-
醛酮类羰基化合物是环境中一类重要污染物[1-4],是我国及世界许多国家和地区重点监测的环境污染物之一。环境中醛酮类化合物一次污染源主要来源于工业排放、植被释放、香烟烟雾、烹饪油烟以及化石燃料和植物燃烧[5-9] 等。大气中VOCs的光氧化反应是醛酮类羰基化合物二次排放的主要来源[10]。在水[11-12]、土壤和沉积物[13-14],尤其是大气[15-16]等介质中均有不同程度的检出。
测定醛酮类羰基化合物主要有GC法[17]、GC/MS(/MS)法[7-18],毛细管电泳-紫外法[19]、HPLC法[13-15, 20]以及HPLC/MS法[12, 21]等。空气样品醛酮类化合物中最常用的方法是用涂敷2,4-二硝基苯肼(DNPH)的硅胶管采集,使目标物与DNPH在酸性介质中发生衍生化反应生成醛酮-DNPH腙衍生物,用反相液相色谱法分离,紫外检测器检测。
LC对比GC和GC/MS法的缺点是分辨率低、分析时间长、溶剂用量大,但GC或GC/MS只能分析样品在进样系统不发生分解且能产生较高不受干扰色谱或质谱峰的羰基化合物或羰基化合物衍生物,而大部分高沸点羰基化合物衍生物容易分解,使得GC或GC/MS分析的目标物种类受到很大限制。LC-MS(/MS)对比LC-UV主要优势在于其选择性和灵敏度,但LC法仪器成本低、方法稳定性好也是其不可被替代的优势。Ochs等[22-23] 建立了RRLC-APCI(-)-MS/MS法测定31种羰基化合物,并与RRLC-UV法的线性、相关系数、检测限和灵敏度进行了比较,RRLC-UV法灵敏度更高;RRLC-APCI(-)-MS/MS检测限为0.71—10.3 µg·L−1,略低于RRLC-UV法,但方法定性准确更高。Zurek等[24]对比了APCI和ESI正离子模式下测定羰基化合物与4-二甲氨基-6-(4-甲氧基-1-萘基)-1,3,5-三嗪-2-肼(DMNTH) 的Hantzsch衍生反应产物。随着高分辨质谱技术的发展,化合物定性准确性又有了大幅提高。孟志娟等[25] 将Orbitrap GC-MS用于农产品中70种农药残留的快速筛查分析,陈溪等[26]用UPLC-Q-Orbitrap MS对水中112种药品及个人护理品进行了筛查和定量测定,112 种PPCPs 的定量限可达 0.002—0. 8 µg·L−1,准确性和灵敏度大大提高。目前还未发现使用液相色谱/高分辨质谱分析醛酮类化合物的报道。
本论文深入研究了25种羰基化合物-DNPH衍生物的HPLC-UV、UPLC-ESI-MS/MS和UPLC-ESI-Q- Orbitrap MS的最佳分析条件,建立了各自对应分析方法,并对分析结果的分离度、分辨率、检出限、线性范围和定性准确性等分析特征进行了比较评价。使用3种方法分别对加标模拟样及天津市大气实际样品进行了分析测定,明确3种方法各自特点和适用范围,Orbitrap MS还可对样品中非靶标污染物进行准确识别,并可通过理化性质相似的已知浓度化合物响应因子比较,提供半定量结果,为复杂环境样品污染物精准识别和准确检测提供了有力技术支持。
-
高效液相色谱仪,带二极管阵列检测器(Agilent1260,美国Agilent公司);超高效液相色谱-串联质谱仪,带电喷雾离子源(Agilent 1290/6460,美国Agilent公司);超高效液相色谱-电喷雾离子源-四极杆/静电场轨道阱高分辨质谱仪(Ultimate 3000-Q Exactive Focuse,美国Thermo Fisher公司);N-EVAP111氮吹仪(Organomation公司);Milli-Q超纯水系统(美国Millipore公司);色谱柱1:Agilent ZORBAX Extend-C18,4.6 mm×250 mm,5 µm;色谱柱2 Agilent ZORBAX Eclipse Plus C18,2.1 mm×100 mm,1.8 μm (美国Agilent公司);色谱柱3:Thermo Accucore RP-MS,2.1 mm×100 mm,2.6 µm(美国Thermo公司);Cleanert DNPH-Silica醛酮气体采样管 (200 mg·3mL−1)及臭氧去除管(240 mg·3mL−1)(天津艾杰尔飞诺美公司);0.22 µm尼龙水相针式滤膜(美国Supelco公司)。
25种醛酮-DNPH标准溶液,15 mg·L−1(美国ChemTek公司),分别为甲醛、乙醛、丙烯醛、丙酮、糠醛、丙醛、巴豆醛/丁烯醛、丁酮、甲基丙烯醛、丁醛、苯甲醛、异戊醛、环己酮、戊二醛、戊醛、对,间,邻-甲基苯甲醛、甲基异丁基酮、己醛、2,5-二甲基苯甲醛、庚醛、辛醛、壬醛和癸醛的DNPH衍生物;16种醛酮类标准液1000 mg·L−1(包括甲醛、乙醛、丙烯醛、丙酮、丙醛、丁烯醛、丁酮、丁醛、苯甲醛、异戊醛、戊醛、对,间,邻-甲基苯甲醛、己醛、2,5-二甲基苯甲醛,CHEM SERVICE公司),其余9种醛酮为标准品,均为色谱纯 (DIKMA公司);2,4-二硝基苯肼(DNPH) (99.5%,百灵威公司);甲醇、乙腈(液相色谱纯,MERCK公司);超纯水,经Milli-Q超纯水器纯化得到。
-
分别取9种醛酮类标准品用乙腈配制成质量浓度为1000 mg·L−1的混合储备溶液,与16种醛酮类标准溶液一起,各取100 µL于10 mL容量瓶中,用乙腈定容,配制成10 mg·L−1的25种醛酮类混合标准工作溶液,低于4 ℃冰箱保存。
-
环境空气按照HJ 683—2014和EPA TO-11的规定采集样品,在真空泵的作用下,气体样品经除臭氧小柱去除臭氧后,用 DNPH柱吸附,样品中醛酮类化合物与DNPH发生衍生化反应。采样流量0.5 L·min−1,采气体积100 L。采样管用密封帽将两端管口封闭,放入避光密封袋中,低于4 ℃保存,时间不超过7 d。
-
用乙腈洗脱采样管,洗脱方向与采样时气流方向相反,将洗脱液收集于5 mL容量瓶中,用氮吹仪浓缩至≤0.5 mL(液相色谱法浓缩至≤1.0 mL),若存在未溶解的橘红色颗粒物,用少量乙腈溶解,乙腈含量应≤0.5 mL,用纯水定容至1.0 mL,混匀,经针头过滤器过滤,滤液收集于2 mL棕色样品瓶中,待测。
-
(1)色谱柱1;柱温:40 ℃;流动相:A相为纯水,B相为乙腈;检测波长:360 nm;进样量:10 μL。流速:1.0 mL·min−1。
(2)梯度洗脱程序:0—15 min,60%B; 15—23 min,60%—100%B;23—34 min,100%B;34—34.1 min,100%—60%B;34—36 min,60%B。
-
(1)色谱柱2;柱温:40 ℃;流动相 A:1 mmol·mol−1乙酸铵/水溶液;流动相B:乙腈;流速:0. 3 mL·min−1;进样量:10 µL。
(2)梯度洗脱程序:0—18 min,60%—70%B;18—23 min,70%—90%B;23—26 min,90%B;26—26.1 min,95%—60%B;26.1—30 min,60%B。
(3)质谱条件:离子源:ESI,负离子模式;干燥气流量:10 L·min−1,温度:300 ℃;毛细管电压:4000 V;监测方式:MRM(多反应监测)。优化后得到25种醛酮腙的MRM条件见表1。
-
(1)色谱柱3;柱温:40 ℃;流动相A:1 mmol·mol−1乙酸铵/水溶液;流动相B:乙腈;流速:0.3 mL·min−1;进样量:10 µL。
(2)梯度洗脱:0—5 min, 50% B; 5—15 min, 50%—60% B; 15—20 min, 60%—70% B; 20—24 min, 70%—80% B; 24—25 min, 80%—95% B; 25—28 min, 95% B; 28—28.1 min, 95%—50% B; 28.1—30 min, 50% B。
(3)离子化模式:HESI电离源;扫描模式:负离子模式;毛细管温度: 325 ℃;加热温度: 350 ℃;鞘气: N2,流速 40 arb;辅助气: N2,流速10 arb;喷雾电压:2800 V; 透镜电压:60 V。
(4)扫描模式:全扫描/数据依赖二级(Full MS/dd-MS2)用于筛查、定性和定量,Full MS参数:分辨率 70000,自动增益控制目标(AGC target) 1×106,最大驻留时间(maximum IT) 100 ms,扫描范围 m/z50—600;dd-MS2参数:分辨率17500,AGC target2×105,最大驻留时间60 ms,动态排除8.0 s;NCE/Stepped NCE 25、45、65,Loop count 3,Isolation window 1.0 m/z。
-
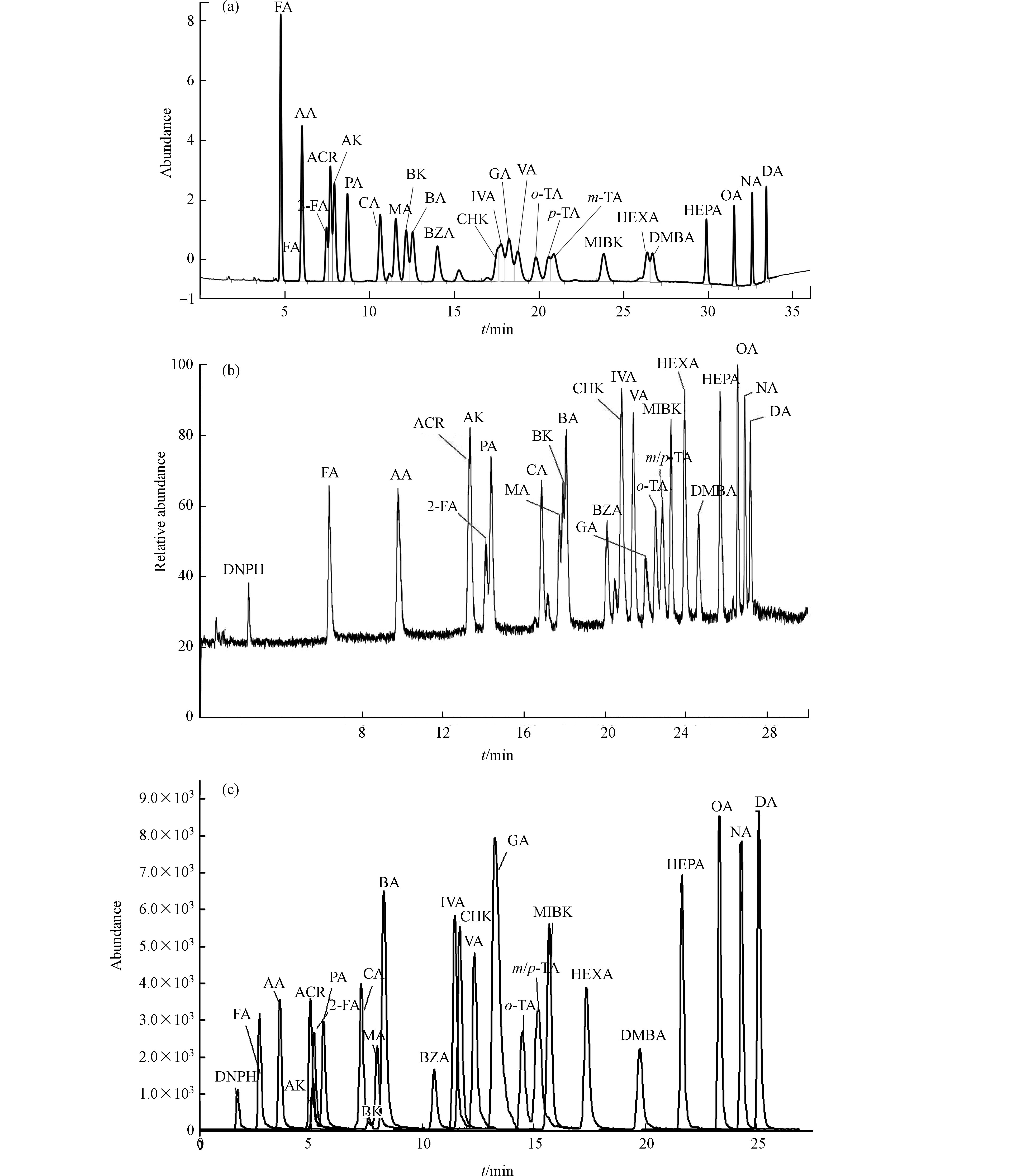
对25种浓度为150 µg·L−1羰基化合物-DNPH混合标准溶液用3种分析仪器在优化的质谱条件下分别进行分离测定,通过优化各自的最佳流动相梯度,各自分别考察了柱1:Agilent ZORBAX Extend-C18、柱2:Agilent ZORBAX Eclipse Plus C18和柱3:Thermo Accucore RP-MS的分离效果。25种目标物中含丙烯醛、丙酮、糠醛;环己酮、戊二醛、异戊醛、戊醛;邻,间,对-甲基苯甲醛等3组难分离物质。
UV法用化合物的特征紫外吸收波长以及其保留时间定性,大部分化合物最佳吸收波长均为360 nm,难分离物质需要单标辅助定性,否则容易造成目标峰识别错误,这对化合物分离的要求更高。通过调整流动相梯度,只有柱1能够实现所有化合物识别(对,间-甲基苯甲醛能够在峰顶分离,环己酮和戊二醛分离最差,仅能分辨出是两种物质接近共流出),其他两根色谱柱均完全不能实现这两组难分离物质对的识别,因此UV法选择柱1进行分析测定。
质谱法均为串联超高效液相色谱,色谱柱容量比常压液相色谱小,溶剂效应影响较大,因此待分析物质溶剂中有机相比例不能高于流动相的初始比例,否则容易出现色谱峰变形,使分离变差。柱2和柱3均为超高压液相色谱柱,适用于质谱的分析。通过优化各自最佳的分离条件,两根色谱柱在各自质谱仪上分辨率相差不大,最终MS/MS法选择柱2,Orbitrap MS法以柱3为分析柱。柱2和柱3除对,间-甲基苯甲醛不能实现分离外,其余化合物在30 min之内均可获得良好的分离度和尖锐对称的色谱峰形.
由于MS/MS法是以特征离子对和保留时间定性、特征子离子定量,Orbitrap MS法以保留时间和具有精确质量数的二级特征碎片离子定性、母离子定量,因此对,间-甲基苯甲醛合并定量外,其他保留时间接近化合物均可不受对方干扰,即可准确定性定量。具体色谱分析条件优化结果见1.5节,本研究3根色谱柱25种目标物出峰顺序略有不同,具体见图1—(a),(b),(c)。
-
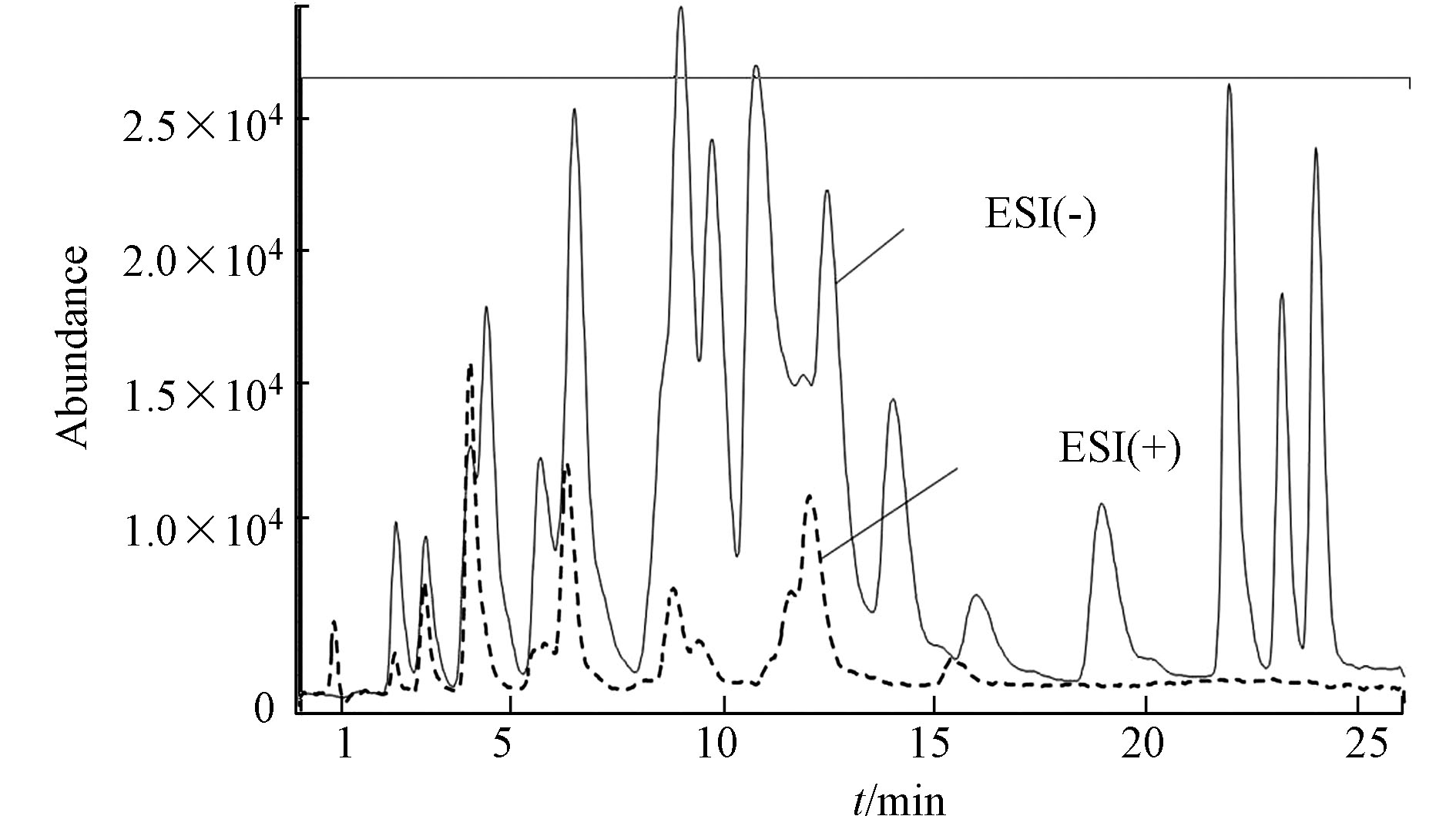
羰基化合物-DNPH结构使得ESI离子源采用正/负离子模式都有可能,通过负离子模式以甲醇−1 mmol/mol乙酸铵/水溶液为流动相,正离子模式以甲醇−0.1%甲酸/水溶液为流动相,在MS/MS法分别优化最佳子离子及最佳离子响应、最佳framgenger和CE值,Orbitrap MS优化了最佳NCE值条件下,测定各自ESI(+/−)模式下的分离度和响应值,结果发现在相同的分离情况下,负离子模式对大多数目标物具有更高的响应值。图2即为MS/MS法ESI(+/−)总离子流图的比较。Orbitrap MS ESI(+/−)母离子提取质谱图对比发现同样是ESI(−)比ESI(+)具有更高的响应值,更适合分析25种醛酮类羰基化合物。具体质谱分析优化条件结果见1.5节。
-
分别量取一定量的羰基化合物-DNPH标准溶液于1.0 mL棕色进样瓶中,用纯水定容,混匀。配制成质量浓度分别为0.45、0.75、1.50、3.00、4.50、7.50、15.0、30.0、45.0、75.0、150、300、450、750、1500 µg·L−1的标准系列,在各自优化的色谱和质谱条件下,UV以目标物色谱峰面积、MS/MS法以母离子对应的定量子离子峰面积、Orbitrap MS以母离子的提取色谱峰面积为纵坐标,以目标物的质量浓度为横坐标,绘制标准曲线,具体线性范围、相关系数测定结果见表2。由测定结果可见,3组曲线相关系数r均大于 0.990,UV法比MS/MS法线性范围更宽,UV法所有目标物在15—1500 µg·L−1内线性关系良好,而MS/MS法线性最高点仅为300 µg·L−1。Orbitrap MS法测定浓度最低,约为0.45—300 µg·L−1。方法检出限测定按照HJ168—2010规定的方法,采用向空白醛酮采样管中加标的方式(不同检测方法加标量分别为各化合物线性最低点对应的量,按采样量为100 L,定容体积为1.0 mL计算),测定7组模拟样品,按照样品测定方法进行测定,并计算标准偏差,以3.14倍标准偏差计算方法检出限,测定结果见表2。由表2可见,各目标物检出限 UV法略高于MS/MS法,分别在0.12—0.40 µg·m−3和0.08—0.60 µg·m−3之间,Orbitrap MS法最低,在1.1—13 ng·m−3之间,更适用于环境低浓度样品的测定。
-
向3个6组空白醛酮采样管口分别加入25种醛酮标准混合溶液,按照样品采集方法,用高纯氮气代替实际样品,模拟采集环境空气中醛、酮类化合物,连续采样 1 h,制成加标量分别为75 ng的模拟样品,按照样品测定方法进行测定,计算加标回收率平均值和相对标准偏差。具体测定结果见表3。25种目标物的UV法、MS/MS法和Orbitrap MS测得平均回收率范围分别为68.9%—98.8%,67.9%—97.6%和66.5%—107%,相对标准偏差范围分别为4.9%—10%,6.9%—18%和5.6%—11%,精密度和准确度均能够满足测定要求。
-
将所建立的方法应用于来自天津市区某采样点于2020年3月9日(样品1)和3月27日(样品2)采集的环境空气样品,采用相同的前处理方法,制备好的试样分别分成3份,使用不同分析仪器针对25种羰基化合物分别进行了定性和定量测定。
测定结果见根据测定结果可知,2个样品中除戊二醛未检出、样品2苯甲醛未检出外,Orbitrap法24种目标物均有检出,且其保留时间及一级母离子精确质量数测定值与理论值相差均<5×10−6,二级质谱5个碎片与筛查谱库离子碎片一致,测得24种目标物平均浓度在0.007—4.84 µg·m−3之间,其中浓度最高分别为丙酮、甲醛、乙醛、壬醛和己醛,浓度均高于1.00 µg·m−3;浓度最低依次是甲基苯甲醛、甲基丙烯醛、糠醛、丁烯醛和甲基异丁基酮。3种方法UV法和MS/MS法检出的化合物均远少于Orbitrap法,UV法检出最少。3种方法均能检出的目标物相对偏差均小于20%。
-
本研究通过优化流动相梯度、最佳离子化模式和离子对、最佳碰撞能量等色谱质谱条件,建立了分别用HPLC-UV、UPLC-ESI-MS/MS和UPLC-ESI-Q-Orbitrap MS法同时测定空气中25种醛酮类羰基化合物的分析方法并进行了比较。研究结果表明3种检测方法线性相关系数、精密度和准确度均良好,MS方法定性准确性高,检出限较UV法低,而Orbitrap MS法最低,可达ng·m−3水平。UV法适用于检测相对高浓度样品,线性范围最宽;MS/MS法适用于测定中等浓度样品;而Orbitrap MS法适用于环境中低浓度样品检测,同时通过分析碎片离子,根据裂解规律、母离子和子离子精确质量,还可扩展筛查其他可能存在的非靶标羰基化合物。3种方法应用于实际样品测定发现Orbitrap MS法能够准确定性定量测定的化合物种类最多,UV法最少,但3种法均能检出的目标物相对偏差均小于20%。3种方法各有侧重,相互补充,为环境醛酮类羰基化合物准确定性定量提供了完善的技术手段,同时为其他适用于液相色谱和液相色谱质谱测定的化合物方法建立提供了可靠的例证。
液相色谱和液相色谱质谱测定空气中25种醛酮类化合物
Determination of 25 aldehydes and ketones in air by HPLC and UHPLC-MS
-
摘要: 本研究建立了高效液相色谱-紫外检测法(HPLC-UV,UV)、超高效液相色谱-电喷雾离子源-串联四极杆质谱法(UPLC-ESI-MS/MS,MS/MS)和超高效液相色谱-电喷雾离子源-三重四极杆/静电场轨道阱高分辨质谱法(UPLC-ESI-Q-Orbitrap MS,Orbitrap MS)分别同时测定空气中25种醛酮类羰基化合物的分析方法。优化了流动相梯度、碎裂电压和碰撞能等参数。结果发现,3种方法标准曲线相关系数r均大于 0.990,UV法比MS/MS法线性范围更宽,在30—1500 µg·L−1内线性关系良好,而MS法线性最高点仅为300 µg·L−1内,Orbitrap MS法适合检测浓度最低,约为0.45—300 µg·L−1。方法检出限UV法略高于MS/MS法,分别在0.12—0.40 µg·m−3和0.08—0.60 µg·m−3之间,Orbitrap MS法最低,在1.1—13 ng·m−3之间。加标量为75 ng的模拟样品UV法、MS/MS法和Orbitrap MS测得平均回收率范围分别为68.9%—98.8%、67.9%—97.6%和66.5%—107%,相对标准偏差范围分别为4.9%—10%、6.9%—18%和5.6%—11%。3种方法检测实际样品,UV法和MS/MS法检出化合物分别为9种和14种,均远少于Orbitrap MS法的24种。3种方法均能检出的目标物测定结果相对偏差均小于20%,均可用于醛酮类羰基化合物检测,但MS法定性较UV法更加准确,UV法可以测定高浓度样品,MS/MS法适合测定中等浓度样品,而Orbitrap MS不仅适用于测定极低浓度样品,同时还可用于筛查非靶标目标物,为污染物识别和防治提供了更加有力的技术手段。Abstract: Three methods of qualitative and quantitative determination of 25 aldehydes and ketones in air by high performance liquid chromatography with ultraviolet detection (HPLC-UV), ultrahigh performance liquid chromatography with electrospray ion source and tandem quadrupole mass spectrometry (UPLC-ESI-MS/MS), and ultrahigh performance liquid chromatography with electrospray ion source and quadrupole/electrostatic field orbitrap high resolution mass spectrometry (UPLC-ESI-Q-Orbitrap MS) were developed.Some key test conditions like gradient of mobile phase, mode of positive and negative ions, fragmentation voltage, collision energy (CE) and Orbitrap MS screening library were optimized. The results showed that the regression coefficients of the calibration curves (r) were all greater than 0.990. The linear ranges of UV method were wider than those of MS/MS method, with the range of 30—1500 µg·L−1, while the highest linear point of MS method was only 300 µg·L−1. The liner ranges of Orbitrap MS method were about 0.45—300 µg·L−1. The method detection limits (MDL) of UV and MS/MS were between 0.12—0.40 µg·m−3 and 0.08—0.60 µg·m−3 respectively, while the Orbitrap MS was between 1.1—13 ng·m−3, which was the lowest of the three methods. The average 75 ng spiked recoveries for UV, MS/MS and orbitrap MS methods were between 68.9%—98.8%, 67.9%—97.6% and 66.5%—107%, and the relative standard deviations were 4.9%—10%, 6.9%—18% and 5.6%—11% respectively. The compounds numbers of field samples detected by UV method and MS/MS method were 9 and 14 respectively, far less than 24, which detected by Orbitrap MS method. The relative deviations of the test results for the targets detected out by all three methods were less than 20%. However, MS methods were more accurate than UV method. UV method could be used to determine high concentration samples, and MS/MS method was suitable for the determination of medium concentration samples. Furthermore Orbitrap MS method is not only suitable for determining very low concentration samples, but also for screening non-target objects, which provides a more powerful techniques for pollutants identification and control.
-
Key words:
- aldehydes and ketones /
- optimization and comparison /
- high performance liquid chromatography with ultraviolet detection (HPLC-UV) /
- ultrahigh performance liquid chromatography with electrospray ion source and tandem quadrupole mass spectrometry (UPLC-ESI-MS/MS) /
- ultrahigh performance liquid chromatography with electrospray ionization / quadrupole / electrostatic field orbitrap high resolution mass spectrometry (UPLC-ESI-Q-Orbitrap MS)
-

-
表 1 目标化合物的多反应监测条件
Table 1. MRM parameters for target compounds
序号
No.化合物缩写
Compound
abbreviation化合物
Compound分子式
Molecular
formula母离子
Precursor
ions(m/z)子离子
Product
ions(m/z)碎裂电压/V
Fragmenter碰撞能/eV
CE1 DNPH 2,4-二硝基苯肼
2,4-dinitrophenylhydrazineC6H6N4O4 197 137 60 8 167.1 4 2 FA 甲醛-DNPH
Formaldehyde-DNPHC7H6N4O4 209 163.1 100 0 151.1 0 3 AA 乙醛-DNPH
Acetaldehyde-DNPHC8H8N4O4 223 46.1 100 16 163.1 0 4 ACR 丙烯醛-DNPH
Acrolein-DNPHC9H8N4O4 235 158.2 100 4 163.1 28 5 PA 丙醛-DNPH
Propionaldehyde-DNPHC9H10N4O4 237.1 46.1 100 20 163.1 16 6 AK 丙酮-DNPH
Actone-DNPHC9H10N4O4 237.1 46.1 100 20 122.0 0 7 CA 巴豆醛/丁烯醛-DNPH
Crotonaldehyde-DNPHC10H10N4O4 249.1 172.1 100 4 46.2 12 8 MA 甲基丙烯醛-DNPH
Methacrolein-DNPHC10H10N4O4 249.1 46.2 100 12 172.1 4 9 BK 丁酮-DNPH
Butanone-DNPHC10H12N4O4 251.1 152.1 100 8 122.0 16 10 BA 丁醛-DNPH
Butylaldehyde-DNPHC10H12N4O4 251.1 152.1 100 8 122.0 16 11 IVA 异戊醛-DNPH
Isovaleraldehyde-DNPHC11H14N4O4 265.1 152.1 100 12 163.1 4 12 VA 戊醛-DNPH
Valeraldehyde-DNPHC11H14N4O4 265.1 152.1 100 12 163.1 4 13 2-FA 糠醛-DNPH
2-Furaldehyde-DNPHC11H8N4O5 275 46.1 100 12 228.0 4 14 CHK 环己酮-DNPH
Cyclohexanone-DNPHC12H14N4O4 277.1 247.2 100 4 231.1 12 15 HEXA 己醛-DNPH
Hexaldehyde-DNPHC12H16N4O4 279.1 152.0 100 12 122.0 28 16 MIBK 甲基异丁基酮-DNPH
Methyl Isobutyl Ketone-DNPHC12H16N4O4 279.1 152.0 100 12 122.0 28 17 BZA 苯甲醛-DNPH
Benzaldehyde-DNPHC13H10N4O4 285.1 46.2 100 20 163.1 20 18 HEPA 庚醛-DNPH
Heptaldehyde-DNPHC13H18N4O4 293.1 152.1 100 12 163.2 4 19 o-TA 邻-甲基苯甲醛-DNPH
o-Tolualdehyde-DNPHC14H12N4O4 299.1 162.9 100 8 252.1 8 20,21 p,m-TA 对,间-甲基苯甲醛-DNPH
p,m-Tolualdehyde-DNPHC14H12N4O4 299.1 162.9 100 8 252.1 8 22 OA 辛醛-DNPH
Octanal-DNPHC14H20N4O4 307.1 152.1 100 20 163.0 8 23 DMBA 2,5-二甲基苯甲醛
2,5-Dimethylbenzaldehyde-DNPHC15H14N4O4 313.1 181.0 140 20 163.0 20 24 NA 壬醛-DNPH
Nonanal-DNPHC15H22N4O4 321.1 152.0 140 20 46.1 56 25 DA 癸醛-DNPH
Decanal-DNPHC16H24N4O4 335.2 152.1 140 20 163.0 8 26 GA 戊二醛-DNPH
Glutaraldehyde-DNPHC17H16N8O8 459.1 182.1 140 12 179.0 8 表 2 不同方法测定25种醛酮类化合物线性方程、相关系数、方法检出限比较
Table 2. Comparison of linear equations, correlation Coefficients, method detection limits of 25 carbonyl derivatives by different methods
化合物compound HPLC-UV法 HPLC-ESI-MS/MS法 HPLC-ESI-Q-Orbitrap MS法 线性方程
Linear
equations线性范围/ (µg·L−1)
Linear range相关系数r 检出限/ (µg·m−3)
MDL线性方程
Linear
equations线性范围/(µg·L−1)
Linear range
相关
系数r检出限/ (µg·m−3)
MDL线性方程
Linear
equations线性范围/(µg·L−1)
Linear range相关
系数r检出限/ (ng·m−3)
MDLFA y=0.362x+0.807 3.0—1500 0.999 0.12 y=226x+55.6 15—300 0.999 0.15 y=7.43e6x+2.026e6 0.75—300 0.999 7.5 AA y=0.267x+0.670 7.5—1500 0.999 0.15 y=259x+130 7.5—300 0.997 0.08 y=1.0917x−3.0536 0.75—300 0.999 4.6 2-FA y=0.0981x+0.256 15—1500 0.999 0.25 y=143x+181 7.5—300 0.996 0.08 y=1.06e7x−6.78e6 0.75—300 0.999 6.5 ACR y=0.231x+0.679 15—1500 0.999 0.15 y=213x+80.2 7.5—300 0.994 0.08 y=1.47e6x−1.57e6 0.75—300 0.999 4.5 AK y=0.209x+0.486 15—1500 1.000 0.16 y=121x+176 7.5—300 0.995 0.08 y=6.87e6x−1.16e6 1.50—300 0.999 15 PA y=0.207x+0.524 15—1500 0.999 0.16 y=430x+545 7.5—300 0.997 0.08 y=1.16e7x−5.58e6 0.75—300 0.999 8.5 CA y=0.182x+0.365 15—1500 0.999 0.20 y=342x+396 7.5—300 0.997 0.08 y=1.53e7x−5.54e6 0.75—150 0.999 9.1 MA y=0.184x+0.722 15—1500 0.999 0.20 y=352x+290 7.5—300 0.999 0.08 y=1.51e7x−6.12e5 0.45—300 0.999 5.1 BK y=0.153x+0.338 15—1500 0.999 0.21 y=764x−10.6 7.5—300 0.994 0.08 y=7.04e6x−3.74e6 1.50—300 0.999 15 BA y=0.160x+0.388 15—1500 0.999 0.21 y=889x+109 7.5—300 0.998 0.08 y=1.47e7x−9.78e4 0.75—300 0.999 7.5 BZA y=0.125x+0.255 15—1500 0.999 0.25 y=165x+70.3 15—300 0.998 0.15 y=1.58e7x-4.47e6 0.45—75.0 0.999 4.5 CHK y=0.0911x+0.310 30—1500 0.999 0.28 y=191x+172 30—300 0.998 0.30 y=7.20e6x-2.61e6 0.45—150 0.999 3.8 IVA y=0.113x+1.16 30—1500 0.999 0.30 y=538x+109 7.5—300 0.998 0.08 y=3.14e7x−9.02e6 0.45—300 0.999 4.1 GA y=0.191x+1.10 30—1500 0.999 0.30 y=1939x1549 7.5—300 0.998 0.08 y=1.69e7x−2.16e6 1.50—75.0 0.999 15 VA y=0.119x+1.33 30—1500 0.999 0.35 y=798x+29.3 7.5—300 0.999 0.08 y=1.50e7x+1.90e6 0.45—300 0.999 4.5 o−TA y=0.105x+1.50 30—1500 0.999 0.38 y=325x+276 30—300 0.995 0.30 y=1.63e7x−6.86e6 0.75—75.0 0.999 5.5 p−TA y=0.0879x+0.496 30—1500 0.999 0.38 y=567x+283 60—600 0.997 0.60 y=2.66e7x+4.00e6 1.5—150 0.999 5.5 m−TA y=0.121x−0.0047 30—1500 0.999 0.38 MIBK y=0.120x+0.258 30—1500 0.999 0.38 y=838x+346 7.5—300 0.998 0.08 y=1.09e7x−6.70e6 0.45—300 0.999 4.5 DMBA y=0.102x+0.927 30—1500 0.999 0.40 y=278x+266 30—300 0.998 0.30 y=1.48e7x−4.44e6 0.45—75.0 0.999 3.5 HEXA y=0.0951x+0.241 30—1500 0.999 0.40 y=521x+266 15—300 0.993 0.15 y=1.58e7x+6.15e6 0.45—300 0.999 3.8 HEPA y=0.108x+0.378 15—1500 0.999 0.20 y=509x+111 7.5—300 0.997 0.08 y=1.53e7x+2.39e6 0.45—300 0.999 2.5 OA y=0.0954x+0.192 15—1500 0.999 0.20 y=553x+738 7.5—300 0.996 0.08 y=1.74e7x−4.66e6 0.45—75.0 0.999 1.1 NA y=0.0897x+0.117 15—1500 1.000 0.21 y=638x+116 7.5—300 0.994 0.08 y=1.81e7x−2.58e6 0.45—75.0 0.999 1.1 DA y=0.0840x+0.709 15—1500 0.999 0.21 y=854x+308 7.5—300 0.997 0.08 y=1.82e7x+2.24e6 0.45—75.0 0.999 1.1 表 3 UV法、MS/MS法和Orbitrap法精密度、准确度和实际样品测定结果比较
Table 3. Comparison of the accuracy, precision and real sample test results by UV, MS/MS and Orbitrap methods respectively
化合物
Compound加标样/% Spiked sample(n=6) 样品1/(µg·m−3) Sample 1 样品2/(µg·m−3) Sample 2 UV MS/MS Orbitrap MS UV MS/MS Orbitrap MS UV MS/MS Orbitrap MS 回收率
RecoveryRSD 回收率
RecoveryRSD 回收率
RecoveryRSD FA 94.7 10.4 90.3 12 97.9 7.1 2.31 2.06 2.25 2.45 2.70 2.56 AA 98.8 7.3 90.7 8.1 107 8.9 2.05 1.78 1.96 1.80 1.76 1.92 2-FA 83.6 7.1 86.1 7.9 88.5 5.6 ND ND 0.052 ND ND 0.017 ACR 75.8 7.5 67.9 6.9 74.1 6.2 ND 0.146 0.136 ND ND 0.049 AK 81.3 7.4 70.3 12 74.5 11 3.27 3.13 3.80 2.78 2.67 2.84 PA 90.9 5.6 89.5 13 94.5 7.1 0.33 0.25 0.300 0.25 0.22 0.229 CA 84.1 5.9 78.7 8.8 81.5 8.9 ND ND 0.10 ND ND 0.013 MA 85.3 6.6 80.2 9.1 83.3 7.8 ND ND 0.033 ND ND 0.016 BK 77.4 9.7 78.6 15 66.5 6.7 0.64 0.702 0.656 0.35 0.41 0.364 BA 68.9 5.5 75.6 8.2 85.3 7.9 0.41 0.468 0.477 0.22 0.26 0.235 BZA 91.1 5.6 92.5 9.6 97.4 9.7 ND ND 0.114 ND ND 0.043 CHK 75.6 8.8 70.7 14 86.7 8.2 ND 0.46 0.048 ND ND 0.040 IVA 80.9 7.4 78.9 17 94.8 9.9 ND 0.12 0.108 ND ND 0.027 GA 71.7 9.8 72.5 18 77.1 11 ND ND ND ND ND ND VA 79.3 5.8 75.1 12 99.4 7.5 ND 0.33 0.274 ND 0.12 0.117 o−TA 79.3 4.9 80.1 11 98.4 8.1 ND ND 0.015 ND ND ND p−TA 92.9 7.6 97.6 12 101 8.7 ND ND 0.009 ND ND ND m−TA 90.5 6.7 ND ND MIBK 82.2 5.4 78.4 8.8 77.7 6.2 ND ND 0.040 ND ND 0.015 DMBA 90.8 7.1 91.2 16 93.2 8.6 ND ND 0.024 ND ND 0.007 HEXA 84.8 7.6 83.4 8.8 79.4 9.2 ND 0.40 0.334 0.50 0.63 0.536 HEPA 86.5 7.3 78.3 11 81.0 7.8 ND 0.16 0.192 0.31 0.29 0.322 OA 93.2 8.4 87.8 9.3 90.9 5.7 0.47 0.54 0.518 0.83 0.78 0.895 NA 90.9 6.7 88.7 10 86.4 9.1 2.29 2.12 2.40 4.45 4.37 4.84 DA 93.4 7.9 92.2 15 90.4 7.7 0.43 0.31 0.336 0.77 0.71 0.820 注:ND., 未检出.
ND., not detected. -
[1] O’BRIEN P J, SIRAKI A G, SHANGARI N. Aldehyde sources, metabolism, molecular toxicity mechanisms, and possible effects on human health [J]. Crit Rev Toxicol, 2005, 35: 609-662. doi: 10.1080/10408440591002183 [2] SKYBOVA M, LENICEK J, RYCHTECKA A N, et al. Determination of volatile organic compounds in the atmosphere and their influence on ozone formation [J]. Fresenius Environmental Bulletin, 2006, 15(12): 1616-1623. [3] ATKINSON R. Gas-phase tropospheric chemistry of organic compounds: A review [J]. Atmos Environ, 1990, 24A: 1-41. [4] GARAYCOECHEA J I, CROSSAN G P, LANGEVIN F, et al. Alcohol and endogenous aldehydes damage chromosomes and mutate stem cells [J]. Nature, 2018, 553: 171-177. doi: 10.1038/nature25154 [5] PANG X, LEE X. Temporal variations of atmospheric carbonyls in urban ambient air and street canyons of a Mountainous city in Southwest China [J]. Atmospheric Environment, 2010, 44: 2098-2106. doi: 10.1016/j.atmosenv.2010.03.006 [6] HUANG J A, FENG Y L, FU J M, et al. A method of detecting carbonyl compounds in tree leaves in China [J]. Environmental Science and Pollution Research, 2010, 17: 1129-1136. doi: 10.1007/s11356-009-0277-3 [7] 崔婷惠, 李恒, 唐石云, 等. 超临界流体色谱与气相色谱-质谱联用测定卷烟主流烟气中醛酮类化合物的研究 [J]. 分析测试学报, 2018, 39(7): 766-771. doi: 10.3969/j.issn.1004-4957.2018.07.002 CUI T H, LI H, TANG S Y, et al. Determination of aldehydes and ketones compounds in mainstream cigarette smoke by supercritical fluid chromatography and gas chromatography-mass spectrometry [J]. Journal of Instrumental Analysis, 2018, 39(7): 766-771(in Chinese). doi: 10.3969/j.issn.1004-4957.2018.07.002
[8] 史纯珍, 姜锡, 姚志良, 等. 烹饪油烟羰基化合物排放特征 [J]. 环境工程学报, 2015, 9(3): 1376-1380. doi: 10.12030/j.cjee.20150364 SHI C Z, JIANG X, YAO Z L, et al. Carbonyl compounds emission characteristics in cooking fumes [J]. Chinese Journal of Environmental Engineering, 2015, 9(3): 1376-1380(in Chinese). doi: 10.12030/j.cjee.20150364
[9] KIM K H, CHOI B, PARK S, et al. Emission characteristics of compression ignition (CI) engine using diesel blended with hydrated butanol [J]. Fuel, 2019, 257(1): 1-9. [10] LI J, CHEN J Y, JI Y M, et al. Solar light induced transformation mechanism of allyl alcohol to monocarbonyl and dicarbonyl compounds on different TiO2: A combined experimental and theoretical investigation [J]. Chemosphere, 2019, 232(9): 287-295. [11] SUKHAREV S, MARIYCHUK R, ONYSKO M, et al. Fast determination of total aldehydes in rainwaters in the presence of interfering compounds [J]. Environmental Chemistry Letters, 2019, 17(3): 1405-1411. doi: 10.1007/s10311-019-00875-z [12] ZWIENER C, FRIMMEL F H. LC-MS analysis in the aquatic environment and in water treatment technology–a critical review [J]. Analytical and Bioanalytical Chemistry, 2004, 378: 862-874. doi: 10.1007/s00216-003-2412-1 [13] 崔连喜, 李利荣, 吴宇峰, 等. 液相色谱法测定土壤和沉积物中15种醛酮类化合物 [J]. 理化检验:化学分册, 2018, 54(4): 484-487. CUI L X, LI L R, WU Y F, et al. LC determination of 15 carbonyl compounds in soil and sediment [J]. Physical Testing and Chemical Analysis part B:Chemical Analysis, 2018, 54(4): 484-487(in Chinese).
[14] 李利荣, 关玉春, 吴宇峰, 等. 2, 4-二硝基苯肼衍生-固相萃取-液相色谱法测定环境固体基质中15种醛酮类羰基化合物的含量 [J]. 理化检验:化学分册, 2018, 54(7): 841-846. LI L R, GUAN Y C, WU Y F, et al. Determination of 15 aldehyde and ketone carbonyl compounds in environmental solid matrix by liquid chromatography with 2, 4-dinitrophenylhydrazine derivatization and solid phase extraction [J]. Physical Testing and Chemical Analysis part B:Chemical Analysis, 2018, 54(7): 841-846(in Chinese).
[15] QIAN X, SHEN H Q, CHEN Z M. Characterizing summer and winter carbonyl compounds in Beijing atmosphere [J]. Atmospheric Environment, 2019, 214: 116845. doi: 10.1016/j.atmosenv.2019.116845 [16] 景盛翱. 上海市典型区域大气羰基化合物水平研究 [J]. 环境污染与防治, 2017, 39(7): 713-716. SHENG J A. Study on the level of ambient carbonyl compounds in typical regions of Shanghai [J]. Environmental Pollution & Control, 2017, 39(7): 713-716(in Chinese).
[17] MANDIC A I, SEDEJ I J, SAKAC M B, et al. Static headspace gas chromatographic method for aldehyde determination in crackers [J]. Food Analytical Methods, 2013, 6(1): 61-68. doi: 10.1007/s12161-012-9415-5 [18] CALEJO I, MOREIRA N, ARAUJO A M, et al. Optimization and validation of a HS-SPME-GC-IT/MS method for analysis of carbonyl volatile compounds as biomarkers in human urine: Application in a pilot study to discriminate individuals with smoking habits [J]. Talanta, 2016, 148: 486-493. doi: 10.1016/j.talanta.2015.09.070 [19] PEREIRA E A, REZENDE M O O, TAVARES M F M. Analysis of low molecular weight aldehydes in air samples by capillary electrophoresis after derivatization with 4-hydrazinobenzoic acid [J]. J Sep Sci, 2004, 27(1-2): 28-32. doi: 10.1002/jssc.200301665 [20] GUO S J, CHEN M, TAN J H. Seasonal and diurnal characteristics of atmospheric carbonyls in Nanning, China [J]. Atmospheric Research, 2016, 169: 46-53. doi: 10.1016/j.atmosres.2015.09.028 [21] TIE C, HU T, JIA Z X, Zhang J L. Derivatization strategy for the comprehensive characterization of endogenous fatty aldehydes using HPLC-multiple reaction monitoring [J]. Anal Chem, 2016, 88: 7762-7768. doi: 10.1021/acs.analchem.6b01756 [22] OCHS S M, FASCIOTTI M, BARRETO R P, et al. Optimization and comparison of HPLC and RRLC conditions for the analysis of carbonyl-DNPH derivatives [J]. Talanta, 2010, 81(1-2): 521-529. doi: 10.1016/j.talanta.2009.12.036 [23] OCHS S M, FASCIOTTI M, NETTO A D P. Analysis of 31hydrazones of carbonyl compounds by RRLC-UV and TTLC-MS(/MS): A comparison of methods [J]. Journal of Spectroscopy, 2015(6b): 1-11. [24] ZUREK G, KARST U. Liquid chromatography-mass spectrometry method for the determination of aldehydes derivatized by the hantzsch reaction [J]. J Chromatogr A, 1999, 864: 191-197. doi: 10.1016/S0021-9673(99)01041-9 [25] 孟志娟, 孙文毅, 赵丽敏, 等. 气相色谱-静电场轨道阱高分辨质谱快速筛查农产品中 70 种农药残留 [J]. 分析化学, 2019, 47(8): 1227-1234. MENG Z J, SUN W Y, ZHAO L M, et al. Rapid screening of 70 kinds of pesticide residues in agricultural products by gas chromatography-electrostatic field orbit trap high resolution mass spectrometry [J]. Chinese Journal of Analytical Chemistry, 2019, 47(8): 1227-1234(in Chinese).
[26] 陈溪, 吴慈, 王龙祥, 等. 基于超高效液相色谱-高分辨质谱技术的水中 112 种药品和个人护理用品的高通量筛查和定量方法 [J]. 色谱, 2019, 36(11): 1147-1157. CHEN X, WU C, WANG L X, et al. High-throughput screening and quantitative analysis method of 112 pharmaceutical and personal care products in water based on ultra-high performance liquid chromatography with high-resolution mass spectrometry [J]. Chinese Journal of Chromatography, 2019, 36(11): 1147-1157(in Chinese).
-



 下载:
下载: