-
近年来废水处理过程中成本控制越来越受到关注,为了在不增加运行成本的情况下获得更高的出水水质,同步硝化反硝化工艺(simultaneous nitrification and denitrification,SND)的研究受到学者们的青睐,目前国内外对于SND工艺的研究已取得较好效果[1-3]。研究发现,SND的效率取决于3个因素:氧浓度、絮体大小和足够的有机碳基质的可用性[4-5]。Third等[1,6]基于SBR系统,通过控制溶解氧实现了SND,研究认为低溶解氧浓度(<2 mg·L−1)和大絮体是最佳SND的必要条件;Holman等[7]采用两组SBR系统,研究认为增加碳源浓度(100—1000 mg·L−1)有利于SND的实现;张可方等[8]研究发现,C/N为3.3—10时,C/N越高出水硝氮浓度越低,SND效果越好;同时刘军等[9]在序批式活性污泥反应器内,以模拟的城市污水为处理对象,研究认为DO=1.60—1.80 mg·L−1,COD/
${\rm{NH}}_4^{+} $ -N=6.5或BOD5/${\rm{NH}}_4^{+} $ -N=4时,TN的去除率分别达到最大;此外有学者采用侧沟式一体化OCO反应器,研究发现C/N约为8时SND速率最高,此时TN去除率达到最高(82%),当C/N小于8或大于8时SND率都呈现出下降趋势[10]。Iannaconea等[11]采用移动床膜生物反应器(C/N为2.7、4.2和5.6;HRT为2 d与1 d;溶解氧浓度,1.0 mg·L−1)研究认为,C/N为4.2时,总无机氮去除率达到68%,而C/N分别为2.7和5.6时,脱氮效率较低且不稳定;以及Zhou等[12]研究认为,降低C/N(20、10和5),TN的去除效率表现为降低的趋势,同时发现改变C/N将导致具有脱氮除磷功能的微生物种类有较大变化。由于SND的形成原因较为复杂,对于SND的机理性研究还应进一步深入,特别是微生物学的研究,现已发现的好氧反硝化菌约50多个属,130多个种[13],其中普遍存在的好氧反硝化菌为产碱杆菌属(Alcaligenes)[14-15]、Diaphorobacter[16]、不动杆菌属(Acinetobacter)[17-18]、气单胞菌属(Aeromonas)[19]、假单胞菌属(Pseudomonas)[20-21]、克雷伯氏菌(Klebsiella)[22]、根瘤菌属(Rhizobium)[23]、芽孢杆菌属(Bacillus)[24]、红球菌属(Rhodococcus)[25]和农杆菌属(Agrobacterium)[26]等,这就使得硝化和反硝化在同一反应器、同一条件下进行成为可能。
关于C/N对SND工艺微生物多样性影响的研究较少,同时基于SBR系统易实现SND现象,因此本文基于4种C/N(0、5、10和15)系统,考察了C/N对SND的影响,同时采用高通量测序技术探究了C/N对SBR活性污泥系统微生物群落分布的影响。
-
SBR反应器由有机玻璃制成,有效容积为5 L,实验用水采用人工模拟污水,无水乙醇配300 mg·L−1 COD,NH4Cl配20—60 mg·L−1
${\rm{NH}}_4^{+} $ -N,及KH2PO4配6 mg·L−1${\rm{PO}}_4^{3-} $ -P和微量元素浓缩液[27],每升废水加入1mL微量元素。活性污泥取自甘肃兰州市污水处理厂曝气池。接种污泥驯化20 d后,均分至4个SBR反应器,每个反应器初始活性污泥浓度为3100 mg·L−1。以化学需氧量(COD)与总氮(TN)的比值计算碳氮比(C/N),4个反应器的C/N分别为0、5、10和15,分别以R0、R5、R10和R15代表,其中R0系统内的碳源浓度为0。具体运行参数见表1。
-
${\rm{NH}}_4^{+} $ -N、${\rm{NO}}_3^{-} $ -N、${\rm{NO}}_2^{-} $ -N、COD、MLSS和MLVSS等常规分析项目均采用国家标准方法[28]。pH值、DO和温度(T)采用WTW-Multi3620测定仪监测。 -
采用Water DNA Isolation Kit 试剂盒(成都福际生物技术有限公司)对污泥样品中的细菌基因组DNA 进行提取,以TE缓冲液为空白对照。将活性污泥样品中提取到的基因组DNA,对16SrRNA V4 区进行扩增,通用引物序列为:F:520F:(5-AYTGGGYDTAAAGNG-3),R:802R:(5-TACNVGGGTATCTAATCC-3)。PCR 反应条件为:98℃预变性3 min;98℃变性30 s;50℃退火30 s;72℃延伸30 s后保温5 min。如此27个循环,提取后于4℃保存。委托上海派森诺生物科技有限公司进行Illumina MiSeq高通量测序。
-
SND率和SND反应速率用以表示在SBR系统好氧段的TN损失率和损失速率,其计算方法如下:
式中,
$ \Delta \rm{NH}_{4}^{+}{\text{、}}\Delta \mathrm{N}{O}_{2}^{-}{\text{和}}\Delta \mathrm{N}{O}_{3}^{-} $ 分别为系统$ \mathrm{N}{\mathrm{H}}_{4}^{+}{\text{、}}\mathrm{N}{\mathrm{O}}_{2}^{-}{\text{和}}\mathrm{N}{\mathrm{O}}_{3}^{-} $ 浓度的变化量,mg·L−1;MLSS为系统活性污泥浓度,g·L−1;T为硝化反应时间,min;SND反应速率 单位为mg · (gSS · d)−1 。 -
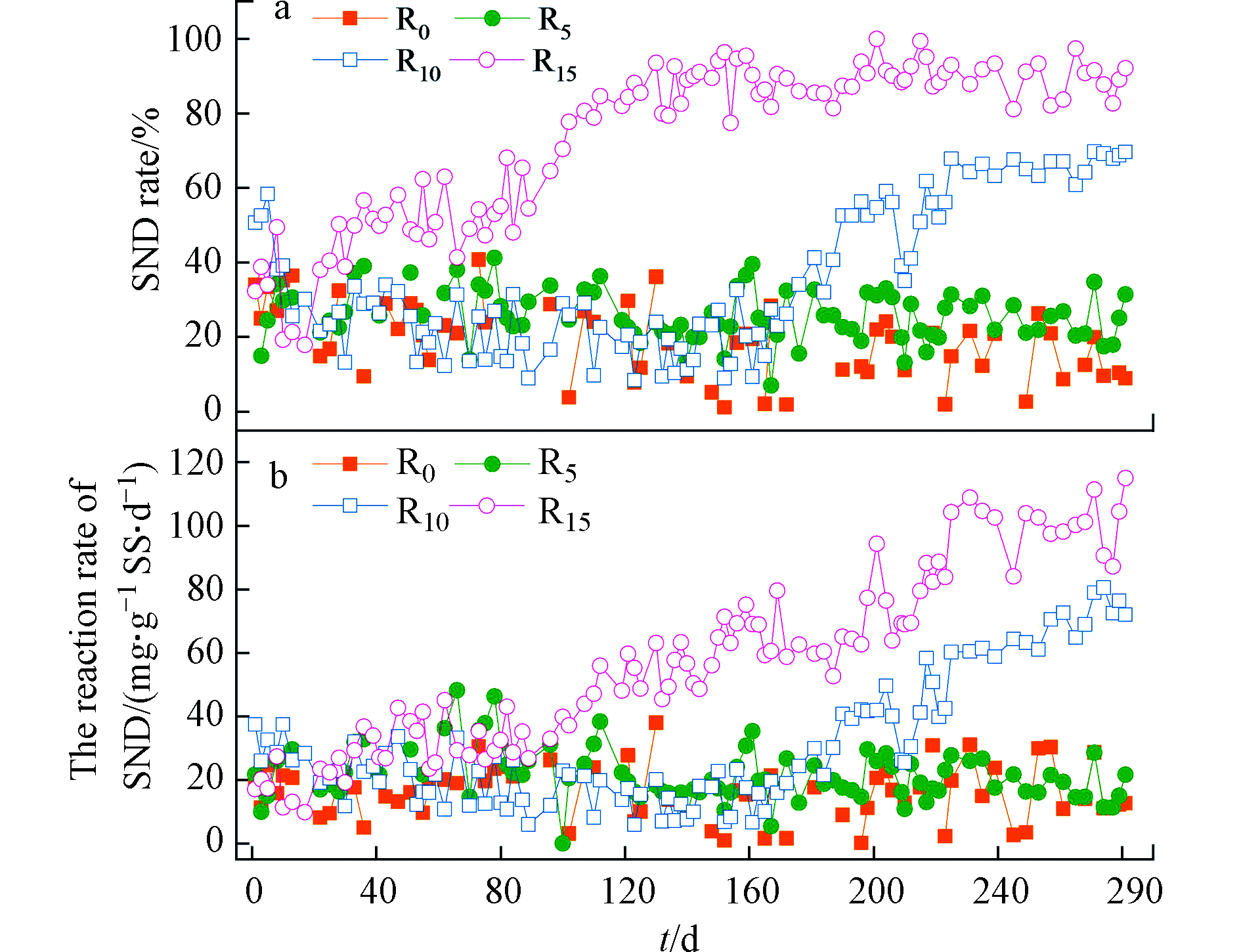
图1为4种C/N条件下SBR系统硝化结束时SND的变化规律(每天运行1周期,运行281 d),随着运行周期数的增加,R15系统SND率和反应速率显著增长,R10在第160周期后有明显的上升的趋势,而R0和R5系统SND率和反应速率无明显的变化,4种C/N系统在整个试验期间SND率均值分别为(19.2±9.8)%、(25.7±6.8)%、(35.0±19.4)%和(74.0±21.1)%(图1a);SND反应速率均值分别为(16.1±8.6)、(21.4±8.2)、(30.2±20.0)、(57.2±27.3)mg·(gSS·d)−1(图1b)。由上可得到随着C/N的升高,SBR系统SND率和反应速率显著升高,即高C/N有利于SND效能的实现及提高[29-30]。由图1(a)所示R10系统在第215周期后,有63.7%总氮去除归咎于SND,R15系统第120周期后SND率趋于稳定,有89%总氮去除归咎于SND。而Holman等[7]和Gogina等[31]以溶解氧为影响因素,研究认为有75%和85%总氮去除率是归咎于SND。
关于SND的解释,研究者认为(1)宏观环境理论:SND污水处理工艺优化主要是由于溶解氧在微生物絮体中的扩散受到限制,体系中微生物絮体的结构、溶解氧的含量及其扩散速率在很大程度上影响同步硝化反硝化的反应过程[32]。(2)微生物学理论:随着脱氮理论研究的不断深入,国内外研究者陆续发现了一些新型脱氮菌株,这些菌株在进行硝化反应同时,还可以进行反硝化[33]。(3)中间产物理论:好氧反硝化所呈现出的最大特征是好氧阶段总氮的损失(图1a),除以上两个方面理论解释外,目前有研究者认为硝化、反硝化过程均可以产生中间产物NO和N2O,而且其比例可高达氮去除率的10%以上,如果C/N较低,DO较低或SRT较小,都能导致N2O释放量增大[34]。
由于SND形成原因较为复杂,基于上述理论并结合本研究结果,低C/N条件下(C/N为0和5)SBR系统内TN并无明显的损失(无大量NO和N2O释放),同时由于絮凝体微缺氧区的形成往往会出现不稳定的现象,导致SND的处理效果会出现波动,而R10和R15系统均在反应的不同阶段实现了稳定的TN去除(图1),因此笔者推测本研究中SND现象主要是基于SBR系统内微生物絮体缺氧环境下的反硝化和系统内好氧反硝化细菌共同作用的结果。
-
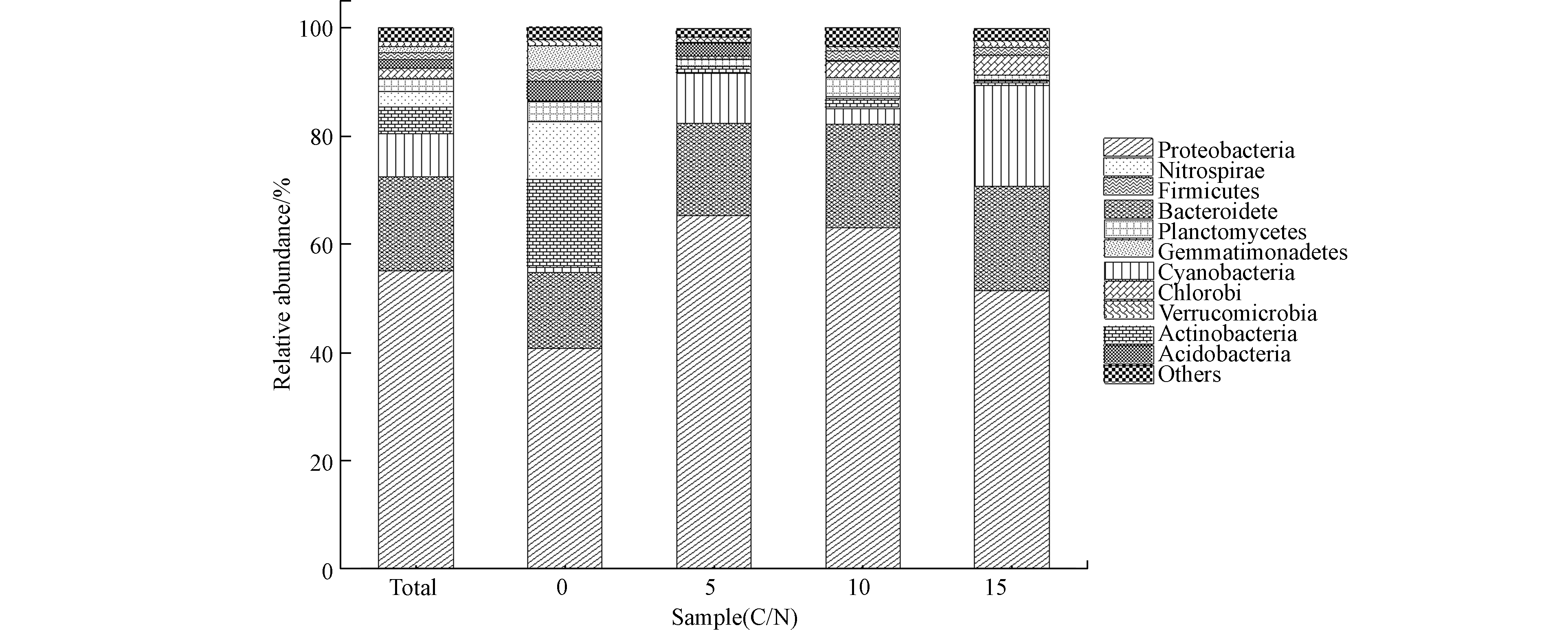
图2所示为4种C/N系统门水平微生物相对丰度,具有较高的多样性,达到19门。其中包括变形菌门(Proteobacteria,55.10%),拟杆菌门(Bacteroidetes,17.40%),蓝细菌门(Cyanobacteria,7.90%),疣微菌门(Verrucomicrobia,5.00%),硝化螺旋菌门(Nitrospirae,2.80%),浮霉状菌门(Planctomycetes,2.40%),绿菌门(Chlorobi,1.90%),放线菌门(Actinobacteria,1.60%),厚壁菌门(Firmicutes,1.30%),芽单胞菌门(Gemmatimonadetes,1.10%)和酸杆菌门(Acidobacteria,1.00%)。其中,变形门是4种C/N条件下活性污泥系统内的优势菌种,其中,变形门是4种C/N条件下活性污泥系统内的优势菌种,这与Zhang等[35]和Sanapareddy等[36]研究现象相同,同时硝化螺旋菌门是污水处理厂中执行亚硝酸盐氧化功能类群[37];此外其他相对丰度较低的微生物为绿弯菌门(Chloroflexi),OD1,装甲菌门(Armatimonadetes),衣原体(Chlamydiae),WPS-2,TM6,黏胶球形菌门(Lentisphaerae),螺旋体门(Spirochaetes),OP11,OP3软皮菌类(Tenericutes and Elusimicrobi)也在4种C/N系统内被检测到,相对丰度在0.01%—0.7%之间。
如图2所示,对于变形菌门,4种C/N系统内相对丰度分别为65.20%(R5),63.10%(R10),51.43%(R15)和40.67%(R0),4种SBR系统内其他主要的门分别为拟杆菌门(14.07%,17.23%,19.00%和19.30%),蓝细菌门(0.87%,9.13%,3.00%,和18.67%),疣微菌门(16.27%,1.30%,1.77%和0.83%)和硝化螺旋菌门(10.67%,0.10%,0.27%和0.10%)。同时,R15内的蓝细菌门分别是R5、R10、和R0的2,6和21倍;且在R0系统内,对于疣微菌门,分别是R10、R5和R15系统内的9,12和20倍,对于硝化螺旋菌门,分别是R10、R5、R15的40、106、106倍。此外,对于某些相对丰度较低的门,黏胶球形菌门只在R15系统内检测出,软皮菌类只在R10系统内被检测出,且仅仅只在R0和R10系统内检测出螺旋体门。
由上可得,不同C/N条件下,门水平微生物群落分布的相对丰度具有显著差异,因此C/N对活性污泥门水平微生物的种类具有显著影响,且C/N对硝化螺旋菌门影响尤为显著,同时可得C/N为0—15时,SBR活性污泥系统内优势菌种均是变形门。
-
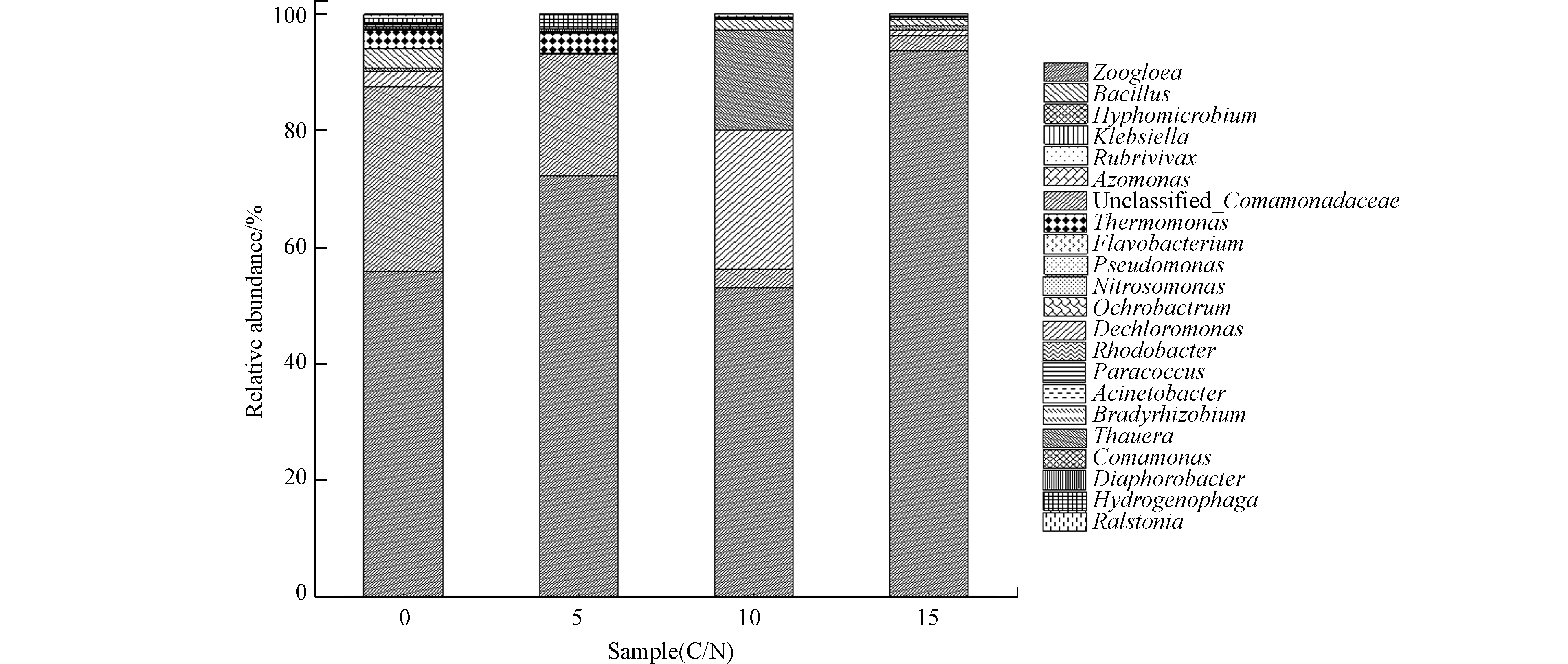
4种C/N条件下活性污泥微生物细菌群落结构在属分类水平上具有较高的多样性(图3),数量均达到112种以上,其中动胶菌属(Zoogloea)为优势菌属,相对丰度为30.80%,这是由于Zoogloea在整个运行期间种群数不断增长,同时对合成胞外聚合物与形成颗粒污泥起到重要促进作用[38],其他主要属包括Dechloromonas(3.10%),硝化螺旋菌属(Nitrospira,2.80%),陶厄氏菌属(Thauera,2.20%),Cryocola(1.20%),突柄杆菌属(Prosthecobacter,0.90%),独岛菌属(Dokdonella,0.90%),热单胞菌属(Thermomonas,0.70%),芽孢杆菌(Bacillus,0.60%),苯基杆菌(Phenylobacterium,0.50%),噬氢菌属Hydrogenophaga(0.40%),乳球菌属(Lactococcus,0.40%),Sediminibacterium(0.40%),浮霉状菌属(Planctomyces,0.40%),出芽菌属(Gemmata,0.30%),Salinibacterium(0.30%),藤黄色单胞菌属(Luteimonas,0.10%),侏囊菌属(Nannocystis,0.20%),红长命菌属(Rubrivivax,0.10%),丰佑菌属(Opitutus,0.20%)和其他相对丰度较低的属(4.10%)。其中Dechloromonas[39]和Thauera[40]具有反硝化功能,且Rubrivivax在光照下条件下具有脱氮的功能[41]。此外,未分类属的相对丰度为49.40%,其中unclassified_MLE1-12为主要的属,相对丰度为7.90%,且其他未分类的属分别属于丛毛单胞菌科(Comamonadaceae),Chitinophagaceae,腐螺旋菌科(Saprospiraceae)和丰佑菌科(Opitutaceae)。
如图3所示,不同C/N活性污泥系统内属水品微生物具有较大的差异性,对于Zoogloea,R5的相对丰度分别是R0、R10和R15的3、1.7、1倍;R10系统内Dechloromonas分别是R0和R15的17.7倍和48.6倍,R10系统内Thauera分别是R0和R15的25.9倍和24.3倍,而Dechloromonas和Thauera未在R5系统内检测到。此外R0系统内Nitrospira均是其他系统内的106倍,且Cryocola分别是R5和R10的2倍和45.5倍,而R15系统内并未监测出这类菌。
基于上述分析,不同C/N条件下,属水平微生物群落分布的相对丰度具有显著差异,因此C/N对活性污泥属水平微生物的种类具有显著影响,且C/N对Dechloromonas[39]、Thauera[40]、Nitrospira和Cryocola影响尤为显著,其中Dechloromonas、Thauera和Nitrospira在氮污染去除过程中发挥作用,即C/N对脱氮功能菌的影响较为显著。同时可得C/N为0—15时,SBR活性污泥系统内优势菌种均是动胶菌属。
-
不同C/N系统污泥样品中包含反硝化细菌的门和属呈现出非常高的多样性,本研究中检测到具有反硝化功能的微生物由Proteobacteria、Bacteroidetes和Firmicutes中的22个菌属所组成[42],包括Zoogloea,Unclassified_Comamonadaceae,Dechloromonas[39],Thauera[40],Bacillus,Thermomonas[43],Rhodobacter,Comamonas,Hyphomicrobium,Flavobacterium,Paracoccus,Diaphorobacter,Klebsiella,Pseudomonas,Acinetobacter,Hydrogenophaga,Rubrivivax,Nitrosomonas,Bradyrhizobium,Ralstonia,Azomonas和Ochrobactrum。而其中大部分包含反硝化菌的属在活性污泥法中的作用并未得到印证,结合国内外学者的研究成果,本研究SBR系统中的Bacillus[24],Diaphorobacter[16],Klebsiella[22],Pseudomonas[20-21]和Acinetobacter[17-18]为活性污泥中出现频率较高的好氧反硝化菌。
表2所示,本研究中不同C/N条件下SBR系统中包含反硝化细菌的属相对丰度均高于25%,C/N由0升高至5时,具有反硝化功能微生物的相对丰度明显的升高,即表明多数反硝化细菌需要有机碳作为电子供体。而在SBR系统内好氧反硝化菌相对丰度低于1%,即好氧反硝化菌在系统内微生物的占比较低,R0、R10和R15系统内相对优势好氧反硝化菌为Bacillus,而R5系统内相对优势好氧反硝化菌为Diaphorobacter。
不同C/N条件下包含反硝化细菌的属相对丰度存在一定差异性,本研究中Zoogloea为优势菌,其相对丰度为(52.9%—93.7%)(Zoogloea相对丰度与所有包含反硝化细菌的属相对丰度之和的比,下同),其中还包括Unclassified_Comamonadaceae(2.4%—31.8%),Dechloromonas(0.05%—23.9%),Thauera(0.03%—17.2%),Bacillus(0.1%—3.3%)和Thermomonas(0.01%—3.3%),此外其他相对丰度较低包含反硝化细菌的属相对丰度为(0.8%—3.2%)。
R0、R5、R10和R15系统中包含反硝化细菌的主要属为Zoogloea(55.7%、72.2%、52.9%和93.7%)和Unclassified_Comamonadaceae(31.8%、20.8%、3.1%和2.4%),此外R10系统中包含反硝化细菌的主要属还包括Dechloromonas(23.9%)和Thauera(17.2%)。
由上述可得,R0系统内包含反硝化细菌的属的相对丰度较低,即无外加碳源抑制了此类细菌的生长,但与其他提供碳源的系统相比其系统内好氧反硝化菌相对丰度相对较高(0.9%),这是由于R0系统内异养菌相对丰度较低,则好氧反硝化菌的相对丰度占比略微升高;R5系统内包含反硝化细菌的属相对丰度较高,但在本系统中R5系统并未实现完全的同步硝化反硝化现象,而在R10和R15系统内虽然包含反硝化细菌的属相对丰度与R5系统相比表现为略微下降的趋势,但均实现了稳定的SND(图1),这是由于在好氧反应阶段R5系统碳源不够充足,不足以实现SND,大部分竞争力较强的异养菌抑制了好氧反硝化细菌的生长(相对丰度为0.3%)[44-45],说明碳源是SND实现的限制性因素[46],且由表2可得R10和R15系统好氧反硝化菌相对丰度均高于R5系统,因此SND的实现由碳源、好氧反硝化菌和缺氧环境共同决定的,且碳源是SND实现的基础。同时有学者认为好氧反硝化菌更喜欢某种特定C/N[44-45],低碳浓度容易导致细菌能量缺乏从而降低细菌增殖及代谢能力,而高碳浓度可能会抑制细菌的生长,Chen等[26]表明好氧反硝化菌喜欢的特定C/N为9—10,这与本文中R15系统内好氧反硝化细菌相对丰度有略微降低的现象是一致的,而Joo等[47]研究认为好氧反硝化通常需要的最佳C/N约为5,不同与本研究结果。
此外由R5系统内包含反硝化细菌的属相对丰度较高(表2和图4),以及Zoogloea和Unclassified_Comamonadaceae(包含反硝化细菌的优势属)相对丰度在SBR系统中占比较高,但对SND的实现影响并不显著,则可推测本研究中SBR活性污泥系统内大部分包含反硝化细菌的属和以上两种优势属并未发挥反硝化的功能,Du等[38]在活性污泥系统内并未发现Zoogloea发挥脱氮的功能,而是对污泥的沉降性能具有一定影响。
-
(1)高C/N有利于SND效能的实现及提高。随着C/N的升高,SBR系统SND率(19.2%→25.7%→35.0%→74.0%)和SND反应速率[(16.1→21.4→30.2→57.2)mg·(gSS·d)−1]显著升高。R10系统在第215周期后有63.7%总氮去除归咎于SND,R15系统第120周期后SND率趋于稳定,有89%总氮去除归咎于SND。
(2)4种C/N系统门水平微生物具有较高的多样性,达到19门。其中主要包括变形菌门(55.10%),拟杆菌门(17.40%),蓝细菌门(7.90%),疣微菌门(5.00%)和硝化螺旋菌门(2.80%),其中变形门是活性污泥系统内的优势菌种,同时硝化螺旋菌门相对丰度分别为R0(10.67%)、R5(0.27%)、R10(0.10%)和R15(0.10%),R0系统内硝化螺旋菌门相对丰度分别是R10、R5和R15的40、106和106倍。
(3)4种C/N条件下活性污泥微生物细菌群落结构在属分类水平上具有较高的多样性,数量达到112种以上,其中动胶菌属为优势菌属。
(4)不同C/N系统污泥中包含反硝化菌的门和属呈现出非常高的多样性,由变形门、拟杆菌门和厚壁菌门中的22个菌属组成了SBR活性污泥系统包含反硝化菌的属;而SBR系统中的Zoogloea和Unclassified_Comamona-daceae(包含反硝化菌的优势属)相对丰度较高,但对SND的实现影响并不显著。
间歇式活性污泥法(SBR)系统碳氮比对同步硝化反硝化微生物群落分布及脱氮效能的影响
Effect of C/N ratio on the microbial community of simultaneous nitrification and denitrification (SND) and the biological nitrogen removal in sequencing batch reactor (SBR)
-
摘要: 同步硝化反硝化(simultaneous nitrification and denitrification,SND)是一种节能型废水处理工艺,但C/N对SBR系统SND及活性污泥微生物种群的影响机理尚不清楚。本试验以人工模拟废水为研究对象,采用4组不同碳氮比(C/N)系统(R0、R5、R10和R15)对比分析了C/N对SBR系统SND及活性污泥微生物种群的影响,并通过高通量测序测定了SBR活性污泥系统微生物多样性。结果表明,C/N与SND效能的实现及提高显著正相关,随着C/N的升高,SBR系统SND率(19.2%→25.7%→35.0%→74.0%)和SND反应速率[(16.1→21.4→30.2→57.2) mg·(gSS·d)−1]显著升高,且R10和R15系统分别在第215和120周期后,SND对总氮去除贡献率达到63.7%和89%。高通量测序结果表明,门水平微生物具有较高的多样性,达到19门,主要包括变形菌门(55.10%),拟杆菌门(17.40%),蓝细菌门(7.90%),疣微菌门(5.00%)和硝化螺旋菌门(2.80%),其中变形门是优势菌种,并且属水平上也具有较高的多样性,数量达到112种以上,其中动胶菌属为优势菌属,且R0系统内硝化螺旋菌属的相对丰度是R5、R10和R15的106倍。此外,SBR活性污泥系统中包含反硝化菌的门和属主要由变形门、拟杆菌门和厚壁菌门中的22个菌属所组成。Abstract: Simultaneous nitrification and denitrification (SND) is an energy-saving process of wastewater treatment, but the mechanism of C/N ratio effect on the SND and the microbial population of activated sludge in sequencing batch reactor (SBR) system are not clear. In this study, the long-term effect of C/N ratio on SND and bacterial community compositions of activated sludge in SBR system treating synthetic wastewater was investigated under four C/N ratio conditions (0, 5, 10, 15), besides Illumina high-throughput sequencing technology was applied to investigate microbial communities of SBR system. The results showed that the C/N ratio was significantly positively related to the realization and improvement of SND performance. With the increase of C/N ratio (0→5→10→15), SND rate(19.2%→25.7%→35.0%→74.0%) and SND reaction rate[(16.1→21.4→30.2→57.2) mg·(gSS·d)−1] observably increased, meanwhile, after cycles of 215 and 120, 63.7% and 89% of total nitrogen removal in R10 and R15 systems were attributed to SND. And the results of high-throughput sequencing technology showed that the level of phylum was diversities and richness and 19 phylums, which mainly included Proteobacteria (55.10%), Bacteroidetes (17.40%), Cyanobacteria (7.90%), Verrucomicrobia (5.00%) and Nitrospirae (2.80%), among which, the Proteobacteria was dominant phylum. Meanwhile, the level of genus was diversities and richness and 112 genera, among which Zoogloea was the dominant genus, besides the relative abundance of Nitrospira in R0 system was 106 times of R5, R10 and R15. In addition, the genus containing denitrifying bacteria in SBR system were mainly composed of 22 genera that belonged to the phylums of the Proteobacteria, Bacteroidetes and Firmicutes.
-

-
表 1 4个SBR反应器运行条件
Table 1. Operating conditions of four SBR reactors
反应器
React-ors周期时间/min
HRT进水/min
Influent
times曝气/min
Aeration
times沉淀排水/min
Sedimentation
and drainageCOD/
(mg·L−1)${\rm{NH}}_4^{+} $
(mg·L−1)MLSS/
(mg·L−1)T/℃ DO/
(mg·L−1)pH R0 300 5 270 25 0 20 2540 25±2.0 1.0—2.5 7.5±0.2 R5 340 5 300 35 300 60 3345 25±2.0 1.0—2.5 7.5±0.2 R10 330 5 270 55 300 30 3154 25±2.0 1.0—2.5 7.5±0.2 R15 270 5 210 55 300 20 2989 25±2.0 1.0—2.5 7.5±0.2 表 2 4个SBR系统内反硝化细菌的相对丰度
Table 2. The relative abundance of denitrifying bacterium communities of samples at genus level
反应器
Reactors包含反硝化细菌的属/%
The genus of denitrifying
bacterium好氧反硝化菌/%
Aerobic denitrifying
bacterium好氧反硝化菌/%
Aerobic denitrifying bacteriumBacillus Diaphorobacter Klebsiella Pseudomonas Acinetobacter R0 25.4 0.9 0.85 0.02 0.01 0.008 0.004 R5 59.5 0.3 0.07 0.23 0.003 0.003 0.007 R10 47.0 0.9 0.83 0.004 0.004 0.006 0 R15 44.1 0.6 0.54 0.004 0.006 0.004 0.004 -
[1] THIRD K A, GIBBS B, NEWLAND M, et al. Long-term aeration management for improved N-removal via SND in a sequencing batch reactor [J]. Water Research, 2005, 39(15): 3523-3530. doi: 10.1016/j.watres.2005.06.014 [2] 王建龙, 彭永臻, 王淑莹, 等. 复合生物反应器亚硝酸型同步硝化反硝化 [J]. 北京工业大学学报, 2007, 33(12): 1310-1314. WANG J L, PENG Y Z, WANG S Y, et al. Simultaneous nitrification and denitrification via nitrite in a sequence hybrid biological reactor [J]. Journal of Beijing University of Technology, 2007, 33(12): 1310-1314(in Chinese).
[3] CHAI H, XIANG Y, CHEN R, et al. Enhanced simultaneous nitrification and denitrification in treating low carbon-to-nitrogen ratio wastewater: treatment performance and nitrogen removal pathway [J]. Bioresource Technology, 2019, 280: 51-58. doi: 10.1016/j.biortech.2019.02.022 [4] KLANGDUEN P, KELLER JÜRG. Study of factors affecting simultaneous nitrification and denitrification (SND) [J]. Water Science and Technology, 1999, 39(6): 61-68. doi: 10.2166/wst.1999.0262 [5] KELLER J, SUBRAMANIAM K, GÖSSWEIN J, et al. Nutrient removal from industrial wastewater using single tank sequencing batch reactors [J]. Water Science and Technology, 1997, 35(6): 137-144. doi: 10.2166/wst.1997.0252 [6] THIRD K A, BURNETT N, CORD-RUWISCH R. Simultaneous nitrification and denitrification using stored substrate (PHP) as the electron donor in an SBR [J]. Biotechnology and Bioengineering, 2003, 83(6): 706-720. doi: 10.1002/bit.10708 [7] HOLMAN J B, WAREHAM D G. COD, ammonia and dissolved oxygen time profiles in the simultaneous nitrification/denitrification process [J]. Biochemical Engineering Journal, 2005, 22(2): 125-133. doi: 10.1016/j.bej.2004.09.001 [8] 张可方, 杜馨, 张朝升, 等. DO C/N对同步硝化反硝化影响的试验研究 [J]. 环境科学与技术, 2007, 30(6): 3-5. doi: 10.3969/j.issn.1003-6504.2007.06.002 ZHANG K F, DU X, ZHANG C S, et al. Influences of DO and C/N on Simultaneous Nitrification and Denitrification [J]. Environmental Science & Technology, 2007, 30(6): 3-5(in Chinese). doi: 10.3969/j.issn.1003-6504.2007.06.002
[9] 刘军, 潘登, 王斌, 等. SBR工艺中DO和C/N对同步硝化反硝化的影响[J]. 北京工商大学学报(自然科学版), 2003, 21(2): 7-10. LIU J, PAN D, WANG B, et al. Effect of DO and C/N on simultaneous nitrification and denitrification in SBR technique[J], Journal of Beijing Technology and Business University(Natural Science Edition)2003, 21(2): 7-10(in Chinese).
[10] 马志华, 李德豪, 周如金, 等. 侧沟式一体化OCO工艺中DO和C/N对同步硝化反硝化的影响 [J]. 环境工程学报, 2012, 6(5): 1513-1517. MA Z H, LI D H, ZHOU R J, et al. Influence of DO and C/N on SND by integral side-ditch OCO process [J]. Techniques and Equipment for Environmental Pollution Control, 2012, 6(5): 1513-1517(in Chinese).
[11] IANNACONEA F, CAPUAB F D, GRANATAA F, et al. Effect of carbon-to-nitrogen ratio on simultaneous nitrification denitrification and phosphorus removal in a microaerobic moving bed biofilm reactor [J]. Journal of Environmental Management, 2019, 250: 1-9. [12] ZHOU J, SUN Q. Performance and microbial characterization of aerobic granular sludge in a sequencing batch reactor performing simultaneous nitrification, denitrification and phosphorus removal with varying C/N ratios [J]. Bioprocess and Biosystems Engineering, 2020, 43(4): 663-672. doi: 10.1007/s00449-019-02264-w [13] 周石磊, 黄廷林, 白士远, 等. 贫营养好氧反硝化菌的分离鉴定及其脱氮特性[J]. 中国环境科学, 2016, 36(1): 238-248. ZHOU S L, HUANG T L, BAI S Y, et al. Isolation, Identification, and nitrogen removal characteristics of oligotrophic aerobic denitrifiers[J]. China Environmental Science, 2016, 36(1): 238-248(in Chinese).
[14] ZHAO B, TIAN M, AN Q, et al. Characteristics of a heterotrophic nitrogen removal bacterium and its potential application on treatment of ammonium-rich wastewater [J]. Bioresource Technology, 2017, 226: 46-54. doi: 10.1016/j.biortech.2016.11.120 [15] LIU Y X, WANG Y, LI Y, et al. Nitrogen removal characteristics of heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis C16 [J]. Chinese Journal of Chemical Engineering, 2015, 23(5): 827-834. doi: 10.1016/j.cjche.2014.04.005 [16] GE Q L, YUE X P, WANG G Y. Simultaneous heterotrophic nitrification and aerobic denitrification at high initial phenol concentration by isolated bacterium Diaphorobacter sp. PD-7 [J]. Chinese Journal of Chemical Engineering, 2015, 23(5): 835-841. doi: 10.1016/j.cjche.2015.02.001 [17] REN Y X, YANG L, LIANG X. The characteristics of a novel heterotrophic nitrifying and aerobic denitrifying bacterium, Acinetobacter junii YB [J]. Bioresource Technology, 2014, 171: 1-9. doi: 10.1016/j.biortech.2014.08.058 [18] HUANG X F, LI W G, ZHANG D Y, et al. Ammonium removal by a novel oligotrophic Acinetobacter sp. Y16 capable of heterotrophic nitrification-aerobic denitrification at low temperature [J]. Bioresource Technology, 2013, 146(10): 44-50. [19] CHEN M X, WANG W C, FENG Y, et al. Impact resistance of different factors on ammonia removal by heterotrophic nitrificationaerobic denitrification bacterium Aeromonas sp. HN-02 [J]. Bioresource Technology, 2014, 167: 456-461. doi: 10.1016/j.biortech.2014.06.001 [20] HE T X, LI Z L, SUN Q, et al. Heterotrophic nitrification and aerobic denitrification by Pseudomonas tolaasii Y-11 without nitrite accumulation during nitrogen conversion [J]. Bioresource Technology, 2016, 200(01): 493-499. [21] 张培玉, 曲洋, 于德爽, 等. 菌株qy37的异养硝化/好氧反硝化机制比较及氨氮加速降解特性研究 [J]. 环境科学, 2010, 31(8): 1819-1826. ZHANG P Y, QU Y, YU D S, et al. Comparison of heterotrophic nitrification and aerobic denitrification system by Strain qy37 and its accelerating removal characteristic of
${\rm{NH}}_4^{+} $ -N [J]. Chinese Journal of Environmental Science, 2010, 31(8): 1819-1826(in Chinese).[22] 孙庆花, 于德爽, 张培玉, 等. 1株海洋异养硝化–好氧反硝化菌的分离鉴定及其脱氮特性 [J]. 环境科学, 2016, 37(2): 647-654. SUN Q H, YU D S, ZHANG P Y, et al. Identification and nitrogen removal characteristics of a heterotrophic nitrification-aerobic denitrification strain isolated from marine environment [J]. Environmental Science, 2016, 37(2): 647-654(in Chinese).
[23] 肖继波, 江惠霞, 褚淑祎. 不同氮源下好氧反硝化菌Defluvibacter lusatiensis str. DN7的脱氮特性 [J]. 生态学报, 2012, 32(20): 6463-6470. doi: 10.5846/stxb201203090318 XIAO J B, JAING H X, ZHU S W. Denitrification characteristics of an aerobic denitrifying bacterium Defluvibacter lusatiensis str. DN7 using different sources of nitrogen [J]. Acta Ecologica Sinica, 2012, 32(20): 6463-6470(in Chinese). doi: 10.5846/stxb201203090318
[24] 赵惊鸿, 黄少斌. 一株耐高温好氧反硝化菌的筛选及特性研究 [J]. 环境科学与技术, 2015, 38(1): 6-10. ZHAO J H, HUANG S B. Isolation and characteristics of a thermophilic aerobic denitrifier [J]. Environmental Science & Technology, 2015, 38(1): 6-10(in Chinese).
[25] CHEN P, LI J, LI Q X, et al. Simultaneous heterotrophic nitrification and aerobic denitrification by bacterium Rhodococcus sp CPZ24 [J]. Bioresource Technology, 2012, 32(116): 266-270. [26] CHEN Q, NI J. Ammonium removal by Agrobacterium sp. LAD9 capable of heterotrophic nitrification-aerobic denitrification [J]. Journal of Bioscience and Bioengineering, 2012, 113(5): 619-623. doi: 10.1016/j.jbiosc.2011.12.012 [27] YE F X, PENG G, LI Y. Influences of influent carbon source on extracellular polymeric substances (EPS) and physicochemical properties of activated sludge [J]. Chemosphere, 2011, 84(9): 1250-1255. doi: 10.1016/j.chemosphere.2011.05.004 [28] 国家环境保护局. 水和废水监测分析方法[M]. 北京: 中国环境科学出版社, 2002. National Environmental Protection Agency. Water and wastewater monitoring and analysis methods [M]. Beijing: China Environmental Press, 2002(in Chinese).
[29] 孙洪伟, 郭英, 尤永军, 等. 不同碳氮比(C/N)条件下驯化微生物的反硝化特性 [J]. 环境化学, 2014, 33(5): 770-775. doi: 10.7524/j.issn.0254-6108.2014.05.001 SUN H W, GUO Y, YOU Y J, et al. Denitrification characteristic of microbial population tamed at different C/N ratios [J]. Environmental Chemistry, 2014, 33(5): 770-775(in Chinese). doi: 10.7524/j.issn.0254-6108.2014.05.001
[30] 柯国华, 汪苹, 杨志. 好氧反硝化脱氮气态中间产物的研究分析 [J]. 环境科学与技术, 2006, 29(7): 45-46. doi: 10.3969/j.issn.1003-6504.2006.07.018 KE G H, WANG P, YANG Z. Analysis of intermediates during aerobic denitrification [J]. Environmental Science & Technology, 2006, 29(7): 45-46(in Chinese). doi: 10.3969/j.issn.1003-6504.2006.07.018
[31] GOGINA E, GULSHIN I. Simultaneous nitrification and denitrification with low dissolved oxygen level and C/N ratio [J]. Procedia Engineering, 2016, 153: 189-194. doi: 10.1016/j.proeng.2016.08.101 [32] LUKASSE L J S, KEESMAN K J, KLAPWIJK A, et al. Optimal control of N-removal in ASPs [J]. Water Science and Technology, 1998, 38(3): 255-262. doi: 10.2166/wst.1998.0219 [33] HUANG G D, OU L M, PAN F, et al. Isolation of a novel heterotrophic nitrification-aerobic denitrification bacterium Serratia marcescens CL1502 from deep-sea sediment [J]. Environ-mental Engineering Science, 2017, 34(6): 453-459. doi: 10.1089/ees.2016.0363 [34] THAKUR I S, MEDHI K. Nitrification and denitrification processes for mitigation of nitrous oxide from waste water treatment plants for biovalorization: Challenges and opportunities [J]. Bioresource Technology, 2019, 282: 502-513. doi: 10.1016/j.biortech.2019.03.069 [35] ZHANG T, SHAO M, YE L. 454 pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants [J]. Isme Journal, 2012, 6(6): 1137-1147. doi: 10.1038/ismej.2011.188 [36] SANAPAREDDY N, HAMP T J, GONZALEZ L C, et al. Molecular diversity of a North Carolina wastewater treatment plant as revealed by pyrosequencing [J]. Applied and Environmental Mic-Robiology, 2009, 75(6): 1688-1696. doi: 10.1128/AEM.01210-08 [37] GILBERT E M, AGRAWAL S, BRUNNER F, et al. Response of different Nitrospira species to anoxic periods depends on operational do [J]. Environmental Science & Technology, 2014, 48(5): 2934-2941. [38] DU R, CAO S, LI X, et al. Efficient Partial-Denitrification/Anammox (PD/A) process through gas-mixing strategy: System evaluation and microbial analysis [J]. Bioresource Technology, 2020: 122675. [39] Horan N J. Biological Wastewater Treatment Systems[M]. New York: Marcel Dekker Inc, 1990. [40] ZHAO Y G, HUANG J, ZHAO H, et al. Microbial community and N removal of aerobic granular sludge at high COD and N loading rates [J]. Bioresource Technology, 2013, 143: 439-446. doi: 10.1016/j.biortech.2013.06.020 [41] 康鹏亮, 黄廷林, 张海涵, 等. 西安市典型景观水体水质及反硝化细菌种群结构 [J]. 环境科学, 2017, 38(12): 5174-5183. KANG P L, HUANG T L, ZHANG H H, et al. Water quality and diversity of denitrifier community structure of typical scenic water bodies in Xi'an [J]. Environmental Science, 2017, 38(12): 5174-5183(in Chinese).
[42] 郑平, 徐向阳, 胡宝兰. 新型生物脱氮理论与技术[M]. 北京: 科学出版社, 2004. ZHENG P, XU X Y, HU B L. Theory and technology of new biological nitrogen removal[M]. Beijing: Science Press, 2004(in Chinese).
[43] MECHICHI T, PATEL B K C, SAYADI S. Anaerobic degradation of methoxylated aromatic compounds by Clostridium methoxybenzovorans and a nitrate-reducing bacterium Thauera sp strain Cin3, 4 [J]. International Biodeterioration & Biodegradation, 2005, 56(4): 224-230. [44] HUANG H K, TSENG S K. Nitrate reduction by Citrobacter diversus under aerobic environment [J]. Applied Microbiology and Biotechnology, 2001, 55(1): 90-94. doi: 10.1007/s002530000363 [45] KIM M, JEONG S Y, YOON S J, et al. Aerobic denitrification of pseudomonas putida AD-21 at different C/N ratios [J]. Journal of Bioscience and Bioengineering, 2008, 106(5): 498-502. doi: 10.1263/jbb.106.498 [46] 王佳乐. 高盐废水强化多路径耦合脱氮技术及机理研究[D]. 重庆: 重庆大学, 2018. WANG J L. The multi-path coupled technologies and mechanisms in nitrogen removal process treating saline wastewater[D]. Chongqing: Chongqing University, 2018(in Chinese).
[47] JOO H S, HIRAI M, SHODA M. Characteristics of ammonium removal by heterotrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4 [J]. Journal of Bioscience and Bioengineering, 2005, 100(2): 184-191. doi: 10.1263/jbb.100.184 -



 下载:
下载: