-
随着现代工业化的蓬勃发展,大量水资源被用于各行各业,其中大部分作为废水排放,这些废水通常含有大量有机物和复杂的基质[1]。目前生化处理工艺是最主要的处理技术,可以有效去除废水中有机物,然而工业废水中含有许多难降解有机污染物,因此,这些处理工艺的出水通常难以满足环境法规及回用需求[2-4]。因此,亟需开发更先进的方法来高效去除工业废水中的难降解有机污染物。
最近,基于多孔阳极开发的新型反应性电化学膜(reactive electrochemical membrane, REM)氧化技术在去除废水中的难降解有机物方面显示出潜力[5-6]。REM氧化系统不仅保留了传统电化学氧化系统(即采用平行板电极设置,溶液仅在电极之间流动)的优点(如无需添加化学试剂、反应条件温和、易于扩大规模等),还通过依靠其微孔结构压缩边界扩散层,强制废水在多孔阳极微孔中的对流传输从而解决了传统系统受传质限制的问题[7]。同时,废水的对流传输也激活了传统系统中难以接触的内部活性位点[8]。因此,与传统系统相比,REM氧化系统能将污染物的氧化动力学提高几倍到几十倍[9]。而且,除了•OH介导的间接氧化过程外,阳极氧化还存在另一种氧化途径,即直接电子转移(direct electron transfer,DET)过程[7]。因此,与基于•OH的高级氧化技术(如光催化氧化、芬顿氧化等)相比,REM氧化工艺的效率受废水基质的影响较小[10]。同时,通过调节电位降低DET过程的活化能垒,阳极可以有效降解各种难降解污染物,甚至是极稳定的全氟辛基磺酸[11]。
当前的研究已经充分证实REM氧化技术能够高效地处理各种实际废水(如垃圾填埋场渗滤液、反渗透浓缩液等)[6,12]。然而,废水中普遍存在的Cl‒虽然可通过形成活性氯提高处理效率,但Cl‒的过度氧化存在生成氯化副产物(主要指ClO3‒和ClO4‒)的风险[13-14]。这些氯化副产物会危害人体健康,如ClO4‒会损害甲状腺功能[14]。因此,对REM氧化过程中形成的氯化副产物有必要进行严格审查和控制。最近,阳极氧化系统中通常被忽略的阴极反应过程越来越受到重视,许多将阳极氧化(anodic oxidation,AO)和阴极还原(cathodic reduction,CR)耦合的系统已被频繁报道[15]。有研究[16-17]表明,氯化副产物能够在阴极被还原为无毒的Cl‒,从而有效降低处理后废水的生物毒性。因此,可以推测使用合适的电极材料构建将AO和CR耦合的REM系统(AO-CR-REM),处理含氯工业废水可能在保证有机物去除的同时抑制含氯副产物的生成。
基于此,本研究旨在系统地评估AO-CR-REM系统对生化处理过的工业废水的处理效果。首先,选择了2种价格低廉、性能稳定、具备工程化应用的REM阳极材料,即Ti4O7和SnO2-Sb,从有机物去除对其处理效率进行比较,并评估配备筛选过的阳极的REM氧化系统中含氯副产物的生成情况。随后,研究了REM氧化系统对工业废水中有机物的氧化机理。最后,结合筛选的多孔阳极和商业化的多孔RuO2阴极,设计了一个双功能AO-CR-REM系统,评估了其在去除有机物的同时抑制氯化副产物的生成的情况,以期为反应性电化学膜技术在高效、安全地处理高含氯、难处理的工业废水方面的应用提供参考。
-
本研究中工业废水样品采集自山东某工业园区污水处理站,该工业园区主要包括基础化工原料、农药及其制剂、医药原料、食品添加剂等生产企业。采集的废水为经厌氧工艺和膜生物反应器(membrane bioreactor, MBR)处理后出水,废水中的悬浮颗粒及胶体已被MBR有效拦截。采集的废水水质特征如下:COD值为230 mg·L−1、Cl‒含量和电导率分别高达2 300 mg·L−1和13 000 μS·cm−1、pH为8.2、NH4+-N和NO3‒-N分别为8.5 mg·L−1和9.2 mg·L−1。其中,废水出水的COD值远超过处理规定限值(100 mg·L−1) 。
-
本研究中使用的REM电极(即Ti4O7和SnO2-Sb)由直径50 mm、厚度2 mm的多孔钛基底制备。SnO2-Sb电极采用溶胶-凝胶法制备,同时制备出SnO2-Sb电极也被用作制备Ti4O7电极的中间体,通过真空等离子喷涂技术进一步负载Ti4O7催化层[9,18-19]。
使用德国Zeiss公司的Sigma 300型扫描电子显微镜(scanning electron microscope,SEM)、日本RigakuD公司的max-3C型X射线衍射仪(X-ray diffractometer,XRD)和Micromeritics公司的AutoPoreIV 9500型的汞孔度计分别对两种电极的表面形态、结晶相和孔隙结构进行表征。采用三电极系统在0.1 mmol·L−1 KH2PO4溶液中以20 mV·s−1的扫描速率进行线性扫描伏安法(linear sweep voltammetry, LSV)和循环伏安法(cyclic voltammetry, CV)测试,获得电极的析氧电位(oxygen evolution potential, OEP)及与电活性位点相关的电流面积。
-
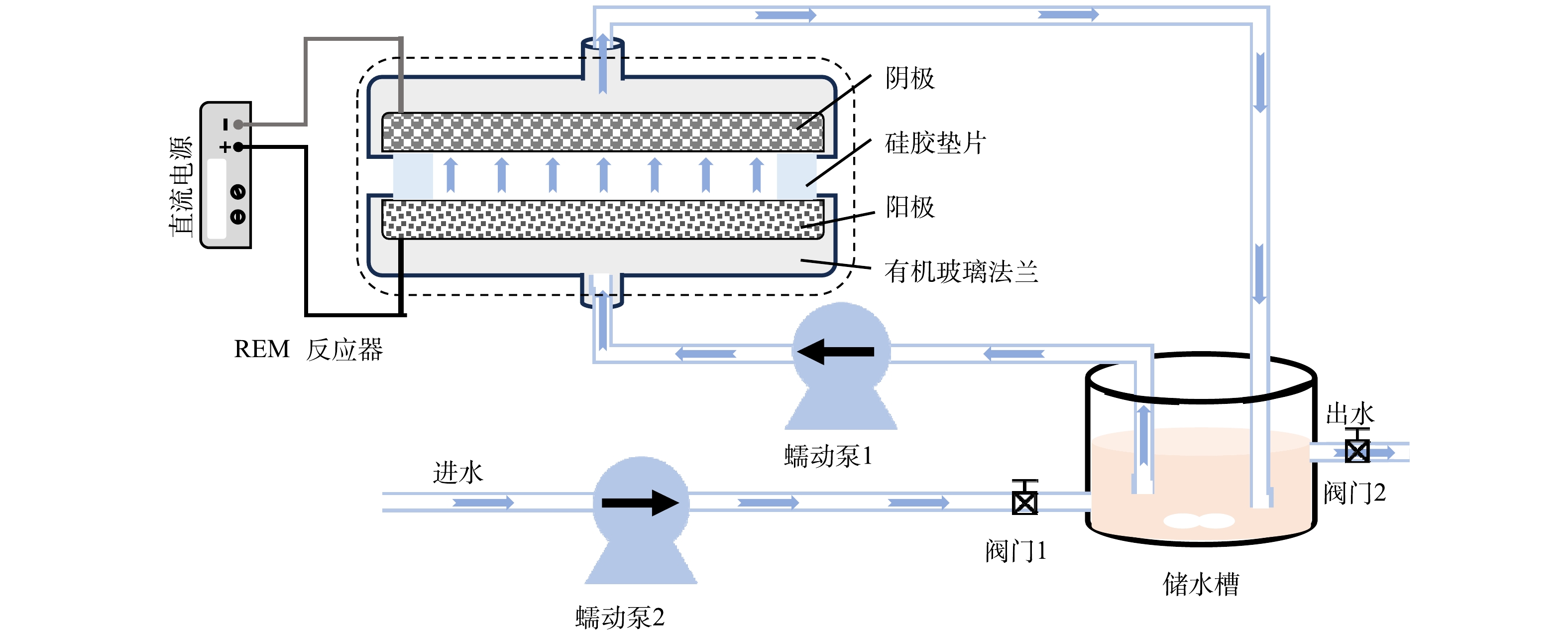
REM反应系统原理图如图1所示,反应系统包括1个REM反应器、1个直流电源、1个储水槽以及2个蠕动泵。其中,REM反应器由阴极、阳极、2个有机玻璃法兰和相应的硅胶垫圈组成。硅胶垫片的厚度、外径和内径分别为6、50和40 mm。制作的多孔Ti4O7和SnO2-Sb REM电极被用作阳极,每个电极的有效表观面积约为12.56 cm2。REM氧化系统运行时,阴极为不锈钢穿孔板,其直径为50 mm,厚度为1 mm。在进行耦合的AO-CR-REM工艺时,阴极为商用的多孔RuO2电极(直径=50 mm,厚度=2 mm)。该反应系统可在循环或连续模式下运行,循环模式下,蠕动泵1驱动废水进入REM反应器中,流经多孔电极后返回储水槽,待测样品从储水槽中获取;连续模式下,保持循环模式继续运行,打开阀门1和2,同时蠕动泵2将原始废水不断送入储水槽,然后在溢流的作用下从出水口流出储水槽,待测样品从出水口获取。
-
收集的工业废水样品首先通过REM氧化工艺在循环模式下进行处理,以评估两种阳极材料(即Ti4O7和SnO2-Sb)去除有机物的效率以及选定的多孔阳极生成氯化副产物的情况。实验使用150 mL工业废水,水循环通量为 150 mL·min−1,电流密度为10~50 mA·cm−2。随后,进一步评估循环模式下AO-CR-REM工艺处理废水的效率以及对氯化副产物抑制情况。实验使用150 mL工业废水,水循环通量为 75~300 mL·min−1。电流密度为20 mA·cm−2和30 mA·cm−2。最后,对AO-CR-REM工艺在连续模式下的应用进行了评估。储水槽中的废水以150 mL·min−1的水循环通量进行循环,原废水以4 mL·min−1的水通量持续注入储水槽,电流密度设定为30 mA·cm−2。
在含有4 mmol·L−1 K4Fe(CN)6、250 μmol·L−1 TPA或2 300 mg·L−1 Cl‒的150 mL溶液中进行模型探针氧化实验,以评估AO-CR-REM工艺在循环模式下去除废水中有机物的机理。溶液的电导率用KH2PO4调节到与收集的工艺废水相同的水平(13 000 μS·cm−1)。实验在水循环通量为150 mL·min−1和电流密度为10~30 mA·cm−2的条件下进行。
-
工业废水中的COD值使用美国Hach公司的DR1900型COD仪测定;ClO3‒和ClO4‒浓度采用美国Thermo Fisher Scientific公司的离子色谱仪测定;活性氯通过美国Hach公司的DPD试剂粉包测定(1407099-CN); NH4+-N和NO3−-N采用国标法测定;K4Fe(CN)6浓度采用紫外可见分光光度计(Hach,DR6000)在420 nm波长下测定;对苯二甲酸(terephthalic acid,TPA)使用配备2489 UV-Vis检测器和XBridge C18柱的高效液相色谱仪进行分析,检测波长为254 nm,柱温保持在35 ℃。流动相为0.1%甲酸溶液和甲醇(40:60),流速设置为1 mL·min−1。
去除有机物的电流效率根据式(1)计算得出,TPA或K4Fe(CN)6氧化的电流效率根据式(2)计算。
式中:η为电流效率,%;C0和Ct分别为0和t时的COD值,g·L−1;F为法拉第常数,96 485.3 C·mol−1;V为待处理溶液体积,0.15 L;I为外加电流,A;t为处理时间,h;n=8为氧气当量。
式中:C0和Ct分别为0和t时的TPA或K4Fe(CN)6的浓度,mol·L−1;x代表反应中的电子转移次数。参与TPA氧化的•OH是水分子通过单电子转移反应在阳极表面生成的;类似地,K4Fe(CN)6氧化也是单电子转移反应。因此,在计算TPA或K4Fe(CN)6氧化的电流效率时,x为1。根据式(3)计算出TPA或K4Fe(CN)6的氧化动力学速率常数,连续运行模式下处理工业废水的电能消耗根据式(4)或(5)计算。
式中:Kobs 为氧化动力学速率常数,m·s−1;A为REM阳极的反应面积,0.125 6×10−2 m2;Cb为K4Fe(CN)6或TPA的质量浓度,mg·L−1。
式中:E为电能消耗,kWh·m−3 或 kWh·g−1(以COD计);U为反应池电压,V;Q为连续废水流量,m3·h−1;Cef为出水中的COD值,mg·L−1。
-
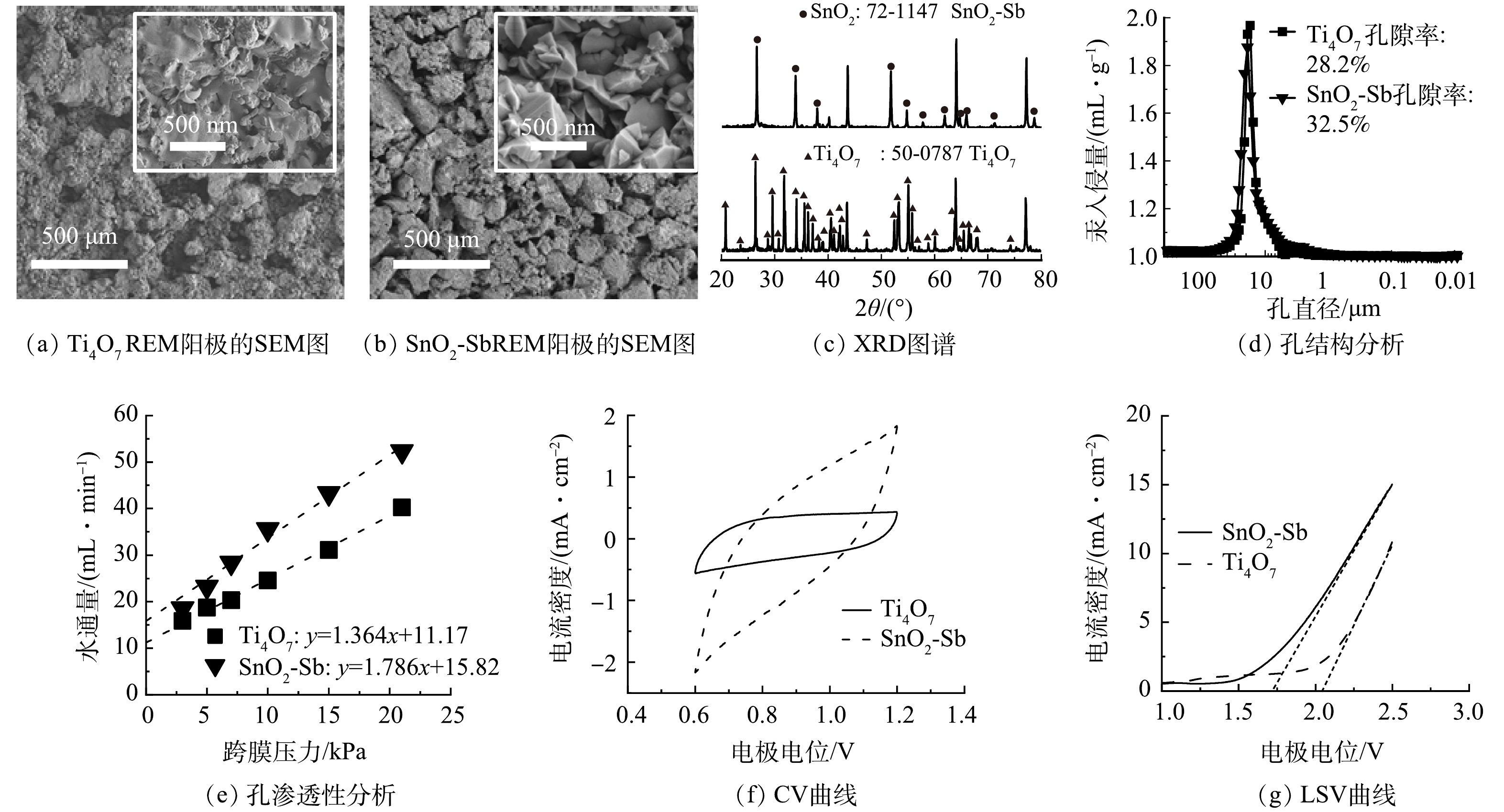
由图2(a)和图2(b)可见, SnO2-Sb和Ti4O7电极均具有发达的孔隙结构,表面形态粗糙。由XRD图谱(图2(c))可见,2个电极上分别出现SnO2和Ti4O7特征峰,证明电极成功负载了SnO2-Sb和Ti4O7催化层。由图2(d)可知,2种电极显示出类似的孔结构,2种电极显示出类似的孔结构,孔径分布、中值孔径分别为5~50 μm和20.0 μm,Ti4O7和SnO2-Sb电极孔隙率分别为28.2和32.5%。。发达的孔隙结构促使两种电极在极低的压力下也能获得极佳的渗透性(图2(e))。同时,SnO2-Sb和Ti4O7电极的孔径远大于传统意义上膜材料(如微滤膜(0.1~1 μm)和纳滤膜(< 2 nm))的孔径促使制备的电极材料几乎不具备拦截水中胶体(1~100 nm)的能力,为后续连续运行实验提供了良好的基础条件[6]。由图2(f)可见,SnO2-Sb电极对应的循环伏安曲线(CV)的电流面积大于Ti4O7电极,表明SnO2-Sb电极可能比Ti4O7电极具有更多的电化学反应活性位点。由图2(g)可知,以Ag/AgCl为参比电极,SnO2-Sb和Ti4O7的析氧电位分别约为1.73 V和2.05 V,符合已报道的SnO2-Sb和Ti4O7电极材料的特征[9,20]。上述表征结果证实目标REM电极被成功制备。
-
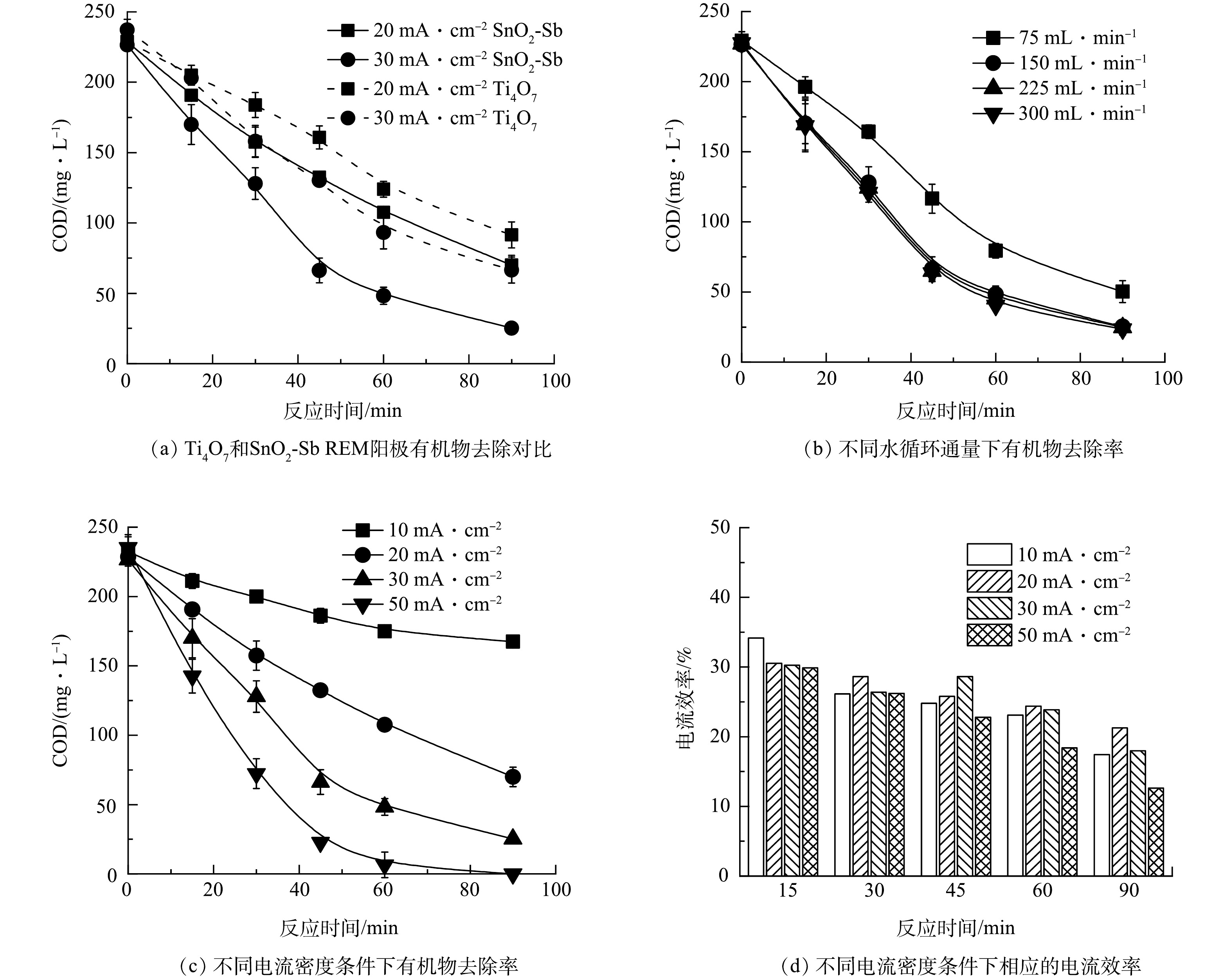
1)有机物的去除。图3(a)对比了2个电极材料处理废水的效率。在相同电流密度下,SnO2-Sb REM阳极对有机物(以COD计)的去除率明显高于Ti4O7,表明SnO2-Sb REM阳极在去除难降解有机物方面更具优势。因此,后续的研究选择SnO2-Sb REM阳极作为研究对象。水通量和电流密度是REM氧化系统运行的2个关键参数,前者影响污染物的传质过程,后者制约着电极的反应动力学.因此,为了进一步优化反应系统的处理效率,研究了水通量和电流密度对反应系统处理工业废水效率的影响。如图3(b)所示,当水循环通量从75 mL·min−1增加到150 mL·min−1时,由于传质增强,90 min处理结束时,有机物去除率从78.1%提升到88.8%;当水循环通量进一步增加到225 mL·min−1和300 mL·min−1时, 有机物去除率几乎没有变化,这表明REM氧化系统达到传质的极限。图3(c)显示了不同电流密度下废水COD的去除情况。随着电流密度的增加,由于电极电子转移能力的提升,有机物去除率显著提升。而由图3(d)可知,当电流密度在10~30 mA·cm−2内,有机物去除的电流效率保持在较高水平,而随着电流密度进一步增加到50 mA·cm−2,由于析氧副反应显著增加,电流效率大幅降低。例如,当电流密度从 30 mA·cm−2增加到50 mA·cm−2时,电流效率由18.0%降低到12.6%。因此,在SnO2-Sb REM阳极采用150 mL·min−1的水循环通量及30 mA·cm−2的电流密度来处理收集的工业废水更为合理。值得注意的是,当有机物去除率在45 min内达到国内的处理规定(100 mg·L−1)的情况下,SnO2-Sb REM阳极的电流效率接近30%,该值远高于达到类似处理效果的传统系统(< 20%)[21-23]。由此可见,新型REM氧化技术能够高效处理工业废水。
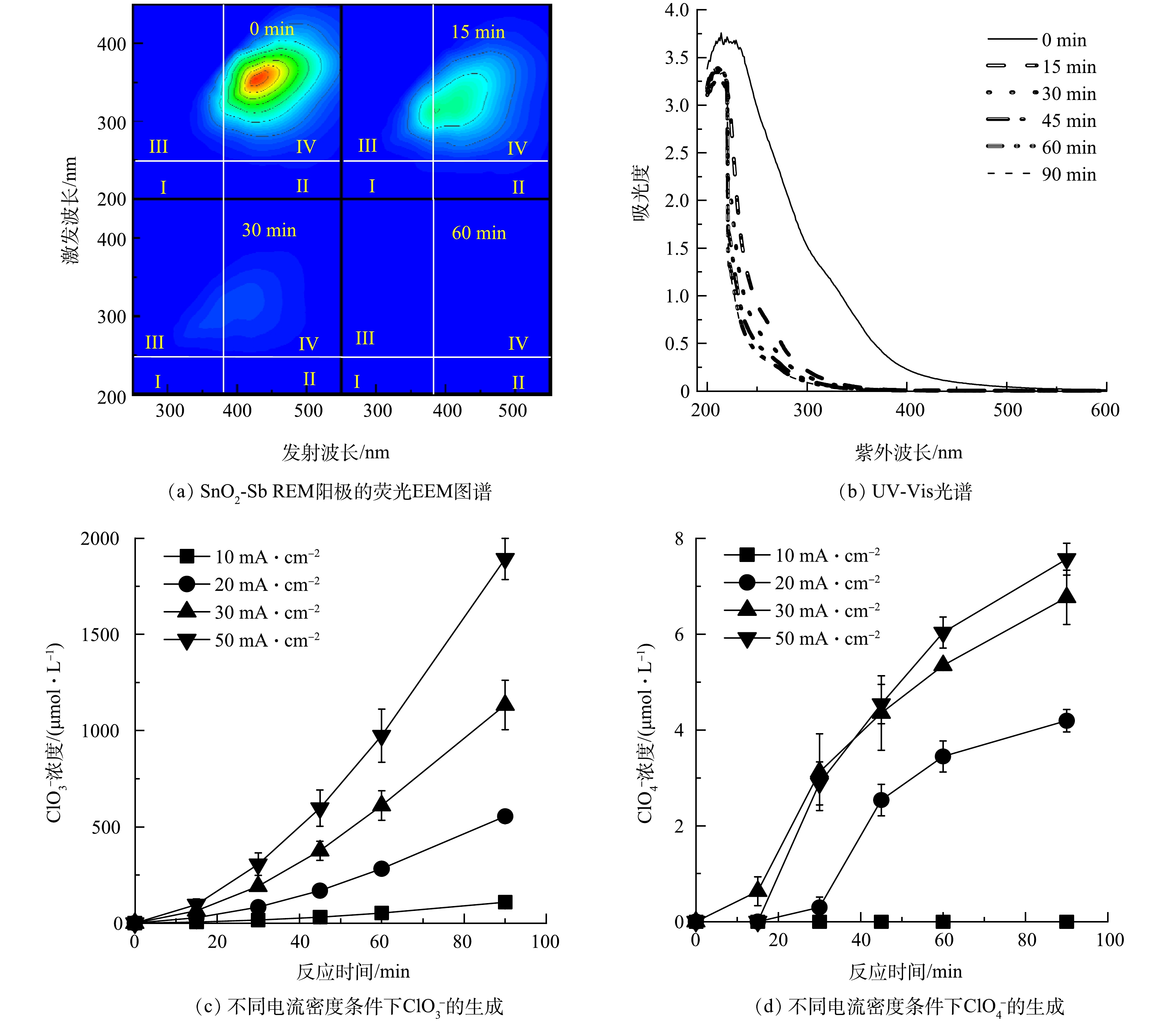
2)有机质转化及氯化副产物生成。为了分析工业废水处理前后有机物特征,进行了三维荧光光谱和UV-Vis吸光光谱分析(图4)。根据不同的激发波长/发射波长(Ex/Em)区域,三维荧光光谱主要反映了不同类型的溶解有机质(dissolved organic matter, DOM):芳香族蛋白区(I),Ex/Em=220~250 nm/280~380 nm;富勒烯酸区(II),Ex/Em=220~250 nm/380~500 nm;可溶性微生物副产物区(III),Ex/Em=250~280 nm/280~380 nm;腐殖酸区(IV), Ex/Em=250~400 nm/380~500 nm[23]。此外,紫外可见光光谱根据特定波长对应的吸光度值或比率同样可以反映有机物的类型。通常,UV250/UV365比值代表腐殖酸类物质,UV254和UV410代表芳香族化合物和共轭大分子[6,24]。如图4(a)和图4(b)所示,在电流密度为30 mA cm−2时,三维荧光强度和UV-Vis吸光度随时间变化与COD有机物去除趋势一致。未经处理的废水荧光区域位于Ex/Em=275~425 nm/350~550 nm的区域内,表明该原始废水中的DOM主要为腐殖酸类物质。随着处理过程,IV区域的荧光强度逐渐消失,III区域荧光强先略微增强随后消失。这些结果表明,工业废水中腐殖酸类物质被氧化分解,并可能产生少量可溶性微生物副产物类物质,这些物质最终被进一步分解。同时,UV250/UV365比值从5.64显著增加到42.90,也表明腐植酸类物质向低分子质量有机物的转化;UV254和UV410经过处理后均大幅降低,说明其中的芳香族化合物和共轭大分子发生了分解。上述结果表明REM氧化工艺能够有效地去除工业废水中的DOM。
图4(c)和图4(d)为处理过程中检测到氯化副产物的生成情况。废水经过处理后同时检测到ClO3‒和ClO4‒的生成,其生成量均随处理时间和电流密度的增加而增加。值得注意的是,本研究中的REM氧化系统中检测到的ClO3‒和ClO4‒的产量远远低于传统系统[25-27]。而且,REM氧化系统中的ClO4‒浓度比低毒的ClO3‒浓度低约2个数量级,而在传统系统中,ClO4‒通常是主要产物[25-26,28]。本研究团队最近的研究还证明,在REM氧化系统中不仅产生的ClO3‒远远少于传统系统,在很短的处理时间内也检测不到ClO4‒的生成[6]。这些结果表明,REM氧化系统与传统系统相比在处理含氯废水时在氯化副产物生成方面具有很大的优势。
-
阳极氧化工艺去除有机物的机理主要涉及DET过程及•OH介导的间接氧化(式(6)~(8))[29]。当未处理废水中存在的Cl−可在阳极表面转化为活性氯,从而参加有机物的氧化(式(9)~(12))[28]。
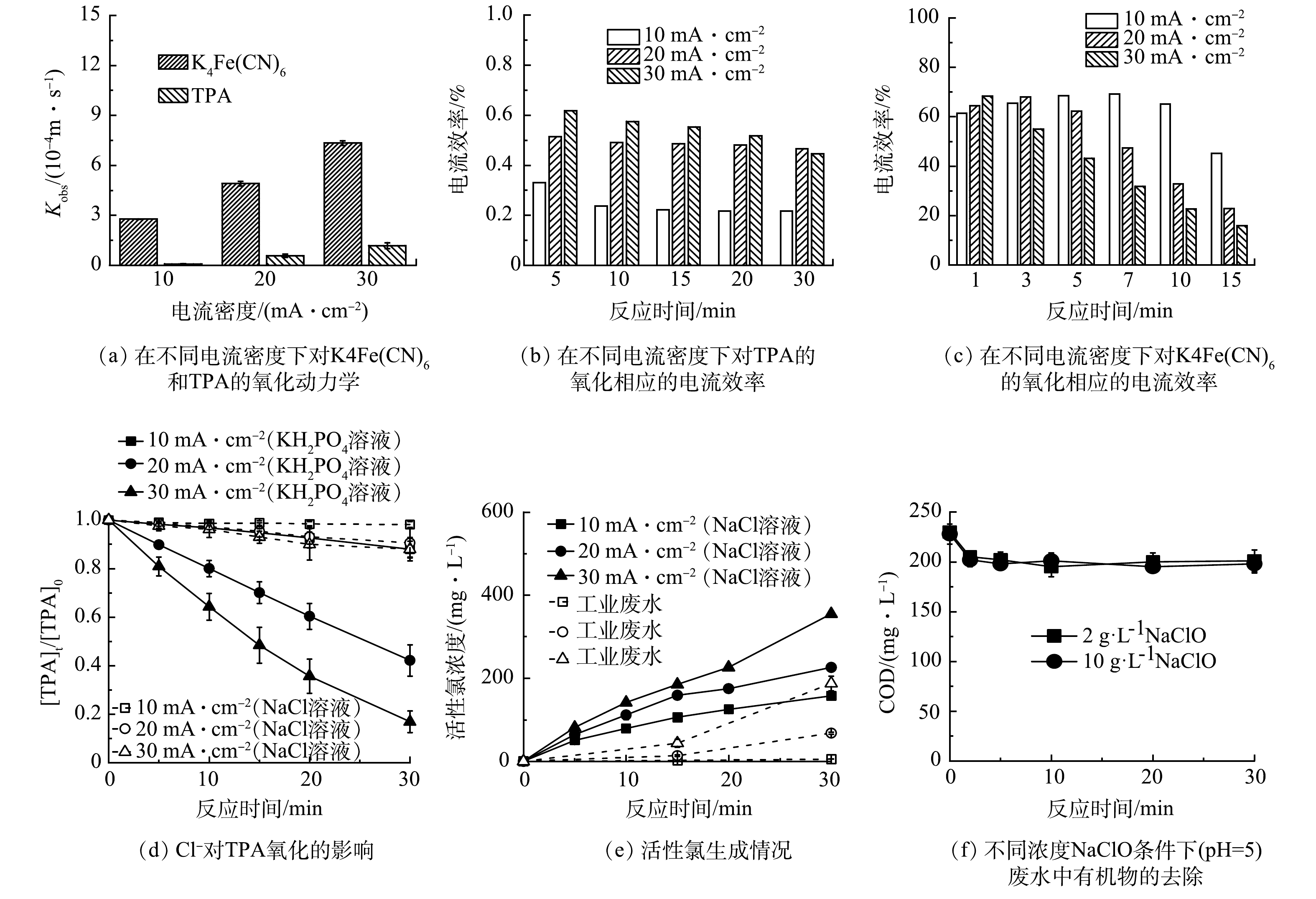
首先,选择K4Fe(CN)6作为探针分子评估了SnO2-Sb REM氧化系统的电化学反应性能,这是因为K4Fe(CN)6不仅极易发生单电子转移反应(Fe(CN)64‒ → Fe(CN)63‒+e‒, E0 = 0.358 V),而且与•OH的反应活性极高(1.2×1010 L·(mol·s)−1)[20]。接着,由于对苯二甲酸(TPA)对•OH具有高选择性(4×109 L·(mol·s)−1),选择TPA作为•OH探针来评估•OH的生成情况[30]。如图5(a)所示,随着电流密度增加,K4Fe(CN)6的Kobs显著增加,这与有机物去除结果一致。在REM氧化系统中,氧化K4Fe(CN)6的Kobs高达2.8~7.3×10−4 m·s−1,比TPA高数倍至数十倍(0.1~1.2×10−4 m·s−1),表明REM氧化具有强大的DET能力,这一结论已在最近的研究报道[6,20,31]。同时, 由图5(b)可知,氧化TPA的电流效率不足1%,与工业废水处理的电流效率(图3(d))相差甚远,表明生成的•OH远无法实现观察到的高有机物去除率。然而,氧化K4Fe(CN)6的电流效率大大提高(图5(c)),与处理工业废水时的电流效率匹配。同时,由于•OH与Cl‒的高反应活性(4.3×109 mol·(L·s)−1),生成的•OH将会被废水中的Cl‒淬灭,如图5(d)中所示,NaCl溶液中TPA氧化动力学与相同电导条件下KH2PO4溶液中相比大幅降低也证明了这一结论。因此,根据上述结果,DET过程在REM氧化工艺处理废水过程中发挥重要作用。为了进一步评价生成的活性氯在废水处理过程的作用,我们测试了处理与工业废水电导率和Cl‒浓度相同的KH2PO4替代溶液时活性氯的生成情况。由图5(e)可见,替代溶液中产生的活性氯浓度远高于处理工业废水时生成的活性氯,尤其是在初始阶段,这表明活性氯参与了有机物的去除。更进一步,我们在工业废水中添加2~10 g·L−1 NaClO,同时用2%稀H2SO4溶液调节pH至5,以确保NaClO转化为活性氯。如图5(f)所示,仅在初始阶段观察到约10%的有机物去除率,而且增加NaClO浓度对有机物的去除几乎没有影响。这一结果表明,氧化能力较低的活性氯难以有效氧化收集的工业废水中大部分原始有机化合物。
根据上述分析,REM氧化工艺去除废水中的有机质机理可能遵循以下过程:首先,工业废水中原始有机物主要依靠DET过程分解;随后,分别形成的二次产物在DET过程和生成的活性氯共同作用下被进一步去除。由于废水中含有大量的Cl‒,生成的•OH会被废水中存在的Cl‒淬灭掉,导致•OH对处理收集的工业废水有机物去除的贡献较小。
-
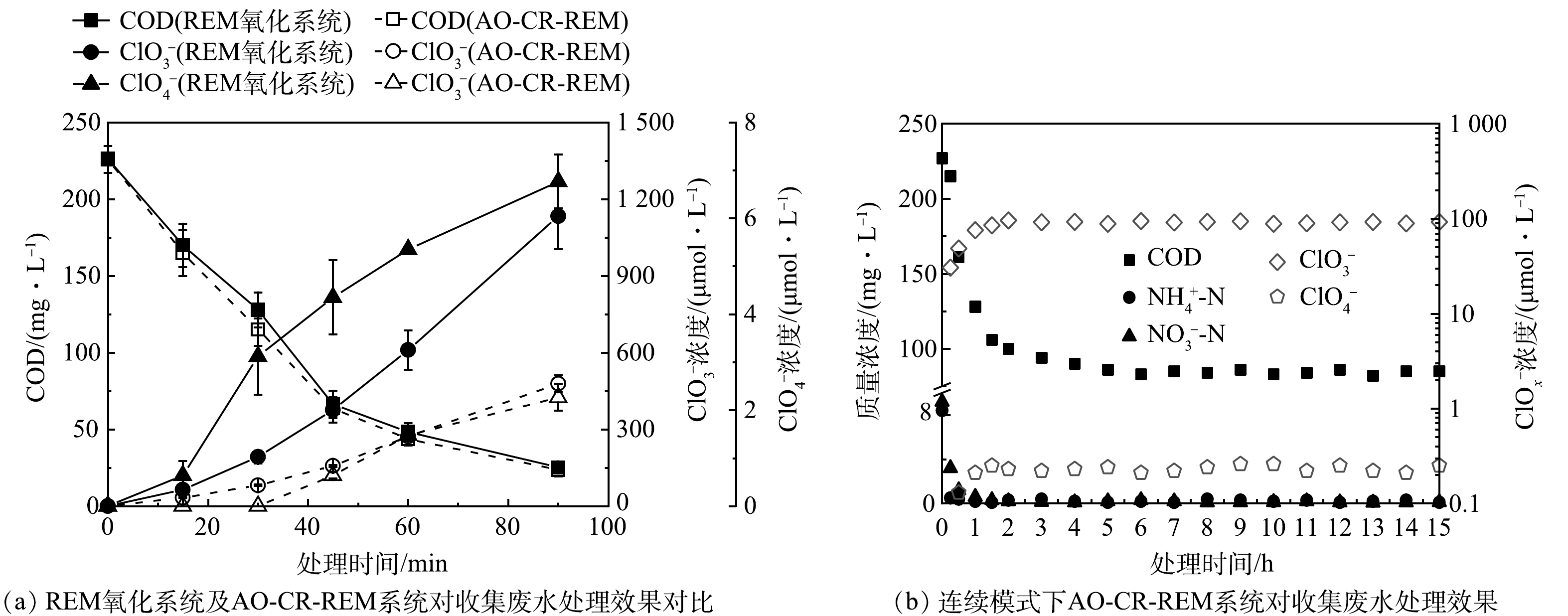
上述结果表明,REM氧化工艺能够高效去除难处理工业废水中的有机物,但伴随着也产生了一定量氯化副产品ClO3‒和ClO4‒[14]。因此,为了去除有机物的同时抑制或者减少氯化副产物的产生,我们进一步采用图1描述的兼具电氧化-还原于一体的双功能AO-CR-REM系统来处理收集的工业废水。如图6(a)所示,在相同的实验条件下,AO-CR-REM系统对于有机物的去除效果与REM氧化系统几乎保持一致,而ClO3‒和ClO4‒的产量则大大减少。例如,在AO-CR-REM系统中,处理30 min后仅测定到约81.5 μmol·L−1的ClO3‒,仅为REM氧化系统的42.3%(约192.3 μmol·L−1),且未检测到ClO4‒的生成。这些结果证明AO-CR-REM系统可以同时去除有机物并显著抑制氯化副产物的生成。需要指出的是,处理过的废水中的ClO3‒仍处于较高水平,因此,今后仍需设计和开发更先进的阴极材料,以进一步抑制氯化副产品的生成。
在实际的废水处理中,通常希望采用连续运行反应系统。目前,主要通过减少水通量或串联多级REM系统来设计连续运行的REM氧化系统。然而,降低水通量会大幅降低污染物的传质效率,从而削减反应系统的处理效率。串联多级REM系统虽然可以保证处理效率,但设备投资成本将急剧上升。据估计,REM氧化技术应用过程中设备的投资成本约占总成本的90%[6]。在此,我们尝试采用一种新的连续运行模式来处理废水,即图1中蠕动泵1以150 mL·min−1水循环通量运行,以确保使AO-CR-REM系统反应不受传质限制,蠕动泵2将原废水不断输送到系统中。由于循环模式下,AO-CR-REM去除有机物遵循伪一级动力学(R2=0.99),对应的动力学常数计算为0.026 min−1。由此可以估算当处理时间为31.4 min时,AO-CR-REM系统能够将150 mL废水的COD削减至接近排放标准(100 mg·L−1),即在连续运行模式下,废水的连续进水速率约为4.8 mL·min−1。为了确保出水水质满足排放标准,设置蠕动泵2以4 mL·min−1的流速不断将原始工业废水输送到反应系统,实验结果如图6(b)所示。处理4 h之后,出水的COD稳定在80 mg·L−1,ClO3‒和ClO4‒分别稳定在90 mg·L−1和0.25 mg·L−1。在连续运行模式下,对废水的处理效果基本维持稳定,反应系统未出现多孔电极堵塞污染问题。出水符合中国的处理规定的情况下,所需电能为7.91 kWh·m−3。同时,还观察到NH4+-N和NO3‒-N几乎被完全去除,这分别归因于活性氯的氧化作用和REM阴极的电还原作用[32-34]。此外,值得注意的是, MBR工艺能够有效去除水中的悬浮物和胶体,避免多孔阳极堵塞和污染,但该工艺无法有效去除废水中普遍存在的硬度离子(Ca2+、Mg2+等),而阴极区域由于析氢反应聚集了大量OH‒,废水中的硬度离子将可能会以氧化物或盐的形式在阴极附近沉降,从而造成阴极结垢。尽管本研究在15 h的运行过程中并未观察到阴极结垢堵塞的情况,但在未来的研究中有必要进一步聚焦于阴极反应过程,研究阴极结垢情况以及应对措施。
-
本研究从电极材料、处理效能、副产物生成与抑制等全面评估了REM工艺处理含氯量高的难降解工业废水的可行性,所得主要结论如下。
1)在Ti4O7和SnO2-Sb 2种常见的电极材料中,SnO2-Sb REM阳极对有机物的去除率比Ti4O7 REM阳极高约9%~17%。
2)废水中的腐殖酸类物质、芳香族化合物和共轭大分子均能够被有效氧化分解。由于Cl‒对生成的•OH有较强的淬灭作用,有机物主要由DET过程分解,接着再由DET过程和生成的活性氯结合进一步去除。
3)进一步开发的AO-CR-REM系统可有效去除有机物,出水符合中国的处理规定的情况下,可有效抑制ClO3‒ 和ClO4‒的生成,同时废水中的NH4+-N和NO3‒-N也被有效去除。
基于耦合电氧化-还原的反应性电化学膜工艺处理高含氯难处理工业废水
Treatment of high chlorine-containing refractory industrial wastewater using reactive electrochemical membrane coupled with electro-oxidation and reduction processes.
-
摘要: 由于传统以生化为主的处理工艺通常难以有效去除工业废水中难降解有机物,出水通常无法满足排放标准及回用需求,因此,本研究从电极材料、处理效能、副产物生成与抑制等全面评估了新型反应性电化学膜(REM)技术处理含氯量高的难降解工业废水的可行性。结果表明,在Ti4O7和SnO2-Sb 2种常见的电极材料中,SnO2-Sb REM阳极对有机物的去除率比Ti4O7 REM阳极高约9%~17%。光谱分析结果表明,废水中的腐殖酸类物质、芳香族化合物和共轭大分子均能够被有效氧化分解。在氧化机理方面,高浓度Cl‒的淬灭作用削弱了氧化过程中•OH的贡献,原始有机物首先主要通过直接电子转移((DET)过程被分解,随后DET过程与生成的活性氯结合实现了废水的进一步氧化。进一步开发的耦合电氧化和还原过程的反应性电化学膜系统可有效去除有机物并抑制ClO3‒ 和ClO4‒的生成,处理4 h之后,出水的COD稳定在80 mg·L−1,ClO3‒和ClO4‒分别稳定在90 mg·L−1和0.25·mg·L−1。该系统的出水符合中国的处理规定的情况下,所需电能仅为7.91 kWh·m−3,同时废水中的NH4+-N和NO3‒-N的去除率也达到100%。以上研究结果表明,反应性电化学膜技术是一项具备前景的处理高含氯量难处理工业废水的技术。Abstract: Traditional biochemical-derived treatment processes struggle to effectively remove refractory organic matter from industrial wastewater, and the effluents often fail to meet discharge standards and reuse requirements. In this study, the feasibility of employing a novel reactive electrochemical membrane (REM) technique to treat industrial wastewater containing high levels of chlorine was assessed in terms of electrode material, treatment efficiency, byproduct generation and inhibition. The results indicated that among the commonly used electrode materials, SnO2-Sb REM anodes exhibited an approximately 9%~17% higher removal rate of organic matter than Ti4O7 REM anodes. Spectroscopic analysis indicated effective oxidation of humic acids, aromatic compounds, and conjugated macromolecules. During the oxidation process, the quenching effects of high concentrations of chloride ions (Cl‒) weakened the contributions of •OH radicals. As a result, the original organic compounds were primarily decomposed through direct electron transfer (DET). Subsequently, the DET process combined with the generated active chlorine realized further wastewater oxidization. The developed REM system, which coupled electrooxidation and reduction processes, could effectively remove organic matter and suppress the generation of ClO3‒ and ClO4‒. After 4 h treatment, the effluent COD stabilized at 80 mg·L−1, and ClO3‒ and ClO4‒ stabilized at 90 mg·L−1 and 0.25 mg·L−1, respectively. With energy consumption of only about 7.91 kWh·m−3, the effluent could meet China’s discharge regulations, and 100% removal of NH4+-N and NO3‒-N from the wastewater occurred. Overall, the above results indicated that REM system is a promising technique for treating refractory industrial wastewater with high chlorine content.
-

-
-
[1] KIM S Y, PARK J W, NOH J H, et al. Potential organic matter management for industrial wastewater guidelines using advanced dissolved organic matter characterization tools[J]. Journal of Water Process Engineering, 2022, 46: 102604. [2] RIBEIRO J P, NUNES M I. Recent trends and developments in Fenton processes for industrial wastewater treatment: A critical review[J]. Environmental Research, 2021, 197: 110957. [3] 陈斌. Fenton法处理焦化废水的试验研究[J]. 能源环境保护, 2017, 31(5): 12-14. doi: 10.3969/j.issn.1006-8759.2017.05.003 [4] 尹星, 郭雲, 任乐辉, 等. Pd/Ti阳极电催化氧化处理焦化废水反渗透浓缩液的研究[J]. 能源环境保护, 2023, 37(6): 12-22. [5] GAYEN P, CHEN C, ABIADE J T, et al. Electrochemical oxidation of atrazine and clothianidin on Bi-doped SnO2-TinO2n–1 electrocatalytic reactive electrochemical membranes[J]. Environmental Science & Technology, 2018, 52(21): 12675-12684. [6] LIN H, PENG H, FENG X, et al. Energy-efficient for advanced oxidation of bio-treated landfill leachate effluent by reactive electrochemical membranes (REMs): Laboratory and pilot scale studies[J]. Water Research, 2021, 190: 116790. [7] TRELLU C, CHAPLIN B P, COETSIER C, et al. Electro-oxidation of organic pollutants by reactive electrochemical membranes[J]. Chemosphere, 2018, 208: 159-175. [8] YANG K, ZHANG X, ZU D, et al. Shifting emphasis from electro-to catalytically active sites: Effects of pore size of flow-through anodes on water purification[J]. Environmental Science & Technology, 2023, 57(48): 20421-20430. [9] YANG K, LIN H, FENG X, et al. Energy-efficient removal of trace antibiotics from low-conductivity water using a Ti4O7 reactive electrochemical ceramic membrane: Matrix effects and implications for byproduct formation[J]. Water Research, 2022, 224: 119047. [10] YANG K, FENG X, LIN H, et al. Insight into the rapid elimination of low-concentration antibiotics from natural waters using tandem multilevel reactive electrochemical membranes: Role of direct electron transfer and hydroxyl radical oxidation[J]. Journal of Hazardous Materials, 2022, 423: 127239. [11] SHI H, WANG Y, LI C, et al. Degradation of perfluorooctanesulfonate by reactive electrochemical membrane composed of magneli phase titanium suboxide[J]. Environmental Science & Technology, 2019, 53(24): 14528-14537. [12] CHEN M, ZHAO X, WANG C, et al. Electrochemical oxidation of reverse osmosis concentrates using macroporous Ti-ENTA/SnO2-Sb flow-through anode: Degradation performance, energy efficiency and toxicity assessment[J]. Journal of Hazardous Materials, 2021, 401: 123295. [13] LIN M-H, BULMAN D M, REMUCAL C K, et al. Chlorinated byproduct formation during the electrochemical advanced oxidation process at Magnéli phase Ti4O7 electrodes[J]. Environmental Science & Technology, 2020, 54(19): 12673-12683. [14] YANG S, FERNANDO S, HOLSEN T M, et al. Inhibition of perchlorate formation during the electrochemical oxidation of perfluoroalkyl acid in groundwater[J]. Environmental Science & Technology Letters, 2019, 6(12): 775-780. [15] PENG H, FENG X, YANG Y, et al. Flow-through electrochemical activation of persulfate for efficient wastewater treatment using Ti4O7 reactive electrochemical membranes[J]. ACS ES& T Water, 2023, 3(5): 1294-1304. [16] YAO F, ZHONG Y, YANG Q, et al. Effective adsorption/electrocatalytic degradation of perchlorate using Pd/Pt supported on N-doped activated carbon fiber cathode[J]. Journal of Hazardous Materials, 2017, 323: 602-610. [17] 李守彪, 吴亚品, 徐凤麒, 等. ClOx-产物对电氧化COD去除性能评价及水体毒性的影响[J]. 工业水处理: 1-13. [18] YANG K, LIN H, LIANG S, et al. A reactive electrochemical filter system with an excellent penetration flux porous Ti/SnO2–Sb filter for efficient contaminant removal from water[J]. RSC Advances, 2018, 8(25): 13933-13944. [19] YANG K, XU J, LIN H, et al. Developing a low-pressure and super stable electrochemical tubular reactive filter: Outstanding efficiency for wastewater purification[J]. Electrochimica Acta, 2020, 335: 135634. [20] YANG K, LIN H, JIANG J, et al. Enhanced electrochemical oxidation of tetracycline and atrazine on SnO2 reactive electrochemical membranes by low-toxic bismuth, cerium doping[J]. Separation and Purification Technology, 2022, 297: 121453. [21] UKUNDIMANA Z, OMWENE P, GENGEC E, et al. Electrooxidation as post treatment of ultrafiltration effluent in a landfill leachate MBR treatment plant: Effects of BDD, Pt and DSA anode types[J]. Electrochimica Acta, 2018, 286: 252-263. [22] PANIIZZA M, MARTINEZ-HUITLE C A. Role of electrode materials for the anodic oxidation of a real landfill leachate-Comparison between Ti-Ru-Sn ternary oxide, PbO2 and boron-doped diamond anode[J]. Chemosphere, 2013, 90(4): 1455-1460. [23] LE LUU T. Post treatment of ICEAS-biologically landfill leachate using electrochemical oxidation with Ti/BDD and Ti/RuO2 anodes[J]. Environmental Technology & Innovation, 2020, 20: 101099. [24] YAN S, LIU Y, LIAN L, et al. Photochemical formation of carbonate radical and its reaction with dissolved organic matters[J]. Water Research, 2019, 161: 288-296. [25] JASPER J T, YANG Y, HPFFMANN M R. Toxic byproduct formation during electrochemical treatment of latrine wastewater[J]. Environmental Science & Technology, 2017, 51(12): 7111-7119. [26] WANG L, WNAG Y, SUI Y, et al. Formation of chlorate and perchlorate during electrochemical oxidation by Magnéli phase Ti4O7 anode: inhibitory effects of coexisting constituents[J]. Scientific Reports, 2022, 12(1): 15880. [27] WANG Y, LI L, HUANG Q. Electrooxidation of per-and polyfluoroalkyl substances in chloride-containing water on surface-fluorinated Ti4O7 anodes: Mitigation and elimination of chlorate and perchlorate formation[J]. Chemosphere, 2022, 307: 135877. [28] WANG L, LU J, LI L, et al. Effects of chloride on electrochemical degradation of perfluorooctanesulfonate by Magnéli phase Ti4O7 and boron doped diamond anodes[J]. Water Research, 2020, 170: 115254. [29] FU R, ZHANG P S, JIANG Y X, et al. Wastewater treatment by anodic oxidation in electrochemical advanced oxidation process: Advance in mechanism, direct and indirect oxidation detection methods[J]. Chemosphere, 2023, 311: 136993. [30] GUO L, JING Y, CHAPLIN B P. Development and characterization of ultrafiltration TiO2 Magnéli phase reactive electrochemical membranes[J]. Environmental Science & Technology, 2016, 50(3): 1428-1436. [31] YANG K, ZU D, ZHANG Z, et al. Mechanistic insight into the spatial scale of nonuniform oxidation of micropollutants in reactive electrochemical membranes for water purification[J]. ACS ES& T Engineering, 2023, 3(12): 2194-2201. [32] LIU Z, TAO Y, ZHANG Z, et al. Active chlorine mediated ammonia oxidation in an electrified SnO2-Sb filter: Reactivity, mechanisms and response to matrix effects[J]. Separation and Purification Technology, 2023, 312: 123369. [33] ZHOU J, PAN F, YAO Q, et al. Achieving efficient and stable electrochemical nitrate removal by in-situ reconstruction of Cu2O/Cu electroactive nanocatalysts on Cu foam[J]. Applied Catalysis B: Environmental, 2022, 317: 121811. [34] 李智卓, 姚福兵, 吴星, 等. 硝酸盐废水电化学选择性还原产氨的研究进展[J]. 能源环境保护, 2023, 37(4): 56-67. -



 下载:
下载: