-
重金属是造成我国水环境污染的主要因素之一[1]。传统处理重金属污水的方法包括物理化学技术和微生物法均存在一定的局限性[1-3]。人工湿地(constructed wetlands, CWs)是人为建造和管控的污水处理系统[4-5],投资少、污水净化效果好,近年来受到大量关注[6-8]。
研究发现,基质吸附是CWs去除重金属的主要途径[9-10]。碎石、河砂、砾石等传统基质具有来源广、造价低等特点受到广泛关注。陈银萍等[11]为期10 d的实验发现粉煤灰对Cd去除效果较好但不利于植物生长;余关龙等[12]发现短期内,碎石基人工湿地对营养物及Cd2+、Zn2+ 去除率较高好。但传统基质在长期运行下对重金属的去除能力仍有限[9]。因此,需要对人工湿地基质配置进行优化,以实现长期最佳的污水处理效果。沸石与生物炭因具有多孔的结构、丰富的官能团[13-15]以及较强的静电效应和吸附性能[16],在污水处理中具有较高的应用价值[17-19],但目前关于添加生物炭能否提高含重金属污水的去除率存在一定的争议。CHEN[20]与CHANG等[21]认为生物炭基CWs具有较好的重金属固定能力并能促进植物生长,而FENG[22]认为添加生物炭会增加重金属对β-变形菌、甲型变形菌纲的毒性导致脱氮效率下降。
多种基质组合使用能发生协同作用,从而提高净化效果[9]。张晓斌等[18]研究发现沸石与炉渣组合添加对电镀废水中Cr、Zn的去除效果优于单独添加;ABEDI等[13]采用响应面分析评估了沸石-生物炭基人工湿地处理模拟污水的效果,发现3个月运行期内Pb、Mn的去除效果优于对照。目前,关于不同基质配置对复合重金属污水长期处理效果的研究较少。鉴于此,本研究构建以沸石+砾石、生物炭+砾石、沸石+生物炭+砾石为基质的CWs,探究其对含有机物、氮磷营养物、多种重金属的复合污水的长期处理效果及重金属的去除途径,以期为CWs处理复合重金属污水提供一定的数据支撑。
-
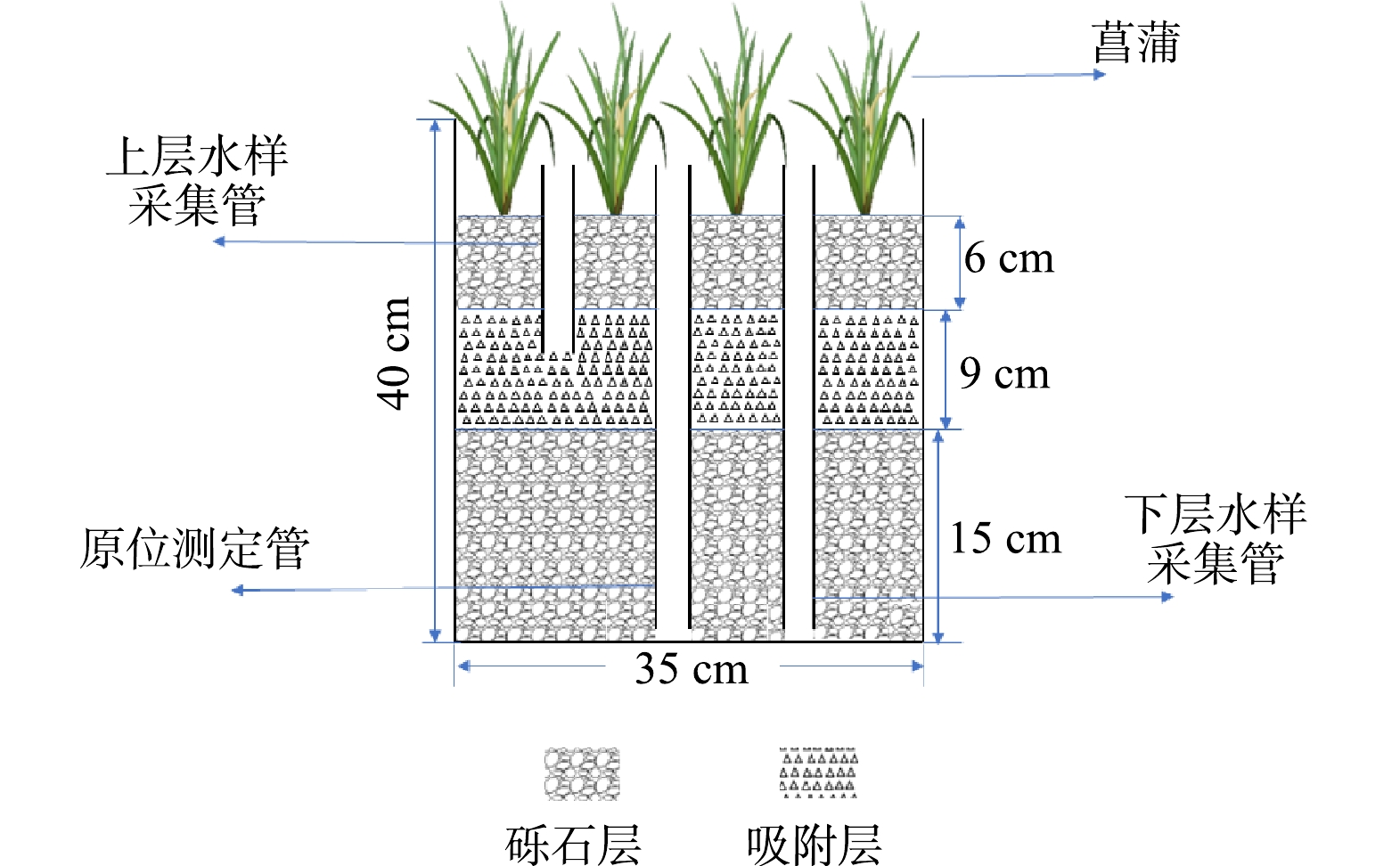
本研究在西南大学资源环境学院温室大棚进行,棚内通风、光照条件良好。模拟装置采用底面内径为35 cm、高40 cm 的PVC圆桶(图1)。桶内基质填料总高度为30 cm(底部填充15 cm砾石,中间9 cm的重金属吸附层及顶部6 cm的砾石层)。依据填充的重金属吸附材料不同,设置4组CWs:对照组(CK)为100%砾石;沸石组(F)为体积比30%沸石+70%砾石;生物炭组(S)为体积比30%生物炭+70%砾石;沸石-生物炭组(FS)为体积比15%沸石+15%生物炭+70%砾石,每个处理设置2个重复。各CWs中部放置3根长30 cm,内径5 cm的PVC多孔管,用于进水、虹吸排水、采集水样以及原位指标的测定。
实验所用砾石为普通建筑青石,粒径为1~3 cm,购自北碚某石料厂;沸石为天然斜发沸石,粒径为1~3 cm,购自河北某公司;生物炭为椰壳生物炭,粒径为0.8~1 cm,购自广东某公司。微生物采用挂膜法接种,将采自重庆市某污水处理厂曝气池的活性污泥使用人工配制的污水驯化,设置进水pH为7.26±0.015,溶解氧为(7.8±0.11) mg·L−1,水力停留时间1 d,待出水稳定后一次性接种至实验装置。湿地植物为野生菖蒲(Acorus Calamus L.),以30株·m−2即每个装置4株的密度种植。
-
本研究采用间歇式进水,设置水力停留时间为4 d,进水pH、溶解氧(DO)分别为6.62±0.08和(7.85±0.73) mg·L−1、C/N比固定为4:1,共计运行161 d。
将人工配制的复合重金属污水由装置顶部倒入,单次进水12.4 L,水质如表1所示,此外每L进水中还包含22.50 mg KH2PO4、100 mg MgSO4·7H2O、58.28 mg CaCl2、10 mg蛋白胨及0.1 mL微量元素。每毫升微量元素中包括:3.50 mg EDTA-Na2、1.69 mg H3BO3、1.08 mg MnCl2·4H2O、1.32 mg ZnSO4·7H2O、0.39 mg CuSO4·5H2O、0.05mg H2MoO4·4H2O。COD、NH4+-N、NO3−-N和TP分别由蔗糖、NH4Cl、KNO3和KH2PO4提供。
-
系统运行稳定后,每4 d采集1次水样,采样时间固定为上午8:00-10:00,采集进水与每个装置的出水。使用SG98型梅特勒-多参数测定仪(瑞士)原位测定DO、水温、pH;COD值、NH4+-N、NO3−-N、TN、TP以及全量Cu、Zn、Pb、Cd、Cr的测定按照水和废水的国家标准方法进行[23]。
实验开始与结束时从每组装置中随机挑选3株长势相似的植株,分成地上部分(茎与叶)和地下部分(根)分别测定鲜质量与干质量,烘干研磨成粉后过100目筛,测定重金属的含量。实验过程中,每3周记录各装置中植物株高和叶片数。
实验结束后,拆除装置,取基质间的沉积物,风干后测定重金属含量。基质与植物体内重金属含量测定方法参照《土壤农化分析与环境监测》[24]。
-
生物吸收系数(biological absorbing factors, BAF)为植物体内某种重金属的含量(mg·kg−1)与溶液中该种重金属含量(mg·kg−1)的比值。生物转移系数(biotransfer factors, BTF)为植物地上部分某种重金属的含量(mg·kg−1)与植物地下部分中该种重金属含量(mg·kg−1)的比值。本文使用Microsoft Excel 2015进行数据处理,使用SPSS 23.0进行统计学检验,使用origin 2017进行相关图表的绘制。
-
1) 出水水质变化及脱氮除磷效果(表2)。各装置的pH逐渐降低,在4组CWs中,生物炭组的pH高于沸石组,而沸石-生物炭组高于前两者,说明沸石与生物炭的联合添加(以下简称联合添加)能显著提高出水pH。湿地CK、F、S和FS出水中DO质量浓度均显著低于进水(P<0.05),分别为(0.59±0.34)、(0.57±0.37)、(0.46±0.36)和(0.51±0.55) mg·L−1,表现为低氧状态,对应的出水COD值分别为(14.83±4.37)、(12.58±4.15)、(11.15±5.12)和(7.60±6.01) mg·L−1,去除率分别达到91.93%、93.15%、93.93%和95.85%(表3)。表明添加沸石与生物炭能显著提高耗氧有机物的去除率(P<0.01),且联合添加的去除效果最好。
由图2可见,4组CWs均具有良好的脱氮除磷效果。TN的平均出水质量浓度分别为(7.55±3.20)、(7.58±4.69)、(8.46±5.37)和(5.95±3.70) mg·L−1,平均去除率均达到80%以上,联合添加实验组的去除效果最好。NH4+-N是出水中TN的主要形态,其变化趋势与TN一致,前65 d内质量浓度呈波动下降,65~117 d内维持较低浓度水平,后期有缓慢上升的现象。从去除率上看,4组CWs均呈上升趋势,在9月达到峰值,湿地F、S和FS去除率显著高于对照(P<0.05),且FS高于F和S,说明联合添加能有效提高人工湿地对NH4+-N的去除效率。NO3−-N出水质量浓度显著低于进水,平均去除率分别为97.6%、96.21%、95.01%和96.15%。所有装置中TP出水质量浓度变化趋势相似,平均去除率为60%左右。
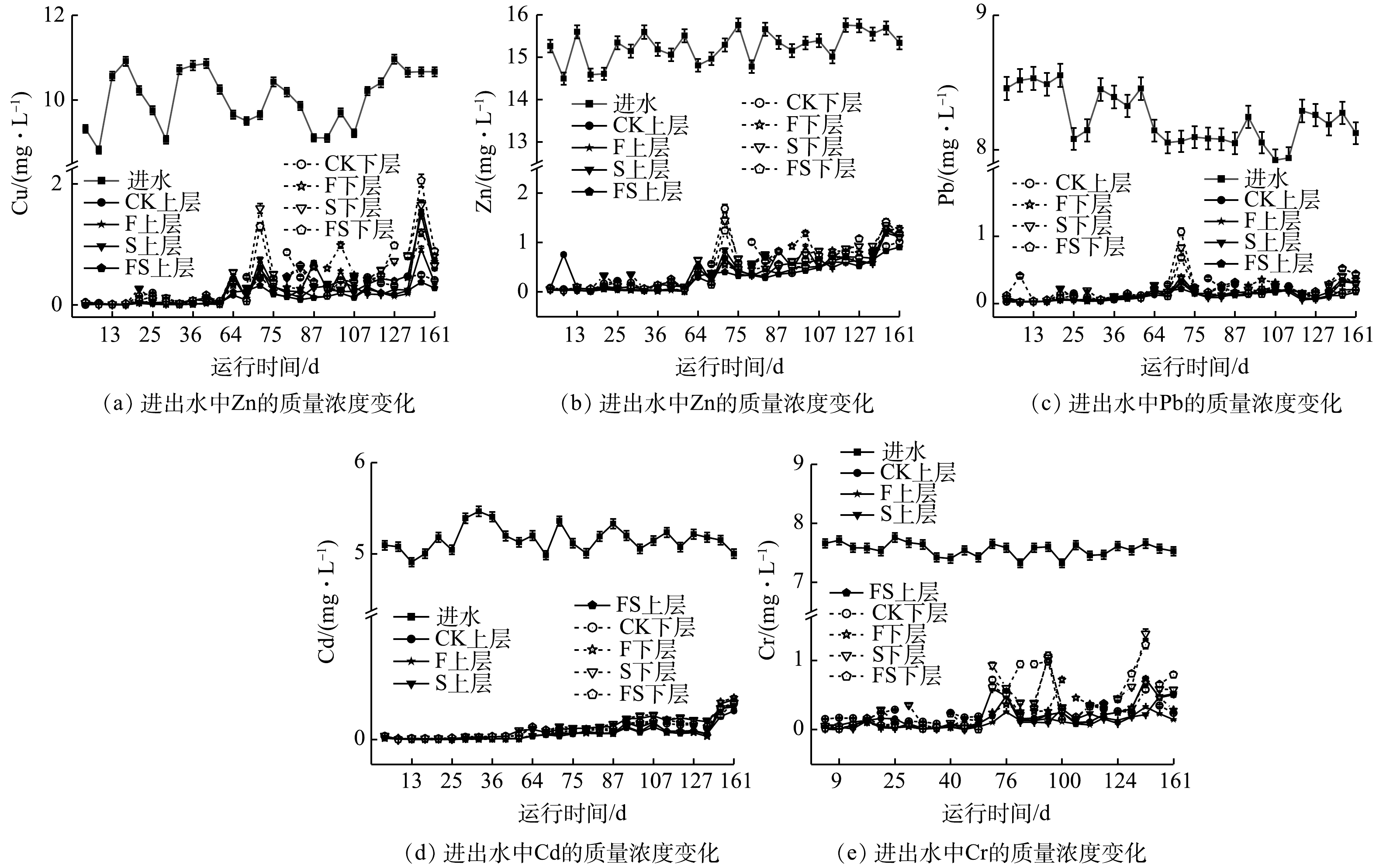
2) 重金属去除效果。不同基质配置对复合污水中的重金属均具有良好的去除效果(图3),且上层基质的去除效果优于下层(P<0.01)。4组CWs出水质量浓度变化趋势呈现出高度的相似性,前65 d内较低,后期有波动上升的现象。
稳定运行期间,4组CWs重金属的去除效果均满足污水综合排放标准(GB 8978-1996)。上下层出水中Cu的平均质量浓度均低于5 mg·L−1。与CK相比,湿地FS对Cu去除率最高,达98.72%,说明联合添加对更有利于对Cu的去除。但在湿地下层中不同基质配置之间无显著性差异。Zn质量浓度除个别天数外,均低于1 mg·L−1。除湿地CK外,其余3组湿地上下层出水中Zn质量浓度均存在显著性差异(P<0.01),组间两两比较,湿地FS显著降低了Zn出水质量浓度(P<0.01),其余3组无显著性差异。Pb出水质量浓度均低于污水综合排放最高标准(1 mg·L−1)。湿地FS上层对Pb去除率最高,而下层中F去除率最高。Cd前期出水质量浓度低于0.1 mg·L−1,湿地FS与湿地F显著降低了出水中Cd的质量浓度,但两者之间无显著性差异。与CK相比,湿地FS和湿地F显著地提高了Cr的去除率(P<0.01),而湿地S与之无显著性差异,此外,湿地FS去除率最高。这说明单独添加生物炭未能显著提升CWs中Cr的去除率,而与沸石联合添加能形成协同作用,且比单独添加沸石更能提高Cr的去除率。
-
5种重金属在菖蒲体内积累量均较少(表4),且主要富集在地下部分(P<0.05)。菖蒲对5种重金属的生物吸收系数分别为:地上2.06~3.16、0.8~1.99、1.36~1.84、1.05~1.5;地下9.46~15.85、4.44~7.67、4.62~7.63,其中,湿地CK最大,约为其余3组人工湿地的2倍,说明添加沸石与生物炭均能减小菖蒲对重金属的吸收量(P<0.05)。5种重金属的转移系数均<1,说明重金属在菖蒲体内的流通性较小,主要积累在地下部分。湿地CK、F和FS中,菖蒲对Cu的BTF高于Zn,而湿地S中则是Zn高于Cu。所有装置中Cr、Pb和Cd的BTF均最小。说明Cr、Pb和Cd主要以根际截留的方式去除,以减少对地上部的危害,Zn和Cu在满足生长代谢所需的前提下避免地上部受过高浓度的危害,这与实验结束时,所有装置中菖蒲的株高与地上、地下部的生物量均有所增长的结果一致。
不同基质中重金属的吸附量存在显著差异(P<0.05),与砾石相比,沸石、生物炭以及联合添加显著提高了重金属的吸附量,其中联合添加的吸附量最高,对Cu、Zn、Pb、Cd、Cr的吸附量分别提高了114.02%、61.3%、36.11%、100.3%和69.2%(表5)。
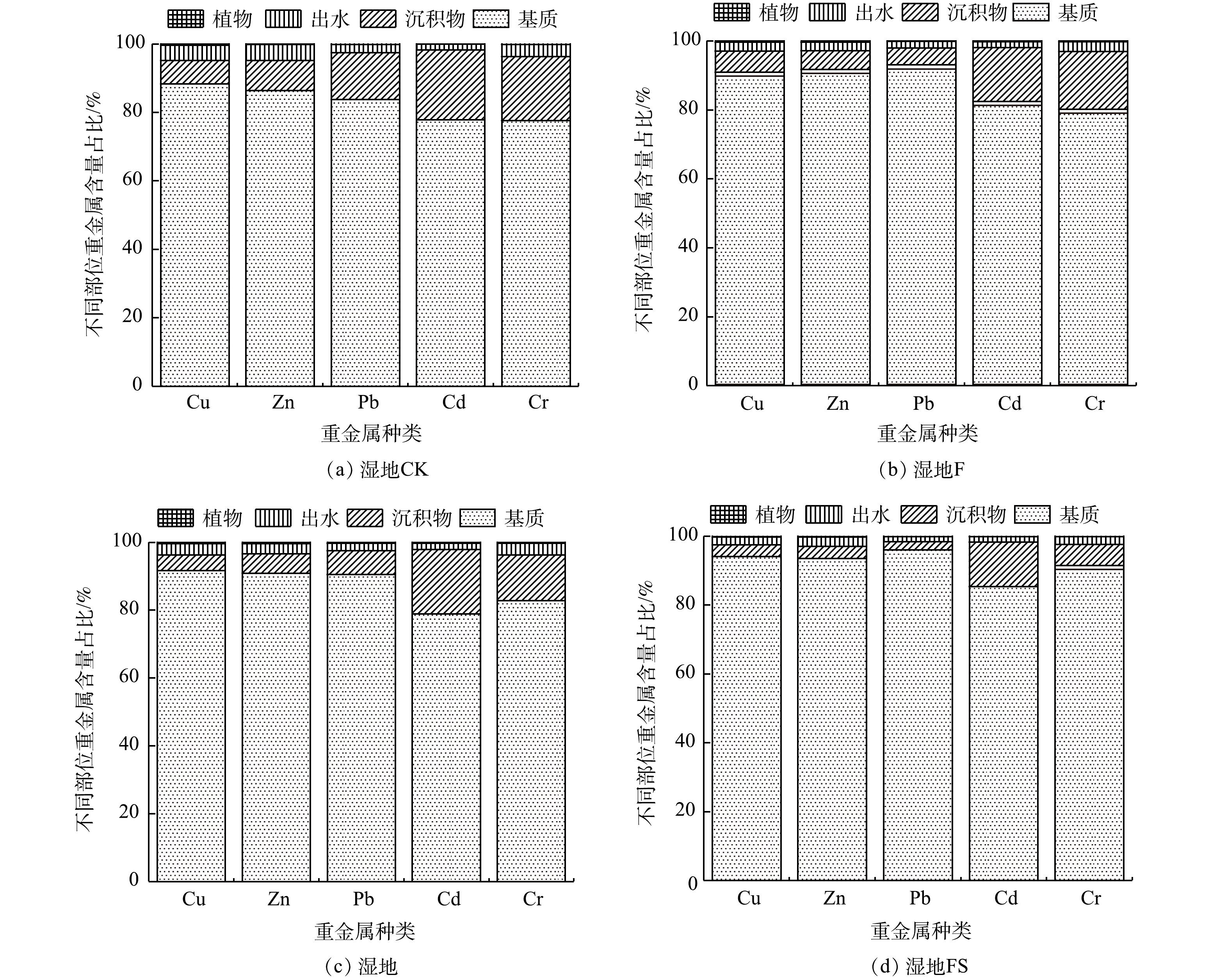
为量化重金属的空间分布情况,引入占比系数ρ。ρ表示系统各部分中重金属的富集量与进水总量的比值。其中,基质占比最高(77%~96%),其后依次是沉积物、出水和植物体内。植物吸收量最小,占比不超过0.5% (图4),进一步说明基质是人工湿地去除重金属的主要场所。此外,湿地FS中重金属累积占比显著高于其余人工湿地(P<0.05),结合湿地FS对复合重金属污水的去除效果,可以得出联合添加通过提高基质吸附量进而提高了污水的处理能力。
-
CWs运行过程中pH受植物、硝化作用以及微生物代谢活动等作用的影响[25]。微生物与有机物的络合反应,消耗水中的阳离子析出OH−[26],使出水pH高于进水。这与本研究结果一致。但随装置的运行,出水pH呈下降趋势。这是因为NH4+-N的氧化伴随着碱度的消耗[27-28],且由于本研究未对装置进行曝气处理,系统长期处于一种低氧状态,导致酸性物质的积累[29]。污水中有机物的去除主要依靠微生物的代谢作用,而基质填料主要通过影响系统微生物从而改变对有机物的去除效果,直接依赖基质的吸附去除的有机物的量很少[30]。生物炭与沸石具有比表面积大、孔隙率高等优点,能够为微生物的附着提供充足的环境[31]。此外,沸石与生物炭都是强静电吸附材料[32],有利于将有机物吸附在其表面,促进去除。
微生物降解是CWs脱氮的主要途径,运行前期由于多种重金属的输入对菖蒲和微生物的生长代谢产生了不利影响,因此,前36 d内TN与NH4+-N的出水质量浓度表现出波动现象[33]。随着装置的运行,湿地植物对重金属的抗性增强,且耐重金属的优势菌种得到大量繁殖,从而使去除率平稳回升。这一结果与付永江[1]的研究相一致。运行125 d后去除率达到峰值后缓慢下降,说明基质已经达到吸附平衡[34]。CWs除磷的方式包括基质吸附、微生物吸收转化和植物吸收利用,其中基质的吸附尤其是化学吸附是主要方式[35-37]。本研究中,前21 d内出水TP质量浓度较高,这可能是因为重金属的输入限制了基质对磷的吸附能力,从而降低了CWs对TP的去除率[22,38]。系统运行89 d后,各装置对P的去除率迅速上升达到80%而之后又缓慢下降,这是因为基质对P的去除率随着接触时间的增加而增加,达到峰值后下降[39-40]。
本研究中,湿地F、S、FS与湿地CK相比显著提高了CWs对氮磷的去除能力,这与本团队[41-42]之前研究发现的沸石、生物炭基CWs均能显著改善出水水质的结果相一致。总的说来沸石与生物炭的联合添加对水质净化效果优于单独添加,说明了两者在污水处理过程中能产生协同作用。
-
本研究中5种重金属的去除效果,前期(67 d)较好,后期有所波动下降。这可能是因为,基质吸附是CWs去除重金属的主要方式[43],装置运行后期基质活性吸附点位逐渐饱和,且由于酸化与腐化现象,pH和DO受到影响,导致重金属释放重新回到水体[44],从而使得后期处理效果下降,这与本研究结果中pH和DO的变化一致。
基质对重金属的吸附能力存在差异。本研究中沸石与生物炭的联合添加组对重金属的去除效果优于单独添加,这可能是因为联合添加提高了出水pH,可促进基质的吸附作用。出水中5种重金属,其中Cd和Pb的去除率最高,Cu、Zn去除率最低,但吸附量为Zn>Cu>Pb>Cr>Cd (湿地CK、S)、Zn>Cu>Cr>Pb>Cd (湿地F、FS)。这是因为,当溶液中发生竞争吸附时,重金属离子的吸附量受到离子浓度、自身电荷等特性影响[45],使部分离子优先占据吸附位点[46]。本研究模拟污水中Zn、Cu的质量浓度大于其他元素,而Cr能在沸石表面及空隙中形成沉淀,且Cr的化合价高于Pb和Cd[47]。同时,Cu、Zn在CWs中主要以可交换态存在,具有较高的浸出性,易释放重新回到水中[48]。这与MEI等[49]、FENG等[22]、MOSLEY等[50]的研究结果一致。
-
污水中重金属被CWs截留后并不能被分解去除,而是保留在系统中,分布于基质、沉积物以及植物体内。本研究中,重金属在基质中的积累量最大,其后依次是沉积物>出水>植物。这与NGUYEN等[51]和LIANG等[52]的研究结果一致,进一步说明了基质对污水中重金属的去除占主导地位。
植物地下部分向地上部分的物质转运受到很多因素的限制[52],一方面植物的根区会分泌一种黏性物质,使得过量的重金属在根系表面附着,阻止高浓度重金属离子进入植物体内;另一方面根系的泌氧作用,使重金属在根区形成沉淀物质。由于本研究未对CWs进行曝气处理,系统长期处于低氧状态,根系的泌氧作用并不能改善装置的溶氧环境。此外,重金属在植物体内分布的规律与组织器官的代谢能力有显著的相关性[53]。根系作为植物重要的营养器官,新陈代谢的能力相较地上部更为旺盛,使重金属在植物根部富集量大于地上部分。植物对重金属的转运存在着复杂的选择性,营养元素、与植物亲和力大的离子容易转移,而对植物有较强的毒害作用的离子会受到根系积累、元素之间的拮抗作用等影响,进而向上的转运受到抑制。Zn、Cu作为植物生长需要的元素,其BTF系数高于其余重金属,这与本研究中BAF以及植物体内的吸附量相符合。
模拟系统运行161 d后,所有装置中的菖蒲的生物量比运行前有一定的增长,且地上部分的生物量大于地下部分。这与李庆华[54]、付永江[1]等的研究结果一致。鉴于菖蒲生物量的变化及重金属的空间分布情况,建议每年收割整株菖蒲或者定期更换基质以维持人工湿地较高水平的处理能力。
-
1) 在161 d的运行期内,所有装置均能长期保持较好的出水水质,出水NH4+-N、TN和COD均达到《城镇污水处理厂污染物排放标准(GB 18918-2002)》一级A标,出水TP达到二级标准。出水Zn满足《污水综合排放标准(GB 8978-1996)》一级标准,Cu在装置运行的前95 d内满足的一、二级标准,后期满足三级标准;一类污染物中除Cd个别天数外均满足最高浓度限值。
2) 沸石与生物炭均能显著提高人工湿地对有机物和NH4+-N的去除率,且联合添加的效果更好;不同基质配置对NO3−-N的去除无显著影响;单独添加沸石对TN的去除效果优于生物炭,但联合添加的去除率更高;联合添加提高了系统对重金属的去除能力,5种重金属的去除率均达到95%以上。
3) 重金属在人工湿地中的空间分布情况为:基质>沉积物>出水>植物体内,联合添加显著提高了基质的吸附量。综合人工湿地系统对复合污水的去除效果以及重金属的空间分布情况,沸石与生物炭的联合添加可作为人工湿地处理复合重金属污水的最佳基质配置。
人工湿地不同基质配置对复合重金属污水的长期处理效果
Long term treatment effect of combined heavy metal wastewater by the constructed wetlands with different substrates
-
摘要: 为探究不同基质对含有复合重金属污水的长期处理效果及重金属在人工湿地中的分布特征,通过温室内构建基质分别为砾石(CK)、沸石(F)、生物炭(S)和沸石-生物炭(FS)的4组垂直潜流人工湿地污水处理模拟装置,连续运行161 d,观测出水水质变化并研究湿地各系统中重金属的浓度分布特征。结果表明,所有装置出水均呈低溶解氧状态,pH呈中性或弱碱性;4组人工湿地均具有良好的脱氮除磷及重金属去除效果,有机物、TN、NO3--N、NH4+-N和TP的平均去除率分别为91.93%~95.85%、86.08%~89.80%、94.99%~97.60%、75.22%~83.58%和60.35%~64.61%,Cu、Zn、Pb、Cd和Cr 5种重金属的去除率均在90%以上;菖蒲体内重金属的质量浓度表现为地下部分>地上部分,但菖蒲体内富集的重金属总量相对较少,整个系统中重金属主要富集在基质中(77%~96%);与湿地CK相比,沸石与生物炭的联合添加显著提高了污水的净化能力(P<0.01),但运行后期,装置对复合污水的去除率呈一定的下降趋势。Abstract: In order to explore the long term treatment effect of combined heavy metal wastewater by the constructed wetlands with different substrates and the distribution characteristics of heavy metals in constructed wetlands, four wastewater treatment simulators of vertical submerged constructed wetlands with gravel(CK), zeolite(F), biochar(S) and zeolite-biochar(FS) substrate were constructed in the greenhouse and ran continuously for 161 days. The changes in effluent quality were tested and the concentrations distribution of heavy metals in each part of the wetland were analyzed. The results showed that the dissolved oxygen contents in the effluent of all devices were low, and pH values were neutral or weakly alkaline. Four wetlands had good removal performance on N, P and heavy metals, the average removal rates of organics, TN, NO3--N, NH4+-N and TP were 91.93%~95.85%, 86.08%~89.80%, 94.99%~97.60%, 75.22%~83.58% and 60.35%~64.61%, respectively. And the removal rates of Cu Zn, Pb, Cd and Cr were all above 90%. The accumulation of heavy metals in the underground part of acorus calamus L. was higher than that overground part, but the total amounts of heavy metals accumulated in acorus calamus L. was relatively low, the heavy metals were mainly accumulated in the substrates(77%~96%). The combined addition of zeolite and biochar increased the purification ability of the wetlands extremely significantly compared to CK(P<0.01), but the removal rate showed a certain downward trend at the later stages of the experiment.
-
Key words:
- vertical subsurface flow constructed wetland /
- zeolite /
- biochar /
- heavy metals removal
-
重金属是造成我国水环境污染的主要因素之一[1]。传统处理重金属污水的方法包括物理化学技术和微生物法均存在一定的局限性[1-3]。人工湿地(constructed wetlands, CWs)是人为建造和管控的污水处理系统[4-5],投资少、污水净化效果好,近年来受到大量关注[6-8]。
研究发现,基质吸附是CWs去除重金属的主要途径[9-10]。碎石、河砂、砾石等传统基质具有来源广、造价低等特点受到广泛关注。陈银萍等[11]为期10 d的实验发现粉煤灰对Cd去除效果较好但不利于植物生长;余关龙等[12]发现短期内,碎石基人工湿地对营养物及Cd2+、Zn2+ 去除率较高好。但传统基质在长期运行下对重金属的去除能力仍有限[9]。因此,需要对人工湿地基质配置进行优化,以实现长期最佳的污水处理效果。沸石与生物炭因具有多孔的结构、丰富的官能团[13-15]以及较强的静电效应和吸附性能[16],在污水处理中具有较高的应用价值[17-19],但目前关于添加生物炭能否提高含重金属污水的去除率存在一定的争议。CHEN[20]与CHANG等[21]认为生物炭基CWs具有较好的重金属固定能力并能促进植物生长,而FENG[22]认为添加生物炭会增加重金属对β-变形菌、甲型变形菌纲的毒性导致脱氮效率下降。
多种基质组合使用能发生协同作用,从而提高净化效果[9]。张晓斌等[18]研究发现沸石与炉渣组合添加对电镀废水中Cr、Zn的去除效果优于单独添加;ABEDI等[13]采用响应面分析评估了沸石-生物炭基人工湿地处理模拟污水的效果,发现3个月运行期内Pb、Mn的去除效果优于对照。目前,关于不同基质配置对复合重金属污水长期处理效果的研究较少。鉴于此,本研究构建以沸石+砾石、生物炭+砾石、沸石+生物炭+砾石为基质的CWs,探究其对含有机物、氮磷营养物、多种重金属的复合污水的长期处理效果及重金属的去除途径,以期为CWs处理复合重金属污水提供一定的数据支撑。
1. 材料与方法
1.1 实验材料及装置
本研究在西南大学资源环境学院温室大棚进行,棚内通风、光照条件良好。模拟装置采用底面内径为35 cm、高40 cm 的PVC圆桶(图1)。桶内基质填料总高度为30 cm(底部填充15 cm砾石,中间9 cm的重金属吸附层及顶部6 cm的砾石层)。依据填充的重金属吸附材料不同,设置4组CWs:对照组(CK)为100%砾石;沸石组(F)为体积比30%沸石+70%砾石;生物炭组(S)为体积比30%生物炭+70%砾石;沸石-生物炭组(FS)为体积比15%沸石+15%生物炭+70%砾石,每个处理设置2个重复。各CWs中部放置3根长30 cm,内径5 cm的PVC多孔管,用于进水、虹吸排水、采集水样以及原位指标的测定。
实验所用砾石为普通建筑青石,粒径为1~3 cm,购自北碚某石料厂;沸石为天然斜发沸石,粒径为1~3 cm,购自河北某公司;生物炭为椰壳生物炭,粒径为0.8~1 cm,购自广东某公司。微生物采用挂膜法接种,将采自重庆市某污水处理厂曝气池的活性污泥使用人工配制的污水驯化,设置进水pH为7.26±0.015,溶解氧为(7.8±0.11) mg·L−1,水力停留时间1 d,待出水稳定后一次性接种至实验装置。湿地植物为野生菖蒲(Acorus Calamus L.),以30株·m−2即每个装置4株的密度种植。
1.2 人工湿地的运行
本研究采用间歇式进水,设置水力停留时间为4 d,进水pH、溶解氧(DO)分别为6.62±0.08和(7.85±0.73) mg·L−1、C/N比固定为4:1,共计运行161 d。
将人工配制的复合重金属污水由装置顶部倒入,单次进水12.4 L,水质如表1所示,此外每L进水中还包含22.50 mg KH2PO4、100 mg MgSO4·7H2O、58.28 mg CaCl2、10 mg蛋白胨及0.1 mL微量元素。每毫升微量元素中包括:3.50 mg EDTA-Na2、1.69 mg H3BO3、1.08 mg MnCl2·4H2O、1.32 mg ZnSO4·7H2O、0.39 mg CuSO4·5H2O、0.05mg H2MoO4·4H2O。COD、NH4+-N、NO3−-N和TP分别由蔗糖、NH4Cl、KNO3和KH2PO4提供。
表 1 人工湿地进水水质Table 1. Water quality of CWs inlet water成分 质量浓度/(mg·L−1) 成分 质量浓度/(mg·L−1) COD 183.69±12.73 CuSO4 10.05±0.66 TP 5.02±0.17 ZnSO4 15.26±0.66 TN 47.52±0.64 CdCl2 5.16±0.14 NH4+-N 24.30±1.20 Cr(NO3)3 7.56±0.11 NO3−-N 23.08±0.42 Pb(NO3)2 8.23±0.19 1.3 样品采集与测定
系统运行稳定后,每4 d采集1次水样,采样时间固定为上午8:00-10:00,采集进水与每个装置的出水。使用SG98型梅特勒-多参数测定仪(瑞士)原位测定DO、水温、pH;COD值、NH4+-N、NO3−-N、TN、TP以及全量Cu、Zn、Pb、Cd、Cr的测定按照水和废水的国家标准方法进行[23]。
实验开始与结束时从每组装置中随机挑选3株长势相似的植株,分成地上部分(茎与叶)和地下部分(根)分别测定鲜质量与干质量,烘干研磨成粉后过100目筛,测定重金属的含量。实验过程中,每3周记录各装置中植物株高和叶片数。
实验结束后,拆除装置,取基质间的沉积物,风干后测定重金属含量。基质与植物体内重金属含量测定方法参照《土壤农化分析与环境监测》[24]。
1.4 数据处理
生物吸收系数(biological absorbing factors, BAF)为植物体内某种重金属的含量(mg·kg−1)与溶液中该种重金属含量(mg·kg−1)的比值。生物转移系数(biotransfer factors, BTF)为植物地上部分某种重金属的含量(mg·kg−1)与植物地下部分中该种重金属含量(mg·kg−1)的比值。本文使用Microsoft Excel 2015进行数据处理,使用SPSS 23.0进行统计学检验,使用origin 2017进行相关图表的绘制。
2. 结果与分析
2.1 不同基质配置对复合重金属污水的去除效果
1) 出水水质变化及脱氮除磷效果(表2)。各装置的pH逐渐降低,在4组CWs中,生物炭组的pH高于沸石组,而沸石-生物炭组高于前两者,说明沸石与生物炭的联合添加(以下简称联合添加)能显著提高出水pH。湿地CK、F、S和FS出水中DO质量浓度均显著低于进水(P<0.05),分别为(0.59±0.34)、(0.57±0.37)、(0.46±0.36)和(0.51±0.55) mg·L−1,表现为低氧状态,对应的出水COD值分别为(14.83±4.37)、(12.58±4.15)、(11.15±5.12)和(7.60±6.01) mg·L−1,去除率分别达到91.93%、93.15%、93.93%和95.85%(表3)。表明添加沸石与生物炭能显著提高耗氧有机物的去除率(P<0.01),且联合添加的去除效果最好。
表 2 人工湿地出水水质Table 2. Effluent quality of CWs处理 COD/(mg·L−1) pH DO/(mg·L−1) NO3−-N/(mg·L−1) NH4+-N/(mg·L−1) TN/(mg·L−1) TP/(mg·L−1) CK 14.83±4.37a 6.69±0.20c 0.59±0.33a 0.55±0.37c 5.91±3.18a 6.34±2.88a 1.99±1.33a F 12.58±4.15b 6.65±0.22d 0.57±0.37a 0.87±1.25c 5.14±4.50b 5.96±4.21a 1.97±1.48a S 11.15±5.12c 6.77±0.13b 0.46±0.36a 1.15±1.20a 5.66±4.80a 6.60±4.90a 1.92±1.11a FS 7.60±6.01d 6.83±0.14a 0.51±0.55a 0.89±0.75b 3.90±3.30c 4.84±3.43b 1.78±0.96b 注:表中不同小写字母表示同一指标在不同CWs间的差异性(P<0.05) 表 3 出水水质指标平均去除率Table 3. Average removal rate of water quality indices in effluent% 处理 COD NO3−-N NH4+-N TN TP CK 91.93 97.60 75.22 86.63 60.35 F 93.15 96.21 78.30 87.42 60.57 S 93.93 94.99 76.15 86.08 61.83 FS 95.85 96.12 83.58 89.80 64.61 由图2可见,4组CWs均具有良好的脱氮除磷效果。TN的平均出水质量浓度分别为(7.55±3.20)、(7.58±4.69)、(8.46±5.37)和(5.95±3.70) mg·L−1,平均去除率均达到80%以上,联合添加实验组的去除效果最好。NH4+-N是出水中TN的主要形态,其变化趋势与TN一致,前65 d内质量浓度呈波动下降,65~117 d内维持较低浓度水平,后期有缓慢上升的现象。从去除率上看,4组CWs均呈上升趋势,在9月达到峰值,湿地F、S和FS去除率显著高于对照(P<0.05),且FS高于F和S,说明联合添加能有效提高人工湿地对NH4+-N的去除效率。NO3−-N出水质量浓度显著低于进水,平均去除率分别为97.6%、96.21%、95.01%和96.15%。所有装置中TP出水质量浓度变化趋势相似,平均去除率为60%左右。
2) 重金属去除效果。不同基质配置对复合污水中的重金属均具有良好的去除效果(图3),且上层基质的去除效果优于下层(P<0.01)。4组CWs出水质量浓度变化趋势呈现出高度的相似性,前65 d内较低,后期有波动上升的现象。
稳定运行期间,4组CWs重金属的去除效果均满足污水综合排放标准(GB 8978-1996)。上下层出水中Cu的平均质量浓度均低于5 mg·L−1。与CK相比,湿地FS对Cu去除率最高,达98.72%,说明联合添加对更有利于对Cu的去除。但在湿地下层中不同基质配置之间无显著性差异。Zn质量浓度除个别天数外,均低于1 mg·L−1。除湿地CK外,其余3组湿地上下层出水中Zn质量浓度均存在显著性差异(P<0.01),组间两两比较,湿地FS显著降低了Zn出水质量浓度(P<0.01),其余3组无显著性差异。Pb出水质量浓度均低于污水综合排放最高标准(1 mg·L−1)。湿地FS上层对Pb去除率最高,而下层中F去除率最高。Cd前期出水质量浓度低于0.1 mg·L−1,湿地FS与湿地F显著降低了出水中Cd的质量浓度,但两者之间无显著性差异。与CK相比,湿地FS和湿地F显著地提高了Cr的去除率(P<0.01),而湿地S与之无显著性差异,此外,湿地FS去除率最高。这说明单独添加生物炭未能显著提升CWs中Cr的去除率,而与沸石联合添加能形成协同作用,且比单独添加沸石更能提高Cr的去除率。
2.2 重金属在人工湿地中的分布
5种重金属在菖蒲体内积累量均较少(表4),且主要富集在地下部分(P<0.05)。菖蒲对5种重金属的生物吸收系数分别为:地上2.06~3.16、0.8~1.99、1.36~1.84、1.05~1.5;地下9.46~15.85、4.44~7.67、4.62~7.63,其中,湿地CK最大,约为其余3组人工湿地的2倍,说明添加沸石与生物炭均能减小菖蒲对重金属的吸收量(P<0.05)。5种重金属的转移系数均<1,说明重金属在菖蒲体内的流通性较小,主要积累在地下部分。湿地CK、F和FS中,菖蒲对Cu的BTF高于Zn,而湿地S中则是Zn高于Cu。所有装置中Cr、Pb和Cd的BTF均最小。说明Cr、Pb和Cd主要以根际截留的方式去除,以减少对地上部的危害,Zn和Cu在满足生长代谢所需的前提下避免地上部受过高浓度的危害,这与实验结束时,所有装置中菖蒲的株高与地上、地下部的生物量均有所增长的结果一致。
表 4 菖蒲不同部位重金属含量Table 4. Enrichment of heavy metals in different parts of acorus calamus L.处理 部位 Cu/(mg·kg−1) Zn/(mg·kg−1) Pb/(mg·kg−1) Cd/(mg·kg−1) Cr/(mg·kg−1) CK 地上 358.75±15.910 439.25±13.08 111.25±13.79 49.75±6.72 106.00±7.70 地下 1 281.00±216.38 1 673.25±375.83 813.75±269.76 370.50±70.71 729.00±8.49 F 地上 245.25±37.12 352.75±90.86 65.75±21.57 36.25±6.72 90.75±11.67 地下 744.75±19.45 1 147.00±41.01 460.00±50.91 348.75±1.06 540.00±22.63 S 地上 374.50±70.71 465.00±11.31 111.25±3.18 59.75±1.06 127.00±13.31 地下 944.75±270.47 1 203.50±142.84 435.50±82.02 276.00±57.89 491.25±19.45 FS 地上 326.50±14.14 390.00±16.97 106.25±2.48 50.75±1.77 116.25±2.48 地下 1 176.25±182.79 1 448.00±258.09 540.75±86.62 387.50±74.95 588.25±12.37 注:小写字母a、b表示同一装置某种重金属地上部分与地下部分的显著差异性(P<0.05) 不同基质中重金属的吸附量存在显著差异(P<0.05),与砾石相比,沸石、生物炭以及联合添加显著提高了重金属的吸附量,其中联合添加的吸附量最高,对Cu、Zn、Pb、Cd、Cr的吸附量分别提高了114.02%、61.3%、36.11%、100.3%和69.2%(表5)。
表 5 不同基质中重金属的吸附量Table 5. Heavy metals adsorption in different substrates处理 Cu/(mg·kg−1) Zn/(mg·kg−1) Pb/(mg·kg−1) Cd/(mg·kg−1) Cr/(mg·kg−1) CK 113.38±3.63c 176.88±0.88d 53.50±2.75a 24.13±0.38b 46.00±0.25b F 167.63±0.88b 252.63±13.88c 62.13±0.13a 45.50±1.75a 70.88±5.38a S 204.38±4.38b 271.88±19.13b 82.13±3.38a 37.13±0.13a 72.35±2.15a FS 242.65±3.53a 285.13±16.75a 72.81±1.19a 48.33±2.05a 77.813±1.44a 注:不同小写字母表示同一种金属在不同基质CW间的差异性(P<0.05) 为量化重金属的空间分布情况,引入占比系数ρ。ρ表示系统各部分中重金属的富集量与进水总量的比值。其中,基质占比最高(77%~96%),其后依次是沉积物、出水和植物体内。植物吸收量最小,占比不超过0.5% (图4),进一步说明基质是人工湿地去除重金属的主要场所。此外,湿地FS中重金属累积占比显著高于其余人工湿地(P<0.05),结合湿地FS对复合重金属污水的去除效果,可以得出联合添加通过提高基质吸附量进而提高了污水的处理能力。
3. 讨论
3.1 不同基质配置对水质净化及脱氮除磷的影响
CWs运行过程中pH受植物、硝化作用以及微生物代谢活动等作用的影响[25]。微生物与有机物的络合反应,消耗水中的阳离子析出OH−[26],使出水pH高于进水。这与本研究结果一致。但随装置的运行,出水pH呈下降趋势。这是因为NH4+-N的氧化伴随着碱度的消耗[27-28],且由于本研究未对装置进行曝气处理,系统长期处于一种低氧状态,导致酸性物质的积累[29]。污水中有机物的去除主要依靠微生物的代谢作用,而基质填料主要通过影响系统微生物从而改变对有机物的去除效果,直接依赖基质的吸附去除的有机物的量很少[30]。生物炭与沸石具有比表面积大、孔隙率高等优点,能够为微生物的附着提供充足的环境[31]。此外,沸石与生物炭都是强静电吸附材料[32],有利于将有机物吸附在其表面,促进去除。
微生物降解是CWs脱氮的主要途径,运行前期由于多种重金属的输入对菖蒲和微生物的生长代谢产生了不利影响,因此,前36 d内TN与NH4+-N的出水质量浓度表现出波动现象[33]。随着装置的运行,湿地植物对重金属的抗性增强,且耐重金属的优势菌种得到大量繁殖,从而使去除率平稳回升。这一结果与付永江[1]的研究相一致。运行125 d后去除率达到峰值后缓慢下降,说明基质已经达到吸附平衡[34]。CWs除磷的方式包括基质吸附、微生物吸收转化和植物吸收利用,其中基质的吸附尤其是化学吸附是主要方式[35-37]。本研究中,前21 d内出水TP质量浓度较高,这可能是因为重金属的输入限制了基质对磷的吸附能力,从而降低了CWs对TP的去除率[22,38]。系统运行89 d后,各装置对P的去除率迅速上升达到80%而之后又缓慢下降,这是因为基质对P的去除率随着接触时间的增加而增加,达到峰值后下降[39-40]。
本研究中,湿地F、S、FS与湿地CK相比显著提高了CWs对氮磷的去除能力,这与本团队[41-42]之前研究发现的沸石、生物炭基CWs均能显著改善出水水质的结果相一致。总的说来沸石与生物炭的联合添加对水质净化效果优于单独添加,说明了两者在污水处理过程中能产生协同作用。
3.2 不同基质配置对重金属的去除效果
本研究中5种重金属的去除效果,前期(67 d)较好,后期有所波动下降。这可能是因为,基质吸附是CWs去除重金属的主要方式[43],装置运行后期基质活性吸附点位逐渐饱和,且由于酸化与腐化现象,pH和DO受到影响,导致重金属释放重新回到水体[44],从而使得后期处理效果下降,这与本研究结果中pH和DO的变化一致。
基质对重金属的吸附能力存在差异。本研究中沸石与生物炭的联合添加组对重金属的去除效果优于单独添加,这可能是因为联合添加提高了出水pH,可促进基质的吸附作用。出水中5种重金属,其中Cd和Pb的去除率最高,Cu、Zn去除率最低,但吸附量为Zn>Cu>Pb>Cr>Cd (湿地CK、S)、Zn>Cu>Cr>Pb>Cd (湿地F、FS)。这是因为,当溶液中发生竞争吸附时,重金属离子的吸附量受到离子浓度、自身电荷等特性影响[45],使部分离子优先占据吸附位点[46]。本研究模拟污水中Zn、Cu的质量浓度大于其他元素,而Cr能在沸石表面及空隙中形成沉淀,且Cr的化合价高于Pb和Cd[47]。同时,Cu、Zn在CWs中主要以可交换态存在,具有较高的浸出性,易释放重新回到水中[48]。这与MEI等[49]、FENG等[22]、MOSLEY等[50]的研究结果一致。
3.3 重金属在CWs中的分布
污水中重金属被CWs截留后并不能被分解去除,而是保留在系统中,分布于基质、沉积物以及植物体内。本研究中,重金属在基质中的积累量最大,其后依次是沉积物>出水>植物。这与NGUYEN等[51]和LIANG等[52]的研究结果一致,进一步说明了基质对污水中重金属的去除占主导地位。
植物地下部分向地上部分的物质转运受到很多因素的限制[52],一方面植物的根区会分泌一种黏性物质,使得过量的重金属在根系表面附着,阻止高浓度重金属离子进入植物体内;另一方面根系的泌氧作用,使重金属在根区形成沉淀物质。由于本研究未对CWs进行曝气处理,系统长期处于低氧状态,根系的泌氧作用并不能改善装置的溶氧环境。此外,重金属在植物体内分布的规律与组织器官的代谢能力有显著的相关性[53]。根系作为植物重要的营养器官,新陈代谢的能力相较地上部更为旺盛,使重金属在植物根部富集量大于地上部分。植物对重金属的转运存在着复杂的选择性,营养元素、与植物亲和力大的离子容易转移,而对植物有较强的毒害作用的离子会受到根系积累、元素之间的拮抗作用等影响,进而向上的转运受到抑制。Zn、Cu作为植物生长需要的元素,其BTF系数高于其余重金属,这与本研究中BAF以及植物体内的吸附量相符合。
模拟系统运行161 d后,所有装置中的菖蒲的生物量比运行前有一定的增长,且地上部分的生物量大于地下部分。这与李庆华[54]、付永江[1]等的研究结果一致。鉴于菖蒲生物量的变化及重金属的空间分布情况,建议每年收割整株菖蒲或者定期更换基质以维持人工湿地较高水平的处理能力。
4. 结论
1) 在161 d的运行期内,所有装置均能长期保持较好的出水水质,出水NH4+-N、TN和COD均达到《城镇污水处理厂污染物排放标准(GB 18918-2002)》一级A标,出水TP达到二级标准。出水Zn满足《污水综合排放标准(GB 8978-1996)》一级标准,Cu在装置运行的前95 d内满足的一、二级标准,后期满足三级标准;一类污染物中除Cd个别天数外均满足最高浓度限值。
2) 沸石与生物炭均能显著提高人工湿地对有机物和NH4+-N的去除率,且联合添加的效果更好;不同基质配置对NO3−-N的去除无显著影响;单独添加沸石对TN的去除效果优于生物炭,但联合添加的去除率更高;联合添加提高了系统对重金属的去除能力,5种重金属的去除率均达到95%以上。
3) 重金属在人工湿地中的空间分布情况为:基质>沉积物>出水>植物体内,联合添加显著提高了基质的吸附量。综合人工湿地系统对复合污水的去除效果以及重金属的空间分布情况,沸石与生物炭的联合添加可作为人工湿地处理复合重金属污水的最佳基质配置。
-
表 1 人工湿地进水水质
Table 1. Water quality of CWs inlet water
成分 质量浓度/(mg·L−1) 成分 质量浓度/(mg·L−1) COD 183.69±12.73 CuSO4 10.05±0.66 TP 5.02±0.17 ZnSO4 15.26±0.66 TN 47.52±0.64 CdCl2 5.16±0.14 NH4+-N 24.30±1.20 Cr(NO3)3 7.56±0.11 NO3−-N 23.08±0.42 Pb(NO3)2 8.23±0.19 表 2 人工湿地出水水质
Table 2. Effluent quality of CWs
处理 COD/(mg·L−1) pH DO/(mg·L−1) NO3−-N/(mg·L−1) NH4+-N/(mg·L−1) TN/(mg·L−1) TP/(mg·L−1) CK 14.83±4.37a 6.69±0.20c 0.59±0.33a 0.55±0.37c 5.91±3.18a 6.34±2.88a 1.99±1.33a F 12.58±4.15b 6.65±0.22d 0.57±0.37a 0.87±1.25c 5.14±4.50b 5.96±4.21a 1.97±1.48a S 11.15±5.12c 6.77±0.13b 0.46±0.36a 1.15±1.20a 5.66±4.80a 6.60±4.90a 1.92±1.11a FS 7.60±6.01d 6.83±0.14a 0.51±0.55a 0.89±0.75b 3.90±3.30c 4.84±3.43b 1.78±0.96b 注:表中不同小写字母表示同一指标在不同CWs间的差异性(P<0.05) 表 3 出水水质指标平均去除率
Table 3. Average removal rate of water quality indices in effluent
% 处理 COD NO3−-N NH4+-N TN TP CK 91.93 97.60 75.22 86.63 60.35 F 93.15 96.21 78.30 87.42 60.57 S 93.93 94.99 76.15 86.08 61.83 FS 95.85 96.12 83.58 89.80 64.61 表 4 菖蒲不同部位重金属含量
Table 4. Enrichment of heavy metals in different parts of acorus calamus L.
处理 部位 Cu/(mg·kg−1) Zn/(mg·kg−1) Pb/(mg·kg−1) Cd/(mg·kg−1) Cr/(mg·kg−1) CK 地上 358.75±15.910 439.25±13.08 111.25±13.79 49.75±6.72 106.00±7.70 地下 1 281.00±216.38 1 673.25±375.83 813.75±269.76 370.50±70.71 729.00±8.49 F 地上 245.25±37.12 352.75±90.86 65.75±21.57 36.25±6.72 90.75±11.67 地下 744.75±19.45 1 147.00±41.01 460.00±50.91 348.75±1.06 540.00±22.63 S 地上 374.50±70.71 465.00±11.31 111.25±3.18 59.75±1.06 127.00±13.31 地下 944.75±270.47 1 203.50±142.84 435.50±82.02 276.00±57.89 491.25±19.45 FS 地上 326.50±14.14 390.00±16.97 106.25±2.48 50.75±1.77 116.25±2.48 地下 1 176.25±182.79 1 448.00±258.09 540.75±86.62 387.50±74.95 588.25±12.37 注:小写字母a、b表示同一装置某种重金属地上部分与地下部分的显著差异性(P<0.05) 表 5 不同基质中重金属的吸附量
Table 5. Heavy metals adsorption in different substrates
处理 Cu/(mg·kg−1) Zn/(mg·kg−1) Pb/(mg·kg−1) Cd/(mg·kg−1) Cr/(mg·kg−1) CK 113.38±3.63c 176.88±0.88d 53.50±2.75a 24.13±0.38b 46.00±0.25b F 167.63±0.88b 252.63±13.88c 62.13±0.13a 45.50±1.75a 70.88±5.38a S 204.38±4.38b 271.88±19.13b 82.13±3.38a 37.13±0.13a 72.35±2.15a FS 242.65±3.53a 285.13±16.75a 72.81±1.19a 48.33±2.05a 77.813±1.44a 注:不同小写字母表示同一种金属在不同基质CW间的差异性(P<0.05) -
[1] 付永江. 水平潜流人工湿地对Cd2+、Zn2+及营养物的处理性能研究[D]. 长沙: 长沙理工大学, 2019. [2] 付丰连. 物理化学法处理重金属废水的研究进展[J]. 广东化工, 2010, 37(4): 115-117. doi: 10.3969/j.issn.1007-1865.2010.04.056 [3] 沈懿静. 污水重金属去除研究进展[J]. 科学技术创新, 2018, 22(12): 20-22. doi: 10.3969/j.issn.1673-1328.2018.12.010 [4] 秦玉, 李慧莉, 尹晓雪, 等. 人工湿地抗生素与重金属的去除及其环境影响[J]. 环境保护科学, 2021, 47(6): 121-126. [5] YU G L, WANG G L, CHI T Y, et al. Enhanced removal of heavy metals and metalloids by constructed wetlands: A review of approaches and mechanisms[J]. Science of the Total Environment, 2022, 821(153516): 1-17. [6] 葛光环, 寇坤, 喻苏慧, 等. 人工湿地香蒲中重金属分布规律研究[J]. 湖北农业科学, 2021, 60(8): 141-145. [7] 葛光环, 陈爱侠, 寇坤, 等. 人工湿地基质和植物中重金属的分布规律研究[J]. 江西农业学报, 2020, 32(1): 115-119. [8] 罗倩, 林凤莲, 袁锋, 等. 人工湿地植物根际微生物对4种污水重金属元素的净化能力[J]. 西南农业学报, 2022, 35(1): 176-185. [9] 孙鹤洲, 刘骅, 田甜, 等. 人工湿地基质处理效果分析及发展趋势研究[J]. 绿色科技, 2022, 24(2): 156-158. doi: 10.3969/j.issn.1674-9944.2022.02.039 [10] YANG Y, ZHAO Y Q, LIU R B, et al. Global development of various emerged substrates utilized in constructed wetlands[J]. Bioresource Technology, 2018, 261(85): 441-452. [11] 陈银萍, 颉海帆, 柯昀琪, 等. 不同基质人工湿地去除重金属Cd的研究[J]. 环境污染与防治, 2019, 41(9): 999-1005. [12] 余关龙, 付永江, 彭海渊, 等. 水平潜流人工湿地对水中Cd2+、Zn2+及营养物的去除[J]. 环境工程, 2019, 37(10): 116-120. [13] ABEDI T, MOJIRI A. Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment[J]. Environmental Technology & Innovation, 2019, 16(100472): 1-11. [14] ZHUANG L L, LI M, LI Y, et al. The performance and mechanism of biochar-enhanced constructed wetland for wastewater treatment[J]. Journal of Water Process Engineering, 2022, 45(102522): 1-10. [15] DENG S J, CHEN J Q, CHANG J J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits[J]. Journal of Cleaner Production, 2021, 293(126156): 1-14. [16] 张家利, 张翠玲, 党瑞. 沸石在废水处理中的应用研究进展[J]. 环境科学与管理, 2013, 38(3): 75-79. doi: 10.3969/j.issn.1673-1212.2013.03.018 [17] SAARAFRAZ S, MOHAMMAD A T, MEGAT J, et al. Wastewater treatment using horizontal subsurface flow constructed wetland[J]. American Journal of Environmental Sciences, 2009, 5(1): 99-105. doi: 10.3844/ajessp.2009.99.105 [18] 张晓斌, 刘鹏, 李星. 不同基质人工湿地去除电镀重金属(Cr, Zn)的研究[J]. 工业安全与环保, 2016, 42(9): 83-85. doi: 10.3969/j.issn.1001-425X.2016.09.026 [19] 张翔凌. 不同基质对垂直流人工湿地处理效果及堵塞影响研究[D]. 武汉: 中国科学院大学(水生生物研究所), 2007. [20] CHEN J Q, DENG S J, JIA W, et al. Removal of multiple heavy metals from mining-impacted water by biochar-filled constructed wetlands: Adsorption and biotic removal routes[J]. Bioresource Technology, 2021, 331(125061): 1-9. [21] CHANG J J, PENG D L, DENG S J, et al. Efficient treatment of mercury (Ⅱ) - containing wastewater in aerated constructed wetland microcosms packed with biochar[J]. Chemosphere, 2022, 290(133302): 1-9. doi: 10.1016/j.chemosphere.2021.133302 [22] FENG L K, HE S F, ZHAO W X, et al. Can biochar addition improve the sustainability of intermittent aerated constructed wetlands for treating wastewater containing heavy metals?[J]. Chemical Engineering Journal, 2022, 444(133636): 1-11. doi: 10.1016/J.CEJ.2022.136636 [23] 国家环保总局. 水和废水监测分析方法(第四版)[M]. 北京: 中国环境科学出版社, 2002. [24] 杨剑虹, 王成林, 代亨林. 土壤农化分析与环境监测[M]. 北京: 中国大地出版社, 2008. [25] 杨波. 模拟人工湿地对废水中Zn的去除效果和机制研究[D]. 兰州: 兰州交通大学, 2018. [26] 陈文慧. 模拟人工湿地处理含镉无机废水的研究[D]. 南宁: 广西大学, 2008. [27] 王清华, 赵亚琦, 黄磊, 等. 间歇曝气生物炭湿地中污染物的去除特征及微生物种群结构[J]. 环境工程学报, 2021, 15(6): 2118-2125. doi: 10.12030/j.cjee.202101043 [28] 王泉, 吴献花, 吴珊, 等. 垂直潜流人工湿地中ORP、DO和pH与脱氮过程的关系[J]. 玉溪师范学院学报, 2011, 27(12): 24-27. doi: 10.3969/j.issn.1009-9506.2011.12.006 [29] 陈世杰. 赤铁矿和生物炭对潜流人工湿地污水处理效果及N2O和CH4排放的影响[D]. 重庆: 西南大学, 2020. [30] LEE S, MANIQUIZ R M C, KIM L H. Settling basin design in a constructed wetland using TSS removal efficiency and hydraulic retention time[J]. Journal of Environmental Sciences, 2014, 26(9): 1791-1796. doi: 10.1016/j.jes.2014.07.002 [31] 吕婉琳. 生物炭对潜流人工湿地N2O排放的影响机理研究[D]. 重庆: 西南大学, 2018. [32] 张杏锋, 冯健飞, 姚航, 等. 美洲商陆生物炭对Zn、Pb、Cd和Cu的吸附特性分析[J]. 环境工程, 2019, 37(8): 88-94. [33] ZHU T, JENSSEN P D, MAHLUMT T, et al. Phosphorus sorption and chemical characteristics of lightweight aggregates (LWA) - potential filter media in treatment wetlands[J]. Water Science and Technology, 1997, 35(5): 103-108. doi: 10.2166/wst.1997.0175 [34] 张璐, 杨忆新, 罗明生, 等. 天然沸石去除煤化工废水中氨氮的研究[J]. 工业水处理, 2018, 38(7): 46-49. doi: 10.11894/1005-829x.2018.38(7).046 [35] YANG L, CHANG H T, HUANG M N L. Nutrient removal in gravel- and soil-based wetland microcosms with and without vegetation[J]. Ecological Engineering, 2001, 18(1): 91-105. doi: 10.1016/S0925-8574(01)00068-4 [36] 徐祖信, 谢海林, 叶建锋, 等. 模拟煤灰渣垂直潜流人工湿地的除磷性能分析[J]. 环境污染与防治, 2007, 2007(4): 241-243. doi: 10.3969/j.issn.1001-3865.2007.04.001 [37] VOHLAC, KOIV M, BAVOR H J, et al. Filter materials for phosphorus removal from wastewater in treatment wetlands: A review[J]. Ecological Engineering, 2009, 37(1): 70-89. [38] SOCHACKI A, SURMACZ G J, FAURE O, et al. Polishing of synthetic electroplating wastewater in microcosm upflow constructed wetlands: Metals removal mechanisms[J]. Chemical Engineering Journal, 2014, 242(75): 43-52. [39] 左思敏, 荆肇乾, 陶梦妮, 等. 天然沸石和改性沸石在废水处理中的应用研究[J]. 应用化工, 2019, 48(5): 1136-1139. doi: 10.3969/j.issn.1671-3206.2019.05.035 [40] 李辉, 左金龙, 王军霞. 天然沸石及其改性对污水中磷的吸附[J]. 哈尔滨:哈尔滨商业大学学报(自然科学版), 2015, 31(3): 311-314. [41] 邹旭青, 郝庆菊, 赵茂森, 等. 铁矿石和生物炭添加对潜流人工湿地污水处理效果及温室气体排放的影响[J]. 环境工程学报, 2021, 15(2): 588-598. doi: 10.12030/j.cjee.202005025 [42] 赵仲婧, 郝庆菊, 张尧钰, 等. 铁碳微电解及沸石组合人工湿地的废水处理效果[J]. 环境科学, 2021, 42(6): 2875-2884. doi: 10.13227/j.hjkx.202010200 [43] 曹笑笑, 吕宪国, 张仲胜, 等. 人工湿地设计研究进展[J]. 湿地科学, 2013, 11(1): 121-128. doi: 10.3969/j.issn.1672-5948.2013.01.018 [44] 戴保琳. 利用农作物秸秆强化人工湿地对降雨径流中氮及重金属去除的研究[D]. 南京: 东南大学, 2015. [45] 宁可佳. 重金属在新型复合型人工湿地中的去除、迁移及累积规律[D]. 重庆: 重庆大学, 2011. [46] GAO Z M, Bancosz T J, Zhao Z, et al. Investigation of factors affecting adsorption of transition metals on oxidized carbon nanotubes[J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 357-365. [47] ALLENDE K L, MCCARTHY D T, FLETSHER T D. The influence of media type on removal of arsenic, iron and boron from acidic wastewater in horizontal flow wetland microcosms planted with Phragmites australis[J]. Chemical Engineering Journal, 2014, 246(35): 217-228. [48] ZHOU Y C, GU T, YI W, et al. The release mechanism of heavy metals from lab-scale vertical flow constructed wetlands treating road runoff[J]. Environ Sci Pollut Res Int, 2019, 26(16): 16588-16595. doi: 10.1007/s11356-019-05097-y [49] MEI H, LING Y Y, YAN D W, et al. Heavy metal adsorption with zeolites: The role of hierarchical pore architecture[J]. Chemical Engineering Journal, 2019, 359(363): 363-372. [50] MOSELY L M, WILLSON P, HAMILTON B, et al. The capacity of biochar made from common reeds to neutralise pH and remove dissolved metals in acid drainage[J]. Environmental Science and Pollution Research International, 2015, 22(19): 15113-15122. doi: 10.1007/s11356-015-4735-9 [51] NGUYEN T T, HUANG H, NGUYEN T A H, et al. Recycling clamshell as substrate in lab-scale constructed wetlands for heavy metal removal from simulated acid mine drainage[J]. Process Safety and Environmental Protection, 2022, 165(26): 950-958. [52] LIANG Y X, ZHU H, BANUELOS G, et al. Preliminary study on the dynamics of heavy metals in saline wastewater treated in constructed wetland mesocosms or microcosms filled with porous slag[J]. Environmental Science and Pollution Research International, 2019, 26(33): 33804-33815. doi: 10.1007/s11356-018-2486-0 [53] 李冰, 舒艳, 李科林, 等. 人工湿地宽叶香蒲对重金属的累积与机理[J]. 环境工程学报, 2016, 10(4): 2099-2108. doi: 10.12030/j.cjee.20160483 [54] 李庆华. 人工湿地植物重金属分布规律及富集性研究[D]. 西安: 长安大学, 2014. 期刊类型引用(1)
1. 张扬,于晓桐,唐旭东. 黑水虻幼虫处理人工湿地固体废弃物效果研究. 环境生态学. 2024(07): 129-134+142 .  百度学术
百度学术
其他类型引用(4)
-






 下载:
下载: