-
受控生态生保系统(controlled ecological life support system,CELSS)依据地球生态圈循环原理构建,在密闭空间实现食物、氧气和水等全部生保物资的持续再生供应,是目前公认的解决长期载人深空探测任务过程中人生命保障问题的有效途径[1-3]。CELSS系统中生活废水污染强度大,尿液和各类洗涤剂的浓度高,氨氮浓度高,碳氮比(C/N)低,如何高效低耗的完成生活废水的净化处理是系统水循环的重点和难点[4]。而通过微生物硝化反应将高含量的氮素转化成植物易于直接吸收利用的硝酸盐氮,是实现废水中氮素等矿物质营养元素的循环的有效途径[5-6]。微生物硝化反应是将氮素转化为硝酸盐氮的有效途径,pH会影响氨氧化菌(ammonia oxidizing bacteria,AOB)和亚硝酸盐氧化菌(nitrite-oxidizing bacteria,NOB)的生长和代谢,是生物硝化反应器效能的关键影响因素[7-9]。前期研究表明生物处理工艺能完成尿液废水、高强度生活废水的转化处理,并初步探究了pH对氮素转化性能的影响[10-11]。而针对CELSS系统中特征性生活废水的全程硝化工艺研究相对较少,尤其在pH对于硝化过程中碱度消耗的影响与减量化措施方面缺少系统性的研究。为了优化工艺运行条件和物质消耗,本研究在课题组前期构建BF-MBR(biofilm-membrane bioreactor)工艺的基础上,设计不同pH水平的长期硝化实验和短期硝化实验,考察好氧系统硝化性能与动力学,并评估硝化过程碱度的消耗情况,为CELSS系统中BF-MBR工艺运行参数选择提供依据,以求最大限度降低物质消耗的同时实现氮素的资源化处理。
-
本研究采用的前期构建的厌氧BF-MBR与好氧BF-MBR组合工艺(anBF-MBR+aBF-MBR)废水处理装置[12]。工艺流程如图1所示,主要由原水水箱、厌氧反应器、好氧反应器等组成。原水水箱、厌氧和好氧反应器(圆筒形)容积均为30 L,直径为300 mm,高450 mm,厌氧和好氧反应器中投放直径25 mm的轻质塑料环填料(有效比表面积>500 m2·m−3),采用外置式PVDF管式膜组件(尺寸6 mm×500 mm,内径30 nm)完成厌氧和好氧反应器的泥水分离,在好氧反应器内设传感器对溶解氧(dissolved oxygen,DO)和pH进行实时监测,并通过pH完成碱液投加和控制。此外,通过调节各水箱液位和出水流量调节水力停留时间(hydraulic retention time,HRT)。
实验采用1/5尿液强度的生活废水(废水中尿液的稀释倍数为5),根据前期CELSS集成实验中生活废水量和水质特征,采用新鲜尿液和各类洗涤剂配制而成[13],废水配方见表1。本研究新鲜尿液收集对象为某研究机构男性工作人员,收集时间为冬季(2021年12月—2022年1月),单次收集10 L于4 ℃低温存储后按需使用。
-
1) pH对好氧硝化性能及碱液消耗量的影响。根据前期研究结果,AOB的适宜生存pH为7.0~8.5,NOB为6.0~7.5[14],本文目的是实现氮素的全程硝化,更加侧重将pH控制在NOB的适宜范围。因此,本实验设置6.0~6.1、6.4~6.5、7.1~7.2 3个pH水平,研究pH对好氧反应器的硝化性能,并考察碱度的消耗情况,为系统pH控制水平优化提供依据。
实验期间通过投加KHCO3溶液补充碱度并调节好氧反应器内pH。实验前8 d为启动期,当出水中氮素含量稳定后,开始控制单个反应器中HRT为(2.5±0.1) d,开始在3个pH水平下的阶段运行实验,实验过程维持好氧反应器中DO大于3.0 mg·L−1。为了降低pH突变对系统性能的影响,在第26~29天设置pH=6.4~6.5的过渡期,第29天开始进入pH=6.0~6.1阶段。在运行期间,根据好氧系统所消耗碱液的量和处理水量进行统计,计算出不同pH条件下单位质量氮素转换消耗碱液的质量,进而评估碱度消耗情况。具体各运行阶段及进水水质如表2所示。因新鲜尿液的收集过程未对具体人员及其饮食结构做出严格限定,因此,实际收集的尿液会存在变化,这也是各阶段水质指标有小幅波动的主要原因。
2)不同pH水平硝化动力学研究。收集好氧反应器中活性污泥开展烧杯实验,考察pH短期效应对硝化过程的影响,并研究不同pH条件下的硝化反应动力学。具体方法为:取一定量的好氧反应器中层填料、混合液至4个烧杯中,静置沉淀排出上清液后得到泥膜混合物,加入模拟废水(以氯化铵为氮源、葡萄糖为碳源,氨氮和总有机碳(total organic carbon,TOC)质量浓度均为50 mg·L−1),调节各烧杯中的初始pH至设定范围,开始实验,实验过程中通过添加KHCO3溶液维持各烧杯的pH,同时记录碱液消耗量,各烧杯中溶解氧维持在2 mg·L−1以上,实时取样检测硝酸盐氮(NO3−-N)、亚硝酸盐氮(NO2−-N)、氨氮(NH4+-N)的质量浓度。
氨氧化过程和亚硝酸盐氧化过程的动力学参数可依据Monod方程建立的对质量浓度与时间之间的函数关系根据式(1)和式(2)进行拟合求得[15]。
式中:N、Y分别为底物氨氮和产物硝酸盐氮的质量浓度,mg·L−1;μmax、vmax分别为最大氨氧化速率和最大亚硝酸盐氧化速率,mg·(L·d)−1;K1、K2分别为氨氧化反应和亚硝酸盐氧化反应过程中半饱和常数,mg·L−1;m、n为常数。
单位污泥质量浓度单位时间内最大比氨氧化速率U1、单位氨氧化菌质量浓度单位时间内最大氨氧化速率U2、以及氨氧化菌最大比增长速率Umax动力学参数根据式(3)、式(4)和式(5)计算,同理可得出单位污泥质量浓度单位时间内最大比亚硝酸盐氧化速率V1、单位亚硝酸盐氧化菌质量浓度单位时间内亚硝酸盐氧化速率V2和亚硝酸盐氧化菌最大比增长速率Vmax。
式中:U1为单位污泥质量浓度单位时间内最大比氨氧化速率,g·(g·d)−1; U2为单位氨氧化菌质量浓度单位时间内最大氨氧化速率,g·(g·d)−1;Umax为氨氧化菌最大比增长速率,g·(g·d)−1;C1为体系中污泥质量浓度,2 000 mg·L−1,C2为AOB和NOB的质量浓度,分别为24 mg·L−1和14 mg·L−1;Y为产率系数,取0.1。
-
好氧反应器中pH和DO均采用工业级传感电极监测;TOC通过燃烧氧化非分散红外吸收法进行分析(SHIMADZU TOC-VCPH);氨氮(NH4+-N)含量通过阳离子色谱仪(Thermo Fishe Aquion,Dionox IonPac-CS12A阳离子色谱柱)分析;亚硝酸盐氮(NO2−-N)和硝酸盐氮(NO3−-N)含量通过阴离子色谱仪(DIONEX ICS-90,IonPac-AS14阴离子色谱柱)分析;氢氧根(OH−)、碳酸根(CO32-)和碳酸氢根(HCO3−)含量采用电位滴定法检测分析。总无机氮(total inorganic nitrogen,TIN)含量为氨氮、亚硝酸盐氮和硝酸盐氮含量之和;全程硝化率为好氧出水中硝态氮占总无机氮的百分比;硝化率为好氧出水中硝态氮和亚硝态氮之和占总无机氮的百分比;游离氨(free ammonia,FA)和游离亚硝酸(free nitrous acid,FNA)质量浓度参照DOWNING等研究给出的计算公式[16]。
-
图2反应了不同pH下好氧反应器氮素和TOC的转化情况。如图2(a)和图2(b)所示,好氧系统pH发生变化时,其出水中NO2−-N、NH4+-N质量浓度以及硝化率会出现先升后降而后趋于稳定的变化趋势,且变化幅度随pH的增加而增大。在pH=6.4~6.5的阶段,稳定时平均全程硝化率和硝化率分别为97.8%和100%。当pH=7.1~7.2时,前期NO2−-N迅速上升,全程硝化率最低降至60.5%,说明此时好氧系统中NOB活性受到抑制,这与杨宏等[17]的研究结果相似。经过约5 d后,NO2−-N氧化性能得以恢复,平均全程硝化率上升至93.9%。pH调至6.0~6.1时,出水NH4+-N有轻微波动而后趋于稳定,稳定后出水NH4+-N低于5 mg·L−1,NO2−-N基本为0,该阶段的全程硝化率最高达到99.3%。程振敏等[10]通过废水处理再利用系统(wastewater treatment and reuse system, WTRS)处理1/10尿液质量浓度的模拟CELSS系统生活废水,结果表明,pH维持在6.5~7.0时,出水NH4+-N质量浓度低于20 mg·L−1,NO2−-N低于2 mg·L−1,能够实现有机化合物的矿化和氮素的转化,与本实验结果近似。
图2(c)为不同pH下好氧出水中TOC变化情况。各阶段出水平均TOC质量浓度均低于20 mg·L−1,TOC去除率均达到97%以上,说明pH对好氧系统TOC的去除率无明显影响。这点与NIU等[18]研究不同pH下好氧颗粒污泥处理高盐废水的性能时所得结果一致。
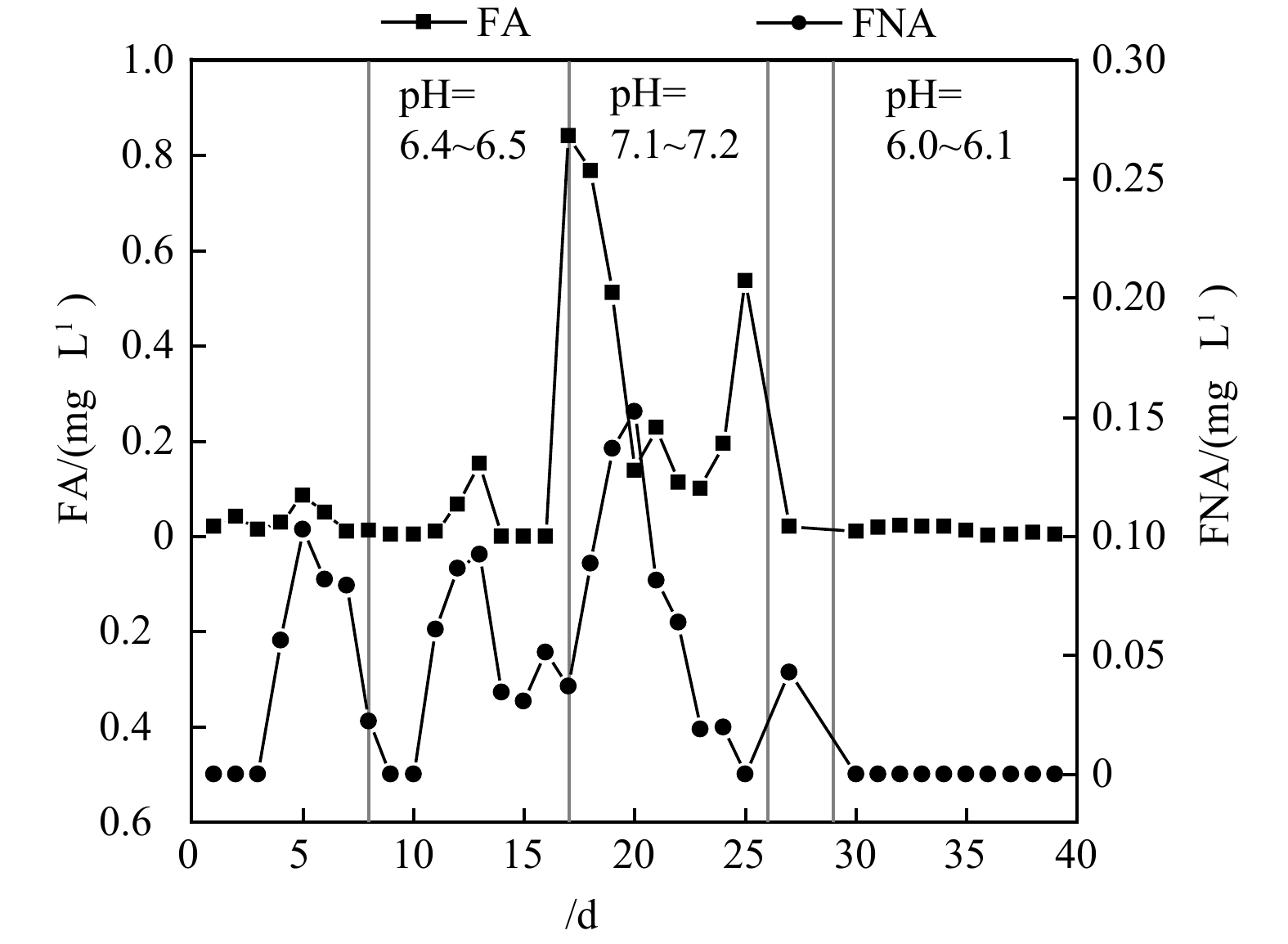
FA和FNA质量浓度与系统硝化性能密切相关,质量浓度到达一定水平时会抑制AOB和NOB的活性,且对这两类细菌的抑制程度不同[19]。ANTHONISEN等[20]研究表明发现,FA对NOB的抑制质量浓度为0.1~1.0 mg·L−1,对AOB的抑制质量浓度为10~150 mg·L−1。FNA对NOB的抑制质量浓度为0.011~0.07 mg·L−1,对AOB的抑制质量浓度为0.42~1.72 mg·L−1[21]。通过计算本文好氧反应器中的FA和FNA质量浓度,得出不同pH下好氧系统中FA、FNA质量浓度变化,如图3所示。
如图3所示,在pH=6.4~6.5阶段中期以及pH=7.1~7.2阶段前期,好氧系统中FA的质量浓度均短暂上升至0.110 mg·L−1,导致系统中NOB活性受到抑制,从而出现亚硝酸盐的短暂上升,但FNA的质量浓度并未达到形成抑制的水平。CIUDAD等[22]的研究也表明,pH的升高使FA质量浓度上升,进而抑制NOB活性,使亚硝酸盐积累。当pH下调至6.0~6.1时,好氧系统中FA和FNA质量浓度均很低,好氧系统硝化性能恢复良好,说明本实验水平的FA对NOB的抑制作用是可逆的。FAN等[8]开展的FA对NOB的抑制作用研究结果也表明,NOB能够适应FA的抑制,能在36.06~50.66 mg·L−1的高质量浓度条件下保持活性。
-
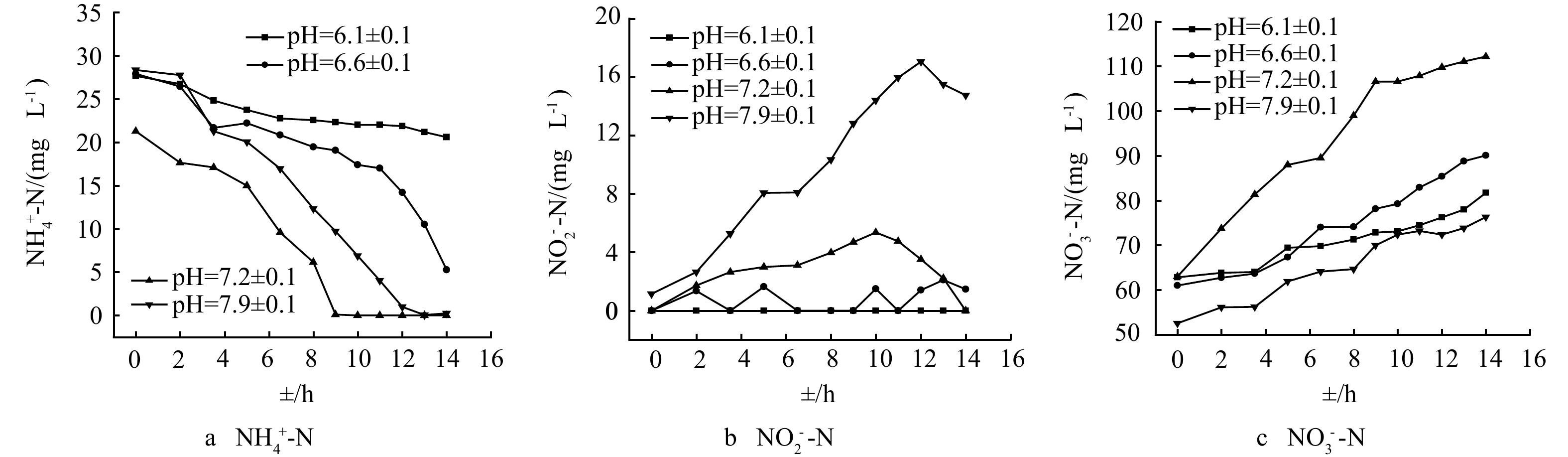
通过烧杯实验考察了不同pH条件下好氧反应器中活性污泥的硝化动力学,各pH体系中氮素质量浓度的变化如图4所示。由图4(a)可以看出,在不同pH的体系中初始氨氮质量浓度均低于废水起始质量浓度50 mg·L−1,说明活性污泥对氨氮起到一定的吸附作用,导致废水中氨氮质量浓度迅速降低[23]。随着反应时间的增加,在pH为7.2±0.1体系中最先将氨氮消耗完,其次为pH=7.9±0.1体系,而在2个酸性体系中氨转换速率最慢,初步说明碱性环境有利于氨氧化反应。这与WANG等[24]的研究结果相似,其氨化率在pH=7.5时达到最大。由图4(b)可知,碱性越强越易形成亚硝酸盐的积累,而所积累的亚硝酸盐在后期均会下降。ALBINA等[25]的研究中也得到了相似的变化趋势。其中pH=7.2±0.1的体系可在短时间内完成NO2−-N的氧化,这与图2(a)所示的母反应器中氮素变化趋势一致。由图4(c)可初步判断,pH=7.2±0.1体系的NO3−-N生成速率最快。由此说明NOB在弱碱性条件下活性较强。张昕等[26]的研究结果也表明,当pH=7.0时NOB的活性最高。
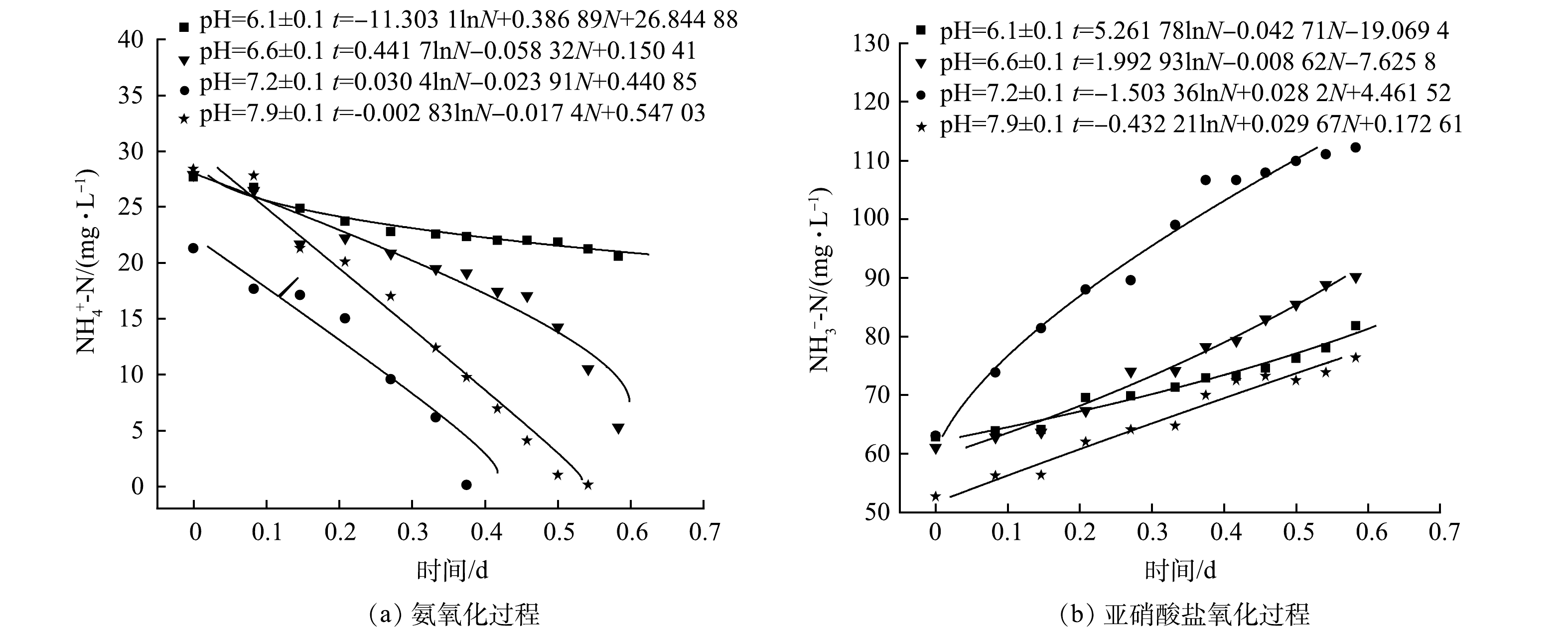
在生物硝化过程中,氨氧化速率与氨氮质量浓度的关系以及亚硝酸盐氧化速率与硝酸盐氮质量浓度的关系可用Monod方程来描述[27],利用式(1)、式(2)进行拟合,所得拟合结果如图5所示。根据式(3)、式(4)和式(5)计算不同pH下氨氧化和亚硝酸盐氧化过程的动力学参数,结果见表3和表4。由拟合结果可知,各pH体系中函数拟合度均可达0.96以上,说明Monod动力学方程能很好地描述本文好氧反应器中活性污泥的硝化过程。对氨氧化过程的计算结果表明,氨氧化速率随着pH的升高而增加,在碱性条件下各动力学参数值远大于酸性条件,表明碱性环境有利于提高AOB的活性并促进氨氧化过程。WANG等[28]的研究结果也得到了同样的趋势。由表4可知,NOB活性在pH为6.6±0.1时达到最高,此时NOB的最大比增长速率为0.829 d−1,最有利于实现全程硝化,该值与遇光禄等的研究结果相近[29]。
由此可见,本文好氧反应器中获得的AOB适宜pH为7.8~8.0,NOB最适宜pH为6.4~6.7;当系统要实现氮素的全程硝化过程,避免亚硝的积累,较优的pH条件为6.6±0.1;当系统要通过短程硝化反硝化完成脱氮,则较优的pH条件为7.9±0.1。
-
1)好氧反应器碱液消耗量。实验过程中首先采用KHCO3调节好氧反应器中的碱度,其理论耗碱量为14.29 g·g−1,碱度消耗为7.14 g·g−1。好氧反应器中KHCO3消耗量统计如表5所示。随着好氧反应器中pH的增大,单位质量氮素转换所消耗KHCO3的量也增加。pH为7.1~7.2时耗碱量最高,但仍远低于理论值,可能的原因为厌氧出水补充了好氧硝化所需部分碱度,同时也为好氧系统提供了良好的缓冲能力。经测试,厌氧出水pH均在9.05±0.96,出水中碳酸根为2.64 mol·L−1,碳酸氢根为43.92 mol·L−1。PAEPE等[30]通过微生物电解池-膜曝气生物膜反应器(microbial electrolysis cell-membrane-aerated biofilm reactor,MEC-MABR)处理源分离尿液的研究中也证实了这点。因在pH为6.0~6.1和6.4~6.5的条件下的碱液消耗量差别较小,又考虑到pH为6.4~6.5时更有利于硝化细菌生长和活性表达,因此,工艺运行较优pH应为6.4~6.5。此外,对好氧出水中的氢氧根、碳酸根和碳酸氢根进行检测,结果表明,好氧出水中含有6.24 mol·L−1碳酸氢根,说明好氧系统处理后的出水仍具备一定的缓冲能力。
2)短期效应实验碱液消耗量。在短期硝化实验中,同样对各pH体系中单位质量氮素转换所消耗的KHCO3的质量进行了统计。如表6所示,碱度的消耗随pH的增大而增大,且pH为7.9±0.1时的耗碱量超过了理论值。在pH为6.1±0.1、6.6±0.1的体系中耗碱量均较低且较为接近,这与好氧反应器实验结果一致。与好氧反应器长期运行KHCO3消耗情况比较可知,在不同pH体系中单位氮素转换所消耗KHCO3的量约为好氧反应器的2倍,进一步表明厌氧系统为后续的好氧系统提供了一定的碱度,节省了工艺运行物料的损耗。
3)不同类型碱液消耗情况。为进一步减少物料的损耗,在pH为6.4~6.5的条件下,分别采用KHCO3和KOH作为碱液,对比考察碱液投加量和好氧反应器的运行情况。根据统计结果可知,KHCO3作为碱液时的消耗量为7.02 g·g−1,当量碱度消耗(以CaCO3计)为3.51 g·g−1;KOH作为碱液时的消耗量为3.74 g·g−1,当量碱度消耗为3.34 g·g−1,KOH作为碱液时的消耗量远低于KHCO3。此外,在系统稳定运行期间,以KHCO3为碱液时好氧系统的硝化率为91.2%,以KOH为碱液时硝化率略有提升至94.6%,说明使用KOH作为碱液时不仅能够减少质量消耗,还能维持良好的硝化性能。
-
1)好氧生物反应系统能适应pH=6.0~7.2的变化,弱酸性条件下有利于实现氮素的全程硝化;系统中短时间内出现的FA抑制是可逆的,不会影响系统中NOB的活性。
2)从硝化动力学结果来看,pH=7.8~8.0时,系统中AOB活性最强,有利于实现短程硝化。pH=6.4~6.7时,NOB活性最强,有利于实现全程硝化。
3)氮素硝化过程所消耗碱液量随pH的上升而增加,综合考虑全程硝化性能和碱液减量化,好氧系统最佳pH为6.4~6.5,选用KOH补充硝化过程所需碱度。
pH对BF-MBR工艺处理高强度生活废水的影响
Effect of pH on the treatment of high strength domestic wastewater by BF-MBR process
-
摘要: 受控生态生保系统中生活废水污染强度大,生物转化后回用于植物培养是废水资源化的有效途径,但面临氮素稳定转化难、碱度消耗较大的问题。以BF-MBR工艺(生物膜耦合膜生物工艺,biofilm-membrane bioreactor)为研究对象,研究了不同pH条件下的好氧硝化性能及硝化动力学,并考察了硝化过程的碱度消耗情况。结果表明,在pH=6.0~7.2内,好氧生物反应器均能获得良好的氨氧化效果,而在pH=6.0~6.5的条件下更有利于全程硝化的维持;氨氧化速率随pH的增加而增大,而亚硝氧化速率在pH 6.6时达到最高;酸性条件下的碱度消耗量远低于碱性条件,而氢氧化钾作为碱液时的消耗量比碳酸氢钾低3.28 g·g-1。从物料损耗和工艺处理效果综合考虑,硝化系统中最佳的pH可调控在6.4~6.5,此时全程硝化率可达97.8%。以上研究结果可为受控生态生保系统中生活废水处理系统的设计和运行提供参考。Abstract: The pollution of domestic wastewater in the controlled ecological life support system (CELSS) is serious. The reuse for plant culture after biological transformation of this domestic wastewater is considered an effective way to resource utilization. However, there are still challenges such as the stability of nitrogen transformation and high alkalinity consumption in the process. In the present study, the biofilm-membrane bioreactor (BF-MBR) process was used to investigate the performance of aerobic nitrification and nitrification kinetics under different pH conditions, and the alkalinity consumption in the nitrification process. The results demonstrated that the aerobic bioreactor had a good ammonia oxidation capability in the range of pH=6.0~7.2, and pH=6.0~6.5 was more conducive to the maintenance of full nitrification. The rate of ammonia oxidation rose as pH increased, while the rate of nitrite oxidation reached its maximum at pH=6.6. The consumption of alkalinity under acidic conditions was much lower than that under alkaline conditions, and the consumption of KOH as alkali liquor was 3.28 g·g-1 lower than that of KHCO3. The optimal pH for the aerobic nitrification system could be adjusted to the range of 6.4~6.5 based on the comprehensive analysis of material consumption and treatment effect by the process, then the full nitrification rate could reach 97.8%. The findings of the study can provide a reference for the design and operation of domestic wastewater treatment system in CELSS.
-

-
表 1 1/5尿液强度生活废水配方用量
Table 1. The composition of domestic wastewater with 1/5 urine concentration
项目 品牌 配水用量 牙膏 冷酸灵 2 cm 洗发水 飘柔长效清爽去屑 2 mL 沐浴露 舒肤佳 2 mL 洗衣液 蓝月亮深层洁净护理 2 mL 洗面奶 曼秀雷敦控油抗痘洁面乳(男士) 2 mL 洗手液 蓝月亮抑菌洗手液 3 mL 新鲜尿液 新鲜采集 4 L 自来水 约16 L 注:生活废水每次配水总量为20 L。 表 2 废水进水水质表
Table 2. The water quality of influent wastewater
阶段 时间段/d TOC/(mg·L−1) TN/(mg·L−1) NH4+-N/(mg·L−1) C/N 启动期 1~8 744±10 815±150 490±200 0.94 pH=6.4~6.5 9~16 831±15 865±50 519±300 0.96 pH=7.1~7.2 17~25 892±50 875±50 524±400 0.95 过渡期 26~28 831±15 865±50 519±300 0.96 pH=6.0~6.1 29~38 954±10 1089±50 714±200 0.88 表 3 不同pH体系中氨氧化过程中动力学参数值
Table 3. Kinetic parameter values of ammonia oxidation process in different pHs
pH 拟合度R12 μmax/(mg·(L·d)−1) K1/(mg·L−1) U1/(g·(g·d)−1) U2/(g·(g·d)−1) Umax/d−1 6.1±0.1 0.965 2.58 29.22 0.0013 0.108 0.011 6.6±0.1 0.982 17.15 7.57 0.0086 0.714 0.071 7.2±0.1 0.968 41.82 1.27 0.0209 1.743 0.174 7.9±0.1 0.966 57.47 0.16 0.0287 2.395 0.239 表 4 不同pH体系中亚硝酸盐氧化过程中动力学参数值
Table 4. Kinetic parameter values of nitrite oxidation process in different pHs
pH 拟合度R22 vmax/(mg·(L·d)−1) K2/(mg·L−1) V1/(g·(g·d)−1) V2/(g·(g·d)−1) Vmax/d−1 6.1±0.1 0.966 23.41 123.20 0.0117 1.672 0.167 6.6±0.1 0.958 116.01 231.20 0.0580 8.286 0.829 7.2±0.1 0.962 35.46 53.31 0.0177 2.533 0.253 7.9±0.1 0.982 33.70 14.57 0.0169 2.407 0.241 表 5 好氧反应器中不同pH下的耗碱量
Table 5. Alkali consumption in aBF-MBR at different pHs
pH KHCO3消耗量/(g·g−1) 当量碱度消耗(以CaCO3计)/(g·g−1) 6.4~6.5 5.65 2.82 7.1~7.2 7.44 3.72 6.0~6.1 5.59 2.80 表 6 短期效应实验中不同pH体系的耗碱量
Table 6. Alkali consumption in short-term effect test at different pHs
pH KHCO3消耗量/(g·g−1) 当量碱度消耗(以CaCO3计)/(g·g−1) 6.1±0.1 10.58 5.29 6.6±0.1 11.46 5.73 7.2±0.1 14.23 7.12 7.9±0.1 22.78 11.39 -
[1] LI T, ZHANG L, AI W, et al. A modified MBR system with post advanced purification for domestic water supply system in 180-day CELSS: Construction, pollutant removal and water allocation[J]. Journal of Environmental Management 2018, 222: 37-43. [2] GUO S S, MAO R X, ZHANG L L, et al. Progress and prospect of research on controlled ecological life support technique[J]. Reach, 2017, 6: 1-10. doi: 10.1016/j.reach.2017.06.002 [3] 张良长, 李婷, 余青霓, 等. 4人180天集成实验环控生保系统设计及运行概况[J]. 航天医学与医学工程, 2018, 31(2): 273-281. [4] ZHANG L, LI T, AI W, et al. Water management in a controlled ecological life support system during a 4-person-180-day integrated experiment: Configuration and performance[J]. Science of the Total Environment, 2019, 651: 2080-2086. doi: 10.1016/j.scitotenv.2018.10.080 [5] ZABEL P, BORNEMANN G, TAJMAR M, et al. Yield of dwarf tomatoes grown with a nutrient solution based on recycled synthetic urine[J]. Life Sciences in Space Research, 2019, 20: 62-71. doi: 10.1016/j.lssr.2019.01.001 [6] DENG S, XIE B, LIU H. The recycle of water and nitrogen from urine in bioregenerative life support system[J]. Acta Astronautica, 2016, 123: 86-90. doi: 10.1016/j.actaastro.2016.03.007 [7] HAGOPIAN D S, RILEY J G. A closer look at the bacteriology of nitrification[J]. Aquacultural Engineering, 1998, 18: 223-244. doi: 10.1016/S0144-8609(98)00032-6 [8] ZHANG F, YANG H, WANG J, et al. Effect of free ammonia inhibition on NOB activity in high nitrifying performance of sludge[J]. RSC Advances, 2018, 8(56): 31987-31995. doi: 10.1039/C8RA06198J [9] SEUNTJENS D, HAN M, KERCKHOF F M, et al. Pinpointing wastewater and process parameters controlling the AOB to NOB activity ratio in sewage treatment plants[J]. Water Research, 2018, 138: 37-46. doi: 10.1016/j.watres.2017.11.044 [10] CHENG Z, WEI Y, GAO M, et al. Development of a combined anaerobic and aerobic membrane bioreactor for wastewater treatment and reclamation in terrestrial-based Controlled Ecological Life Support System[J]. Water Science & Technology:Water Supply, 2018, 19(3): 718-724. [11] OOSTERHUIS M, LOOSDRECHT V, M C M. Nitrification of urine for H2S control in pressure sewers[J]. Water Practice and Technology, 2009, 4(3):WPT2009059. [12] 王优, 汪形艳, 张良长, 等. 两级MBfR工艺处理高强度生活废水能力研究[J]. 化工学报, 2020, 71(5): 2363-2372. [13] VEROSTKO C E, CARRIER C, GB TECH I. Ersatz wastewater formulations for testing water recovery systems[J]. Sae Technical Papers, 2004, 113: 1008-1024. [14] ALLEMAN J E. Elevated nitrite occurrence in biological wastewater treatment systems[J]. Water Science and Technology, 1985, 17(2/3): 409-419. doi: 10.2166/wst.1985.0147 [15] DOWNING A L, PAINTER H A, KNOWLES G. Nitrification in the activated sludge process[J]. Journal of the Institute of Sewage Purification, 1964: 130-153. [16] PENG L, QIU H, LI S, et al. The mitigation effect of free ammonia and free nitrous acid on nitrous oxide production from the full-nitrification and partial-nitritation systems[J]. Bioresource Technology, 2022: 128564. [17] 杨宏, 姚仁达. pH和硝化细菌浓度对氨氮氧化速率的影响[J]. 环境工程学报, 2017, 11(5): 2660-2665. doi: 10.12030/j.cjee.201512155 [18] NIU X, HAN X, JIN Y, et al. Aerobic granular sludge treating hypersaline wastewater: Impact of pH on granulation and long-term operation at different organic loading rates[J]. Journal Environmental Management, 2023, 330: 117164. [19] LIU Y, NGO H H, GUO W, et al. The roles of free ammonia (FA) in biological wastewater treatment processes: A review[J]. Environment International, 2019, 123: 10-19. doi: 10.1016/j.envint.2018.11.039 [20] ANTHONISEN A, LOEHR R, PRAKASAM T, et al. Inhibition of nitrification by ammonia and nitrous acid[J]. Water Pollution Control Federation, 1976, 48(5): 835-852. [21] ZHOU Y, OEHMEN A, LIM M, et al. The role of nitrite and free nitrous acid (FNA) in wastewater treatment plants[J]. Water Research, 2011, 45(15): 4672-4682. doi: 10.1016/j.watres.2011.06.025 [22] CIUDAD G, GONZALEZ R, BORNHARDT C, et al. Modes of operation and pH control as enhancement factors for partial nitrification with oxygen transport limitation[J]. Water Res, 2007, 41(20): 4621-4629. doi: 10.1016/j.watres.2007.06.036 [23] 高兰, 钟振兴, 艾庆华, 等. CIBR污泥对生活污水中氨氮的吸附性能分析[J]. 环境工程, 2019, 37(8): 66-69. doi: 10.13205/j.hjgc.201908012 [24] WANG L, ZHENG P, ABBAS G, et al. Enrichment and characterization of acid-tolerant nitrifying sludge[J]. Journal of Environmental Management, 2016, 184: 196-203. [25] ALBINA P, DURBAN N, BERTRON A, et al. Nitrate and nitrite bacterial reduction at alkaline pH and high nitrate concentrations, comparison of acetate versus dihydrogen as electron donors[J]. Journal Environmental Management, 2021, 280: 111859. [26] 张昕, 吴长峰, 于雪, 等. pH值对亚硝酸盐氧化菌动力学及功能基因的影响[J]. 中国环境科学, 2020, 40(4): 1537-1544. doi: 10.3969/j.issn.1000-6923.2020.04.019 [27] DOWNING L S, NERENBERG R. Total nitrogen removal in a hybrid, membrane-aerated activated sludge process[J]. water research, 2008, 42: 3697-3708. doi: 10.1016/j.watres.2008.06.006 [28] WANG Z, MENG Y, ZHU-BARKER X, et al. Responses of nitrification and ammonia oxidizers to a range of background and adjusted pH in purple soils[J]. Geoderma, 2019, 334: 9-14. doi: 10.1016/j.geoderma.2018.07.038 [29] 遇光禄, 喻立军, 唐颖栋. pH对高浓度氨氮短程硝化抑制动力学的影响[J]. 环境污染与防治, 2013, 35(6): 65-68. doi: 10.3969/j.issn.1001-3865.2013.06.013 [30] PAEPE J D, PAEPE K D, GòDIA F, et al. Bio-electrochemical COD removal for energy-efficient, maximum and robust nitrogen recovery from urine through membrane aerated nitrification[J]. Water Research, 2020, 185: 116-223. -




 下载:
下载: