-
人类活动使得大量的铅(Pb)进入土壤环境,Pb在土壤中累积不仅会抑制植物生长,还会被作物吸收通过食物链对人类健康构成威胁[1]. 有研究报道美国农田与欧洲牧场土壤中Pb最大值分别达到135 mg·kg−1和1090 mg·kg−1[2-3]. 作为农业大国,我国土壤Pb污染比例为1.5%,加强修复技术体系研究,保障生态环境与食品安全,已成为重大的现实需求[4]. 植物修复作为一种原位修复技术,具有广泛的适用性和环境友好等优点,其中植物提取能够利用植物对重金属的吸收并通过收获地上部生物质清除土壤中的重金属,尤其适合漫污染区的修复[5]. 已发现多种Pb超积累植物能从土壤中提取大量的Pb并转运至地上部[6]. 然而超积累植物在Pb污染土壤修复的实际应用受其生长速率,实用价值和适生范围的限制[7]. 因此,有研究者提出种植具备金属耐受性、高生物量、农艺技术纯熟的非食用经济作物来修复污染土壤,兼顾生态与经济价值,让时间成本不再成为植物修复的限制因素,满足可持续发展需要,这已成为植物修复研究领域关注的热点[8].
在当今世界能源短缺的背景下,油料作物可以作为工业、医药原料且能制备生物能源而具有极高的经济价值,将油料作物开发与Pb污染土壤植物修复相结合,能够弥补植物修复的不足并充分利用Pb污染土壤发展生物能源[9]. 有关单一油料作物在应对土壤Pb污染方面已多有研究,Adesodun等[10]发现向日葵不同生长阶段对Pb的吸收积累不同,生长4周时植株地上部Pb积累效率最高. 张守文等[11]研究了油菜对Pb的耐性与富集效应,认为油菜具备修复Pb污染土壤的潜力. 然而,不同栽培物种的植物对Pb的耐受与积累存在差异,有效的植物修复需要考虑污染区的栽培历史和种植条件,选择特定的修复作物并形成农民驱动的修复模式[12],受限于现有研究间因环境条件、金属水平、栽培时间等方面的不同,难以比较并筛选出对Pb具有耐受与积累能力的修复型作物.
鉴于此,本文以种植范围广泛的大豆、向日葵和油菜等3种油料作物为试材,以生长状况和光合能力表征耐受性,以植株中Pb浓度、Pb积累量、生物富集系数BCF及转运系数TF评价Pb积累能力,且为避免作物因生育期不同引起的Pb积累差异,采用土培盆栽试验研究不同浓度土壤Pb污染对3种油料作物早期生长阶段的耐受性和Pb积累能力并对含油率和籽实中Pb含量进行评价,为筛选修复型油料作物以提高土壤污染植物修复技术的经济性提供新思路.
全文HTML
-
供试大豆(Glycine max)、向日葵(Helianthus annuus Linn.)和油菜(Brassica napus L.)品种分别为“中黄25”、“中科茂华矮大盘”和“中油821”. 试验用土采集自江南大学0—20 cm表层花园土,土壤基本理化性质为:pH:6.47,有机质:0.3%,总氮:8.86 g·kg−1,速效磷:0.43 g·kg−1,速效钾:0.94 g·kg−1,未有重金属Pb检出.
-
土壤采集后分拣除去石块和动植物残体,自然风干并粉碎过5 mm筛,移至直径24 cm,高15 cm的花盆,每盆装土2.5 kg,称取相应质量的分析纯Pb(NO3)2溶于水与土壤充分混合、拌匀,回填至花盆,配置成100、250、500、1000 mg·kg−1等4个浓度梯度Pb模拟污染土壤,并以无Pb处理为对照组(CK). 土壤老化15 d,保持田间持水量为60%—70%.以每千克干土加入N 0.25 g、P2O5 0.15 g、K2O 0.1 g计算,施入尿素,磷酸二氢钾和氯化钾作为底肥. 挑选籽粒饱满的种子于0.1% HgCl2溶液中浸泡消毒10 min,取出后用去离子水冲洗,浸泡8 h后于蛭石中((25±2)°C)萌发,采用育苗移栽的方式,待第一片真叶出现,取长势一致的幼苗移栽入花盆中,每盆定植3株. 植物在自然光照下培养,培养30 d后收获植株用于比较作物生长、光合和Pb积累能力,培养至成熟期收获籽实以评估作物含油率和籽实中Pb含量. 整个培养期间随机排列不同处理组的花盆,定期松土浇水,每个处理重复3次.
-
植物收获时尽可能避免根的损失,用自来水冲洗植株后再用去离子水洗净并沥干水分. 植株样品于105°C杀青,85°C烘干至恒重,分别称量根与地上部干重,参照Shibuya等[13]通过测定收获时与收获7 d前的两次地上部干重计算相对生长速率RGR.在晴天9:00—11:00时间段内选取不同处理下生长30 d植物相同叶位的叶片(倒二叶)进行光合指标测定,通过GFS-3000光合仪(WALZ, Effeltrich, Germany)测定净光合速率Pn,设定参数为:相对湿度70%、CO2浓度:385 μmol·mol−1、叶室温度:25 °C、光照强度:800 μmol·m−2·s−1、气体流速:750 μmol·s−1. 通过FluorCam荧光成像仪(FluorCam, Drasov, Czech)测定初始荧光F0、潜在光合活性Fv/F0和PSII最大光化学量子产量QYmax. 参照Yang等[14]的方法消解植株与籽实样品,采用导津AA-7000型火焰原子吸收光谱仪测定Pb浓度,并参照Lam等[15]计算地上部生物富集系数BCF,转运系数TF以及单株根与地上部Pb积累量. 参照黎红亮等[16]采用索氏提取法测定籽实中含油率.
-
所有数据均以3次独立试验的平均值±标准误差(Mean±SD)表示,采用SPSS 20.0软件处理数据分析显著性差异(P<0.05).
1.1. 供试材料
1.2. 试验设计
1.3. 指标测定
1.4. 数据处理
-
如表1所示,100 mg·kg−1 Pb处理对3种油料作物根与地上部干重无影响(P>0.05).250 mg·kg−1 Pb处理增加了大豆干重(P<0.05),对向日葵地上部和油菜根与地上部干重无显著影响. 500 mg·kg−1 Pb处理对大豆干重无影响,但降低了向日葵根与地上部以及油菜地上部干重. 1000 mg·kg−1 Pb处理下大豆、向日葵和油菜干重均降低,根与地上部干重降幅分别为对照的24.0%、31.8%、32.1%和11.5%、12.2%、23.8%.以上结果表明,Pb对大豆生长的抑制程度低于向日葵和油菜且其耐受Pb胁迫的浓度临界值更高.
与本研究相似的是,徐应明等[17]报道了100 mg·kg−1和400 mg·kg−1 Pb处理对大豆生长发育具备刺激作用,且能够提高大豆产量. 另外,3种作物根干重的降幅高于地上部,表明Pb对3种作物根系生长的抑制大于地上部,这可能是由于根系作为植物直接接触土壤中Pb的器官,更易受Pb的毒害. 已有研究发现Pb对水培条件下玉米、黑麦草和紫花苜蓿根长的抑制作用大于株高[18],与本研究结果一致.曹莹等[19]同样发现400—1000 mg·kg−1 Pb胁迫下花生根生物量的受抑制程度高于地上部.
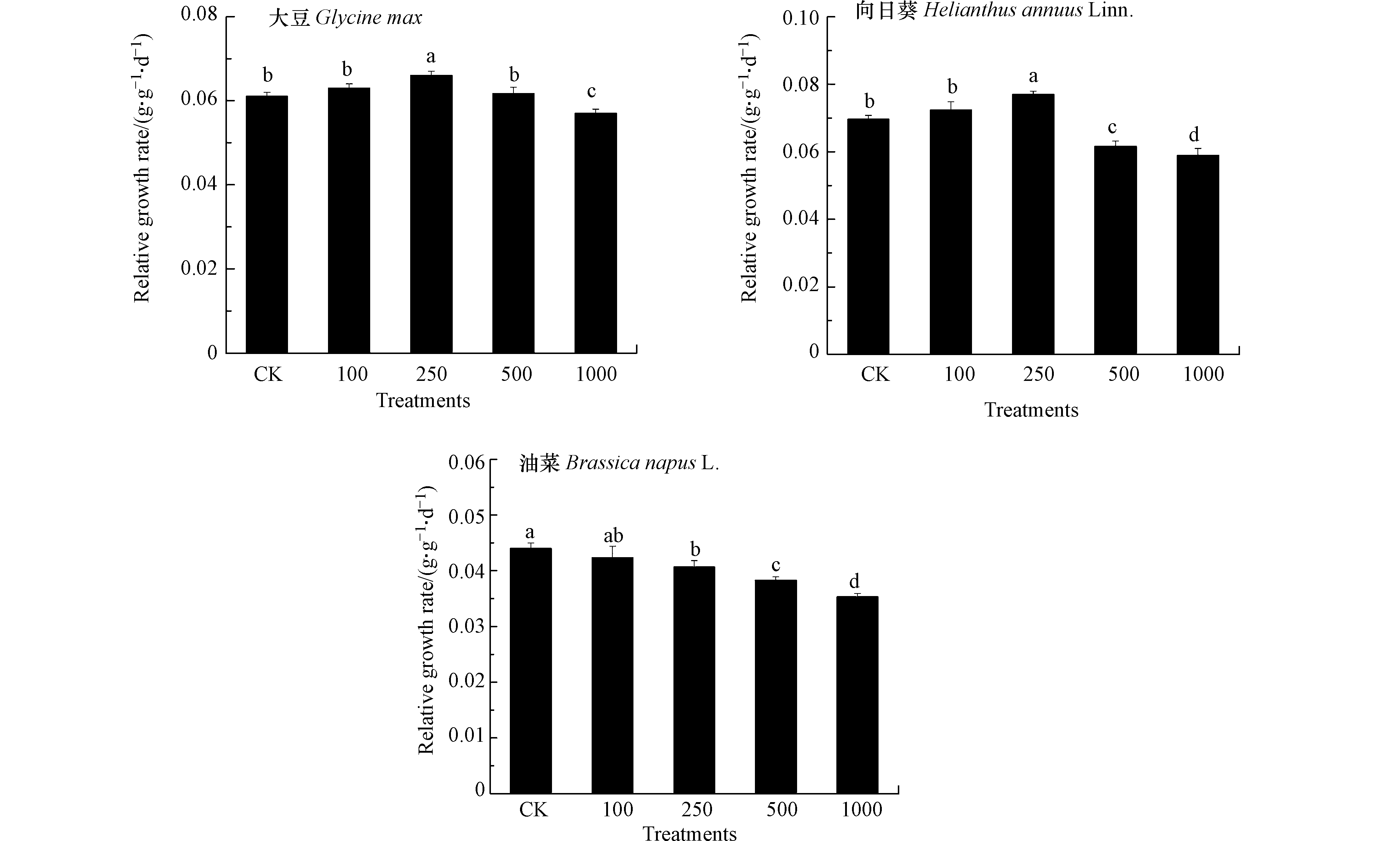
相对生长速率(RGR)被用来量化不同浓度Pb处理下植物生长变化情况,能够避免3种油料作物因个体形态大小差异引起的不同影响[20]. 结合3种油料作物生物量的变化情况能够一定程度反映植物对Pb的耐受性. 如图1所示,100 mg·kg−1 Pb处理对3种油料作物地上部RGR无影响. 250 mg·kg−1 Pb处理增加了大豆与向日葵RGR,降低了油菜RGR.500 mg·kg−1 Pb处理下大豆RGR与对照相比仍无差异,而向日葵和油菜RGR降低. 1000 mg·kg−1 Pb处理下3种油料作物RGR均降低,且油菜降幅最大,其次是向日葵和大豆.对照生物量的变化状况,可以发现大豆、向日葵和油菜地上部生物量在Pb处理下的变化差异是由Pb对3种油料作物生长速率的不同抑制程度引起的,可以推测随Pb处理时间延长,3种油料作物生物量对Pb的响应差异可能进一步扩大. 结果表明,在生长层面3种作物对于Pb的耐受顺序为大豆>向日葵>油菜. 赵鲁等[21]研究发现在175—1050 mg·kg−1 Pb处理下大豆对Pb的耐受性高于小麦,并随种植时间延长而增强. Zhi等[22]对23个大豆栽培品种的Pb耐受性进行评估,发现大部分大豆对土壤中的Pb具有高耐受性.
-
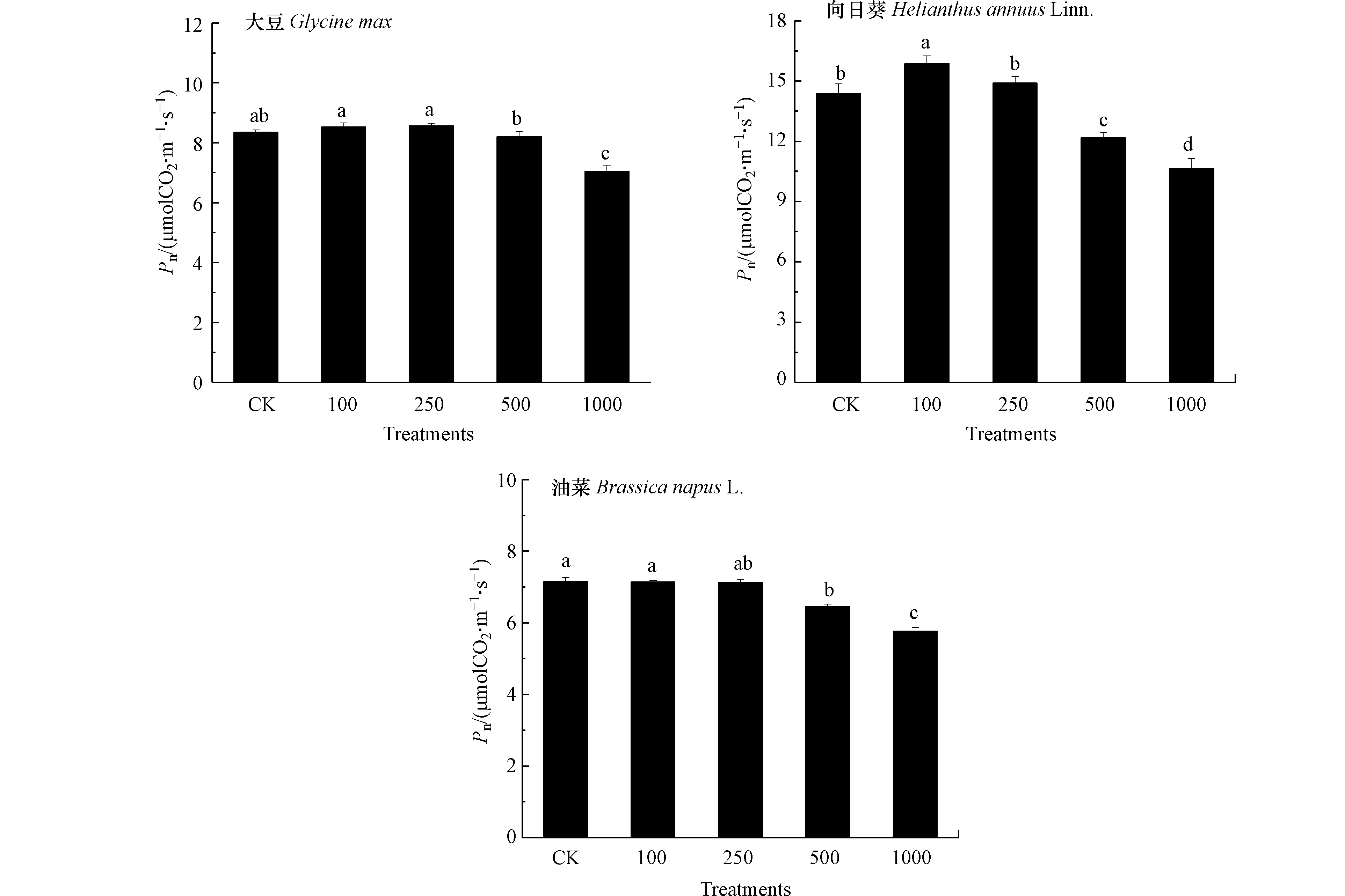
重金属对植物生长的影响与生理过程直接或间接相关,植物生长发育的生理基础和物质前提是光合作用和其生产的有机物[23].如图2所示,100 mg·kg−1 Pb处理对3种油料作物Pn无显著影响,250 mg·kg−1 Pb处理下大豆和向日葵Pn升高,而油菜Pn降低,与该处理下3种油料作物生长表现出的差异一致. 500 mg·kg−1 Pb处理下大豆Pn仍处于对照水平,向日葵和油菜Pn低于对照,1000 mg·kg−1 Pb处理下3种油料作物Pn均降低,且向日葵和油菜降幅高于大豆. 结果表明大豆光合作用受抑制程度低于向日葵和油菜,且Pb对3种油料作物光合作用的不同影响是造成Pb胁迫下作物生长具有差异的原因. 有研究发现[24],Pb能够降低植物PSI光系统活性,加剧PSII光系统供体侧和受体侧损伤,从而抑制光合作用.
叶绿素荧光不仅反映了光合作用中能量的吸收和转化,而且与光化学反应中的电子传递过程密切相关[25]. 如表2所示,3种油料作物初始荧光F0,潜在光合活性Fv/F0和PSⅡ最大光化学量子产量QY_max在不同浓度Pb处理下表现出种属特异性. 作为光系统Ⅱ(PSⅡ)反应中心处于完全开放时的荧光产量,3种油料作物F0随Pb处理浓度升高呈现增加趋势,且增幅为向日葵>油菜>大豆,F0的增大代表PSII能量耗散增强,PSII反应中心失活,表明3种油料作物光合机构受损程度为向日葵>油菜>大豆. Fv/F0和QY_max分别代表天线色素到PSII的光能传递能力和原初光能转换效率[26]. 100 mg·kg−1 Pb处理对3种油料作物Fv/F0和QY_max无影响,250 mg·kg−1 Pb处理降低了向日葵与油菜Fv/F0和QY_max,对大豆无影响.500 mg·kg−1和1000 mg·kg−1 Pb处理下3种油料作物Fv/F0和QY_max均降低,且Fv/F0降幅高于QY_max,表明Fv/F0和QY_max对Pb的敏感性不同,高浓度Pb抑制了3种油料作物PSII能量传递效率与光能转化效率,以传能效率受抑更为严重.
另外,大豆Fv/F0和QY_max的降幅低于向日葵和油菜,表明与向日葵和油菜相比,大豆光合机构不易受Pb胁迫损伤,这可能是大豆对Pb具有较高耐受性的原因.
-
如表3所示,大豆、向日葵和油菜根与地上部Pb浓度随Pb处理浓度升高显著增加且种间差异显著. 100 mg·kg−1 Pb和250 mg·kg−1 Pb处理下大豆和向日葵根与地上部Pb浓度处于相同水平,500 mg·kg−1 Pb和1000 mg·kg−1 Pb处理下大豆根中Pb浓度低于向日葵,而地上部Pb浓度高于向日葵,表明随Pb处理浓度升高,大豆根系积累Pb的能力弱于向日葵,但将Pb从根转运至地上部能力更强. 王帅等[9]报道了水培条件下Pb在苗期大豆组织中的迁移率高于向日葵和蓖麻,但低于花生,这与本研究结果一致.对于油菜,其根与地上部Pb浓度均低于大豆和向日葵,表现出最低的Pb积累效率. Marchiol等[27]比较了2种十字花科植物对土壤重金属的吸收积累能力,发现与油菜相比,萝卜地上部对土壤中重金属的积累更为有效. 另外,3种油料作物根中Pb浓度均高于地上部,表明Pb主要在3种油料作物根部积累,这可能是根系生长受抑制程度高于地上部的原因. 杜兵兵等[28]在茶树中发现了与本研究结果相似的Pb累积分布规律,具体表现为根>茎>老叶>嫩叶. Pb在植物地上部积累受限可能与植物根系对Pb的防御机制有关. 研究表明,被植物根系吸收的Pb会在内皮层凯氏带附近累积,且植物根系多种亚细胞结构也可以固定截留Pb以阻碍其向地上部迁移[29].
植物体内Pb的积累量与植株中Pb浓度和光合作用产生的生物量有关,评估3种油料作物中Pb积累量能直观反映出3种作物对土壤中Pb的植物提取差异. 3种油料作物根与地上部Pb积累量与Pb处理浓度显著正相关,且地上部Pb积累量高于根. 在100—1000 mg·kg−1 Pb处理下大豆Pb总积累量为13.09—43.14 µg,高于向日葵(14.75—29.42 µg)和油菜(2.30—7.24 µg). 1000 mg·kg−1 Pb处理下大豆地上部Pb积累量最高为34.64 µg,占大豆植株Pb总积累量的84.93%,分别是相同处理下向日葵和油菜地上部Pb积累量的1.89倍和6.41倍,表明在相同生长时间内大豆对Pb的植物提取能力最强,能够通过收获植株地上部去除土壤中较多的Pb. 另外,油菜在3种作物中Pb积累量最低应归因于油菜植株最低的Pb浓度与生物量.研究表明,田间条件下单位面积大豆地上部Pb积累总量高于向日葵、油菜和芝麻,且大豆和油菜轮作系统对土壤中Pb的提取效率最高[14]. 因此,在实际修复过程中可选择植物提取能力强的油料作物,通过采取适当的农艺措施并调整种植结构以获得最好的修复效果.
为了阐明3种油料作物对于Pb的吸收和转运特性以比较植物提取Pb的潜力,引入了生物富集系数BCF和转运系数TF,分别代表植物地上部Pb积累能力以及Pb从根转运到地上部的能力[30]. 本研究中,3种油料作物BCF均低于1,且随Pb处理浓度升高呈现降低趋势. 不同Pb处理下大豆BCF高于向日葵和油菜,表明大豆能够在其地上部积累更多的Pb. 对于TF,3种油料作物中大豆最高(0.55—0.76),其次是油菜(0.41—0.71)和向日葵(0.18—0.75),表明大豆对Pb的转运能力最强,使其与向日葵和油菜相比能将更多的Pb转运至地上部积累. Ali等[31]回顾了适合植物提取的作物应具备生长速率大、生物量高、耐受目标金属并将其积累转运至地上部的能力强、易于栽培和收获等特征. 考虑到在实际生产过程中的种植时间,意味着与向日葵和油菜相比,通过持续收获具有较高Pb积累能力的大豆将土壤中Pb浓度降低至无害水平所用的时间更短. 结合3种油料作物对Pb的耐受与积累差异,大豆是适用于Pb污染土壤植物修复的潜在油料作物.
-
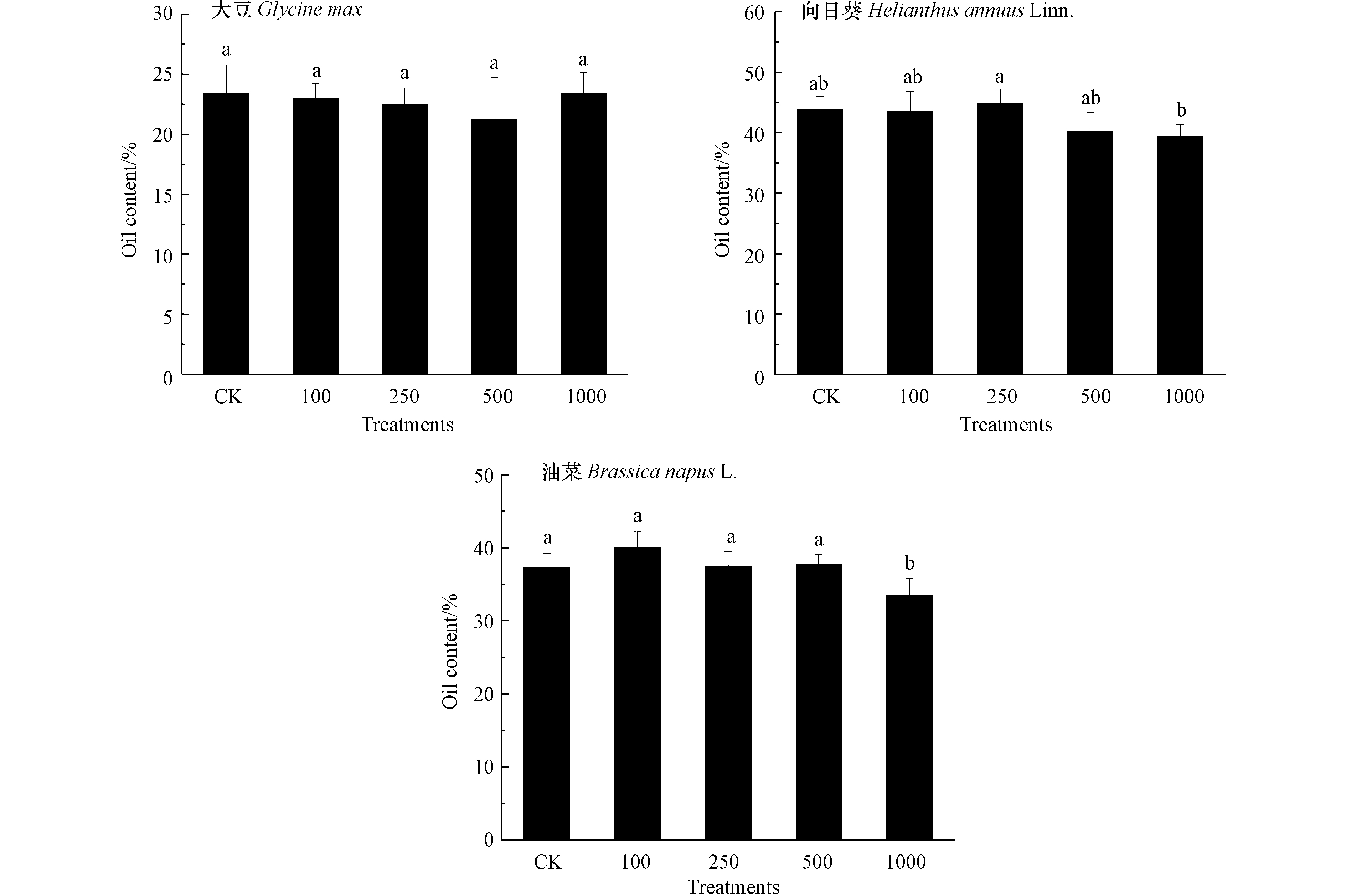
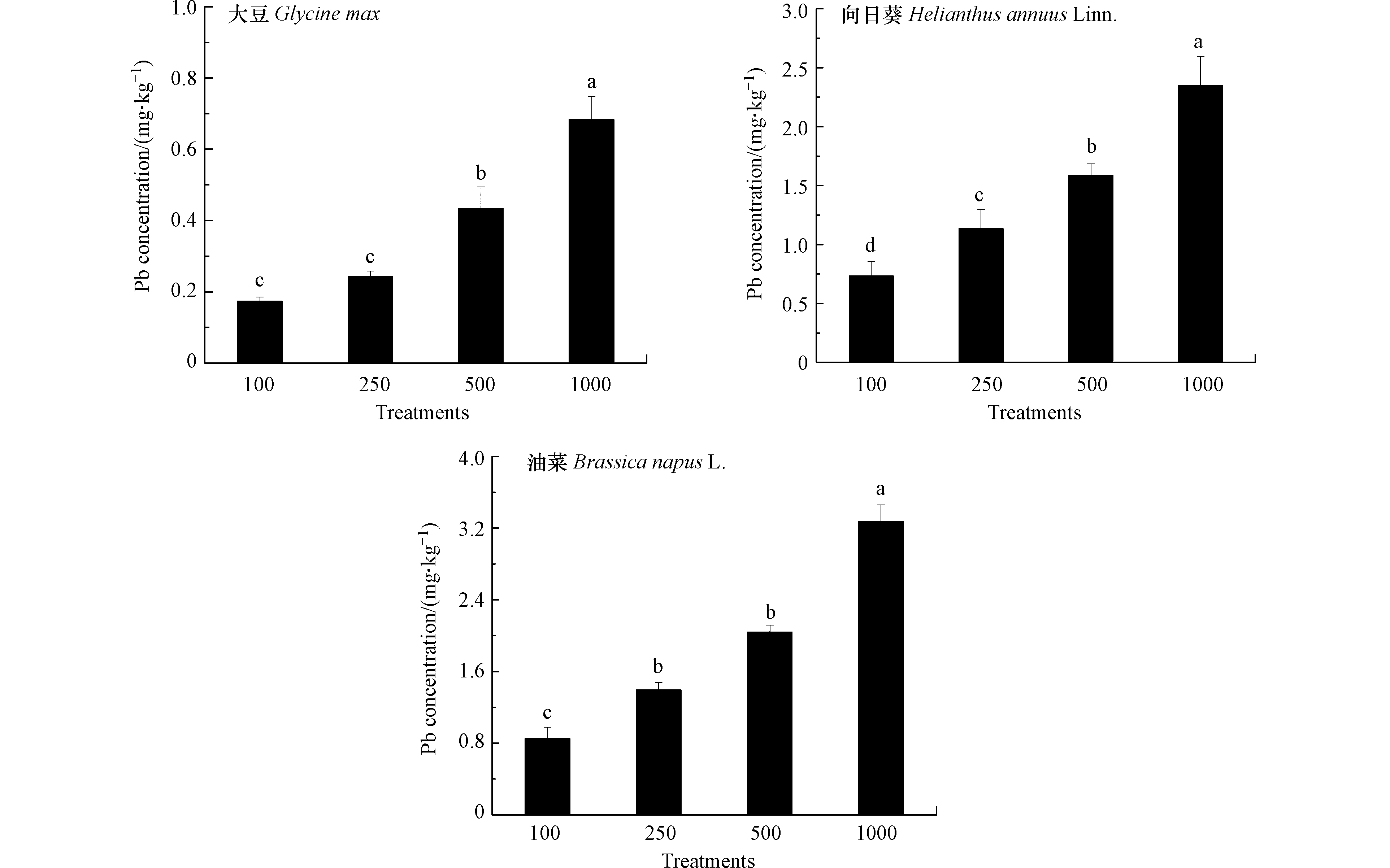
评估3种油料作物含油率与籽实中Pb含量是利用油料作物修复Pb污染土壤获取经济效益的重要参考. 如图3所示,100 mg·kg−1到500 mg·kg−1 Pb处理下3种油料作物含油率与对照相比无显著差异,经济性状未受土壤中Pb影响.1000 mg·kg−1 Pb处理导致向日葵和油菜含油率降低,而大豆籽实含油率仍处于对照水平,表明与向日葵和油菜相比,选择大豆提取土壤中的Pb更能保障经济价值的实现. 曹莹等[32]发现,随Pb处理浓度升高,玉米籽实中脂肪含量呈现降低趋势. 如图4所示,大豆、向日葵和油菜籽实中Pb含量随Pb处理浓度升高显著增加,在1000 mg·kg−1 Pb处理下分别达到0.68、2.35、3.27 mg·kg−1. 研究表明,油料作物经油脂提取后Pb主要在油粕中残留,而油分中Pb含量较低,可用作生物柴油或生物燃料[33]. 结合本研究结果可以发现,与向日葵和油菜相比,大豆籽实中较低的Pb含量意味着将其用于修复污染土壤时,获取经济价值的安全风险较低. 另外,油料作物籽实凭借其低纤维高蛋白特征还能够作为畜禽饲料的主要来源[34]. 本研究条件下3种油料作物籽实中Pb含量均符合饲料卫生标准(GB13078-2017)制定的10 mg·kg−1 Pb限值,这也为利用油料作物修复Pb污染土壤的同时获取经济效益提供了可行的途径.
2.1. Pb对3种油料作物生长的影响
2.2. Pb对3种油料作物净光合速率和叶绿素荧光的影响
2.3. 3种油料作物对重金属Pb的吸收与积累
2.4. 3种油料作物籽实中Pb含量及含油率
-
(1) 比较同一Pb处理下3种油料作物生长状况和光合能力的变化,发现大豆生长对Pb的耐受能力最强,其次是向日葵和油菜.
(2) Pb对3种油料作物光合效率抑制程度不同,其中Pb通过抑制植物光能转换与传递效率降低光合效率,造成生物量积累减少. 与向日葵和油菜相比,大豆对Pb较强的耐受能力可能与其光合作用受Pb抑制较小有关.
(3) 本研究实验培育期内,3种油料作物中大豆对Pb的积累与转运能力强于向日葵和油菜,结合其较强的耐受性及经济价值的实现,是Pb污染土壤植物修复的较好选择.对于3种油料作物成熟期对Pb的耐受与积累状况及在实地污染土壤中的应用有待进一步研究.



 下载:
下载: