-
随着经济的不断发展,资源环境在满足人们不断增加的物质文化需求的同时也受到了相当大的危害,并带来了一系列环境问题,如重金属污染、有机污染物污染以及其他污染[1-2]。零价铁作为一种还原剂,广泛存在于自然界,自身价格低廉、无毒害作用,在环境修复中有巨大潜能,但其比表面积较小,影响处理效果。而还原性更强、比表面积更高的纳米零价铁的出现,有效解决了零价铁作用效果不明显的问题,使其成为土壤和地下水修复最有效的技术之一[3-4]。近年来,纳米零价铁已被广泛用于多种污染物的去除,其中包括 (氯代烃,硝基芳烃,多氯联苯,有机磷酸盐,重金属等),在环境污染的净化中展现出显著的作用效果[5]。但纳米零价铁自身存在易团聚、易氧化、难回收以及潜在生物毒性等问题,限制了在实际中的广泛应用[6]。针对纳米零价铁存在的问题,不同研究者提出了很多新的制备方法 (负载型稳定化处理与表面改性稳定化处理等),显著提高了纳米零价铁的稳定性与处理效果[7-8]。
据调查,已超过 50 种不同类型的纳米零价铁的试点或大规模应用于实际场地,但仍存在一些关键问题需要改进,才能使其大规模的商业化 [6, 9-10]。目前,在纳米零价铁的推广应用方面存在的主要问题就是纳米零价铁的量产问题。以往采用传统的水热法制备的纳米零价铁的量较少,且仅限于实验室研究阶段。而球磨法的出现,一方面可以使得纳米材料以 kg 量级产出[11],为纳米零价铁工业化量产提供了全新的思路;另一方面,通过球磨制备出一种 Fe 基复合体系,进一步提高了纳米零价铁体系整体的作用效果。
本文针对球磨法的作用机理、影响因素及其在纳米零价铁体系的制备以及应用方面做了简要概述,并对未来球磨法在纳米零价铁制备方面提供一些思路。
-
球磨法制备过程较为复杂,影响球磨产物的因素包括球磨时间、球磨转速、球料比、球磨气氛、磨球大小及比例、球磨温度、工艺控制剂、研磨原料的性质、球磨的间歇时间以及球磨机的类型等。一种或者多种参数的变化,都会对球磨产物的颗粒粒径、形貌特征以及类型产生影响,因此球磨参数的设置主要根据球磨原料以及生成物的特性来确定。
通常来说,球磨时间的长短主要取决于磨机类型、转速、球料比。球磨时间的延长,能够使晶体颗粒不断细化并逐渐趋于均匀化。然而,当球磨时间超过所需时间,粉末污染会增加,使得动力学结晶加强,在纳米晶形成过程中使平均粒径增加[17]。球磨机的转速越高对粉末施加能量越高[18]。当转速大时,碰撞的频率快,能量转化大,局部的快速升温转为整体温升,会使非晶体再结晶,进而使粒径增大;球磨转速过小,则会导致研磨不充分。在高球料比条件下,磨球之间碰撞的频率增加,使粉末能量增加,晶体单位时间粒径变化大,过程引起的粉末温升现象,可能会使得非晶向晶体转变[19-21]。另外,不同的粒径的磨球混合使用时,球磨的效果会更显著。
球磨过程中因机械摩擦、碰撞,产生大量的热,同时原料粒径达到纳米尺寸后,活性更强,会与球磨罐内所通气体反应,导致粉末产物污染[22]。因此,在合金化过程中,球磨罐抽真空或冲入惰性气体(氩气或氦气)是有必要的。为了控制球磨中的冷焊、粘结作用,通常会添加一定量的工艺控制剂(有机化合物或轻质有机溶剂),使其吸附在粉末表面,延缓颗粒间的冷焊作用,增强粉末分散效果,使得球磨所需时间变短,并且制备出粒径更小的粉末颗粒。但是过量的工艺调控剂不仅会影响原子扩散,还会使产物污染[23]。
-
纳米零价铁的制备主要有物理制备法和化学制备法,其中物理制备法主要有高能球磨法、蒸汽冷凝法、溅射法和等离子体法等 (表 1),化学制备法包括有液相还原法、固相还原法、气相还原法以及电沉积法等 (表 2),并且大部分集中在液相还原制备。
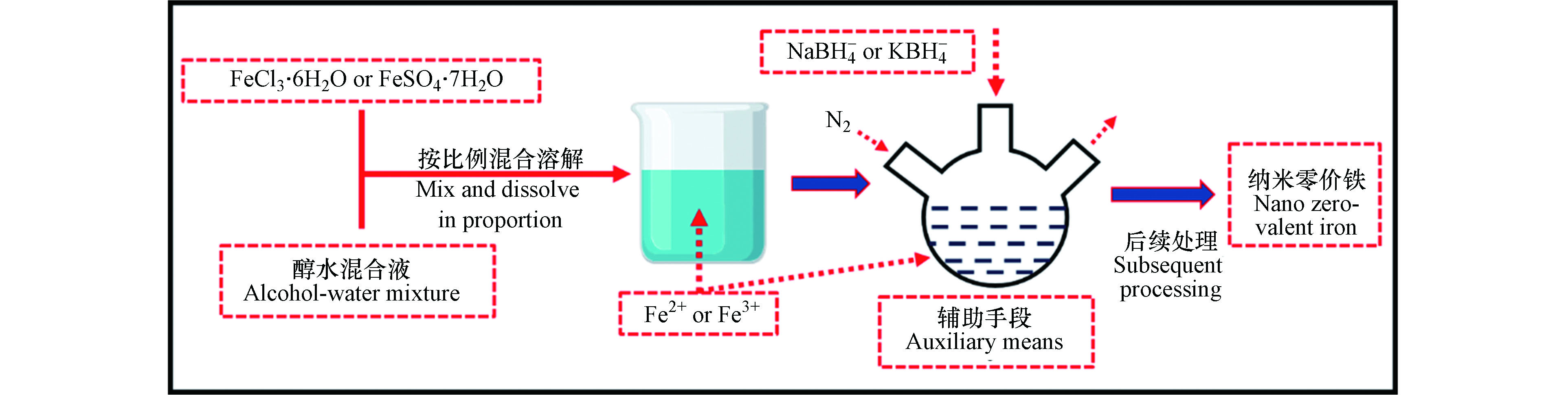
液相还原法制备纳米零价铁,主要以 Fe2+ 或 Fe3+ 为前驱体,在惰性气体 (氮气或氩气) 保护下,以液相环境为反应介质,利用液相还原剂还原制备。实验室制备通常选用 NaBH4− 或 KBH4− 溶液作为还原剂,流程 (图 2)。Fu 等[33]和 Zhang 等[34]利用此法,在还原反应开始前,分别添加经处理后的沸石、改性稻壳,最终制得稳定性与分散性更强的两种负载型纳米零价铁,其中前者应用于水体污染物中的酸性橙7的处理中,后者应用于对硝基苯的去除,并指出经负载后的纳米零价铁体系对污染物的去除效果更佳。
然而,利用 BH4− 还原制备纳米零价铁的成本高[35],且产物溶液中含有高浓度硼污染物[36],倘若后续处理不当,易造成二次污染。近些年来,将一些从植物里提取出的绿色还原剂应用到纳米零价铁的制备中也逐渐被注意[37-38],作为 BH4− 的取代物,虽然有效解决了 BH4− 价格昂贵和产物溶液的高硼污染问题,但其产量依旧满足不了大规模的推广应用。
除此之外,液相还原制备过程易混入杂质,且颗粒粒径分布不均。尽管球磨法制备纳米零价铁的过程中也存在易混入杂质、易黏结的问题,但通过调控球磨参数可以缓解这些问题。对比这两种制备方法的经济和环境效益,液相还原法具有较好的环境效益,而球磨法的经济效益较为明显[39-40]。在量产方面,球磨法具有液相还原法不可比拟的优势。
鉴于纳米零价铁的高活性及应用前景,综合考虑其制备成本、设备要求、能耗、产量、产率与制备过程中操作复杂度以及所制得颗粒的分散性、粒径、形貌、结晶程度等因素,球磨法制备或协同制备纳米零价铁及其复合体系具有较好的应用前景。
-
由于纳米零价铁易团聚以及因表面钝化而很快失去其活性,其单独用于污染物的修复,处理效果不佳。但是,通过一些方法的改进,可以得到不同种类的纳米复合体系,从而明显提高其活性。一是制备方法的改进,通过用负载材料负载、改性物质稳定化处理等方法 (图 3),从而达到提高纳米零价铁的分散性,降低其氧化产物的生成速率的目的。二是在应用过程中,通过一些现有技术的支撑,来提高纳米零价铁的处理效果,如利用超声辅助法[41]的热力作用与空化作用使其分散均匀,提高反应活性;利用弱磁场能有效地增强纳米零价铁的降解能力[32];利用恒温搅拌,提高纳米零价铁体系的处理效果等等。
负载型稳定化处理,一般选用一些孔隙多、比表面积大的多孔材料,如无机材料、有机高分子材料、多孔纳米材料等。为了降低环境修复成本,提高重复利用率,使用后的回收也是需要注意的问题。当负载材料为磁性物质,如 Fe3O4 等,可以提高纳米零价铁的回收率,还可以降低纳米零价铁的环境毒性效应[42-43]。与负载型稳定化处理有一些区别的纳米双金属体系[44]的应用也比较广泛,该体系是在零价铁表面镀上适量的还原电位高的金属,从而起到增加活性位点、加速氢化反应、缓解纳米零价铁钝化速率等作用。表面改性稳定化处理,一般选用一些表面活性剂之类的有机物[45]。另外,改性物质膜处理中改性物质有一部分具有导电性,除了提高体系分散性、降低体系粒径的作用,还可以提高纳米零价铁的对污染物的处理效果。与表面改性稳定化处理有一定区别的包埋型纳米零价铁体系[46]也有人关注,该体系中特殊的包埋材料可以有效防止纳米零价铁的流失,同时又允许污染物分子进入体系内与纳米零价铁发生降解反应,还有利于固液分离、提高出水水质。
为了更好地提高纳米零价铁的处理能力,目前的研究已不仅仅局限于单一的复合体系,将多种复合体系的优点结合到一起已成为一种新趋势。利用生物炭 (BC) 负载经聚乙二醇 (PEG) 稳定化处理后得到的 PEG-nZVI@BC 体系对重金属 Cr(Ⅵ) 的修复效果远远优于 BC-nZVI (nZVI@BC)[47]。采用绿色还原合成法合成了纳米 Fe-Cu 双金属体系与膨润土负载后的纳米 Fe-Cu 体系对四环素的去除能力进行对比,发现两者去除率都较好。但膨润土负载后的纳米复合体系较于纳米 Fe-Cu 双金属体系在水体中存在更稳定[36]。
-
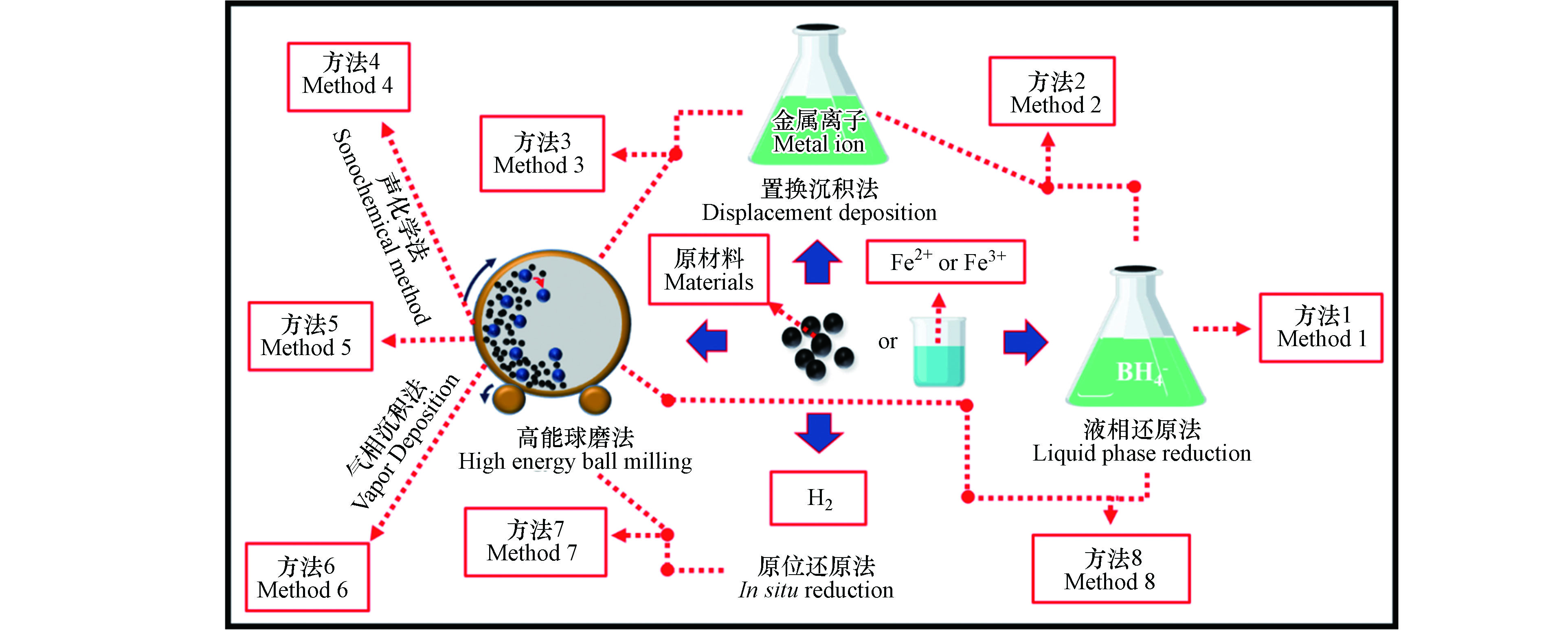
由于不同的制备方法各有优缺点,综合利用多种方法协同制备纳米零价铁复合体系已经成为一种趋势 (图 4),除了可以提高纳米零价铁体系的产量,还可以改善纳米零价铁的纯度,同时还有可能提高复合体系的作用效果。
液相还原法 (方法1) 通常只能制备出纯的纳米零价铁,但纳米零价铁复合体系却已不再局限于单一体系,为了更明显地提高体系的作用效果,使其兼备多种优势,在制备方法上也必须有所改进。
在液相还原制备的基础之上,结合置换沉积法 (方法2),可制备功能更强的纳米零价铁复合体系 (图 5)。Li 等[48]采用此法利用此法制备出生物炭负载纳米 Fe-Ni 双金属体系,并指出生物炭与 Ni/Fe 质量比从 0 增加到 1.0 后,1, 1, 1三氯乙烷的去除率由 42.3% 提高到 99.3%。潘煜等[45]利用类似办法制备了纳米 Fe-Cu 复合体系,并在此基础上加入了不同质量的羧甲基纤维素 (CMC) 改性处理,制备了粒径更小、反应活性更高、分散性更强的 CMC 改性纳米 Fe-Ni 双金属体系。
以液相还原法为基础,结合球磨法协同制备 (方法8) 的纳米零价铁复合体系似乎要更优 (图 6)。De 等[49]先利用过量的 NaBH4 溶液还原 Fe2+ 制备了纳米零价铁,并指出该法制备产率近 70%,平均粒径约为 32 nm。另外,又将螯合配体-聚芳醚酮作为改性物质对其进行改性处理,使平均粒径减少到18.3 nm。最后经过在球料比为 40:1,球磨时间为 3 h,转速为 300 r·min−1 的球磨条件下,对液相还原制得的纳米零价铁进行球磨,整个体系的平均粒径降为 13.8 nm。
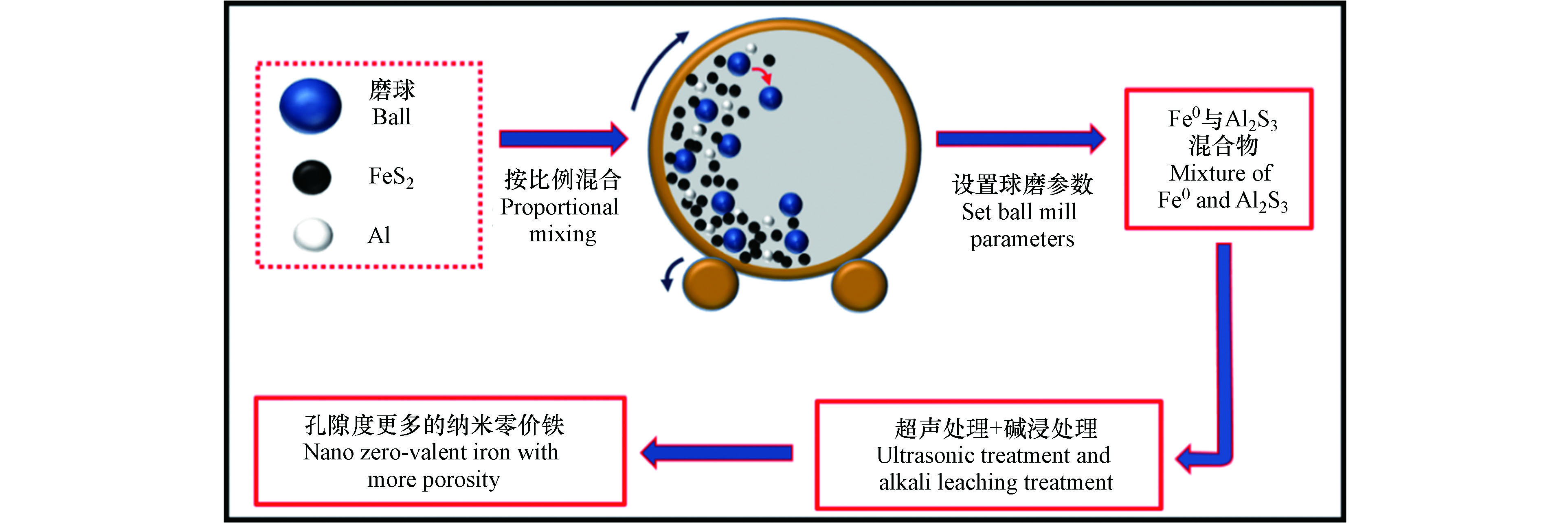
与液相还原法不同的是,由于球磨法其特有的机械力化学特性,可以制备出液相还原无法制备出的复合材料。除此之外,球磨法制备纳米零价铁及其复合体系所需用的原料相较于液相还原法更加广泛,可以是以氧化铁、四氧化三铁、羟基氧化铁等为主要成分的含铁矿物,也可以是经过进一步提纯后得到的还原铁粉,甚至还可以是液相还原法所用的 Fe2+ 或 Fe3+ 溶液。以天然黄铁矿和铝金属粉末为原材料,利用两步反应法 (方法4) 制备出孔隙度更多、比表面积更大的纳米零价铁,制备流程见图 7[50]。由此研究基础,可将实验所需的原材料进一步拓展,利用机械力化学反应有效降低反应活化能,诱导热化学反应无法实现的化学反应这一突出优势,同时结合其他辅助手段来制备多孔纳米零价铁也不失为一种新的思路。但是,该体系中球磨原料均为固体,由于固体与固体之间的扩散速率远远低于固体与液体之间的扩散速率,其反应过程中所需要的能量更高,反应速率更慢。为提高产物制备效率,优化产物特性,固液反应球磨制备也是一种有巨大应用潜力的纳米颗粒制备技术[51]。
球磨法单独制备 (方法5) 单一的纳米零价铁并不常见,极大可能是由于纳米零价铁的特性限制。为了克服这一局限,在球磨之前,通过加入 Al2O3 进行调控,最终制备出的颗粒分散性好,几乎无团聚现象,而且产物比表面积较大、产率较高[10]。另外,加入混合料(碳化硼、碳化硅、氧化铝、石英砂等),经过球磨合成粒径为微米级的不同Fe基复合材料,并将其用于 2-CP 的去除,发现 SiO2-Fe 对 2-CP 脱氯效率最高[52]。
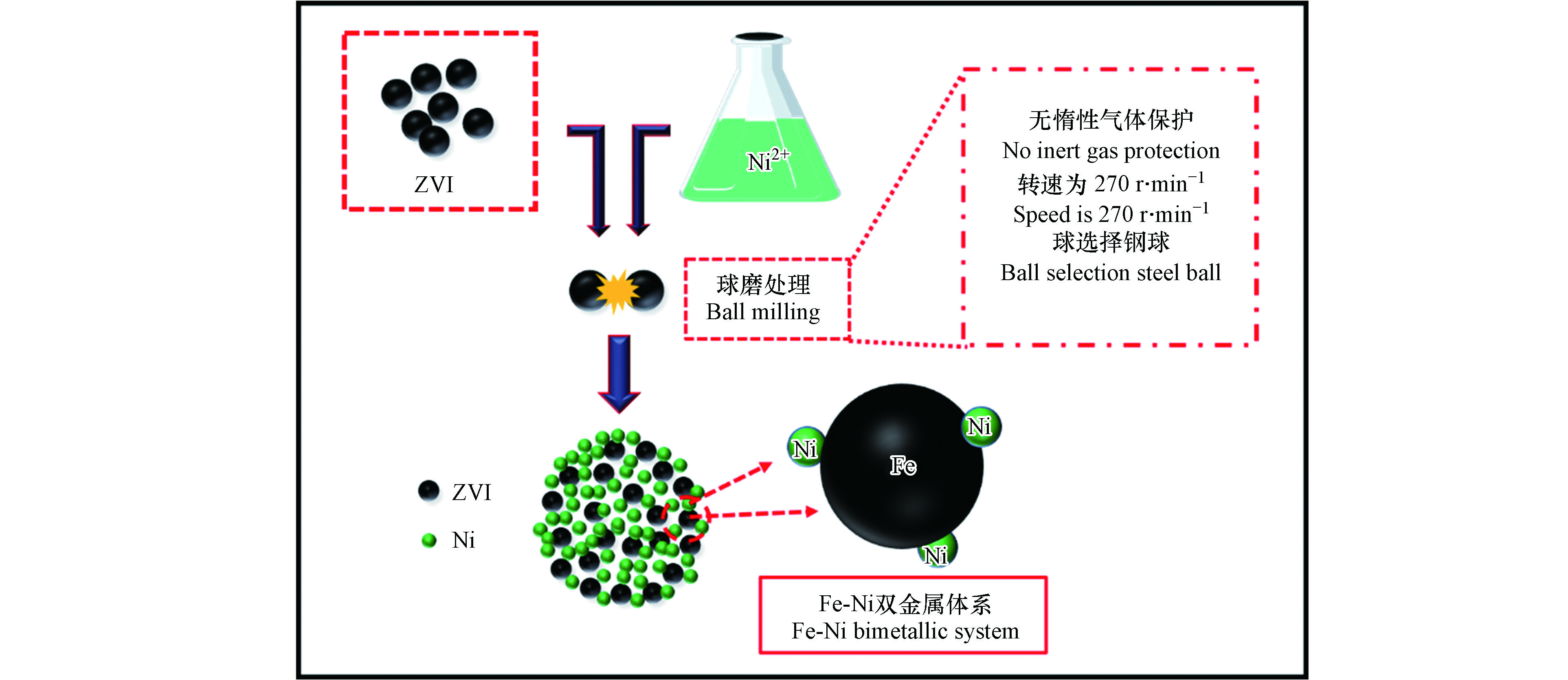
球磨法在制备纳米 Fe 基合金方面的应用更为广泛,机理(见图 1)。Zhang 等[53]以球磨法为基础,结合置换沉积法 (方法3),制备出纳米 Fe-Ni 双金属体系(图 8),将其应用到氯化有机污染物的处理中,并指出该法制备的双金属可以有效减小催化剂的尺寸,同时可以更好地控制 Ni 在 Fe 表面的均匀分布,而且 Fe-Ni 体系脱氯活性随着球磨时间的延长而显著增加,在球磨时间为 4 h 时显示出最大活性,并在 90 min 的反应时间内可实现对 4-CP 快速完全脱氯。刘银等[54]选用纯度高于 99% 的 Fe2O3 和 NiO 为原材料,在确定原料最佳配比后,将原料放置到球磨罐中,在球磨转速分别为250、280 r·min−1、球料比为 6:1、工艺控制剂为无水酒精、磨球 (直径为10 mm和20 mm混合) 的条件下进行球磨,经干燥后,利用氢气原位还原法进行还原 (方法7),最后经烧结制备出平均粒径约为 35 nm Fe-Ni 合金。但由于原位还原法的高耗能、高污染的缺点,不适合广泛应用[40]。而 Aaoullar 等[55]采用低压气相沉积法替代氢气原位还原法 (方法6),有效解决了氢气还原法高污染的问题,并结合球磨法,利用两种不同气体 (H2和CH4) 为还原剂 (CH4的存在还可以促进纳米颗粒周围石墨壳的形成),在合适的温度和气体流速条件下,可将球磨制备的 Fe2O3-C 体系中的 Fe2O3 完全还原为 Fe0,进而制备出 Fe0-C 复合体系。
以球磨法为基础,结合置换沉积法与原位还原法,却制备出不同类型的物质,原因可能是原位还原法提供的能量远远高于置换沉积所提供的。对比两种不同类型的物质,作者认为纳米 Fe 基合金与纳米 Fe 基双金属体系有类似,但也有所区别。在定义范围上,纳米双金属体系是无定型合金的一种,属于二元合金的范畴,而合金包括二元以及多元合金;在结构上,填隙型的纳米 Fe 基合金比置换型的纳米 Fe 基双金属更为复杂,结合度更强;在应用上,纳米 Fe 基合金常常被用于工业、建筑业等,仅有少部分人将合金应用于环境污染修复中[56-57],但纳米 Fe 基双金属则主要应用于环境污染修复中。纳米双金属体系的结合度并不是很高,可能会影响去除效果。所以,将结合度更高的二元甚至多元合金应用于环境污染修复中可能会成为一个新的研究方向。
-
球磨法是一种纳米材料制备方法,突破了传统的冶炼合金的局限,并且在制备新型材料以及改善材料性能方面有巨大潜力。纳米零价铁优越的吸附性能和还原活性,在环境污染修复领域也备受关注。针对球磨法在纳米零价铁领域中的应用以及未来球磨法以及纳米零价铁的发展有如下建议:
(1)球磨法在制备纳米合金的研究较多,然而球磨法制备的纳米合金在环境污染控制领域的应用研究甚少;
(2)在纳米零价铁制备方面,球磨法有望与其他新型的制备方法结合,进一步提高纳米零价铁及其复合体系的活性,从而提高其处理效果;
(3)除了利用球磨法协同制备和活化纳米零价铁及其复合体系,提高重复利用率以外,球磨法有望制备新型环保可回收纳米多孔材料以及新型可降解无污染的有机高分子材料来稳定化处理纳米零价铁,可能有更好的处理效果。然而,球磨法的作用机制较为复杂,迫切需要更为完善的理论支撑。
球磨法制备纳米零价铁的研究进展
Research progress of preparation of nano zero-valent iron by ball milling
-
摘要: 纳米零价铁作为一种新型的环境修复材料,兼备纳米材料巨大的表面能与零价铁的还原特性,在环境修复方面已展现出巨大的潜力。然而由于常规方法制备的纳米零价铁的产量较少,无法满足其大范围的实地推广应用。而球磨法作为一种超细纳米材料制备方法,在纳米零价铁的制备方面具有明显优势,除了可以实现纳米零价铁的工业量产,还可以通过构成一种铁基复合体系,增强体系活性,进而提高其对污染物的选择性。本文综述了球磨法制备纳米零价铁及其复合体系,主要从以下三方面进行概述:(1)球磨法的作用机理和主要影响因素;(2)纳米零价铁及其复合体系的制备;(3)球磨法目前存在的问题以及未来的研究方向。
-
关键词:
- 球磨法 /
- 纳米零价铁及其复合体系 /
- 制备
Abstract: Nano zero-valent iron, as a new type of environmental remediation material, combines the huge surface energy of nano-materials with the reduction characteristics of zero-valent iron, and has shown great potential in environmental remediation. However, due to the low yield of nano-zero iron prepared by conventional methods, it cannot meet its large-scale field application. As a preparation method of ultra-fine nano-materials, the ball milling method has obvious advantages in the preparation of nanometer zero-valent iron. The ball milling method can not only realize the industrial mass production of nano zero-valent iron, but also can form an iron-based composite system to enhance the activity of its surface system, thereby improving its selectivity for pollutants. This article summarized the preparation of nano zero-valent iron and its composite system by ball milling, mainly summarized from the following three aspects: (1) The action mechanism and main influencing factors of the ball milling method; (2) The preparation of nano zero-valent iron and its composite system; (3) The current problems and future research directions of the ball milling method. -

-
表 1 不同物理制备法及其优缺点
Table 1. Different physical preparation methods and their advantages and disadvantages
物理制备法
Physical preparation method制备过程
Preparation Process优点
Advantages缺点
Disadvantages高能机械球磨法[24] 利用磨球对金属原料的撞击、压缩、断裂、研磨等作用制备 产量大;成本低;工艺简单 颗粒易粘结氧化;易混入杂质 蒸汽冷凝法[25] 将金属原料蒸发,在通过蒸汽的冷却凝结作用制备 颗粒粒径小且均匀;结晶程度好;分散性好 操作难度高;耗能大;难控制 溅射法[26] 利用辉光放电在惰性气体或活性气体中,使放电的离子冲击材料靶,使其上面的原子蒸发出,后经冷凝处理制备 产物粒径较小,形貌均匀;纯度高 设备要求极高,产率低 等离子体法[27] 利用含有氢气等离子体与金属间电弧产热效应,使得金属熔融,经惰性气体吹出、收集后制得 产物纯度高;颗粒粒径小且分布均匀 设备要求高;能量消耗大 表 2 不同种化学制备法及其优缺点
Table 2. Different chemical preparation methods and their advantages and disadvantages
化学制备法
Chemical preparation method制备过程
Preparation Process优点
Advantages缺点
Disadvantages液相还原法[28] 以含铁盐为前驱体,在惰性气体保护下,以去离子水为介质,用液相还原剂还原制备 制备的颗粒形貌相对统一,粒径较为均匀,活性较高;制备条件温和,操作简单,用时较少 还原剂的成本较高;前驱体的环境危害效应;制备的颗粒分散不均容易粘结团聚 气相化学反应法[29] 以易于气化和分解的含铁材料为前驱体,经高温分解制备 颗粒形貌、粒径、分散性效果最佳;颗粒粒径可调节 操作过程复杂;工艺、设备要求高 固相还原法[30] 以铁氧化物为前驱体,在还原气氛条件下煅烧制备 颗粒结晶度高;性质较为稳定 熔融态下颗粒易团聚;
工艺要求高电沉积法[31] 以含铁离子溶液为介质,在通电条件下,通过电结晶沉积在阴极上制备 密度高,成本低,设备简单;晶粒大小和成核速度可调控 容易混入杂质,纯度不高 溶剂热法[32] 以有机物为溶剂,加入一种或多种前驱体,在密闭体系中以及一定温度和压力下反应制备 过程简单易于控制;防止有毒物质的挥发;产物分散性好,颗粒均匀 原料性质活波,危险性大 -
[1] YANG P, DROHAN P J, YANG M, et al. Spatial variability of heavy metal ecological risk in urban soils from Linfen, China [J]. Catena, 2020, 190: 104554. doi: 10.1016/j.catena.2020.104554 [2] LU F, ASTRUC D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants [J]. Coordination Chemistry Reviews, 2020, 408: 213180. doi: 10.1016/j.ccr.2020.213180 [3] LYU H H, TANG J C, CUI M K, et al. Biochar/iron (BC/Fe) composites for soil and groundwater remediation: Synthesis applications, and mechanisms [J]. Chemosphere, 2020, 246: 125609. doi: 10.1016/j.chemosphere.2019.125609 [4] ZHAO X, LIU W, CAI Z, et al. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation [J]. Water Research, 2016, 100: 245-266. doi: 10.1016/j.watres.2016.05.019 [5] CHANG D, CHEN T, LIU H, et al. A new approach to prepare ZVI and its application in removal of Cr(VI) from aqueous solution [J]. The Chemical Engineering Journal, 2014, 244: 264-272. doi: 10.1016/j.cej.2014.01.095 [6] YAN W, LIEN H L, KOEL B E, et al. Iron nanoparticles for environmental clean-up: recent developments and future outlook [J]. Environmental ence: Processes & Impacts, 2013, 15(1): 63-77. [7] SHEKARRIZ M, TAGHIPOOR S, ALIAKBARI F H, et al. Optimal synthesis and nitrate and mercury removal ability of microemulsion-made iron nanoparticles [J]. International Journal of Nanoparticles, 2010, 3(2): 123-137. doi: 10.1504/IJNP.2010.034846 [8] ZHANG Y, LI Y, LI J, et al. Enhanced removal of nitrate by a novel composite: nanoscale zero valent iron supported on pillared clay [J]. Chemical Engineering Journal, 2011, 171(2): 526-531. doi: 10.1016/j.cej.2011.04.022 [9] MUELLER N C, BRAUN J, BRUNS J, et al. Application of nanoscale zero valent iron (NZVI) for groundwater remediation in Europe [J]. Environmental Science & Pollution Research, 2012, 19(2): 550-558. [10] RIBAS D, CERNIK M, MARTÍ V, et al. Improvements in nanoscale zero-valent iron production by milling through the addition of alumina [J]. Journal of Nanoparticle Research, 2016, 18(7): 181. doi: 10.1007/s11051-016-3490-2 [11] 刘银, 王静, 张明旭, 等. 机械球磨法制备纳米材料的研究进展 [J]. 材料导报, 2003, 17(7): 20-22. doi: 10.3321/j.issn:1005-023X.2003.07.006 LIU Y, WANG J, ZHANG M X, et al. Research progress in the preparation of nanomaterials by mechanical ball milling [J]. Materials Herald, 2003, 17(7): 20-22(in Chinese). doi: 10.3321/j.issn:1005-023X.2003.07.006
[12] LYU J, LIDER A and KUDIIAROV V. Using ball milling for modification of the hydrogenation/dehydrogenation process in magnesium-based hydrogen storage materials: an overview [J]. Matels, 2019, 9(7): 768. [13] REN T F, YANG S Y, WU S, et al. High-energy ball milling enhancing the reactivity of microscale zero-valent aluminum toward the activation of persulfate and the degradation of trichloroethylene [J]. Chemical Engineering Journal, 2019, 374: 100-111. doi: 10.1016/j.cej.2019.05.172 [14] PETERSON S C, JACKSON M A, KIM S, et al. Increasing biochar surface area: Optimization of ball milling parameters [J]. Powder Technology, 2012, 228: 115-120. doi: 10.1016/j.powtec.2012.05.005 [15] GHARSALLAH H I, MAKHLOUF T, SAURINA J, et al. Effect of boron addition on structural and magnetic properties of nanostructured Fe75Al25 alloy prepared by high energy ball milling [J]. Materials Letters, 2016, 181: 21-24. doi: 10.1016/j.matlet.2016.05.190 [16] AMBIKA S, DEVASENA M, NAMBI I M. Synthesis, characterization and performance of high energy ball milled meso-scale zero valent iron in Fenton reaction [J]. Journal of Environmental Management, 2016, 181: 847-855. doi: 10.1016/j.jenvman.2016.06.054 [17] 魏德强, 史绍远, 李新凯. 高能球磨混粉技术制备Cu/SiC混合粉末的工艺 [J]. 特种铸造及有色合金, 2015, 35(6): 565-568. WEI D Q, SHI S Y, LI X K. High energy ball milling powder mixing technology for the preparation of music mixed powder [J]. Special Casting and Nonferrous Alloys, 2015, 35(6): 565-568(in Chinese).
[18] 邹伟东. 转速和填充率对球磨机粉磨效果的影响[D]. 广州: 华南理工大学, 2016. ZOU W D. The effect of rotation speed and filling rate on the grinding effect of ball mill[D]. Guangzhou: South China University of Technology, 2016(in Chinese).
[19] 郝万军, 田秋娟, 蔡云光. 球料比对球磨机工作性能的影响数值分析 [J]. 矿山机械, 2010, 38(23): 72-75. HAO W J, TIAN Q J, CAI Y G. Numerical analysis of the effect of ball-material ratio on the performance of ball mill [J]. Mining Machinery, 2010, 38(23): 72-75(in Chinese).
[20] NOVÁK F, PRŮŠA K, NOVÁ, et al. Application of mechanical alloying in synthesis of intermetallics [J]. Acta Physica Polonica A, 2018, 134(3): 120-123. [21] MHADHBI M, KHITOUNI M, ESCODA L, et al. Characterization of mechanically alloyed nanocrystalline Fe(Al): Crystallite size and dislocation density [J]. Journal of Nanomaterials, 2010, 2010: 1-8. [22] SADEGHI A R, MOSTAJABODAVEH H, BABAKHANI A, et al. Effects of milling and heat treatment on the synthesis of NiTi powders [J]. Journal of Wuhan University of Technology, 2017, 32(5): 172-178. [23] 许宝松, 陈琦, 邱奔, 等. 过程控制剂对球磨法制备纳米硅粉的影响 [J]. 功能材料, 2018, 49(12): 211-222. XU B S, CHEN Q, QIU B, et al. Effect of process control agent on the preparation of nanometer silicon powder by ball milling [J]. Functional Materials, 2018, 49(12): 211-222(in Chinese).
[24] 刘培, 刘博古, 张倩倩, 等. 机械球磨法在纳米储氢材料制备中的应用 [J]. 化工新型材料, 2019, 47(3): 15-19. LIU P, LIU B G, ZHANG Q Q, et al. Application of mechanical ball milling method in the preparation of nano hydrogen storage materials [J]. New Chemical Materials, 2019, 47(3): 15-19(in Chinese).
[25] HAHN H. Gas phase synthesis of nanocrystalline materials [J]. Nanostructured Materials, 1997, 9(1-8): 0-12. [26] 张林, 张昌文, 王永娟. Fe-ZnSe纳米复合颗粒膜的制备和磁电阻效应 [J]. 微细加工技术, 2008(6): 14-33. ZHANG L, ZHANG C W, WANG Y J. Preparation of Fe-ZnSe nanocomposite particle film and magnetoresistance effect [J]. Microfabrication Technology, 2008(6): 14-33(in Chinese).
[27] SUN L, WANG C, ZHOU Y, et al. Flowing nitrogen assisted-arc discharge synthesis of nitrogen-doped single-walled carbon nanohorns [J]. Applied Surface Science, 2013, 277: 88-93. doi: 10.1016/j.apsusc.2013.04.006 [28] 肖凯军, 代佳丽, 何其, 等. 非离子表面活性剂作用下纳米零价铁的制备及其表征 [J]. 现代食品科技, 2015, 31(9): 138-144. XIAO K J, DAI J L, HE Q, et al. Preparation and characterization of nano-zero-valent iron under the action of nonionic surfactants [J]. Modern Food Science and Technology, 2015, 31(9): 138-144(in Chinese).
[29] SUNKARA B, ZHAN J, HE J, et al. Nanoscale zerovalent iron supported on uniform carbon microspheres for the in situ remediation of chlorinated hydrocarbons [J]. ACS Applied Materials & Interfaces, 2010, 2(10): 2854-2862. [30] COSTA, MOURA, ARDISSON, et al. Highly active heterogeneous Fenton-like systems based on Fe0/Fe3O4 composites prepared by controlled reduction of iron oxides [J]. Applied Catalysis B Environmental, 2008, 83(1-2): 131-139. doi: 10.1016/j.apcatb.2008.01.039 [31] 王宁, 金明江, 李家瑶, 等. 脉冲电沉积制备Fe-Pd合金薄膜 [J]. 材料科学与工程学报, 2014, 32(3): 324-330. WANG N, JIN M J, LI J Y, et al. Fe-Pd alloy thin film prepared by pulse electrodeposition [J]. Journal of Materials Science and Engineering, 2014, 32(3): 324-330(in Chinese).
[32] 王薇, 金朝晖, 李铁龙. 包覆型纳米零价铁的制备及其去除三氯乙烯的研究 [J]. 中国环境科学, 2009, 29(8): 811-815. doi: 10.3321/j.issn:1000-6923.2009.08.006 WANG W, JIN Z H, LI T L. Preparation of coated nano-zero-valent iron and its research on removal of trichloroethylene [J]. Environmental Science in China, 2009, 29(8): 811-815(in Chinese). doi: 10.3321/j.issn:1000-6923.2009.08.006
[33] FU X, ZHANG J, ZHAO H, et al. Enhanced peroxymonosulfate activation by coupling zeolite-supported nano-zero-valent iron with weak magnetic field [J]. Separation and Purification Technology, 2020, 230: 115886. doi: 10.1016/j.seppur.2019.115886 [34] ZHANG D, LI Y, SUN A, et al. Enhanced nitrobenzene reduction by modified biochar supported sulfidated nano zerovalent iron: Comparison of surface modification methods [J]. Science of The Total Environment, 2019, 694: 133701. doi: 10.1016/j.scitotenv.2019.133701 [35] IRAVANI, SIAVASH. Green synthesis of metal nanoparticles using plants [J]. Green Chemistry, 2011, 13(10): 2638. doi: 10.1039/c1gc15386b [36] MARAT I, ARSTAN M, TIMUR J, et al. Impact of chromium and boron compounds on the reproductive function in rats [J]. Toxicology and Industrial Health, 2018, 34(6): 365-374. doi: 10.1177/0748233718759162 [37] GOPAL G, SANKAR H, NATARAJAN C, et al. Tetracycline removal using green synthesized bimetallic nZVI-Cu and bentonite supported green nZVI-Cu nanocomposite: A comparative study [J]. Journal of Environmental Management, 2020, 254: 109812. doi: 10.1016/j.jenvman.2019.109812 [38] AKBAR, SOLIEMANZADEH, MAJID, et al. The application of green tea extract to prepare bentonite-supported nanoscale zero-valent iron and its performance on removal of Cr(VI): Effect of relative parameters and soil experiments [J]. Microporous & Mesoporous Materials, 2017, 239: 60-69. [39] VISENTIN C, Braun A B, THOMÉ A, et al. Lifecycle assessment of environmental and economic impacts of nano-iron synthesis process for application in contaminated site remediation, Journal of Cleaner Production, 2019, 231: 307-319. [40] QUARESMA S, ANDRE V, FERNANDES A, et al. Mechanochemistry – a green synthetic methodology leading to metallodrugs, metallopharmaceuticals and bio-in-spired metal-organic frameworks [J]. Inorganica Chimica Acta, 2017, 455: 309-318. doi: 10.1016/j.ica.2016.09.033 [41] 周华, 张晓华, 熊丽凤, 等. 超声波辅助纳米零价铁-牡蛎壳材料处理As(Ⅲ)废水的研究 [J]. 水处理技术, 2017, 43(9): 47-51. ZHOU H, ZHANG X H, XIONG L F, et al. Ultrasound-assisted treatment of As(Ⅲ) wastewater with nano-zero-valent iron-oyster shell materials [J]. Water Treatment Technology, 2017, 43(9): 47-51(in Chinese).
[42] 葛兴彬, 王振虹, 郭楚奇, 等. 纳米零价铁的生态毒性效应研究进展 [J]. 生态毒理学报, 2015, 10(3): 28-37. GE X B, WANG Z H, GUO C Q, et al. Research progress on the ecotoxic effect of nano-zero-valent iron [J]. Journal of Ecotoxicology, 2015, 10(3): 28-37(in Chinese).
[43] 李虹, 吕小凡, 马溢阳, 等. 超声协同Fe-0@Fe3O4降解四氯化碳 [J]. 环境科学学报, 2018, 38(7): 2650-2658. LI H, LÜ X F, MA Y Y, et al. Ultrasound cooperates with Fe-0 @ Fe3O4 to degrade carbon tetrachloride [J]. Journal of Environmental Science, 2018, 38(7): 2650-2658(in Chinese).
[44] DANISH M, GU X, LU S, et al. An efficient catalytic degradation of trichloroethene in a percarbonate system catalyzed by ultra-fine heterogeneous zeolite supported zero valent iron-nickel bimetallic composite [J]. Applied Catalysis A General, 2017, 531: 177-186. doi: 10.1016/j.apcata.2016.11.001 [45] 潘煜, 孙力平, 陈星宇, 等. CMC改性纳米Fe/Cu双金属模拟PRB去除地下水中2, 4-二氯苯酚 [J]. 中国环境科学, 2019, 39(9): 3789-3796. doi: 10.3969/j.issn.1000-6923.2019.09.023 PAN Y, SUN L P, CHEN X Y, et al. Removal of 2, 4-dichlorophenol in groundwater by CMC modified nano Fe/Cu bimetallic simulated PRB [J]. Chinese Journal of Environmental Science, 2019, 39(9): 3789-3796(in Chinese). doi: 10.3969/j.issn.1000-6923.2019.09.023
[46] 郭汶俊, 张永祥, 井琦, 等. CMS包覆纳米零价铁去除2, 4-二氯酚的条件优化 [J]. 环境工程学报, 2018, 12(12): 3289-3296. doi: 10.12030/j.cjee.201806032 GUO W J, ZHANG Y X, JING Q, et al. Optimization of conditions for removal of 2, 4-dichlorophenol by CMS-coated nano-zero iron [J]. Journal of Environmental Engineering, 2018, 12(12): 3289-3296(in Chinese). doi: 10.12030/j.cjee.201806032
[47] WU H H, WEI W X, XU C, et al. Polyethylene glycol-stabilized nano zero-valent iron supported by biochar for highly efficient removal of Cr(VI) [J]. Ecotoxicology and Environmental Safety, 2020, 188: 109902. doi: 10.1016/j.ecoenv.2019.109902 [48] LI H, QIU Y, WANG X, et al. Biochar supported Ni/Fe bimetallic nanoparticles to remove 1, 1, 1-trichloroethane under various reaction conditions [J]. Chemosphere, 2017, 169: 534-541. doi: 10.1016/j.chemosphere.2016.11.117 [49] DE A, DE A K, PANDA G S, et al. Synthesis of zero valent iron nanoparticle and its application as a dephenolization agent for coke oven plant wastewater situated in West Bengal: India [J]. Environmental Progress & Sustainable Energy, 2017, 36(6): 1700-1708. [50] AKHGAR B N, POURGHAHRAMANI P. Implementation of sonochemical leaching for preparation of nano zero-valent iron (NZVI) from natural pyrite mechanochemically reacted with Al [J]. International Journal of Mineral Processing, 2017, 164: 1-5. doi: 10.1016/j.minpro.2017.05.002 [51] CHEN D, NI S, CHEN Z. Synthesis of Fe3O4 nanoparticles by wet milling iron powder in a planetary ball mill [J]. China Particuology, 2007, 5(5): 357-358. doi: 10.1016/j.cpart.2007.05.005 [52] ZHANG Y, YANG B, FAN J, et al. A mechanically synthesized SiO2-Fe metal matrix composite for effective dechlorination of aqueous 2-chlorophenol: the optimum of the preparation conditions [J]. RSC Advances, 2016, 6(80): 76867-76873. doi: 10.1039/C6RA12889K [53] ZHANG S S, YANG N, NI S Q, et al. One-pot synthesis of highly active Ni/Fe nano-bimetal by simultaneous ball milling and in situ chemical deposition [J]. RSC advances, 2018, 8(47): 26469-26475. doi: 10.1039/C8RA04426K [54] 刘银, 秦晓英, 张明旭. 纳米γ-Ni-Fe合金的磁电阻 [J]. 材料研究学报, 2003, 17(1): 19-24. doi: 10.3321/j.issn:1005-3093.2003.01.004 LIU Y, QIN X Y, ZHANG M X. Magnetoresistance of Nanometer γ-Ni-Fe Alloy [J]. Journal of Materials Research, 2003, 17(1): 19-24(in Chinese). doi: 10.3321/j.issn:1005-3093.2003.01.004
[55] AAOULLAR D, MADSEN S J, GÜT B, et al. Synthesis and characterization of graphite-encapsulated iron nanoparticles from ball milling-assisted low-pressure chemical vapor deposition [J]. Carbon, 2017, 124: 170-179. doi: 10.1016/j.carbon.2017.08.043 [56] XU J, PU Y, QI W K, et al. Chemical removal of nitrate from water by aluminum-iron alloys [J]. Chemosphere, 2017, 166: 197-202. doi: 10.1016/j.chemosphere.2016.09.102 [57] LIU X L, YANG Z C, ZHAO M Y, et al. Preparation of silica-supported nanoFe/Ni alloy and its application in viscosity reduction of heavy oil [J]. Micro & Nano Letters, 2015, 10(3): 167-171. -




 下载:
下载: