-
大气过氧化氢(H2O2)是重要的光化学二次产物,其主要是由HO2与HO2自由基结合生成的[1]。此外,无光情况下已知的能够形成过氧化氢的途径为O3与烯烃的反应以及NO3与有机化合物的反应[2]。过氧化氢唯一的直接排放源为生物质燃烧[3-4]。过氧化氢本身是大气中重要的氧化剂,同时又作为HOx自由基的储库分子,参与HO2和OH自由基的循环[5-6]。作为大气中最重要的氧化剂之一,H2O2在污染大气化学中扮演着重要的作用。一方面,在pH<5的云、雾和雨水中,H2O2被认为是S (Ⅳ)氧化生成硫酸盐最重要的氧化剂,对大气降水酸化和颗粒物的质量增长有重要影响[7-8]。而且,近年来随着研究手段的进步以及对过氧化氢认识的不断深入,有研究发现过氧化氢与气溶胶颗粒物之间存在着复杂的关系。有研究通过外场观测以及模型模拟发现气溶胶表面非均相反应对H2O2可能同时存在源和汇的双重作用[4, 6, 9]。另一方面,有研究将H2O2/HNO3的比值用做评估O3生成对VOCs或NOx敏感性的指示剂,且在多种指示剂评估方法中,认为H2O2/HNO3比值的指示剂方法对O3生成敏感性的评估最合适[10-11]。此外,毒理学研究表明,H2O2能够随细颗粒物进入肺部,进而损伤肺泡,从而对人类健康产生不利影响[12]。因此,对大气中过氧化氢的浓度水平、污染特征、生消过程及影响因素等进行深入研究,对掌握区域光化学氧化剂的污染水平和研究光化学与颗粒物复合污染的形成以及人类健康具有重要意义。
本研究对大气过氧化氢的浓度水平、气象因素和相关环境污染物的大气浓度开展了同步观测,旨在分析影响H2O2生成的因素。着重关注高NOx以及颗粒物污染情况下H2O2的污染特征,通过模式对过氧化氢的源汇进行分析。
全文HTML
-
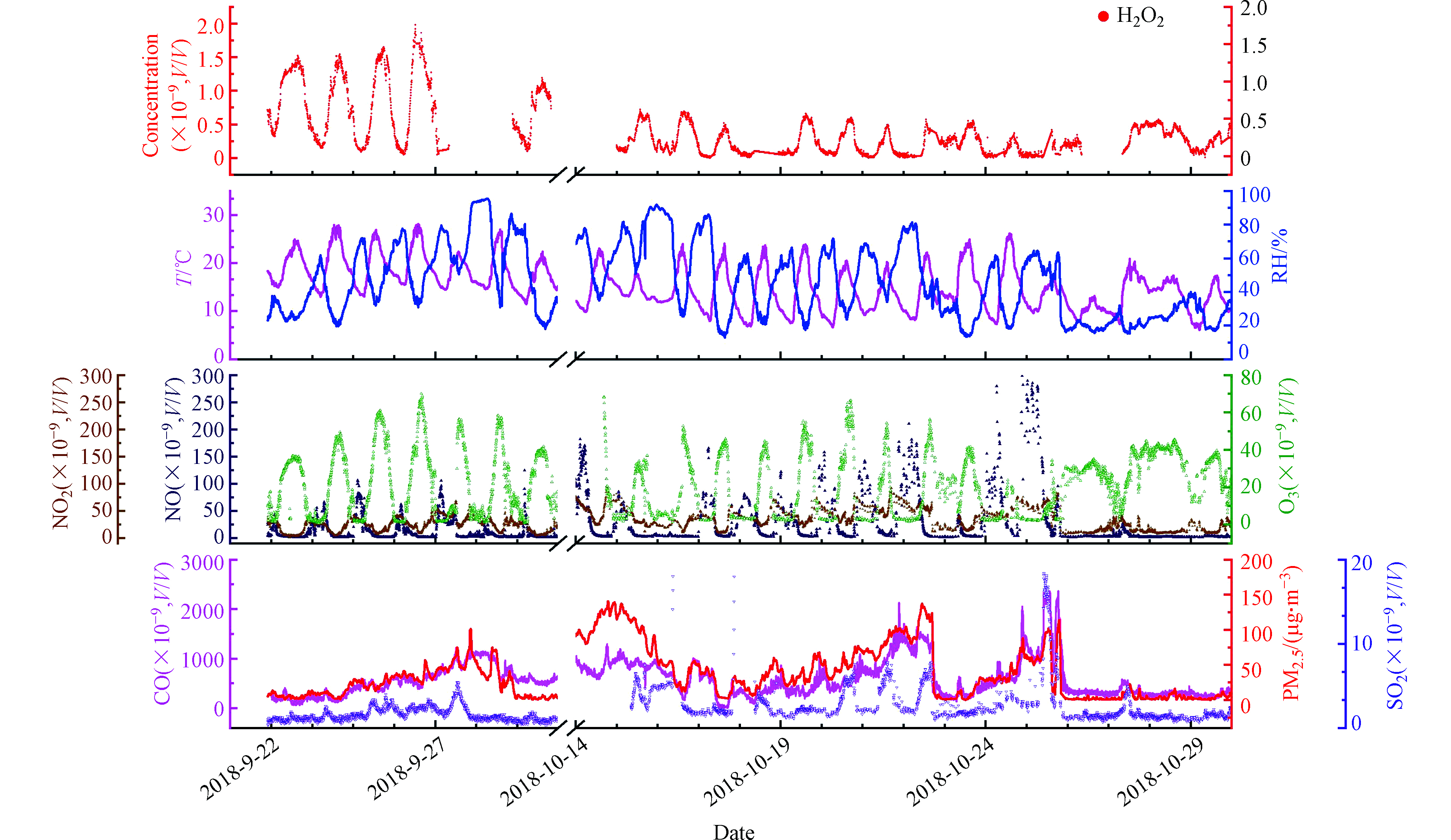
本研究的大气过氧化氢观测地点位于北京市海淀区中国科学院生态环境研究中心(40.00°N,116.33°E)综合楼顶楼,采样口距地面20 m。采样点正南方向有一条东西方向公路,采样点周围无工业源,主要为教学园区以及居民区。观测时间为2018年秋季9月22日—10月30日,其中在10月初由于设备故障有部分观测数据缺失。
-
本研究使用的大气过氧化氢分析仪器为美国EPA制造的大气过氧化氢分析设备原机,并在使用过程中对设备的数据采集和流量控制部分进行了改进和更换。研究组利用美国NI6002数据采集卡并编写labview程序进行数据采集,并更换了质量流量控制部分的组件。采样气体流速控制在1 L·min−1。仪器对大气过氧化氢的检测原理见文献[13],利用过氧化物与被过氧化物酶催化的对羟基苯乙酸的液相反应,在326 nm波长下激发该反应产生的荧光二聚体,以400—420 nm波长下检测到的荧光强度定量过氧化物浓度。过氧化氢与有机过氧化物的测定通过两个平行通道(Channal A和Chanal B)的设计实现,其中Channal B加入过氧化氢酶选择性破坏过氧化氢。过氧化氢的浓度通过两通道的信号差值计算得到。
观测期间除大气过氧化氢外,配套观测的污染物种类还包括SO2、NOx、CO以及O3等气态污染物。利用Thermo scientific – TEOM1405F实时在线测量大气气溶胶质量浓度;利用URG9000D-AIM分析仪在线测量大气PM2.5上无机水溶性离子及气态氨气、硝酸气和硫酸气浓度;利用TSI-SMPS分析仪实时测量大气气溶胶粒径分布。并在天气晴朗、光化学条件较好的情况下,利用不锈钢采样罐采集全空气样品,使用三级冷阱预浓缩-Dean-switch-GC-FID/FID方法进行了VOCs光化学活性组分(107种)的分析。仪器参数信息见表1。
-
基于观测的模型(observation-based model,OBM)是一种以观测数据为基础进行光化学污染过程分析的方法[14]。与传统的空气质量模型相比,其具有不依赖源清单的优势。此外,OBM作为空气质量模型的重要补充,其与化学机理耦合能够模拟光化学产物的生成及衰减过程,获得人们关注的一些光化学产物如O3、H2O2和HNO3等物种生成及消耗途径以及速率的信息。Cardelino和Chameides、以及Wang等[15-17]的研究中对OBM模型在O3生成与前体物关系的应用方面做了详细介绍,而Wang、Qin等[4, 18]的研究中参照O3研究中相对增量反应活性的概念,对H2O2和其前体自由基HO2与NOx、CO和VOC前体物的关系进行了探究。因此,参照相对增量反应活性概念,本研究利用OBM对H2O2生成对前体物变化的敏感性进行了探究,研究中OBM模型采用实测污染物(O3、NO、NO2、SO2、CO、VOCs)和气象因子(T、RH)5 min间隔数据作为限制条件输入。H2O2的净光化学生成速率为H2O2生成速率与损失速率之差。H2O2生成潜势定义为观测地点12 h H2O2净光化学生成速率的总和。特定污染物相对增量反应活性计算见2.2节(式1)。
1.1. 观测地点
1.2. 方法与设备
1.3. 基于观测的模型(OBM)
-
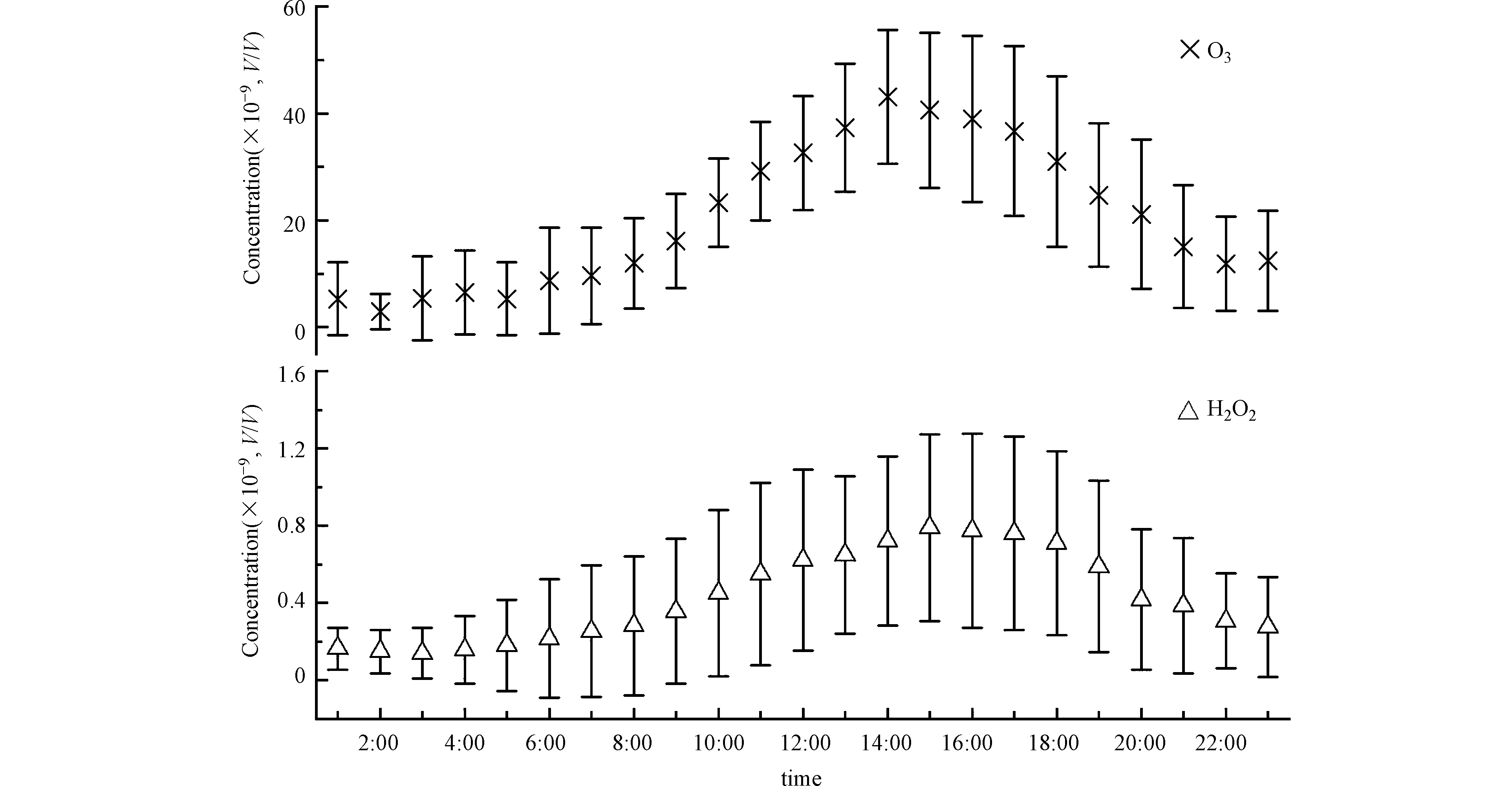
北京2018年9—10月份观测期间H2O2的平均体积浓度为(0.29±0.36)×10−9。观测期间H2O2的最大体积混合比为1.98×10−9。大气过氧化氢表现出明显的日变化规律(图1),变化规律与同为光化学产物的O3一致,不过H2O2峰值出现时间略晚于O3(图1),过氧化氢在午后15:00左右达到日间峰值。
北京地区秋季的H2O2观测浓度与国际和国内其他观测所给出的大气过氧化氢的浓度范围基本一致(表2)。文献报告的H2O2的体积浓度范围在0.01×10−9—11.3×10−9[4]之间,由于夏季太阳辐射的增加而导致光化学过程的增强,文献中同一地区的大气过氧化氢的浓度水平常在夏季较高,冬季较低[18]。本次观测期间北京地区以晴朗和多云天气为主导,9月主要为晴朗天气,10月多为多云天气。共捕捉到4次颗粒物污染过程,其中3次发生在10月。观测期间9月H2O2和O3浓度显著高于10月(图2),NOx在10月浓度显著高于9月。10月份较高的NOx浓度通过与HO2自由基结合,可能产生抑制HO2与HO2自由基的结合,而减少H2O2的生成的效应。
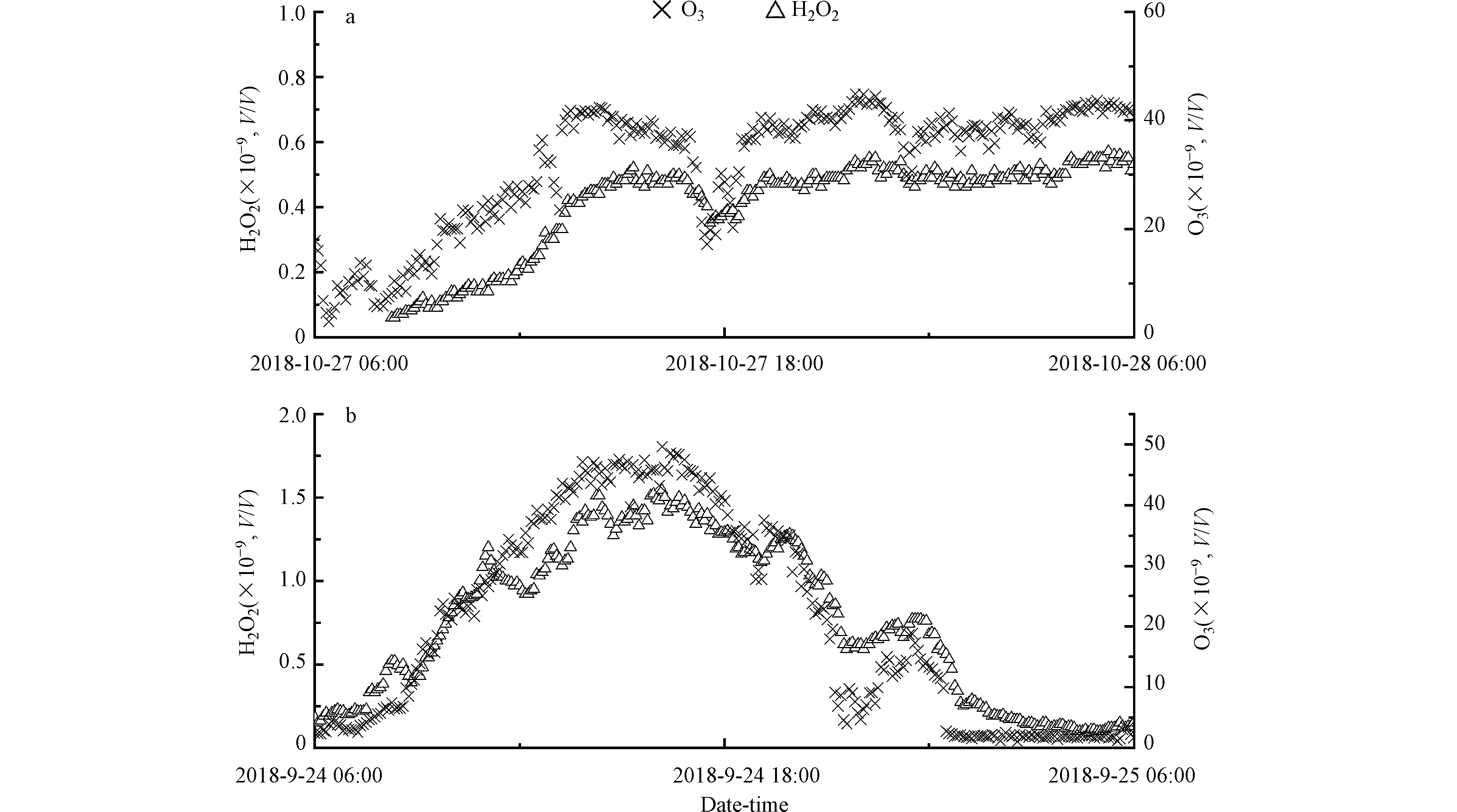
H2O2单日浓度变化曲线并不平滑,经常会有较大的凸起,说明影响大气中H2O2浓度因素的复杂性。一般来说,随着太阳辐射逐渐减弱,光化学产物H2O2以及O3的生成会随着光化学反应活性的降低而逐渐减少。本研究在部分日期的光化学活性较弱或光化学停止的时段观测到明显的H2O2与O3的同步增加的情况(图3)。不同于其它研究观测到的H2O2浓度夜间上升而O3浓度下降,而被推测为臭氧与烯烃的反应或NO3与有机化合物的反应的情况[2],本研究中的O3与H2O2在下午及夜间是同时涨落,更可能是污染气团区域传输的结果。以往对北京城区及近郊地区的大气H2O2的观测表明,北京郊区的夜间O3和H2O2浓度显著高于城区[20],由于北京的特殊地形,山谷风导致平原地区风场日变化为午后偏南风,夜间偏北风的规律,因而图3中夜间H2O2和O3的同步升高现象有可能是北京北部山区气团的水平或垂直方向传输造成的。
-
大气H2O2主要是HO2与HO2自由基结合生成的,但大气HO2的浓度水平和生成速率则受到其前体物CO、NOx以及VOCs的大气浓度和光照情况影响。有研究发现,霾天TVOC相比非霾天具有显著增加,其中VOCs不同类别烷烃、烯烃、芳烃和炔烃等具有不同的增加比例,增加最快的为烯烃[31]。且也有研究表明,霾天较高水平的VOCs、O3和水汽对H2O2的生成具有较大贡献[18]。考虑到霾天较高VOCs、O3水平和气溶胶非均相作用对H2O2生成产生影响,以及霾天较高相对湿度对H2O2氧化SO2生成硫酸盐对SOA的贡献,了解VOCs各类别及主要单一组分对H2O2生成的影响对提出有效的污染控制措施具有指示意义。目前,对H2O2前体物尤其是VOCs各类别以及主要单一组分对H2O2生成贡献的研究相对较少。相对增量反应活性方法广泛用来研究O3生成对前体物的敏感性。研究中H2O2生成的相对增量反应活性计算参考O3生成的相对增量反应活性计算方法,H2O2相对增量反应活性的计算为,改变H2O2特定前体物源强后,H2O2生成速率变化的百分比与H2O2特定前体物源强变化百分比的比率(式1)[4]。
式中,X为特定污染物种,S(X)为污染物X的源效应,ΔS(X)为源强变化量,
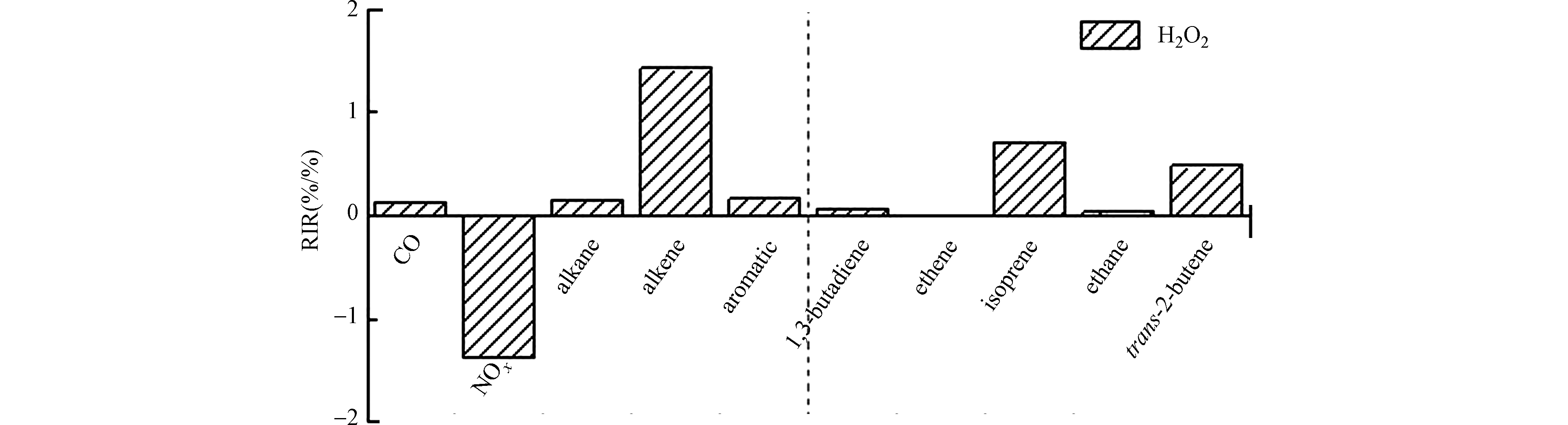
${P_{{{\rm{H}}_2}{{\rm{O}}_2}}} $ (X)为7:00—19:00 H2O2生成潜势,Δ${P_{{{\rm{H}}_2}{{\rm{O}}_2}}} $ (X)为源效应变化ΔS(X)后${P_{{{\rm{H}}_2}{{\rm{O}}_2}}} $ (X)的变化量。OBM耦合RACM区域大气化学机理用来模拟计算前体物对H2O2生成的相对增量反应活性。RACM中根据排放速率的大小、官能团的相似性以及化合物与OH的反应性对VOCs进行分类。具体分类和机理中涉及的237个反应见文献[32]。按照RACM机理中对VOCs的分类方法将VOCs分为烷烃(ETH、HC3、HC5和HC8)、烯烃(ETE、DIEN、OLI和OLT)和芳烃(XYL和TOL)。各前体物源强削减为10%,不同前体物对H2O2生成的相对增量反应活性结果如图4。结果表明,北京地区秋季H2O2的生成对烯烃最为敏感,在单一前体物中对异戊二烯和反式-2-丁烯最为敏感,此结果与Wang等[4]的研究结果一致,提示在光化学条件较好且具有较高烯烃水平的条件下有利于大气H2O2的生成。反式-2-丁烯主要为机动车排放物种,有效的机动车控制策略可能除了降低大气臭氧和颗粒物污染,也有助于减少H2O2的生成。异戊二烯为植物源排放主要产物,其对大气H2O2的生成有较大的贡献作用。
-
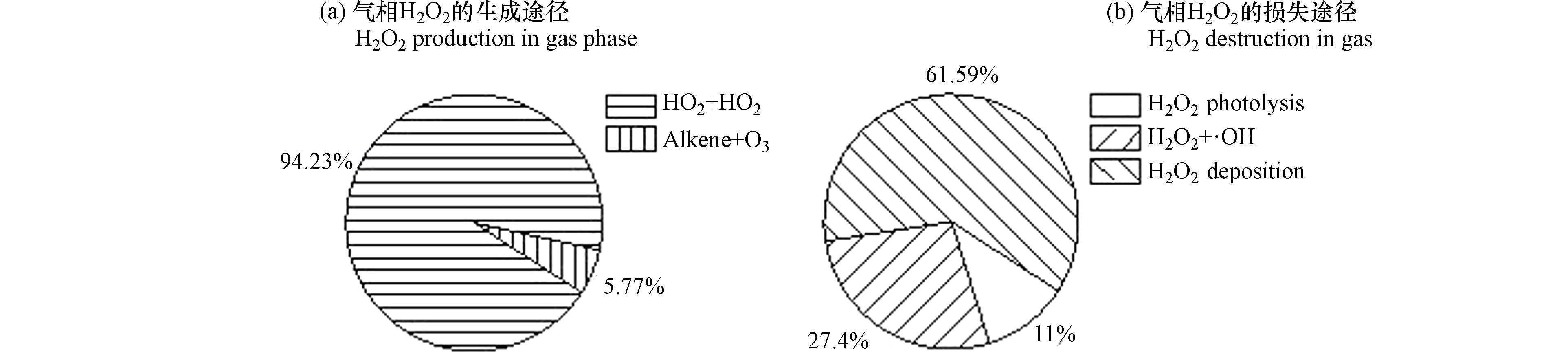
为了解不同反应途径对大气H2O2生成及损耗的贡献,研究采用了基于观测的OBM模型(observation-based model),耦合气相化学RACM机制(regional atmospheric chemistry mechanism)计算了不同气相反应途径对H2O2生成及损耗的贡献。模式中H2O2的生成途径主要包括HO2与HO2结合的反应、O3和烯烃的反应,损失途径包括H2O2与OH自由基的反应、H2O2的光解反应以及H2O2的干沉降。结果表明,HO2与HO2自由基结合的反应是大气H2O2的主要气相生成途径,贡献了总H2O2气相生成途径的94.23%,O3与烯烃的反应仅占总H2O2生成的5.77%。不同H2O2的去除途径对H2O2消耗的贡献依次为H2O2干沉降、H2O2与OH反应和H2O2光解,依次贡献了总H2O2损失的61.59%、27.40%和11.00%(图5)。
不过,模式中对H2O2生成速率的模拟对比观测结果存在低估的情况。本研究中基于实测环境大气VOCs和NOx浓度限制的RACM机理模拟所给出的HO2自由基日最高浓度为3.61×108 molecule·cm−3,其对应的日最高H2O2生成体积速率约为0.2×10−9·h−1。由模式模拟得到的日间过氧化氢生成体积速率
${P_{{{\rm{H}}_2}{{\rm{O}}_2}}} $ 的累积仅约1.54×10−9,在去除了光解和沉降的去除情况下不足以达到观测到的H2O2峰值体积浓度1.98×10−9。此前在华北地区也有观测发现气溶胶上HO2的非均相转化可能是H2O2的重要源[9]。也有观测表明生物质燃料燃烧可能是局地大气过氧化氢的重要来源[4]。这些额外来源可能贡献了部分的日间过氧化氢生成途径。此外,近年来有研究通过对自由基HO2实测值和模拟值的比较,认为高NOx条件下,模式中对HO2自由基的低估可能高达5倍,且HO2观测值与模拟值的比值随着NOx的增加呈上升的趋势[33]。本研究模拟中H2O2的低估也可能与模式中HO2自由基源的缺失有关。由于本研究未开展气溶胶成分和大气过氧自由基的测量,有关H2O2实测值与模拟值之间的差异,还需未来通过更多观测和模型计算进行深入研究。
2.1. 浓度水平和变化规律
2.2. H2O2生成对前体物变化的敏感性
2.3. H2O2源、汇分析
-
本研究对北京秋季9—10月份的大气过氧化氢进行了观测。结果表明,观测期间其体积浓度水平为(0.29±0.36)×10−9;日变化明显,早晚较低,最大值出现在午后15:00—16:00,白天的日变化与O3相似,说明观测期间本地光化学作用主导H2O2生成;夜间H2O2上升,似与污染气团的区域传输有关。H2O2生成速率对光化学前体物的敏感性分析表明,烯烃对H2O2的生成最敏感,光照条件好且烯烃水平高有利于H2O2的生成。大气H2O2生成及损耗的模拟表明,HO2与HO2结合反应是H2O2的主要已知生成途径,H2O2干沉降为主要损失途径。H2O2观测与模拟值的差异可能与机制中缺失颗粒物上的非均相源/汇有关,高NOx条件下模式中HO2自由基源的缺失也可能是导致这种结果的原因。



 下载:
下载: