-
挥发性有机物 (volatile organic compounds,VOCs) 是近地面O3的主要反应物之一。部分VOCs的化学性质活跃,进入大气后可能会导致光化学烟雾的产生[1-3],生成二次污染物,对大气质量环境和人类生产活动产生严重危害[4-5]。VOCs主要来源于汽车尾气排放、工业排放源、煤炭及生物燃料燃烧、柴汽油或天然气泄漏与挥发等[6-9]。常见VOCs控制技术主要有破坏性消除法和回收法[10-12]。破坏性消除法包括生物降解[13-15]、焚烧[16-17]、催化氧化[18-20] 和等离子体[21-22] 等;回收法包括吸附[23-25]、吸收[26-27] 和冷凝[28-30] 等。在回收方法中,吸附技术被认为是最有效的方法之一,主要是由于其灵活性、一次投资成本低、耗能低、净化效率高和资源可回收,故在工程中被广泛应用[31-33]。
吸附法包含了活性炭对废气中污染物的吸附过程,也包含了活性炭的脱附再生过程[34]。活性炭脱附是指通过能量或物质的引入,达到减弱活性炭与吸附物质之间作用力的目的,使得吸附组分分子获得一定的能量后脱离固体表面而解吸出来[35]。活性炭脱附VOCs方法有变温脱附法、变压脱附法和微波再生法等。变温脱附法具有再生时间短、效率高、无选择性等优点,故在实际工程中被广泛应用。常见的变温脱附法采用水蒸气脱附再生、热氮气脱附再生等方式[36]。
变温脱附工艺在实际工程的设计中,需要参考的评价因子较多。常见评价因子有吸附剂的脱附率及脱附气体浓缩比等。荀志萌等[37]在研究大风量低浓度VOCs气体二次吸附浓缩选取净化技术开发时,选取能源节约率和脱附浓缩比作为变温脱附VOCs的评价因子。李照海等[38]在研究活性炭和沸石分子筛处理非稳定排放VOCs气体的性能时,选取脱附浓缩比、脱附量和再生单位质量二甲苯所需能耗作为变温脱附VOCs的评价因子。大风量、低浓度的排放通过旋转浓缩后处理的运行费用低,需考虑浓缩比;中高浓度的排放一般不采用旋转浓缩,主要考虑脱附率和能耗比等因素[39]。
本研究以甲苯为VOCs类代表性的目标污染物,搭建实验装置以模拟活性炭吸附脱附处理VOCs工艺中的脱附阶段,通过常见评价因子探讨不同脱附工况下气体对各床层的脱附情况,进一步提高现有脱附工艺的能量利用效率,提高大风量、低浓度工况下的浓缩比,以及各工况下的脱附率,降低各工况下的能耗比,以期得到最佳脱附工艺条件,并降低活性炭吸附净化工艺的运行维护成本,促进资源回收,为变温脱附工程化应用的低碳化技术优化提供参考。
-
选择市售的CTC 90煤制柱状活性炭作为吸附剂,直径为4 mm,其四氯化碳吸附值为92.15%。选择市售的甲苯 (分析纯,体积分数≥99.5%) 作为吸附质。
-
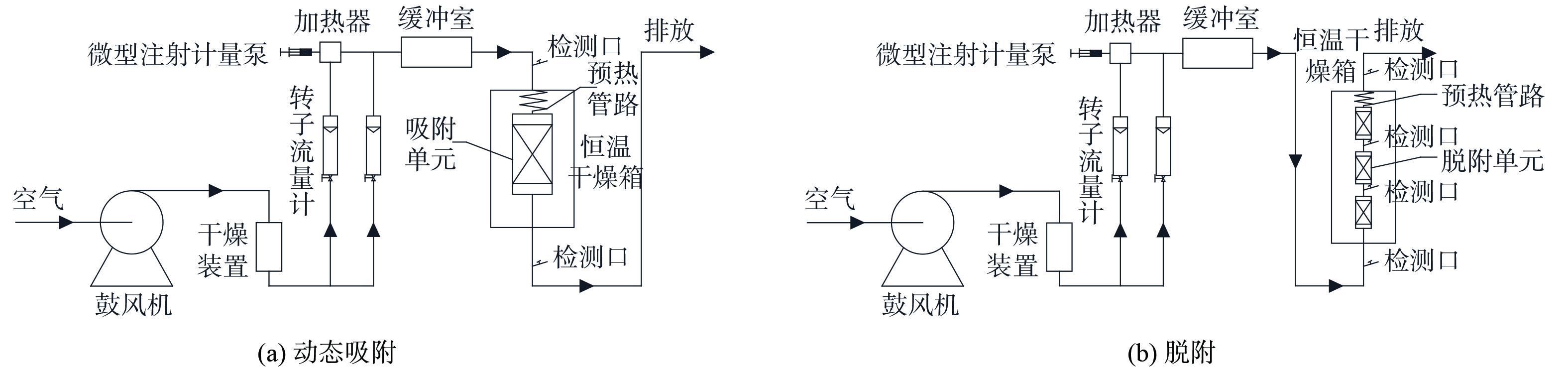
1) 吸附实验装置。采用动态吸附法,测定出甲苯在活性炭吸附床上的吸附平衡等温线,实验装置如图1 (a) 所示。该装置以实验室环境空气为气源,由鼓风机向其内部送入空气。在硅胶干燥装置中将空气中的水蒸气去除后,通过数显质量流量计的控制,按计算好的比例分成两路。一路气体进入设定好浓度的气体发生装置,作为挥发后甲苯的载气,与另一路稀释气体在气体缓冲室中进行充分的混合后进入吸附系统。混合后的甲苯气体先经过恒温干燥箱中的气体预热管路达到实验温度,然后进入填满活性炭的吸附柱中进行吸附实验。
2) 脱附实验装置。使用脱附装置进行实验,观察甲苯气体在活性炭层的脱附过程,装置如图1 (b) 所示。使用活性炭吸附质量浓度为15 g·m−3的甲苯气体至饱和,使用干燥且洁净的空气脱附饱和活性炭床。选择上流模式作为进气模式,将活性炭床填充至30 cm高度。脱附单元为3个柱长为100 mm、内径为40 mm的石英玻璃柱。在不同的工况下脱附2 h,用Smart FID对每个检测口连续监测。在实验结束后,取出脱附后的活性炭,用天平进行称量。
-
本研究的代表性VOCs物质选取甲苯为单一检测对象。使用德国Ersatec公司的便携式总烃分析仪中的氢火焰离子化检测器 (flame ionization detector,FID) 对甲苯的浓度进行定量分析。通过定期制作甲苯的标准曲线,将体积分数折算成对应的质量浓度。
-
根据本研究的要求,进气质量浓度设置为5~50 g·m−3,温度设置为25~180 ℃。测定不同温度和质量浓度下甲苯在活性炭上的饱和吸附容量,并采用重量法验证吸附等温线。通过该装置获取吸附饱和的活性炭。吸附后的气体经过检测口进入检测系统,便携式总烃分析仪通过连续采样来实时测定甲苯的出口质量浓度。当便携式总烃分析仪检测的甲苯出口质量浓度与由气体发生装置发生的甲苯入口质量浓度一致时,吸附柱的活性炭达到吸附平衡。
-
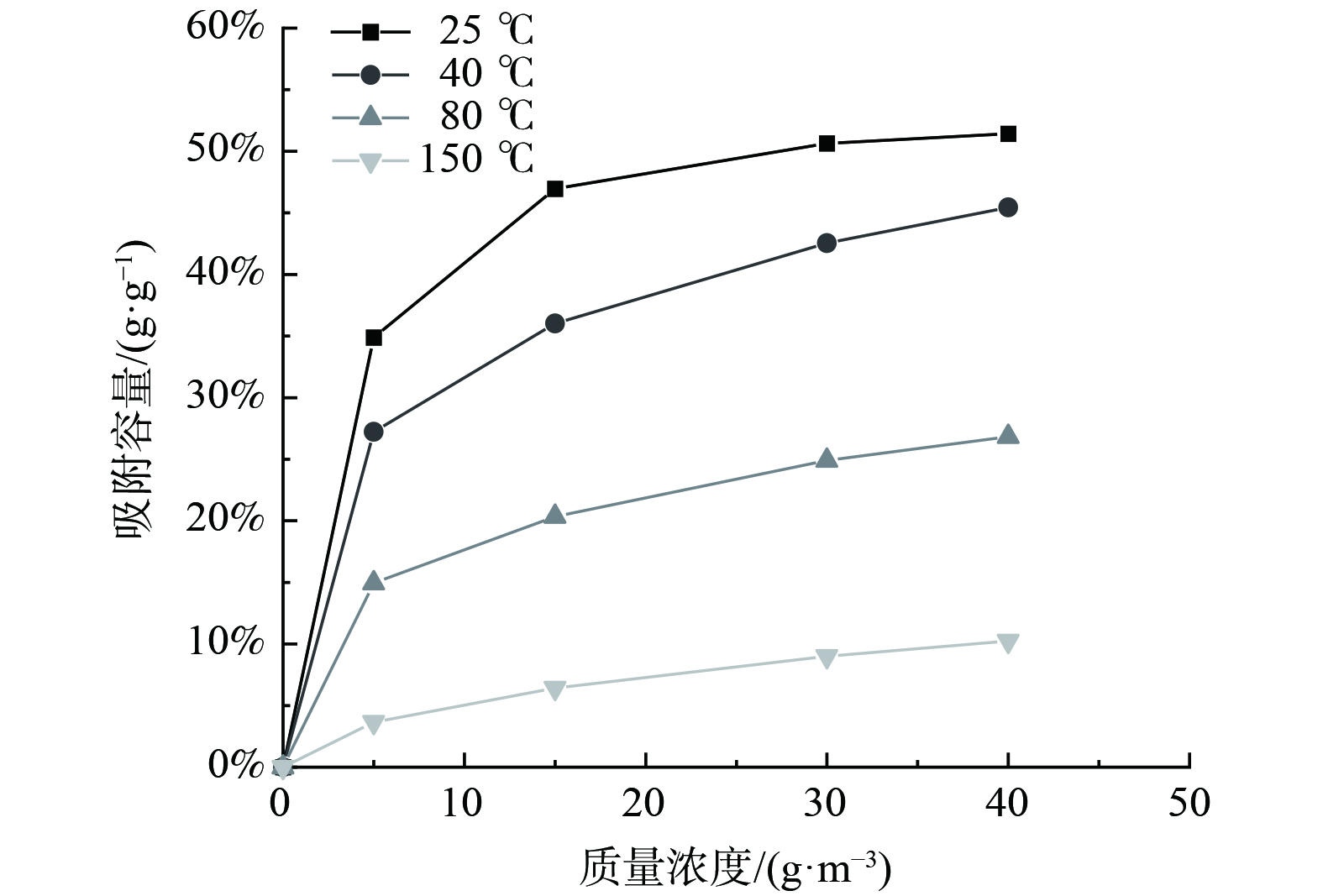
在不同实验温度下,活性炭对甲苯的吸附等温曲线如图2所示。该等温曲线符合单分子层吸附的第I类吸附等温线[40]。吸附容量为单位质量吸附剂达到最大吸附量时,所吸附吸附质的质量。在温度不变的条件下,随着甲苯质量浓度的上升,甲苯的吸附容量呈上升趋势。而甲苯质量浓度不变时,随着温度的上升,甲苯吸附容量呈下降趋势,故该过程为放热过程。
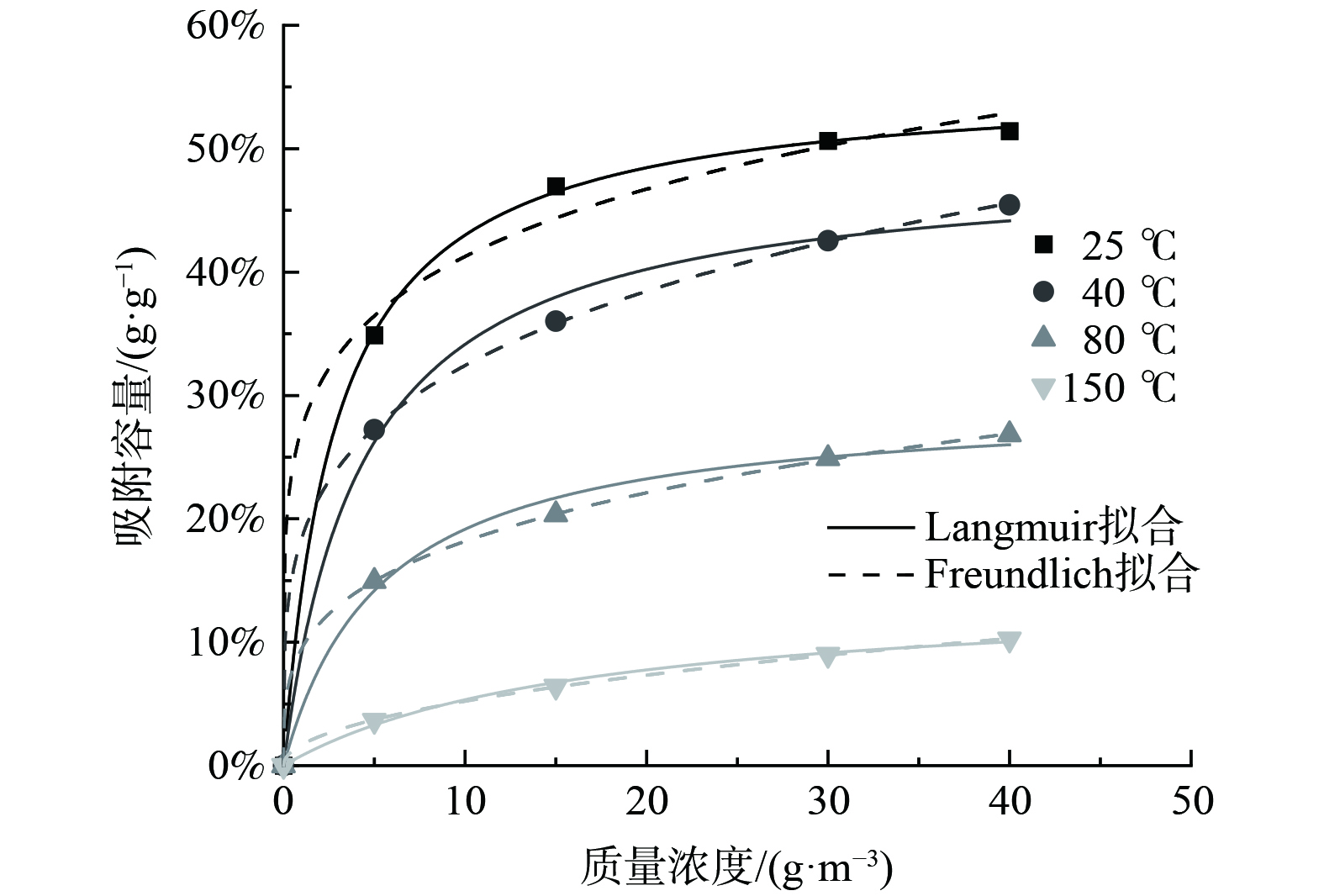
图2反映了活性炭对甲苯吸附的吸附行为,并较好地拟合了Freundlich和Langmuir吸附等温方程。将CTC 90活性炭对甲苯的吸附等温线进行拟合,结果如表1所示,拟合曲线如图3所示。b为Langmuir方程的经验吸附平衡常数,其大小表征了气体分子在固体表面上被吸附能力的强弱。b随温度的升高而下降,这与实验中随着温度的升高,活性炭对甲苯吸附容量下降的结论相符。Freundlich方程中的k为吸附剂和吸附质特性及温度有关的常数,n为温度相关常数,两者大小皆可表征吸附剂对吸附质的吸附能力,这2个参数数值也随温度的升高而下降。活性炭吸附甲苯的Freundlich吸附等温方程的R2基本高于Langmuir等温方程,且更加稳定,基本都在0.99以上,故CTC 90活性炭对甲苯的吸附用Freundlich等温方程拟合效果更好。甲苯在活性炭上的吸附等温线是研究变温脱附的基础。通过吸附等温线,及Langmuir和Freundlich拟合方程计算可知,低温吸附与中高温脱附条件下对应的平衡吸附容量的变化可实现活性炭的脱附,两者的差值与实际甲苯的脱附量相近,该差值为理论的甲苯脱附量。
-
结合活性炭吸附前后的重量法进行分析,甲苯脱附率按公式 (1)计算。
式中:x1为活性炭的甲苯脱附率;m0为预处理后未吸附的活性炭质量,g;mt为t时刻脱附实验的活性炭质量,g;m1为吸附后未脱附的活性炭质量,g。
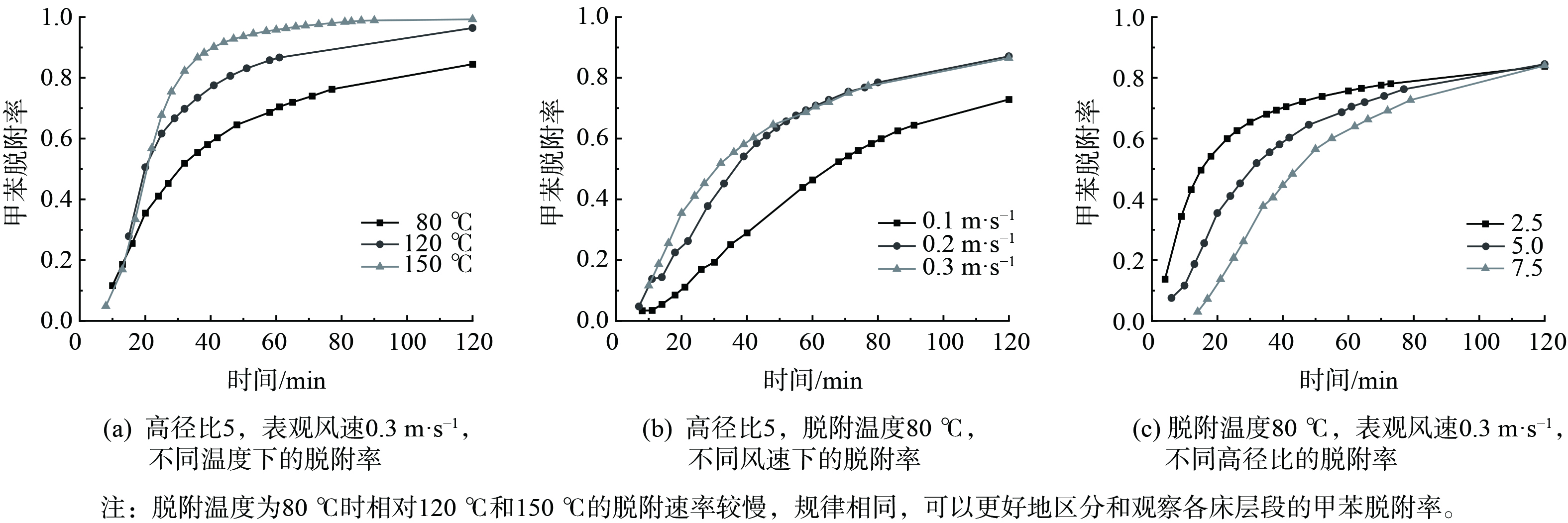
将不同工况下不同高径比在不同时刻的脱附率进行计算,结果如图4所示。分析120 min时各工况下的甲苯脱附率,可得到在中高温 (80~150 ℃) 条件下,3个床层在120 min时的脱附量基本相同。这说明此时的脱附接近结束。在表观风速相同时,随着脱附温度的增加,甲苯的脱附率也随之增加。同时,在脱附温度相同时,随着表观风速的增加,甲苯脱附率也随之增加。当脱附温度为150 ℃,表观风速为0.3 m·s−1时,甲苯脱附率最高,为97.7%。
结果表明,高径比为2.5时的甲苯脱附率到达速度平缓的拐点时间最短,且到脱附实验结束时的脱附率一直最高。当高径比为7.5时到达拐点时间最慢,且到脱附实验结束时的脱附率一直最低。从各床层的脱附情况可见,在活性炭脱附时,每层都在进行脱附,然而第2层和第3层炭床受到第1层炭床脱附出的高质量浓度甲苯的影响,同时又在进行吸附作用。当第1层炭床的脱附浓度快速下降后,第2层炭床不再受到第一层炭床脱附的影响。
当脱附温度为80 ℃、脱附率达60%时,脱附率的上升速度显著降低,脱附率开始渐渐平稳。而当脱附温度为120 ℃时,脱附率达80%;当脱附温度为150 ℃时,脱附率达84%。将这些到达一定脱附率后上升速度显著降低、脱附率渐渐平稳的点作为拐点。在相同的脱附温度下,随着表观风速的增加,脱附率到达拐点的时间越短。在相同的表观风速条件下,随着脱附温度增加,脱附率到达拐点的时间也越短。甲苯脱附率的研究规律基本符合同类报道[40-43]。
-
甲苯浓缩比是脱附实验出口累计的平均质量浓度与吸附时进口质量浓度的比例[37-39]。甲苯浓缩比越高,浓缩倍数越大,低质量浓度、大风量的VOCs变为高质量浓度、低风量后的处理成本减少。甲苯浓缩比按式 (2) 计算。
式中:x2为活性炭的甲苯浓缩比;m1为吸附后未脱附的活性炭质量,g ;c0为吸附实验中吸附甲苯的质量浓度,为15 g·m−3;mt为t时刻脱附实验的活性炭质量,g;Qt为t时通入的风量,m3。
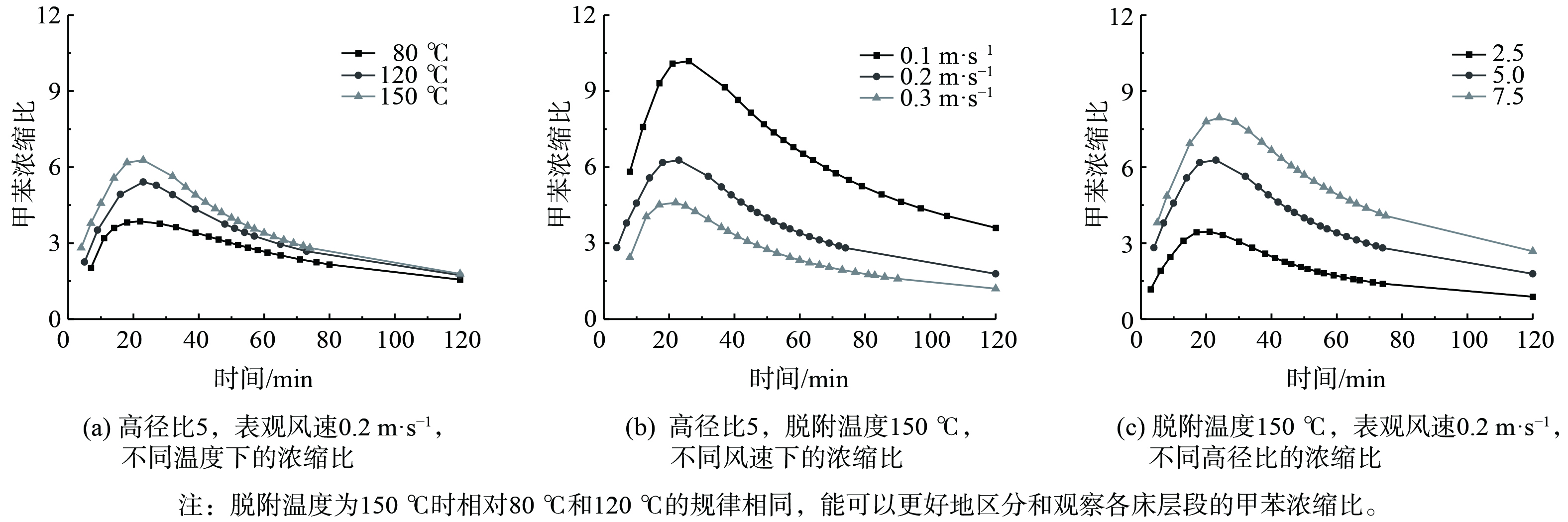
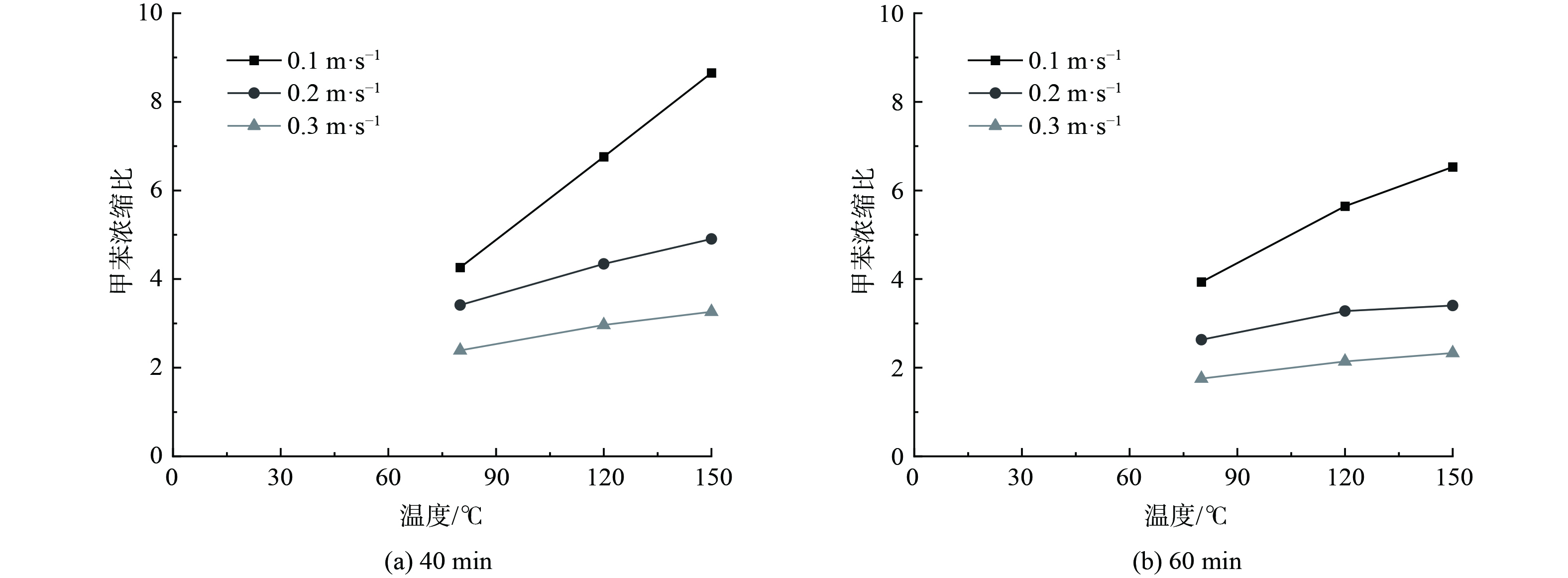
计算分析各工况下的甲苯浓缩比,结果如图5所示。在实验开始时,各床层的甲苯浓缩比先快速上升到最高点,然后开始缓慢下降。同时,高径比越大,甲苯的浓缩比越大,到达最高点的时间越慢。在相同脱附温度下,随着表观风速的增加,甲苯浓缩比到达最高点的时间越短,且浓缩比越低。同时,在相同的表观风速条件下,随着脱附温度增加,甲苯浓缩比越高。当脱附温度150 ℃,表观风速0.1 m·s−1时,甲苯浓缩比最高为10.185。
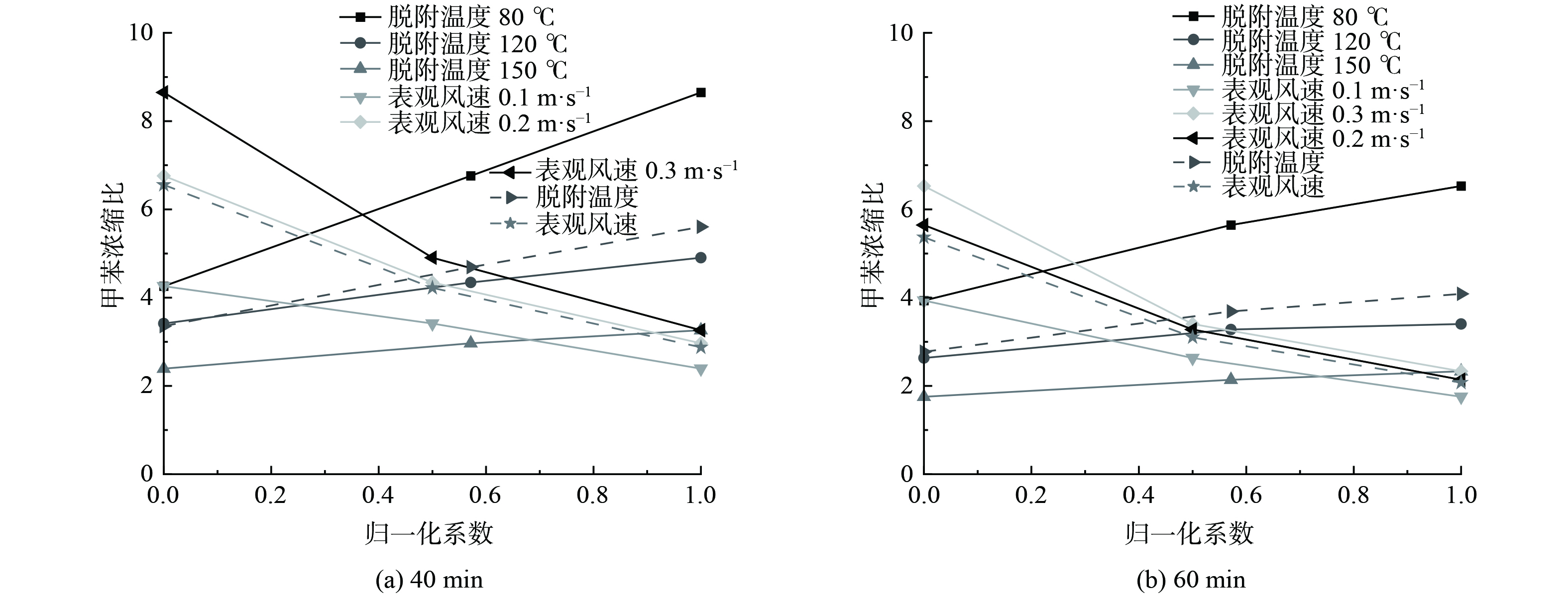
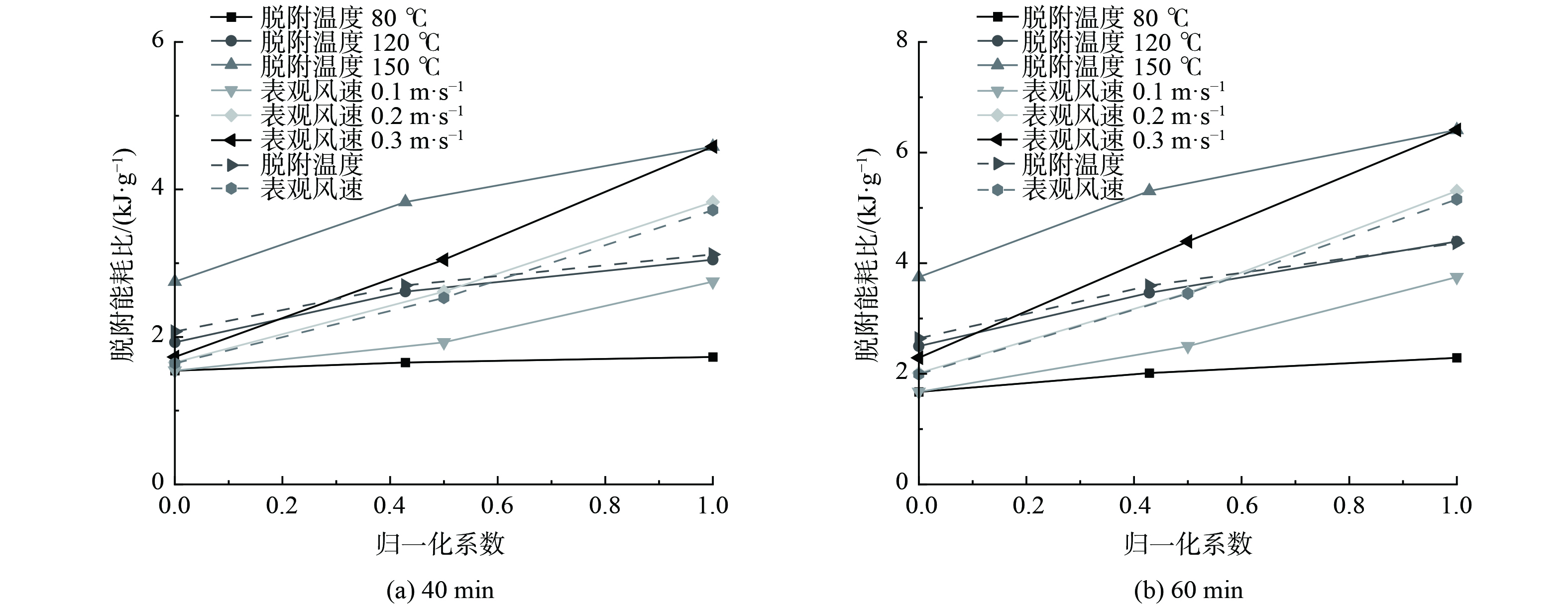
将不同实验工况下高径比为5的40 min和60 min的浓缩比进行分析,结果如图6所示。为使原始客观指标数据进行预处理以消除数据间数量级差异,将脱附温度与表观风速分别进行线性函数归一化处理[44],结果作为横坐标,将40 min和60 min时的浓缩比作为纵坐标,结果如图7所示。
图6表明,脱附实验进行到40 min和60 min时,甲苯浓缩比的规律相同,均随脱附温度的增加和表观风速的减小而增加。当脱附温度为150 ℃,表观风速为0.1 m·s−1时,40 min和60 min的甲苯浓缩比最高,分别为8.651 0和6.529 4。图7表明,不同表观风速归一化处理40 min时刻浓缩比和60 min时刻浓缩比的斜率总体上大于不同脱附温度归一化处理的斜率。这说明在整个脱附的过程中,表观风速是浓缩比的主要控制因素。当表观风速越大、脱附温度越低,浓缩比受到的影响越大。
将不同脱附温度下低风速 (表观风速为0.1 m·s−1) 、高径比 (高径比为2.5) 的浓缩比与高风速 (表观风速为0.3 m·s−1) 、高径比 (高径比为7.5) 的浓缩比进行比较分析,结果如图8所示。在中温 (80~120 ℃) 条件下,低风速、高径比的甲苯浓缩比小于高风速、高径比;在高温 (120~150 ℃) 条件下,低风速、高径比的甲苯浓缩比仅在峰值段大于高风速、高径比,其他时段基本一样。
-
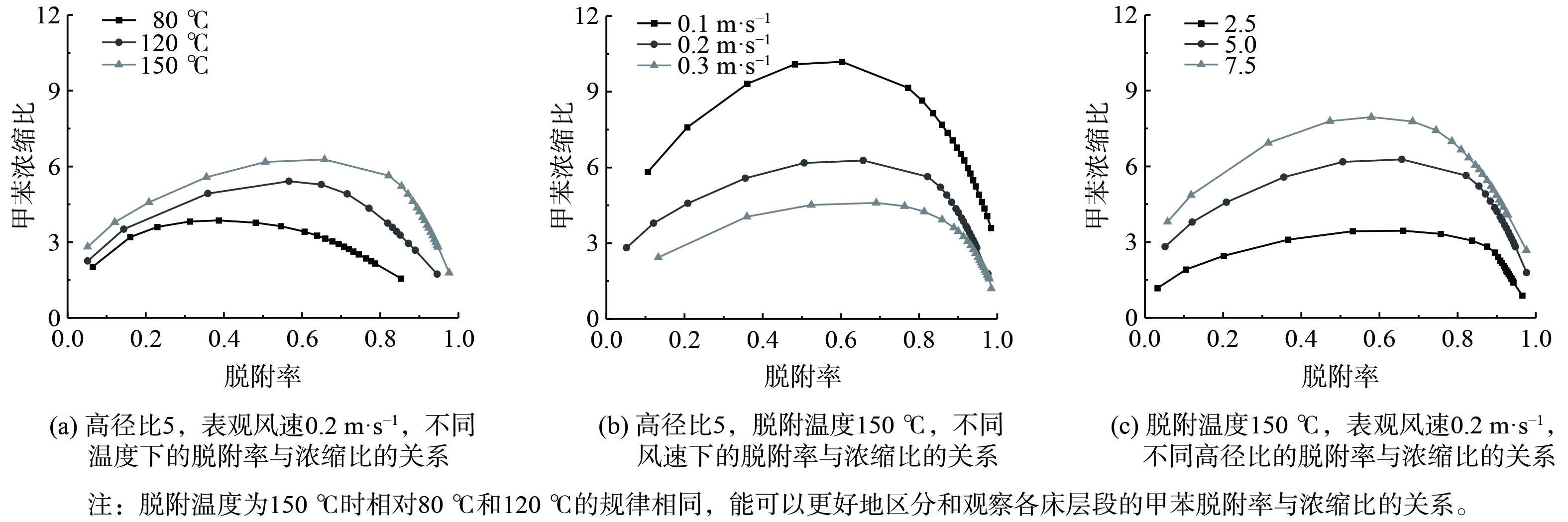
以脱附率作为横坐标,浓缩比作为纵坐标分析甲苯脱附率与浓缩比,结果如图9所示。各床层的甲苯浓缩比在脱附率从零增长时,先上升到一个最高点,然后随着脱附率的增加开始快速下降。同时,脱附温度越高,表观风速越小,活性炭床的装填长度越长,相同脱附率下的甲苯浓缩比越大。将不同工况下高径比为5,脱附率为80%时的脱附时间和浓缩比进行整理并分析,结果如表2所示。
表2表明,表观风速从0.1 m·s−1增至0.2 m·s−1时,高径比为5时120 ℃和150 ℃的脱附时间分别缩短了30.6%和26.8%,浓缩比减少了29.3%和33.9%;表观风速从0.2 m·s−1增至0.3 m·s−1时,高径比为5时80 ℃、120 ℃和150 ℃的脱附时间分别缩短了2.4%、16.3%和6.7%,浓缩比减少了35.6%、18.1%和25.6%。结合表2与图9分析得到,当脱附率达到80%时,在相同的脱附温度下,随着表观风速的增加,浓缩比越低。在相同的表观风速下,随着脱附温度增加,浓缩比越高。浓缩比较高的区域集中在脱附温度为150 ℃,表观风速为0.1 m·s−1的位置。在此操作条件下,脱附率达到80%时的浓缩比较高。综上所述,在工程实践中,当高径比为7.5、脱附温度为150 ℃、表观风速为0.1 m·s−1时,脱附时间为40min时的脱附率为80%,此时的甲苯浓缩比最高,为10.571。
-
脱附单位质量甲苯所需热量的能耗比根据公式 (3)和 (4) 计算。
式中:Q为通入热气体所消耗的热量,kJ;c为气体的比热,kJ·(kg· ℃)−1;
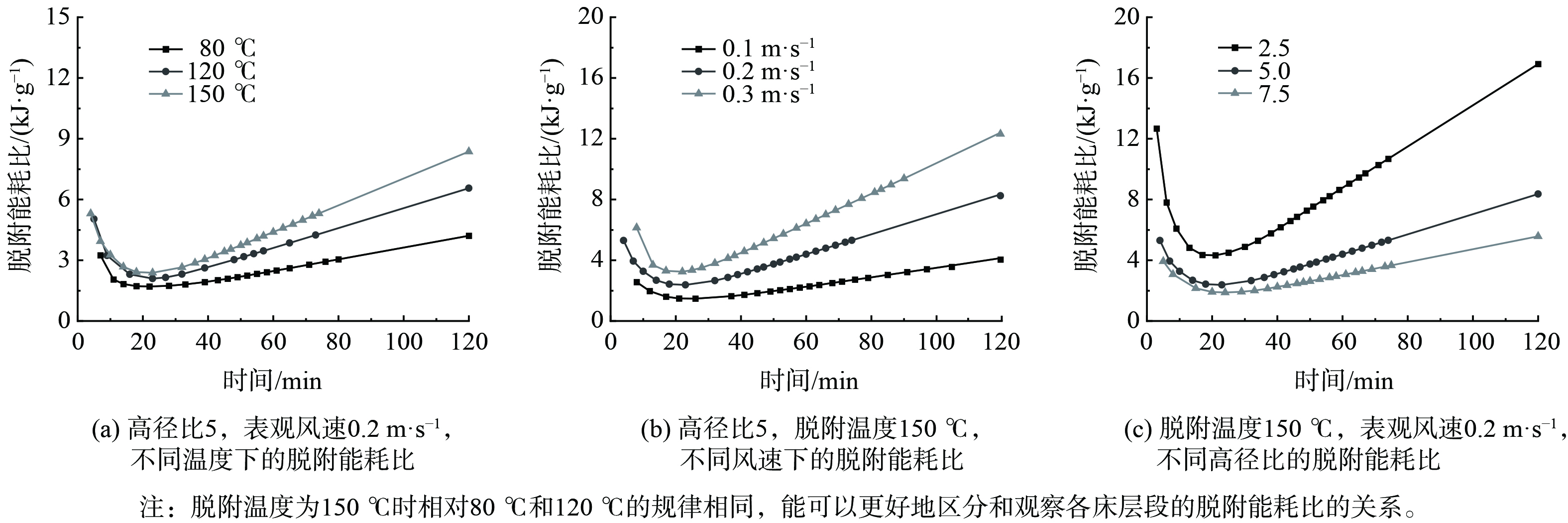
$ \rho $ 为热气体的密度,kg·m−3;t为脱附实验的时间,min;T为脱附实验所需的脱附温度, ℃; x3为活性炭的甲苯脱附的能耗比;m为脱附出的甲苯质量,g。不同工况下各床层脱附单位质量甲苯所需热量的能耗比进行计算,结果如图10所示。当实验开始时,各床层脱附单位质量甲苯的能耗比较高。这是由于温度传热过程比活性炭脱附甲苯的浓度传质过程快,此时甲苯脱附量低,消耗热量多。接着能耗比快速下降至最低点,甲苯瞬时脱附速度达到最大,脱附量快速上升,然后能耗比开始上升。这是由于脱附接近结束,脱附量渐渐稳定,而持续通入的热气体所消耗的能量不变,排放出大量未利用的热量,导致排气温度高,能耗比持续增加,最后呈线性增长。其中,高径比越大,脱附单位质量甲苯所消耗的能量就越低。
在相同的脱附温度下,随着表观风速的增加,各床层脱附单位甲苯的能耗比到达最低点的时间最短,且最低点能耗比的数值越高。经过最低点后,表观风速的越大,各床层能耗比上升的速度越快。这是由于表观风速越大,通入热气体的流量越大,加热气体所消耗的热量越多。在相同的表观风速条件下,经过最低点后,随着脱附温度增加,各床层能耗比上升的速度相对较快。将高温低风速 (即温度为150 ℃,风速为0.1 m·s−1时) 与中温高风速 (即温度为80 ℃,风速为0.3 m·s−1时) 进行比较分析,得出高温低风速时的能耗比一直比中温高风速时的能耗比低,即在输入相同的热量时,高温低风速的脱附量更高。当脱附温度为80 ℃,表观风速为0.3m·s−1时,脱附能耗比最低为1.170 6 kJ·g−1。
已有研究对相同脱附率下的脱附能耗比进行过讨论[37-38]。此次将脱附温度与表观风速分别进行归一化处理,作为横坐标,40 min和60 min的甲苯能耗比作为纵坐标,讨论能耗比的主要影响因素,结果如图11所示。不同表观风速归一化处理40 min时刻浓缩比和60 min时刻浓缩比的斜率总体上大于不同脱附温度归一化处理的斜率。这说明在整个脱附的过程中,表观风速是能耗比的主要控制因素。当表观风速越大,脱附温度越高,能耗比受到的影响越大。当脱附温度为80 ℃,表观风速为0.1 m·s−1时,40 min和60 min的脱附能耗比最低,分别为1.543 1和1.671 4 kJ·g−1。
-
1) 随着脱附温度和表观风速的增加,甲苯脱附率随之增加。当脱附温度150 ℃,表观风速0.3 m·s−1时,甲苯脱附率最高为97.7%。随着表观风速和脱附温度的增加,脱附率到达拐点的时间越短。2) 随着脱附温度和高径比的增加,表观风速的减小,甲苯浓缩比随之增加。当脱附温度为150 ℃,表观风速为0.1 m·s−1时,甲苯浓缩比最高为10.185。其中,表观风速是浓缩比的主要控制因素。表观风速越大,脱附温度越低,浓缩比受到的影响越大。3) 在中温条件下,低风速、高径比的甲苯浓缩比小于高风速、高径比。在高温条件下,低风速、高径比的甲苯浓缩比仅在峰值段大于高风速、高径比,其他时段基本一致。4) 脱附温度越高,表观风速越小,高径比越大,相同脱附率下的甲苯浓缩比越大。当脱附率达到80%时,随着脱附温度的增加,表观风速的减少,浓缩比越高。在脱附温度为150 ℃、表观风速为0.1 m·s−1的工况下,脱附率达到80%时的浓缩比较高。5) 脱附温度越低,表观风速越小,高径比越大,脱附单位质量甲苯所消耗的能量就越低。高温低风速时的能耗比比中温高风速时的能耗比低。当脱附温度为80 ℃,表观风速为0.3 m·s−1时,脱附能耗比最低为1.170 6 kJ·g−1。表观风速是能耗比的主要控制因素。表观风速越大,脱附温度越高,能耗比受到的影响越大。6) 在工程实践中,当高径比为7.5脱附温度为120 ℃表观风速为0.2 m·s−1时,脱附时间在54 min时,脱附率达到80%,此时能获得最适宜的甲苯浓缩比和能耗比,分别为4.985 9和2.365 2 kJ·g−1。
活性炭变温脱附高浓度VOCs的工艺参数优化与评价
Optimization and evaluation of process parameters of thermal desorption of high concentration VOCs using activated carbon at variable temperature
-
摘要: 以甲苯为VOCs类代表性的目标污染物,通过搭建实验装置模拟活性炭吸附脱附处理VOCs工艺,并在不同工艺条件下 (脱附温度、表观风速、高径比) ,选取常见评价因子 (甲苯脱附率、甲苯浓缩比、脱附能耗比) 以探讨不同脱附工况下气体对各床层的脱附情况进行研究。结果表明,甲苯脱附率随脱附温度和表观风速的增加而增加。甲苯浓缩比随脱附温度和高径比的增加,随表观风速的减小而增加,即表观风速是浓缩比的主要控制因素。在中温范围内,低风速、高径比的甲苯浓缩比小于高风速、高径比。在高温范围内,低风速、高径比的甲苯浓缩比仅在峰值段大于高风速、高径比。脱附温度越高,表观风速越小,高径比越大,相同脱附率下的甲苯浓缩比越大。脱附能耗比随脱附温度和表观风速的减小,高径比的增加而降低,即表观风速是能耗比的主要控制因素。高温低风速时的能耗比比中温高风速时的能耗比低。当脱附温度80 ℃、表观风速0.3 m·s−1时,脱附能耗比最低为1.170 6 kJ·g−1。本研究可为变温脱附工程化应用的低碳化工艺优化提供参考。Abstract: An experiment device was built to simulate the adsorption and desorption process of activated carbon to treat VOCs, by taking toluence as the reprentative target pollutant of VOCs. Common evaluation factors (toluene desorption rate, toluene concentration ratio, desorption energy consumption ratio) in the process were selected to study the gas desorption of each bed under different desorption conditions (desorption temperature, cross-section wind speed, height to diameter ratio). The results showed that the toluene desorption rate increased with the increase of desorption temperature and cross-section wind speed. The concentration ratio of toluene increased with the increase of desorption temperature and height to diameter ratio, and the decrease of cross-section wind speed, which was the main control factor of concentration ratio. In the range of middle temperature , the concentration ratio of toluene at low wind speed and height diameter ratio was smaller than that at high wind speed and height-diameter ratio. In the range of high temperature, the concentration ratio of toluene at low wind speed and high diameter ratio was higher than that at high wind speed and height-diameter ratio only at the peak stage. At the same desorption rate, greater toluene concentration ratio would be achieved under higher desorption temperature, lower cross-section wind speed, and higher height-diameter ratio. The desorption energy consumption ratio decreased with the decrease of desorption temperature and cross-section wind speed, and the increase of height-diameter ratio. The cross-section wind speed was the main control factor of energy consumption ratio. The desorption energy consumption ratio at high temperature and low wind speed was lower than that at medium temperature and high wind speed. When the desorption temperature was 80 ℃ and the apparent wind speed was 0.3 m·s−1, the lowest desorption energy consumption ratio was 1.170 6 kJ·g−1. This study can provide reference for low-carbon improvement in the engineering application of variable temperature desorption.
-

-
表 1 CTC 90活性炭对甲苯吸附等温曲线的Freundlich和Langmuir方程参数
Table 1. Freundlich and Langmuir equation parameters of the adsorption isotherm curve of CTC 90 activated carbon for toluene
温度/℃ Freundlich Langmuir k/ Pa−n n R2 qs/(g·g−1) b/(m³·g−1) R2 25 0.273 2 5.581 7 0.991 8 0.555 1 0.343 5 0.996 9 40 0.184 2 4.071 6 0.999 9 0.489 4 0.230 8 0.950 1 80 0.094 9 3.540 3 1.000 0 0.295 3 0.184 7 0.944 6 150 0.016 9 2.039 7 0.999 7 0.141 8 0.060 4 0.983 0 表 2 变温脱附各工况下高径比5、脱附率80%时的脱附时间和甲苯浓缩比
Table 2. Desorption time and toluene concentration ratio at the desorption rate of 80% and a height-diameter ratio of 5 under various conditions of variable thermal desorption
温度/ ℃ 表观风速/(m·s−1) 脱附时间/min 浓缩比 80 0.1 — — 0.2 84 2.016 7 0.3 84 1.299 2 120 0.1 62 5.644 0 0.2 43 3.990 9 0.3 36 3.267 8 150 0.1 41 8.651 0 0.2 30 5.718 9 0.3 28 4.253 1 -
[1] CARDELINO C A, CHAMEIDES W L. An observation-based model for analyzing ozone precursor relationships in the urban atmosphere[J]. Journal of the Air & Waste Management Association, 1995, 45(3): 161-180. [2] UTEMBE S R, WATSON L A, SHALLCROSS D E, et al. A common representative intermediates (CRI) mechanism for VOC degradation. part 3: development of a secondary organic aerosol module[J]. Atmospheric Environment, 2009, 43(12): 1982-1990. doi: 10.1016/j.atmosenv.2009.01.008 [3] 潘昕, 张巍, 黄银芝, 等. 典型涂料制造企业VOCs排放量核算与排放特征分析[J]. 环境工程学报, 2021, 15(3): 1049-1059. doi: 10.12030/j.cjee.202008022 [4] DU Z, MO J, ZHANG Y. Risk assessment of population inhalation exposure to volatile organic compounds and carbonyls in urban China[J]. Environment International, 2014, 73: 33-45. doi: 10.1016/j.envint.2014.06.014 [5] DU Z, MO J, ZHANG Y, et al. Benzene, toluene and xylenes in newly renovated homes and associated health risk in Guangzhou, China[J]. Building and Environment, 2014, 72: 75-81. doi: 10.1016/j.buildenv.2013.10.013 [6] TSAI W Y, CHAN L Y, BLAKE D R, et al. Vehicular fuel composition and atmospheric emissions in South China: Hong Kong, Macau, Guangzhou, and Zhuhai[J]. Atmospheric Chemistry and Physics Discussions, 2006, 6(3): 3687-3707. [7] MONOD A, SIVE B C, AVINO P, et al. Monoaromatic compounds in ambient air of various cities: a focus on correlations between the xylenes and ethylbenzene[J]. Atmospheric Environment, 2001, 35(1): 135-149. doi: 10.1016/S1352-2310(00)00274-0 [8] LESON G, WINER A M. Biofiltration: An innovative air pollution control technology for VOC emissions[J]. Journal of the Air & Waste Management Association, 1991, 41(8): 1045-1054. [9] DUMANOGLU Y, KARA M, ALTIOK H, et al. Spatial and seasonal variation and source apportionment of volatile organic compounds (VOCs) in a heavily industrialized region[J]. Atmospheric Environment, 2014, 98: 168-178. doi: 10.1016/j.atmosenv.2014.08.048 [10] KHAN F I, GHOSHAL A K. Removal of volatile organic compounds from polluted air[J]. Journal of Loss Prevention in the Process Industries, 2000, 13(6): 527-545. doi: 10.1016/S0950-4230(00)00007-3 [11] PARMAR G R, RAO N N. Emerging control technologies for volatile organic compounds[J]. Critical Reviews in Environmental Science and Technology, 2008, 39(1): 41-78. doi: 10.1080/10643380701413658 [12] 张丹庆, 卜龙利, 陈瑾, 等. 微波催化燃烧技术处理印刷包装行业VOCs[J]. 环境工程学报, 2022, 16(2): 524-534. doi: 10.12030/j.cjee.202012005 [13] ZHOU Q, ZHANG L, CHEN J, et al. Enhanced stable long-term operation of biotrickling filters treating VOCs by low-dose ozonation and its affecting mechanism on biofilm[J]. Chemosphere, 2016, 162: 139-147. doi: 10.1016/j.chemosphere.2016.07.072 [14] MOHAMED E F, AWAD G, ANDRIANTSIFERANA C, et al. Biofiltration technology for the removal of toluene from polluted air using Streptomyces griseus[J]. Environmental Technology, 2015, 131(9/10/11/12): 1197-1207. [15] GARCIA-PEREZ T, AIZPURU A, ARRIAGA S. By-passing acidification limitations during the biofiltration of high formaldehyde loads via the application of ozone pulses[J]. Journal of Hazardous Materials, 2013, 262: 732-740. doi: 10.1016/j.jhazmat.2013.09.053 [16] CHOI B S, YI J. Simulation and optimization on the regenerative thermal oxidation of volatile organic compounds[J]. Chemical Engineering Journal, 2000, 76(2): 103-114. doi: 10.1016/S1385-8947(99)00118-7 [17] ORDONEZ S, BELLO L, SASTRE H, et al. Kinetics of the deep oxidation of benzene, toluene, n-hexane and their binary mixtures over a platinum on γ-alumina catalyst[J]. Applied Catalysis B:Environmental, 2002, 38(2): 139-149. doi: 10.1016/S0926-3373(02)00036-X [18] JEON E C, KIM K J, Kim J C, et al. Novel hybrid technology for VOC control using an electron beam and catalyst[J]. Research on Chemical Intermediates, 2008, 34(8/9): 863-870. [19] LI J, LI B, SUI G, et al. Removal of volatile organic compounds from air using supported ionic liquid membrane containing ultraviolet-visible light-driven Nd-TiO2 nanoparticles[J]. Journal of Molecular Structure, 2021, 1231: 130023. doi: 10.1016/j.molstruc.2021.130023 [20] CHOUDHARY T V, BANERJEE S, CHOUDHARY V R. Catalysts for combustion of methane and lower alkanes[J]. Applied Catalysis A General, 2002, 234(1/2): 1-23. [21] FUTAMURA S, ZHANG A H, YAMAMOTO T. The dependence of nonthermal plasma behavior of VOCs on their chemical structures[J]. Journal of Electrostatics, 1997, 42(1/2): 51-62. [22] ZHOU A, LIU J L, ZHU B, et al. Plasma catalytic removal of VOCs using cycled storage-discharge (CSD) mode: An assessment methodology based on toluene for reaction kinetics and intermediates[J]. Chemical Engineering Journal, 2022, 433: 134338. doi: 10.1016/j.cej.2021.134338 [23] DAS D, GAUR V, VERMA N. Removal of volatile organic compound by activated carbon fiber[J]. Carbon, 2004, 42(14): 2949-2962. doi: 10.1016/j.carbon.2004.07.008 [24] LEE D G, KIM J H, LEE C H. Adsorption and thermal regeneration of acetone and toluene vapors in dealuminated Y-zeolite bed[J]. Separation and Purification Technology, 2011, 77(3): 312-324. doi: 10.1016/j.seppur.2010.12.022 [25] SERRANO D P, CALLEJA G, BOTAS J A, et al. Adsorption and hydrophobic properties of mesostructured MCM-41 and SBA-15 materials for volatile organic compound removal[J]. Industrial & Engineering Chemistry Research, 2004, 43(22): 7010-7018. [26] LIU L, TIAN S, NING P. Phase behavior of tweens/toluene/water microemulsion systems for the solubilization absorption of toluene[J]. Journal of Solution Chemistry, 2010, 39(4): 457-472. doi: 10.1007/s10953-010-9519-8 [27] LAWSON R B, ADAMS C D. Enhanced VOC absorption using the ozone/hydrogen peroxide advanced oxidation process[J]. Journal of the Air & Waste Management Association, 1999, 49(11): 1315-1323. [28] DAVIS R J, ZEISS R F. Cryogenic condensation: A cost-effective technology for controlling VOC emissions[J]. 2002, 21(2): 111-115. [29] GUPTA V K, VERMA N. Removal of volatile organic compounds by cryogenic condensation followed by adsorption[J]. Chemical Engineering Science, 2002, 57(14): 2679-2696. doi: 10.1016/S0009-2509(02)00158-6 [30] XU H, XU X, CHEN L, et al. A novel cryogenic condensation system based on heat-driven refrigerator without power input for volatile organic compounds recovery[J]. Energy Conversion and Management, 2021, 238: 114157. doi: 10.1016/j.enconman.2021.114157 [31] WANG S, LIANG Z, CHAO L, et al. Enhanced adsorption and desorption of VOCs vapor on novel micro-mesoporous polymeric adsorbents[J]. Journal of Colloid & Interface Science, 2014, 428(428): 185-190. [32] GHOSHAL A K, MANJARE S D. Selection of appropriate adsorption technique for recovery of VOCs: an analysis[J]. Journal of Loss Prevention in the Process Industries, 2002, 15(6): 413-421. doi: 10.1016/S0950-4230(02)00042-6 [33] 党小庆, 王琪, 曹利, 等. 吸附法净化工业VOCs的研究进展[J]. 环境工程学报, 2021, 15(11): 3479-3492. doi: 10.12030/j.cjee.202011052 [34] DAVINI P. Adsorption and desorption of SO2 on active carbon: The effect of surface basic groups[J]. Carbon, 1990, 28(4): 565-571. doi: 10.1016/0008-6223(90)90054-3 [35] 杨晓娜, 任晓玲, 严孝清, 等. 活性炭对VOCs的吸附研究进展[J]. 材料导报, 2021, 35(17): 17111-17124. doi: 10.11896/cldb.21010141 [36] BERCIC G, PINTAR A, LEVEC J. Desorption of phenol from activated carbon by hot water regeneration. Desorption Isotherms[J]. Industrial & Engineering Chemistry Research, 1996, 35(12): 4619-4625. [37] 荀志萌, 李照海, 何娇, 等. 大风量低浓度VOCs气体二次吸附浓缩净化技术开发[J]. 环境工程学报, 2016, 10(1): 283-288. doi: 10.12030/j.cjee.20160146 [38] 李照海, 羌宁, 刘涛, 等. 活性炭和沸石分子筛处理非稳定排放VOCs气体的性能比较[J]. 环境工程学报, 2017, 11(5): 2933-2939. doi: 10.12030/j.cjee.201611026 [39] 羌宁, 史天哲, 缪海超. 挥发性有机物污染控制方案的运行费用效能比较[J]. 环境科学, 2020, 41(2): 638-646. doi: 10.13227/j.hjkx.201907029 [40] 党小庆, 敬开锐, 马红周, 等. 吸附VOCs活性炭真空热再生及影响因素实验[J]. 环境工程, 2022, 40(8): 62-68. [41] 徐胜男, 羌宁, 裴冰. 活性炭处理甲苯气体吸附再生实验研究[J]. 环境污染与防治, 2008, 29(1): 57-59. doi: 10.3969/j.issn.1001-3865.2008.01.017 [42] 李慧, 陈哲, 钟缘, 等. 乙酸丁酯废气在活性炭上的吸附和脱附行为研究[J]. 离子交换与吸附, 2020, 36(1): 31-40. doi: 10.16026/j.cnki.iea.2020010031 [43] 刘义鑫. VOCs吸附箱结构及其吸附剂脱附效果优化研究[D]. 宁波: 宁波大学, 2019. [44] 陈君毅, 陈磊, 蒙昊蓝, 等. 基于神经网络的车辆交通协调性评价模型[J]. 同济大学学报(自然科学版), 2021, 49(1): 135-141. doi: 10.11908/j.issn.0253-374x.20243 -



 下载:
下载: