部分样品进行了BET分析,如表1和图1所示。Bi3+-TiO2/MnCeOx催化剂比表面积随TiO2负载量的增加先降低后增加,而孔体积和孔径持续降低,其中10.0%Bi3+-TiO2/MnCeOx催化剂展示出最低比表面积(54.0 m2·g−1),却显示出最佳的催化氧化活性。这表明催化剂比表面积与催化剂氧化活性并非正相关。负载有1.0和10.0%TiO2的催化剂显示出II型气体等温吸附线,且相对压力P/P0在0.8~1.0具有H3型滞后环[22],高压区吸附量上升较快。这说明孔径尺寸较大、分布相对较宽且以大孔居多[23],这有利于催化剂对气态甲醛吸附及反应产物的排出,而当TiO2负载量达到20.0%,催化剂显示出Ⅴ型气体吸附等温线,亦伴有滞后环,以小孔径为主(平均孔径3.88 nm)。这表明MnCeOx活性氧化物孔道被TiO2大量占据,致使孔容孔径降低,这不利于气态甲醛在孔道内扩散,且孔道内的Bi3+-TiO2因无法接收光源致使催化剂无法充分发挥其光催化性能,故TiO2负载量不是越多越好。
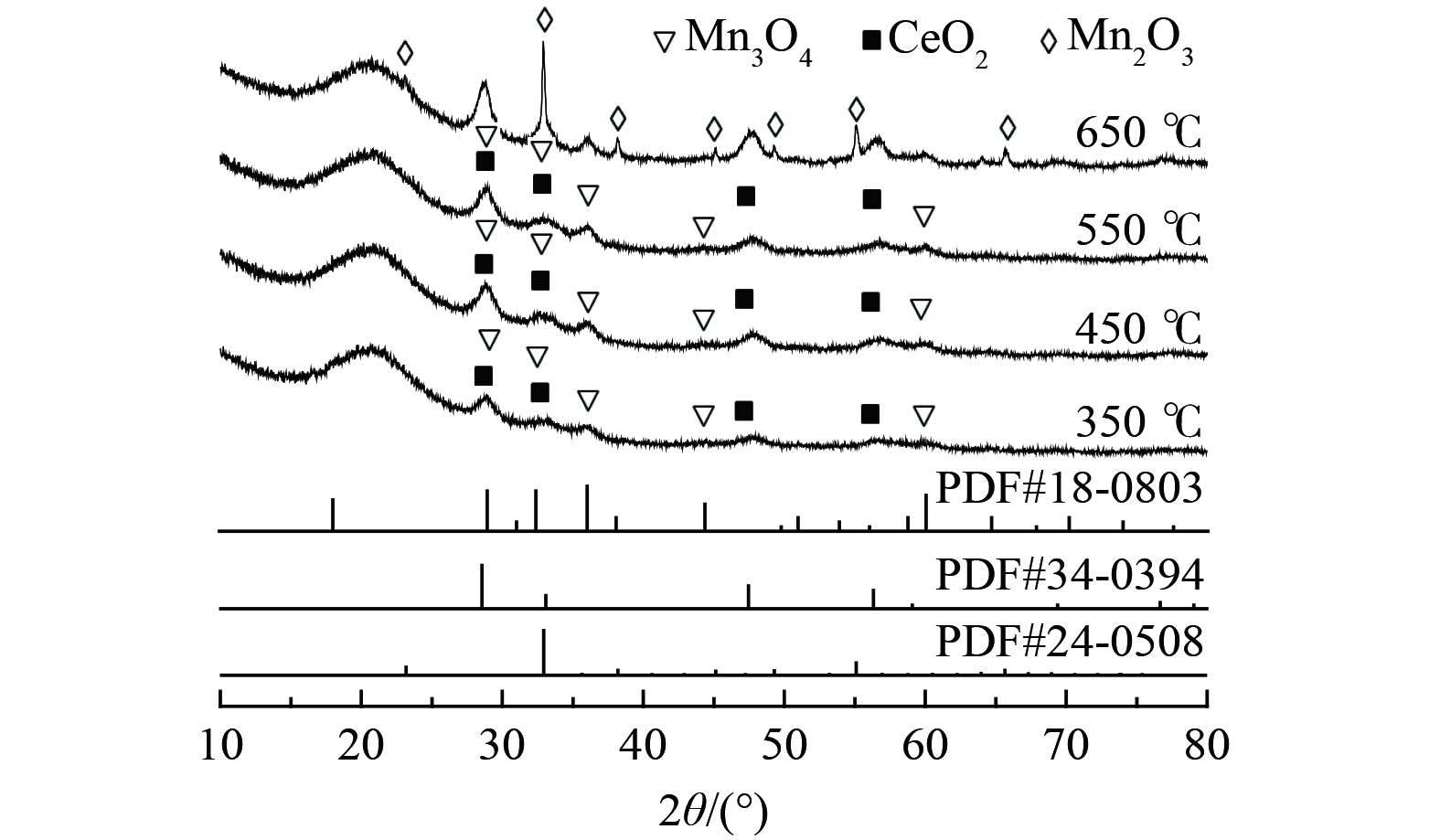
对经不同煅烧温度处理的Bi3+-TiO2/MnCeOx催化剂晶型结构进行了XRD分析,如图2所示。随着煅烧温度的升高,样品中氧化物晶体结晶度不断增强,其晶体粒径逐渐增大。2θ为28.9、35.9、44.3和59.9°归属为Mn3O4特征衍射峰(PDF #18-0803),位于28.8、47.8和56.6°处特征衍射峰为CeO2晶体(PDF #34-0394) [24],其所展示的氧化锰和氧化铈晶体衍射峰强度较低,衍射峰较宽,Mn3O4和CeO2衍射角分别向小角度(0.1°)和大角度(0.3°)偏移。考虑到Mn4+的离子半径(0.25 nm)小于Ce4+的半径(0.31 nm),锰离子易被引入到氧化铈晶格中形成固溶体。这表明氧化铈抑制了Mn3O4晶体粒径的增大,且氧化锰易进入CeO2晶格中形成缺陷[19]。多价态的Mn3O4(+2和+3)提高了氧的流动性,两者间的协同作用有利于氧空位数量的增加,进而提升催化剂常温催化氧化活性。在以上结果中均未发现TiO2特征衍射峰,可能仍为无定形态或粒径较小、高度分散于MnCeOx表面而低于XRD检出限。随着煅烧温度提升至650 ℃时, 2θ为23.1、32.9、38.2、45.1、49.3、55.1和65.7°归属为Mn2O3的特征衍射峰(PDF #24-0508);晶面分别为(211)、(222)、(400)、(323)、(413)、(044)和(622);衍射峰逐渐由宽峰演变为尖峰,Mn2O3晶体粒径逐渐增大。这表明高温致使氧化物晶型及晶体粒径发生显著变化。
利用SEM和TEM对催化剂的表观结构形貌进行了分析,如图3所示。MnCeOx粉末以无规则形态存在,表面结构蓬松,富含大量孔隙,属介孔材料,但其内部富有大量类似球状颗粒物,为MnCeOx的氧化物,其晶格间距0.27和0.31 nm分别归属为Mn2O3 (2 2 2)和CeO2 (1 1 1)。而Bi3+-TiO2/MnCeOx底部为密实结构的MnCeOx氧化物,部分展示出长条状结构,其表面富含类似球状TiO2。MnCeOx和球状TiO2通过长条状结构或直接担载紧密结合,而催化剂表面未被TiO2完全覆盖,其边缘富含MnCeOx氧化物,晶格间距0.25、0.27和0.31 nm分别归属为Mn3O4 (2 1 1)、Mn2O3 (2 2 2)和CeO2 (1 1 1),且晶格条纹中均存在大量凹陷状,即晶格缺陷。与此同时,实验采用纯乙醇代替乙醇和丙三醇混合溶液制备了Bi3+-TiO2/MnCeOx(EtOH)催化剂,其SEM如图3(e)所示。催化剂底部为具有密实结构的MnCeOx氧化物,其上负载有短棒状的TiO2,且相互搭接分布于MnCeOx氧化物上。同时,对Bi3+-TiO2/MnCeOx使用NaOH改性处理,结果表明(图3(f)),其表观结构遭受不同程度的侵蚀,且表面呈现出大量纳米片状结构。
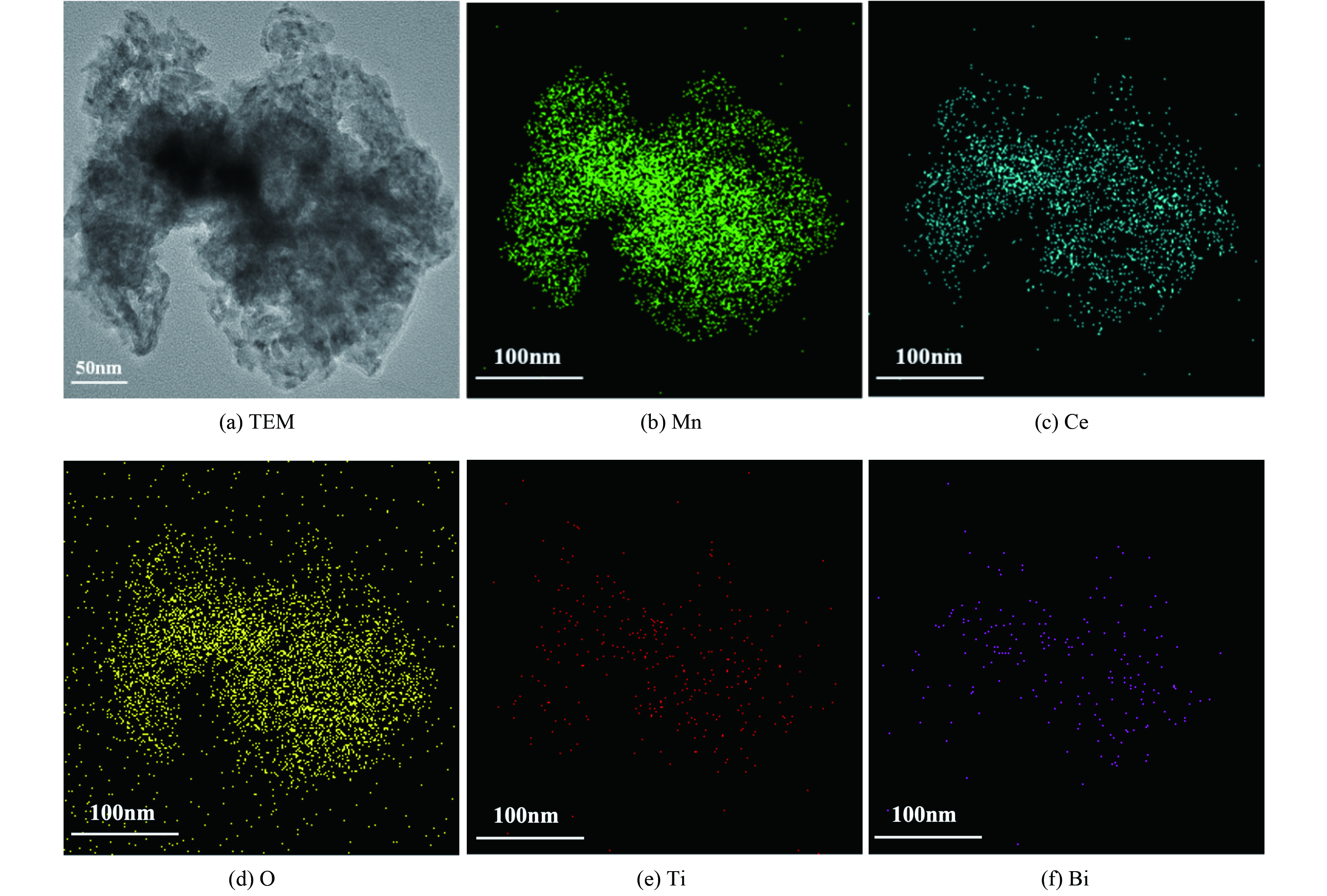
为考察Mn、Ce、O、Ti和Bi元素在催化剂中分布情况,对Bi3+-TiO2/MnCeOx催化剂进行了TEM-EDS分析,如图4所示。Mn、Ce、O、Ti和Bi5种元素分布与催化剂形态完全一致,其元素含量依次为O、Mn、Ce、Ti和Bi。这与所制催化剂实际结果相一致,氧化锰与氧化铈均匀紧密结合,形成混、复合氧化物,彼此间发挥协同常温催化作用[25]。与此同时,掺Bi的TiO2亦均匀分布与MnCeOx上,且Bi原子部分取代Ti原子[26]。两者紧密结合,发挥协同光催化性能。由于负载量低,Bi3+-TiO2未将MnCeOx表面全部覆盖,这有利于Bi3+-TiO2/MnCeOx催化剂发挥光催化耦合常温催化氧化HCHO性能。
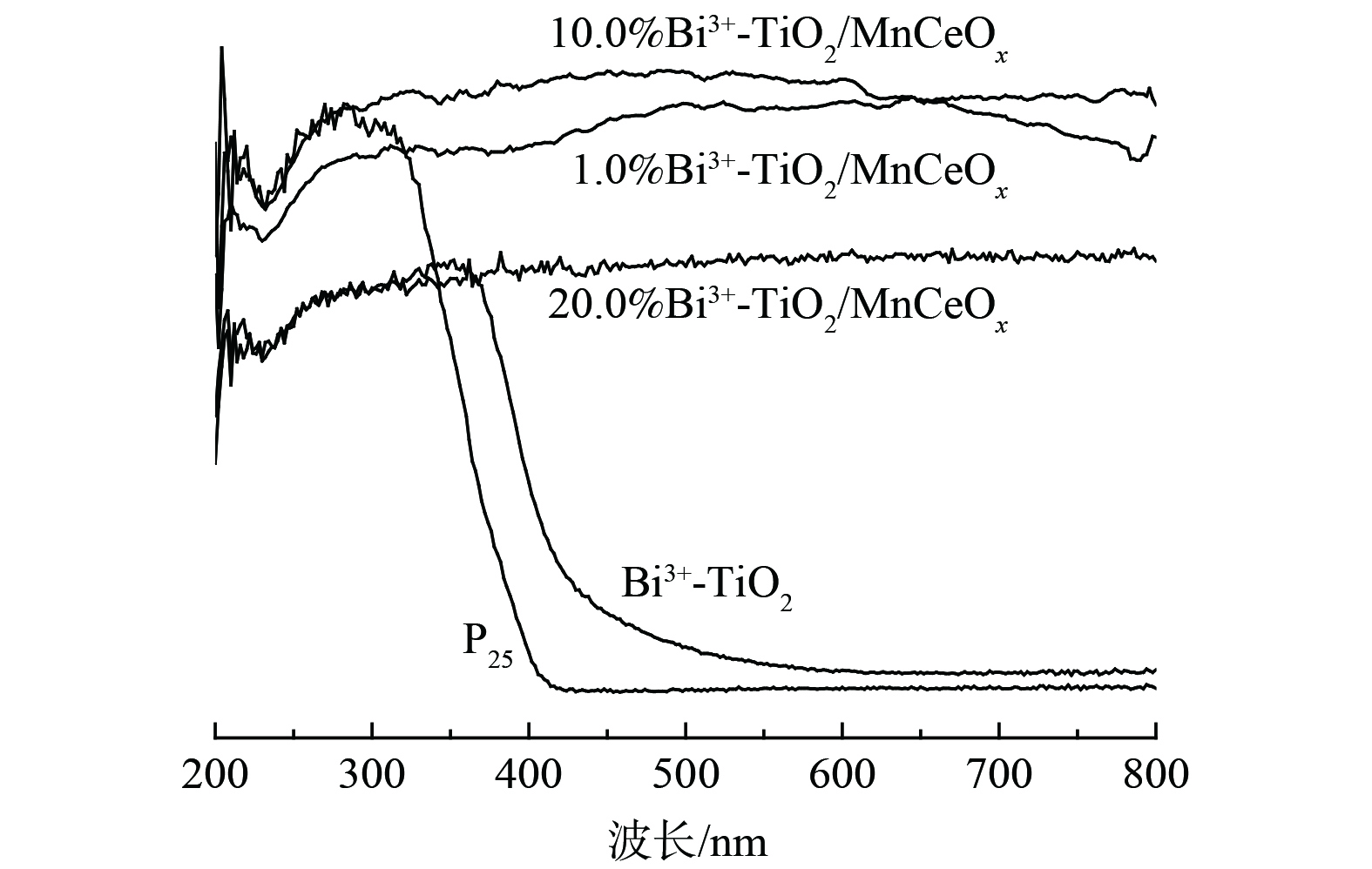
2) UV-vis DRS图谱。图5为450 ℃下制备的Bi3+-TiO2/MnCeOx催化剂的UV-vis DRS图。相较于P25,Bi3+-TiO2催化剂显示出明显的可见光吸收,吸收光发生红移。通过计算其带隙能分别为3.1 eV和2.8 eV,因此,Bi3+-TiO2光催化氧化HCHO性能明显优于P25,这与实际测试结果相一致。而Bi3+-TiO2/MnCeOx催化剂显示出在200~800 nm波长范围内全波段吸收,且吸收度随着Bi3+-TiO2负载量的增加先增加后降低。其中,10.0%Bi3+-TiO2/MnCeOx催化剂最佳,优于Bi3+-TiO2及MnCeOx催化剂,而展示出最佳的氧化活性。这与实际结果相一致。10.0% Bi3+-TiO2/MnCeOx复合催化剂在紫外可见光区域全波段吸收可能与载体MnCeOx氧化物为黑色组成有关,可充分吸收紫外和可见光。尤其在可见光区域吸收明显增强,产生热能,亦可致使氧化锰价态的变化[27],进而有利于催化氧化活性的提升。因此,以MnCeOx为载体,Bi3+-TiO2为活性组分可进一步优化催化剂催化氧化甲醛性能。
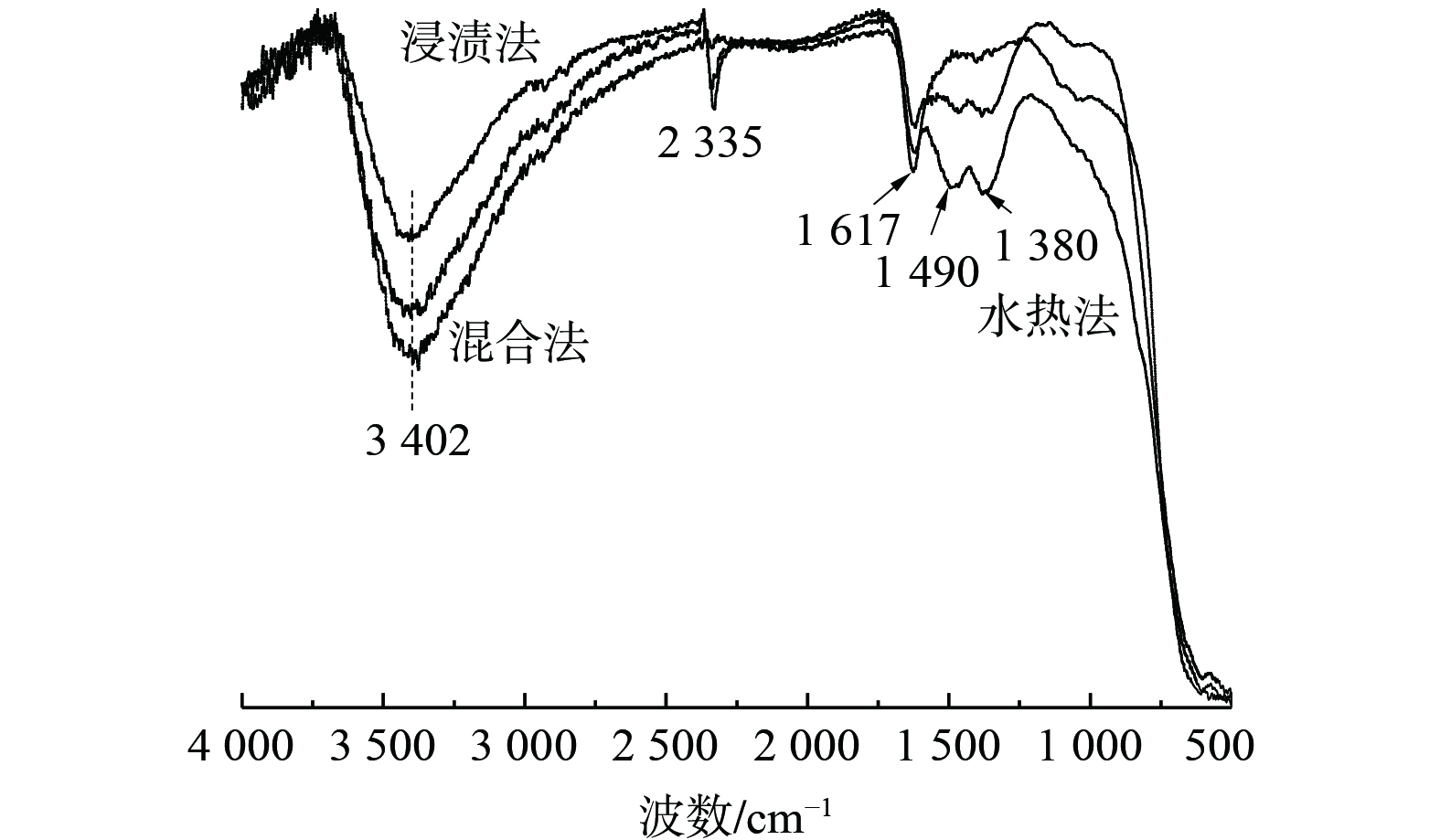
3) IR光谱。图6为3种采用不同制备方法所制的Bi3+-TiO2/MnCeOx催化剂的傅里叶红外光谱图。波数为3 402 cm−1宽峰和1 617 cm−1处特征峰均归属为催化剂上羟基自由基或含水物种的对称伸缩振动νs(—OH)峰,这些羟基通过氢键参与对气态HCHO吸附[28]。在2 335 cm−1处的特征峰可归属为C—O(νs (HCO3−))的拉伸或弯曲振动,而在1 380 cm−1处特征峰被归属为甲酸盐的对称伸缩振动νs(COO−)[29],1 490 cm−1处特征峰归属为参与催化氧化反应生成的二氧甲基(DOM)的δ(CH−)振动峰[30]。结果表明,3种方法所制催化剂吸水性能依次为混合法>水热法>浸渍法,其与催化氧化活性成反比。这表明过多水蒸气和HCHO之间存在明显的竞争吸附关系[31],而适量羟基通过氢键参与对气态HCHO吸附有利于催化剂氧化性能的提升。通过采用水热法和浸渍法所制催化剂的IR光谱图发现:有DOM和甲酸的存在,且均为催化氧化HCHO中间产物[32],还有碳酸氢的存在。这表明催化氧化HCHO反应最终形成CO2和H2O,而采用混合法所制催化剂未发现上述情况。
-
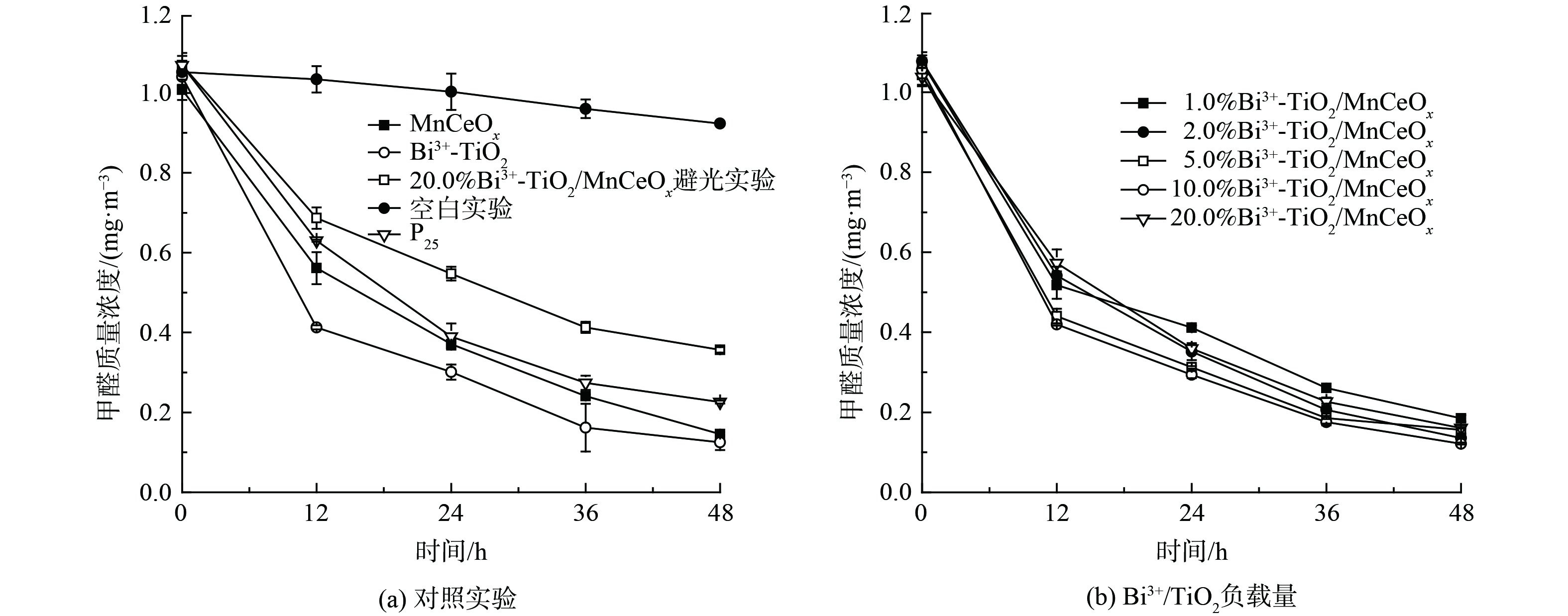
为研究探索Bi3+-TiO2/MnCeOx催化剂光催化耦合常温催化氧化HCHO性能,在同一测试条件下(36W LED灯,温度:20±4 ℃) 进行了一组对比实验,结果如图7所示。空白对照实验(图7(a))显示玻璃反应器内甲醛浓度略有下降,反应48 h后由1.052 mg·m−3降至0.923 mg·m−3。这是由于昼夜温差及吸附导致,但未见显著性下降趋势,亦说明玻璃反应器密封性良好,完全满足实验测试要求。为对比分析,对P25,Bi3+-TiO2和MnCeOx三者还进行了活性测试。结果表明:三者均展示出良好的吸附及催化氧化性能,反应48 h后甲醛降解率分别为78.9%、88.0%和85.5%。其中,Bi3+-TiO2优于P25和MnCeOx,其性能提升可能与Bi3+-TiO2展示出一定的可见光吸收特性有关。同时,对10.0% Bi3+-TiO2/MnCeOx催化剂进行避光性能测试。结果表明,在无光的条件下催化剂性能较差。这可能与Bi3+-TiO2的光催化性能受光照条件限制有关。催化剂在48 h内吸附及催化降解率均展示出逐渐降低的趋势,这是由于吸附气态低浓度甲醛是催化反应限速步骤,且空气中水蒸气与甲醛间存在竞争吸附所致[33]。为进一步提升催化剂的性能,将光催化剂Bi3+-TiO2和具有常温催化氧化性能的MnCeOx相结合(图7(b)),制备了不同Bi3+-TiO2负载量的Bi3+-TiO2/MnCeOx催化剂。结果显示,450 ℃焙烧所得Bi3+-TiO2/MnCeOx催化氧化HCHO性能较Bi3+-TiO2略有提升,其催化氧化性能随着Bi3+-TiO2负载量的增加先提升后降低。一般来言,活性组分越多所提供的活性位越多,其比表面积越大,越有利于反应的进行,但当活性组分负载量超过分散阈值后,多余晶相会覆盖MnCeOx活性位,并堵塞起表面孔道[34],这不利于气态甲醛及反应产物在孔道内扩散,会阻碍氧化反应的进行致使催化剂氧化活性降低。这与BET及孔径分布分析结果相一致。其中,10% Bi3+-TiO2/MnCeOx的性能最佳,其反应48 h后甲醛质量浓度为0.122 mg·m−3,但仍高于《民用建筑工程室内环境污染控制规范》(GB50325-2001)所规定的甲醛限值(0.08 mg·m−3),性能仍需优化。
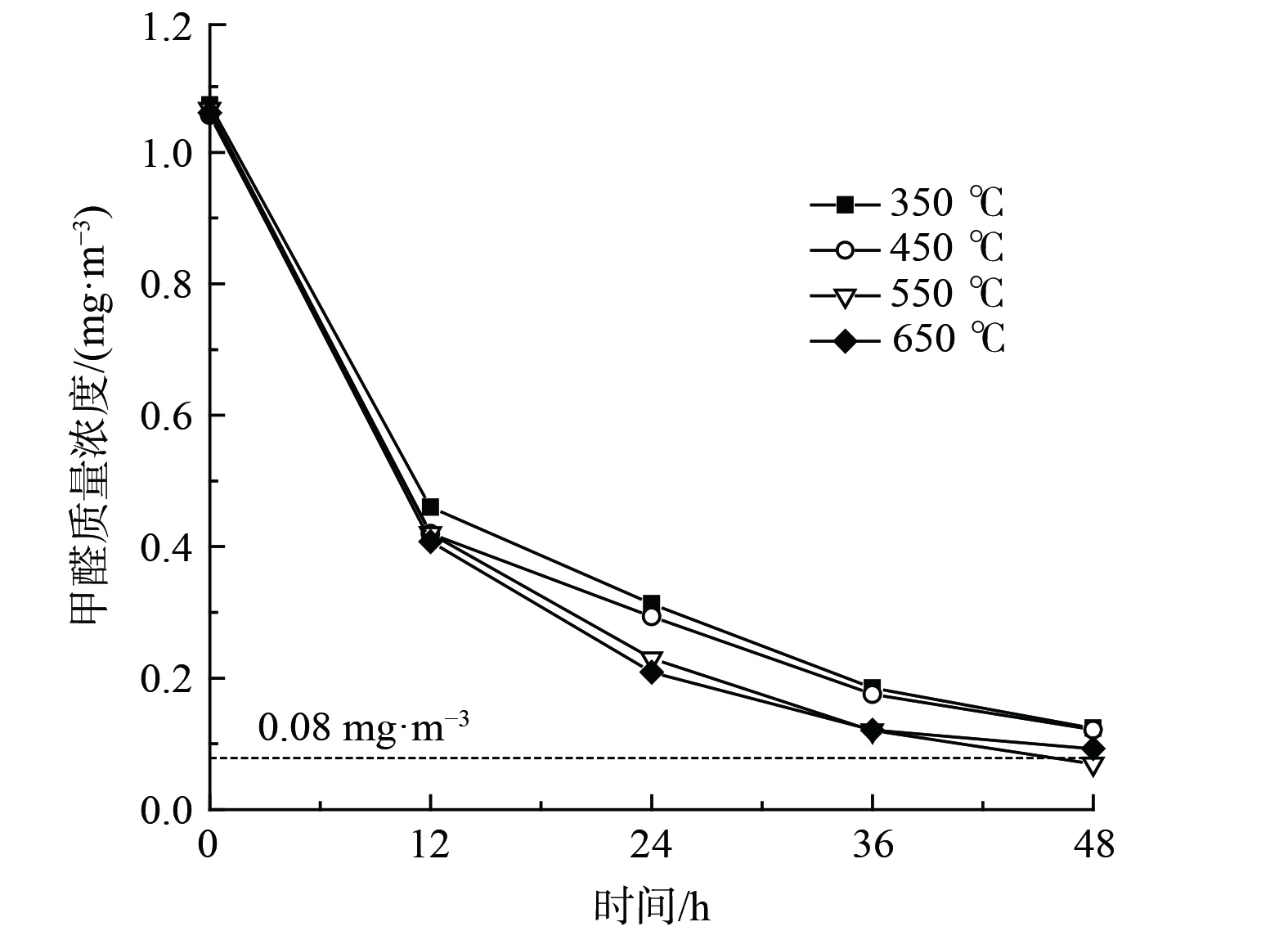
煅烧温度能影响金属氧化物晶型、粒径及孔道结构,进而影响催化剂氧化性能[35]。为进一步优化催化剂性能,考察了煅烧温度对催化剂氧化性能的影响,如图8所示。随着煅烧温度的增加,催化氧化HCHO降解率呈现出先增加后降低的趋势。经550 ℃和650 ℃煅烧所制催化剂明显优于350 ℃和450 ℃。其中,经550 ℃煅烧所制10.0%Bi3+-TiO2/MnCeOx催化剂HCHO降解率(48 h)最佳,HCHO去除率达93.4%,其质量浓度由1.066 mg·m−3 降至0.070 mg·m−3,性能较Bi3+-TiO2和MnCeOx有显著提升,低于室内HCHO浓度控制限值。随着煅烧温度的提升,二氧化钛逐渐由无定形态转变为锐钛矿型,氧化锰由Mn3O4逐渐转变为Mn2O3,且晶体粒径逐渐增大。其晶型转变、粒径增大且大量的晶格缺陷是催化剂氧化性能提升的重要原因,MnCeOx氧空位及流动性和掺Bi3+的锐钛矿TiO2利于催化剂常温催化及光催化氧化作用的发挥,故后续实验均以经550 ℃焙烧所制催化剂为研究对象。
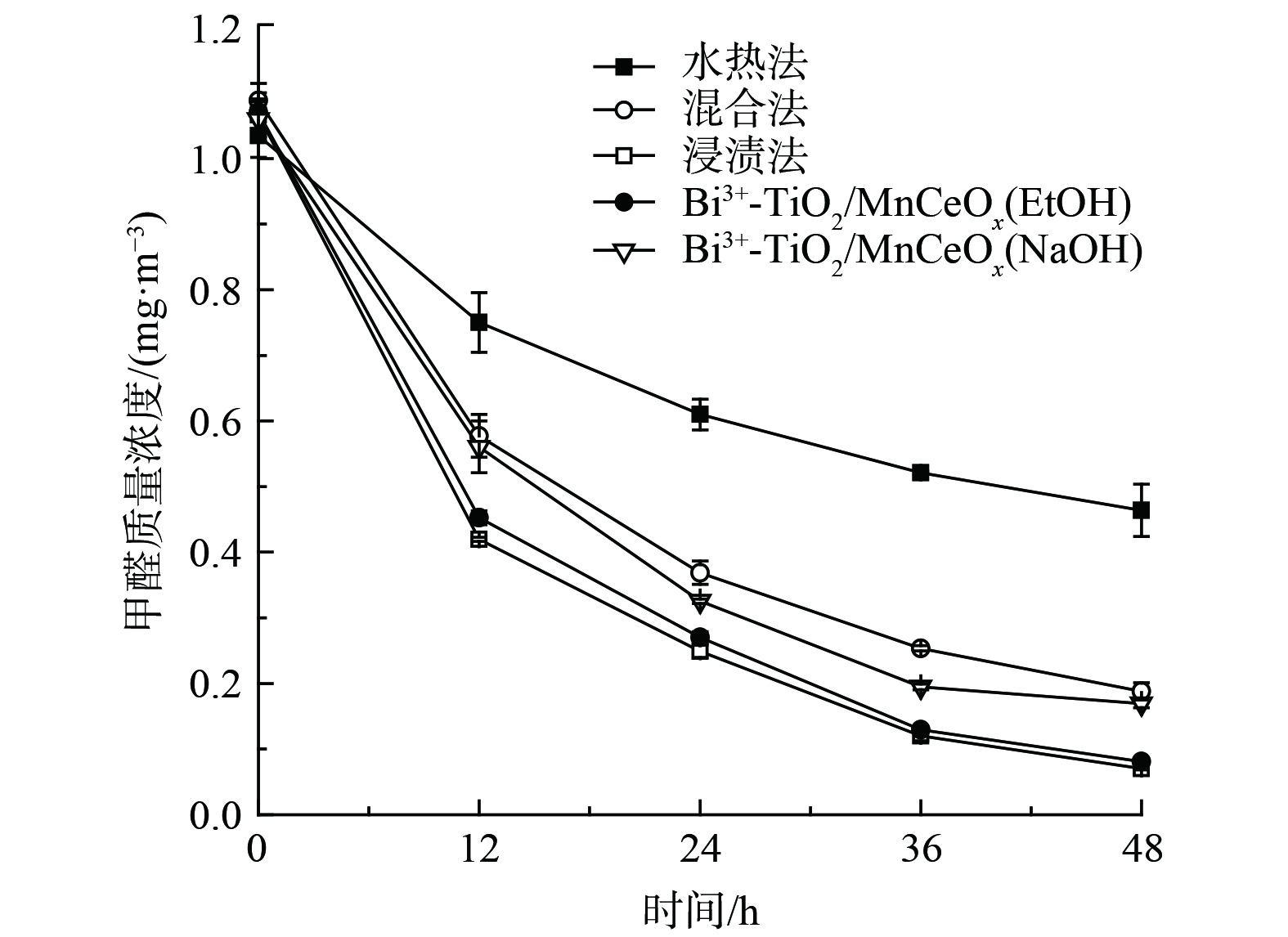
还考察了制备方法(水热法,混合法和浸渍法)以及改性处理(NaOH和EtOH)对Bi3+-TiO2/MnCeOx催化剂氧化性能的影响,结果如图9所示。经浸渍法所制催化剂(550 ℃)表现较佳,其氧化效率较混合法和水热法分别提升了11%和38%,实现了Bi3+-TiO2与MnCeOx间的协同耦合作用。促使催化剂性能提升的原因可能与Bi3+-TiO2/MnCeOx催化剂展示出良好的抗水蒸气性能有关。这一结果与红外光谱分析结果相吻合。与此同时,由于纳米结构的TiO2可通过影响孔结构的排列及比表面积对复合材料的光催化性能产生显著影响[36-37]。采用浸渍法制备的以乙醇替代乙醇和丙三醇混合溶液的Bi3+-TiO2/MnCeOx(EtOH)也显示出较佳的氧化性能(92.5%),其TiO2表观结构由球状转变为短棒状,且相互搭接分布于MnCeOx氧化物上,其结构未发生明显变化。而采用浸渍法所制催化剂经NaOH改性处理后的Bi3+-TiO2/MnCeOx(NaOH)样品的48 h HCHO去除率为84.1%,其氧化性能显著降低。这与催化剂表观结构遭受不同程度的侵蚀有关,其表面存有大量纳米片状结构,可能为Na2O晶体,其结构可由SEM表征结果证实。
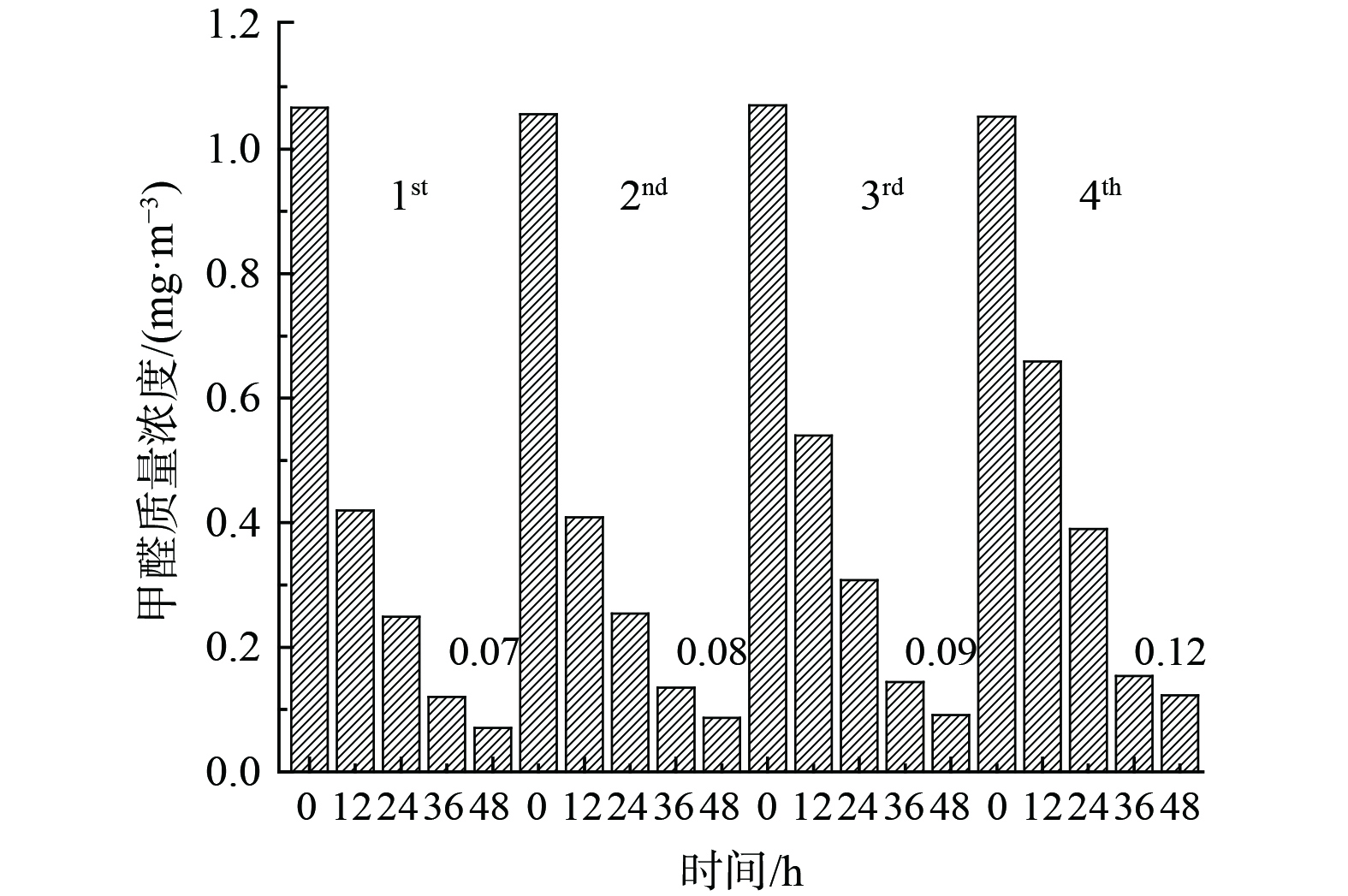
催化剂可吸附空气中大量水蒸气而导致催化剂氧化性能(竞争吸附)及稳定性能下降。为考察Bi3+-TiO2/MnCeOx催化剂的稳定性,对经550 ℃煅烧处理所制的10.0%Bi3+-TiO2/MnCeOx催化剂进行4次平行测定 (图10) 发现,催化剂稳定性良好,未见显著性氧化活性下降趋势,仅表现为小幅度降低,其48 h内HCHO质量浓度由0.07 mg·m−3升至0.12 mg·m−3。分析其主要原因可能与催化剂吸收空气中水分有关,HCHO和水蒸气间存在竞争性吸附致使催化剂氧化性能降低。这表明Bi3+-TiO2/MnCeOx催化剂显示出良好的抗水蒸气性能。
-
基于上述研究结果,推测Bi3+-TiO2/MnCeOx去除HCHO的催化氧化机理如图11所示。Bi3+-TiO2/MnCeOx表面羟基通过氢键参与对气态HCHO吸附,形成吸附态。在Bi3+-TiO2(光生电子e−和空穴h+)的光催化氧化和MnCeOx (Oads和Olatt)的常温催化氧化的共同作用下,O2在催化剂表面解离,立即形成活性氧物种或表面羟基等(O2−, O− , ·OH)。吸附的HCHO与活性氧或表面羟基反应形成DOM和甲酸盐,进而在Bi3+-TiO2/MnCeOx晶格氧的作用下进一步氧化为CO2和H2O[38]。
-
1) 将具备常温催化氧化性能的MnCeOx与光催化性能的Bi3+-TiO2相结合,采用浸渍法制备了一系列Bi3+-TiO2/MnCeOx催化剂。采用浸渍法所制的10.0%Bi3+-TiO2/MnCeOx(550 ℃)性能最佳,其48 h可将气态HCHO降低至0.07 mg·m−3,低于室内HCHO控制标准,且稳定性良好。
2) 基于具备常温催化氧化性能的MnCeOx氧化物为载体,其氧化锰和氧化铈间的协同作用及晶格缺陷有利于氧空位和氧的流动性的增加,进而提升其常温氧化性能。同时,以具备可见光催化氧化能力的Bi3+-TiO2为活性组分,其表观结构形态、晶型、粒径、孔道、掺杂、可见光吸收以及抗水性为Bi3+-TiO2/MnCeOx提升其氧化性能提供必要条件。两者间的多重协同耦合作用是核心,研究可为光催化耦合常温催化氧化新材料的制备提供参考。
光催化耦合常温催化氧化HCHO的Bi3+-TiO2/MnCeOx催化剂性能
Catalytic oxidation of HCHO over Bi3+-TiO2/MnCeOx catalyst based on photocatalytic coupled catalytic oxidation at ambient temperature
-
摘要: 常用净化室内挥发性有机物(VOCs)的方法主要有吸附、低温等离子体、光催化氧化、常温催化氧化等,而鲜有基于光催化耦合常温催化催化剂的报道。以具备常温催化氧化性能的MnCeOx为载体,以具有可见光催化性能的Bi3+-TiO2为活性组分,考察负载量、煅烧温度、制备方法以及结构形态等对Bi3+-TiO2/MnCeOx催化氧化甲醛(HCHO)性能的影响,并利用XRD、BET、SEM、TEM、UV-vis DRS和IR等技术对催化剂进行微观表征与分析。结果表明,催化剂表观结构形态、晶型、粒径、孔道、掺杂、可见光吸收及抗水性为Bi3+-TiO2/MnCeOx氧化性能提升提供了必要条件,两者间的多重协同耦合作用是核心,其中采用浸渍法所制的负载量10.0%Bi3+-TiO2/MnCeOx(550 ℃)表现最佳,48 h催化降解率高达93.4%,其HCHO浓度低于室内控制标准(GB50325-2001),且稳定性良好。本研究结果可为室内HCHO高效控制耦合光催化和常温催化技术提供参考。Abstract: The common methods for removing indoor volatile organic compounds (VOCs) mainly included adsorption, low-temperature plasma, photocatalytic oxidation, catalytic oxidation at ambient temperature, etc. However, there are few research reports based on the combination of photocatalytic oxidation and catalytic oxidation at ambient temperature. In this paper, MnCeOx were used as supports to catalytic oxidation of formaldehyde at ambient temperature, and Bi3+-TiO2 as the active component were utilized to photocatalytic performance under visible light. The effects of supporting, calcination temperature, preparation method and structure on the performance of formaldehyde (HCHO) oxidation were investigated, and their physicochemical properties were characterized by XRD, BET, SEM, TEM, UV-vis DRS and IR. The results exihibited that the apparent structural morphology, crystal type, particle size, pore, doping, absorption under visible light and water resistance provided the necessary conditions, and the potentially synergistic multiple effect of Bi3+-TiO2 and MnCeOx was the critical factor for improving the performance of Bi3+-TiO2/MnCeOx. Among them, 10.0% Bi3+-TiO2/MnCeOx (550 ℃) prepared by the impregnation method exhibited the highest activity and stability, and the degradation rate was as high as 93.4% at 48 h. Finally, the concentration of HCHO was lower than the indoor control standard (GB50325-2001). The synergistic effects are responsible for the enhanced indoor HCHO removing and it provides some reference for the research.
-

-
表 1 部分样品比表面积及孔容孔径
Table 1. Physical properties of these catalysts in the orthogonal experiment
样品名称 比表面积/
(m2·g−1)孔体积/
(cm3·g−1)平均
孔径/nmBi3+-TiO2 27.3 0.07 6.73 MnCeOx 87.0 0.21 38.9 1.0% Bi3+-TiO2/MnCeOx 63.4 0.334 15.3 10.0% Bi3+-TiO2/MnCeOx 54.0 0.235 13.3 20.0%Bi3+-TiO2/MnCeOx 84.4 0.112 3.88 -
[1] SALTHAMMER T, MENTESE S, MARUTZKY R. Formaldehyde in the indoor environment[J]. Chemical Reviews, 2010, 110(4): 2536-2572. doi: 10.1021/cr800399g [2] 李省吾, 吴晓航, 黄荣珠, 林建标. 室内空气中甲醛污染去除技术进展研究[J]. 广东化工, 2022, 49(8): 148-149. doi: 10.3969/j.issn.1007-1865.2022.08.047 [3] 徐倩, 曲振平. 介孔氧化硅材料负载Au、Ag催化剂及其甲醛催化氧化性能研究[D]. 大连: 大连理工大学, 2021. [4] 关圣楠, 张琦. 锰基催化剂催化氧化甲醛脱除的研究[D]. 合肥: 中国科学技术大学, 2021. [5] ZHANG G X, SUN Z M, DUAN Y W, et al. Synthesis of nano-TiO2/diatomite composite and its photocatalytic degradation of gaseous formaldehyde[J]. Applied Surface Science, 2017, 412: 105-112. doi: 10.1016/j.apsusc.2017.03.198 [6] LI X, QIAN X R, AN X H, et al. Preparation of a novel composite comprising biochar skeleton and “chrysanthemum” g-C3N4 for enhanced visible light photocatalytic degradation of formaldehyde[J]. Applied Surface Science, 2019, 487: 1262-1270. doi: 10.1016/j.apsusc.2019.05.195 [7] ZHANG G K, XIONG Q, WEI X, et al. Synthesis of bicrystalline TiO2 supported sepiolite fibers and their photocatalytic activity for degradation of gaseous formaldehyde[J]. Applied Clay Science, 2014, 102: 231-237. doi: 10.1016/j.clay.2014.10.001 [8] MALAYERI M, HAGHIGHAT F, LEE C S. Modeling of volatile organic compounds degradation by photocatalytic oxidation reactor in indoor air: A review[J]. Building and Environment, 2019, 154: 309-323. doi: 10.1016/j.buildenv.2019.02.023 [9] YANG Y, LI X J, CHEN J T, et al. Effect of doping mode on the photocatalytic activities of Mo/TiO2[J]. Journal of Photochemistry and Photobiology A:Chemistry, 2004, 163(3): 517-522. doi: 10.1016/j.jphotochem.2004.02.008 [10] PAN X Y, XU Y J. Defect-mediated growth of noble-metal (Ag, Pt, and Pd) nanoparticles on TiO2 with oxygen vacancies for photocatalytic redox reactions under visible light[J]. The Journal of Physical Chemistry C, 2013, 117(35): 17996-18005. doi: 10.1021/jp4064802 [11] LI J, ZHANG M, LI Q Y, et al. Enhanced visible light activity on direct contact Z-scheme g-C3N4-TiO2 photocatalyst[J]. Applied Surface Science, 2017, 391: 184-193. doi: 10.1016/j.apsusc.2016.06.145 [12] HUANG Q, WANG P, FAN Y Z, et al. Synthesis and photocatalytic activity of N-doped BixTi1-xO2 photocatalysts under energy saving lamp illumination[J]. Indoor and Built Environment, 2017, 26(6): 785-795. doi: 10.1177/1420326X16641177 [13] HUANG Y F, WEI Y L, WANG J, et al. Controllable fabrication of Bi2O3/TiO2 heterojunction with excellent visible-light responsive photocatalytic performance[J]. Applied Surface Science, 2017, 423: 119-130. doi: 10.1016/j.apsusc.2017.06.158 [14] HAMDI A, FERRARIA A M, BOTELHO DO REGO A M, et al. Bi–Y doped and co-doped TiO2 nanoparticles: Characterization and photocatalytic activity under visible light irradiation[J]. Journal of Molecular Catalysis A:Chemical, 2013, 380: 34-42. doi: 10.1016/j.molcata.2013.09.005 [15] TIAN H, HE J H, ZHANG X D, et al. Facile synthesis of Porous manganese oxide K-OMS-2 materials and their catalytic activity for formaldehyde oxidation[J]. Microporous and Mesoporous Materials, 2011, 138(1/2/3): 118-122. [16] 何小云, 葛笑, 宋留名, 等. 室温下MnOx/HZSM-5催化氧化甲醛的性能和机理分析[J]. 材料工程, 2021, 49(1): 144-152. [17] YANG P, YANG S S, SHI Z N, et al. Deep oxidation of chlorinated VOCs over CeO2-based transition metal mixed oxide catalysts[J]. Applied Catalysis B:Environmental, 2015, 162: 227-235. doi: 10.1016/j.apcatb.2014.06.048 [18] TANG X F, LI Y G, HUANG X M, et al. MnOx-CeO2 mixed oxide catalysts for complete oxidation of formaldehyde: Effect of preparation method and calcinations temperature[J]. Applied Catalysis B:Environmental, 2006, 62(3/4): 265-273. [19] TANG X F, CHEN J L, HUANG X M, et al. Pt/MnOx CeO2 catalysts for the complete oxidation of formaldehyde at ambient temperature[J]. Applied Catalysis B:Environmental, 2008, 81(1/2): 115-121. [20] ZHANG Y, CHEN M X, ZHANG Z X, et al. Simultaneously catalytic decomposition of formaldehyde and ozone over manganese cerium oxides at room temperature: Promotional effect of relative humidity on the MnCeOx solid solution[J]. Catalysis Today, 2019, 327: 323-333. doi: 10.1016/j.cattod.2018.04.027 [21] SEKINE Y, NISHIMURA A. Removal of formaldehyde from indoor air by passive type air-cleaning materials[J]. Atmospheric Environment, 2001, 35(11): 2001-2007. doi: 10.1016/S1352-2310(00)00465-9 [22] 陶涛, 肖瑶, 胡宇欣, 等. MnCeOx/凹凸棒土催化剂的制备、表征及常温催化氧化性能[J]. 功能材料, 2020, 12(51): 12001-12008. [23] WANG T, YAN X Q, ZHAO S S, et al. Preparation, characterization and photocatalytic activity of three-dimensionally ordered mesoporous /macroporous TiO2 microspheres[J]. Journal of Molecular Catalysis, 2014, 28(4): 359-366. [24] 黄琼, 白梦天, 任超, 等. Mn基金属氧化物催化剂常温催化氧化甲醛[J]. 中国环境科学, 2018, 38(1): 103-111. doi: 10.3969/j.issn.1000-6923.2018.01.013 [25] LI H J, QI G S, TAN A, et al. Low-temperature oxidation of ethanol over a Mn0.6Ce0.4O2 mixed oxide[J]. Applied Catalysis B:Environmental, 2011, 103(1/2): 54-61. [26] HUANG Q, YE J, SI H, et al. Differences of characteristics and performance with Bi3+ and Bi2O3 doping over TiO2 for photocatalytic oxidation under visible light[J]. Catalysis Letters, 2020, 150: 1098-1110. doi: 10.1007/s10562-019-03017-w [27] 朱立才, 袁中直, 李伟善. 现场紫外-可见吸收光谱研究电解二氧化锰的还原过程[J]. 电化学, 2004, 10(2): 168-174. doi: 10.13208/j.electrochem.2004.02.008 [28] CHEN B B, SHI C, CROKER M, et al. Catalytic removal of formaldehyde at room temperature over supported gold catalysts[J]. Applied Catalysis B: Environmental, 2013, 132–133: 245-255. [29] 庞光龙. MnOx基催化剂上甲醛室温催化氧化反应的研究 [D]. 北京: 北京理工大学, 2015. [30] 朱杰, 孙月吟, 顾名扬, 等. TiO2负载MnFeOx催化剂的制备及常温催化氧化甲醛性能研究[J]. 功能材料, 2022, 53(4): 4011-4019. [31] LIU F, RONG S P, ZHANG P Y, et al. One-step synthesis of nanocarbon-decorated MnO2 with superior activity for indoor formaldehyde removal at room temperature[J]. Applied Catalysis B:Environmental, 2018, 235: 158-167. doi: 10.1016/j.apcatb.2018.04.078 [32] SUN D, WAGEH S, Al-GHAMDI A A, et al. Pt/C@MnO2 Composite hierarchical hollow microspheres for catalytic formaldehyde decomposition at room temperature[J]. Applied Surface Science, 2019, 466: 301-308. doi: 10.1016/j.apsusc.2018.10.044 [33] 李妍, 张舸, 蒋贞. 锐钛矿型TiO2吸附甲醛影响因素的模拟与实验研究[J]. 现代化工, 2019, 39(4): 207-210. [34] 段宝庆, 丁雅萍, 陈英文等. 铈基催化剂上CVOCs催化研究进展[J]. 现代化工, 2017, 37(12): 24-27. doi: 10.16606/j.cnki.issn0253-4320.2017.12.006 [35] 张萍, 许丽, 王莉. 水热法合成二氧化钛纳米管的晶型与形貌控制的研究[J]. 当代化工, 2018, 47(5): 893-896. doi: 10.13840/j.cnki.cn21-1457/tq.2018.05.005 [36] YUAN Z Y, SU B L. Titanium oxide nanotubes, nanofibers and nanowires[J]. Colloids and Surfaces A:Physicochemical and Engineering Aspects, 2004, 241(3): 173-183. [37] 赵红花, 马树平. 负载型 TiO2光催化降解含酚废水的研究[J]. 兰州理工大学学报, 2007, 33(1): 74-78. [38] 朱杰. MnFeOx氧化物催化剂常温催化氧化低浓度HCHO性能研究[D]. 南京: 南京信息工程大学, 2022. -



 下载:
下载: