-
氯化石蜡(chlorinated paraffins, CPs)是一类工业合成的正构烷烃氯代衍生物,化学通式为CmH2m+2-nCln,氯含量(质量分数)通常在30%—72%之间。根据碳链长度,CPs 可以分为短链氯化石蜡(C10-13, short chain chlorinated paraffins, SCCPs)、中链氯化石蜡(C14-17, medium chain chlorinated paraffins, MCCPs)和长链氯化石蜡(C18-30, long chain chlorinated paraffins, LCCPs)。由于具有挥发性低、阻燃性和电绝缘性好、稳定性高等优点,氯化石蜡被广泛应用于金属加工润滑剂、聚氯乙烯(PVC)的增塑剂、油漆、涂料和橡胶等高分子材料中的阻燃剂等[1-2]。由于具有持久性、长距离迁移性、生物累积性和潜在的生物毒性等持久性有机污染物(persistent organic pollutants, POPs)特性,以质量计算氯化程度超过48%的SCCPs已于2017年5月列入到《关于持久性有机污染物的斯德哥尔摩公约》附件A的受控名单中。我国是世界上最大的CPs生产国、消费国和出口国,2013年的产量为105万t,占到全球产量的15%,并且产量还在逐年增加[3]。由于全球对SCCPs的限制和禁令,CPs工业品的生产已经从SCCPs转向MCCPs [4],而我国也逐渐限制了CPs工业品中SCCPs的含量。与中长链的CPs相比,SCCPs的毒性效应相对较大,但是研究发现氯含量超过46%的MCCPs同样具有环境持久性和潜在的生物毒性[5],可见MCCPs的环境问题同样需要关注。
目前,SCCPs和MCCPs在多种环境介质和生物体内被频繁检出[6-8],但对于昆虫这种低等级无脊椎动物的研究还未见报道。昆虫是生态系统中的重要组成部分,是连接生产者和消费者的重要纽带,在水生和陆生生态系统中具有重要的生态地位。而水生昆虫作为环境污染的生物指示性物种早就受到关注和重视,如Evenset等[9]在北极圈两个湖泊中摇蚊幼虫体内监测到多种持久性有机污染物;Van Praet等[10]通过蜻蜓(细蟌科)幼虫监测了比利时不同土地利用方式下16个池塘水体中POPs的污染状况。但是对于SCCPs和MCCPs这类新型持久性有机污染物在昆虫中的富集特征还并不清楚。另外,有研究表明昆虫在迁移和转运污染物中起到关键作用,认为水生昆虫(如蜻蜓、蚊和蜉蝣)是污染物重要的携带者和中间载体,它们能跨越水生生态系统边界,将污染物传递到陆生生态系统中[11]。
本研究以广东清远龙塘镇一个受电子垃圾严重污染的池塘为研究区域,采集不同种类的水生昆虫和环境介质(水体)样品。前期的研究表明,这个池塘的沉积物和电子垃圾残渣中含有高浓度的SCCPs(1.6—47 μg·g−1)和MCCPs(7.3—215 μg·g−1)[12]。本文通过气相色谱质谱联用仪(GC/MS)分析水生昆虫和水体中SCCPs和MCCPs的浓度水平和组成模式,研究水生昆虫体内SCCPs和MCCPs的生物富集规律及其影响机制,进一步理解电子垃圾回收区氯化石蜡的环境行为和归趋。
-
2016年6月在广州清远电子垃圾回收区的池塘中采集了3个目的4种水生昆虫,样品采用水网的方法采集,分别为蜻蜓目幼虫(蜻科和蟌科,Libellulidae和Coenagrionoidea)、半翅目(负子蝽,Belostomatidae)和鞘翅目(水龟虫,Hydrophilidae)。昆虫样品采集后立即带回实验,用去离子水清洗晾干后,于电子天平称取湿重,用锡箔纸包装保存于−20 ℃冰箱内待测。由于昆虫个体较小,不适合解剖分析,所以昆虫样品均作整样处理,且多个个体混合成1个混合样,具体样本量见表1。同时还采集了水生昆虫栖息池塘的水样,装于4 L棕色玻璃瓶中保存。
样品的前处理方法详述见参考文献[12-13]。昆虫样品经过冷冻干燥后,充分研磨至粉末状。然后称取1 g干重样品,添加回收率指示物(13C10-trans-chlordane),在60 ℃水浴锅中,用200 mL二氯甲烷/正己烷(1∶1,V/V)溶剂进行索式抽提48 h。提取液加入10 mL浓硫酸除脂肪后,过弗罗里硅胶复合层析柱(自下而上:20∶2∶5 cm,弗罗里硅土:中性硅胶:酸性硅胶)进一步净化,先用 80 mL正己烷淋洗(弃去),后用50 mL二氯甲烷洗脱,收集目标物(SCCPs和MCCPs)。旋转蒸发浓缩后氮吹至近干,加入内标指示物(ε-HCH)后用异辛烷定容至100 μL。水样通过0.7 μm玻璃纤维滤膜(GF/F, Whatman)过滤,将水样分为溶解相和颗粒相。过滤后的水样(溶解相)通过液液萃取的方法提取,净化及后续处理同昆虫样品处理方法。滤膜(颗粒相)经过冷冻干燥后,其前处理过程同昆虫样品。
-
SCCPs和MCCPs的含量分析采用安捷伦气相色谱-质谱联用仪(Agilent 6890GC- 5975MS)负化学离子源(NCI),选择离子检测(SIM)模式检测,色谱柱为DB-5HT(15 m×0.25 mm×0.10 μm),载气为高纯氦气。不分流进样,进样体积1 μL,进样口温度为250 ℃,接口温度为280 ℃,离子源温度为200 ℃,柱流速1.5 mL·min−1。升温程序为80 ℃保持3 min,以25 ℃·min−1升温到160 ℃,保持6 min,再以20 ℃·min−1升温到300 ℃,保持15 min。为增加仪器灵敏度和较少SCCPs和MCCPs之间的干扰,每个样品分4次进样,分组分别为C10和C15,C11和C16,C12和C17,C13和C14,具体仪器和数据分析方法见参考文献[13]。
整个实验过程所用玻璃器皿在450 ℃下灼烧后依次用色谱纯溶剂丙酮,二氯甲烷和正己烷清洗。质量控制与保证包括方法空白及每个样品添加回收率替代物等措施。方法空白中有痕量的CP单体检出,其含量低于样品中相应化合物含量的1%,报道的数据都经过空白校正。生物样品计算浓度以湿重(ww)表示,水样浓度以体积(L)表示。方法检出限定义为3倍信噪比时实际样品的浓度,SCCPs和MCCPs的方法检出限分别为5.6、4.2 ng·g−1 ww。13C10-trans-chlordane的回收率为81% — 116%。
-
两组数据间的比较采用独立样本t检验法,两组以上数据进行比较用单因素方差分析。采用Pearson检验法对正态分布的数据进行相关分析,非正态分布的数据在分析前进行对数转化。主成分分析(Principal component analysis, PCA)取前两个因子进行分析,因子得分与因子载荷结果分别讨论。以上统计分析均由于Origin 8.5和SPSS 19.0软件完成,P < 0.05认为具有统计学意义。
-
4种水生昆虫中SCCPs和MCCPs的氯含量范围分别为61.3%—62.1%和55.9%—57.9%,∑SCCPs的浓度为52—410 ng·g−1 ww,∑MCCPs的浓度为40—740 ng·g−1 ww,如表1所示。其中,蜻蜓目蟌科幼虫∑SCCPs的浓度最高,其中值为280 ng·g−1 ww,负子蝽的浓度最低(98 ng·g−1 ww)。对于MCCPs,水龟虫具有最高的浓度(300 ng·g−1 ww),最低值(51 ng·g−1 ww)出现在蟌科幼虫中。不同种类昆虫中SCCPs和MCCPs的浓度差异较大,造成这种差异的原因可能是食性及摄食习性的不同,蜻蜓目幼虫为肉食性昆虫,捕食小型水生生物;水龟虫取食藻类和浮游生物,一般为植食性;负子蝽的口器为刺吸式,以猎物的组织液为食。与前期的研究相比,蜻科幼虫体内∑SCCPs(97—410 ng·g−1 ww)和∑MCCPs(40—340 ng·g−1 ww)的浓度与多氯联苯(∑PCBs,170—270 ng·g−1 ww)和多溴联苯醚(∑PBDEs,79—490 ng·g−1 ww)相当[14],说明CPs也是电子垃圾污染区重要的POPs。目前,还未见到有关水生昆虫体内SCCPs和MCCPs的报道,因此只能与其生境相似的底栖动物进行比较。该电子垃圾回收区水生昆虫体内∑SCCPs和∑MCCPs的浓度,与同一研究区域[15]和辽东湾[16]采集的底栖动物(虾和螃蟹)中SCCPs的浓度相当(分别为230—610 ng·g−1 ww和240—500 ng·g−1 ww);高于香港海域软体动物和甲壳纲动物(SCCPs和MCCPs:11—52 ng·g−1 ww和 19—88 ng·g−1 dw)[17]和加拿大安大略湖和密歇根湖中糠虾(SCCPs和MCCPs:2.4—7.5 ng·g−1 ww和未检出)[18];但低于另一电子垃圾区(石角镇)采集的贝类和虾类(SCCPs和 MCCPs:37—120 μg·g−1 lw和36—130 μg·g−1 lw),广东省市场采集的水产品(包括鱼类,虾和牡蛎;SCCPs和MCCPs:1400 ng·g−1 ww和590 ng·g−1 ww)[19]和辽河口海域软体动物(螺和贝类;SCCPs:6100 ng·g−1 dw)[20]中的污染水平。
水体(溶解相和颗粒相)中∑SCCPs和∑MCCPs的中值浓度分别为2800 ng·L−1和5600 ng·L−1。其中,颗粒相的浓度贡献占97%以上(∑SCCPs:2700 ng·L−1和∑MCCPs:5500 ng·L−1),这主要是由于有机质对CPs具有很强的吸附能力,在水体中CPs更易于吸附在悬浮颗粒物上,而不是自由溶解态[21]。水体(溶解相和颗粒相)中∑SCCPs和∑MCCPs的浓度明显高于∑PCBs(90 ng·L−1)和∑PBDEs(1100 ng·L−1)[14],表明SCCPs和MCCPs在受电子垃圾污染的水体中广泛存在,其浓度甚至超过传统的POPs。本研究水体(溶解相)中SCCPs和MCCPs的浓度,低于北京地区河水和湖水(SCCPs:120、460 ng·L−1)[22],高碑店湖中表层水体(SCCPs:160—180 ng·L−1)[23]和英国受工业污染的河流水体(SCCPs:200—1700 ng·L−1)[24]中的浓度;但高于加拿大安大略湖水体(SCCPs和MCCPs:1.2 ng·L−1和0.9 pg·L−1)[18],辽东湾海水(SCCPs:4.1—13 ng·L−1)[16]和日本河流水体(SCCPs:7.6—31 ng·L−1)[25]中的浓度。目前,对环境水体(颗粒相)中SCCPs和MCCPs的相关研究较少,仅有Li等[26]报道黄河流域水体(颗粒相)中SCCPs和MCCPs的均值浓度分别为17 μg·g−1 dw和2.6 μg·g−1 dw,明显低于本研究水体(颗粒相)中SCCPs和MCCPs的浓度(500、1400 μg·g−1 dw)。水体(溶解相和颗粒相)中SCCPs和MCCPs的浓度高于预测的最低无影响浓度(SCCPs和MCCPs:500、1000 ng·L−1)[27],说明粗犷的电子垃圾拆解回收活动已经给当地水生生态系统造成了严重的污染。
-
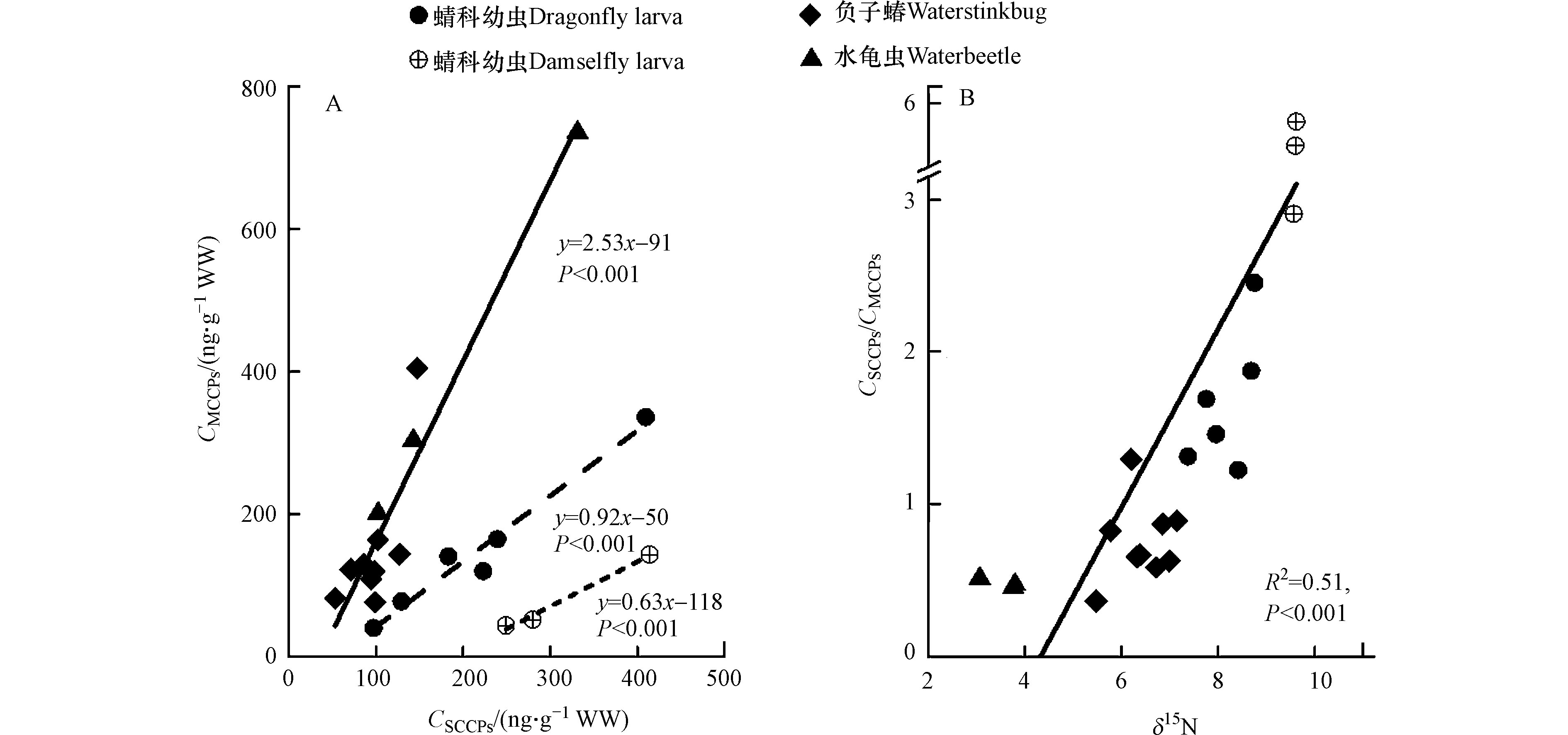
4种水生昆虫中SCCPs与MCCPs的浓度比值为0.47—5.50,如表1所示。其中,蟌科幼虫(5.50)和蜻科幼虫(1.57)中SCCPs与MCCPs的比值均大于1,明显高于水体中的比值(0.37—0.50),表明SCCPs比MCCPs更易于在它们体内富集。而负子蝽和水龟虫中SCCPs与MCCPs的比值分别为0.66和0.47,略高于水体中的比值,说明不同种类昆虫对SCCPs和MCCPs的富集能力有明显差异。通过对SCCPs与MCCPs浓度的相关分析也发现,不同种类昆虫中SCCPs与MCCPs线性拟合方程的斜率差异明显,结果如图1A所示。其中,负子蝽和水龟虫的斜率为2.53,明显高于蜻科幼虫(0.92)和蟌科幼虫(0.63),造成这种差异的原因可能是不同种类昆虫的食性、摄食习性和生境不同。另外发现,水生昆虫体内SCCPs与MCCPs的浓度比值与其氮稳定同位素值(δ15N)呈显著的正相关关系(图1B,P < 0.001),说明SCCPs比MCCPs更易于通过生物放大过程(食物链)累积到生物体内。这与Houde等[18]研究发现水生食物链上SCCPs比MCCPs具有更高的营养级放大潜力的结果一致,但也有研究表明MCCPs在水生食物链上具有更高的营养级放大潜力[17],这可能与高等级生物(如哺乳动物、鸟类等)对SCCPs具有较强的代谢能力有关[28]。
-
水生昆虫和水体(溶解相和颗粒相)中SCCPs和MCCPs同系物的组成模式如图2所示。蜻蜓幼虫(蜻科和蟌科)和负子蝽中SCCPs同系物的组成以C10-11(64%—86%)和Cl6-8(77%—85%)为主,与淀山湖底栖动物[29]、大连市海产品[30]、渤海湾软体动物[31]、辽东湾海洋生物[16]和香港海域软体动物和甲壳纲动物[17]中SCCPs的组成模式一致。水龟虫中SCCPs的组成特征是以C12-13(61%)和Cl6-8(76%)为主,这与我们前期在同一区域所研究的鱼体(鲮鱼)内SCCPs组成模式相似[15],这可能是由于水龟虫和鲮鱼均是植食性生物,取食的藻类和浮游生物,它们具有相似的污染来源。水体中SCCPs不同碳数同系物的占比较为平均(均为20%左右),与同一区域所研究的沉积物的组成模式相似[12],但是水体中低氯代同系物的丰度更高(75%),这主要是由化合物的理化性质决定的,低氯代单体具有更高的溶解度。
对于MCCPs而言,其同系物在水生昆虫和水体中均以C14(34%—53%)和Cl7-9(64%—75%)为主(图2),与法国河流中淡水鱼[32]和黄河水体(颗粒相)[26]中MCCPs的组成特征相似。但本研究的水生昆虫中C14的丰度要低于来自加拿大湖泊和河流[33]、辽东湾[34]、波罗的海[35]和挪威北极圈海域[36]的鱼类中C14的丰度(贡献60%以上)。Zeng等[17, 37]对中国香港海域水生生物的研究发现,脂肪含量越高和营养级越高的水生生物中C14的丰度越高,认为C14更倾向于累积在高脂肪含量的捕食者中。如表1所示,本研究的水生昆虫具有较低的脂肪含量(1.8%—3.2%),也是低营养级生物,同时它们具有较低的C14丰度。这可能是由化合物的理化性质决定,MCCPs的中长碳链组分具有更高的辛醇/水分配系数(KOW)和更大的分子量,因而生物富集和生物放大潜力较低,但是这还需要进一步研究。
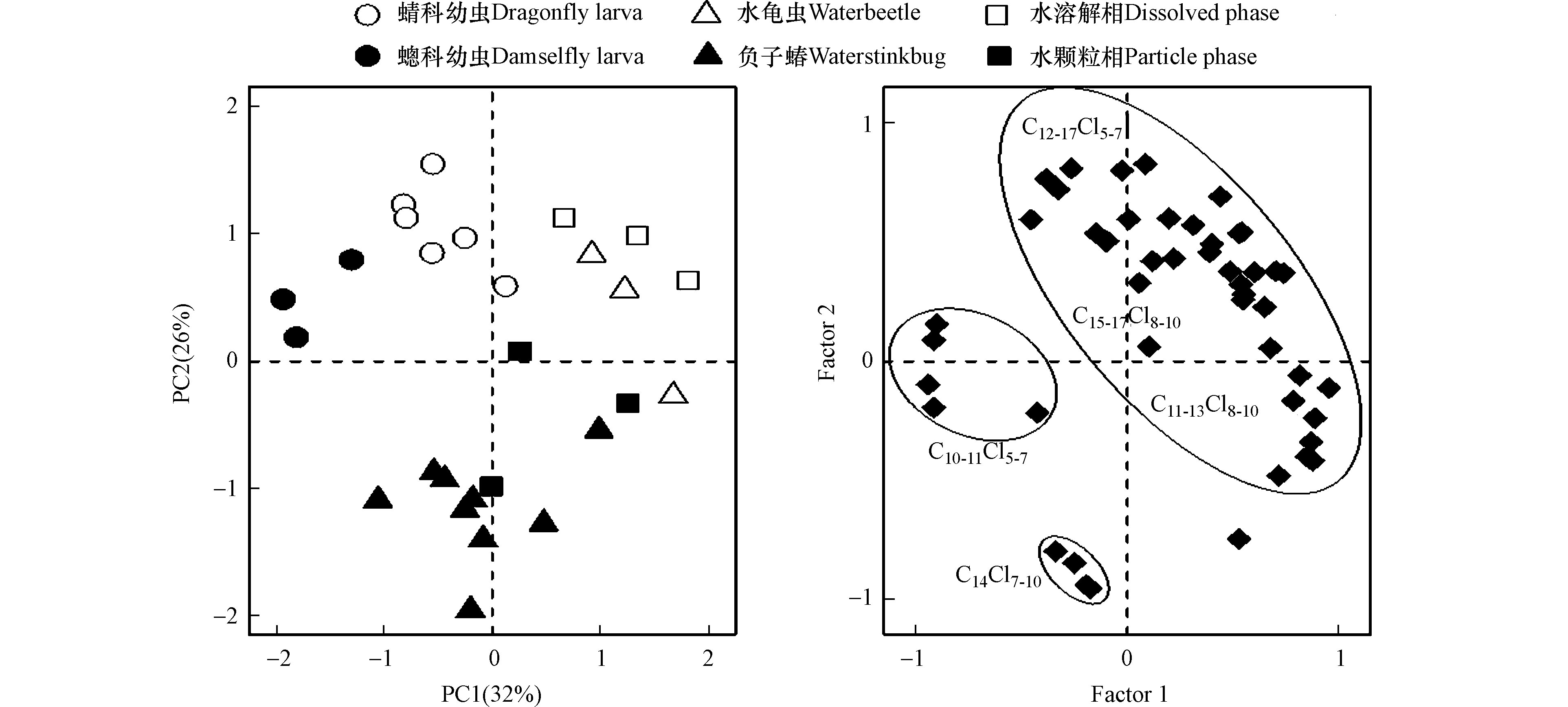
利用主成分分析(PCA)对4种水生昆虫和水体中SCCPs和MCCPs同系物的丰度进行了特征分类,提取占总变量58% 的前两个变量作为第一主成分(PC1:32%)和第二主成分(PC2:26%),结果如图3所示。蜻蜓幼虫(蜻科和蟌科)聚集在得分图的左上方,以低碳低氯代(C10-11Cl5-7)单体为主;水龟虫和水体(溶解相和颗粒相)聚集在得分图右上方,以高碳高氯代(C13-17Cl8-10)单体为主;负子蝽则聚集在得分图下方,以C14Cl7-10单体为主。结果表明,水生昆虫中SCCPs和MCCPs的组成特征表现出种间特异性,与前期发现不同种类昆虫体内HOPs的组成模式不同的研究结果一致[14],这主要是由于食性、摄食习性或者富集能力的不同,从而造成它们体内污染物组成模式的差异。
-
水生昆虫中SCCPs和MCCPs的生物富集因子(Bioaccumulation factor, BAF)采用以下公式计算[18]:
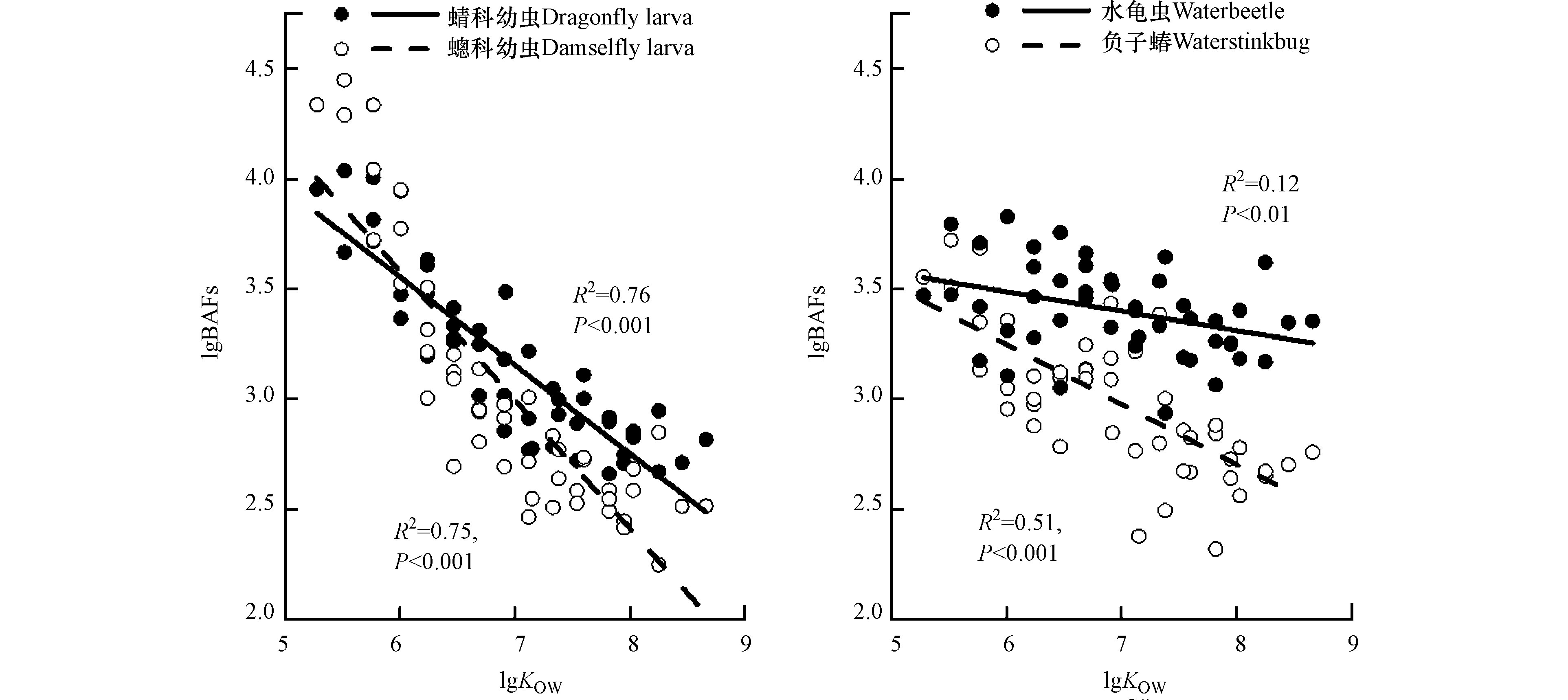
式中,Ci为水生昆虫体内SCCPs或MCCPs的湿重浓度(ng·g−1 ww),Cw为水体(溶解相)中SCCPs或MCCPs的体积浓度(ng·L−1)。如图4所示,四种水生昆虫中SCCP和MCCP同系物的lg BAF值范围分别为2.70—4.45和2.25—3.64。∑SCCPs的lg BAF值最大的是蟌科幼虫(3.79),最小值是负子蝽(3.27),而∑MCCPs的lg BAF值最大的是水龟虫(3.34),最小值是蟌科幼虫(2.62)。与前期的研究相比,蜻科幼虫中∑SCCPs和∑MCCPs的lg BAF值(3.59和2.86)均小于∑PCBs(4.87)和∑PBDEs(4.65),说明在水生昆虫中SCCPs和MCCPs的生物富集潜力低于PCBs和PBDEs。本研究中SCCPs的lg BAF值与同一区域所研究的鱼类的BAF值(2.46—3.45)[15]相近,高于珠江口鱼类和底栖动物(螃蟹、虾和牡蛎)的BAF值(1.6—3.0)[38],但是低于辽东湾底栖动物(螃蟹、虾、螺和贝类,4.5—5.5)[16]和鱼类(4.69—6.05)[34],加拿大安大略湖鱼类(6.0—6.6)[18]和北京高碑店湖鱼类的 BAF值(4.75—5.45)[23]。目前对于水生生物中MCCPs的BAF值的研究较少,仅有Houde等[18]报道了安大略湖鱼类中MCCPs的lg BAF值为6.6—7.3,明显高于本研究中水生昆虫的BAF值(2.62—3.34)。但是在辽东湾、安大略湖和高碑店湖鱼类中SCCPs和MCCPs的lg BAF值的计算方法是以污染物的脂肪归一化浓度为基础,而本研究中BAF值的计算是以湿重浓度为基础,从而造成计算结果的不同。
对于SCCPs和MCCPs,分析了4种水生昆虫的lg BAF值与化合物理化性质的关系。如表2和图4所示,4种水生昆虫中SCCP和MCCP同系物的BAF值与其辛醇/水分配系数(KOW)和碳原子数(C10-17)均呈显著负相关(P < 0.05)。对于氯代程度,蜻蜓幼虫(蜻科和蟌科)的BAF值与氯原子取代数(Cl5-10)呈显著负相关(P < 0.05),但是负子蝽和水龟虫的BAF值与氯原子取代数没有明显的相关性。这些结果表明化合物的理化性质影响了水生昆虫对SCCPs和MCCPs的积累,低KOW和低碳链长度的同系物更容易被生物富集。Sun等[15]和Huang等[38]也报道底栖动物(螃蟹和虾类)中SCCPs的BAF值与其KOW值呈显著负相关,但与鱼类的研究结果不同的是,在鱼类中SCCPs的BAF值与其KOW值和氯原子取代数呈显著正相关[23-34]。可能的原因是,对于底栖动物(包括昆虫、螃蟹和虾类)而言,水体并不是污染物富集的主要途径,它们更容易从沉积物中累积污染物。另一种可能的解释是,与鱼类不同,昆虫、螃蟹和虾的生长发育过程中都需要多次蜕壳(外骨骼),而高KOW值或长碳链的同系物累积在外骨骼中被清除,但是这一过程对污染物生物富集的影响还需深入研究。
-
(1)在受电子垃圾污染的池塘水体和水生昆虫中均检测到较高浓度的SCCPs和MCCPs,说明电子垃圾回收活动已经给当地水生生态系统造成了CPs的严重污染。
(2)不同种类水生昆虫中SCCPs和MCCPs的浓度和组成均表现出明显的种间差异,这可能是食性、摄食习性或富集能力不同造成的。
(3)SCCPs和MCCPs的理化性质(KOW值、碳原子数和氯原子取代数)影响污染物的生物富集效应,低KOW和低碳链长度的同系物更倾向在水生昆虫体内累积。
电子垃圾回收区水生昆虫体内氯化石蜡的污染水平和富集特征
Bioaccumulation of short- and medium-chain chlorinated paraffins in aquatic insects from an e-waste recycling site
-
摘要: 通过GC/MS法测定了广东清远龙塘镇电子垃圾回收区水生昆虫和水体中短链氯化石蜡(SCCPs)和中链氯化石蜡(MCCPs)的浓度水平。结果显示,4种水生昆虫体内∑SCCPs和∑MCCPs的浓度范围分别为52—410 ng·g−1 ww(湿重)和40—740 ng·g−1 ww(湿重),其中,蟌科幼虫的∑SCCPs浓度最高,水龟虫的∑MCCPs浓度最高。主成分分析表明,不同种类水生昆虫中SCCP和MCCP同系物的组成特征表现出种间特异性,可能是由于食性、摄食习性或富集能力的不同。水生昆虫中∑SCCPs和∑MCCPs的生物富集因子(lg BAFs值)的范围分别为3.27—3.79和2.62—3.34。蜻科幼虫中∑SCCPs和∑MCCPs的lg BAFs值均小于多氯联苯(∑PCBs,4.87)和多溴联苯醚(∑PBDEs,4.65),说明在水生昆虫中SCCPs和MCCPs的生物富集潜力低于PCBs和PBDEs。SCCPs和MCCPs同系物的BAFs值与辛醇/水分配系数(KOW)和碳链长度(C10-17)均呈显著负相关(P < 0.05),表明化合物的理化性质影响了水生昆虫对SCCPs和MCCPs的积累,低KOW和低碳链长度的同系物更容易被生物富集。
-
关键词:
- 短链氯化石蜡(SCCPs) /
- 中链氯化石蜡(MCCPs) /
- 水生昆虫 /
- 生物富集因子 /
- 电子垃圾
Abstract: The concentrations of short-chain chlorinated paraffins (SCCPs) and medium-chain chlorinated paraffins (MCCPs) in aquatic insects and water from an electronic waste recycling site in Longtang Town, Qingyuan County of Guangdong Province were determined using gas chromatographic-mass spectrometric (GC/MS). The results showed that ∑SCCPs and ∑MCCPs concentrations in aquatic insects were range from 52 ng·g−1 to 410 ng·g−1 and 40 ng·g−1 to 740 ng·g−1 wet weight (ww), respectively. Damselfly larvae have the highest level of ∑SCCPs and the highest level of ∑MCCPs was found in waterbeetles. Principal component analysis was conducted, and species-specific different composition patterns of SCCP and MCCP congeners were seen among aquatic insects. This may be due to differences in diet, feeding habits and bioaccumulation ability among different species. Bioaccumulation factor (BAF) values of ∑SCCPs and ∑MCCPs in aquatic insects were range from 3.27 to 3.79 and 2.62 to 3.34, respectively. BAFs of ∑SCCPs and ∑MCCPs in dragonfly larvae were lower than that of ∑PCBs (4.87) and ∑PBDEs (4.65), suggesting the bioaccumulation potential of SCCPs and MCCPs was below that of PCBs and PBDEs in aquatic insects. Significant negatively correlations (P < 0.05) were observed between BAF values and octanol-water partition coefficient (KOW) and carbon-chain length (C10-17) of SCCPs and MCCPs. Those results indicated that the physicochemical properties of compounds affected the bioaccumulation of SCCPs and MCCPs by aquatic insects, and homologues with low KOW and low carbon chain length were preferred to bioaccumulation. -

-
表 1 水生昆虫和水体中脂肪含量、氯含量及SCCPs和MCCPs的浓度水平
Table 1. Lipid content, chlorine content, and concentrations of SCCPs and MCCPs in aquatic insects and water
化合物
Compounds蜻蜓目
Odonata/(ng g−1 ww)半翅目Hemiptera/
(ng·g−1 ww)鞘翅目 Coleoptera/
(ng·g−1 ww)水体
Water/(ng·L−1)蜻科幼虫
Dragonfly larva蟌科幼虫
Damselfly larva负子蝽
Waterstinkbug水龟虫
Waterbeetle溶解相
Dissolved phase颗粒相
Particle phaseNc 6 (75) 3 (40) 9 (130) 3 (45) 3 3 Lipid /% 1.8 ± 0.11d 2.0 ± 0.05 3.2 ± 0.08 3.0 ± 0.60 NDe ND 短链氯含量/% 61.3 ± 0.21 61.3 ± 0.06 62.1 ± 0.16 61.4 ± 0.30 62.5 ± 0.48 61.7 ± 0.33 ∑C10 75 (45—130)f 150 (140—220) 37 (19—48) 25 (18—28) 12 (11—13) 710 (490—1200) ∑C11 50 (20—110) 89 (78—110) 25 (13—28) 33 (21—68) 14 (13—22) 710 (500—1600) ∑C12 44 (19—97) 25 (23—54) 18 (12—35) 40 (28—100) 12 (11—21) 670 (390—1800) ∑C13 33 (13—69) 11 (7.5—31) 14 (8.9—41) 44 (35—130) 13 (12—17) 650 (340—1600) ∑SCCPs 200 (97—410) 280 (250—410) 98 (52—150) 140 (100—330) 50 (49—72) 2700 (1700—6200) 中链氯含量 /% 55.9 ± 0.40 56.1 ± 0.23 57.9 ± 0.30 56.6 ± 0.43 56.1 ± 0.15 57.4 ± 0.19 ∑C14 50 (12—140) 23 (18—55) 69 (43—160) 120 (78—290) 53 (45—65) 3100 (1200—11000) ∑C15 39 (14—97) 14 (11—42) 27 (16—110) 81 (57—230) 52 (42—57) 1100 (690—6800) ∑C16 24 (8.2—60) 6.5 (6.4—28) 18 (10—76) 55 (36—110) 34 (30—39) 750 (530—5000) ∑C17 16 (5.3—41) 7.2 (6.9—17) 9.0 (6.4—62) 48 (29—98) 24 (17—24) 540 (360—3200) ∑MCCPs 130 (40—340) 51 (43—140) 120 (76—400) 300 (200—740) 160 (130—190) 5500 (2700—26000) ∑SCCPs/∑MCCPs 1.57 ± 0.41 5.50 ± 1.29 0.66 ± 0.25 0.47 ± 0.02 0.37 ± 0.04 0.50 ± 0.16 c N为混合后的样品数量,括号内为采集的样品数量;d平均值 ± 标准偏差;e 未检测;f 中值(范围).
a Unit (ng g−1 ww); b Unit (ng L−1); c N means number of composite samples analyzed and figures in brackets indicate the number of individuals collected; d Mean ± standard deviation; e Not detected; f Median (range).表 2 水生昆虫中SCCP和MCCP同系物的BAFs值与其辛醇水分配系数(KOW)、碳链长度(C10-17)和氯代程度(Cl5-10)的相关性
Table 2. Correlation between the lg BAFs and octanol-water partition coefficient (KOW), carbon chain length (C10-17) and chlorinated degree (Cl5-10) for SCCPs and MCCPs in aquatic insects
种类
Species辛醇水分配系数(KOW)
Octanol-water partition coefficient碳链长度(C10-17)
Carbon chain length氯代程度(Cl5-10)
Chlorinated degree蜻蜓目 蜻科幼虫 −0.873 (< 0.001)** −0.807 (< 0.001)** −0.337 (0.019)* 蟌科幼虫 −0.869 (< 0.001)** −0.781 (< 0.001)** −0.349 (0.015)* 半翅目 负子蝽 −0.725 (< 0.001)** −0.708 (< 0.001)** 0.052 (0.727) 鞘翅目 水龟虫 −0.370 (0.010)** −0.313 (0.030)* −0.057 (0.701) ** 在0.01水平(双侧)上显著相关;* 在0.05水平(双侧)上显著相关.
** Correlation was significant at P < 0.01; * Correlation was significant at P < 0.05. -
[1] TOMY G T, STEM G A, LOCKHART W L, et al. Occurrence of C10-C13 polychlorinated n-alkanes in Canadian midlatitude and arctic lake sediments [J]. Environmental Science & Technology, 1999, 33(17): 2858-2863. [2] 王亚韡, 王莹, 江桂斌. 短链氯化石蜡的分析方法、污染现状与毒性效应 [J]. 化学进展, 2017, 29(9): 919-929. doi: 10.7536/PC170504 WANG Y W, WANG Y, JIANG G B. Analytical methods, environmental pollutions and toxicity of short chain chlorinated paraffins [J]. Progress in Chemistry, 2017, 29(9): 919-929(in Chinese). doi: 10.7536/PC170504
[3] VAN MOURIK L M, GAUS C, LEONARDS P E G, et al. Chlorinated paraffins in the environment: A review on their production, fate, levels and trends between 2010 and 2015 [J]. Chemosphere, 2016, 155: 415-428. doi: 10.1016/j.chemosphere.2016.04.037 [4] KRATSCHMER K, SCHACHTELE A, MALISCH R, et al. Chlorinated paraffins (CPs) in salmon sold in southern Germany: Concentrations, homologue patterns and relation to other persistent organic pollutants [J]. Chemosphere, 2019, 227: 630-637. doi: 10.1016/j.chemosphere.2019.04.016 [5] TOMY G T, FISK A T, WESTMORE J B, et al. Environmental chemistry and toxicology of polychlorinated n-alkanes [J]. Reviews of Environmental Contamination and Toxicology, 1998, 158: 53-128. [6] GLUGE J, SCHINKEL L, HUNGERBUHLER K, et al. Environmental risks of medium-chain chlorinated paraffins (MCCPs): A review [J]. Environmental Science & Technology, 2018, 52(12): 6743-6760. [7] WEI G L, LIANG X L, LI D Q, et al. Occurrence, fate and ecological risk of chlorinated paraffins in Asia: A review [J]. Environment International, 2016, 92-93: 373-387. doi: 10.1016/j.envint.2016.04.002 [8] BAYEN S, OBBARD J P, THOMAS G O. Chlorinated paraffins: a review of analysis and environmental occurrence [J]. Environment International, 2006, 32(7): 915-929. doi: 10.1016/j.envint.2006.05.009 [9] EVENSET A, CHRISTENSEN G N, KALLENBORN R. Selected chlorobornanes, polychlorinated naphthalenes and brominated flame retardants in Bjornoya (Bear Island) freshwater biota [J]. Environmental Pollution, 2005, 136(3): 419-430. doi: 10.1016/j.envpol.2005.01.018 [10] VAN PRAET N, COVACI A, TEUCHIES J, et al. Levels of persistent organic pollutants in larvae of the damselfly Ischnura elegans (Odonata, Coenagrionidae) from different ponds in Flanders, Belgium [J]. Science of the Total Environment, 2012, 423: 162-167. doi: 10.1016/j.scitotenv.2012.02.045 [11] KRAUS J M, WALTERS D M, WESNER J S, et al. Metamorphosis alters contaminants and chemical tracers in insects: implications for food webs [J]. Environmental Science & Technology, 2014, 48(18): 10957-10965. [12] 路风辉, 陈满英, 陈燕舞, 等. 电子垃圾拆解区氯化石蜡和多氯联苯的分布特征——以广东清远龙塘镇为例 [J]. 环境化学, 2015, 34(7): 1297-1303. doi: 10.7524/j.issn.0254-6108.2015.07.2014111103 LU F H, CHEN M Y, CHEN Y W, et al. Distribution of chlorinated paraffins and polychlorinated biphenyls in e-waste, residues and sediment from e-waste areas of Qingyuan [J]. Environmental Chemistry, 2015, 34(7): 1297-1303(in Chinese). doi: 10.7524/j.issn.0254-6108.2015.07.2014111103
[13] CHEM M Y, LUO X J, ZHANG X L, et al. Chlorinated paraffins in sediments from the Pearl River Delta, South China: spatial and temporal distributions and implication for processes [J]. Environmental Science & Technology, 2011, 45(23): 9936-9943. [14] LIU Y, LUO X J, HUANG L Q, et al. Bioaccumulation of persistent halogenated organic pollutants in insects: Common alterations to the pollutant pattern for different insects during metamorphosis [J]. Environmental Science & Technology, 2018, 52(9): 5145-5153. [15] SUN R X, LUO X J, TANG B, et al. Bioaccumulation of short chain chlorinated paraffins in a typical freshwater food web contaminated by e-waste in south china: Bioaccumulation factors, tissue distribution, and trophic transfer [J]. Environmental Pollution, 2017, 222: 165-174. doi: 10.1016/j.envpol.2016.12.060 [16] MA X D, ZHANG H J, WANG Z, et al. Bioaccumulation and trophic transfer of short chain chlorinated paraffins in a marine food web from Liaodong Bay, North China [J]. Environmental Science & Technology, 2014, 48(10): 5964-5971. [17] ZENG L X, LAM J C W, CHEN H, et al. Tracking dietary sources of short- and medium-chain chlorinated paraffins in marine mammals through a subtropical marine food web [J]. Environmental Science & Technology, 2017, 51(17): 9543-9552. [18] HOUDE M, MUIR D C, TOMY G T, et al. Bioaccumulation and trophic magnification of short-and medium-chain chlorinated paraffins in food webs from Lake Ontario and Lake Michigan [J]. Environmental Science & Technology, 2008, 42(10): 3893-3899. [19] WANG R H, GAO L R, ZHENG M H, et al. Short- and medium-chain chlorinated paraffins in aquatic foods from 18 Chinese provinces: Occurrence, spatial distributions, and risk assessment [J]. Science of The Total Environment, 2018, 615: 1199-1206. doi: 10.1016/j.scitotenv.2017.09.327 [20] CHEN C, MA X D, GUO W, et al. Congener specific distribution and bioaccumulation of short-chain chlorinated paraffins in Liao estuary [J]. Chinese Science Bulletin (Chinese Version), 2014, 59(7): 578-585. doi: 10.1360/972013-801 [21] XU C, ZHANG Q, GAO L R, et al. Spatial distributions and transport implications of short- and medium-chain chlorinated paraffins in soils and sediments from an e-waste dismantling area in China [J]. Science of The Total Environment, 2019, 649: 821-828. doi: 10.1016/j.scitotenv.2018.08.355 [22] 王迎军, 王亚韡, 江桂斌. 固相萃取法测定水中短链氯化石蜡 [J]. 分析化学, 2018, 46(7): 1102-1108. doi: 10.11895/j.issn.0253-3820.181008 WANG Y J, WANG Y W, JIANG G B. Determination of short chain chlorinated paraffins in water by solid-phase extraction [J]. Chinese Journal of Analytical Chemistry, 2018, 46(7): 1102-1108(in Chinese). doi: 10.11895/j.issn.0253-3820.181008
[23] ZENG L X, WANG T, WANG P, et al. Distribution and trophic transfer of short-chain chlorinated paraffins in an aquatic ecosystem receiving effluents from a sewage treatment plant [J]. Environmental Science & Technology, 2011, 45(13): 5529-5535. [24] NICHOLLS C R, ALLCHIN C R, and LAW R J. Levels of short and medium chain length polychlorinated n-alkanes in environmental samples from selected industrial areas in England and Wales [J]. Environmental Pollution, 2001, 114(3): 415-430. doi: 10.1016/S0269-7491(00)00230-X [25] IINO F, TAKASUGA T, SENTHILKUMAR K, et al. Risk assessment of short-chain chlorinated paraffins in Japan based on the first market basket study and species sensitivity distributions [J]. Environmental Science & Technology, 2005, 39(3): 859-866. [26] LI Q L, CHENG X H, CUI Y R, et al. Short- and medium-chain chlorinated paraffins in the Henan section of the Yellow River: Occurrences, fates, and fluxes [J]. Science of The Total Environment, 2018, 640-641: 1312-1319. doi: 10.1016/j.scitotenv.2018.05.344 [27] KOBETICOVA K and CEMY R. Ecotoxicity assessment of short- and medium-chain chlorinated paraffins used in polyvinyl-chloride products for construction industry [J]. Science of the Total Environment, 2018, 640-641: 523-528. doi: 10.1016/j.scitotenv.2018.05.300 [28] LI H J, BU D, Fu J J, et al. Trophic dilution of short-chain chlorinated paraffins in a plant-plateau pika-eagle food chain from the Tibetan Plateau [J]. Environmental Science & Technology, 2019, 53(16): 9472-9480. [29] ZHOU Y H, YIN G, DU X Y, et al. Short-chain chlorinated paraffins (SCCPs) in a freshwater food web from Dianshan Lake: Occurrence level, congener pattern and trophic transfer [J]. Science of The Total Environment, 2018, 615: 1010-1018. doi: 10.1016/j.scitotenv.2017.10.026 [30] 虞俊超, 王宝盛, 王亚韡, 等. 大连市海产品中短链氯化石蜡的含量与分布研究 [J]. 环境科学, 2014, 35(5): 1955-1961. YU J C, WANG B S, WANG Y W, et al. Levels and distribution of short chain chlorinated paraffins in seafood from Dalian, China [J]. Environmental Science, 2014, 35(5): 1955-1961(in Chinese).
[31] YUAN B, WANG T, ZHU N L, et al. Short chain chlorinated paraffins in mollusks from coastal waters in the Chinese Bohai Sea [J]. Environmental Science & Technology, 2012, 46(12): 6489-6496. [32] LABADIE P, BLASI C, LE MENACH K, et al. Evidence for the widespread occurrence of short- and medium-chain chlorinated paraffins in fish collected from the Rhone River basin (France) [J]. Chemosphere, 2019, 223: 232-239. doi: 10.1016/j.chemosphere.2019.02.069 [33] SABORIDO BASCONCILLO L, BACKUS S M, MCGOLDRICK D J, et al. Current status of short- and medium chain polychlorinated n-alkanes in top predatory fish across Canada [J]. Chemosphere, 2015, 127: 93-100. doi: 10.1016/j.chemosphere.2015.01.016 [34] HUANG H T, GAO L R, XIA D, et al. Bioaccumulation and biomagnification of short and medium chain polychlorinated paraffins in different species of fish from Liaodong Bay, North China [J]. Scientific Reports, 2017, 7(1): 10749. doi: 10.1038/s41598-017-06148-5 [35] RETH M, ZENCAK Z, and OEHME M. First study of congener group patterns and concentrations of short- and medium-chain chlorinated paraffins in fish from the North and Baltic Sea [J]. Chemosphere, 2005, 58(7): 847-854. doi: 10.1016/j.chemosphere.2004.09.036 [36] RETH M, CIRIC A, CHRISTENSEN G N, et al. Short- and medium-chain chlorinated paraffins in biota from the European Arctic - differences in homologue group patterns [J]. Science of the Total Environment, 2006, 367(1): 252-260. doi: 10.1016/j.scitotenv.2005.12.014 [37] ZENG L X, LAM J C, WANG Y W, et al. Temporal rrends and pattern changes of short- and medium-chain chlorinated paraffins in marine mammals from the South China sea over the past decade [J]. Environmental Science & Technology, 2015, 49(19): 11348-11355. [38] HUANG Y M, CHEN L G, JIANG G, et al. Bioaccumulation and biomagnification of short-chain chlorinated paraffins in marine organisms from the Pearl River Estuary, South China [J]. Science of the Total Environment, 2019, 671: 262-269. doi: 10.1016/j.scitotenv.2019.03.346 -



 下载:
下载: